Abstract
Background
Electrophysiological remodeling and increased susceptibility for cardiac arrhythmias are hallmarks of heart failure (HF). Ventricular action potential (AP) duration (APD) is typically prolonged in HF, with reduced repolarization reserve. However, underlying K+ current changes are often measured in non-physiological conditions (voltage-clamp, low pacing rates, cytosolic Ca2+ buffers).
Methods and Results
We measured the major K+ currents (IKr, IKs, IK1) and their Ca2+- and beta-adrenergic dependence in rabbit ventricular myocytes in chronic pressure/volume overload-induced HF (vs. age-matched controls). APD was significantly prolonged only at lower pacing rates (0.2–1 Hz) in HF under physiological ionic conditions and temperature. However, when cytosolic Ca2+ was buffered, APD prolongation in HF was also significant at higher pacing rates. Beat-to-beat variability of APD was also significantly increased in HF. Both IKr and IKs were significantly upregulated in HF under AP-clamp, but only when cytosolic Ca2+ was not buffered. CaMKII inhibition abolished IKs upregulation in HF, but it did not affect IKr. IKs response to beta-adrenergic stimulation was also significantly diminished in HF. IK1 was also decreased in HF regardless of Ca2+ buffering, CaMKII inhibition or beta-adrenergic stimulation.
Conclusions
At baseline Ca2+-dependent upregulation of IKr and IKs in HF counterbalances the reduced IK1, maintaining repolarization reserve (especially at higher heart rates) in physiological conditions, unlike conditions of strong cytosolic Ca2+ buffering. However, under beta-adrenergic stimulation, reduced IKs responsiveness severely limits integrated repolarizing K+ current and repolarization reserve in HF. This would increase arrhythmia propensity in HF, especially during adrenergic stress.
Journal Subject Terms: Electrophysiology, Ion Channels/Membrane Transport, Heart failure
Keywords: Heart failure, electrophysiology, potassium channels, action potential, calcium/calmodulin-dependent protein kinase II, delayed rectifier potassium current, inward rectifier potassium current, beta-adrenergic stimulation
INTRODUCTION
Heart failure (HF) is known to induce profound electric remodeling affecting the repolarization process of the cardiac action potential (AP) resulting in increased risk for cardiac arrhythmias. HF patients with prolonged heart rate-corrected QT interval (QTc) in their electrocardiogram are thought to have higher risk for electric abnormalities and sudden cardiac death;1–3 although details are debated,4, 5 and more than half of HF patients exhibit normal QTc.2–6 Meanwhile, in isolated ventricular myocytes AP duration (APD) is typically reported to be prolonged both in human HF7, 8 and animal HF models.9–12 However, in those studies APD prolongation was most pronounced at low pacing rates and when cytosolic Ca2+ was buffered (i.e. non-physiological conditions). Moreover, no APD prolongation was found above 1 Hz pacing frequency in human,8 canine,13 and rabbit14 failing myocytes, in line with observations that differences in QT intervals between healthy controls and HF patients was abolished at high heart rates.15
Earlier studies demonstrated that reduced K+ currents, enhanced Na+/Ca2+ exchange and increased late Na+ currents contribute to APD prolongation in HF.7–14, 16 But these data were obtained in non-physiological conditions (rectangular voltage clamp pulses vs. AP clamp, long cycle lengths and with cytosolic Ca2+ transients eliminated by Ca2+ buffers). In addition to direct Ca2+effects on channel gating, Ca2+/calmodulin-dependent protein kinase II (CaMKII) activation requires locally high [Ca2+]i levels. Importantly, CaMKII is also known to be upregulated and more active in HF17 and has been shown to modulate Na+ and K+ channel gating and can also alter channel expression levels18, 19 that shape the cardiac AP and contribute to arrhythmogenesis in HF.20, 21 This regulatory action of CaMKII on ionic currents might have been underestimated in previous HF studies using non-physiological conditions for recordings.
In this study, we aimed to measure the actual repolarizing K+ currents that occur under physiological AP-clamp recording conditions to assess the modulatory effect of CaMKII by [Ca2+]i transients and β-adrenergic receptor (β-AR) activation. We hypothesized that increased [Ca2+]i and CaMKII activity under physiological conditions play a role in the attenuated AP prolongation at higher pacing rates in HF vs. control. Specifically, we measured APs and three major repolarizing K+ currents during phase 3 of the AP: the rapid and slow components of delayed rectifier K+ currents (IKr and IKs, respectively) and the inward rectifier K+ current (IK1) in HF and age-matched control rabbit ventricular myocytes. We used a previously well-characterized chronic non-ischemic HF rabbit model (combined volume and pressure overload), which is also arrhythmogenic.11, 17, 21–25 We used the AP-clamp technique at 2 Hz pacing both with preserved [Ca2+]i transients26, 27 and with [Ca2+]i buffered below the diastolic level. Modulation of these K+ currents by Ca2+, CaMKII and β-AR stimulation was also examined to assess the changes of the repolarization reserve in pathophysiological settings characteristic of HF.11, 20
METHODS
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
All animal handling and laboratory procedures were in accordance with the approved protocols of the local Institutional Animal Care and Use Committee confirming to the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (8th edition, 2011).
Arrhythmogenic rabbit non-ischemic HF model
HF was induced in New Zealand White rabbits (all male, 2.5–3 kg, 3–4-month-old) by combined aortic insufficiency and afterwards abdominal aortic stenosis as previously described.22 HF progression was monitored by echocardiography and myocytes were isolated when left ventricular end-systolic dimension exceeded 1.55 cm (Table 1). Similar to our previous studies in this rabbit model, the hearts used here were increased in weight by ~100%, dilated (LVEDD) and exhibited reduced fractional shortening (FS). HF animals also exhibited abdominal ascites fluid and evidence of lung edema (LW and LW/BW).
Table 1. Morphometric data of HF and age-matched control rabbits.
Heart failure was induced in 10 rabbits and progression was assesed periodically by echocardiography. Data of HF rabbits at the time when cardiac myocytes were isolated are compared to 5 age-matched healthy control rabbits.
| Age-matched control | HF | |
|---|---|---|
| Age (years) | 2.40±0.17 | 2.46±0.35n.s. |
| Body weight (kg) | 3.99±0.14 | 3.98±0.13n.s |
| Heart weight (g) | 10.46±0.59 | 21.86±2.54*** |
| HW/BW (g/kg) | 2.63±0.15 | 5.52±0.71** |
| Lung weight (g) | 13.86±0.25 | 18.94±1.16** |
| LW/BW (g/kg) | 3.49±0.12 | 4.95±0.21** |
| LVESD (cm) | 1.08±0.07 | 1.63±0.11*** |
| LVEDD (cm) | 1.71±0.07 | 2.29±0.09*** |
| FS (%) | 36.70±2.02 | 28.39±1.13* |
All data represent mean±SEM. Unpaired, two-tailed Student’s t test;
p<0.05,
p<0.01,
p<0.001.
(HW/BW, heart weight–to–body weight ratio; LW/BW, lung weight–to–body weight ratio LVESD, left ventricular end-systolic diameter; LVEDD, left ventricular end-diastolic diameter; FS, fractional shortening calculated as FS=(LVEDD-LVESD)/LVEDD*100)
For myocyte isolation, rabbits were subjected to general anesthesia (induction with propofol 2 mg/kg followed by 2–5% isoflurane in 100% oxygen). After thoracotomy, the heart was quickly excised and rinsed in cold nominally Ca2+-free MEM. The right atrium was removed and the aorta opened to visualize the left coronary ostium which was then cannulated using a 5F Judkins right catheter (Performa; Merit Medical Systems). Perfusion of the LV and LA was established before removal of the RV free wall and application of a purse-string suture to secure the catheter in place. The remainder of the isolation procedure was then essentially as previously described.28 Cells isolated from healthy age-matched rabbits were used for control experiments.
Electrophysiology
Isolated cells were transferred to a temperature controlled plexiglass chamber (Cell Microsystems) and continuously superfused with a bicarbonate containing Tyrode solution with the following composition (in mmol/L): NaCl 124, NaHCO3 25, KCl 4, CaCl2 1.2, MgCl2 1, HEPES 10, Glucose 10, with pH = 7.4. APs and underlying ion currents were recorded in whole-cell configuration of patch-clamp technique. Electrodes were fabricated from borosilicate glass (World Precision Instruments) with tip resistances of 2–2.5 MΩ when filled with internal solution containing (in mmol/L): K-Aspartate 110, KCl 25, NaCl 5, Mg-ATP 3, HEPES 10, cAMP 0.002, phosphocreatine-K2 10, and EGTA 0.01, with pH = 7.2. This composition preserved the physiological [Ca2+]i transient and contraction of the myocytes.29 To study the Ca2+-dependence of ionic currents in HF, [Ca2+]i was buffered to nominally zero by adding 10 mM BAPTA to the above listed pipette solution. The electrodes were connected to the input of an Axopatch 200B amplifier (Axon Instruments). Outputs from the amplifier were digitized at 50 kHz using Digidata1440A A/D card (Molecular Devices) under software control (pClamp 10). The series resistance was typically 3–5 MΩ and it was compensated by 85%. Experiments were discarded when the series resistance was high or increased by more than 10% during the experiment. Reported AP voltages are already corrected to the liquid junction potentials. All experiments were conducted at 37±0.1°C.
