Abstract
Rapid climatic changes and increasing human influence at high elevations around the world will have profound impacts on mountain biodiversity. However, forecasts from statistical models (e.g. species distribution models) rarely consider that plant community changes could substantially lag behind climatic changes, hindering our ability to make temporally realistic projections for the coming century. Indeed, the magnitudes of lags, and the relative importance of the different factors giving rise to them, remain poorly understood. We review evidence for three types of lag: “dispersal lags” affecting plant species’ spread along elevational gradients, “establishment lags” following their arrival in recipient communities, and “extinction lags” of resident species. Variation in lags is explained by variation among species in physiological and demographic responses, by effects of altered biotic interactions, and by aspects of the physical environment. Of these, altered biotic interactions could contribute substantially to establishment and extinction lags, yet impacts of biotic interactions on range dynamics are poorly understood. We develop a mechanistic community model to illustrate how species turnover in future communities might lag behind simple expectations based on species’ range shifts with unlimited dispersal. The model shows a combined contribution of altered biotic interactions and dispersal lags to plant community turnover along an elevational gradient following climate warming. Our review and simulation support the view that accounting for disequilibrium range dynamics will be essential for realistic forecasts of patterns of biodiversity under climate change, with implications for the conservation of mountain species and the ecosystem functions they provide.
Keywords: biotic interactions, climate change, climatic debt, migration, alpine ecosystems, novel interactions, range dynamics, range expansion
Introduction
Mountains are experiencing extensive changes in land-use and climate and increasing levels of biological invasion, with temperature increasing faster than global averages at high elevations in many mountain ranges (Mountain Research Initiative, 2015). Such rapid changes in temperature are expected to result in extinctions of cold-adapted plant species in mountains (e.g. Dirnböck et al., 2011), coupled with a dramatic turnover in alpine plant communities (Dullinger et al., 2012a; Hülber et al., 2016). In some regions, projections using species distribution models (SDMs) predict up to 100 % species turnover in alpine plant communities by 2100 (Engler et al., 2009; Engler et al., 2011). Nonetheless, time lags in species’ responses to changing climate over the next 50-200 years could be substantial (Bertrand et al., 2011; Corlett & Westcott, 2013; Svenning & Sandel, 2013). Lags could lead to a discrepancy between realised community changes and expectations from SDMs that do not account for time lags in biotic responses (“disequilibrium dynamics”; Svenning & Sandel, 2013). Indeed, while range expansions to higher elevation have occurred in many regions (e.g. Lenoir et al., 2008), so far losses of cold-adapted species appear to have been relatively few, at least for plants on boreal-temperate mountains of Europe (Kulonen, 2017; Pauli et al., 2012). Therefore, to more accurately forecast the nature and temporal dynamics of mountain plant community change over the next century, it will be necessary to understand the processes contributing to lags in species’ responses to environmental change (Bertrand et al., 2016).
Empirical studies reveal considerable variation in species’ range dynamics along elevational gradients, with asynchrony in the rate, or even direction, of range expansions and contractions (e.g. Crimmins et al., 2011; Lenoir et al., 2008). Partly this variation in response can be explained by individualistic responses of species to a complex suite of climatic changes, with alterations to the mean, variation and seasonality of temperature and precipitation, as well as land-use changes (Crimmins et al., 2011). But even accounting for this, species vary inherently in their rates of spread and consequently in their ability to track climate change (“dispersal lags”; Essl et al., 2015; Svenning & Sandel, 2013), their ability to establish in communities at higher elevation (“establishment lags”) and in their ability to persist at their trailing range edge (“extinction lags”; Dullinger et al., 2012a; Lenoir & Svenning, 2013).
Several processes can influence the magnitude of dispersal, establishment and extinction lags, and therefore the rate of community turnover in mountain ecosystems following environmental change. These include: (1) intrinsic species attributes, such as dispersal ability (Engler et al., 2009), physiology and demographic rates (Kroiss & HilleRisLambers, 2014), (hereafter “physiology and demography”); (2) biotic interactions (HilleRisLambers et al., 2013; Kaarlejärvi et al., 2013); and (3) features of the physical environment (Elsen & Tingley, 2015; Randin et al., 2009; Scherrer & Körner, 2011). Recent theoretical (Urban et al., 2012), empirical (reviewed by Wisz et al., 2013) and experimental (e.g. Alexander et al., 2015) work has emphasised the potentially crucial role for biotic interactions to influence range dynamics. This is especially likely in mountains, where steep environmental gradients give rise to abrupt transitions between bioclimatic zones, most apparent at the subalpine-alpine ecotone between forest and alpine vegetation (Descombes et al., 2017; Körner, 2003; Mayor et al., 2017). Species’ range expansions across this ecotone will therefore precipitate interactions between alpine taxa and novel competitors (Alexander et al., 2015; Le Roux & McGeoch, 2008) and natural enemies (Rasmann et al., 2014), which might strongly influence alpine species’ persistence and ecosystem properties. Nonetheless, the possible consequences of such altered interactions remain poorly studied, and difficult to integrate into predictive biodiversity models.
Our aim here is to outline how different demographic, community and physical processes might affect the dynamics of plant community change in mountain – and especially alpine – ecosystems following climate change. We focus on mountains because of their inherent societal and conservation value (Körner, 2004), but also because the compressed climatic gradients in mountains make them ideal model systems to study impacts of climate change, and its interaction with other global change drivers such as land-use changes and non-native species (Pauchard et al., 2016; Sundqvist et al., 2013). In particular, we expect community changes to occur more rapidly across compressed elevational climatic gradients, providing opportunities to develop and test predictions about key processes that might be applied to more extensive lowland areas. First, we outline how different processes contribute to disequilibrium dynamics in mountain plant communities. We show that while the importance of dispersal lags is generally well appreciated, comparatively much less is known about how biotic interactions could influence range dynamics. Therefore, we next develop a process-based and dynamic community model to illustrate how dispersal lags and competitive interactions could influence expectations for range shifts and rates of community turnover along an elevational gradient under climate change. Finally, we discuss the possible implications of disequilibrium dynamics for the future of mountain ecosystems, and highlight areas for further research.
Lags in range shifts of plant species with climate change
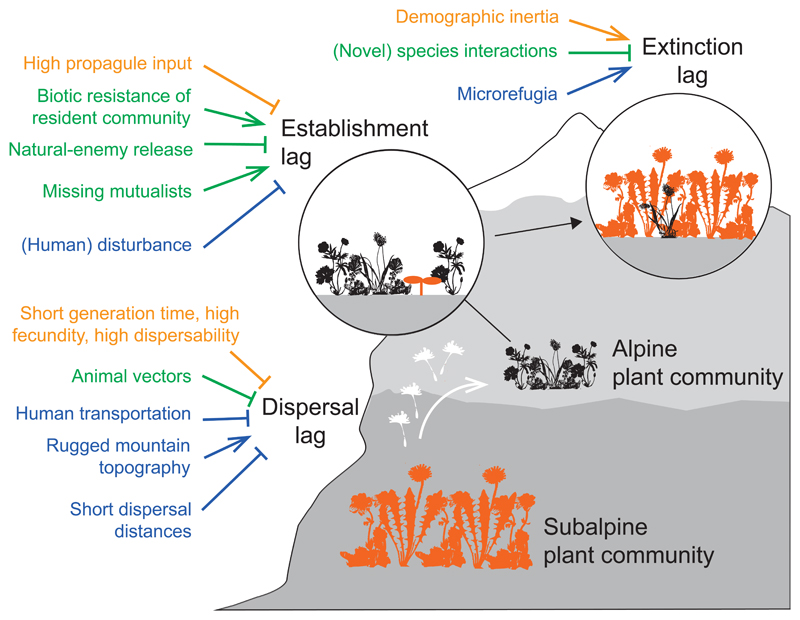
Species’ range shifts occur as the result of population expansion at the leading range edge and/or contraction at the trailing range edge (Lenoir & Svenning, 2013). The pace at which range shifts occur following climate change will depend on factors influencing: (1) dispersal at the leading edge; (2) the likelihood of a species establishing populations and increasing in abundance (hereafter “establishment”) beyond their current range edge once propagules arrive; and (3) local extinction at the trailing edge (Svenning & Sandel, 2013; Urban et al., 2012) (Fig. 1). The extent to which species show lags in these three processes – dispersal, establishment and extinction – determines the rate and synchronicity of species’ range shifts, and ultimately community turnover. Three broad classes of factors increase and/or decrease the magnitude of these lags and how they vary across species: (a) intrinsic physiological and demographic responses of the range-shifting species themselves; (b) interactions with other species; (c) and characteristics of the physical environment (Fig. 1). In the following sections we consider how these factors (a, b and c) could influence each type of lag (1, 2 and 3), and review examples from the recent literature demonstrating these processes operating in mountains.
