To assess the effectiveness of first‐line azacitidine in red blood cell transfusion‐dependent lowerrisk myelodysplastic syndrome, a prospective meta‐analysis of azacitidine treatment was performed in this population.
Keywords: Meta‐analysis, Azacitidine, Lower‐risk myelodysplastic syndromes
Abstract
Background.
After erythropoiesis‐stimulating agent (ESA) failure, lenalidomide and hypomethylating agents are the only remaining treatment options for most patients with lower‐risk myelodysplastic syndromes (LR‐MDS). Optimal choice of these agents as front‐line therapy in non‐del(5q) LR‐MDS is unclear. Because azacitidine clinical data mainly describe experience in higher‐risk MDS, we performed a meta‐analysis of patient‐level data to evaluate azacitidine in patients with red blood cell (RBC) transfusion‐dependent LR‐MDS.
Materials and Methods.
We searched English‐language articles for prospective phase II and III azacitidine clinical trials and patient registries published between 2000 and 2015, and Embase abstracts from 2015 conferences. Patient‐level data from identified relevant studies were provided by investigators. Meta‐analyses followed Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines. Efficacy endpoints were RBC transfusion independence (TI) and Clinical Benefit (RBC‐TI, erythroid response, and complete or partial remission, per International Working Group 2006 criteria for MDS).
Results.
Data for 233 patients from 6 clinical studies and 1 registry study met criteria for inclusion in analyses. Overall, 90.3% of patients had non‐del(5q) LR‐MDS. Pooled estimates from random‐effects models of RBC‐TI and Clinical Benefit were 38.9% and 81.1%, respectively; for the ESA‐refractory subgroup, they were 40.5% and 77.3%; and for patients with isolated anemia, they were 41.9% and 82.5%. In multivariate analyses, planned use of ≥6 azacitidine treatment cycles was significantly predictive of response.
Conclusion.
Azacitidine effects in these patients, most with non‐del(5q) LR‐MDS, were promising and generally similar to those reported for lenalidomide in similar patients. The choice of initial therapy is important because most patients eventually stop responding to front‐line therapy and alternatives are limited.
Implications for Practice.
Lower‐risk myelodysplastic syndromes (LR‐MDS) are primarily characterized by anemia. After erythropoiesis‐stimulating agent (ESA) failure, lenalidomide and hypomethylating agents are the only remaining treatment options for most patients. This meta‐analysis of 233 azacitidine‐treated red blood cell (RBC) transfusion‐dependent patients with LR‐MDS (92.3% non‐del[5q]) from 7 studies showed 38.9% became RBC transfusion‐independent. There is no clear guidance regarding the optimal choice of lenalidomide or hypomethylating agents for patients with non‐del(5q) LR‐MDS following ESA failure. Clinical presentation (e.g., number of cytopenias) and potential outcomes after hypomethylating agent failure are factors to consider when making initial treatment decisions for LR‐MDS patients.
Introduction
Myelodysplastic syndromes (MDS) comprise a heterogenous group of clonal myeloid neoplasms characterized by ineffective hematopoiesis and by increased risk of transformation to acute myeloid leukemia (AML) [1]. Lower‐risk MDS (LR‐MDS; i.e., International Prognostic Scoring System [IPSS]–defined Low or Intermediate‐1 risk) is characterized mainly by peripheral cytopenias [2]. Approximately 80% of patients with MDS are initially diagnosed because of symptoms related to anemia [3]. Although not approved by the U.S. Food and Drug Administration for treatment of MDS, erythropoiesis‐stimulating agents (ESAs) and growth factors (e.g., granulocyte colony‐stimulating factor [G‐CSF]) are widely used as initial therapy for patients with LR‐MDS with symptomatic anemia or who are red blood cell (RBC) transfusion‐dependent [4]. However, many patients show primary resistance to ESAs, and those who respond to these agents typically become resistant to them within about 2 years [4], [5], [6].
Goals of LR‐MDS treatment include reducing cytopenias (primarily anemia) and improving quality of life [7]. Because RBC transfusion dependence is an independent risk factor for decreased survival [8], [9], a primary objective is to eliminate the need for blood product transfusions. Currently, there are only three treatment modalities available for patients with MDS who are resistant or unlikely to respond to ESAs: lenalidomide, the hypomethylating agents (HMAs), azacitidine and decitabine, and immunosuppressive therapy (IST) with antithymocyte globulins with or without cyclosporine for younger patients below 60 years of age or patients with RBC transfusion dependence of short duration [10].
Lenalidomide is an immunomodulatory drug and the disease‐modifying treatment of choice for patients with LR‐MDS with a deletion in chromosome 5 (del[5q]) [10]; for those patients, lenalidomide is associated with durable erythroid responses and RBC transfusion‐independence (TI) rates of 60%–70% [11], [12]. Lenalidomide has also been studied in LR‐MDS patients without the del(5q) karyotype in two large controlled trials. In the phase II MDS‐002 study of lenalidomide in RBC transfusion‐dependent patients with non‐del(5q) LR‐MDS, lenalidomide was associated with an RBC‐TI rate of 26% with median duration of 41 weeks [13]. Additionally, in the randomized, phase III MDS‐005 study, lenalidomide was associated with significantly higher rates of RBC‐TI compared with placebo (26.9% vs. 2.5%, respectively; p < .001) in RBC transfusion‐dependent patients with non‐del(5q) LR‐MDS who were ineligible for or refractory to ESAs [14]. Median duration of RBC‐TI in patients with non‐del(5q) LR‐MDS in the MDS‐005 study was ∼31 weeks [14].
HMAs are the standard of care for treatment of higher‐risk MDS [15], [16], [17]. The therapeutic effects of azacitidine and decitabine in hematologic disorders are believed to be due in part to methylation reductions in promotor regions of DNA, leading to re‐expression of tumor suppressor genes [18]. Both HMAs are indicated in the U.S. for treatment of all French‐American‐British (FAB) [19] subtypes of MDS and for low‐blast‐count AML (i.e., 20%–30% bone marrow [BM] blasts) [20]. Azacitidine is approved in the European Union for treatment of higher‐risk MDS and for adult patients with AML (any BM blast count) who are not candidates for allogeneic hematopoietic stem cell transplantation [21], and decitabine is approved for adult patients with newly diagnosed AML who are not eligible for induction chemotherapy [22]. In the randomized, phase III AZA‐001 clinical trial in patients with higher‐risk MDS and low‐blast‐count AML, azacitidine was shown to significantly prolong overall survival (OS) compared with conventional care regimens [15], [16]. Although azacitidine clinical data are primarily based on use in patients with higher‐risk MDS, it has also shown efficacy in patients with LR‐MDS, with or without del(5q) [23], [24], including in patients who were refractory to ESAs [25], [26].
National Comprehensive Cancer Network (NCCN) guidelines for treatment of MDS recommend azacitidine for patients with non‐del(5q) LR‐MDS who have clinically relevant thrombocytopenia or neutropenia, or increased BM blasts, and for those with LR‐MDS with serum erythropoietin (EPO) >500 mU/mL who are unlikely to respond to immunosuppressive therapy [10]. NCCN guidelines also recommend lenalidomide for all patients with del(5q) LR‐MDS and symptomatic anemia, and for patients with non‐del(5q) LR‐MDS with symptomatic anemia who are not likely to respond to ESAs [10].
