This article reports the outcomes of a comprehensive specialized interdisciplinary team intervention that was designed to help manage patients with cancer with aberrant opioid‐related behavior.
Keywords: Interdisciplinary approach, Team, Intervention, Opioids, Cancer, Aberrant behavior
Abstract
Background.
Data on the development and outcomes of effective interventions to address aberrant opioid‐related behavior (AB) in patients with cancer are lacking. Our outpatient supportive care clinic developed and implemented a specialized interdisciplinary team approach to manage patients with AB. The purpose of this study was to report clinical outcomes of this novel intervention.
Materials and Methods.
The medical records of 30 consecutive patients with evidence of AB who received the intervention and a random control group of 70 patients without evidence of AB between January 1, 2015, and August 31, 2016, were reviewed.
Results.
At baseline, pain intensity (p = .002) and opioid dose (p = .001) were significantly higher among patients with AB. During the course of the study, the median number of ABs per month significantly decreased from three preintervention to 0.4 postintervention (p < .0001). The median morphine equivalent daily dose decreased from 165 mg/day at the first intervention visit to 112 mg/day at the last follow‐up (p = .018), although pain intensity did not significantly change (p = .984). “Request for opioid medication refills in the clinic earlier than the expected time” was the AB with the highest frequency prior to the intervention and the greatest improvement during the study period. Younger age (p < .0001) and higher Edmonton Symptom Assessment System anxiety score (p = .005) were independent predictors of the presence of AB.
Conclusion.
The intervention was associated with a reduction in the frequency of AB and opioid utilization among patients with cancer receiving chronic opioid therapy. More research is needed to further characterize the clinical effectiveness of this intervention.
Implications for Practice.
There are currently no well‐defined and evidence‐based strategies to manage cancer patients on chronic opioid therapy who demonstrate aberrant opioid‐related behavior. The findings of this study offer a promising starting point for the creation of a standardized strategy for clinicians and provides valuable information to guide their practice regarding these patients. The study results will also help clinicians to better understand the types and frequencies of the most common aberrant behaviors observed among patients with cancer who are receiving chronic opioid therapy. This will enhance the process of timely patient identification, management, or referral to the appropriate specialist teams.
Introduction
Although chronic opioid therapy is the main treatment of cancer‐related pain [1], [2], its aberrant use has resulted in significant challenges in cancer pain management. Prescription opioids account for 18.9% of all drug‐related deaths [3]. The magnitude of the issue prompted the implementation of certain key nationwide measures, including the recent release of a U.S. Centers for Disease Control and Prevention guideline for prescribing opioids for chronic non‐cancer‐related pain [4], an unprecedented letter from the Surgeon General to U.S. physicians regarding the cautious use of opioids [5], and an American Society of Clinical Oncology policy statement on access to opioids for cancer pain [6]. With the support of the U.S. Congress, the Obama administration signed into law the Comprehensive Addiction and Recovery Act of 2016, intended to develop programs and increase access to treatment as part of efforts to combat the opioid epidemic [7]. There has been an inherent challenge in the medical community to maintain the complex harmony between ensuring legitimate access to opioids and minimizing complications from its aberrant use by patients with chronic pain and the general public.
Patients with advanced cancer were previously felt to be at lower risk for opioid abuse [8], [9]. However, recent evidence indicates that it may be more prevalent than was previously thought [10], [11], [12], [13], [14]. Although such patients represent a minority of patients with cancer receiving chronic opioid therapy [15], they typically consume a significant amount of time and resources at the expense of opioid‐adherent and critically ill patients who need the clinic staff the most. In view of these significant challenges, there is a need for clinicians to develop strategies to address this issue more effectively in routine clinical practice.
Various measures have been suggested, such as universal screening using validated risk assessment tools [16], ongoing monitoring of aberrant opioid‐related behavior (AB) [17], [18], and the use of the prescription monitoring program database [19] and urine drug screens (UDS) [20], [21], [22]. However, there are limited data regarding outcome assessment of these measures’ impact on aberrant opioid use. Our outpatient supportive care clinic (SCC) developed and implemented a comprehensive specialized interdisciplinary team intervention to help manage patients with AB. The purpose of this study was to report outcomes of this intervention. We sought to determine the frequency and type of common ABs and examine the changes in patient behaviors, symptoms, and opioid use within 3 months following the intervention. We also obtained exploratory data on the predictors of AB among patients with cancer receiving opioid therapy.
Materials and Methods
Study Participants and Procedure
The medical records of 100 patients seen at the University of Texas MD Anderson SCC from January 1, 2015, to August 31, 2016, were reviewed. Patients were eligible if they were 18 years or older, had a current or past diagnosis of cancer, and were receiving chronic opioid therapy (defined as the treatment of pain with opioids for 7 or more days [18]). The sample consisted of two different cohorts. Cohort A was a purposeful sample of 30 consecutive patients with documented evidence of AB who received the specialized interdisciplinary team intervention. Cohort B was a random sample of 70 patients without documented evidence of AB who were seen in the clinic during the same period. This sample was selected for comparison of key clinical characteristics.