Figure 1.
The magnitude and pace of alpine plant community turnover with climate change will be influenced by rates of species dispersal from lower elevations (“dispersal lags”), their rates of establishment and population growth in alpine communities (“establishment lags”) and extinction rates of resident alpine species (“extinction lags”). We include factors highlighted by our review that will increase (pointed arrows) or decrease (flat arrows) the magnitude of lags (factors related to focal species physiology and demography, biotic interactions, and the physical environment coloured orange, green and blue, respectively, in the online version).
1. Dispersal lags
(a). Physiology and demography
As research into biological invasions has shown (e.g. Pyšek et al., 2009), variation in dispersal lags across plant species will depend to a large extent on functional traits influencing dispersal and population spread (Matteodo et al., 2013), particularly the length of the juvenile period, fecundity and dispersal ability. On average, an extended time to maturity will increase the likelihood of a dispersal lag (Kroiss & HilleRisLambers, 2014; Lenoir & Svenning, 2013), because spread rates will be less likely to keep pace with the rate of climate change. Indeed, herbaceous plants with shorter life cycles have shifted further towards higher elevation than have woody plants with longer life cycles under contemporary climate change (Lenoir et al., 2008). Spread rates also depend on propagule production by reproductive adults (Molau & Larsson, 2000) and the dispersal ability of those propagules (i.e. the shape of their dispersal kernel) (Muller-Landau et al., 2002). In general, plants producing many small seeds will spread faster than those producing few heavy seeds, which could explain the greater elevation range shifts of small-seeded plants in the Italian Alps (Parolo & Rossi, 2008). Species more associated with human-induced dispersal, and particularly lowland non-native species, might also have an advantage as mountains become increasingly connected by roads and other human corridors (e.g. power lines; Pauchard et al., 2016). Lower elevation native species are also transported to alpine areas by agricultural or recreational activities (Pickering et al., 2011). Finally, evolution of traits conferring greater dispersal ability will also tend to reduce dispersal lags. While there is some evidence of this occurring in plants (e.g. Williams et al., 2016), we are not aware of any empirical examples from mountain environments.
(b). Biotic interactions
Biotic interactions could influence dispersal lags for plants relying on animal vectors for dispersal (Svenning et al., 2014). The high mobility of most animals compared to plants (Lenoir & Svenning, 2013) means that interactions with animal vectors are unlikely to limit rates of plant dispersal, assuming that the vector species can migrate independently of the plant species it transports (i.e. it is not a specialist on that plant species) (but see Neuschulz et al., in press). Indeed, animal-dispersed plants are perhaps better able to track climate change than those relying on wind or passive dispersal. For example, bears disperse seeds of cherry trees upwards several hundred meters in elevation as they follow plant phenology during the summer season (Naoe et al., 2016), and birds regularly disperse seeds across large distances (Viana et al., 2016). Interactions with animal vectors could therefore accentuate variation among plants in spread rates.
(c). Physical environment
The magnitude of dispersal lags will further depend on barriers to dispersal such as landscape configuration and heterogeneity. When suitable habitat patches are isolated, even species with high dispersal potential could have a low probability of reaching them. The rugged topography of mountains, with suitable habitat patches separated by valleys or regions of different bedrock, can present significant dispersal barriers (but see Elsen & Tingley, 2015; Thiel-Egenter et al., 2011). In the European Alps, nearly half of 183 alpine plants have failed to fill their potential climatic niche, suggesting substantial post-glacial recolonization lags, and this is more pronounced for species for which suitable habitat patches (calcareous substrate) are more isolated (Dullinger et al., 2012b).
Other evidence suggests that dispersal lags along elevational gradients could be small due to the compressed climatic gradients in mountains. Engler et al. (2009) found that dispersal did not strongly limit the speed of mountain species’ responses to climate change; simulations of unlimited dispersal provided a similar outcome to projections constrained by dispersal kernels. Empirical studies have found smaller lags in range expansion along elevational than latitudinal gradients (Bertrand et al., 2011; Jump et al., 2009, but see Chen et al., 2011), or even little lag at all (Beckage et al., 2008). Furthermore, short dispersal distances mean that many propagules might already be present outside of the current range of climatically suitable areas of adult plants. This is the case, for example, for alpine areas in Sweden, where 11 species were found in the seed bank up to 500 m higher in elevation than the upper limit of established populations (Molau & Larsson, 2000). These species might experience no dispersal lag once climate becomes permissive for population establishment, depending on how long dormant propagules remain viable in the seed bank. Finally, high rates of dispersal to higher elevations might be expected due to greater land area at, and so relatively higher propagule pressure from, lower elevations, although over two-thirds of mountain ranges do not show monotonic declines in area with elevation (Elsen & Tingley, 2015). Altogether, these lines of evidence suggest that dispersal lags might only moderately constrain the rate of range expansion, especially for species with broad habitat requirements.
2. Establishment lags
(a). Physiology and demography
Having arrived at a new site, the probability of a plant species establishing will depend on traits such as seed size and germination requirements, mode of reproduction (selfing vs. outcrossing) and propagule number. Germination and seedling establishment are major bottlenecks in arctic and alpine plant life history (Graae et al., 2011; Shevtsova et al., 2009) and show very low rates under field conditions, even when propagule inputs are high. For instance, along an elevational gradient in northern Sweden, average seedling emergence of 17 species was 7.5 %, and their subsequent mortality rate 80 %, when growing without competition (Milbau et al., 2013); under high-arctic conditions on Svalbard, germination was generally below 5 %, compared to c. 80 % under optimal lab conditions for the same seed source (Müller et al., 2011). Therefore, propagules must arrive in sufficient numbers to increase the probability of establishment, and overcome other limitations of small population size such as Allee effects or a limited capacity to adapt genetically to the new environmental conditions.
(b). Biotic interactions
The probability of establishment in alpine communities will depend on interactions with the resident plant community, natural enemies and mutualists (HilleRisLambers et al., 2013). Vegetation cover facilitates alpine plants by creating soil and microclimatic conditions favourable for plant growth (Körner, 2003; Michalet et al., 2014). In particular, strong facilitative effects have been demonstrated after experimental neighbour removal (Callaway et al., 2002; Choler et al., 2001; Olsen et al., 2016), or observed for plants growing in the presence versus absence of nurse plants (Cavieres et al., 2014). Facilitation can also be density dependent, as when higher canopy cover of adult trees promotes tree seedling recruitment near treeline (Maher & Germino, 2006). Altogether, facilitation in alpine vegetation could promote the establishment of populations towards species’ high elevation range limits (Choler et al., 2001; le Roux et al., 2012), something that has also been observed for some non-native plants (Cavieres et al., 2008).