APs were recorded in current-clamp experiments where cells were stimulated with supra-threshold depolarizing pulses (2 ms duration) delivered via the patch pipette at various pacing frequencies between 0.2 Hz and 5 Hz. After reaching steady-state at each frequency (3 min pacing at a given frequency), 50 consecutive APs were recorded to examine the average behaviour. APD at 95% of repolarization (APD95) was used to assess precisely the influence of NCX function – which is known to be altered in HF22 - on AP profile when measured under physiological conditions.29 Series of 50 consecutive APs were analysed to estimate short-term variability (STV) of AP duration according to the following formula: STV = Σ (│APDi+1 − APDi│)/[(nbeats −1) × √2], where APDn and APDn+1 indicate the durations of the ith and (i+1)th APs, and nbeats denotes the number of consecutive beats analysed.30, 31 Changes in STV are presented as Poincaré plots, where 50 consecutive APD95 values are plotted, each against the duration of the previous AP.
AP-clamp Sequential Dissection experiments were conducted as previously described.26 All currents were recorded after their specific blocker had reached steady-state effect (~2 min perfusion). The following sequence of blockers was used to measure the major K+ currents: 1 μM HMR-1556 for IKs, 1 μM E-4031 for IKr, 100 μM BaCl2 for IK1. Experiments were performed both when Ca2+ cycling was preserved (Physiol) and when [Ca2+]i was buffered below the diastolic level using 10 mM BAPTA in the pipette solution (BAPTAi) to compare the Ca2+-sensitivity of these K+ currents during AP. To test the effect of CaMKII, cells were pretreated for ~2 hours with the specific CaMKII inhibitor, autocamtide-2-related inhibitory peptide (AIP, cell-permeable myristoylated form, 1 μM) before starting the experiment, and both the perfusion and pipettes solutions were also supplemented with AIP. In experiments examining the effect of β-AR stimulation on K+ currents, isoproterenol (ISO, 3–300 nM) was applied on AP-clamped cells. When ISO reached a steady-state effect (~2 min), then the K+ current blockers were added to the perfusion solution in a cumulative manner to measure IKs, IKr and IK1. Experiments were excluded from analysis if significant rundown of L-type Ca2+ current was observed (in periodic tests) or the membrane current following ISO stimulation did not reach steady-state.
Ion currents were normalized to cell capacitance, determined in each cell using short (10 ms) hyperpolarizing pulses from −10 mV to −20 mV. Cell capacitance was 191.68±3.60 pF in HF (n=116 cells/10 animals) vs. 144.52±1.45 pF in age-matched controls (75 cells/5 animals) using two sample Student’s t test, p<0.001.
Chemicals and reagents were purchased from Sigma-Aldrich, if not specified otherwise. E-4031 and HMR-1556 were from Tocris Bioscience.
Statistical analysis
Data are expressed as Mean±SEM. The number of cells in each experimental group was reported in the figures and/or figure captions as n = number of cells/number of animals. Cells in each group came from at least three individual animals. Statistical significance of differences was evaluated using one-way or two-way ANOVA to compare multiple groups and a Bonferroni posttest was used for pairwise comparisons. Differences were deemed significant if p<0.05.
RESULTS
Frequency- and Ca2+-dependent changes of AP in HF
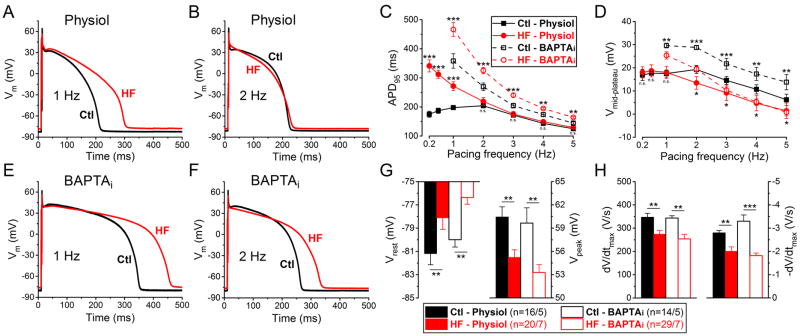
Figure 1 shows representative APs and group analysis in HF and age-matched Control (abbreviated as Ctl in Figs) myocytes, and first we consider the physiological case with normal Ca2+ cycling (Physiol; Fig. 1A–B). APD95 was significantly longer in HF vs. Control at 1 Hz pacing (271.8±15.2 ms vs. 197.8±6.9 ms, respectively, p<0.001) (Fig. 1C). As pacing rate was reduced, APD95 progressively increased in HF, but not in Control, making the Control-HF difference larger (Fig. 1C). The anomalous APD shortening in rabbit ventricle at very low pacing rates has been attributed to slowly-recovering transient outward K+ current (Ito,s) that is normally suppressed at physiological heart rates.32 Notably, at higher physiological stimulation rates (2–5 Hz) APD95 was not significantly different in HF vs. Control (218.9±13. ms vs. 204.2±7.7 ms at 2 Hz in HF and Control, respectively).
Figure 1. Frequency- and Ca2+-dependent changes of AP parameters in HF.
(A–B) Representative action potentials (APs) recorded at 1 Hz and 2 Hz pacing in heart failure (HF) and healthy age-matched control cells with physiological solutions (Physiol). (C–D) Frequency dependence of AP duration measured at 95% of repolarization (APD95) and mid-plateau potential (Vmid-plateau). (E–F) Representative APs when cytosolic Ca2+ was buffered to nominally zero using 10 mM BAPTA in pipette solution (BAPTAi) at 1 Hz and 2 Hz pacing. (G) Resting membrane potential (Vrest) is less negative in HF in line with decreased AP peak (Vpeak) at 1 Hz. (H) Both maximal rate of rise (dV/dtmax) and maximal rate of phase 3 repolarization (-dV/dtmax) are significantly decreased in HF at 1 Hz. Columns and bars represent mean±SEM. n refers to cells/animals measured in each group. ANOVA with Bonferroni posttest, Ctl vs. HF, *p<0.05, **p<0.01, ***p<0.001.
Buffering [Ca2+]i to very low levels with 10 mM BAPTA in the pipette (BAPTAi) also prevented [Ca2+]i transients, and resulted in longer APD in general compared to the physiological solution (Fig. 1C, E-F). Moreover, with BAPTAi significant APD prolongation was observed in HF vs. Control at all pacing frequencies studied (Fig. 1C), although the absolute difference became smaller with increasing frequency.
AP plateau height, characterized as mid-plateau potential (Vmid-plateau) was less positive in HF at all frequencies studied for the BAPTAi case, and for the physiological case as well (except for 0.2–1 Hz; Fig. 1D). The extent of plateau depression at 2–5 Hz was greater with buffered [Ca2+]i than for physiological pipette solution (HF-Physiol vs. HF-BAPTAi, Fig. 1D). Consistent with this, early repolarization rate and magnitude (AP phase 1) were both reduced in HF under both pipette conditions (Fig. 1A–B, 1E-F). Resting membrane potential (Vrest) was slightly more positive in HF and AP peak voltage (Vpeak) was significantly lower in HF under both conditions (Fig. 1G). In line with this, the AP maximum rate of rise (dV/dtmax) was also decreased by ~25% in HF (Fig. 1H), which might reflect the altered Na+ channel availability at more positive Vrest, but contributions of decreased Na+ channel functional expression or elevated [Na+]i cannot be ruled out. Importantly here, the maximum rate of repolarization (-dV/dtmax) during AP phase 3 was also significantly decreased in HF vs. Control myocytes under physiological conditions and more markedly so with BAPTAi (Fig. 1H).
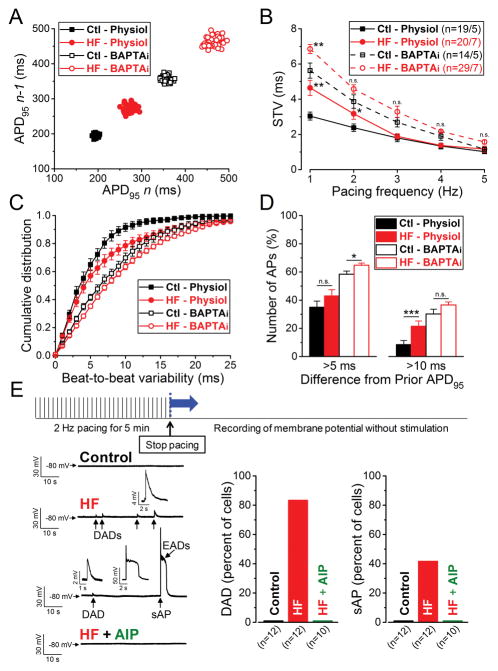
HF rabbit myocytes also exhibited increased temporal variability of APD95 compared to myocytes from Control hearts (Ctl; Fig. 2A). The short-term variability (STV) calculated using 50 consecutive APD95 values was significantly increased at 1 Hz pacing in HF vs. Control (HF-Physiol vs. Ctl-Physiol, 4.65±0.43 ms vs. 3.04±0.23 ms, respectively, p<0.01), and at 2 Hz as well, where mean APD95 did not differ in HF vs. Control (Fig. 2B). However, these differences became smaller at higher frequencies. We also analyzed the cumulative distribution of beat-to-beat changes in APD95. More than half of consecutive beats have less than 5 ms difference (both Control and HF), but for HF a larger percentage of beats exhibited more than 5 and 10 ms difference from beat to beat, apparent as “long-tails” in the cumulative distribution curves (Fig. 2C–D). This difference was less with BAPTAi.