Data Collection
Patient demographic and clinical characteristics were obtained at the initial consultation visit, intervention visits, and last visit within 3 months following the date of intervention. Characteristics included age, gender, race, cancer diagnosis, cancer stage, educational status, insurance status, morphine equivalent daily dose (MEDD), Edmonton Symptom Assessment System (ESAS) [23], [24], [25], performance status, Memorial Delirium Assessment Scale [26], [27], and Cut Down, Annoyed, Guilty, and Eye Opener questionnaire adapted to include drugs (CAGE‐AID) score [14], [28], [29]. For those who received the intervention, we carefully reviewed each of their patient encounters at the University of Texas MD Anderson Center within the preceding month and each visit within 3 consecutive months following the intervention in order to obtain information regarding ABs. These encounters included all inpatient and outpatient visits with any physician, emergency room visits, and telephone calls.
Data on the various aberrant opioid‐related events for all patients were independently gathered by two different investigators; both had the clinical experience and expertise to recognize the presence of those events in the patients’ medical charts. These two investigators then met with a third investigator to compare their results, and any discrepancy was discussed in detail until a mutual agreement was reached among the three investigators.
We identified and tabulated the following eight commonly identified ABs based on literature [17], [18], [30], [31]: “Request for opioid medication refills in the clinic (either via phone or in person) earlier than the expected time,” “request for excessive dose increase of the opioid medication not consistent with patient's pain syndrome (either via phone or in person),” “reports of lost or stolen opioid prescription/medication,” “seeking opioids from multiple providers (e.g., primary oncologists, emergency room physicians, outside physicians),” “request for specific opioid medications,” “resistance to changes in the opioid regimen,” “reports of the use of street drug,” and “abnormal UDS result if not accounted for above.” Any other AB was documented as “other” (such as reports of impaired functioning in daily activities due to opioid use, family member concerns about patient's inappropriate opioid use, and reports of tampering with or forging opioid prescriptions).
The Supportive Care Clinic Process
The University of MD Anderson SCC sees mainly patients with advanced cancer and a smaller number with early‐stage disease or in early remission [32]. In accordance with the routine delivery of care, the patient and family are initially assessed by the nurse, who gathers pertinent clinical information with the use of a template. The findings are discussed with the palliative care physician, who then conducts an interview with the patient and family, does a physical examination, and subsequently formulates the assessment and plan. Other members of the team are involved in the care of the patient when necessary.
Intervention
The intervention in this study is an interdisciplinary intervention provided by a specialized team called the Compassionate High Alert Team (CHAT). The team consists of a palliative care physician and two or more of the following members: a palliative care trained registered nurse, psychologist or counsellor, pharmacist, social worker, and patient advocate. Representatives from the risk management department or security personnel may be involved when legal or safety issues become imminent. When the physician and/or clinic nurse identifies AB based on history, physical examination, risk assessment tools and/or UDS, they will involve the team. Prior to the patient visit, the team will meet to debrief on the case, derive strategies, and formulate a plan. They will then collectively have a “chat” with the patient during his or her clinic visit to address any concerning issues related to the patient's opioid use, openly discussing the goals of opioid therapy, expectations, potential risks, and alternatives related to their pain management. The encounter is conducted in a supportive and nonjudgmental manner with emphasis on the need for patient and family safety. Certain measures to ensure patient safety may be instituted when necessary, such as decreasing the time interval between follow‐ups for refills, limiting the opioid quantity and doses at each visit, and transitioning a patient from opioid analgesics to nonopioid analgesics or nonpharmacological interventions.
At the end of the visit, the attending physician will document the outcome of the encounter, overall assessment, and plan of care. The physician will then determine the need for the patient to continue receiving the intervention at subsequent clinic visits until a positive behavioral change is observed.
Roles of the Specialized Interdisciplinary Team Members
SCC physician: determines and approves the need for the team approach, leads and facilitates the discussion, reinforces education on opioid safety and guidelines, documents the outcome of the patient visit, and formulates and outlines the subsequent treatment plan related to the patient's care.
SCC nurse: coordinates patient care, performs initial patient screening using risk assessment tools, conducts baseline medication review, helps to identify and coordinate pertinent team members, and provides education on opioid safety.
SCC psychologist or counselor: provides supportive counselling to the patient and patient's family and provides coping techniques and nonpharmacologic means for pain management.
SCC social worker: assesses patient, family, and caregiver needs, provides counselling, facilitates logistical issues, and explores community resources available for patients.
SCC pharmacist: assists with medication review and patient education, monitors the state prescription drug monitoring program database, and assists with interpretation of UDS results.
Patient advocate: provides patient support and facilitates optimal communication between the team members and patient or caregiver.
These roles are applied when all team members are available. When a team member is absent, his or her duties will be reassigned among those present, if possible, to ensure that all the aspects of the interdisciplinary intervention are delivered to the patient during the CHAT encounter.
Statistical Analysis
Descriptive statistics of patients’ demographics, clinical characteristics, the number of ABs (per patient per type of AB for each defined period), and the change in the number of ABs were provided using mean, standard deviation, median, range, frequency, and percentage. The Wilcoxon rank sum test was used to examine the difference in patients’ characteristics in continuous measures between two groups (intervention vs. no intervention). The chi‐square test or Fisher's exact test, as appropriate, was used to examine the difference in patients’ categorical characteristics between two groups. The Wilcoxon signed‐rank test was used to examine patients’ change of ESAS measures between two time points (at intervention and last follow‐up visit within the study period) and also to examine patients’ change in number of ABs before and after intervention (standardized as “per month”). Univariate and multicovariate logistic analyses were used to examine the association between patients’ characteristics and the presence of AB.
