Although nivolumab has shown clinical efficacy in platinum efractory recurrent metastatic head and neck squamous cell carcinoma, its value is less clear given the high price of the drug. This article discusses the cost‐effectiveness of nivolumab compared with docetaxel at the current listed price in Canada and considers whether there are any patient subgroups for whom nivolumab could be cost‐effective.
Keywords: Cost‐effectiveness analysis; Nivolumab; Carcinoma, squamous cell; Carcinoma, metastatic; Drug therapy; Programmed cell death 1 ligand 2 protein; Docetaxel
Abstract
Background.
Treatment options for patients with platinum‐refractory, recurrent, metastatic head and neck squamous cell carcinoma (r/m HNSCC) are limited and prognosis is poor. The recent CheckMate 141 clinical trial demonstrated that nivolumab, an anti‐programmed cell death protein 1 monoclonal antibody, was efficacious in extending the median overall survival (OS) in this patient population compared with standard therapies. We conducted a cost‐effectiveness analysis to determine whether nivolumab is a cost‐effective treatment in this patient population and examined various subgroups to determine for which, if any, the treatment is more cost‐effective.
Materials and Methods.
We implemented a state transition model for HNSCC with a patient cohort who had tumor progression 6 months after the last dose of platinum‐containing chemotherapy and compared the cost‐effectiveness of nivolumab with docetaxel. Treatment effect estimates and adverse event rates were obtained from CheckMate 141. Costs, utilities, and other model inputs were gathered from published sources. We used a Canadian perspective, a 5‐year time horizon, and a 1.5% discount rate for the analysis.
Results.
Nivolumab extended mean OS by 4 months compared with docetaxel and resulted in fewer treatment‐related adverse events, producing an incremental effectiveness of 0.13 quality‐adjusted life years (QALY). The incremental cost of treatment with nivolumab was $18,823. At a willingness‐to‐pay threshold of $100,000/QALY, nivolumab was not a cost‐effective treatment option for r/m HNSCC, with an incremental cost‐effectiveness ratio of $144,744/QALY. Nivolumab would be cost‐effective if its price was reduced by 20%. Our subgroup analysis seemed to indicate that nivolumab might be cost‐effective for tumors with expression of programmed death‐ligand 1 >5%.
Conclusion.
We conclude that although nivolumab offers clinical benefit for the treatment of r/m HNSCC over current regimens, it is not cost‐effective based on its list price. We have also established a value‐based price estimate for nivolumab to be cost‐effective in this patient population. Further study is required to draw a definitive conclusion on biomarkers for cost‐effectiveness.
Implications for Practice.
In health care settings in which cost considerations are a constraint on choice of therapy, patient selection should be carefully considered to maintain efficiency in the system. Until a biomarker for response to therapy is identified for nivolumab, this medication is unlikely to be cost‐effective for most patients with recurrent, metastatic head and neck squamous cell carcinoma.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a cancer originating in the epithelial cells of the oral cavity, oropharynx, nasopharynx, hypopharynx, and larynx. Approximately 95% of all head and neck cancers originate in the squamous cells, while only 5% originate the soft tissue such as the salivary gland. In 2016 there were an estimated 4,600 cases of head and neck cancer in Canada, of which over 4,300 cases were HNSCC [1]. An estimated 560,000 cases of head and neck cancer affected patients globally in 2016, and in the U.S., an estimated 49,670 Americans will develop head and neck cancer in 2017 and 9,700 will die from the disease [2]. Many of the cases of HNSCC are discovered at an advanced stage and 20%–30% result in death [1]. The incidence of HNSCC in men compared with women is approximately 3:1 [1]. Squamous cell carcinoma of the head and neck is strongly associated with environmental and lifestyle risk factors such as the consumption of tobacco, alcohol, and betel quid (also known as paan) [3]. More recently, oropharyngeal infection with human papilloma virus‐16 (HPV‐16) and HPV‐18 has also been identified as an increasingly important risk factor for developing HNSCC [4]. The prognosis of patients with HNSCC secondary to HPV infection is significantly better [5].
Approximately 50%–60% of patients treated for HNSCC will have recurrent disease [6]. The prognosis for recurrent HNSCC is generally poor, and treatment goals are often to reduce pain and discomfort and increase progression‐free survival (PFS) [6]. Treatment options in these patients may sometimes include salvage surgery or reirradiation. Often, patients are not eligible for these interventions and are treated with chemotherapy with palliative intent. Palliative chemotherapy typically involves the use of single agent or a combination of agents, including cetuximab, cisplatin, 5‐fluorouracil, methotrexate, docetaxel, or paclitaxel [6].
Recent studies have shown that 50%–60% of HNSCC tumors express programmed death ligands (PD‐L1 and PD‐L2) that allow them to evade antitumor activity by T cells that express PD‐1 [7]. Furthermore, even though many of these tumors are infiltrated by PD‐1‐positive T cells, they are still able to evade an immune response [8]. In fact, it has been shown that interferon gamma released by T cells locally induces the expression of PD‐L1 by the tumor cells, inhibiting T‐cell antitumor activity [7]. The presence of T cells in HPV‐associated HNSCC is associated with favorable clinical outcomes [9]. The novel agent nivolumab (NIVO) is a human immunoglobulin G4 monoclonal antibody that binds to PD‐1 receptors and blocks their interaction with PD‐L1 and PD‐L2 [10]. This releases the T‐lymphocyte from inhibition and allows it to express its antitumor activity. Nivolumab was approved in Canada in September 2015 for the treatment of non‐small cell lung cancer (NSCLC). Since then, it has also been approved for the treatment of metastatic melanoma and metastatic renal cell carcinoma with various restrictions in both Canada and the U.S. [10]. It has recently garnered approval for the treatment of patients with recurrent or metastatic squamous cell carcinoma of the head and neck (r/m HNSCC) based on the results of CheckMate 141, a randomized controlled trial of 361 patients with r/m HNSCC, in which 240 patients were treated with nivolumab and 121 patients received investigator's choice standard therapy. In this trial, nivolumab increased median overall survival at 1 year by 2.4 months (7.5 months NIVO vs. 5.1 month standard therapy; hazard ratio [HR] for death 0.69, 97.73% confidence interval [CI] 0.51–0.96, p = .01). Progression‐free survival with nivolumab compared with standard therapy did not achieve significance (median time to progression 2.3 months NIVO vs. 2.0 months standard therapy; HR for progression or death 0.89, 95% CI 0.70–1.13, p = .32) [11].
Although nivolumab has shown clinical efficacy in r/m HNSCC, its value for money is less clear given the high price of the drug. The goal of this study is to determine its cost‐effectiveness compared with docetaxel at the current listed price in Canada. The secondary goal is to determine if there are any patient subgroups for whom nivolumab is cost‐effective.