In the context of these facilitative effects of vegetation cover, competition within closed alpine communities can continue to play an important role even under relatively harsh environmental conditions (Chesson & Huntly, 1997). This appears to be especially the case at the seedling recruitment stage, which can be greatly reduced in closed alpine vegetation, presumably because of competition (Gough, 2006; Graae et al., 2011; Lembrechts et al., 2016; Milbau et al., 2013; Moen, 1993). For example, Graae et al. (2011) observed early establishment rates of 0.9 % in undisturbed and 11 % in disturbed tundra vegetation. The extent to which vegetation cover facilitates or inhibits recruitment will therefore depend on the balance between competition and exposure to severe environmental conditions. As an example of this, a theoretical study suggests that seedling recruitment could be greater in small rather than large gaps, because small gaps balance relaxed competition with a favourable microclimate (Lembrechts et al., 2015). Nonetheless, recruitment from seed occurs frequently in some alpine areas, such as in the Australian Alps (Venn & Morgan, 2009), where negative effects of vegetation on recruitment are also weaker (Venn et al., 2009). Interaction strengths are also likely to change with ontogeny, for example with competitive effects at the seedling stage weakening (Gough, 2006) or becoming increasingly facilitative (le Roux et al., 2013) as the plant grows.
Given the many different ways in which species can interact with each other and their environment, generalization is difficult. Nonetheless, reconciling these empirical and theoretical observations, we suggest that facilitation will most strongly influence climate change range dynamics in sparsely vegetated habitats, such as high alpine screes or in drought-prone mountain regions (Michalet et al., 2014). Here, establishment lags might be protracted by the colonization of pioneer species that can facilitate later-arriving species. By contrast, in already densely vegetated alpine meadows we expect a stronger role for competition, with colonization of new species contingent on disturbance creating “safe-sites” for establishment (Vittoz et al., 2009). The strength of competition from alpine communities could increase with climate warming (e.g. Alexander et al., 2015; Olsen et al., 2016), potentially reducing establishment success further (Hampe, 2011). In addition, it will take time for founding individuals to expand population size towards carrying capacity (cf. “abundance lag” in Essl et al., 2015). This process could be accelerated by traits conferring high competitive ability and/or high relative growth rate, which might trade-off with traits conferring successful dispersal or initial establishment (Turnbull et al., 1999). Furthermore, colonizing species from warmer areas are likely to possess faster growth rates than alpine species under climate change conditions (Körner, 2003), potentially conferring a competitive advantage (Alexander et al., 2015) and promoting population expansion.
Natural enemies accompanying plants as they expand their ranges from lower elevation might also influence establishment lags. Establishment lags should be reduced for species that experience “enemy release” (Engelkes et al., 2008) by dispersing faster than the natural enemies that regulate their population abundance at lower elevation. For example, release from seed predation might promote the spread of tropical trees to higher elevations in the Andes (Hillyer & Silman, 2010). However, natural enemies already present in alpine ecosystems are likely to increase establishment lags of plants arriving from lower elevation. Mammalian herbivores can prevent establishment at the upper range margin of some forbs (Kaarlejärvi et al., 2013) and trees (Brown & Vellend, 2014), contributing to the formation of treelines (Speed et al., 2010). Range expansion might also be constrained by soil pathogens (Brown & Vellend, 2014). For example, the broad host range dieback pathogen Phytophthora cinnamomi, already present in sub-alpine regions of Australia, may negatively impact not only existing flora, but also any range expanding species from lower elevations, including non-native plants (Burgess et al., 2017).
Finally, lagged range shifts of mutualists such as pollinators or soil microbiota might increase plant establishment lags, especially when mutualists have poor dispersal ability and engage in specialized interactions (Corlett & Westcott, 2013). For example, the availability of pollinator services might already limit range expansion of some species at their high elevation range edge (HilleRisLambers et al., 2013). Similarly, the spread of non-native trees into new environments has been slowed or halted by a lack of appropriate mycorrhizal fungi (Nuñez et al., 2009).
(c). Physical environment
As previously noted, low establishment rates in alpine ecosystems are attributed to limiting abiotic conditions like high irradiance, drought and extremes of temperature, and the extent to which these are ameliorated by vegetation cover (Eckstein et al., 2011; Graae et al., 2011; Milbau et al., 2013). In areas where vegetation cover reduces establishment success, successful recruitment might only occur when disturbances create ephemeral safe sites for establishment (Milbau et al., 2013). Although alpine areas are naturally disturbed (e.g. by freeze-thaw dynamics, landslides and animals), certain attributes of human disturbances (e.g. frequency, intensity, nutrient release) cause them to more strongly promote the establishment of colonizing (non-native) plant species from lower elevation (Lembrechts et al., 2016). Therefore, establishment lags might be strongly influenced by the extent of human disturbances in alpine areas, easing the barriers for some species.
Soil development itself will limit the establishment of propagules across the alpine elevational gradient, especially for species that require deep organic soils. In the Swiss Alps, alpine species that are able to colonize scree habitats have increased in frequency markedly over the last century, in contrast to alpine species with a preference for organic soil (Kulonen, 2017). Poorly-developed alpine soils with low water-retaining capacity can limit seedling growth and survival, which is expected to constrain the range expansion of tree and grassland species beyond the current limits of alpine meadows (Ford, 2014; HilleRisLambers et al., 2013). But soil development could also constrain establishment at lower elevations as well. For example, low water-holding capacity in thin alpine soils could explain colonization lags by spruce (Picea abies) in the Swiss Alps during the Holocene, and with future climate change (Henne et al., 2011). Alpine soil development can take hundreds to thousands of years, and be further slowed on steep slopes prone to soil erosion (Theurillat et al., 1998). Therefore in general, soil development is likely to contribute substantially to establishment lags, even given increased rates of soil formation due to warmer climate (Svenning & Sandel, 2013).
3. Extinction lags
(a). Physiology and demography
Populations of alpine species that are physiologically unable to tolerate altered climatic conditions due to climate change, especially those at the low-elevation distribution limit, will rapidly become locally extinct. For example, Ranunculus glacialis does not tolerate warmer conditions when transplanted to lower elevations (Prock & Körner, 1996). Population dieback is occurring in some areas, caused primarily by interactions between extreme climatic conditions (e.g. summer drought or severe frost) and plant pathogens. Examples include the shrub Nematolepis ovatifolia in alpine areas of Australia (Green, 2016), and the cushion plant Azorella macquariensis on the sub-Antarctic Macquarie Island (Bergstrom et al. 2015).
Extinction lags could, however, be substantial for many other alpine species, even if climate change has a negative effect on population dynamics (Dullinger et al., 2012a; Essl et al., 2015; Svenning & Sandel, 2013). Some herbaceous alpine plant species, including those dominating alpine grasslands such as Carex curvula in the European Alps, have persisted many centuries through extensive fluctuations in climate (De Witte et al., 2012). Long-lived species with extensive resources stored in roots, rhizomes and stems can persist for decades or even centuries after cessation of reproduction, as is the case for some trees (Hampe & Jump, 2011). In addition, recruitment might continue from long-lived soil seed banks, or after living individuals have disappeared altogether from the community (Ouburg & Eriksson, 2004). Finally, many alpine species are widely distributed and possess an intrinsic ability to tolerate temperature and water stresses (Körner, 2003), equipping them to tolerate climate changes. Widely distributed species tend to have broad ecological tolerances, making them less vulnerable to extinction following climate change (Slatyer et al., 2013). Such species can also host higher levels of genetic variability in ecologically relevant traits (Sheth & Angert, 2014), potentially fostering adaptation to environmental change and so delaying extinction lags. Together, therefore, there could be considerable inertia in alpine plant populations, delaying local extinction due to unfavourable climate.
