Abstract
Lessons Learned.
Combination regimen with bevacizumab (BEV) and vorinostat is well tolerated in patients with recurrent glioblastoma.
Treatment of recurrent glioblastoma remains challenging as this study and others attempt to improve progression‐free survival and overall survival with BEV‐containing regimens.
Background.
Recurrent glioblastoma (GBM; World Health Organization grade 4) continues to have a very poor prognosis. Bevacizumab (BEV) has been shown to improve progression‐free survival (PFS) in recurrent GBM and is approved by the U.S. Food and Drug Administration for the treatment of recurrent GBM. Combination regimens have been explored, and in this phase II nonrandomized trial, we evaluated the efficacy of BEV combined with histone deacetylase inhibitor vorinostat (VOR) in recurrent GBM.
Materials and Methods.
In this phase II, single‐center, nonrandomized study, subjects with recurrent GBM received BEV 10 mg/kg intravenously (IV) every 2 weeks combined with VOR 400 mg p.o. daily for 7 days on, 7 days off, in a 28‐day cycle. The primary endpoint was 6‐month PFS (PFS6).
Results.
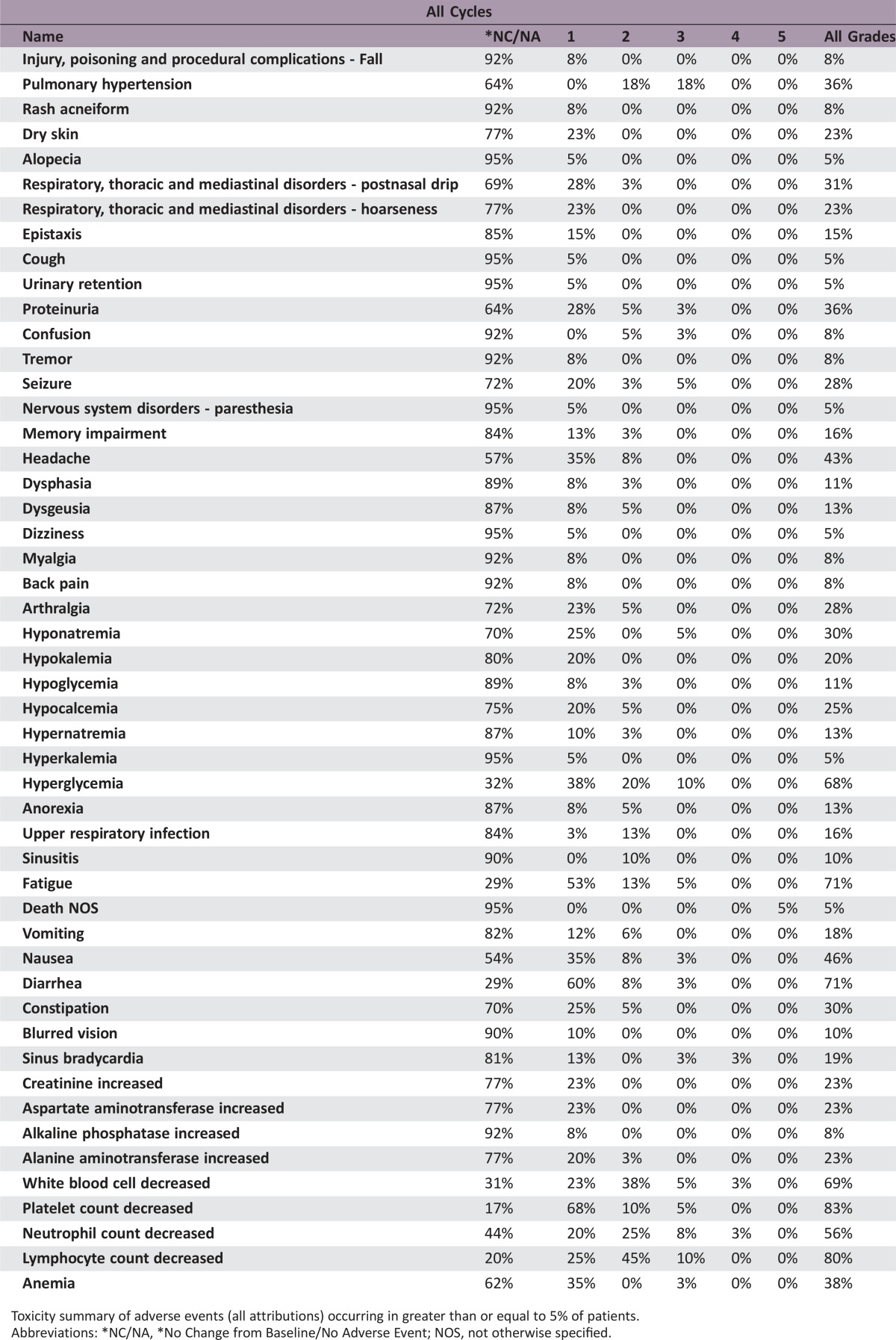
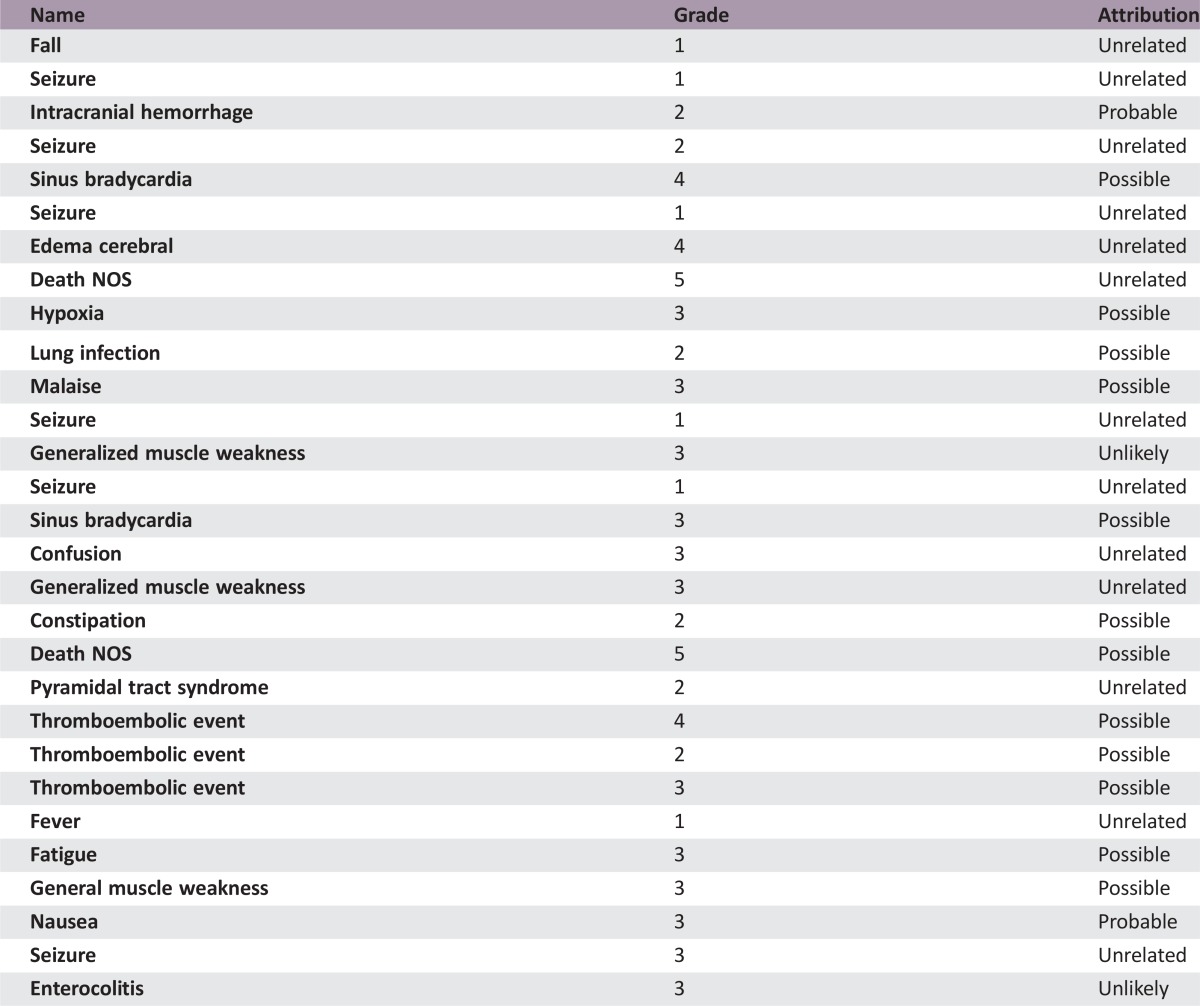
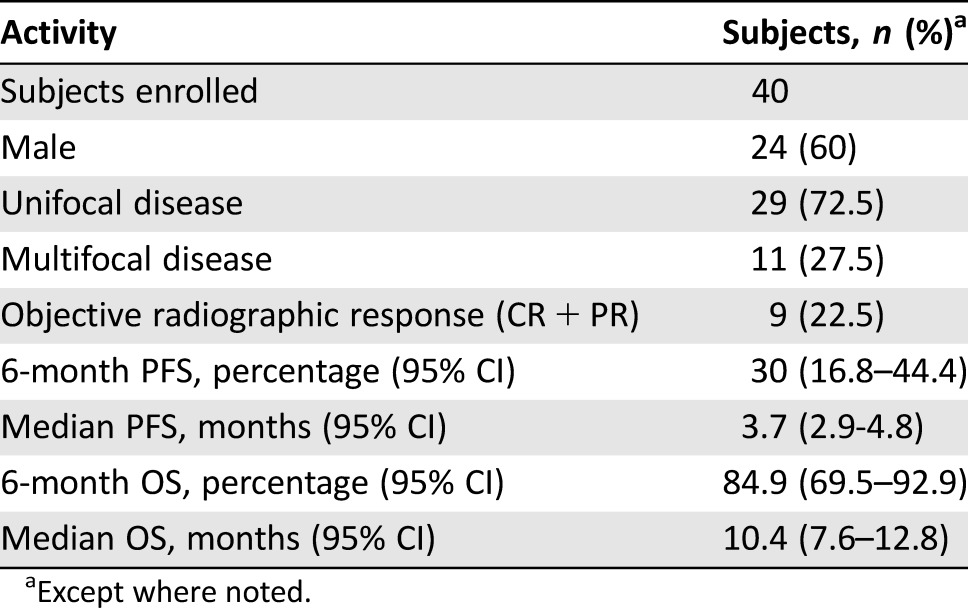
Forty patients with recurrent GBM were enrolled and evaluated. PFS6 was 30.0% (95% confidence interval [CI] 16.8%–44.4%). Median overall survival (OS) was 10.4 months (95% CI 7.6–12.8 months). Overall radiographic response rate was 22.5% based on 9 partial responses. The most common grade 2 and above treatment‐related adverse events were lymphopenia (55%), leukopenia (45%), neutropenia (35%), and hypertension (33%). Grade 4 adverse events were leukopenia (3%), neutropenia (3%), sinus bradycardia (3%), and venous thromboembolism (3%). Two deaths occurred in this study, with one due to tumor progression and another possibly related as death not otherwise specified.
Conclusion.
Combination treatment of BEV and VOR was well tolerated. This combination therapy for this study population did not improve PFS6 or median OS when compared with BEV monotherapy.