Materials and Methods
Study Design
We developed a state‐transition model of end‐stage HNSCC to assess the cost‐effectiveness of treatment with nivolumab versus docetaxel. A cost utility analysis was performed using a state‐transition model comparing patients treated with nivolumab with those on docetaxel. Analyses were performed per the guidelines for economic evaluation by the Canadian Agency for Drugs and Technologies in Health (CADTH) [12]. The input data were primarily derived from the original clinical trial of nivolumab, Checkmate 141. This report is structured according to guidelines set out by the consolidated health economic evaluation reporting standards [33].
Cohort
The cohort of patients included in our model was based on the reported characteristics of patients enrolled in CheckMate 141. Patients had confirmed recurrent or metastatic head and neck squamous‐cell carcinoma of the oral cavity, pharynx, or larynx that was considered incurable. These patients had tumor progression or recurrence 6 months after the last dose of platinum‐containing chemotherapy, a median age of 60 years, and a relatively high performance status (Eastern Cooperative Oncology Group score ≤1) at the start of the analysis. Our cohort was assumed to have adequate bone marrow, hepatic and renal function, and measurable disease and excluded patients with active brain metastases; autoimmune disease; systemic immune suppression; known human immunodeficiency virus, hepatitis B virus, or hepatitis C virus infection; and any previous therapy targeting T‐cell costimulation or immune checkpoint pathways [11].
Strategies
The two treatment strategies evaluated in our model included single‐agent treatment with nivolumab and single‐agent treatment with docetaxel. Cetuximab is not publicly funded in most jurisdictions for recurrent HNSCC and therefore is not a relevant comparator in Canada. In addition, the evidence for the use of cetuximab in the recurrent/metastatic setting is most relevant in combination with platinum and 5‐fluorouracil (5‐FU) in patients who still have platinum‐sensitive disease (EXTREME trial) [13], which is different from the use of monotherapy cetuximab in the setting of our cohort with platinum‐refractory disease. Methotrexate is also not commonly used in recurrent HNSCC because it is not considered to be as effective as other treatment options [14]. A literature review showed that there is no agent that has definitively proven to be superior in recurrent metastatic disease when patients become platinum‐refractory and that choice of therapy depends on previous therapy, patient status, and tumor progression [15]. Our choice of docetaxel as the comparator represents the most relevant agent in Canada.
Decision Model
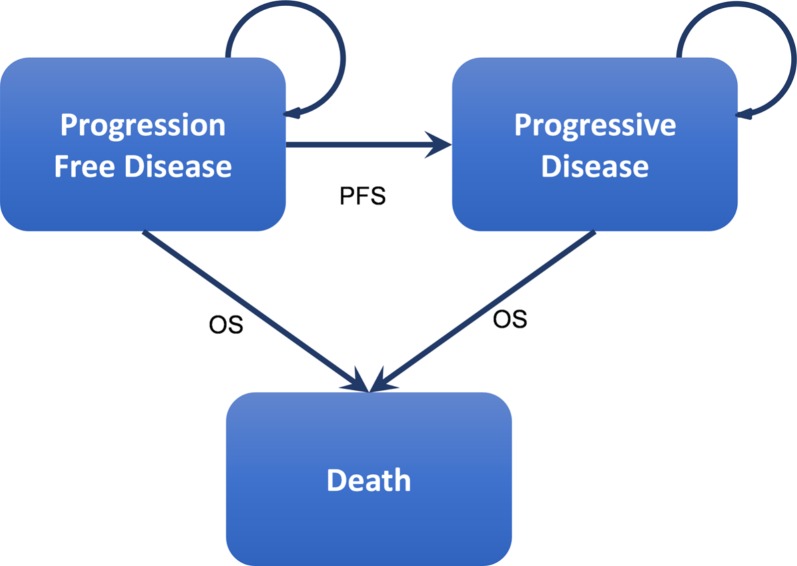
A cohort‐based, state‐transition model was implemented using TreeAge Pro 2017 (TreeAge Software, Inc., Williamston, MA, www.treeage.com) in the form of a cost utility analysis over a 5‐year time horizon. We selected a 5‐year time horizon because survival in this patient population is unlikely to be any longer than 5 years. In each arm of the treatment, patients started in a progression‐free disease state and moved to either progressive disease or death in monthly cycles (Fig. 1). Simulated patients received treatment while they had progression‐free disease and received best supportive care when they transitioned to a progressive disease state. Grade 3 and 4 adverse events for each treatment arm were built into our model in the progression‐free state while patients were on active treatment and, depending on the type of adverse event, patients either continued to receive treatment or therapy was stopped and they transitioned to a progressive disease health state. Transition from a progression‐free disease to a progressive disease followed the PFS curve extracted from the clinical trial data, and similarly, transition to death was based on the OS curve from CheckMate 141 [11] (Fig. 2).
Figure 1.
Health states and progression of recurrent metastatic head and neck squamous cell carcinoma. This figure represents the natural health states of platinum‐refractory, recurrent metastatic head and neck squamous cell carcinoma. The patient is assumed to be in progression‐free disease at the start of the treatment. Treatment is initiated in this stage. At some point the patient transitions to progressive disease and finally death. Patients may also transition to death while in progression‐free disease because of other complications. The PFS curve and OS curve determine the transition probabilities over time.
Abbreviations: OS, overall survival; PFS, progression‐free survival.
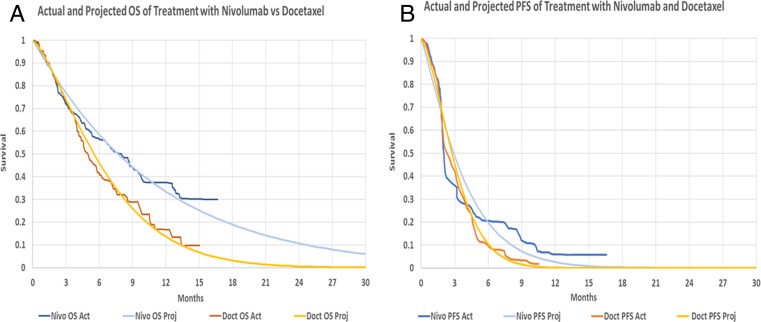
Figure 2.