Figure 2. Increased temporal variability of APD and arrhythmogenesis in HF.
(A) Representative Poincare plots of 50 consecutive APD95 values at 1 Hz steady-state pacing in 4 individual myocytes from HF and age-matched control animals recorded with either preserved or buffered [Ca2+]i. (B) Frequency-dependent short-term variability of APD95 (STV). (C) Cumulative distribution curves of individual beat-to-beat variability values at 1 Hz pacing. (D) Percentage of total APs measured at 1 Hz pacing having beat-to-beat APD95 variability of >5 ms and >10 ms in consecutive beats. Columns and bars represent mean±SEM. n refers to cells/animals measured in each group. ANOVA with Bonferroni posttest, *p<0.05, **p<0.01, ***p<0.001. (E) Arrhythmogenic diastolic activities were tested using the pacing protocol shown above. (Left) Representative records in control, HF and AIP (CaMKII inhibitor, 1 μM) treated HF cells are shown below. HF cells frequently showed delayed afterdepolarizations (DADs, enlarged in insets) and spontaneous AP (sAP, enlarged in inset). Early afterdepolarizations (EADs, enlarged in inset) were also superimposed on the sAP repolarization. (Right) Percentage of cells having DAD and sAP. 10/12 cells and 5/12 cells showed DADs and sAP in HF, respectively, whereas no DAD, sAP or EAD was observed in control and following AIP treatment in HF.
Spontaneous SR Ca2+ release (or leak) and consequent afterdepolarizations are known to contribute to increased beat-to-beat variability of APD,30 and are known to occur more frequently in this HF model at a given SR Ca2+ load (CaMKII-dependently).17, 33, 34 However, at baseline in steady-state pacing neither early nor delayed afterdepolarizations (EAD, DAD) were observed in either Control or HF. Therefore, to test the SR Ca2+ store stability we paced the cells at 2 Hz for 5 min, then paused to record Vm without stimulation. DADs developed in 10/12 HF cells and among them 5 cells also exhibited spontaneous APs likely to be triggered by SR Ca release (Fig. 2E shows an example). Average DAD frequency was 1.14±0.31/min, with average amplitude of 3.15±0.19 mV. Only 15% of DADs were larger than 5 mV in amplitude (not including those that triggered APs). However, neither the age-matched controls cells (0/12 cells) nor HF cells pretreated with CaMKII inhibitory peptide AIP (1 μM) (0/10 cells) showed such arrhythmogenic activities (Fig. 2E). Thus, higher SR Ca2+ instability may contribute to the higher beat-to-beat APD variability in HF (Fig. 2C–D), that was suppressed by BAPTAi.
Changes in magnitude and dynamics of IKr, IKs and IK1 under AP in HF
The APD data suggest complex remodeling in HF and the involvement of Ca2+-dependent processes in the altered repolarization phase at higher physiological rabbit pacing rates. Thus, we studied the major repolarizing K+ currents (IKr, IKs, IK1) during phase 3 of the AP at 2 Hz. All three ionic currents were recorded from the same myocyte, employing the AP-clamp Sequential Dissection technique26 with preserved [Ca2+]i cycling condition (Physiol) and also with [Ca2+]i buffered with 10 mM BAPTA in the pipette (BAPTAi). Involvement of CaMKII pathway was also investigated in the Ca2+-dependent alteration of repolarization. A previously recorded typical rabbit ventricular AP was used as voltage command in all AP-clamp experiments (canonical AP-clamp) at 2 Hz. This typical AP has an APD95 of 205.5 ms, which is not significantly different from the APD95 of either Control or HF cells at 2 Hz (one sample t test). Because significant cellular hypertrophy was found in HF (~33% increase in cell capacitance, see Methods for details) all reported currents are normalized to the corresponding cell capacitance.
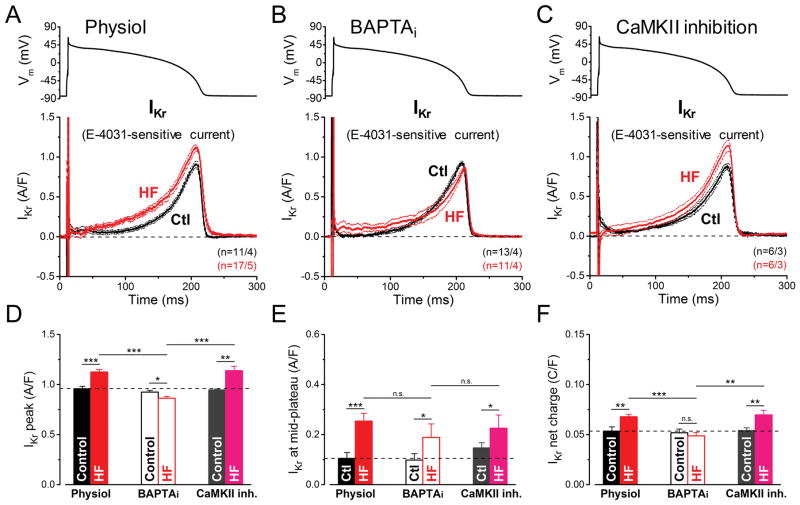
The rapid component of delayed rectifier potassium current (IKr) was measured as E-4031-sensitive current under AP-clamp (Fig. 3A–C). Peak IKr density was 18% higher in HF myocytes compared to age-matched controls when measured in our physiological condition (1.13±0.02 vs. 0.96±0.02 A/F in HF and Control, respectively, p<0.001; Fig. 3A,D). In contrast, with BAPTAi peak IKr density was 7% smaller in HF vs. Control (0.86±0.02 vs. 0.92±0.02 A/F, respectively, p<0.05; Fig. 3B,D). AIP pretreatment to inhibit CaMKII affected neither peak IKr density nor integrated charge carried by IKr, in either Control or HF (Fig. 3C,D,F), suggesting that CaMKII is not involved in acute IKr modulation. On the other hand, IKr is elevated earlier during the AP in HF vs. Control (Fig. 3E), regardless of [Ca2+]i (Fig. 3A vs. B and 3E), indicating altered IKr gating in HF. The slight increase in peak and integrated IKr under physiological conditions here, could easily be missed if IKr is measured under non-physiological conditions (e.g. BAPTAi).
Figure 3. Ca2+-dependent upregulation of IKr in HF under AP-clamp.
The rapid component of delayed rectifier K+ current (IKr) was measured as E-4031 (1 μM)-sensitive current in HF and age-matched control cells. AP-clamp using a prerecorded typical AP (shown above) was applied at 2 Hz. (A) IKr traces (mean±SEM) recorded under preserved [Ca2+]i cycling (Physiol). IKr is not only increased in HF cells having [Ca2+]i transients, but it activates earlier during the AP. (B) IKr traces (mean±SEM) recorded under buffered [Ca2+]i using 10 mM BAPTA in the pipette (BAPTAi). Buffering [Ca2+]i significantly reduced IKr density in HF below the control level, but it had no effect in control. (C) IKr traces (mean±SEM) recorded in cells pretreated with the specific CaMKII inhibitor peptide AIP (1 μM). AIP had no effect on IKr both in control and in HF. (D) Peak IKr density is significantly upregulated in HF under AP by a Ca2+-dependent, but CaMKII-independent pathway. (E) IKr density measured at the mid-plateau increased in HF indicating earlier IKr accumulation during AP, which occurred independent of [Ca2+]i level and CaMKII activity. (F) Net charges carried by IKr in HF and age-matched control. Columns and bars represent mean±SEM. n refers to cells/animals measured in each group. ANOVA with Bonferroni posttest, *p<0.05, **p<0.01, ***p<0.001.
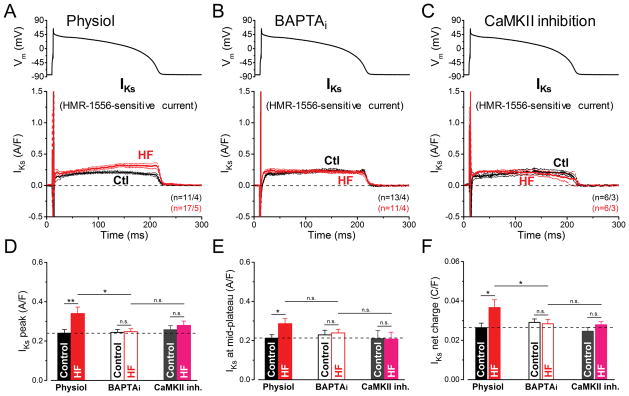
The slow component of delayed rectifier K+ current (IKs) was measured as HMR-1556-sensitive current under AP-clamp (Fig. 4A–C). IKs is a tiny current in the absence of β-AR stimulation in healthy ventricular myocytes (Fig. 4A), consistent with minimal effects of IKs block on baseline APD in rabbit ventricle.35 Surprisingly, we found a 42% increase in basal IKs magnitude in HF vs. Control in our physiological condition (0.34±0.03 vs. 0.24±0.02 A/F, respectively, p<0.01; Fig. 4A,D-F). Furthermore, the increase in IKs was not observed with BAPTAi (Fig. 4B) or when HF cells were pretreated with AIP (Fig. 4C–F). We infer that Ca2+-dependent CaMKII activity may acutely increase IKs in HF.