Abstract
经验总结
• 贝伐珠单抗(BEV)和伏立诺他联合给药方案治疗胶质母细胞瘤复发患者的耐受性良好。
• 复发性胶质母细胞瘤的治疗仍然充满挑战, 本研究和其他研究试图改善含BEV给药方案的无进展生存期和总生存期。
背景.复发性胶质母细胞瘤(GBM;世界卫生组织4级)的预后仍然很差。贝伐珠单抗(BEV)已被证实可以改善复发性GBM的无进展生存率(PFS), 并且已获美国食品药品监督管理局批准用于治疗复发性GBM。联合给药方案已经进行探索。在本项II期非随机化试验中, 我们评价了BEV与组蛋白脱乙酰酶抑制剂伏立诺他(VOR)联合给药治疗复发性GBM的疗效。
材料与方法.在本项II期、单中心、非随机化研究中, 复发性GBM患者每两周通过静脉注射(IV)接受BEV 10 mg/kg, 同时每天口服VOR 400 mg, 共7天, 然后停药7天, 28天为一个给药周期。研究的主要终点为6个月PFS(PFS6)。
结果.40例复发性GBM患者入组研究并参与了评价。PFS6为30.0%[95%置信区间(CI) 16.8%–44.4%]。中位总生存期(OS)为10.4个月(95% CI 7.6–12.8个月)。9例患者达到部分缓解, 总体影像学缓解率为22.5%。最常见的≥2级治疗相关不良事件为淋巴细胞减少症(55%)、白细胞减少症(45%)、中性粒细胞减少症(35%)和高血压(33%)。4级不良事件为白细胞减少症(3%)、中性粒细胞减少症(3%)、窦性心动过缓(3%)和静脉血栓栓塞(3%)。研究期间发生了2例死亡, 1例死于肿瘤进展, 另1例死亡可能与治疗有关(死亡原因未说明)。
结论.BEV和VOR联合给药可良好耐受。与BEV单药疗法相比, BEV和VOR联合给药并未改善本研究人群的PFS6或中位OS。
Discussion
Prognosis for GBM remains very poor, with median OS of 12–16 months. The treatment of recurrent GBM presents further challenges, with PFS6 between 9% and 48%. BEV, a humanized monoclonal IgG1 antibody that inhibits the human vascular endothelial growth factor (VEGF), has shown modest effect in recurrent GBM. The phase II BRAIN trial reported PFS6 with BEV monotherapy to be 42.6% and median OS 9.2 months [1]. Following these positive results, recent studies have examined the role of BEV in combination with other chemotherapy and targeted agents. Traditional cytotoxic chemotherapies have been relatively unsuccessful when combined with BEV. More recently, a large phase III trial reported no difference in overall survival between lomustine alone versus combination lomustine and BEV.
Given the limited treatment options in recurrent GBM, the trend has been to combine novel therapies with agents such as BEV. VOR is a derivative of hydroxamic acid that has antitumor properties acting directly as a histone deacetylase (HDAC) inhibitor and indirectly antiangiogenic. We conducted a phase II, single‐arm, nonrandomized study of combination BEV and VOR for recurrent GBM. Our primary endpoint was PFS6, with secondary endpoints being OS, PFS, radiographic response, and safety/tolerability. The major eligibility criteria included age ≥18 years, Karnofsky Performance Status ≥70, ≥4 weeks’ time interval since most recent treatment, and ≤2 prior progressions. Treatment consisted of BEV 10 mg/kg IV every 2 weeks plus VOR 400 mg p.o. daily for 7 days on, 7 days off, in a 28‐day cycle.
A total of 40 patients were enrolled into the study. Median follow‐up was 23.3 months (95% CI 21.0–32.0). PFS6 was 30.0% (95% CI 16.8%–44.4%), and median OS was 10.4 months (95% CI 7.6–12.8). Nine patients had a confirmed partial response, and none had a complete response. Therefore, the radiographic response rate was 22.5% (95% CI 12.1%–37.7%; Table 1). The most common grade 2 and above treatment‐related adverse events were lymphopenia (55%), leukopenia (45%), neutropenia (35%), and hypertension (33%). Five patients (12.5%) experienced treatment‐related unacceptable toxicities, which the protocol defined as any treatment‐related, nonhematologic grade 4 or 5 toxicity or a grade 2 or greater central nervous system (CNS) hemorrhage. Two patients died during the study, one due to tumor progression and another possibly related as death not otherwise specified. Treatment with combination BEV and VOR was tolerable, but there was no improvement in progression‐free survival at 6 months with this regimen. As the community of neuro‐oncology moves forward with research in antiangiogenic agents in the treatment of recurrent GBM, further studies are warranted to evaluate antiangiogenic agents in other combinations, including with immunotherapy or other targeted agents.
Table 1. Summary of clinical activities.
Except where noted.