Following ESA failure, there is no clear guidance regarding the choice of lenalidomide or an HMA as initial disease‐modifying therapy for patients with non‐del(5q) LR‐MDS, who mainly require treatment to reduce anemia and the need for transfusions. The choice of initial therapy is important because most patients will eventually stop responding to first‐line therapy and alternatives are limited.
No large, randomized, controlled trials or large prospective studies of azacitidine specific to LR‐MDS have yet been reported. To assess the effectiveness of first‐line azacitidine in RBC transfusion‐dependent LR‐MDS, we performed a prospective meta‐analysis of azacitidine treatment in this population in accordance with Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines, using individual patient‐level data obtained from investigators from relevant studies.
Materials and Methods
Data Sources
The PubMed database was searched for English‐language articles published between January 1, 2000, and December 31, 2015. Embase was searched for abstracts from hematology conferences published in 2015. The key words used for this search were azacitidine, azacytidine, 5‐azacitidine, myelodysplastic syndrome, and MDS.
Study Selection, Meta‐Analysis Inclusion Criteria, and Data Extraction
Meta‐analysis data were collected from prospective clinical trials (phase II or phase III) and patient registries that included RBC transfusion‐dependent patients with LR‐MDS. Criteria for including patient data in meta‐analyses were as follows: (a) the study included patients with LR‐MDS defined by IPSS, FAB, or World Health Organization (WHO) treated with azacitidine; (b) rates of RBC‐TI were assessed; and (c) no concomitant chemotherapy, immunotherapy, hematopoietic stem cell transplant, other epigenetic therapy, or investigational treatments were included in the study.
Not included in these analyses were data from review articles, editorials, preclinical studies, retrospective analyses, case reports, studies specific to oral azacitidine, studies in which azacitidine was not used as first‐line treatment, studies with nonclinical endpoints (e.g., with translational focus), transplant‐specific studies, or studies limited to patients with higher‐risk MDS or AML.
Data Collection
Available patient‐level data were obtained from respective study investigators. Although studies included in meta‐analyses required enrollment of patients with IPSS‐, FAB‐, or WHO‐defined LR‐MDS, patient‐level data in some cases did not differentiate LR‐MDS from higher‐risk MDS. Therefore, LR‐MDS was established based on an IPSS score of ≤1, derived from individual patient data, as the sum of BM blasts percentage (BMB) plus cytopenia (C) scores as follows [2]:
BMB < 5%, BMB score = 0
5% ≤ BMB ≤ 10%, BMB score = 0.5
10% < BMB ≤ 20%, BMB score = 1.5
20% < BMB ≤ 30%, BMB score = 2
And
0 or 1 cytopenia, C score = 0
2 or 3 cytopenias, C score = 0.5
Cytopenias were defined as hemoglobin (Hgb) <10 g/dL, platelets <100 × 109/L, and absolute neutrophil count (ANC) <0.5 × 109/L.
Pretreatment patient data collected included age, gender, IPSS prognostic risk (if not provided, this was calculated from subscores as described above), number and depth of cytopenias, Eastern Cooperative Oncology Group (ECOG) performance status score, BM blast percentage, time since MDS diagnosis, presence of del(5q) abnormality, EPO level, and prior use of and responsiveness to ESAs. Treatment variables included azacitidine regimen and schedule in the individual studies, total azacitidine doses, and numbers of treatment cycles prospectively specified in the study designs.
Endpoints
Rates of RBC‐TI and Clinical Benefit were assessed for patients in the individual studies with available data and for the group of all patients with available data. RBC‐TI was defined as no RBC transfusions for a period of 56 consecutive days on‐study. Clinical Benefit was defined as attainment of RBC‐TI, hematologic improvement in the erythroid lineage (HI‐E), complete remission (CR), or partial remission (PR), per International Working Group 2006 criteria for MDS [27].
Statistical Analysis
Baseline characteristics and treatment variables are summarized descriptively.
Three patient cohorts were identified for RBC‐TI and Clinical Benefit outcomes: (a) the overall group of RBC transfusion‐dependent patients with LR‐MDS; (b) the subgroup of patients who had failed prior ESA use or with serum EPO level >500 mU/mL (“ESA refractory”); and (c) the subgroup of patients with isolated anemia (Hgb <10 g/dL with platelets ≥100 × 109/L and ANC ≥0.5 × 109/L).
Univariate analyses were performed using available data from the group of all patients to estimate the association between response and the following baseline variables: age (continuous variable), gender (male vs. female), Hgb level (≥10 vs. <10 g/dL), ANC (≥0.5 vs. <0.5 × 109/L), platelet level (<100 vs. ≥100 × 109/L), BM blast percentage (≥5% vs. <5%), ECOG performance status (0 vs. 1–3), number of cytopenias (0–1 vs. 2–3), presence of del(5q) (no vs. yes), isolated anemia (yes vs. no), prior ESA usage (yes vs. no), and EPO level (≤500 vs. >500 mU/mL). Other factors in the univariate analysis were protocol‐specified planned number of azacitidine cycles (<6 vs. ≥6 cycles) and total azacitidine dose (continuous variable).
Significant variables at p < .1 were included in the multivariate models for further assessment of factors associated with response (significant at p < .05) for all patients and in the ESA‐refractory and isolated anemia patient subgroups.
Heterogeneity of available data from trials included in meta‐analyses was assessed using the chi‐square test of heterogeneity and the I2 measure of inconsistency. Heterogeneity was considered present when the p value of the Cochran Q test was <.05 and the I2 statistic was >50%. Response rates were calculated using random‐effects models in cases of high heterogeneity, and by using fixed‐effects models in cases of low heterogeneity.