Projected versus actual OS and PFS curves using the Weibull distribution. (A): Actual and projected OS curves for nivolumab and docetaxel. Actual data are digitized from the original clinical trial. The projection is based on the method described by Guyot et al. [16] of retrieving IPD data from the Kaplan‐Meier curve and the associated at risk data. Weibull distribution was used to project the data. (B): Actual and projected PFS curves for nivolumab and docetaxel. Actual data are digitized from the original clinical trial and projected in a similar manner to OS curves. The projected data do not capture the extended PFS benefit of nivolumab in a small subgroup of patients; however, our analysis shows that even if PFS remained flat beyond 16 months, it would have no impact on the ICER of treatment.
Abbreviations: Act, actual; Doct, docetaxel; ICER, incremental cost‐effectiveness ratio; IPD, individual patient data; Nivo, nivolumab; OS, overall survival; PFS, progression‐free survival; Proj, projected.
Model Inputs
Efficacy.
The efficacy data of each treatment were derived directly from CheckMate 141 trial data by digitizing the PFS and OS Kaplan‐Meier curves for both treatment arms using the open source software WebPlot Digitizer (Ankit Rohatgi, Austin, TX, arohatgi.info) [34]. We retrieved a close approximation of the individual patient time‐to‐event data using the survival curves and published at‐risk data as per the method described by Guyot et al. [16]. Because trial data only extended out to 16 months, we used a Weibull survival distribution to project the OS and PFS to 5 years (Figs. 2 and 3). For our base case analysis, the actual OS and PFS data from the trial were used for time periods in which they were available and the projected data were only used for time periods beyond the trial follow‐up period. This was done to prevent curve fitting errors for available data. For the OS curve, we considered alternate fitted models for the survival and calculated the impact to the incremental cost‐effectiveness ratio (ICER) using different extrapolation distributions. The results of this analysis are available in supplemental online Appendix 1. For the PFS curve, a separate analysis was performed in which nivolumab PFS remained flat out to 60 months.
Adverse Events.
Clinically important grade 3 and 4 adverse events were identified from the CheckMate 141 trial in both treatment arms. These were combined with data from the product monograph for each treatment option to provide a complete picture of all actual and potential treatment‐related adverse events (Table 1). For the base case analysis, the incidence of each treatment‐related adverse event was based on CheckMate 141 trial data. For the sensitivity analysis, data from the product monographs for nivolumab and docetaxel were used to determine the upper limits of incidence of adverse events. If an adverse event reported in the trial did not appear in the product monograph, we increased the trial incidence by 25% to determine the upper limit. If a clinically important adverse event did not occur in the trial but was present in the product monograph, we added it into our model. We used an incidence of zero for the lower limit of each adverse event in the sensitivity analysis.
Table 1. Incidence of clinically important grade 3 and 4 treatment related adverse events for nivolumab and docetaxel.
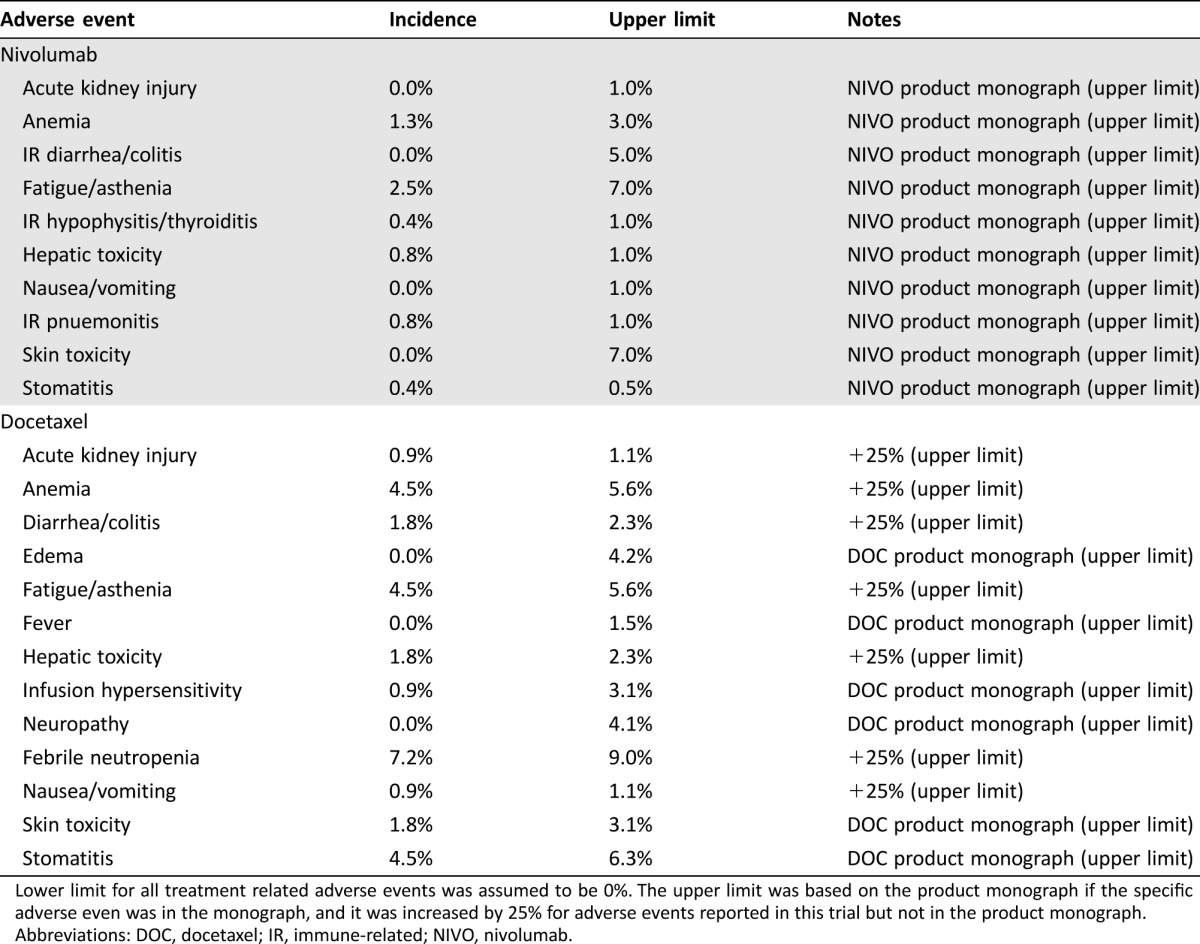
Lower limit for all treatment related adverse events was assumed to be 0%. The upper limit was based on the product monograph if the specific adverse even was in the monograph, and it was increased by 25% for adverse events reported in this trial but not in the product monograph.
Abbreviations: DOC, docetaxel; IR, immune‐related; NIVO, nivolumab.
Costs.