Trial Information
- Disease
Brain cancer – primary
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
Two prior regimens
- Type of Study ‐ 1
Phase II
- Type of Study ‐ 2
Single‐arm
- Primary Endpoint
Progression‐free survival at 6 months
- Secondary Endpoint
Overall survival
- Secondary Endpoint
Progression‐free Survival
- Secondary Endpoint
Overall response rate
- Secondary Endpoint
Toxicity
- Additional Details of Endpoints or Study Design
- The objective of this open‐label phase II study was to assess the efficacy of bevacizumab plus vorinostat for the treatment of patients with recurrent WHO grade IV glioma. The study was designed to have adequate power to compare the efficacy of this regimen with a historical benchmark. The basis for this efficacy assessment is the proportion of patients who survive progression‐free for 6 months. The justification of the sample size requirement for this study is as follows. Vredenburgh [5] reported a 6‐month progression‐free survival rate of 42.6% (97.5% confidence interval 29.6%–55.5%) among patients with recurrent GBM treated with bevacizumab and irinotecan. If the true 6‐month PFS with the combination of bevacizumab and vorinostat were 40% or less, there would be limited interest in developing this combination further. However, if the true 6‐month PFS were 60% or more, there would definitely be interest in further investigation of this treatment regimen. Therefore, within this patient subgroup, the study was designed to differentiate between a 40% and 60% rate of 6‐month PFS. Statistically, the hypothesis that was to be tested was H0: p < 0.40 versus H1: p > 0.60, where p is the proportion of patients who live 6 or more months without disease progression. Forty patients were to be enrolled in this single‐stage study. If 21 or more of these 40 patients lived 6 or more months without disease progression, the treatment regimen would be considered worthy of further investigation. Otherwise, the treatment regimen would be determined not worthy of further investigation within this patient population. The type I and II error rates associated with this testing are 0.074 and 0.13, respectively.
- Investigator's Analysis
Inactive because results did not meet primary endpoint
Drug Information for Phase II Treatment Arm
- Drug 1
- Generic/Working Name
Bevacizumab
- Trade Name
Avastin
- Company Name
Genentech
- Drug Type
Antibody
- Drug Class
Angiogenesis ‐ VEGF
- Dose
10 mg/kg
- Route
IV
- Schedule of Administration
Administered every 2 weeks combined with VOR 400 mg p.o. daily for 7 days, then 7 days off in a 28‐day cycle
- Drug 2
- Generic/Working Name
Vorinostat
- Trade Name
Zolinza
- Company Name
Merck & Co.
- Drug Type
Small molecule
- Drug Class
HDAC
- Dose
400 mg per flat dose
- Route
p.o.
- Schedule of Administration
VOR 400 mg p.o. daily for 7 days, then 7 days off, in a 28‐day cycle
Patient Characteristics for Phase II Treatment Arm
- Number of Patients, Male
24
- Number of Patients, Female
16
- Stage
No stage
- Age
Median (range): 52.4 years (32–74 years)
- Number of Prior Systemic Therapies
Number: 1
- Performance Status: ECOG
-
0 — 1
1 — 35
2 — 2
3 — 0
Unknown — 2
Primary Assessment Method for Phase II Treatment Arm
- Title
PFS6
- Number of Patients Enrolled
40
- Number of Patients Evaluable for Toxicity
40
- Number of Patients Evaluated for Efficacy
40
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 9 (22.5%)
- (Median) Duration Assessments OS
10.4 months, CI 7.6–12.8
Phase II Treatment Arm Adverse Events
Toxicity summary of adverse events (all attributions) occurring in greater than or equal to 5% of patients.
Abbreviations: *NC/NA, *No Change from Baseline/No Adverse Event; NOS, not otherwise specified.
Serious Adverse Events
Assessment, Analysis, and Discussion
- Completion
Study completed
- Pharmacokinetics/Pharmacodynamics
Not collected
- Investigator's Assessment
Inactive because results did not meet primary endpoint
The treatment of recurrent glioblastoma (GBM; World Health Organization grade 4) continues to present a challenge to the neuro‐oncology community. Depending on the use of antiangiogenic agents in recurrence, the 6‐month progression‐free survival (PFS6) ranges from 9% to 48%. Bevacizumab (BEV), a humanized monoclonal IgG1 antibody that inhibits the human vascular endothelial growth factor, has shown modest effect in recurrent GBM [1]. The phase II BRAIN trial reported PFS6 with bevacizumab monotherapy to be 42.6% and median overall survival (OS) 9.2 months, and this trial established the groundwork for the U.S. Food and Drug Administration (FDA) approval of bevacizumab for recurrent GBM [2]. Bolstered by the initial success of bevacizumab in recurrent GBM, other clinical trials explored the role of BEV in combination with other chemotherapy and targeted agents [3]. Diaz and colleagues sought to understand this concept of combinations with bevacizumab and undertook a systemic evaluation of clinical data published from clinical trials for newly diagnosed and recurrent glioblastoma patients treated with bevacizumab [3]. They identified 14 clinical trials in the published literature that examined the use of bevacizumab in combination with other agents for the treatment of recurrent GBM. They concluded that bevacizumab alone and in combination does improve PFS, but that there were no statistically significant changes in OS for patients with recurrent GBM. In the hope that combination therapy could provide improved outcomes for recurrent GBM, we designed our clinical trial on bevacizumab in combination with vorinostat, a derivative of hydroxamic acid that has antitumor properties by inhibiting histone deacetylase (HDAC).