Results
Data Sources
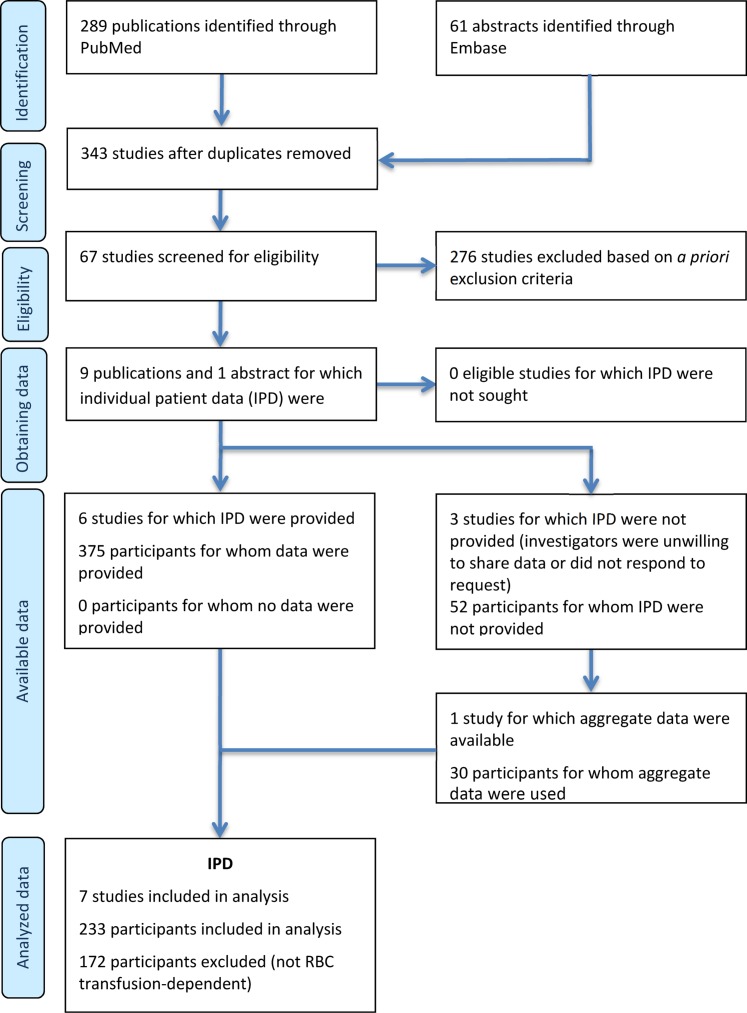
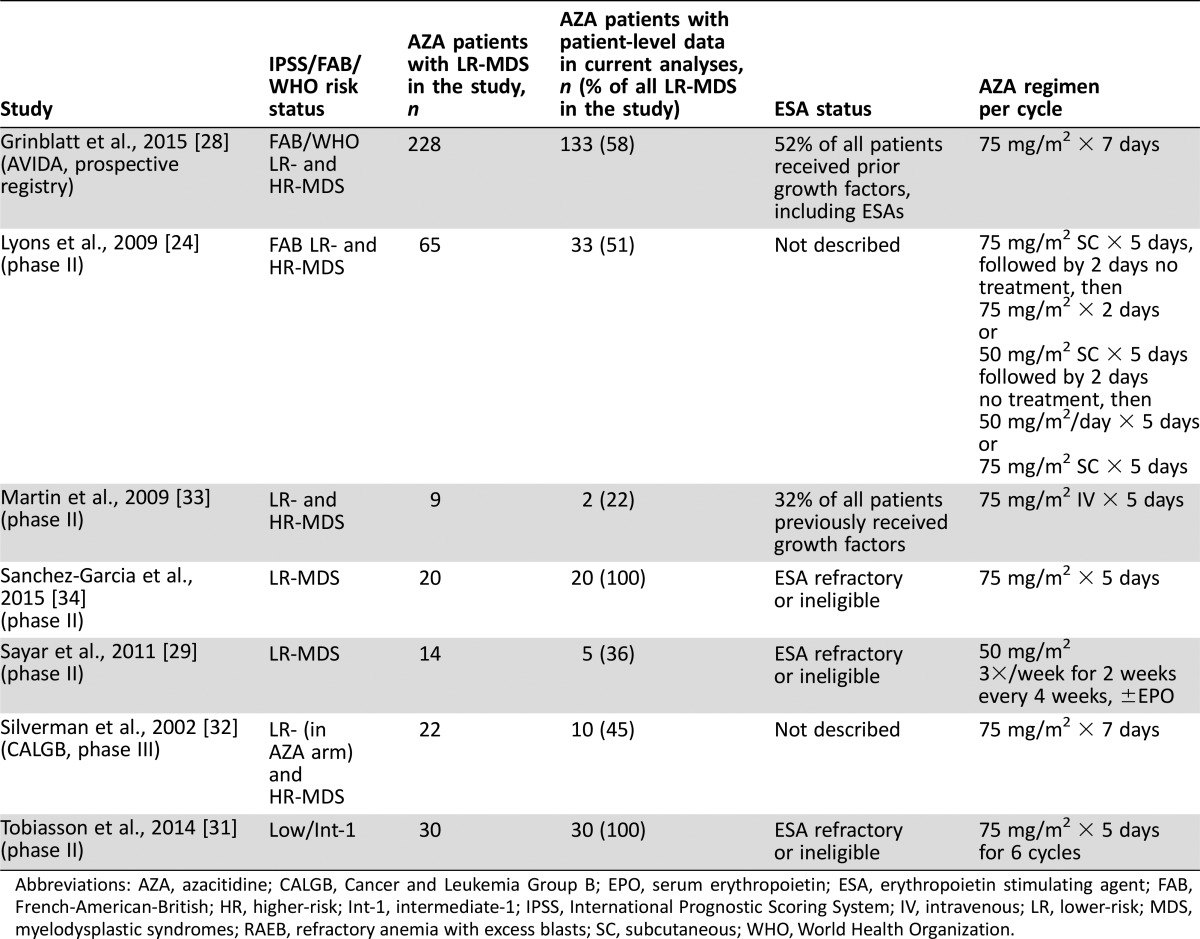
In all, 289 publications and 61 abstracts were initially identified based on literature search parameters (Figure 1). All but nine published studies and one abstract were excluded based on prospective search criteria [24], [25], [26], [28], [29], [30], [31], [32], [33], [34]. Investigators from these 10 studies were contacted to request published and unpublished patient‐level data for inclusion in analyses, which was kindly provided by investigators from 6 studies (Table 1). Appropriate patient‐level data were available in another study reported in abstract form [34] and are also included in analyses. Together, data for 233 patients from 7 studies met the relevant criteria [24], [28], [29], [31], [32], [33], [34]. Of these seven studies, six were clinical trials and one was a prospective community‐based registry study (AVIDA [28]). In the current analysis, the number of patients with data provided from the AVIDA registry (n = 133) was greater than the number from the other six clinical trials combined (n = 100).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses analysis.
Abbreviations: IPD, individual patient data; RBC, red blood cell.
Table 1. Studies from which patient‐level data were provided and included in meta‐analyses.
Abbreviations: AZA, azacitidine; CALGB, Cancer and Leukemia Group B; EPO, serum erythropoietin; ESA, erythropoietin stimulating agent; FAB, French‐American‐British; HR, higher‐risk; Int‐1, intermediate‐1; IPSS, International Prognostic Scoring System; IV, intravenous; LR, lower‐risk; MDS, myelodysplastic syndromes; RAEB, refractory anemia with excess blasts; SC, subcutaneous; WHO, World Health Organization.
All patients in these analyses were RBC transfusion‐dependent before treatment; however, baseline data were missing for other demographic or disease‐related variables in some cases (Table 2). ESA‐refractory and isolated anemia statuses were not mutually exclusive; of patients with available data, 161/165 patients (97.6%) were ESA refractory and 81/200 patients (40.5%) had isolated anemia. The median age of all patients was 74 years (range 37–91). Of patients with available information, 68.1% were male, 90.3% of patients had non‐del(5q) LR‐MDS, and 67.9% had Hgb levels of <10 g/dL at entry into the individual studies.
Table 2. Baseline characteristics for patients included in meta‐analyses.