Unit costs and their sources are listed in Table 2. All costs are reported in 2016 Canadian dollars. If an international data source was used to determine the treatment cost of an adverse event, cost was converted to Canadian dollars using the purchasing power parity index for the given year [35], then increased to 2016 dollars using the consumer price index from Statistics Canada [21]. The cost of therapy was calculated in the following manner. For nivolumab, a biweekly regimen at the recommended dose of 3 mg/kg for an average weight of 75 kg at a cost of $19.56/mg was used and adjusted for 6.5% wastage. For docetaxel, a triweekly regimen at the recommended dose of 75 mg/m2 for an average body surface area (BSA) of 1.8 m2 at a cost of $11.42/mg was used. BSA and patient weight were linked in the analysis by using an average body mass index (BMI) of 26.6, resulting in an average height for our patient population of approximately 1.7 m. This was compared with published data [22] and adjusted for the male‐to‐female ratio of 3:1 in HNSCC. We used the Mosteller formula for BSA estimation. To avoid penalizing nivolumab for increased survival, we applied a one‐time cost of $37,346 for best supportive care prior to death [20]. For the sensitivity analysis, we adjusted all costs by ±25% to derive the upper and lower limits. Similarly, patient weight and BMI were also varied by ±25% to determine upper and lower limits for sensitivity analyses.
Table 2. Unit costs of treatment related adverse events, monthly treatment costs, and best supportive care post progression (palliative care).
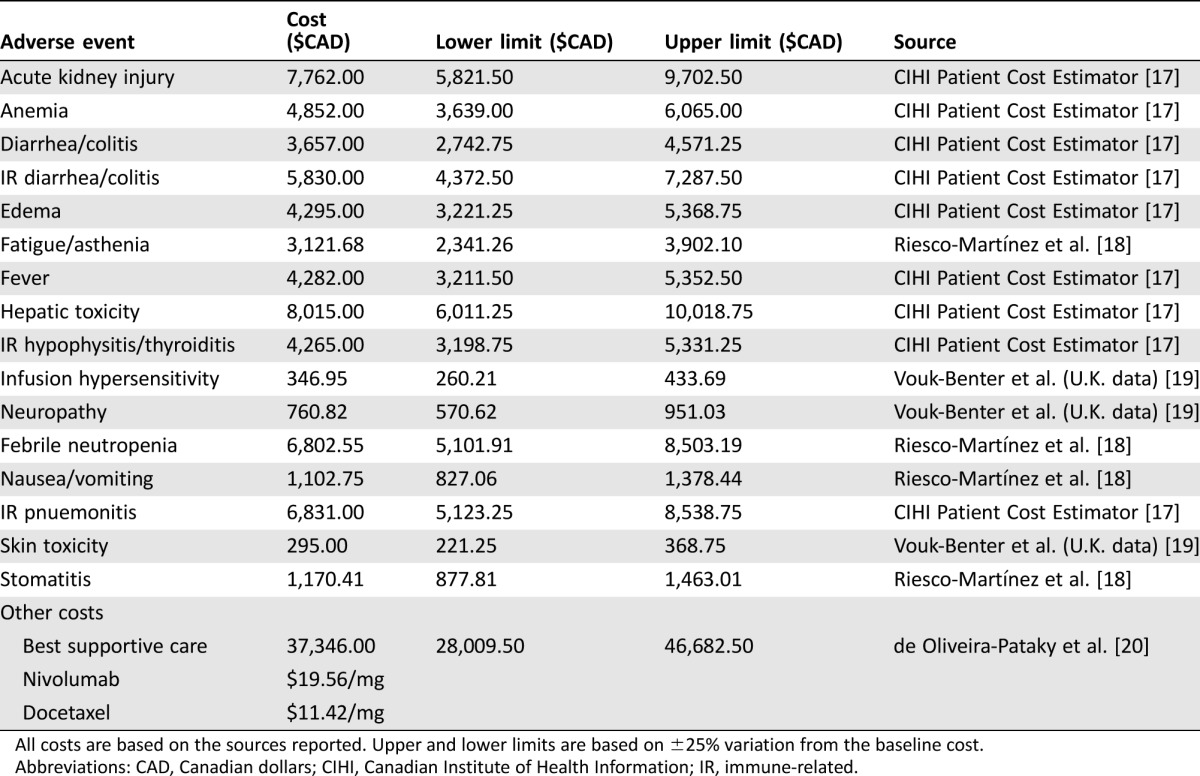
All costs are based on the sources reported. Upper and lower limits are based on ±25% variation from the baseline cost.
Abbreviations: CAD, Canadian dollars; CIHI, Canadian Institute of Health Information; IR, immune‐related.
Utilities.
Utility values and sources are outlined in Table 3. Utilities associated with the baseline health state are based on clinical trial data from CheckMate 141. For each adverse event, a utility deduction was assessed. The utility deductions were based on published Canadian and U.S. studies of chemotherapy‐associated adverse events [18], [23], [24]. The values were calculated as the difference in EQ‐5D scores before the adverse event and after the adverse event as reported in the literature. Lower and upper limits were derived by adjusting utility values by ±25%.
Table 3. Utility values associated with grade 3and 4 treatment related adverse events and health states.