Vorinostat is FDA approved for the treatment of cutaneous T‐cell lymphoma and is an orally available HDAC inhibitor. Common toxicities include bone marrow suppression, fatigue, and diarrhea, and the treatment is generally well tolerated. In a phase II study by Galanis and colleagues, they evaluated the treatment of vorinostat in patients with recurrent GBM [4]. The primary endpoint for this study was 6‐month progression‐free survival with the expectation that the regimen would be considered active if the 6‐month progression‐ free survival were ≥25%, and they achieved this endpoint with 9 of the first 52 patients (of note, 66 patients participated in this study) progression‐free at 6 months. In this study, expected toxicities of vorinostat included fatigue and bone marrow as the most common toxicities. These promising results increased our interest in pursuing a clinical trial in recurrent GBM using the combination of vorinostat and bevacizumab. Using the same endpoint as the aforementioned study, we sought to improve 6‐month progression‐free survival. Vredenburgh and colleagues reported a 6‐month progression‐free survival percentage of 42.6% among patients with recurrent GBM treated with bevacizumab and irinotecan [5]. If the true 6‐month progression‐free survival with the combination of bevacizumab and vorinostat were 40% or less, there would be limited interest in developing this combination further. However, if the true 6‐month progression‐free survival were 60% or more, there would definitely be interest in further investigation of this treatment regimen.
With the early success of bevacizumab and subsequent approval of bevacizumab by the FDA for treatment of recurrent GBM, many studies have sought to find the appropriate partner to improve outcomes beyond bevacizumab. In a randomized, controlled, phase II study, single‐agent bevacizumab or lomustine versus the combination of bevacizumab plus lomustine were studied in patients with recurrent GBM (BELOB trial) [6]. The combination of bevacizumab and lomustine exhibited a 6‐month progression‐free survival of 42% (95% confidence interval [CI] 29%–55%) and is strikingly similar to the study from Vredenburgh and colleagues [5]. Of note, this was superior to the 6‐month progression‐free survival with bevacizumab alone (16%) and lomustine alone (13%). Therefore, our assessment was that a combination therapy with a PFS6 of 60% or more would be worthy of further study. Of note, the data from the BELOB trial did lead to a randomized phase III study of lomustine versus bevacizumab with lomustine (EORTC 26101), and the primary endpoint of an improvement in overall survival was not achieved [7].
We conducted a phase II, single‐arm, nonrandomized study of combination bevacizumab and vorinostat for recurrent GBM. Our primary endpoint was 6‐month progression‐free survival, with secondary endpoints being OS, progression‐free survival, radiographic response, and safety/tolerability. The major eligibility criteria included age ≥18 years, Karnofsky Performance Status ≥70, ≥4 weeks' time interval since most recent treatment, and ≤2 prior progressions. Treatment consisted of bevacizumab 10 mg/kg intravenously every 2 weeks, plus vorinostat 400 mg p.o. daily for 7 days on, 7 days off, in a 28‐day cycle.