Abbreviations: ANC, absolute neutrophil count; AZA, azacitidine; BM, bone marrow; CALGB, Cancer and Leukemia Group B; d, days; ECOG, Eastern Cooperative Oncology Group; EPO, serum erythropoietin; ESA, erythropoietin stimulating agent; FAB, French‐American‐British; Hgb, hemoglobin; HR, higher‐risk; LR, lower‐risk; MDS, myelodysplastic syndromes; NA, not available; SD, standard deviation.
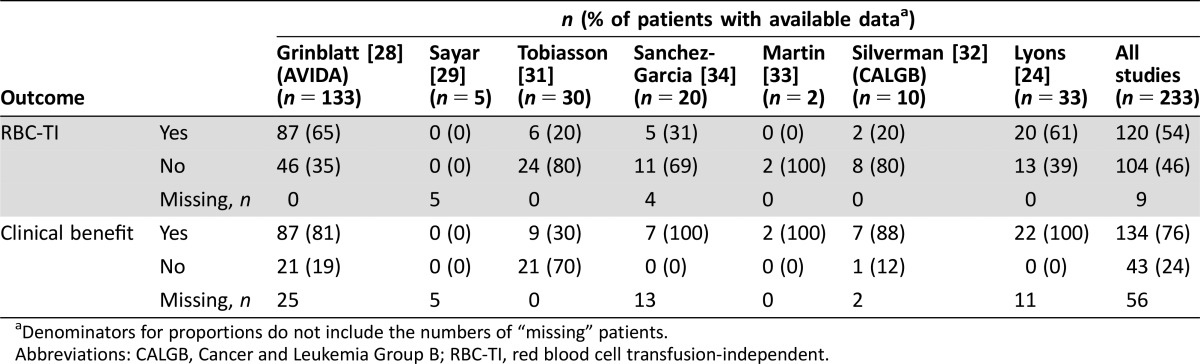
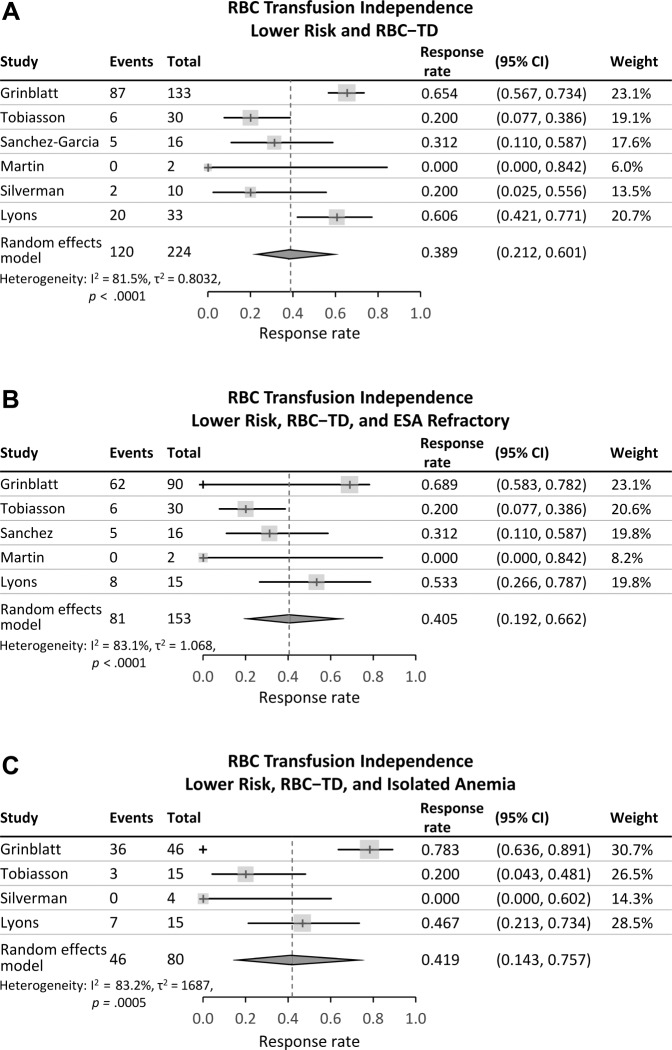
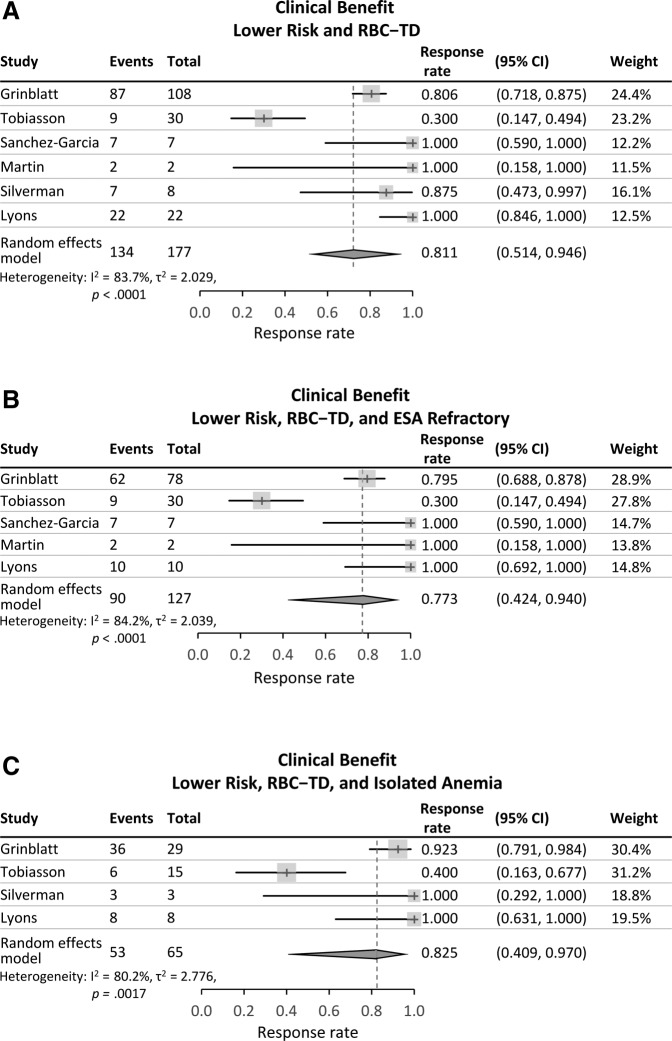
Table 3 shows RBC‐TI and Clinical Benefit rates for included patients in each individual study and a combined response rate. Overall RBC‐TI and Clinical Benefit rates for patients with available data were 54% and 76%, respectively; however, there was significant heterogeneity of response results among the seven studies. The Cochran Q test values were p < .01 for all analyses and I2 values ranged from 81.5% to 83.2% for RBC‐TI and from 80.2% to 84.2% for Clinical Benefit. Thus, using random‐effects models, the pooled estimates of RBC‐TI rate in meta‐analysis was 38.9% for all patients, 40.5% in the ESA‐refractory subgroup, and 41.9% in patients with isolated anemia (Figure 2). Pooled estimates of Clinical Benefit rates were 81.1% for all patients, 77.3% in ESA‐refractory patients, and 82.5% in patients with isolated anemia (Figure 3).