(b). Biotic interactions
Changing species interactions can play a more decisive role in population declines and local extinction following climate change than direct climatic effects (Cahill et al., 2013). Altered biotic interactions could therefore accelerate local extinction, firstly, due to changes in interactions among species already co-occurring in alpine communities today. For instance, some alpine plants might be competitively suppressed under climatic warming by neighbours that respond more favourably to altered climate (Niu & Wan, 2008), such as those already found at lower elevation. Biotic interactions among alpine species can shift from facilitative to competitive as climatic conditions change (Olsen et al., 2016). Climate change could also alter the trophic interactions regulating population size, such as with pollinators, herbivores and pathogens. For example, some species of Phytophthora occur with susceptible hosts in alpine areas of the Australian Alps, but disease outbreak is only expected to occur once climate warms sufficiently to become conducive to disease expression (Burgess et al., 2017), with potentially large impacts on host plant populations (Cahill et al., 2008). Pathogens have also been implicated in population collapses in an alpine ecosystem on the sub-Antarctic Macquarie Island (Bergstrom et al., 2015). In contrast, mutualistic interactions can enhance plant species’ climatic tolerance (Afkhami et al., 2014), potentially delaying or avoiding local extinction, as suggested for effects of ectomycorrhizal fungi on range contraction rates of North American trees (Lankau et al., 2015).
Competitors and natural enemies can restrict a species’ distribution to a subset of the environmental conditions it would physiologically be able to tolerate (i.e. set the limits to its realized climate niche; Fig. 2). Therefore, the local persistence of some alpine plants near their lower range margin might hinge on the outcome of their interactions with new competitors or natural enemies that migrate-in from lower elevations. For example, a plant community from low elevation suppressed the survival and growth of three focal alpine plants much more strongly than an alpine plant community when growing under a warmer low elevation climate (Alexander et al., 2015). Partly this might be driven by functional trait differences between sub-alpine and alpine species, with traits such as taller stature and faster growth conferring a competitive advantage to lower elevation species once climate at high elevations becomes permissive. Although upwards range shifts of lower elevation taxa have been well documented, direct evidence that competitive replacement is already occurring is scant. However, in mountains of China (Zong et al., 2016) and Japan (Kudo et al., 2011), the climate-related range expansion of two grass species into alpine areas has led to local reductions in species richness.
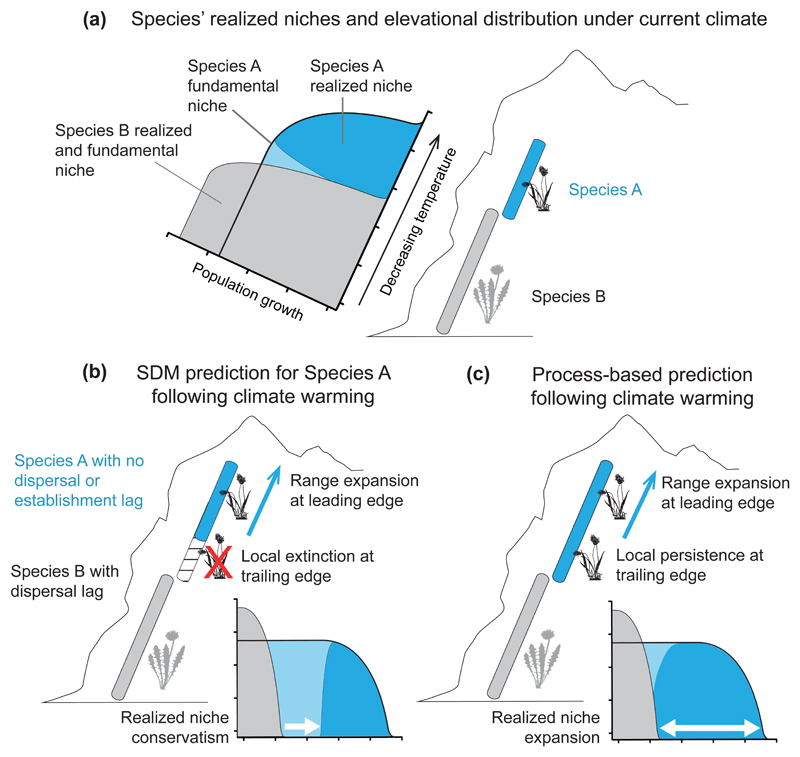
Figure 2.
Range predictions for a focal alpine species (species A) following climate change. Species A shows a trade-off between population growth rate and tolerance of low temperature, such that it can tolerate a greater range of temperature than species B, but is outcompeted by species B under warmer climatic conditions (i.e. it has a broader fundamental niche than species B, but a realized niche restricted to cooler temperatures; panel a). Following climate warming, a simple species distribution modelling (SDM) approach for species A, that assumes realized niche conservatism, would predict high elevation range expansion and low elevation range contraction (panel b). However, if species B is constrained by a dispersal lag, as shown, then the prediction of local extinction at the lower elevation range margin for species A would be incorrect. Instead species A would persist at its current lower elevation range margin, expanding its realized niche, due to the absence of competition from species B (panel c). This outcome would be predicted by process-based models that account independently for effects of climate and competition on population growth rates.
Interactions with new natural enemies could also accelerate the local extinction of alpine plants. Alpine communities can contain a low abundance of certain taxa found more commonly at lower elevations, such as herbivorous insects (Pellissier et al., 2012). As a result, alpine plants tend to be less defended against herbivores than lowland species (Bruelheide & Scheidel, 1999; Pellissier et al., 2012). More generally, the arrival of functionally new herbivores from lower elevations with climate warming might increase the competitive advantage of better-defended, co-evolved lowland plants (Rasmann et al., 2014). This arrival might accelerate the extinction of local alpine plant species, thus favouring the establishment of other species from lower elevation and in the end precipitate rapid community change. Overall, the effect of low elevation species on extinction lags of alpine plants will depend on their rates of dispersal and establishment, as well as their direct (e.g. through competition, herbivory) and indirect (e.g. by facilitating the arrival of other lowland species) effects on alpine plant population dynamics.
(c). Physical environment
The complex topography and spatial heterogeneity of mountain environments contribute to the occurrence of microsites within close proximity that differ strongly in climatic characteristics (Randin et al., 2009; Scherrer & Körner, 2011). This microsite variation might also help increase extinction lags at the landscape scale by providing microrefugia for populations to persist locally, as climatic relics, under deteriorating regional climate (Hampe & Jump, 2011). However, the effectiveness of such microhabitats for long-term species survival relies on long-term microclimate stability (Lenoir et al., 2017). Other features of the physical environment that increase dispersal and establishment lags, such as topographic isolation, will also tend to increase extinction lags by sheltering alpine plants from interactions with lowland taxa. For example, rocky habitats like scree that are difficult for lowland competitors to colonize, and slow soil development more generally, could provide microrefugia for some alpine plants under a warmer climate (Kulonen, 2017). Indeed, populations of some alpine plants can persist at low elevations in habitats, such as rocky slopes, where they are sheltered from competition with lowland species (Spillmann & Holderegger, 2008).
Prevalence and prediction of lagged range dynamics in alpine communities
Based on our review, dispersal lags could be substantial for some species spreading to higher elevations, but on the whole the available evidence suggests that dispersal lags will contribute less to disequilibrium range dynamics than establishment and extinction lags. Existing vegetation could facilitate range expansion into sparsely vegetated alpine habitats (Cavieres et al., 2008), but biotic pressures (e.g. herbivory, competition) in temperate/arctic alpine ecosystems with closed vegetation can impose strong barriers to establishment (e.g. Kaarlejärvi et al., 2013; Milbau et al., 2013). Therefore, even when low elevation species arrive in a climatically suitable area, we could expect a considerable lag before they are able to establish and increase population size. Coupled with this, several lines of evidence suggest that extinction lags in alpine species could also be substantial; some species have persisted in situ through large climatic fluctuations (De Witte et al., 2012), and there appears to have been little local extinction at trailing edges so far (Pauli et al., 2012), in contrast to the many examples of range expansions (but see Cannone et al., 2007). These observations are consistent with the view that species’ low elevation range edges tend not to be set by direct climatic effects, which might be favourable for plant growth following climate change, but rather by negative biotic interactions (Gaston, 2003; Hargreaves et al., 2014; MacArthur, 1972).