We also determined the number of patients who attained complete response, partial response, stable response, or worse response to the intervention in each consecutive month following the intervention. All computations were carried out in SAS 9.3 (SAS Institute, Inc., Cary, NC, www.sas.com).
Definition of Terms.
For the purposes of our study, we defined complete response as the absence of any ABs within the month, partial response as a decrease from baseline in the number of ABs within the month, stable response as no change from baseline in the number of ABs within the month, and worse response as an increase from baseline in the number of ABs within the month. Results were analyzed according to intention‐to‐treat. Therefore, patients who did not return for follow‐up each month were considered nonresponders.
The total symptom distress score (SDS) was calculated as the sum of all the ESAS scores. A higher score indicates higher symptom distress, whereas a lower score indicates less symptom distress [33], [34], [35]. In a previous study, the optimal minimal clinically important difference cutoff for SDS was found to be ≥+2 points for improvement and ≤−1 point for deterioration [34].
Results
Between January 1, 2015, and August 31, 2016, a total of 100 patients were included in the analysis, 30 consecutive patients who underwent the CHAT intervention and, during the same period, a random control group of 70 patients without AB selected for comparison of clinical characteristics. Table 1 presents information on patient demographics and clinical characteristics. The median age was 57 years. Sixty‐one percent were female and 70% were white. Eighty‐three percent of patients had advanced cancer. At baseline, the median ESAS pain intensity (8.5 vs. 6, p = .002), SDS score (47 vs. 35, p = .009), and MEDD (65 mg/day vs. 25 mg/day, p = .001) were higher in the intervention group than the nonintervention group.
Table 1. Baseline patient demographic and clinical characteristics.
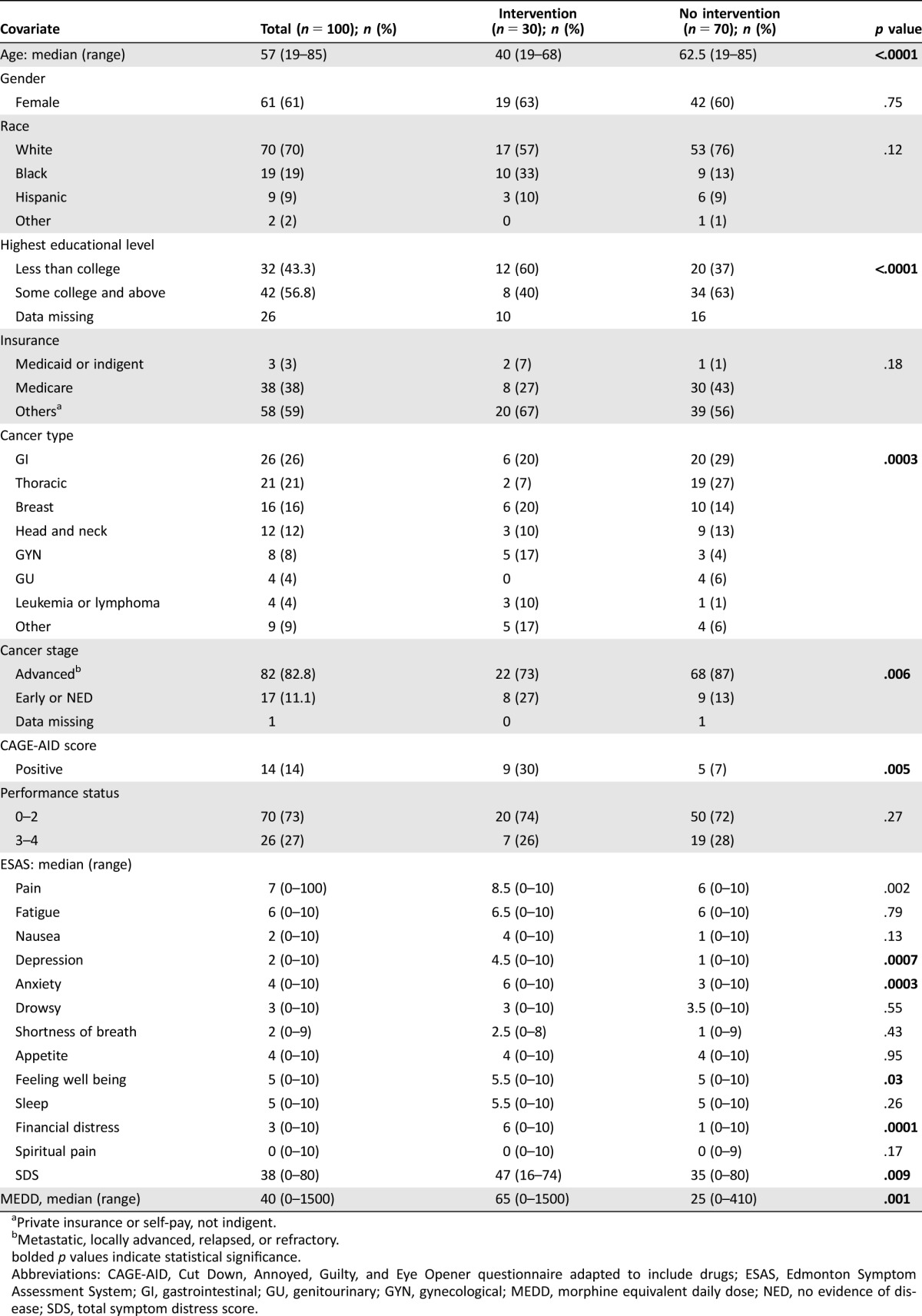
Private insurance or self‐pay, not indigent.