Table 3. Response in individual studies and overall for patients with available data.
Denominators for proportions do not include the numbers of “missing” patients.
Abbreviations: CALGB, Cancer and Leukemia Group B; RBC‐TI, red blood cell transfusion‐independent.
Figure 2.
Transfusion‐independence in patients with RBC transfusion‐dependent lower‐risk myelodysplastic syndromes. (A): All patients. (B): The subgroup of patients refractory to erythropoiesis‐stimulating agents. (C): The subgroup of patients with isolated anemia.
Abbreviations: CI, confidence interval; ESA, erythropoiesis‐stimulating agent; RBC, red blood cell; RBC‐TD, RBC transfusion‐dependent.
Figure 3.
Clinical Benefit in patients with RBC transfusion‐dependent lower‐risk myelodysplastic syndromes. This composite endpoint comprises RBC transfusion independence, hematologic improvement in the erythroid lineage, and complete or partial remission, per the International Working Group 2006 criteria for myelodysplastic syndromes [27]. (A): All patients. (B): The subgroup of patients refractory to erythropoiesis‐stimulating agents. (C): The subgroup of patients with isolated anemia.
Abbreviations: CI, confidence interval; ESA, erythropoiesis‐stimulating agent; RBC‐TD, red blood cell transfusion‐dependence.
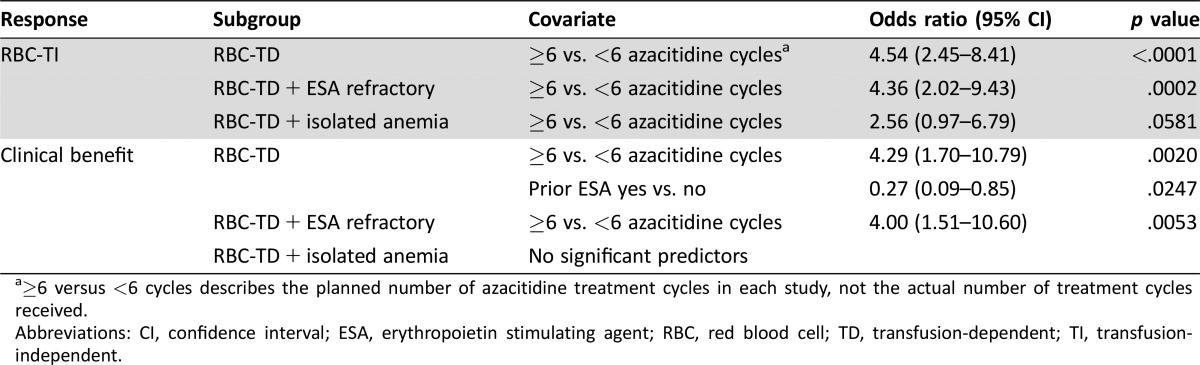
In univariate analysis, significant predictors of RBC‐TI were older age, baseline ANC ≥0.5 × 109/L, higher total azacitidine dose, and studies with ≥6 prospectively planned azacitidine treatment cycles. Significant predictors of Clinical Benefit were no prior ESA usage, baseline presence of 0 or 1 cytopenia, baseline ANC ≥0.5 × 109/L, higher total azacitidine dose, and ≥6 planned azacitidine treatment cycles. These variables were added to multivariate models.
In multivariate analyses, participation in a study with a prospective plan to treat patients with ≥6 azacitidine treatment cycles was a significant predictor of RBC‐TI in all three cohorts (i.e., in the overall patient group and in the ESA‐refractory and isolated anemia subgroups; Table 4). Prospectively planned use of at least 6 azacitidine treatment cycles was also significantly predictive of attaining Clinical Benefit for the overall population and for patients who were ESA refractory. In the group of all patients, no prior ESA use was also significantly predictive of attaining Clinical Benefit. For patients with isolated anemia, no factor emerged as significantly predictive of Clinical Benefit.
Table 4. Significant predictors of response in multivariate analyses of patients with RBC transfusion‐dependent lower‐risk myelodysplastic syndromes.
≥6 versus <6 cycles describes the planned number of azacitidine treatment cycles in each study, not the actual number of treatment cycles received.
Abbreviations: CI, confidence interval; ESA, erythropoietin stimulating agent; RBC, red blood cell; TD, transfusion‐dependent; TI, transfusion‐independent.
Because AVIDA was the only registry study and the number of patients with data from AVIDA was disproportionately large, sensitivity meta‐analyses were performed excluding AVIDA patient data. When AVIDA patients were removed from analyses, response rates decreased, with RBC‐TI ranging from 29.4% to 31.8% and Clinical Benefit rates from 75.3% to 83.7%. In multivariate sensitivity analyses without AVIDA patients, significant predictors of attaining RBC‐TI for the group of all patients were older age, no prior ESA use, Hgb ≥10 g/dL, and ≥6 planned azacitidine treatment cycles. In the subgroup of patients with refractory anemia, Hgb ≥10 g/dL was significantly predictive of achieving RBC‐TI, and no prior ESA use was significantly predictive of RBC‐TI in patients with isolated anemia. In the group of all patients, only planned administration of ≥6 azacitidine cycles was significantly predictive of Clinical Benefit.
Discussion
MDS hemopathies are progressive disorders, and treatment options are limited; therefore, the choice of initial treatment should be made judiciously, with the understanding that at some point, alternate or additional treatment may be necessary. In this meta‐analysis, azacitidine was associated with an RBC‐TI rate of 38.9% in the group of all patients with RBC transfusion‐dependent LR‐MDS, more than 90% of whom had non‐del(5q) disease. The RBC TI rate obtained with lenalidomide treatment in RBC transfusion‐dependent patients with non‐del(5q) LR‐MDS in the MDS‐002 study [13] and rates in patients with non‐del(5q) LR‐MDS in the phase III MDS‐005 study who were RBC transfusion‐dependent and unresponsive or refractory to ESAs was ∼27% [14].
To determine whether the order of treatment made a difference in clinical response rates, Zeidan et al. retrospectively evaluated HI‐E rates for 63 patients with non‐del(5q) LR‐MDS at a single institution who had received sequential treatment with both lenalidomide and azacitidine [35]. The HI‐E rate with lenalidomide as first‐line therapy was 38% compared with 12% when used as second‐line therapy after azacitidine (p = .04), whereas response rates to azacitidine were similar whether it was used before or after lenalidomide (35% and 38%, respectively) [35]. Komrokji et al. reported similar outcomes for 144 patients from multiple sites (including the original 63 patients mentioned above) who had received sequential lenalidomide and azacitidine or decitabine [36], showing a 20% rate of HI‐E with lenalidomide when it was used first‐line compared with 11% HI‐E when lenalidomide was used second‐line after the HMA (p = .046) [36]. Again, there was no significant difference in HI‐E rates when an HMA was used as first‐ or second‐line therapy (39% and 30%, p = .2). In this larger cohort, rate of transformation to AML was also significantly different based on treatment order, with 9% of patients developing AML when lenalidomide was used first‐line compared with 22% of patients when it was used after an HMA (p = .03). Despite differences in response rates, treatment order had no influence on OS [36]. At this time, no randomized prospective data are available to confirm these findings.