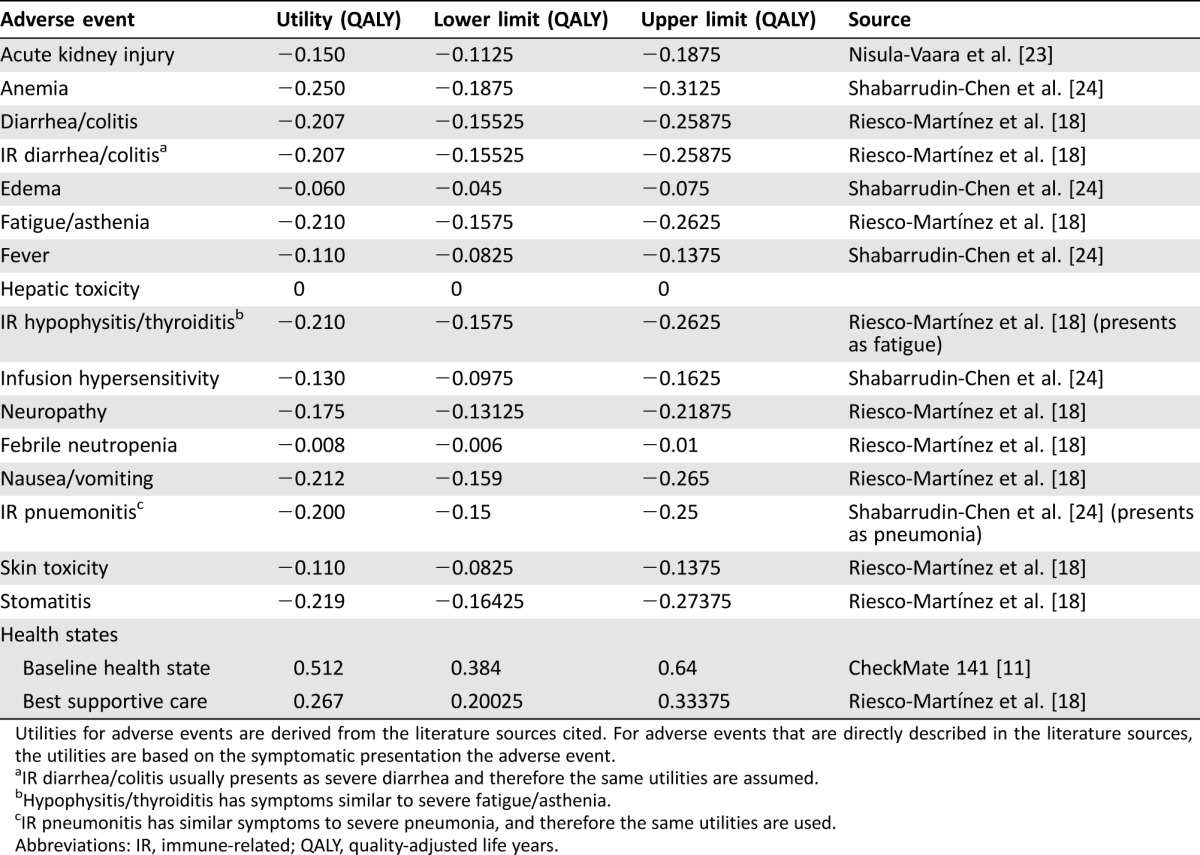
Utilities for adverse events are derived from the literature sources cited. For adverse events that are directly described in the literature sources, the utilities are based on the symptomatic presentation the adverse event.
IR diarrhea/colitis usually presents as severe diarrhea and therefore the same utilities are assumed.
Hypophysitis/thyroiditis has symptoms similar to severe fatigue/asthenia.
IR pneumonitis has similar symptoms to severe pneumonia, and therefore the same utilities are used.
Abbreviations: IR, immune‐related; QALY, quality‐adjusted life years.
Economic Assumptions
The economic analysis was conducted from the perspective of the Canadian health care system and structured as a cost utility analysis. The Canadian health care system is a universal access health care system that covers the cost of care delivered in a hospital setting. In cancer therapy, the cost of a prescribed chemotherapy regimen delivered in a hospital setting is also covered. The primary outcome of interest was the ICER, defined as the incremental cost to gain one quality‐adjusted life year ($/QALY). All costs and benefits were discounted at 1.5% annually, per CADTH guidelines [12]. We used a willingness‐to‐pay (WTP) threshold of $100,000 per QALY for cost‐effectiveness. This value is 1.76 times the Canadian gross domestic product (GDP) per capita of $56,736.58 in the fourth quarter of 2016 [21]. We also reported results for a WTP of $150,000, which is 2.64 times the per‐capita GDP. In 2016, $1 Canadian dollar (CAD) = $.7880 U.S. dollars (USD) using the purchasing power parity index [25].
Analytic Strategy
We conducted a base‐case analysis to determine the ICER of treatment with nivolumab compared with docetaxel. We then completed a deterministic one‐way sensitivity analysis on all variables in our model, using the upper and lower limits previously described, to identify which of these would have the greatest impact on the ICER of treatment. Finally, a probabilistic sensitivity analysis (PSA) was completed using a Monte Carlo simulation for 10,000 iterations to determine the probability that treatment with nivolumab would be cost‐effective at various WTP thresholds. Drug price was not included as a variable in either the deterministic or probabilistic sensitivity analyses; however, we ran our model using a 10%, 20%, and 25% price discount on the cost of nivolumab (vs. the marketed price) to see how that would affect the ICER of treatment.
We also conducted an analysis using the HR for death reported by subgroup in the CheckMate 141 trial, as well as in the exploratory analysis that was conducted according to tumor PD‐L1 expression and p16 status. We calculated the transition probabilities for each patient subgroup using the hazard ratios reported in the clinical trial. We used this new OS curve and the original PFS curve in our state transition model to calculate the ICER for treatment with nivolumab. We compared the ICER of nivolumab with docetaxel for various patient subgroups of interest. A PSA was conducted for each patient subgroup to determine in which subgroups treatment with NIVO would most likely be cost‐effective.
Validation
We validated our model using a Monte Carlo simulation with 10,000 patients. We tracked the time of death and time to progression in our model and plotted Kaplan‐Meier survival curves for both. These were visually compared with the OS and PFS curves from the trial results.
Results
Base Case Analysis
In our base model, nivolumab (mean cost $60,035, mean effectiveness 0.248 QALY, mean OS 10.3 months) was compared with docetaxel (mean cost $41,212, mean effectiveness 0.118 QALY, mean OS 6.2 months), resulting in an incremental average cost of $18,823 and an incremental effectiveness of 0.130 QALY. The calculated ICER for the base case analysis was $144,744 per QALY (Table 4). At a WTP threshold of $100,000 per QALY, nivolumab is not a cost‐effective treatment option for r/m HNSCC; however, at a WTP threshold of $150,000, it is cost‐effective. If PFS for nivolumab was kept flat out to 60 months, the impact on ICER was negligible.
Table 4. Results of the base‐case cost‐effectiveness analysis.
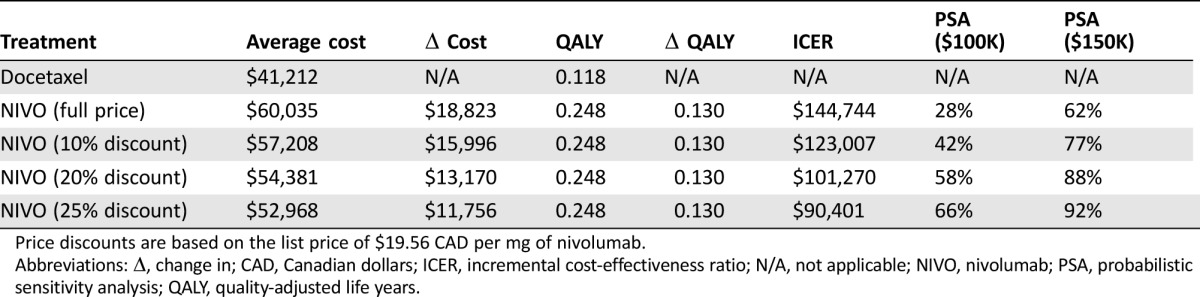
Price discounts are based on the list price of $19.56 CAD per mg of nivolumab.