Metastatic, locally advanced, relapsed, or refractory.
bolded p values indicate statistical significance.
Abbreviations: CAGE‐AID, Cut Down, Annoyed, Guilty, and Eye Opener questionnaire adapted to include drugs; ESAS, Edmonton Symptom Assessment System; GI, gastrointestinal; GU, genitourinary; GYN, gynecological; MEDD, morphine equivalent daily dose; NED, no evidence of disease; SDS, total symptom distress score.
Results from logistic regression models (Table 2) indicate that univariately, younger age (p < .0001), CAGE‐AID‐positive status (p = .004), higher intensity of pain (p = .004), depression (p = .001), anxiety (p = .0003), financial distress (p = .0001), symptom distress score (p = .0126), and higher opioid dose (p = .002) were associated with the presence of AB. In a multivariate analysis, the odds for the presence of AB were 0.93 per 1‐year increase in age (p < .0001) and 1.28 per 1‐point increase in anxiety score (p = .005).
Table 2. Univariate and multivariate analysis of potential predictors of aberrant opioid‐related behavior.
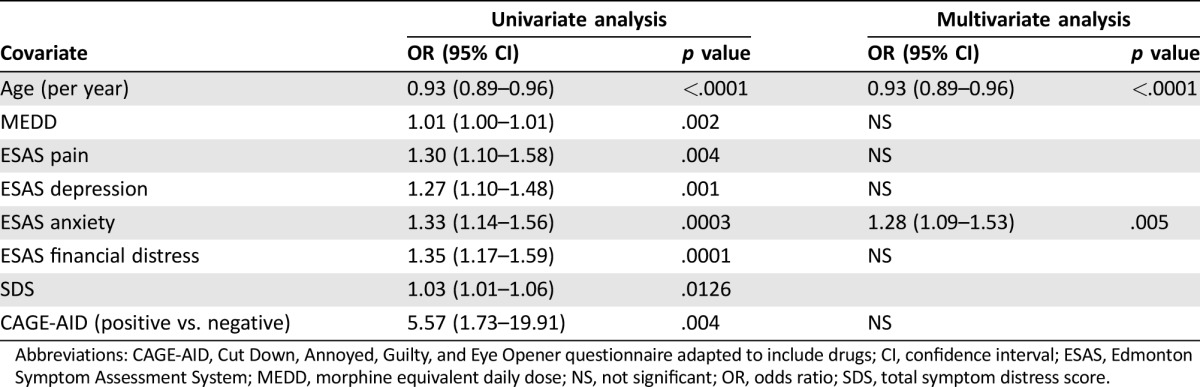
Abbreviations: CAGE‐AID, Cut Down, Annoyed, Guilty, and Eye Opener questionnaire adapted to include drugs; CI, confidence interval; ESAS, Edmonton Symptom Assessment System; MEDD, morphine equivalent daily dose; NS, not significant; OR, odds ratio; SDS, total symptom distress score.
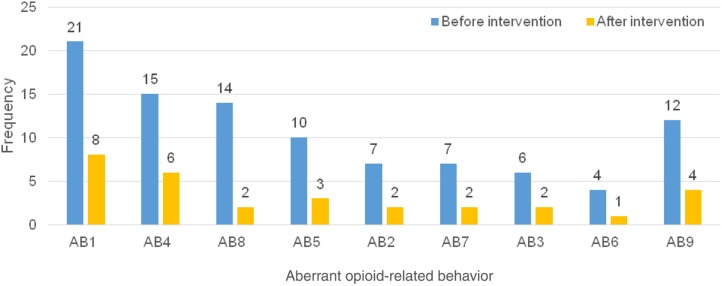
Table 3 shows the change in the number of documented ABs per month per patient before and during the 3 months after the intervention. The median (range) number of behaviors significantly decreased from 3 (1–6) preintervention to 0.4 (0–3) postintervention (p < .0001). Figure 1 also shows the frequency and types of individual ABs observed during the study period. “Request for opioid medication refills in the clinic earlier than the expected time” was the AB with the highest frequency prior to the intervention and the greatest improvement during the study period. The least prevalent preintervention AB was “resistance to changes in opioid regimen.” Every individual AB decreased in frequency over the course of 3 months following the intervention.
Table 3. Average number of aberrant opioid‐related behaviors per patient per month before and after the intervention.