HMAs do not selectively target anemia, but improve platelet and neutrophil counts [15]; thus, they may be a better choice of first‐line therapy for patients with LR‐MDS and anemia accompanied by significant thrombocytopenia or neutropenia. Additionally, the presence of biomarkers of improved response may suggest preferential use of azacitidine for some patients. For example, TET2 mutations, which occur in approximately 20% of patients with MDS, have been found to be predictive of better response to HMAs [37], [38], [39]. Mutations in IDH genes, found in ∼5% of MDS patients, have also been linked to better response to treatment with HMAs [40], [41]. One study showed that although mutations in the histone modulators ASXL1 or EZH2 did not influence response rates, they were associated with significantly prolonged OS in patients with higher‐risk MDS treated with azacitidine [42]. There is contradictory evidence regarding the influence of TP53 mutations on outcomes of azacitidine treatment. Some studies suggest azacitidine‐treated patients with TP53 mutations have poorer OS than those with wild‐type TP53 [39, 43], whereas other data suggest TP53 mutational status has no influence on survival [44]. Response to lenalidomide may also be influenced by mutational status. Preliminary data suggest higher rates of RBC‐TI in lenalidomide‐treated patients with non‐del(5q) LR‐MDS who have DNMT3A mutations (∼8% of MDS patients), compared with patients without this mutation (44% vs. 25%, respectively; p = .13), and significantly lower RBC‐TI rates in patients with ASXL1 mutations (∼20% of MDS patients) compared with wild‐type (10% vs. 32%, respectively; p = .031) [45].
Results of the current multivariate analyses demonstrate the importance of azacitidine treatment duration. The likelihood of attaining RBC‐TI was increased ∼three‐ to four‐fold when patients participated in studies that planned for at least 6 azacitidine treatment cycles. Compared with the rapid myeloablation incurred with intensive chemotherapy regimens, response to azacitidine is more gradual and 4–6 months of azacitidine treatment may be required to induce a response [46], [47].
A limitation of current analyses is the high heterogeneity of results among the seven studies and the large imbalance among the numbers of patients from each study. More patient data came from the AVIDA registry than from all other studies combined. Eligibility criteria for enrollment in the AVIDA registry were (a) having MDS or other hematologic disorder, (b) having had no prior azacitidine exposure, and (c) that patients be “candidates for initiation of azacitidine treatment” [28]. Inclusion of so many patients from AVIDA may have introduced unforeseen biases; for example, concomitant use of ESAs or growth factors may have elevated RBC‐TI rates relative to what might have been achieved with azacitidine alone. Indeed, when AVIDA data were excluded in sensitivity analyses, rates of RBC‐TI dropped by up to 10.4% and Clinical Benefit rate decreased by up to 5.8%. Other potential limitations of these analyses were that data regarding pretreatment disease or demographic characteristics were not available for some patients, increasing the chance that there may not have been a large enough dataset to robustly confirm whether a specific variable that was not significant in univariate or multivariate analyses actually did influence response. Additionally, methods of response assessment (e.g., local vs. central) varied among studies, and in some cases were not reported at all. Finally, information regarding durability of response were available for only a subset of patients from three of the seven studies, precluding robust analysis of those data.
Although current treatment options for patients with LR‐MDS who are refractory to ESAs are limited to injectable HMAs and lenalidomide, there are several drugs now in various stages of clinical development that could substantially expand the LR‐MDS therapeutic landscape. In a phase I/II study of patients with LR‐MDS, CC‐486 (oral azacitidine) was associated with an RBC‐TI rate of 34% [48]. CC‐486 doses under investigation deliver lower cumulative azacitidine doses per cycle than the 7‐days‐per‐cycle injectable regimen, and can be administered over longer periods of the 28‐day treatment cycle to sustain hypomethylating effects [48], [49]. A phase III randomized study is currently underway to evaluate the efficacy and safety of CC‐486 in patients with LR‐MDS who have RBC transfusion‐dependent anemia and thrombocytopenia (≤75 × 109/L platelets; NCT01566695). Another drug in phase III of development for use in LR‐MDS is imetelstat, an intravenous 13‐mer lipid‐conjugated oligonucleotide that competitively inhibits telomerase enzymatic activity (NCT02598661) [50]. In a small pilot study (NCT01731951) with nine patients, most of whom had refractory anemia with ringed sideroblasts (n = 8), three of eight transfusion‐dependent patients (38%) achieved RBC‐TI. All patients experienced at least one (reversible) grade ≥3 cytopenia [50].
Several other drugs are in earlier stages of development. APG101 is a fusion protein comprising the extracellular domain of human CD95 receptor and the Fc domain of the IgG1 antibody [51]. APG101 appears to inhibit the interaction between the CD95 receptor and its cognate ligand to prevent a CD95‐mediated blockade of erythrocyte production in BM. In a small phase I study (NCT01736436), 6 of 20 APG101‐treated patients (30%) showed increases in Hgb levels and 8 (40%) required fewer RBC transfusions [51]. Luspatercept, a modified ActRIIB‐Fc fusion protein, was evaluated in the extension period of a phase II study (PACE‐MDS; NCT02268383) in 32 RBC transfusion‐dependent patients with LR‐MDS who were refractory to or unlikely to respond to ESAs [52]. Twenty‐six patients (81%) attained HI‐E and 11 of 22 patients (50%) attained RBC‐TI [52]. Luspatercept is currently under investigation in a placebo‐controlled, randomized phase III study with RBC transfusion‐dependent patients with LR‐MDS with ringed sideroblasts (NCT02631070). Luspatercept is the only new drug under investigation currently in phase 3 of clinical development. OPN‐305 is an intravenous IgG4 kappa monoclonal antibody directed against the toll‐like receptor type 2 (TLR2); signalling mediated by TLR2 leads to expression of multiple cytokines that can interfere with hematopoiesis [53]. OPN‐305 is currently in a phase I/II dose‐finding and expansion study for use as second‐line treatment of LR‐MDS (NCT02363491). Preliminary outcomes of a dose‐finding trial in a small number of patients with LR‐MDS for whom prior HMA treatment had failed showed a 50% overall response rate (6 of 12 patients) and 17% RBC‐TI [54]. At doses evaluated (4 and 10 mg/kg) no significant effect on cytokine levels was observed suggesting higher doses of OPN‐305 should be evaluated [54].
Conclusion
Ultimately, determining the optimal choice of initial disease‐modifying therapy may require investigation in a prospective, randomized clinical trial. As new treatment options become available, there will be greater flexibility in the choice of initial and subsequent therapies to improve hematopoiesis in LR‐MDS. The impact of HMAs on the natural history of the disease among LR‐MDS patients with higher‐risk features identified by new clinical risk models [55], and on somatic mutations, are important subjects of research. Many factors influence the choice of initial treatment. These analyses suggest azacitidine is an effective therapy for LR‐MDS; however, convenience, side‐effect profiles, and cost may influence the choice of azacitidine as first‐line treatment after ESA failure.