Abbreviations: Δ, change in; CAD, Canadian dollars; ICER, incremental cost‐effectiveness ratio; N/A, not applicable; NIVO, nivolumab; PSA, probabilistic sensitivity analysis; QALY, quality‐adjusted life years.
Subgroup Analysis Based on Hazard Ratio
In the absence of survival curves for each treatment subgroup, we performed an analysis based on the hazard ratio for death to compare the ICER of nivolumab with docetaxel for different patient subgroups. Using this approach, higher levels of tumor PD‐L1 expression correlated with an increased cost‐effectiveness of treatment with nivolumab compared with docetaxel. In fact, for patients with tumor PD‐L1 expression greater than 5%, nivolumab was cost‐effective at a WTP threshold of $100,000. These results do not include the cost of a PD‐L1 immunohistochemical (IHC) 22C3 pharmDx assay. The ICER of nivolumab versus docetaxel was also lower for patients with p16 positive tumors (due to HPV infection) and patients who were cetuximab naïve (Table 5), making nivolumab more cost‐effective for these patient populations. In both patient subgroups, a WTP threshold of $100,000 was not achieved. Nivolumab had the highest ICER and was least cost‐effective for patients over the age of 65.
Table 5. Hazard ratio subgroup analysis.
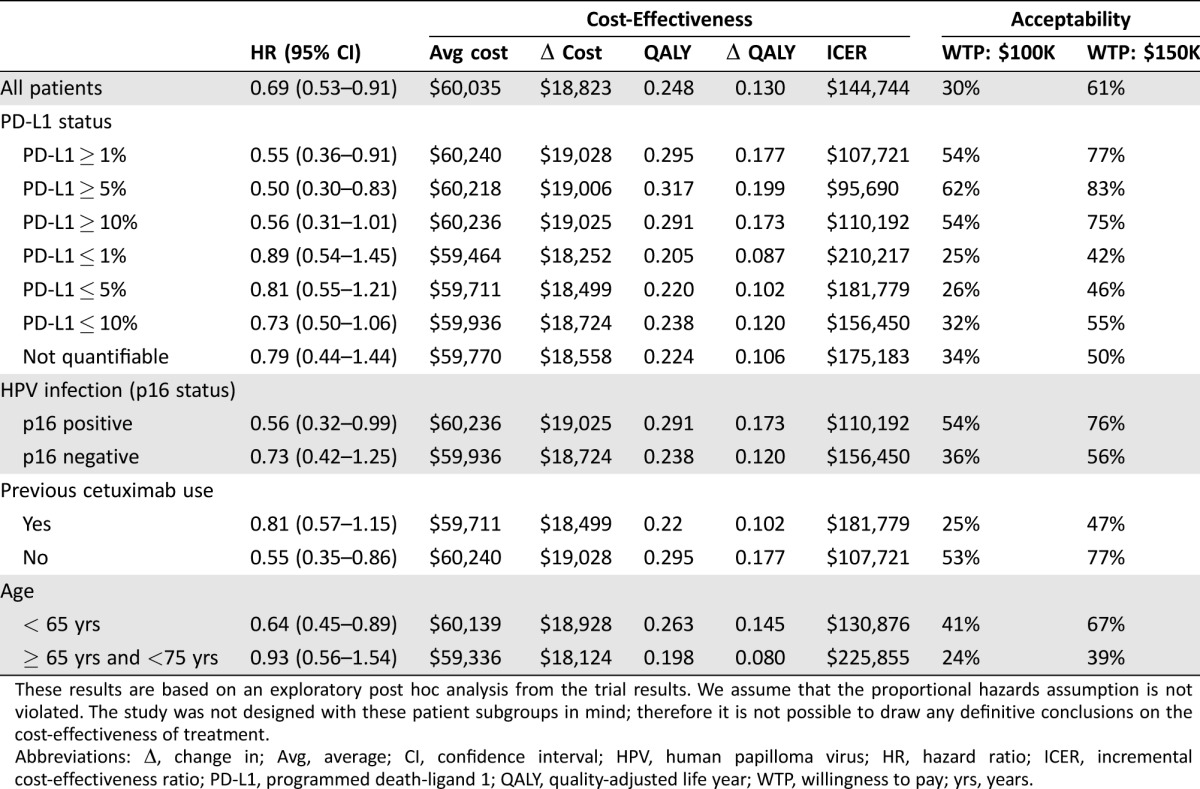
These results are based on an exploratory post hoc analysis from the trial results. We assume that the proportional hazards assumption is not violated. The study was not designed with these patient subgroups in mind; therefore it is not possible to draw any definitive conclusions on the cost‐effectiveness of treatment.
Abbreviations: Δ, change in; Avg, average; CI, confidence interval; HPV, human papilloma virus; HR, hazard ratio; ICER, incremental cost‐effectiveness ratio; PD‐L1, programmed death‐ligand 1; QALY, quality‐adjusted life year; WTP, willingness to pay; yrs, years.
One‐Way Sensitivity Analysis
Detailed results of our one‐way sensitivity analysis, including the tornado diagram, are discussed in supplemental online Appendix 1. Not surprisingly, patient weight is the most sensitive variable affecting the ICER of treatment with nivolumab compared with docetaxel, because it directly affects the amount of drug needed and therefore the cost of treatment. As we varied the weight of the patient by 25% in either direction, the ICER ranged from a low of $98,797 to a high of $191,206. A one‐way sensitivity analysis on the cost of nivolumab shows that this drug would be cost‐effective for the treatment of r/m HNSCC patients at a WTP of $100,000 if it was priced at $15.53/mg instead of $19.56/mg. This represents a 20.6% price reduction from the current list price of nivolumab in Canada.
Probability Sensitivity Analysis
Using a probabilistic model improves the ICER of treatment with nivolumab to $133,348. Even so, a PSA shows that at a WTP of $100,000 nivolumab is only cost‐effective in 28% of cases. Nivolumab starts to become cost‐effective at a price discount of 20%–25% when the probability of cost‐effectiveness is in the range of 58%–66%. At a WTP of $150,000, nivolumab is likely to be cost‐effective in 62% of use cases at the full listed price and 88% of cases at a 20% price discount.