Figure 4. Ca2+/CaMKII-dependent upregulation of IKs in HF under AP-clamp.
The slow component of delayed rectifier K+ current (IKs) was measured as HMR-1556 (1 μM)-sensitive current in HF and age-matched control cells. AP-clamp using a prerecorded typical AP (shown above) was applied at 2 Hz. (A) IKs traces (mean±SEM) recorded under preserved [Ca2+]i cycling (Physiol). Basal IKs is a tiny current under AP, yet it is significantly upregulated in HF cells having [Ca2+]i transients. (B) IKs traces (mean±SEM) recorded under buffered [Ca2+]i using 10 mM BAPTA in the pipette (BAPTAi). Buffering [Ca2+]i reduced IKs in HF back to its control level. (C) IKs traces (mean±SEM) recorded in cells pretreated with the specific CaMKII inhibitor peptide AIP (1 μM). AIP abolished the IKs upregulation in HF. (D) Peak IKs density is significantly upregulated in HF under AP by a Ca2+/CaMKII-dependent pathway. (E) IKs density measured at the mid-plateau of the AP. (F) Net charges carried by IKs in HF and age-matched control. Columns and bars represent mean±SEM. n refers to cells/animals measured in each group. ANOVA with Bonferroni posttest, *p<0.05, **p<0.01.
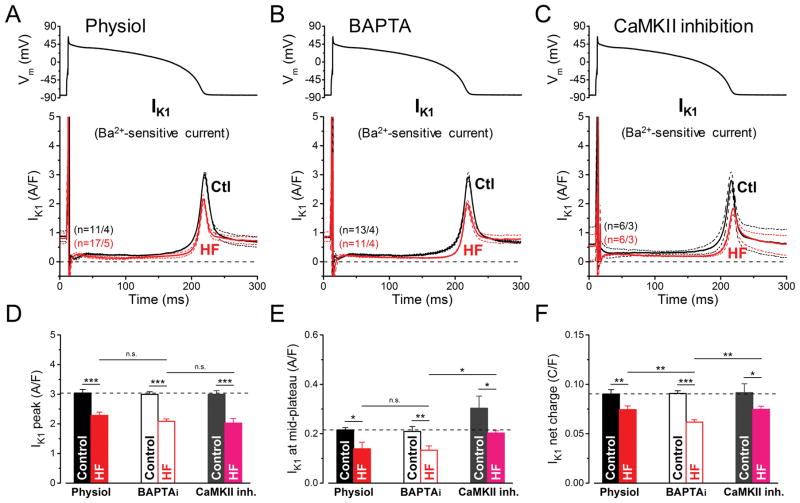
The inward rectifier K+ current (IK1) was measured as Ba2+-sensitive current (Fig. 5A–C). Peak IK1 density during repolarization was 25% smaller in HF vs. Control (2.28±0.11 vs. 3.04±0.12 A/F, respectively, p<0.001; Fig. 5A, D). Mid-plateau and integrated IK1 over the APD was also smaller in HF vs. Control (Fig. 5E–F). Neither buffering [Ca2+]i (BAPTAi) nor CaMKII inhibition (AIP) altered this conclusion (Fig. 5B,C). We conclude that IK1 downregulation in this HF model is not mainly due to acute effects of [Ca2+]i or CaMKII on IK1, but might reflect lower functional expression level of the channel proteins. Since CaMKII is upregulated in HF17 and can cause reduced functional expression of Kir2.1 and IK1,19 this does not preclude an indirect chronic effect of CaMKII on IK1 functional expression in HF.
Figure 5. Reduction of IK1 in HF under AP-clamp.
The inward rectifier K+ current (IK1) was measured as Ba2+ (100 μM)-sensitive current in HF and age-matched control cells. AP-clamp using a prerecorded typical AP (shown above) was applied at 2 Hz. (A–C) IK1 traces (mean±SEM) recorded under preserved [Ca2+]i cycling (Physiol), [Ca2+]i buffering (BAPTAi) and CaMKII inhibition using AIP. IK1 was significantly reduced in HF in all these conditions. (D–F) Statistics for peak IK1 density shows that the reduction is not influenced by [Ca2+]i buffering or acute CaMKII inhibition. (E) IK1 density measured at the mid-plateau of the AP was also reduced. (F) Net charges carried by IK1 was further reduced in HF when measured with [Ca2+]i buffering. Columns and bars represent mean±SEM. n refers to cells/animals measured in each group. ANOVA with Bonferroni posttest, *p<0.05, **p<0.01, ***p<0.001.
Altered β-adrenergic response of K+ currents in HF
Increased sympathetic activation and altered β-AR responses are frequently reported in HF. Therefore, we also tested effects of β-AR stimulation on IKr, IKs and IK1 in HF and Control myocytes using AP-clamp. Because both protein kinase A (PKA) and CaMKII are known to mediate downstream effects of β-AR stimulation and the activity of these kinases are also known to be altered in HF, we again measured ionic currents with physiological preserved [Ca2+]i cycling and with heavily buffered [Ca2+]i.
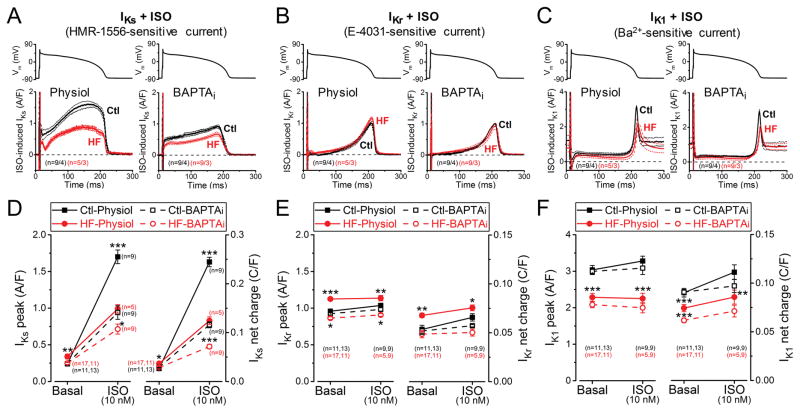
IKs increased robustly upon treatment with the non-specific β-AR agonist isoproterenol (ISO, 10 nM; Fig. 6A,D). Buffering [Ca2+]i limited IKs response, indicating the involvement of Ca2+ and/or CaMKII (as well as PKA) in mediating ISO effect on IKs. However, the ISO-induced increase in IKs peak density was much reduced in HF both with [Ca2+]i cycling (7.25-fold in Control vs. 2.91-fold increase in HF) and with BAPTAi (3.85-fold in Control vs. 2.88-fold in HF; Fig. 6D). The ISO-induced increase in IKs in the presence of Ca2+ was not simply a scaled-up version of the BAPTAi traces, but a more prominent increase during the plateau, resulting in an even greater increment in integrated charge carried by IKs (Fig. 6A,D). Even after ISO, the integrated K+ efflux during the AP in HF was less than half of that in Control (Fig. 6D), which could seriously limit repolarization reserve.
Figure 6. Altered response of K+ currents to β-AR stimulation in HF.
IKs, IKr and IK1 currents were recorded following 2 min pretreatment with beta-adrenergic receptor agonist isoproterenol (ISO, 10 nM). (A) IKs traces (mean±SEM) under canonical AP-clamp at 2 Hz measured with preserved [Ca2+]i cycling (Physiol) and [Ca2+]i buffering using 10 mM BAPTA in the pipette (BAPTAi). (B) IKr traces (mean±SEM) under AP-clamp following ISO pretreatment in HF and age-matched control cells. (C) IK1 traces (mean±SEM) under AP-clamp following ISO pretreatment in physiological solutions and in BAPTAi. (D) Robust upregulation of IKs peak and net charge induced by ISO, which is reduced in BAPTAi indicating a Ca2+-dependent pathway in mediating the response of β-AR stimulation on IKs besides the classical PKA-dependent phosphorylation. HF cells showed significantly reduced IKs accumulation upon ISO application both with and without [Ca2+]i buffering. (E) IKr is slightly modulated by ISO both in control and HF. (F) IK1 peak density is not influenced by ISO, but IK1 net charge is slightly increased in HF due to altered rectification. Symbols and bars represent mean±SEM. n refers to cells measured in each group, and the cells in each group came from three to five individual animals. ANOVA with Bonferroni posttest, *p<0.05, **p<0.01, ***p<0.001.