Acknowledgments
Editorial support was provided by Sheila Truten and Kelly Dittmore of Medical Communication Company, Inc. (Wynnewood, PA), funded by Celgene Corporation. Albert Fliss and Mary M. Sugrue were employed by Celgene Corporation during manuscript drafting.
Author Contributions
Collection and provision of patient data: Rami Komrokji, David Grinblatt, Roger M. Lyons, Magnus Tobiasson, Lewis R. Silverman, Hamid Sayar, Ravi Vij
Data analysis: Arlene S. Swern, Nora Tu
Data interpretation: Rami Komrokji, David Grinblatt, Roger M. Lyons, Magnus Tobiasson, Lewis R. Silverman, Hamid Sayar, Ravi Vij
Manuscript writing: Rami Komrokji, Arlene S. Swern, David Grinblatt, Roger M. Lyons, Magnus Tobiasson, Lewis R. Silverman, Hamid Sayar, Ravi Vij, Albert Fliss, Nora Tu, Mary M. Sugrue
Final approval of manuscript: Rami Komrokji, Arlene S. Swern, David Grinblatt, Roger M. Lyons, Magnus Tobiasson, Lewis R. Silverman, Hamid Sayar, Ravi Vij, Albert Fliss, Nora Tu, Mary M. Sugrue
Disclosures
Rami Komrokji: Celgene Corporation, Novartis (C/A), Novartis (H); Arlene S. Swern: Celgene Corporation (E, OI); David Grinblatt: Celgene Corporation (C/A); Lewis R. Silverman: Celgene Corporation (C/A); Ravi Vij: Celgene Corporation, Bristol‐Myers Squibb, Janssen, Abbvie, Karyopharma (H), Amgen, Takeda (H, RF); Albert Fliss: Celgene Corporation (E, OI); Nora Tu: Celgene Corporation (E, OI); Mary M. Sugrue: Celgene Corporation (E, OI). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med 2009;361:1872–1885. [DOI] [PubMed] [Google Scholar]
- 2. Greenberg P, Cox C, LeBeau MM et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997;89:2079–2088. [PubMed] [Google Scholar]
- 3. Santini V. Anemia as the main manifestation of myelodysplastic syndromes. Semin Hematol 2015;52:348–356. [DOI] [PubMed] [Google Scholar]
- 4. Davidoff AJ, Weiss SR, Baer MR et al. Patterns of erythropoiesis‐stimulating agent use among Medicare beneficiaries with myelodysplastic syndromes and consistency with clinical guidelines. Leuk Res 2013;37:675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kelaidi C, Park S, Sapena R et al. Long‐term outcome of anemic lower‐risk myelodysplastic syndromes without 5q deletion refractory to or relapsing after erythropoiesis‐stimulating agents. Leukemia 2013;27:1283–1290. [DOI] [PubMed] [Google Scholar]
- 6. Jadersten M, Montgomery SM, Dybedal I et al. Long‐term outcome of treatment of anemia in MDS with erythropoietin and G‐CSF. Blood 2005;106:803–811. [DOI] [PubMed] [Google Scholar]
- 7. Fenaux P, Ades L. How we treat lower‐risk myelodysplastic syndromes. Blood 2013;121:4280–4286. [DOI] [PubMed] [Google Scholar]
- 8. Malcovati L. Impact of transfusion dependency and secondary iron overload on the survival of patients with myelodysplastic syndromes. Leuk Res 2007;31(suppl 3):S2–S6. [DOI] [PubMed] [Google Scholar]
- 9. Malcovati L, Germing U, Kuendgen A et al. Time‐dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol 2007;25:3503–3510. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Myelodysplastic Syndromes Version 2.2017. Available at https://www.nccn.org/professionals/physician_gls/pdf/mds.pdf. Accessed October 25, 2017. [DOI] [PubMed]
- 11. List A, Dewald G, Bennett J et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med 2006;355:1456–1465. [DOI] [PubMed] [Google Scholar]
- 12. Fenaux P, Giagounidis A, Selleslag D et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion‐dependent patients with Low‐/Intermediate‐1‐risk myelodysplastic syndromes with del5q. Blood 2011;118:3765–3776. [DOI] [PubMed] [Google Scholar]
- 13. Raza A, Reeves JA, Feldman EJ et al. Phase 2 study of lenalidomide in transfusion‐dependent, low‐risk, and intermediate‐1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood 2008;111:86–93. [DOI] [PubMed] [Google Scholar]
- 14. Santini V, Almeida A, Giagounidis A et al. Randomized phase III study of lenalidomide versus placebo in RBC transfusion‐dependent patients with lower‐risk non‐del(5q) myelodysplastic syndromes and ineligible for or refractory to erythropoiesis‐stimulating agents. J Clin Oncol 2016;34:2988–2996. [DOI] [PubMed] [Google Scholar]
- 15. Fenaux P, Mufti GJ, Hellstrom‐Lindberg E et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher‐risk myelodysplastic syndromes: A randomised, open‐label, phase III study. Lancet Oncol 2009;10:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fenaux P, Mufti GJ, Hellstrom‐Lindberg E et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol 2010;28:562–569. [DOI] [PubMed] [Google Scholar]
- 17. Dombret H, Seymour JF, Butrym A et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015;126:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Christman JK. 5‐Azacytidine and 5‐aza‐2'‐deoxycytidine as inhibitors of DNA methylation: Mechanistic studies and their implications for cancer therapy. Oncogene 2002;21:5483–5495. [DOI] [PubMed] [Google Scholar]
- 19. Bennett JM, Catovsky D, Daniel MT et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol 1982;51:189–199. [PubMed] [Google Scholar]
- 20. Harris NL, Jaffe ES, Diebold J et al. The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November, 1997. Ann Oncol 1999;10:1419–1432. [DOI] [PubMed] [Google Scholar]
- 21.European Medicines Agency . Vidaza (azacitidine). 2016:EMA/450923/452016.
- 22.European Medicines Agency . Dacogen (decitabine). EPAR summary for the public. 2016:EMA/344257/342016.