Discussion
The results of our analysis show that if we consider the conventional WTP threshold of $100,000 as our cut‐off [26], [27], nivolumab at an ICER of $144,744 is not a cost‐effective treatment option for routine use in r/m HNSCC compared with docetaxel. Similar economic evaluations of nivolumab for the treatment of various tumors types have consistently come to the same conclusion. An economic evaluation of nivolumab for the treatment of NSCLC in Canada resulted in an ICER of $151,560 CAD per QALY compared with docetaxel [28]. Similarly, in Switzerland, an evaluation of nivolumab compared with docetaxel for the treatment of advanced NSCLC resulted in an ICER of 177,478 Swiss francs ($245,962 CAD) per QALY [29]. An economic evaluation of nivolumab compared with everolimus for the treatment of metastatic renal cell carcinoma in the U.S. concluded that at an ICER of $151,676 USD per QALY it was unlikely to be a high‐value treatment [30].
Given that patient‐reported quality of life outcomes are higher and treatment‐related adverse events are lower with nivolumab, our analysis shows that it could become a cost‐effective treatment option for r/m HNSCC at a price discount of 20% versus the current list price of $19.59/mg in Canada.
A preliminary subgroup analysis based on hazard ratios seems to indicate that nivolumab is more cost‐effective for patients with high PD‐L1 expression. There is currently conflicting evidence with regard to whether PD‐L1 expression is a predictive biomarker for response to nivolumab therapy [31]. In our analysis, it seems to have a positive correlation with cost‐effectiveness; however, the clinical impact of this is not known. Based on our hazard ratio analysis, using nivolumab to treat patients with tumor PD‐L1 expression greater than 5% results in an ICER of $95,695, making it a cost‐effective treatment compared with docetaxel for these patients. For patients with PD‐L1 expression less than 5%, the ICER of treatment with nivolumab versus docetaxel is $181,779. However, when comparing the ICER of treatment with nivolumab between the two patient subgroups directly, there is only a difference of $5,200/QALY in favor of the high PD‐L1 group. This conflicting evidence seems to support the idea that PD‐L1 levels should not be used as a biomarker to direct patient choice even from a cost‐effectiveness perspective.
Although this evaluation did not find nivolumab to be cost‐effective as a routine treatment option in r/m HNSCC, many other factors must be considered when making the decision to fund a treatment. For example, recent evidence has shown that HNSCC tumors that express high levels of PD‐L1 are resistant to radiation therapy [32]. In these patients, nivolumab may have an important role in treatment and may even be cost‐effective when combined with radiation therapy. Similarly, nivolumab may have a place in therapy for those patients with lower performance status or severe myelosuppression in which docetaxel is not indicated.
Limitations
In the clinical trial of nivolumab in HNSCC (CheckMate 141), the comparator arm consisted of docetaxel, cetuximab, or methotrexate. The latter two drugs are rarely used in Canada for this stage of HNSCC, and they may have had an impact on the effectiveness of treatment in the standard arm of the clinical trial. Furthermore, nivolumab may be used earlier in r/m HNSCC prior to docetaxel, but we had no way to evaluate the economic outcome of this treatment modality compared with single agent nivolumab or single agent docetaxel, because the data were lacking. Finally, the study results seem to indicate that nivolumab provides a longer mortality benefit in a subset of patients with HNSCC. PD‐L1 status may be a determinant of response to treatment; however, without a head‐to‐head comparison of nivolumab in patients expressing high levels of PD‐L1 with those with low PD‐L1 expression, we can draw no definitive conclusions about cost‐effectiveness of therapy in the two patient groups.
Nivolumab has been publicly funded in Canada for its currently approved indications in melanoma, NSCLC, and renal cell carcinoma since March 2017, and it is not yet publicly funded for HNSCC. At this point it is too early to tell if there is a benefit to patients and to the health care system beyond an improved side effect profile. It may well be that patients on nivolumab use fewer health care resources compared with patients on traditional cytotoxic chemotherapy. Once more data are available, a future study should compare patients’ use of health care resources such as supportive care, hospitalizations, urgent care visits, and prophylactic medication with use by patients on traditional cytotoxic chemotherapy. This is particularly true in advanced HNSCC, in which patients struggle with symptoms such as dry mouth, difficulty swallowing, pain, and fatigue. Even speaking and eating may be difficult at times [35]. At this time, it is unclear how much nivolumab may reduce patients’ reliance on ancillary health care services.
Another limitation of this study is that we did not include infusion‐related costs in our model. Docetaxel may be infused weekly for 1 hour or once every 3 weeks for 1 hour. Nivolumab is generally infused once every 2 weeks for 1 hour. Depending on the chosen docetaxel regimen, this may have a small positive or negative impact on the ICER of treatment with nivolumab. Infusion‐related adverse events are generally less frequent with nivolumab, possibly resulting in additional cost savings. More data are required to assess the full impact of infusion‐related costs.
Conclusion
At an ICER of $144,744, this evaluation did not find nivolumab to be cost‐effective as a routine treatment option in r/m HNSCC; however, preliminary evidence seems to suggest nivolumab may be more cost‐effective in a certain subset of patients. Further work needs to be done to find a biomarker that can help identify the patients who will benefit more and have fewer side effects from nivolumab. Doing so will improve the cost‐effectiveness of nivolumab when used in this population. In a publicly funded health care system with finite resources, cost‐effectiveness is an important factor that must be considered when deciding on public funding of treatments to optimize resource allocation and maximize population health outcomes.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
Dr. Wong was supported by the Canadian Liver Foundation, the Canadian Institutes of Health Research, and the Natural Sciences and Engineering Research Council of Canada.
Footnotes
Editor's Note: See the related commentary, “Immunotherapy for Head Neck Cancer in the Era of Exponentially Increasing Health Care Expenditure,” by Rafael Santana‐Davila and Cristina P. Rodriguez on page 147 of this issue.
Author Contributions
Conception/design: Thomas McFarlane, Kelvin K.W. Chan, William W.L. Wong
Collection and/or assembly of data: Mahdi Zargar
Data analysis and interpretation: Mahdi Zargar, Thomas McFarlane, Kelvin K.W. Chan, William W.L. Wong
Manuscript writing: Mahdi Zargar, Thomas McFarlane, Kelvin K.W. Chan, William W.L. Wong
Final approval of manuscript: Mahdi Zargar, Thomas McFarlane, Kelvin K.W. Chan, William W.L. Wong
Disclosures
The authors indicated no financial relationships.
References
- 1.Canadian Cancer Society. Oral cancer statistics. Available at http://www.cancer.ca/en/cancer-information/cancer-type/oral/statistics/?region=on. Accessed January 25, 2017.
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Canadian Cancer Society. Risk Factors for Oral Cavity Cancer. Available at http://www.cancer.ca/en/cancer-information/cancer-type/oral/risks/?region=on. Accessed February 1, 2017.