IKr was affected minimally upon ISO-stimulation (Fig. 6B,E), which achieved statistical significance only in Control cells and physiological solutions (8.1% increase in IKr peak density and 23.1% increase in total charge carried during the AP, p<0.05 in both cases). As in Fig. 3, IKr was higher at baseline in HF vs. Control, but failed to increase significantly upon ISO exposure (Fig. 6E).
IK1 peak density was not affected by beta-adrenergic stimulation. (Fig. 6C,F). However, the IK1 net charge increased significantly (by 23%) following ISO application in Control, but not in HF cells, when measured with cycling [Ca2+]i under AP-clamp (Fig. 6F), possibly reflecting some Ca2+-dependent rectification of IK1 as previously described.27, 36
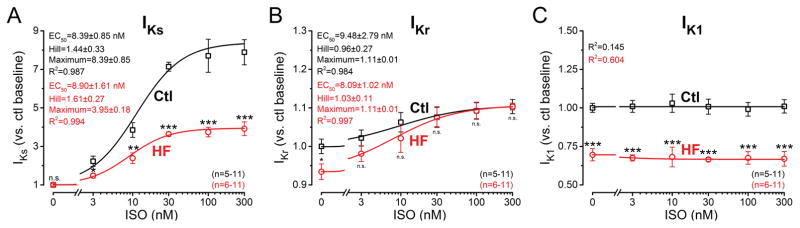
Because the ISO effect on IKs was blunted in HF vs. Control, we tested whether the β-AR effects were due to decreased ISO-sensitivity or limited maximal response. We varied ISO between 3 and 300 nM in AP-clamped HF and Control cells (Fig. 7). Since steady-state contracting AP-clamped myocytes were unstable at the higher ISO concentrations without [Ca2+]i buffering, these experiments were performed with 10 mM BAPTA in the pipette. ISO dose-dependently increased IKs both in Control and HF cells with nearly identical EC50 values (8.39±0.85 nM and 8.90±1.61 nM, respectively, n.s.) and Hill coefficients (Fig. 7A). However, the maximum response was only half as much in HF vs. Control (4.0-fold vs. 8.4-fold increase in peak IKs density, respectively) indicating unchanged ISO-sensitivity, but significant hyporesponsiveness of IKs to ISO. IKr increased only following ISO application and no difference was seen between Control and HF regarding EC50 values and maximal responses (Fig. 7B). Finally, peak IK1 density during the AP did not show any change, even at high concentration of ISO, but IK1 was again significantly reduced in HF (Fig. 7C).
Figure 7. β-AR responsiveness of K+ currents in HF.
Dose-response effect of isoproterenol (ISO) on IKs, IKr and IK1 peak densities under AP-clamp at 2 Hz. Pipette solution contained 10 mM BAPTA. (A) IKs measured as HMR-sensitive current showed a robust accumulation following ISO application. IKS sensitivity (EC50) to ISO was unchanged in HF, whereas the response was significantly reduced in HF. (B) IKr was minimally affected by ISO treatment; however, neither the ISO-sensitivity nor the magnitude of the response were altered in HF. (C) IK1 peak density did not change following ISO application under buffered [Ca2+]i conditions and IK1 was uniformly decreased in HF. EC50 values, Hill coefficients and maximum responses were determined by fitting data to the Hill equation, indicated by solid lines. Symbols and bars represent mean±SEM. n refers to the number of cells measured in each group, and the cells in each group came from three to five individual animals. ANOVA with Bonferroni posttest, *p<0.05, **p<0.01, ***p<0.001.
Relative contributions of each K+ current to AP phase 3 repolarization
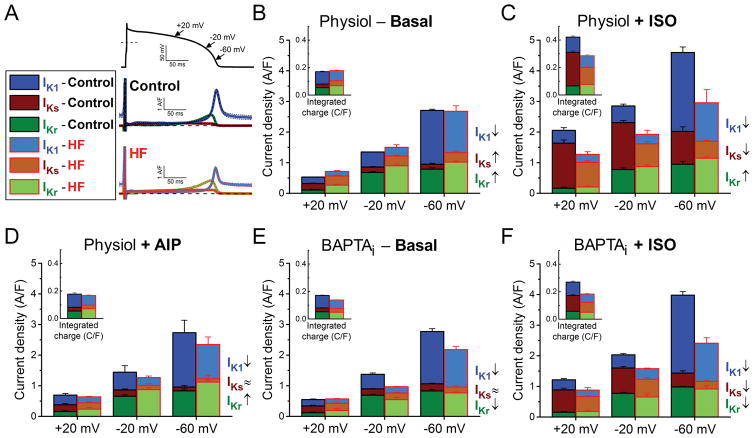
AP repolarization is governed by the dynamic time-and voltage-dependent activation of K+ currents. Each K+ current has its unique fingerprint (current shape under the changing voltages of AP) and different magnitude during the cardiac AP. The relative contributions of the major K+ currents (IKr, IKs and IK1) involved in the phase 3 repolarization of AP in control and in HF are shown in Fig. 8 analyzed at different points of repolarization (+20, −20 and −60 mV) and as integrated K+ flux during the AP (Fig. 8, insets).
Figure 8. Relative contribution of each K+ current to net repolarizing K+ current in HF.
Relative contributions and magnitudes of the major repolarizing K+ currents (IKr, IKs, IK1) during AP are compared in different phases of the repolarization process in HF to those in age-matched control. (A) IKr, IKs and IK1 traces in control and HF measured under AP-clamp at 2 Hz without using any Ca2+ buffer or β-AR agonist. Mean traces and SEM are shown. (B) When [Ca2+]i cycling is preserved, upregulation of IKr and IKs compensates the decrease of IK1 in HF during phase 3 of AP. (C) Stimulation of β-ARs using isoproterenol (ISO, 10 nM) significantly upregulates IKs, thus ISO reverses IKr/IKs dominant pattern of repolarization during phase 3 of AP. However, HF cells are hyporesponsive to ISO-induced stimulation (i.e. IKs increases in a smaller extent than in control) thus the net repolarizing current is largely reduced in HF compared to control. Reversal in IKr/IKs dominance in repolarization following ISO treatment also fail to happen in HF. (D) CaMKII inhibition using the specific inhibitory peptide AIP abolishes the increase in IKs, whereas it does not affect IKr and IK1. (E) When [Ca2+]i is buffered (BAPTAi), the reduction in IK1 without the upregulation of IKr and IKs results in a significant decrease in the net repolarizing current in HF. (F) Under β-adrenergic stimulation in BAPTAi, HF cells show decrease in all three K+ currents compared to control. The contributions of these K+ currents to total net charge are shown in the insets. Columns and bars represent mean±SEM. Statistics and n numbers are shown in Figs. 3–6.
First, we consider physiological solutions with Ca2+ cycling at baseline (Fig. 8A–B) where total repolarizing K+ current is unaltered in HF vs. Control at either −20 mV, −60 mV or the integral. However, the reduced IK1 in HF is largely compensated by increases in both IKr and IKs, and this balance may allow the relatively maintained APD95 observed in HF myocytes at 2–5 Hz stimulation (Fig. 1B–C). In Control myocytes ISO greatly increases IKs and reverses the IKr vs. IKs dominant pattern of AP repolarization. However, in HF myocytes hyporesponsive of IKs to β-AR stimulation, IKs remains less than IKr, such that the combined IKs+IKr do not compensate for the reduced IK1 and integrated K+ current is substantially reduced in HF (Fig. 8C).
CaMKII inhibition with AIP affected only IKs, but because basal IKs is a tiny current under physiological AP, it does not significantly alter the magnitude of the net K+ current (Fig. 8D). Buffering cytosolic Ca2+ below diastolic level abolishes the upregulation of both IKr and IKs in HF, resulting in a pronounced decrease in net K+ current (Fig. 8E). Isoproterenol in the presence of BAPTAi exacerbated the difference between HF and Control because under those conditions IK1, IKs and IKr are all reduced in HF compared to Control (Fig. 8F).
DISCUSSION
Action potential duration changes in HF
There is consensus that HF is associated with lengthening of the ventricular APD (and corrected QT intervals, QTc). This is supported by evidence from numerous experimental studies involving several species and multiple methods to induce HF.7–14 However, HF-associated APD differences in previous studies often used lower pacing rates. In humans HF was associated with a 50 ms difference in baseline QTc, but as heart rate increased during exercise, the QTc values converged and were not different between Control and HF.15 In agreement with these observations, we found longer APD in HF vs. Control in rabbits at 0.2–1 Hz pacing, but not at higher physiological pacing rates for rabbits (Fig. 1). However, when the [Ca2+]i was buffered, APD was prolonged in HF at all stimulation rates, suggesting a role for Ca2+-dependent processes in shaping the AP morphology in HF.