The accumulation of species’ dispersal, establishment and extinction lags will affect the magnitude of community turnover (i.e. changes in species composition and abundance) with climate change (Bertrand et al., 2011). Evidence of alpine community turnover during the last decades of climate change exist (Gottfried et al., 2012; Wipf et al., 2013), but so far the documented change in alpine communities are the result of colonisation of species from lower elevation (i.e. a thermophilisation of the flora) without clear negative effects on resident species. Regions such as the Swiss Alps have already experienced more than 1 °C of warming in the 20th century, yet its effect on the vegetation is sometimes barely perceptible (Vittoz et al., 2009). Taken together then, lags in the dispersal, establishment and local extinction of individual species will collectively determine the rate of community turnover.
The need to forecast the impact of global changes on species assemblages has fuelled the development of a variety of predictive models (e.g. Thuiller et al., 2005). Due to their simplicity of application, statistical models such as species distribution models (SDMs) have been a particularly popular approach to forecast the consequence of global changes on species assemblages (e.g. Descombes et al., 2016; Engler et al., 2011; Thuiller et al., 2005). SDMs fitting relationship between species’ occurrence (or abundance) and climate allow species assemblages to be predicted in space and time (e.g. Guisan & Rahbek, 2011). Though they can integrate dispersal (Engler et al., 2009), SDMs do not usually account explicitly for biotic interactions (Wisz et al., 2013), so they are unable to consider processes such as establishment or extinction lags. Rather, mechanistic approaches that account independently for effects of climate and biotic interactions on population dynamics would be needed to model such disequilibrium situations (Schurr et al., 2012; Zurell et al., 2016). Meta-community models could prove particularly useful in identifying the range of circumstances under which strong lags should arise (Jackson & Sax, 2010). In the next section, we develop a process-based meta-community model that directly manipulates dispersal processes while indirectly manipulating establishment and extinction processes through competition. The model illustrates the effects of lags in dispersal, establishment and extinction on community turnover compared to expectations of species-specific range shifts with unlimited dispersal (e.g. SDMs). Our objective is to illustrate the sort of modelling approach that might be used to predict disequilibrium range dynamics under climate change.
Lagged range dynamics and community turnover along an elevational gradient: a meta-community model
Meta-community models could help provide forecasts of disequilibrium range dynamics (Jackson & Sax, 2010), including dispersal, establishment and extinction lags. Some previous attempts have been made to model mechanisms of plant community responses to climate changes, for example including dispersal kernels (Engler et al., 2009) or demographic effects (Cotto et al., 2017; Dullinger et al., 2012a) within SDMs. Combining SDMs with demographic parameters, Cotto et al. (2017) showed that perennial species can persist in unsuitable habitats longer than predicted by their climatic tolerance, causing delayed range losses. Nevertheless, to integrate the effect of biotic interactions on the turnover of entire communities, a mechanistic model is needed that not only allows dispersal limitation and demographic inertia, but also periods of transient co-occurrence in communities caused by lags in the establishment of new species and their replacement of weaker competitors.
We formulate a model to describe the assembly of a meta-community along a temperature gradient, and the response of the system to climate warming, inspired by a Lotka-Volterra model of interspecific competition. The model does not attempt to consider all processes highlighted by our review; it includes variation among species in demographic rates (dispersal, growth) and impacts of one type of biotic interaction (competition), but for simplicity omits non-climatic impacts of the physical environment on lags.
We developed a 1-dimensional stepping stone model representing a meta-community of 500 communities along a linear temperature (elevation) gradient varying between 0 and 20 °C. A plant species’ distribution along the gradient depends on dispersal, its growth response to temperature, and effects of competitors (see Appendix S1). The model simulates the dispersal and growth dynamics of a set of plant species within a meta-community. The population size of species i in cell j at time t+1, Pi,j,t+1, can be calculated from the population of species i in cell j at time t, Pi,j,t, in two successive operations. In each community, all species export a fraction (d) of their local population to the two adjacent communities in the 1-dimensional landscape:
| (Equation 1) |
Weak dispersal to neighbouring cells will cause dispersal lags under scenarios of warming. Next, we derive the species’ population sizes after taking into account population growth and competitive interactions:
| (Equation 2) |
Competition enters the model in three ways. First, fast-growing species have a competitive advantage over slow-growing species because they more rapidly attain higher abundance within a community. Therefore, growth rate (gi) captures variation among species in their innate competitive ability. A large difference in gi between an invader species and those in the established communities will allow the invader to quickly expand its population with a small establishment lag. Second, species vary in their sensitivity to competition (li), i.e. the extent to which their population size is directly reduced by the abundance of both hetero- and conspecific neighbours. When the invader is a greatly superior competitor (i.e. li invader << li established species), the population size of the established community will offer weak resistance to the invader’s growth, resulting in a small establishment lag for the invader. Combined with a higher growth of the invader, this implies short extinction lags for the established species. Third, population size shows additional sensitivity to the abundance of conspecific neighbours, given by a coefficient ci that is set to be constant across all species and is needed to stabilize the coexistence of multiple species (Chesson, 2000). We assume a fundamental trade-off between competitive ability and tolerance of low temperatures (Jones & Gilbert, 2016; le Roux et al., 2013; Loehle, 1998), so that fast-growing species (high gi) are less sensitive to competition (low li) but restricted to growing under warmer temperatures (high Tmini), while slow-growing species (low gi) suffer greater competitive suppression (high li) but can tolerate lower temperatures for growth (low Tmini) (Grime, 1977). This widely-held assumption in fact remains largely untested (Jones & Gilbert, 2016), but is consistent with observations that species are principally limited by abiotic factors at their colder range edge (Pellissier et al., 2013) but biotic interactions at the warmer edge (Alexander et al., 2015), and is further supported by the successful growth of many alpine plants in lowland botanical gardens (Vetaas, 2002).
We investigate the rate of temporal community turnover, quantified as β-diversity between the communities present at a given point along the gradient before and after a 3 °C increase in temperature, using the β-diversity of Jost (2007) and Tuomisto (2010) (see Appendix S1). We compared the meta-community model with the expectation of community turnover when all species shift their range independently and without dispersal limitation (i.e. by simply stacking SDM projections). In this scenario, communities are simply transposed to higher elevations under the +3 °C climate warming scenario without any dispersal lag or altered competitive interactions. Smaller values for temporal β-diversity in the process-based simulation relative to the SDM-stacking indicate greater time lags in community turnover.
To test the influence of competition and dispersal on the response of the meta-community to climate warming, we simulated a set of 500 scenarios in which we randomly sampled the values of the following three parameters (Appendix S1): dispersal rate d; mean and coefficient of variation in gi in the species pool; and mean and coefficient of variation in li in the species pool (see Table S1 for parameter distributions). We first explored scenarios varying the average values of these parameters across the entire gradient, to quantify how the strength of those processes influence community turnover. Second, we explored scenarios differing in the coefficient of variation (CV) of gi or li along the gradient, hypothesizing that a greater CV (i.e. greater asymmetry in growth or sensitivity to competition between low- and high-elevation species) should promote faster turnover under temperature change. Each meta-community was subjected to an initial burn-in period to reach equilibrium (see Appendix S1), and was then exposed to a gradual climate warming of 3 °C and allowed to reassemble along the elevational gradient. We computed temporal community turnover and fitted a linear mixed effects model to quantify the effects on community turnover of dispersal rate, mean and CV of growth rate and sensitivity to competition, with species pool as a random effect. We also controlled for initial meta-community structure (after the initial burn-in period) by including mean α- and spatial β-diversity across the gradient as fixed effects. To exclude edge effects on our estimates of temporal turnover, we removed low and high elevation communities from the linear model estimation (see Appendix S1). Our baseline for comparing the results of the meta-community model and the SDM-stacking approach were the meta-communities after the initial burn-in period, which differed between each set of parameters (Figs. 3 & 4).