- 4. Kreimer AR, Clifford GM, Boyle P et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol Biomarkers Prev 2005;14:467–475. [DOI] [PubMed] [Google Scholar]
- 5. Elrefaey S, Massaro MA, Chiocca S et al. HPV in oropharyngeal cancer: The basics to know in clinical practice. Acta Otorhinolaryngol Ital 2014;34:299–309. [PMC free article] [PubMed] [Google Scholar]
- 6. Vermorken JB, Specenier P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol 2010;21(suppl 7):vii252–vii261. [DOI] [PubMed] [Google Scholar]
- 7. Moy JD, Moskovitz JM, Ferris RL. Biological mechanisms of immune escape and implications for immunotherapy in head and neck squamous cell carcinoma. Eur J Cancer 2017;76:152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bauman JE, Cohen E, Ferris RL et al. Immunotherapy of head and neck cancer: Emerging clinical trials from a National Cancer Institute Head and Neck Cancer Steering Committee Planning Meeting. Cancer 2017;123:1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Badoual C, Hans S, Merillon N et al. PD‐1‐expressing tumor‐infiltrating T cells are a favorable prognostic biomarker in HPV‐associated head and neck cancer. Cancer Res 2013;73:128–138. [DOI] [PubMed] [Google Scholar]
- 10. Raedler LA. Opdivo (nivolumab): Second PD‐1 inhibitor receives FDA approval for unresectable or metastatic melanoma. Am Health Drug Benefits 2015;8:180–183. [PMC free article] [PubMed] [Google Scholar]
- 11. Ferris RL, Blumenschein GJ, Fayette J et al. Nivolumab for recurrent squamous‐cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canadian Agency for Drugs and Technologies in Health. Guidelines for the Economic Evaluation of Health Technologies . 3rd Edition. Ottawa, Ontario: Canadian Agency for Drugs and Technologies in Health, 2006. Available at http://www.deslibris.ca/ID/203719. Accessed February 1, 2017.
- 13. Vermorken JB, Mesia R, Rivera F et al. Platinum‐based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359:1116–1127. [DOI] [PubMed] [Google Scholar]
- 14. Guardiola E, Peyrade F, Chaigneau L et al. Results of a randomised phase II study comparing docetaxel with methotrexate in patients with recurrent head and neck cancer. Eur J Cancer 2004;40:2071–2076. [DOI] [PubMed] [Google Scholar]
- 15. Peyrade F, Cupissol D, Geoffrois L et al. Systemic treatment and medical management of metastatic squamous cell carcinoma of the head and neck: Review of the literature and proposal for management changes. Oral Oncol 2013;49:482–491. [DOI] [PubMed] [Google Scholar]
- 16. Guyot P, Ades A, Ouwens MJ et al. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan‐Meier survival curves. BMC Med Res Methodol 2012;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canadian Institute for Health Information. Patient Cost Estimator. February 21, 2017. Available at https://www.cihi.ca/en/patient-cost-estimator. Accessed April 9, 2017.
- 18. Riesco‐Martínez MC, Berry SR, Ko YJ et al. Cost‐effectiveness analysis of different sequences of the use of epidermal growth factor receptor inhibitors for wild‐type KRAS unresectable metastatic colorectal cancer. J Oncol Pract 2016;12:e710–e723. [DOI] [PubMed] [Google Scholar]
- 19. Vouk K, Benter U, Amonkar MM et al. Cost and economic burden of adverse events associated with metastatic melanoma treatments in five countries. J Med Econ 2016;19:900–912. [DOI] [PubMed] [Google Scholar]
- 20. de Oliveira C, Pataky R, Bremner KE et al. Phase‐specific and lifetime costs of cancer care in Ontario, Canada. BMC Cancer 2016;16:809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Statistics Canada . Canada: Economic and Financial Data. April 7, 2017. Available at http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/dsbbcan-eng.htm. Accessed April 9, 2017.
- 22. Risk Factor Collaboration NCD. A century of trends in adult human height. Elife; 2016;5:e13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nisula S, Vaara ST, Kaukonen KM et al. Six‐month survival and quality of life of intensive care patients with acute kidney injury. Crit Care 2013;17:R250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shabaruddin FH, Chen LC, Elliott RA et al. A systematic review of utility values for chemotherapy‐related adverse events. Pharmacoeconomics 2013;31:277–288. [DOI] [PubMed] [Google Scholar]
- 25.Organisation for Economic Co‐Operation and Development. Purchasing Power Parities (PPP). 2017. Available at http://data.oecd.org/conversion/purchasing-power-parities-ppp.htm. Accessed February 5, 2017.
- 26. Neumann PJ, Cohen JT, Weinstein MC. Updating cost‐effectiveness–the curious resilience of the $50,000‐per‐QALY threshold. N Engl J Med 2014;371:796–797. [DOI] [PubMed] [Google Scholar]
- 27. Bertram MY, Lauer JA, De Joncheere K et al. Cost‐effectiveness thresholds: Pros and cons. Bull World Health Organ 2016;94:925–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goeree R, Villeneuve J, Goeree J et al. Economic evaluation of nivolumab for the treatment of second‐line advanced squamous NSCLC in Canada: A comparison of modeling approaches to estimate and extrapolate survival outcomes. J Med Econ 2016;19:630–644. [DOI] [PubMed] [Google Scholar]
- 29. Matter‐Walstra K, Schwenkglenks M, Aebi S et al. A cost‐effectiveness analysis of nivolumab versus docetaxel for advanced nonsquamous NSCLC including PD‐L1 testing. J Thorac Oncol 2016;11:1846–1855. [DOI] [PubMed] [Google Scholar]
- 30. Wan XM, Peng LB, Ma JA et al. Economic evaluation of nivolumab as a second‐line treatment for advanced renal cell carcinoma from US and Chinese perspectives. Cancer 2017;123:2634–2641. [DOI] [PubMed] [Google Scholar]
- 31. Fusi A, Festino L, Botti G et al. PD‐L1 expression as a potential predictive biomarker. Lancet Oncol 2015;16:1285–1287. [DOI] [PubMed] [Google Scholar]
- 32. Skinner HD, Giri U, Yang LP et al. Integrative analysis identifies a novel AXL‐PI3 kinase‐PD‐L1 signaling axis associated with radiation resistance in head and neck cancer. Clin Cancer Res 2017;23:2713–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Husereau D, Drummond M, Petrou S et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346:f1049. [DOI] [PubMed] [Google Scholar]
- 34. Rohatgi A. WebPlot Digitizer. Available at http://arohatgi.info/WebPlotDigitizer/. Accessed January 14, 2017.
- 35. Oliveira KG, von Zeidler SV, Podestá JR et al. Influence of pain severity on the quality of life in patients with head and neck cancer before antineoplastic therapy. BMC Cancer 2014;14:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.