Patients with QTc prolongation have increased risk for sudden cardiac death almost independent of the etiology of disease.37 Substantial QTc prolongation, especially in end-stage HF and in ischemic origin, indicates higher risk for cardiac arrhythmias also in HF.4 However, data also suggest that short-term QTc-variability may be a better predictor of sudden cardiac death than QTc interval per se.5, 38 In our arrhythmogenic HF model, STV of APD95 was also significantly increased (Fig. 2). It was reported that STV depends critically on the baseline APD95.31 In our case, STV was increased in HF vs. Control at 2 Hz pacing, despite no difference in baseline APD95. Interestingly, in our HF data a higher fraction of APs showed large difference in APD95 in subsequent beats (Fig. 2C–D) despite unaltered mean APD95. Because strong [Ca2+]i buffering suppressed this effect, it seems likely due to Ca2+-related events (e.g. SR Ca2+ releases) rather than remodeling in ionic currents and their different contribution to STV.31 Accordingly, spontaneous SR Ca2+ leak, Ca2+ sparks and waves can contribute to increased beat-to-beat variability.30 Even without overt EAD or DAD induction during steady-state pacing, local Ca2+ events could readily cause APD variations. Indeed, we saw such events following trains of stimuli in HF that were prevented by CaMKII inhibition, and were not seen in Control cells. This is consistent with higher SR Ca2+ leak and Ca2+ waves measured in this arrhythmogenic rabbit HF model (at matched SR Ca2+ load) which was also sensitive to CaMKII inhibition.17, 24, 33, 34, 39 This higher SR Ca2+ leak associated with HF may contribute to increased beat-to-beat variability of APD, however, further studies are needed to define the precise relationship between these two important parameters of HF.
Changes in repolarizing K+ currents in HF that shape the AP
In this study, we systematic characterize the major K+ currents (IKr, IKs, IK1) underlying phase 3 AP repolarization in Control and HF myocytes, including Ca2+- and β-AR-dependent properties. Decreased transient outward K+ current (Ito) in HF has been reported in several models, including ours.7, 11, 40 Consistent with that, we saw reduced phase 1 AP repolarization in HF (Fig. 1). However, our focus here is on the K+ currents that dominate phase 3 repolarization.
To understand how IKr, IKs and IK1 are altered in shaping the APD in HF, we used our sequential dissection method that allows all three K+ currents to be measured in the same myocyte under relatively physiological AP conditions. Often these individual currents are measured independently, with square voltage clamp pulses and under relatively non-physiologic conditions, including prevention of [Ca2+]i transients. Since Ca2+-dependent properties can influence several K+ currents, and to compare with prior work, we did parallel measurements with strong [Ca2+]i buffering.
In previous studies, most K+ currents were found to be downregulated in HF. In our study, IK1 density was indeed lower in HF under all conditions, regardless of [Ca2+]i buffering, CaMKII inhibition or β-AR activation in agreement with prior work with this rabbit HF model.11 However, some studies suggested that acute CaMKII inhibition could reduce IK1 in myocytes.19, 41 We cannot readily explain the differences, but one of these studies was in rabbit, using less physiological conditions, square voltage clamp pulses, and short-term cell culture to overexpress CaMKIIδ.19 Moreover, these studies showed that CaMKIIδ overexpression in either transgenic mice or in 24 hr in rabbit myocytes induced downregulation of IK1 and Kir2.1. Since CaMKII is upregulated at the protein and activity level in HF, CaMKII-induced downregulation may explain the dominant IK1 downregulation that we saw in HF. Conceivably, our preincubation with the CaMKII inhibitor AIP increased channel expression, to coincidentally offset an acute inhibitory effect on channel function.
Surprisingly, both IKr and IKs densities were increased during the AP in HF, under physiological AP conditions (Fig. 3–4). However, in both cases this enhancement was abolished by strong [Ca2+]i buffering, and for IKs by CaMKII inhibition. Ca2+-sensitivity of delayed rectifier K+ currents is well known, but details in the myocyte environment are still under debate.42–46 For IKs, calmodulin (CaM) is an intrinsic subunit of the channel, and is known to mediate Ca2+-dependent stimulation of IKs amplitude.42–44 So, the BAPTAi effect to reduce IKs during the AP in HF is consistent with the myocyte Ca2+ transient boosting IKs during the AP. But in Control myocytes, [Ca2+]i buffering had no effect on IKr or IKs under AP-clamp. The observation that CaMKII inhibition prevented the increased IKs in HF (but had no effect in Control) might provide a clue explaining this apparent dichotomy. That is, if CaMKII activity (which is elevated in HF),20 promotes the intrinsic Ca2+ effect on IKs gating, AIP could suppress that Ca2+-dependent IKs activation. The lower CaMKII activity in Control could limit that Ca2+-dependent IKs activation, explaining the lack of BAPTAi or AIP on IKs in Control. In any case, basal IKs is small compared to IKr or IK1, so its role in shaping the basal AP is moderate, but slightly stronger in HF.
IKr upregulation was Ca2+-dependent, but CaMKII-independent. IKr is not known to be directly regulated by Ca2+ or CaM, but protein kinase C (PKC) has been reported to increase IKr,47 be increased in HF;48, 49 and its Ca2+-dependence makes this an intriguing possibility (although others found that PKC activation decreased IKr density50). Regardless of Ca2+, IKr is higher early in the AP in HF vs. Control, suggesting potential changes in channel gating. Possible explanations might be HF-associated alterations in subunit composition or splice variants of the channel,51 and chronic β-AR activation (which occurs in HF) positively shifts the HERG activation voltage, independent of actual PKA activity.52 Further studies are needed to elucidate the underlying molecular mechanisms for IKr and IKs upregulation in HF and the exact involvement of Ca2+, CaMKII and PKC in their regulation.
β-adrenergic induced changes in repolarizing K+ currents in HF
β-AR stimulation is critical in the regulation of the heart in health and disease. Increased sympathetic activity is compensatory in early HF, but chronic β-AR activation also contributes to adverse remodeling in HF. On the other hand, cardiac contractile system and Ca2+ handling system are hyporesponsive to β-AR stimulation in HF.11
IKs is known to be increased by both PKA and Ca2+/CaM in ventricular myocytes44 and thus contributes to AP shortening upon β-AR stimulation (compensating for higher inward Ca2+ and Na+/Ca2+ exchange currents).27, 53 We found that the ISO-induced increase in IKs was suppressed in HF (both in physiological and BAPTAi conditions; Fig. 6D). Indeed, the ISO effect without [Ca2+]i transients was smaller in HF, as was the increase by allowing [Ca2+]i transients in HF. The EC50 ISO concentration for IKs (or IKr) activation was not altered in HF, but the maximal effect was less than half in HF vs. age-matched control (Fig. 7). This may be due to the reduced number of sarcolemmal β1-AR in this HF model,54 and lower local cAMP levels in HF55 might explain the reduced ISO-response. Additionally, phosphatases and phosphosdiesterases are also remodeled in HF,17 which may limit IKs target phosphorylation in HF. IKr was much less affected by β-AR stimulation than IKs in our study and in previous reports27, 53 (~10 fold larger change in IKs than in IKr upon β-AR stimulation), but IKr changes are still debated. The β-adrenergic regulation of IKr may have species differences and involve both PKA and PKC.
IKr, IKs and IK1 reach their peak density sequentially during phase 3 of AP and their relative contributions to physiological repolarization and repolarization reserve differ, as demonstrated previously in control ventricular myocytes in detail.27, 56, 57 In this study we demonstrated their detailed contribution to repolarization in a chronic pressure/volume overload-induced HF model (Fig. 8). Important to note, that inward currents, such as late Na+ current16 and Na+/Ca2+ exchanger current,22 are also increased in HF and β-AR further enhances inward Na+/Ca2+ exchange and Ca2+ current. These increase the demand on the repolarization reserve during phase 3 of the AP to limit APD prolongation. Therefore, any change in the delicate balance between these depolarizing and repolarizing currents in HF could have significant impact on phase 3 repolarization velocity and thus on APD. Accordingly, the observed Ca2+-dependent upregulation of IKr and IKs in HF may partly compensate for the loss of IK1 in HF and limits APD prolongation, especially at faster pacing rates when IKr may accumulate.58 In line with our findings QTc prolongation in HF patients occurs preferentially at low but not at high heart rates.15 However, as we have shown in Fig. 8, β-AR stimulation impairs the repolarization reserve capacity because of the hyporesponsive IKs.
These results also put heart rate reducing agents in HF therapy into a new perspective. The rationale of this strategy is to increase diastolic interval by which the myocardial oxygen supply/demand can be improved. Nevertheless, large clinical trials (BEATIFUL59 and SHIFT60 trials) found only a slight benefit of ivabradine in HF patients. Our results also suggest that β-AR blockers might have more benefit regarding K+ currents and AP repolarization reserve, thus susceptibility for cardiac arrhythmias might be more reduced compared to that with heart-rate reducing agents. Another potential clinical implication is that drugs inhibiting delayed rectifier K+ currents may cause even more pronounced QTc prolongation in HF patients by limiting an already diminished repolarization reserve in HF, especially during β-AR stimulation.
Supplementary Material
What is Known?
Ion channel remodeling occurs in heart failure resulting in prolongation of the cardiac action potential (and QT interval on the ECG), predominantly at low heart rates.
Prolonged action potential is associated with increased risk of cardiac arrhythmias.
What the Study Adds?
Calcium/CaMKII-dependent increases of delayed rectifier K+ currents (IKr, IKs) compensate for the decrease of inward rectifier K+ current (IK1) in heart failure (0t higher heart rates).