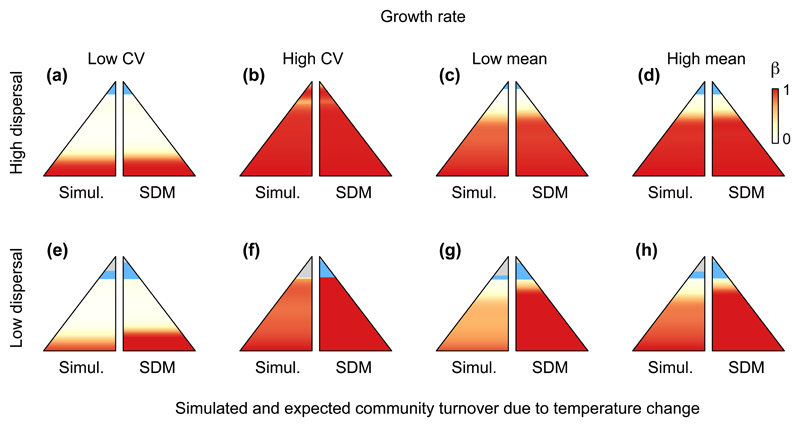
Figure 3.
Temporal community turnover along an elevational temperature gradient (β-diversity, represented by the gradient from white to red, see legend in panel d) following climate warming. The left section of the mountains represents the results of simulations (“Simul.”), while the right section represents the expected turnover obtained by simply stacking species distribution model projections (“SDM”). Shown are eight scenarios that differ depending on dispersal ability (rows) and growth rate within the species pool (a, e: coefficient of variation [CV] = 0.1; b, f: CV = 1/√3; c, g: mean = 0.2; d, h: mean = 0.5; a-d: d = 10-1; e-h: d = 10-4). In each panel, all other parameters except the ones specified in the header of their line and column were set to the average value of their respective distribution (see Table S1). The lowest elevation communities are not displayed (see Appendix S1). Communities at the highest elevations resulting from colonization of previously unoccupied habitat are coloured in blue, while communities that remain empty despite warming are in light grey. Note that outcomes of temporal community turnover obtained from stacking SDM projections can also differ slightly among panels due to different initial conditions (i.e. initial burn-in period that allowed the meta-communities to equilibrate).
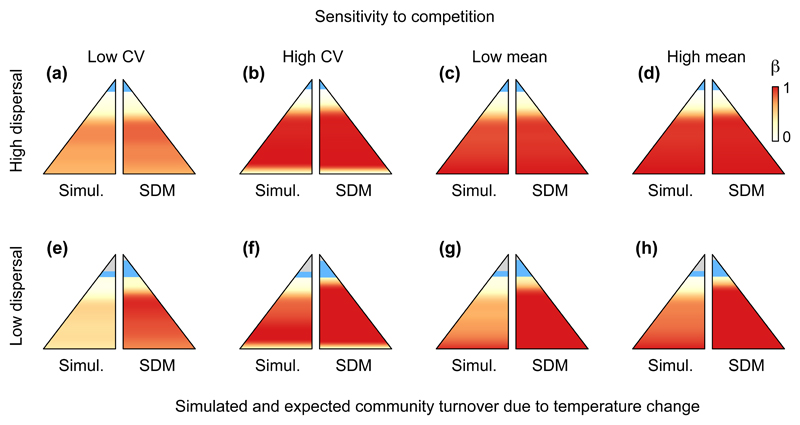
Figure 4.
Temporal community turnover along an elevational temperature gradient (β-diversity, represented by the gradient from white to red, see legend in panel d) following climate warming. The left section of the mountains represents the results of simulations (“Simul.”), while the right section represents the expected turnover obtained by stacking species distribution models projection (“SDM”). Shown are eight scenarios that differ depending on dispersal ability (rows) and sensitivity to competition within the species pool (a, e: coefficient of variation [CV] = 0.1; b, f: CV = 1/√3; c, g: mean = 0.7; d, h: mean = 1.5; a-d: d = 10-1; e-h: d = 10-4). In each panel, all other parameters except the ones specified in the header of their line and column were set to the average value of their respective distribution (see Table S1). The lowest elevation communities are not displayed (see Appendix S1). Communities at the highest elevations resulting from colonization of previously unoccupied habitat are coloured in blue, while communities that remain empty despite warming are in light grey. Note that outcomes of temporal community turnover obtained from stacking SDM projections can also differ slightly among panels due to different initial conditions (i.e. initial burn-in period that allowed the meta-communities to equilibrate).
We documented lags in the dynamics of species dispersal and replacement across the gradient, with an average 10 % lower temporal community turnover (β-diversity) compared to the expectations obtained by simply stacking SDMs (Figs. 3-4, S1c). Both outcomes from our process-based model and expectations obtained by stacking SDM projections followed the same pattern along the temperature gradient: a low turnover at high elevation and a high turnover at low elevations. This reflects the structure of the meta-community in our model; the gradient in species diversity is not linear, since species coexistence results from an interactive effect of temperature-related growth on population sizes acting together with the species’ intrinsic abilities to tolerate competition (Fig. S1d, Fig. S2, Fig. S3).
Both dispersal rate and competition significantly affected the extent of community turnover following climate warming. Temporal turnover increased with higher dispersal rates (Table 1), bringing it closer to the expectations obtained using SDM-stacking (Figs. 3, 4), as expected from previous work (Engler et al., 2009). Under high dispersal meta-communities had a temporal turnover close to the one predicted by SDM stacking regardless of other model parameters (Fig. 3a-d; Fig. 4a-d), and also an accelerated colonisation of previously empty communities at the mountain summits (Figs. 3, 4). However, the effects of competitive ability captured by the mean and coefficient of variation among species in growth rate (gi) were nearly three to five times greater than the effect size of the log-transformed dispersal rate on community turnover over time (Table 1). When growth rates were high across the whole gradient, competitive replacement and hence community turnover occurred more rapidly (Fig. 3c,d,g,h). A greater variation in growth rate between warm- and cool-adapted species also accelerated the rate of temporal turnover in community composition (Fig. 3a,b,e,f). The effects on community turnover over time of species’ sensitivity to competition were qualitatively similar to growth rate but smaller in magnitude (Table 1, Fig. 4). Because in our simulations climate change occurred gradually over time, these results are likely to be conservative with respect to the relative importance of competition and dispersal on temporal community turnover. Nevertheless, the model outputs are constrained by the architecture of the models and the number of time steps considered within the 3°C change and confrontations with empirical data are needed to provide realistic expectations.
Table 1.
Parameter estimates from the linear mixed effects model linking temporal community turnover (β-diversity) to dispersal rate, mean growth rate, the coefficient of variation in growth rate, mean sensitivity to competition, the coefficient of variation in sensitivity to competition, mean α-diversity across the gradient and initial spatial β-diversity across the gradient. Because the covariates and response variable were standardized, the estimates are also effect sizes (*P < 0.05; *** P < 0.001). The marginal R2 = 0.360 and conditional R2 = 0.411 indicate the amount of variance explained by the fixed effects, and by the combination of fixed and random effects, respectively. For all fixed effects, d.f. = 492.
| Parameter | Estimate (± SD) |
|---|---|
| Intercept | 0.000 (0.013)ns |
| Mean of gi | 0.356 (0.018)*** |
| Coefficient of variation of gi | 0.591 (0.031)*** |
| Mean of li | 0.117 (0.013)*** |
| Coefficient of variation of li | 0.216 (0.013)*** |
| Dispersal (log transformed) | 0.124 (0.012)*** |
| Mean α-diversity across the gradient | 0.006 (0.012)ns |
| Initial β-diversity across the gradient | 0.059 (0.027)* |
Our simulation exercise with a meta-community model indicates how the demographic parameters gi, li and d influence lags in community turnover under climate change and how forecasts might differ from single species models based on their realized niche and with unlimited dispersal. Beyond the use of SDMs in community forecasts under climate change, our results advocate the development and calibrations of process-based models to reach more accurate temporal estimates of community changes. This agenda will require methodological developments (e.g. Harsch et al., 2017; Urban et al., 2016) and the collection of appropriate demographic data, e.g. using experiments (Alexander et al., 2016). Despite the complexity of mechanisms described in the review section, our model focuses on competition because of its central importance for regulating plant population dynamics, even under relatively harsh environmental conditions (Chesson & Huntly, 1997). Nonetheless, the model structure might be extended to accommodate a more complex suite of positive and negative interactions (Brooker et al., 2007; Svenning et al., 2014), including interactions with higher trophic levels. In addition, the model as written corresponds to environmental conditions typical for temperate mountains like the European Alps, where water is generally not limiting along the gradient (with the exception of some internal valleys). Further modifications would be required to accommodate other environmental contexts, such as mountains where vegetation is shaped by drought at lower elevations or where climate change increases, rather than decreases, climatic stress at high elevation. Finally, while we illustrate the potential of dispersal, establishment and extinction lags in shaping community turnover over time, we are still far from being able to calibrate such a model with empirical data.