A total of 40 patients were enrolled into the study. Median follow‐up was 23.3 months (95% CI 21.0–32.0). Six‐month progression‐free survival was 30.0% (95% CI 16.8%–44.4%), and median OS was 10.4 months (95% CI 7.6–12.8). Based on our statistical design, we need not meet the desired threshold to deem this regimen active. Nine patients had a confirmed partial response and none had a complete response. Therefore, the radiographic response rate was 22.5% (95% CI 12.1%–37.7%; Table 1). The most common grade 2 and above treatment‐related adverse events were lymphopenia (55%), leukopenia (45%), neutropenia (35%), and hypertension (33%). Five patients (12.5%) experienced treatment‐related unacceptable toxicities, which the protocol defined as any treatment‐related, nonhematologic grade 4 or 5 toxicity or a grade 2 or greater central nervous system hemorrhage. Two patients died while enrolled on this study, one due to tumor progression and another possibly related as death not otherwise specified. In regards to the death not otherwise specified, the patient had been admitted to a local hospital for 1 week of progressive confusion and weakness. Imaging of the brain was obtained, which showed stable disease, and the patient was improving with physical therapy. On date of death, the patient was otherwise at baseline condition and became acutely apneic. Attempts to resuscitate the patient were performed but were unsuccessful. Cause of death is not able to be determined and no autopsy was performed.
Although overall survival remains the critical endpoint, our study affirmed the utility of the PFS6 landmark, progression‐free survival at 6 months. Although the partial responses noted are of interest, one difficulty in assessing response is that bevacizumab can induce a “pseudoresponse” due to improvement in membrane permeability in glioblastoma. At a PFS6 of 30% versus previous reports at 40%, we concluded that, although treatment with combination bevacizumab and vorinostat was tolerable, there was no improvement in progression‐free survival at 6 months with this regimen. Based on the findings of this study, the combination of bevacizumab and vorinostat should not be pursued as an option for patients with recurrent glioblastoma. As the community of neuro‐oncology moves forward with research in antiangiogenics in the treatment of recurrent GBM, further studies are warranted to evaluate other combinations such as immunotherapy or other targeted agents.
Footnotes
ClinicalTrials.gov Identifier: NCT01738646
Sponsors: Merck, Sharp, and Dohme, Corp.; Genentech, Inc.
Principal Investigator: Katherine B. Peters
IRB Approved: Yes
Disclosures
Ashley Ghiaseddin: Monteris Medical (C/A), Orbus Therapeutics (RF); David Reardon: Abbvie, Agenus, Amgen, Bristol‐Meyers Squibb, Cavion, Celldex, EMD Serono, Genentech/Roche, Inovio, Juno Pharmaceuticals, Merck, Midatech, Momenta Pharmaceuticals, Novartis, Novocure, Oncorus, Oxigene, Regeneron, Stemline Therapeutics (C/A, H), Acerta Pharma, Agenus, Celldex Therapeutics, EMD Serono, Incyte, Inovio, Midatech, Tragara (RF); Annick Desjardins: Genentech/Roche (C/A, RF); Henry S. Friedman: Istari Oncology (IP), Genentech/Roche (C/A); Katherine B. Peters: Abbvie, Agios, Nocvocure (C/A), Agios, Eisai, Genentech, Merck, Biomimetix, VBL Therapeutics (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Ghiaseddin A, Peters KB. Use of bevacizumab in recurrent glioblastoma. CNS Oncol 2015;4:157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Friedman HS, Prado MD, Wen PY et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009;27:4733–4740. [DOI] [PubMed] [Google Scholar]
- 3. Diaz RJ, Ali S, Qadir MG et al. The role of bevacizumab in the treatment of glioblastoma. J Neurooncol 2017;133:455–467. [DOI] [PubMed] [Google Scholar]
- 4. Galanis E, Jaeckle KA, Maurer MJ et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: A North Central Cancer Treatment Group Study. J Clin Oncol 2009;27:2052–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vredenburgh JJ, Desjardins A, Herndon JE 2nd et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 2007;25:4722–4729. [DOI] [PubMed] [Google Scholar]
- 6. Taal W, Oosterkamp HM, Walenkamp AME et al. Single‐agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in paients with recurrent glioblastoma (BELOB trial): A randomised controlled phase 2 trial. Lancet Oncol 2014;15:943–953. [DOI] [PubMed] [Google Scholar]
- 7. Wick W, Brandes AA, Gorlia T et al. EORTC 26101 phase III trial exploring the combination of bevacizumab and lomustine in patients with first progression of a glioblastoma. J Clin Oncol 2016;34 (suppl 15):2001. [Google Scholar]