Reduced beta-adrenergic responsiveness of IKs severely limits repolarization reserve in heart failure during sympathetic stimulation, further increasing arrhythmia risk.
Acknowledgments
We thank Logan R. J. Bailey, Johanna M. Borst, Maura Ferrero, Matthew L. Stein, Ian P. Palmer and Maximilien Bergman for their help in animal care and cell isolation.
Sources of Funding: This work was supported by grants from the National Institute of Health R01-HL30077 and P01-080101 (DMB), R01HL90880 (LTI and YCI), R01HL123526 (YCI) and the American Heart Association 14GRNT20510041 (YCI), 15GRNT25860028 (SMP).
Footnotes
Disclosures: None.
References
- 1.Tomaselli GF, Beuckelmann DJ, Calkins HG, Berger RD, Kessler PD, Lawrence JH, Kass D, Feldman AM, Marban E. Sudden cardiac death in heart failure. The role of abnormal repolarization. Circulation. 1994;90:2534–2539. doi: 10.1161/01.cir.90.5.2534. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe E, Arakawa T, Uchiyama T, Tong M, Yasui K, Takeuchi H, Terasawa T, Kodama I, Hishida H. Prognostic significance of circadian variability of RR and QT intervals and QT dynamicity in patients with chronic heart failure. Heart Rhythm. 2007;4:999–1005. doi: 10.1016/j.hrthm.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Arsenos P, Gatzoulis KA, Dilaveris P, Gialernios T, Sideris S, Lazaros G, Archontakis S, Tsiachris D, Kartsagoulis E, Stefanadis C. The rate-corrected QT interval calculated from 24-hour holter recordings may serve as a significant arrhythmia risk stratifier in heart failure patients. Int J Cardiol. 2011;147:321–323. doi: 10.1016/j.ijcard.2010.12.076. [DOI] [PubMed] [Google Scholar]
- 4.Algra A, Tijssen JGP, Roelandt JRTC, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden-death due to cardiac-arrest. Circulation. 1991;83:1888–1894. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- 5.Piccirillo G, Magri D, Matera S, Magnanti M, Torrini A, Pasquazzi E, Schifano E, Velitti S, Marigliano V, Quaglione R, Barilla F. QT variability strongly predicts sudden cardiac death in asymptomatic subjects with mild or moderate left ventricular systolic dysfunction: A prospective study. Eur Heart J. 2007;28:1344–1350. doi: 10.1093/eurheartj/ehl367. [DOI] [PubMed] [Google Scholar]
- 6.Vrtovec B, Knezevic I, Poglajen G, Sebestjen M, Okrajsek R, Haddad F. Relation of b-type natriuretic peptide level in heart failure to sudden cardiac death in patients with and without QT interval prolongation. Am J Cardiol. 2013;111:886–890. doi: 10.1016/j.amjcard.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 7.Beuckelmann DJ, Nabauer M, Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993;73:379–385. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- 8.Li GR, Lau CP, Leung TK, Nattel S. Ionic current abnormalities associated with prolonged action potentials in cardiomyocytes from diseased human right ventricles. Heart Rhythm. 2004;1:460–468. doi: 10.1016/j.hrthm.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Kaab S, Nuss HB, Chiamvimonvat N, O’Rourke B, Pak PH, Kass DA, Marban E, Tomaselli GF. Ionic mechanism of action potential prolongation in ventricular myocytes from dogs with pacing-induced heart failure. Circ Res. 1996;78:262–273. doi: 10.1161/01.res.78.2.262. [DOI] [PubMed] [Google Scholar]
- 10.Tsuji Y, Zicha S, Qi XY, Kodama I, Nattel S. Potassium channel subunit remodeling in rabbits exposed to long-term bradycardia or tachycardia: Discrete arrhythmogenic consequences related to differential delayed-rectifier changes. Circulation. 2006;113:345–355. doi: 10.1161/CIRCULATIONAHA.105.552968. [DOI] [PubMed] [Google Scholar]
- 11.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 12.Rose J, Armoundas AA, Tian Y, DiSilvestre D, Burysek M, Halperin V, O’Rourke B, Kass DA, Marban E, Tomaselli GF. Molecular correlates of altered expression of potassium currents in failing rabbit myocardium. Am J Physiol Heart Circ Physiol. 2005;288:H2077–2087. doi: 10.1152/ajpheart.00526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li GR, Lau CP, Ducharme A, Tardif JC, Nattel S. Transmural action potential and ionic current remodeling in ventricles of failing canine hearts. Am J Physiol Heart Circ Physiol. 2002;283:H1031–1041. doi: 10.1152/ajpheart.00105.2002. [DOI] [PubMed] [Google Scholar]
- 14.Rozanski GJ, Xu Z, Whitney RT, Murakami H, Zucker IH. Electrophysiology of rabbit ventricular myocytes following sustained rapid ventricular pacing. J Mol Cell Cardiol. 1997;29:721–732. doi: 10.1006/jmcc.1996.0314. [DOI] [PubMed] [Google Scholar]
- 15.Davey PP, Barlow C, Hart G. Prolongation of the QT interval in heart failure occurs at low but not at high heart rates. Clin Sci (Lond) 2000;98:603–610. [PubMed] [Google Scholar]
- 16.Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, Makielski JC. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol. 2005;38:475–483. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 18.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner S, Hacker E, Grandi E, Weber SL, Dybkova N, Sossalla S, Sowa T, Fabritz L, Kirchhof P, Bers DM, Maier LS. Ca/calmodulin kinase II differentially modulates potassium currents. Circ Arrhythm Electrophysiol. 2009;2:285–294. doi: 10.1161/CIRCEP.108.842799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoeker GS, Hanafy MA, Oster RA, Bers DM, Pogwizd SM. Reduced arrhythmia inducibility with calcium/calmodulin-dependent protein kinase II inhibition in heart failure rabbits. J Cardiovasc Pharmacol. 2016;67:260–265. doi: 10.1097/FJC.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pogwizd SM, Qi M, Yuan WL, Samarel AM, Bers DM. Upregulation of Na+/Ca2+ exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res. 1999;85:1009–1019. doi: 10.1161/01.res.85.11.1009. [DOI] [PubMed] [Google Scholar]
- 23.Despa S, Islam MA, Weber CR, Pogwizd SM, Bers DM. Intracellular Na+ concentration is elevated in heart failure but Na/K pump function is unchanged. Circulation. 2002;105:2543–2548. doi: 10.1161/01.cir.0000016701.85760.97. [DOI] [PubMed] [Google Scholar]
- 24.Zima AV, Bovo E, Bers DM, Blatter LA. Ca2+ spark-dependent and -independent sarcoplasmic reticulum Ca2+ leak in normal and failing rabbit ventricular myocytes. J Physiol. 2010;588:4743–4757. doi: 10.1113/jphysiol.2010.197913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchinoumi H, Yang Y, Oda T, Li N, Alsina KM, Puglisi JL, Chen-Izu Y, Cornea RL, Wehrens XH, Bers DM. CaMKII-dependent phosphorylation of RyR2 promotes targetable pathological RyR2 conformational shift. J Mol Cell Cardiol. 2016;98:62–72. doi: 10.1016/j.yjmcc.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banyasz T, Horvath B, Jian Z, Izu LT, Chen-Izu Y. Sequential dissection of multiple ionic currents in single cardiac myocytes under action potential-clamp. J Mol Cell Cardiol. 2011;50:578–581. doi: 10.1016/j.yjmcc.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banyasz T, Jian Z, Horvath B, Khabbaz S, Izu LT, Chen-Izu Y. Beta-adrenergic stimulation reverses the I Kr-I Ks dominant pattern during cardiac action potential. Pflugers Arch. 2014;466:2067–2076. doi: 10.1007/s00424-014-1465-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nichols CB, Chang CW, Ferrero M, Wood BM, Stein ML, Ferguson AJ, Ha D, Rigor RR, Bossuyt S, Bossuyt J. Beta-adrenergic signaling inhibits Gq-dependent protein kinase D activation by preventing protein kinase D translocation. Circ Res. 2014;114:1398–1409. doi: 10.1161/CIRCRESAHA.114.303870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banyasz T, Horvath B, Jian Z, Izu LT, Chen-Izu Y. Profile of L-type Ca2+ current and Na+/Ca2+ exchange current during cardiac action potential in ventricular myocytes. Heart Rhythm. 2012;9:134–142. doi: 10.1016/j.hrthm.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson DM, Heijman J, Bode EF, Greensmith DJ, van der Linde H, Abi-Gerges N, Eisner DA, Trafford AW, Volders PG. Diastolic spontaneous calcium release from the sarcoplasmic reticulum increases beat-to-beat variability of repolarization in canine ventricular myocytes after beta-adrenergic stimulation. Circ Res. 2013;112:246–256. doi: 10.1161/CIRCRESAHA.112.275735. [DOI] [PubMed] [Google Scholar]