Disequilibrium dynamics in alpine plant communities: implications and research needs
Two main findings emerge from our review and simulation of disequilibrium dynamics in alpine plant communities. Firstly, they confirm that lags in species’ range responses to recent climate change along elevation gradients occur, and that the conditions promoting lags are common and likely to influence rates of community turnover in the future. This has implications for the future structure and functioning of alpine communities, which are discussed below. Secondly, biotic interactions play a crucial role in mediating disequilibrium dynamics, and likely have a particularly strong influence on establishment and extinction lags. Together, these findings imply that modelling of disequilibrium dynamics in a way that accounts for changing biotic interactions will be necessary to forecast the response of alpine communities to climate change. Our simulation model offers an example of one way forward, and other tools are becoming available (Evans et al., 2016; Pagel & Schurr, 2012; Zurell et al., 2016), rooted in the common demographic basis of both range dynamics and impacts of biotic interactions on population dynamics (Alexander et al., 2016).
Whilst our simulation model is informative, we can as yet say little about the relative contribution of different lags to disequilibrium dynamics without data on demographic rates and biotic interactions for a large number of species. Parameterizing such demographic models will be a huge challenge, since potentially all species can respond differently to changing environmental conditions. One approach might be to measure demographic rates for a subset of species under a range of controlled environmental conditions, either in the field or phytotron, and correlate these with functional trait proxies that are easier to measure for a large number of species (Alexander et al., 2016). For example, such trait-based models have been developed to predict dispersal potential (Tackenberg, 2003), and therefore might also be applied to predict demographic responses to abiotic gradients (i.e. to estimate something approaching a species’ fundamental niche). Traits can also be used as proxies for the outcome of biotic interactions, especially for trophic interactions (Morales-Castilla et al., 2015) and to some extent for competition among plants (e.g. Kraft et al., 2015).
There are a number of further implications of disequilibrium community dynamics not addressed here, which constitute open questions for future research. Firstly, how long will novel communities persist following climate change? Novel communities arising from asynchronous migration might be transient, especially when community turnover is rapid, and converge towards the composition of communities typical of lower elevations today (Cannone et al., 2007). However, they might be more persistent when environmental conditions themselves are novel (Williams & Jackson, 2007), or if priority effects or rapid evolution stabilize a novel community structure. Secondly, what are the consequences of disequilibrium dynamics for alpine ecosystem structure and function? For instance, considerable time will pass before trees invading alpine habitats will deliver the full quantity and quality of forest ecosystem services (e.g. timber production, protection against avalanches) (Essl et al., 2015; Svenning & Sandel, 2013). Similarly, alpine meadows composed of fewer, functionally different species might not deliver ecosystem services in the same way as current alpine meadow communities (Körner, 2004). Thirdly, what are the implications of disequilibrium dynamics for the conservation and management of biodiversity in mountains? The prevalence of lags underscores the need for networks of protection promoting (or sometimes reducing) connectivity, that do more than just protect the highest elevation ecosystems (Plassmann et al., 2016). Understanding the nature and causes of lags at leading and trailing range edges might inform active management strategies for particular species or habitat types of conservation concern. Finally, the examples we have reviewed illustrate the usefulness of elevational gradients, and alpine ecosystems in particular, as model systems to observe disequilibrium dynamics and also to conduct experiments at the scale of species’ ranges. Disequilibrium dynamics are likely to be magnified in lowland areas by the greater spatial scales involved, so studies from mountains should provide lower limits for the magnitude of lags that we should expect following climate change. But one should extrapolate biological responses from mountainous to lowland regions with caution, since climatic changes with latitude covary with numerous factors, such as photoperiod, geological history and regional species pools, that tend to be relatively constant across elevational gradients. Nonetheless, we hope that further insights and methodological approaches developed in mountains will eventually be scaled-up to help predict disequilibrium dynamics across broader latitudinal and longitudinal gradients.
Supplementary Material
Additional Supporting Information may be found in the online version of this article
Acknowledgments
JMA, TIB, SH, JL, NJS and LP initiated discussions on the paper; LC and LP developed the simulation model; JMA led the writing; all authors contributed to discussions and revising drafts of the paper. This paper arose from a workshop “Biosecurity in Mountains and Northern Ecosystems: Current Status and Future Challenges” from 3-5 June 2015, organized by the Mountain Invasion Research Network (MIREN) and supported through funding by the Mountain Research Initiative (MRI) of the University of Bern (Switzerland), the Marcus Wallenberg Foundation for International Scientific Collaboration, the Oscar and Lili Lamms Remembrance Foundation, the Arctic Research Centre at Umeå University (ARCUM), and the Climate Impacts Research Centre (CIRC). JMA received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 678841. FE was supported by the Austrian Science Foundation (FWF, grant I2096-B16). LJR was supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture Hatch: MONB00363. NJS was supported by a National Science Foundation Dimensions of Biodiversity grant (NSF-1136703) and the Carlsberg Foundation through the Carlsberg Foundation's Semper Ardens program. AP was supported by ICM P05-002 and CONICYT PFB-23. LP was supported by the NSF grant Nr. 31003A 162604 (Life3web project).