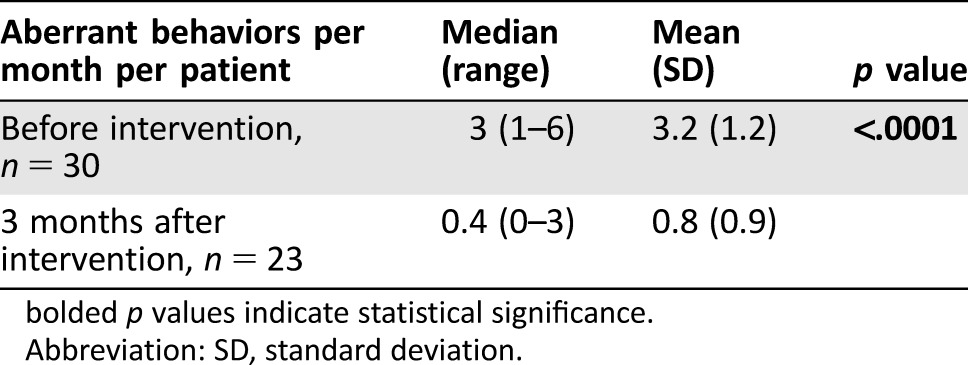
bolded p values indicate statistical significance.
Abbreviation: SD, standard deviation.
Figure 1.
Frequency and type of aberrant opioid‐related behavior preintervention and 3 months postintervention. Describes AB observed among 30 patients preintervention and 23 patients postintervention.
Abbreviations: AB, aberrant opioid‐related behavior; AB1, opioid refill earlier than the expected time; AB2, excessive dose increase of the opioid inconsistent with patient's pain syndrome; AB3, lost or stolen opioid; AB4, seeking opioids from multiple providers; AB5, request for specific opioid medications; AB6, resistance to changes in opioid regimen; AB7, reports of the use of street drugs; AB8, abnormal urine drug test result if not accounted for above; AB9, other behaviors, such as impaired functioning in daily activities due to opioid use, family member concerns about patient's inappropriate opioid use, and tampering or forging opioid prescriptions.
The median MEDD (range) of all intervention patients who had at least one follow‐up visit significantly decreased from 165 mg/day (30–1130) at the intervention to 112 mg/day (0–810) at the final follow‐up (p = .018), although the ESAS pain intensity did not significantly change (7 vs. 7.5, p = .984). The predefined responses to the CHAT intervention among the 30 patients who received it were also assessed for each visit within 3 consecutive months following the intervention. At 1 month, 11 out of 23 patients who returned for follow‐up had complete response. According to intention‐to‐treat, 11/30 (37%) were considered complete responders. By the end of 3 months, 14 out of 16 patients who returned for follow‐up had complete response. According to intention‐to‐treat, 14/30 (47%) were considered complete responders.
Discussion
In this preliminary study, we report on the impact of a novel interdisciplinary team approach to managing patients with cancer receiving chronic opioid therapy who demonstrated evidence of aberrant opioid use. We found that the use of the intervention resulted in a significant reduction in the frequency of ABs and the amount of opioid utilization.
AB has multiple underlying biomedical, psychosocial, financial, and legal factors and therefore requires the expertise of multiple providers working together to address these issues [36]. This approach is likely to prevent burnout in any individual provider who tries to address the issue alone. Programs that do not have all the resources may still adapt the concept and tailor the roles to suit their immediate needs. The finding in this study provides valuable evidence during this era when efforts to establish proven strategies to curb the opioid crisis are much needed.
We found that certain ABs were more frequent and may be more responsive to change than others. This novel analysis provides a key step in our understanding of such patients, as efforts are needed to modify behavior and improve adherence among patients with cancer receiving opioids. Whereas in some patients complete behavioral change is potentially achievable, it may not be so in others; hence goals for such patients may need to be directed at minimizing harm. It is conceivable that some behaviors, such as losing medications, may be relatively less concerning than others, such as injecting or “shooting” oral formulations. More research is needed in order to validate the magnitude of such variations. We are not aware of any study that has reported on the frequencies of individual ABs and their response to well‐defined mitigating strategies in patients with cancer.
The use of the CHAT intervention resulted in a significant decrease in patients’ opioid use without a worsening in pain intensity levels. Previous studies have shown that patients at risk for aberrant opioid use are more likely to have higher opioid requirements and take a longer time to be weaned off opioids [37], [38]. Patients who cope chemically [39], [40] tend to escalate the use of opioids at disproportionate levels that may result in deleterious consequences and safety concerns such as excessive sedation, respiratory depression, overdose [41], or even death. Care is needed in order to avoid undertreatment in patients with cancer [42], [43], [44], but clinicians cannot discount the potential harm and dangers associated with excessive utilization of opioids.
The CHAT intervention, when provided by the supportive care team, aims to mitigate maladaptive chemical coping and reinforce positive coping strategies with an emphasis on physical function, family function, psychosocial care, spiritual wellbeing, and other personal care needs [45]. The team ensures that the intervention is conducted in a supportive and compassionate manner. Our findings suggest that such intervention embedded within the supportive care environment is particularly effective in decreasing excessive opioid use without compromising good patient care.