- 31.Szentandrassy N, Kistamas K, Hegyi B, Horvath B, Ruzsnavszky F, Vaczi K, Magyar J, Banyasz T, Varro A, Nanasi PP. Contribution of ion currents to beat-to-beat variability of action potential duration in canine ventricular myocytes. Pflugers Arch. 2015;467:1431–1443. doi: 10.1007/s00424-014-1581-4. [DOI] [PubMed] [Google Scholar]
- 32.Bassani RA, Altamirano J, Puglisi JL, Bers DM. Action potential duration determines sarcoplasmic reticulum Ca2+ reloading in mammalian ventricular myocytes. J Physiol. 2004;559:593–609. doi: 10.1113/jphysiol.2004.067959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res. 2003;93:592–594. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- 34.Curran J, Brown KH, Santiago DJ, Pogwizd S, Bers DM, Shannon TR. Spontaneous Ca waves in ventricular myocytes from failing hearts depend on Ca2+-calmodulin-dependent protein kinase II. J Mol Cell Cardiol. 2010;49:25–32. doi: 10.1016/j.yjmcc.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lengyel C, Iost N, Virag L, Varro A, Lathrop DA, Papp JG. Pharmacological block of the slow component of the outward delayed rectifier current (IKs) fails to lengthen rabbit ventricular muscle QTc and action potential duration. Br J Pharmacol. 2001;132:101–110. doi: 10.1038/sj.bjp.0703777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaza A, Rocchetti M, Brioschi A, Cantadori A, Ferroni A. Dynamic Ca2+-induced inward rectification of K+ current during the ventricular action potential. Circ Res. 1998;82:947–956. doi: 10.1161/01.res.82.9.947. [DOI] [PubMed] [Google Scholar]
- 37.Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, Deckers JW, Kingma JH, Sturkenboom MC, Stricker BH, Witteman JC. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–367. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 38.Barr CS, Naas A, Freeman M, Lang CC, Struthers AD. QT dispersion and sudden unexpected death in chronic heart failure. Lancet. 1994;343:327–329. doi: 10.1016/s0140-6736(94)91164-9. [DOI] [PubMed] [Google Scholar]
- 39.Grimm M, Ling H, Willeford A, Pereira L, Gray CB, Erickson JR, Sarma S, Respress JL, Wehrens XH, Bers DM, Brown JH. CaMKIIdelta mediates beta-adrenergic effects on RyR2 phosphorylation and SR Ca2+ leak and the pathophysiological response to chronic beta-adrenergic stimulation. J Mol Cell Cardiol. 2015;85:282–291. doi: 10.1016/j.yjmcc.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaab S, Dixon J, Duc J, Ashen D, Nabauer M, Beuckelmann DJ, Steinbeck G, McKinnon D, Tomaselli GF. Molecular basis of transient outward potassium current downregulation in human heart failure - a decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation. 1998;98:1383–1393. doi: 10.1161/01.cir.98.14.1383. [DOI] [PubMed] [Google Scholar]
- 41.Nagy N, Acsai K, Kormos A, Sebok Z, Farkas AS, Jost N, Nanasi PP, Papp JG, Varro A, Toth A. [Ca2+]i-induced augmentation of the inward rectifier potassium current (IK1) in canine and human ventricular myocardium. Pflugers Arch. 2013;465:1621–1635. doi: 10.1007/s00424-013-1309-x. [DOI] [PubMed] [Google Scholar]
- 42.Tohse N. Calcium-sensitive delayed rectifier potassium current in guinea pig ventricular cells. Am J Physiol. 1990;258:H1200–1207. doi: 10.1152/ajpheart.1990.258.4.H1200. [DOI] [PubMed] [Google Scholar]
- 43.Xie Y, Ding WG, Matsuura H. Ca2+/calmodulin potentiates IKs in sinoatrial node cells by activating Ca2+/calmodulin-dependent protein kinase ii. Pflugers Arch. 2015;467:241–251. doi: 10.1007/s00424-014-1507-1. [DOI] [PubMed] [Google Scholar]
- 44.Bartos DC, Morotti S, Ginsburg KS, Grandi E, Bers DM. Quantitative analysis of the Ca2+-dependent regulation of delayed rectifier K+ current IKs in rabbit ventricular myocytes. J Physiol. 2017;595:2253–2268. doi: 10.1113/JP273676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nitta J, Furukawa T, Marumo F, Sawanobori T, Hiraoka M. Subcellular mechanism for Ca2+-dependent enhancement of delayed rectifier K+ current in isolated membrane patches of guinea-pig ventricular myocytes. Circ Res. 1994;74:96–104. doi: 10.1161/01.res.74.1.96. [DOI] [PubMed] [Google Scholar]
- 46.Ghosh S, Nunziato DA, Pitt GS. KCNQ1 assembly and function is blocked by long-QT syndrome mutations that disrupt interaction with calmodulin. Circ Res. 2006;98:1048–1054. doi: 10.1161/01.RES.0000218863.44140.f2. [DOI] [PubMed] [Google Scholar]
- 47.Heath BM, Terrar DA. Protein kinase c enhances the rapidly activating delayed rectifier potassium current, IKr, through a reduction in c-type inactivation in guinea-pig ventricular myocytes. J Physiol. 2000;522:391–402. doi: 10.1111/j.1469-7793.2000.t01-2-00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL, Mintze K, Pickard T, Roden R, Bristow MR, Sabbah HN, Mizrahi JL, Gromo G, King GL, Vlahos CJ. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation. 1999;99:384–391. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- 49.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Bodi I, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 50.Cockerill SL, Tobin AB, Torrecilla I, Willars GB, Standen NB, Mitcheson JS. Modulation of hERG potassium currents in HEK-293 cells by protein kinase C. Evidence for direct phosphorylation of pore forming subunits. J Physiol. 2007;581:479–493. doi: 10.1113/jphysiol.2006.123414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holzem KM, Gomez JF, Glukhov AV, Madden EJ, Koppel AC, Ewald GA, Trenor B, Efimov IR. Reduced response to IKr blockade and altered hERG1a/1b stoichiometry in human heart failure. J Mol Cell Cardiol. 2016;96:82–92. doi: 10.1016/j.yjmcc.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shu L, Zhang W, Su G, Zhang J, Liu C, Xu J. Modulation of hERG K+ channels by chronic exposure to activators and inhibitors of PKA and PKC: Actions independent of PKA and PKC phosphorylation. Cell Physiol Biochem. 2013;32:1830–1844. doi: 10.1159/000356616. [DOI] [PubMed] [Google Scholar]
- 53.Szentandrassy N, Farkas V, Barandi L, Hegyi B, Ruzsnavszky F, Horvath B, Banyasz T, Magyar J, Marton I, Nanasi PP. Role of action potential configuration and the contribution of Ca2+ and K+ currents to isoprenaline-induced changes in canine ventricular cells. Br J Pharmacol. 2012;167:599–611. doi: 10.1111/j.1476-5381.2012.02015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Desantiago J, Ai X, Islam M, Acuna G, Ziolo MT, Bers DM, Pogwizd SM. Arrhythmogenic effects of beta2-adrenergic stimulation in the failing heart are attributable to enhanced sarcoplasmic reticulum ca load. Circ Res. 2008;102:1389–1397. doi: 10.1161/CIRCRESAHA.107.169011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barbagallo F, Xu B, Reddy GR, West T, Wang Q, Fu Q, Li M, Shi Q, Ginsburg KS, Ferrier W, Isidori AM, Naro F, Patel HH, Bossuyt J, Bers D, Xiang YK. Genetically encoded biosensors reveal PKA hyperphosphorylation on the myofilaments in rabbit heart failure. Circ Res. 2016;119:931–943. doi: 10.1161/CIRCRESAHA.116.308964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Banyasz T, Magyar J, Szentandrassy N, Horvath B, Birinyi P, Szentmiklosi J, Nanasi PP. Action potential clamp fingerprints of K+ currents in canine cardiomyocytes: Their role in ventricular repolarization. Acta Physiol (Oxf) 2007;190:189–198. doi: 10.1111/j.1748-1716.2007.01674.x. [DOI] [PubMed] [Google Scholar]
- 57.Jost N, Virag L, Bitay M, Takacs J, Lengyel C, Biliczki P, Nagy Z, Bogats G, Lathrop DA, Papp JG, Varro A. Restricting excessive cardiac action potential and QT prolongation: A vital role for IKs in human ventricular muscle. Circulation. 2005;112:1392–1399. doi: 10.1161/CIRCULATIONAHA.105.550111. [DOI] [PubMed] [Google Scholar]
- 58.Jurkiewicz NK, Sanguinetti MC. Rate-dependent prolongation of cardiac action potentials by a methanesulfonanilide class III antiarrhythmic agent. Specific block of rapidly activating delayed rectifier K+ current by dofetilide. Circ Res. 1993;72:75–83. doi: 10.1161/01.res.72.1.75. [DOI] [PubMed] [Google Scholar]
- 59.Fox K, Ford I, Steg PG, Tendera M, Ferrari R Investigators B. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): A randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807–816. doi: 10.1016/S0140-6736(08)61170-8. [DOI] [PubMed] [Google Scholar]
- 60.Swedberg K, Komajda M, Bohm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L Investigators S. Ivabradine and outcomes in chronic heart failure (SHIFT): A randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.