- 23. Komrokji RS, Al Ali NH, Padron E et al. Azacitidine treatment of lenalidomide‐resistant myelodysplastic syndrome with deletion 5q. Blood 2011;118:2774a. [Google Scholar]
- 24. Lyons RM, Cosgriff TM, Modi SS et al. Hematologic response to three alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes. J Clin Oncol 2009;27:1850–1856. [DOI] [PubMed] [Google Scholar]
- 25. Falantes J, Delgado RG, Calderon‐Cabrera C et al. Multivariable time‐dependent analysis of the impact of azacitidine in patients with lower‐risk myelodysplastic syndrome and unfavorable specific lower‐risk score. Leuk Res 2015;39:52–57. [DOI] [PubMed] [Google Scholar]
- 26. Fili C, Malagola M, Follo MY et al. Prospective phase II Study on 5‐days azacitidine for treatment of symptomatic and/or erythropoietin unresponsive patients with low/INT‐1‐risk myelodysplastic syndromes. Clin Cancer Res 2013;19:3297–3308. [DOI] [PubMed] [Google Scholar]
- 27. Cheson BD, Greenberg PL, Bennett JM et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 2006;108:419–425. [DOI] [PubMed] [Google Scholar]
- 28. Grinblatt DL, Sekeres MA, Komrokji RS et al. Patients with myelodysplastic syndromes treated with azacitidine in clinical practice: The AVIDA registry. Leuk Lymphoma 2015;56:887–895. [DOI] [PubMed] [Google Scholar]
- 29. Sayar H, Chan RJ, Orschell CM et al. Thrice weekly azacitidine does not improve hematological responses in lower‐risk myelodysplastic syndromes: A study of the Hoosier Oncology Group. Leuk Res 2011;35:1108–1110. [DOI] [PubMed] [Google Scholar]
- 30. Musto P, Maurillo L, Spagnoli A et al. Azacitidine for the treatment of lower risk myelodysplastic syndromes: A retrospective study of 74 patients enrolled in an Italian named patient program. Cancer 2010;116:1485–1494. [DOI] [PubMed] [Google Scholar]
- 31. Tobiasson M, Dybedahl I, Holm MS et al. Limited clinical efficacy of azacitidine in transfusion‐dependent, growth factor‐resistant, low‐ and Int‐1‐risk MDS: Results from the nordic NMDSG08A phase II trial. Blood Cancer J 2014;4:e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Silverman LR, Demakos EP, Peterson BL et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the cancer and leukemia group B. J Clin Oncol 2002;20:2429–2440. [DOI] [PubMed] [Google Scholar]
- 33. Martin MG, Walgren RA, Procknow E et al. A phase II study of 5‐day intravenous azacitidine in patients with myelodysplastic syndromes. Am J Hematol 2009;84:560–564. [DOI] [PubMed] [Google Scholar]
- 34. Sanchez‐Garcia J, Falantes J, Medina A et al. Final results of a phase II randomized trial of azacitidine versus support treatment in patients with low risk myelodysplastic syndrome without 5q deletion. Leuk Res 2015;39:114a. [Google Scholar]
- 35. Zeidan AM, Al Ali NH, Padron E et al. Lenalidomide treatment for lower‐risk nondeletion 5q myelodysplastic syndromes patients yields higher response rates when used before azacitidine. Clin Lymphoma Myeloma Leuk 2015;15:705–710. [DOI] [PubMed] [Google Scholar]
- 36. Komrokji R, Sekeres MA, Barnard J et al. Optimal treatment order of lenalidomide and hypomethylating agents for lower‐risk myelodysplastic syndromes: A report on behalf of the MDS Clinical Research Consortium. Blood 2016;128:4322a. [Google Scholar]
- 37. Ganguly BB, Kadam NN. Mutations of myelodysplastic syndromes (MDS): An update. Mutat Res Rev Mutat Res 2016;769:47–62. [DOI] [PubMed] [Google Scholar]
- 38. Itzykson R, Kosmider O, Cluzeau T et al. Impact of TET2 mutations on response rate to azacitidine in myelodysplastic syndromes and low blast count acute myeloid leukemias. Leukemia 2011;25:1147–1152. [DOI] [PubMed] [Google Scholar]
- 39. Bejar R, Lord A, Stevenson K et al. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood 2014;124:2705–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med 2015;373:1136–1152. [DOI] [PubMed] [Google Scholar]
- 41. Emadi A, Faramand R, Carter‐Cooper B et al. Presence of isocitrate dehydrogenase mutations may predict clinical response to hypomethylating agents in patients with acute myeloid leukemia. Am J Hematol 2015;90:E77–E79. [DOI] [PubMed] [Google Scholar]
- 42. Tobiasson M, McLornan DP, Karimi M et al. Mutations in histone modulators are associated with prolonged survival during azacitidine therapy. Oncotarget 2016;7:22103–22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bally C, Ades L, Renneville A et al. Prognostic value of TP53 gene mutations in myelodysplastic syndromes and acute myeloid leukemia treated with azacitidine. Leuk Res 2014;38:751–755. [DOI] [PubMed] [Google Scholar]
- 44. Tang L, Dolnik A, MacBeth KJ et al. Impact of gene mutations on overall survival in older patients with acute myeloid leukemia (AML) treated with azacitidine (AZA) or conventional care regimens (CCR). Blood 2016;128:2859a. 27799161 [Google Scholar]
- 45. Santini V, Fenaux P, Giagounidis A et al. Impact of somatic mutations on response to lenalidomide (LEN) in IPSS lower‐risk myelodysplastic syndromes (MDS) patients (pts) without del(5q) and ineligible for or refractory to erythropoiesis‐stimulating agents (ESAs). Blood 2016;128:225a. [Google Scholar]
- 46. Silverman LR, Fenaux P, Mufti GJ et al. Continued azacitidine therapy beyond time of first response improves quality of response in patients with higher‐risk myelodysplastic syndromes. Cancer 2011;117:2697–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pleyer L, Burgstaller S, Girschikofsky M et al. Azacitidine in 302 patients with WHO‐defined acute myeloid leukemia: Results from the Austrian Azacitidine Registry of the AGMT‐Study Group. Ann Hematol 2014;93:1825–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Garcia‐Manero G, Gore SD, Kambhampati S et al. Efficacy and safety of extended dosing schedules of CC‐486 (oral azacitidine) in patients with lower‐risk myelodysplastic syndromes. Leukemia 2016;30:889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Laille E, Shi T, Garcia‐Manero G et al. Pharmacokinetics and pharmacodynamics with extended dosing of CC‐486 in patients with hematologic malignancies. PLoS One 2015;10:e0135520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tefferi A, Al‐Kali A, Begna KH et al. Imetelstat therapy in refractory anemia with ring sideroblasts with or without thrombocytosis. Blood Cancer J 2016;6:e405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Boch T, Luft T, Mossner M et al. Safety and efficacy of CD95‐ligand inhibitor APG101 in transfusion‐dependent patients with low risk MDS: Results from a phase 1 study. Blood 2016;128:228a. [DOI] [PubMed] [Google Scholar]
- 52. Platzbecker U, Germing U, Gotze K et al. Luspatercept increases hemoglobin and reduces transfusion burden in patients with low‐intermediate risk myelodysplastic syndromes (MDS): Long‐term results from the phase 2 PACE‐MDS study. Blood 2016;128:3168a. [Google Scholar]
- 53. Reilly M, Miller RM, Thomson MH et al. Randomized, double‐blind, placebo‐controlled, dose‐escalating phase I, healthy subjects study of intravenous OPN‐305, a humanized anti‐TLR2 antibody. Clin Pharmacol Ther 2013;94:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Garcia‐Manero G, Montalban‐Bravo G, Yang H et al. A clinical study of OPN‐305, a toll‐like receptor 2 (TLR‐2) antibody, in patients with lower risk myelodysplastic syndromes (MDS) that have received prior hypomethylating agent (HMA) therapy. Blood 2016;128:227a. 27099149 [Google Scholar]
- 55. Garcia‐Manero G. Myelodysplastic syndromes: 2014 update on diagnosis, risk‐stratification, and management. Am J Hematol 2014;89:97–108. [DOI] [PubMed] [Google Scholar]