Our exploratory data showed that younger age and higher anxiety were significant predictors of AB. This is consistent with numerous studies that have constantly identified age as a strong predictor of opioid abuse [14], [46], [47]. Studies have also shown that the coexistence of common psychiatric conditions such as depression and anxiety disorders in patients with a history substance abuse is extremely high [48]. Some patients use opioids in a maladaptive manner as a way of coping with the stress from cancer and other associated mental health conditions that may emerge during the diagnosis and progression of their disease. Sometimes, treatment of such underlying conditions will facilitate recovery from opioid abuse or minimize the likelihood of relapse. The results from this study further underscore the need for clinicians to intensify monitoring of patients with these clinical characteristics.
This study is an innovative effort to appraise the clinical effectiveness of a holistic strategy in managing aberrant opioid use among patients with advanced cancer. Previous studies have recommended strategies that aid in identifying such patients such as the use of risk assessment tools [16], prescription monitoring programs [19], UDS [20], [21], [22], and closer observation [17], [18]. However, once such patients are identified, subsequent management becomes challenging for clinical teams. Even in patients who convert to an opioid‐adherent behavior, the risk of relapse may be high due to the significant stress levels associated with the disease. In a similar study of 195 patients with chronic noncancer pain referred by their primary care physicians to an Opioid Renewal Clinic due to AB who underwent a structured multispecialty opioid risk management program, about 53% did not have resolution of the behavior at 1 year [49], [50].
Despite efforts by the SCC clinicians to detect ABs at every encounter, it is possible that a significant number of patients with AB went undetected during the study period. More research is necessary to better characterize how ABs can be detected more effectively using valuable tools such as random UDS, as is done in some noncancer pain clinics [4], [51]. There are no known formal guidelines regarding this tool's use among patients with advanced cancer [52]. Efforts to standardize its integration and utilization in routine ambulatory clinics are therefore warranted. Because patients with cancer differ from patients without cancer in terms of symptom burden, needs, and expectations, more studies are needed in order to better define the timing, frequency, and extent to which UDS should be implemented in this patient population. Our program is currently conducting various studies on random UDS to determine its feasibility [17] and impact on patients, families, and clinicians.
Limitations of this study include the retrospective design and a relatively smaller sample size. Future studies may need to be conducted prospectively at multiple centers with larger sample sizes in order to improve research efficacy. Regrettably, all the skilled interdisciplinary personnel may not be readily available in smaller cancer centers. Possible ways to approach the care of these complex patients in such situations might include establishing ad hoc teams with experts from other institutions, referring to centers with the required interdisciplinary capability, or merging some of the roles of the intervention to suit immediate needs. More research will be necessary to appraise these modified approaches.
Furthermore, we only reviewed patients in an ambulatory setting with relatively better functional status. Future studies should involve patients in the inpatient setting, because they are likely to have different symptom severities and behavioral expressions and may therefore respond differently to the intervention. Another potential limitation is that part of the information was based on patient self‐report during their visits to our institution. It is possible that patients with AB may inaccurately report on their opioid intake or may purposefully not display certain behaviors in order to avoid detection. Lastly, the observation period is relatively short and hence might have limited our ability to detect changes in patients’ opioid‐related behavior over a longer follow‐up period.
Conclusion
Our study found that the use of the CHAT intervention resulted in a significant reduction in the frequency of ABs and the amount of opioid utilization among patients with cancer receiving chronic opioid therapy. These findings have implications for health care providers’ approach to a complex clinical issue and offer a promising starting point for the creation of a standardized universal strategy for clinical teams dealing with patients on chronic opioid therapy. More research is needed to better characterize aberrant opioid use and further verify the effectiveness of this intervention in patients with cancer.
Contributed equally
Footnotes
For Further Reading: Maxine de la Cruz, Akhila Reddy, Vishidha Balankari et al. The Impact of an Educational Program on Patient Practices for Safe Use, Storage, and Disposal of Opioids at a Comprehensive Cancer Center. The Oncologist 2017;22:115–121; first published on October 14, 2016.
Implications for Practice: Prescription opioid abuse is a fast‐growing epidemic that has become more prominent recently, even in the cancer pain population. A previous study reported that 26% of cancer outpatients seen in the supportive care center either lose their pain medications or share their pain medications with someone else. This study demonstrates that the implementation of an opioid educational program and distribution of educational material on opioid safety brings about an improvement in opioid storage, use, and disposal practices in patients being prescribed opioids for cancer‐related pain. This study highlights the importance of consistent and thorough opioid education at every instance in which opioids are prescribed.
Author Contributions
Conception/design: Joseph Arthur, Tonya Edwards, Suresh Reddy, David Hui, Sriram Yennu, Eduardo Bruera
Provision of study material or patients: Tonya Edwards, Suresh Reddy, Kristy Nguyen
Collection and/or assembly of data: Joseph Arthur, Tonya Edwards, Kristy Nguyen, David Hui
Data analysis and interpretation: Joseph Arthur, Minjeong Park, Diane Liu, Eduardo Bruera
Manuscript writing: Joseph Arthur, David Hui, Sriram Yennu, Eduardo Bruera
Final approval of manuscript: Joseph Arthur, Tonya Edwards, Suresh Reddy, Kristy Nguyen, David Hui, Sriram Yennu, Minjeong Park, Diane Liu, Eduardo Bruera
Disclosures
The authors indicated no financial relationships.
References
- 1. Stjernswärd J. WHO cancer pain relief programme. Cancer Surv 1988;7:195–208. [PubMed] [Google Scholar]
- 2. Stjernswärd J, Colleau SM, Ventafridda V. The World Health Organization Cancer Pain and Palliative Care Program. Past, present, and future. J Pain Symptom Manage 1996;12:65–72. [DOI] [PubMed] [Google Scholar]
- 3.National Center on Addiction and Substance Abuse . Controlled prescription drug abuse at epidemic level. J Pain Palliat Care Pharmacother 2006;20:61–64. [PubMed] [Google Scholar]
- 4. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain ‐ United States, 2016. JAMA 2016;315:1624–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Surgeon General of the United States. The Surgeon General's call to end the opioid crisis. 2016. Available at http://turnthetiderx.org. Accessed January 24, 2017.
- 6.American Society of Clinical Oncology. ASCO Policy Statement on Opioid Therapy: Protecting Access to Treatment for Cancer‐Related Pain May 2016. Available at https://www.asco.org/advocacy-policy/policies-positions-guidance/policy-statements. Accessed January 24, 2017.
- 7.114th Congress. Comprehensive Addiction and Recovery Act of 2016, S 524, 2016. Available at https://www.congress.gov/bill/114th-congress/senate-bill/524. Accessed January 24, 2017.
- 8. World Health Organization. Cancer Pain Relief: With a Guide to Opioid Availability. 2nd ed Geneva, Switzerland: World Health Organization, 1996. [Google Scholar]
- 9. Derogatis LR, Morrow GR, Fetting J et al. The prevalence of psychiatric disorders among cancer patients. JAMA 1983;249:751–757. [DOI] [PubMed] [Google Scholar]
- 10. Arthur JA Edwards T, Lu Z et al. Frequency, predictors, and outcomes of urine drug test among patients with advanced cancer on chronic opioid therapy at an outpatient supportive care clinic. Cancer 2016;122:3732–3739. [DOI] [PubMed] [Google Scholar]
- 11. Arthur JA, Haider A, Edwards T et al. Aberrant opioid use and urine drug testing in outpatient palliative care. J Palliat Med 2016;19:778–782. [DOI] [PubMed] [Google Scholar]
- 12. Rauenzahn S, Sima A, Cassel B et al. Urine drug screen findings among ambulatory oncology patients in a supportive care clinic. Support Care Cancer 2017;25:1859–1864. [DOI] [PubMed] [Google Scholar]
- 13. Barclay JS, Owens JE, Blackhall LJ. Screening for substance abuse risk in cancer patients using the Opioid Risk Tool and urine drug screen. Support Care Cancer 2014;22:1883–1888. [DOI] [PubMed] [Google Scholar]
- 14. Childers JW, King LA, Arnold RM. Chronic pain and risk factors for opioid misuse in a palliative care clinic. Am J Hosp Palliat Care 2015;32:654–659. [DOI] [PubMed] [Google Scholar]
- 15. Nguyen LM, Rhondali W, De la Cruz M et al. Frequency and predictors of patient deviation from prescribed opioids and barriers to opioid pain management in patients with advanced cancer. J Pain Symptom Manage 2013;45:506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moore TM, Jones T, Browder JH et al. A comparison of common screening methods for predicting aberrant drug‐related behavior among patients receiving opioids for chronic pain management. Pain Med 2009;10:1426–1433. [DOI] [PubMed] [Google Scholar]
- 17. Arthur JA, Edwards T, Lu Z et al. Frequency, predictors, and outcomes of urine drug testing among patients with advanced cancer on chronic opioid therapy at an outpatient supportive care clinic. Cancer 2016;122:3732–3739. [DOI] [PubMed] [Google Scholar]
- 18. Anghelescu DL, Ehrentraut JH, Faughnan LG. Opioid misuse and abuse: Risk assessment and management in patients with cancer pain. J Natl Compr Canc Netw 2013;11:1023–1031. [DOI] [PubMed] [Google Scholar]
- 19.Prescription Drug Monitoring Program Training and Technical Assistance Center. State PDMP Websites. Available at http://www.pdmpassist.org/content/state-pdmp-websites. Accessed April 4, 2017.
- 20. Magnani B, Kwong T. Urine drug testing for pain management. Clin Lab Med 2012;32:379–390. [DOI] [PubMed] [Google Scholar]
- 21. Christo PJ, Manchikanti L, Ruan X et al. Urine drug testing in chronic pain. Pain Physician 2011;14:123–143. [PubMed] [Google Scholar]
- 22. Moeller KE, Lee KC, Kissack JC. Urine drug screening: Practical guide for clinicians. Mayo Clin Proc 2008;83:66–76. [DOI] [PubMed] [Google Scholar]
- 23. Bruera E, Kuehn N, Miller MJ et al. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care 1991;7:6–9. [PubMed] [Google Scholar]
- 24. Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer 2000;88:2164–2171. [DOI] [PubMed] [Google Scholar]
- 25. Philip J, Smith WB, Craft P et al. Concurrent validity of the modified Edmonton Symptom Assessment System with the Rotterdam Symptom Checklist and the Brief Pain Inventory. Support Care Cancer 1998;6:539–541. [DOI] [PubMed] [Google Scholar]
- 26. Breitbart W, Rosenfeld B, Roth A et al. The Memorial Delirium Assessment Scale. J Pain Symptom Manage 1997;13:128–137. [DOI] [PubMed] [Google Scholar]
- 27. Fadul N, Kaur G, Zhang T et al. Evaluation of the memorial delirium assessment scale (MDAS) for the screening of delirium by means of simulated cases by palliative care health professionals. Support Care Cancer 2007;15:1271–1276. [DOI] [PubMed] [Google Scholar]
- 28. Dev R, Parsons HA, Palla S et al. Undocumented alcoholism and its correlation with tobacco and illegal drug use in advanced cancer patients. Cancer 2011;117:4551–4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kwon JH, Tanco K, Park JC et al. Frequency, predictors, and medical record documentation of chemical coping among advanced cancer patients. The Oncologist 2015;20:692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pergolizzi JV Jr., Gharibo C, Passik S et al. Dynamic risk factors in the misuse of opioid analgesics. J Pscyhosom Res 2012;72:443–451. [DOI] [PubMed] [Google Scholar]
- 31. Passik SD, Kirsh KL, Donaghy KB et al. Pain and aberrant drug‐related behaviors in medically ill patients with and without histories of substance abuse. Clin J Pain 2006;22:173–181. [DOI] [PubMed] [Google Scholar]
- 32. Dalal S, Bruera S, Hui D et al. Use of palliative care services in a tertiary cancer center. The Oncologist 2016;21:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Follwell M, Burman D, Le LW et al. Phase II study of an outpatient palliative care intervention in patients with metastatic cancer. J Clin Oncol 2009;27:206–213. [DOI] [PubMed] [Google Scholar]
- 34. Hui D, Shamieh O, Paiva CE et al. Minimal clinically important difference in the physical, emotional, and total symptom distress scores of the Edmonton Symptom Assessment System. J Pain Symptom Manage 2016;51:262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arthur J, Tanco K, Haider A et al. Assessing the prognostic features of a pain classification system in advanced cancer patients. Support Care Cancer 2017;25:2863–2869. [DOI] [PubMed] [Google Scholar]
- 36. Passik SD, Portenoy RK, Ricketts PL. Substance abuse issues in cancer patients. Part 1: Prevalence and diagnosis. Oncology (Williston Park) 1998;12:517–521, 524. [PubMed] [Google Scholar]
- 37. Koyyalagunta D, Bruera E, Aigner C et al. Risk stratification of opioid misuse among patients with cancer pain using the SOAPP‐SF. Pain Med 2013;14:667–675. [DOI] [PubMed] [Google Scholar]
- 38. Kwon JH, Hui D, Chisholm G et al. Predictors of long‐term opioid treatment among patients who receive chemoradiation for head and neck cancer. The Oncologist 2013;18:768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bruera E, Moyano J, Seifert L et al. The frequency of alcoholism among patients with pain due to terminal cancer. J Pain Symptom Manage 1995;10:599–603. [DOI] [PubMed] [Google Scholar]
- 40. Del Fabbro E. Assessment and management of chemical coping in patients with cancer. J Clin Oncol 2014;32:1734–1738. [DOI] [PubMed] [Google Scholar]
- 41. Dunn KM, Saunders KW, Rutter CM et al. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med 2010;152:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fisch MJ, Lee JW, Weiss M et al. Prospective, observational study of pain and analgesic prescribing in medical oncology outpatients with breast, colorectal, lung, or prostate cancer. J Clin Oncol 2012;30:1980–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Deandrea S, Montanari M, Moja L et al. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol 2008;19:1985–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Apolone G, Corli O, Caraceni A et al. Pattern and quality of care of cancer pain management. Results from the Cancer Pain Outcome Research Study Group. Br J Cancer 2009;100:1566–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hui D, Bruera E. Integrating palliative care into the trajectory of cancer care. Nat Rev Clin Oncol 2016;13:159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: Preliminary validation of the Opioid Risk Tool. Pain Med 2005;6:432–442. [DOI] [PubMed] [Google Scholar]
- 47. Edlund MJ, Steffick D, Hudson T et al. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non‐cancer pain. Pain 2007;129:355–362. [DOI] [PubMed] [Google Scholar]
- 48. Khantzian EJ, Treece C. DSM‐III psychiatric diagnosis of narcotic addicts. Recent findings. Arch Gen Psychiatry 1985;42:1067–1071. [DOI] [PubMed] [Google Scholar]
- 49. Meghani SH, Wiedemer NL, Becker WC et al. Predictors of resolution of aberrant drug behavior in chronic pain patients treated in a structured opioid risk management program. Pain Med 2009;10:858–865. [DOI] [PubMed] [Google Scholar]
- 50. Wiedemer NL, Harden PS, Arndt IO et al. The opioid renewal clinic: A primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med 2007;8:573–584. [DOI] [PubMed] [Google Scholar]
- 51. Owen GT, Burton AW, Schade CM et al. Urine drug testing: Current recommendations and best practices. Pain Physician 2012;15(suppl 3):ES119–E133. [PubMed] [Google Scholar]
- 52. Tan PD, Barclay JS, Blackhall LJ. Do palliative care clinics screen for substance abuse and diversion? Results of a national survey. J Palliat Med 2015;18:752–757. [DOI] [PubMed] [Google Scholar]