References
- Afkhami ME, McIntyre PJ, Strauss SY. Mutualist-mediated effects on species' range limits across large geographic scales. Ecology Letters. 2014;17:1265–1273. doi: 10.1111/ele.12332. [DOI] [PubMed] [Google Scholar]
- Alexander JM, Diez JM, Hart SP, Levine JM. When climate reshuffles competitors: a call for experimental macroecology. Trends in Ecology & Evolution. 2016;31:831–841. doi: 10.1016/j.tree.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JM, Diez JM, Levine JM. Novel competitors shape species' responses to climate change. Nature. 2015;525:515–518. doi: 10.1038/nature14952. [DOI] [PubMed] [Google Scholar]
- Beckage B, Osborne B, Gavin DG, Pucko C, Siccama T, Perkins T. A rapid upward shift of a forest ecotone during 40 years of warming in the Green Mountains of Vermont. Proceedings of the National Academy of Sciences. 2008;105:4197–4202. doi: 10.1073/pnas.0708921105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom DM, Bricher PK, Raymond B, et al. Rapid collapse of a sub-Antarctic alpine ecosystem: the role of climate and pathogens. Journal of Applied Ecology. 2015;52:774–783. doi: 10.1111/1365-2664.12436. [DOI] [Google Scholar]
- Bertrand R, Lenoir J, Piedallu C, et al. Changes in plant community composition lag behind climate warming in lowland forests. Nature. 2011;479:517–520. doi: 10.1038/nature10548. [DOI] [PubMed] [Google Scholar]
- Bertrand R, Riofrío-Dillon G, Lenoir J, Drapier J, de Ruffray P, Gégout J-C, Loreau M. Ecological constraints increase the climatic debt in forests. Nature Communications. 2016;7:12643. doi: 10.1038/ncomms12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker RW, Travis JMJ, Clark EJ, Dytham C. Modelling species’ range shifts in a changing climate: The impacts of biotic interactions, dispersal distance and the rate of climate change. Journal of Theoretical Biology. 2007;245:59–65. doi: 10.1016/j.jtbi.2006.09.033. [DOI] [PubMed] [Google Scholar]
- Brown CD, Vellend M. Non-climatic constraints on upper elevational plant range expansion under climate change. Proceedings of the Royal Society B: Biological Sciences. 2014;281 doi: 10.1098/rspb.2014.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruelheide H, Scheidel U. Slug herbivory as a limiting factor for the geographical range of Arnica montana. Journal of Ecology. 1999;87:839–848. doi: 10.1046/j.1365-2745.1999.00403.x. [DOI] [Google Scholar]
- Burgess TI, Scott JK, McDougall KL, et al. Current and projected global distribution of Phytophthora cinnamomi, one of the world's worst plant pathogens. Global Change Biology. 2017;23:1661–1674. doi: 10.1111/gcb.13492. [DOI] [PubMed] [Google Scholar]
- Cahill AE, Aiello-Lammens ME, Fisher-Reid MC, et al. How does climate change cause extinction? Proceedings of the Royal Society B: Biological Sciences. 2013;280 doi: 10.1098/rspb.2012.1890. 20121890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill DM, Rookes JE, Wilson BA, Gibson L, McDougall KL. Phytophthora cinnamomi and Australia’s biodiversity: impacts, predictions and progress towards control. Australian Journal of Botany. 2008;56:279–310. doi: 10.1071/BT07159. [DOI] [Google Scholar]
- Callaway RM, Brooker RW, Choler P, et al. Positive interactions among alpine plants increase with stress. Nature. 2002;417:844–848. doi: 10.1038/nature00812. [DOI] [PubMed] [Google Scholar]
- Cannone N, Sgorbati S, Guglielmin M. Unexpected impacts of climate change on alpine vegetation. Frontiers in Ecology and the Environment. 2007;5:360–364. doi: 10.1890/1540-9295(2007)5[360:UIOCCO]2.0.CO;2. [DOI] [Google Scholar]
- Cavieres LA, Brooker RW, Butterfield BJ, et al. Facilitative plant interactions and climate simultaneously drive alpine plant diversity. Ecology Letters. 2014;17:193–202. doi: 10.1111/ele.12217. [DOI] [PubMed] [Google Scholar]
- Cavieres LA, Quiroz CL, Molina-Montenegro MA. Facilitation of the non-native Taraxacum officinale by native nurse cushion species in the high Andes of central Chile: are there differences between nurses? Functional Ecology. 2008;22:148–156. doi: 10.1111/j.1365-2435.2007.01338.x. [DOI] [Google Scholar]
- Chen I-C, Hill JK, Ohlemüller R, Roy DB, Thomas CD. Rapid range shifts of species associated with high levels of climate warming. Science. 2011;333:1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- Chesson P. Mechanisms of maintenance of species diversity. Annual Review of Ecology, Evolution, and Systematics. 2000;31:343–366. doi: 10.1146/annurev.ecolsys.31.1.343. [DOI] [Google Scholar]
- Chesson P, Huntly N. The roles of harsh and fluctuating conditions in the dynamics of ecological communities. The American Naturalist. 1997;150:519–553. doi: 10.1086/286080. [DOI] [PubMed] [Google Scholar]
- Choler P, Michalet R, Callaway RM. Facilitation and competition on gradients in alpine plant communities. Ecology. 2001;82:3295–3308. doi: 10.1890/0012-9658(2001)082[3295:FACOGI]2.0.CO;2. [DOI] [Google Scholar]
- Corlett RT, Westcott DA. Will plant movements keep up with climate change? Trends in Ecology & Evolution. 2013;28:482–488. doi: 10.1016/j.tree.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Cotto O, Wessely J, Georges D, et al. A dynamic eco-evolutionary model predicts slow response of alpine plants to climate warming. Nature Communications. 2017;8:15399. doi: 10.1038/ncomms15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins SM, Dobrowski SZ, Greenberg JA, Abatzoglou JT, Mynsberge AR. Changes in climatic water balance drive downhill shifts in plant species' optimum elevations. Science. 2011;331:324–327. doi: 10.1126/science.1199040. [DOI] [PubMed] [Google Scholar]
- De Witte LC, Armbruster GFJ, Gielly L, Taberlet P, Stöcklin J. AFLP markers reveal high clonal diversity and extreme longevity in four key arctic-alpine species. Molecular Ecology. 2012;21:1081–1097. doi: 10.1111/j.1365-294X.2011.05326.x. [DOI] [PubMed] [Google Scholar]
- Descombes P, Pradervand J-N, Golay J, Guisan A, Pellissier L. Simulated shifts in trophic niche breadth modulate range loss of alpine butterflies under climate change. Ecography. 2016;39:796–804. doi: 10.1111/ecog.01557. [DOI] [Google Scholar]
- Descombes P, Vittoz P, Guisan A, Pellissier L. Uneven rate of plant turnover along elevation in grasslands. Alpine Botany. 2017;127:53–63. doi: 10.1007/s00035-016-0173-7. [DOI] [Google Scholar]
- Dirnböck T, Essl F, Rabitsch W. Disproportional risk for habitat loss of high-altitude endemic species under climate change. Global Change Biology. 2011;17:990–996. doi: 10.1111/j.1365-2486.2010.02266.x. [DOI] [Google Scholar]
- Dullinger S, Gattringer A, Thuiller W, et al. Extinction debt of high-mountain plants under twenty-first-century climate change. Nature Climate Change. 2012a;2:619–622. doi: 10.1038/nclimate1514. [DOI] [Google Scholar]
- Dullinger S, Willner W, Plutzar C, et al. Post-glacial migration lag restricts range filling of plants in the European Alps. Global Ecology and Biogeography. 2012b;21:829–840. doi: 10.1111/j.1466-8238.2011.00732.x. [DOI] [Google Scholar]
- Eckstein RL, Pereira Eva, Milbau A, Graae BJ. Predicted changes in vegetation structure affect the susceptibility to invasion of bryophyte-dominated subarctic heath. Annals of Botany. 2011;108:177–183. doi: 10.1093/aob/mcr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsen PR, Tingley MW. Global mountain topography and the fate of montane species under climate change. Nature Climate Change. 2015;5:772–776. doi: 10.1038/nclimate2656. [DOI] [Google Scholar]
- Engelkes T, Morrien E, Verhoeven KJF, et al. Successful range-expanding plants experience less above-ground and below-ground enemy impact. Nature. 2008;456 doi: 10.1038/nature07474. [DOI] [PubMed] [Google Scholar]
- Engler R, Randin CF, Thuiller W, et al. 21st century climate change threatens mountain flora unequally across Europe. Global Change Biology. 2011;17:2330–2341. doi: 10.1111/j.1365-2486.2010.02393.x. [DOI] [Google Scholar]
- Engler R, Randin CF, Vittoz P, Czáka T, Beniston M, Zimmermann NE, Guisan A. Predicting future distributions of mountain plants under climate change: does dispersal capacity matter? Ecography. 2009;32:34–45. doi: 10.1111/j.1600-0587.2009.05789.x. [DOI] [Google Scholar]
- Essl F, Dullinger S, Rabitsch W, Hulme PE, Pyšek P, Wilson JRU, Richardson DM. Historical legacies accumulate to shape future biodiversity in an era of rapid global change. Diversity and Distributions. 2015;21:534–547. doi: 10.1111/ddi.12312. [DOI] [Google Scholar]
- Evans MEK, Merow C, Record S, McMahon SM, Enquist BJ. Towards process-based range modeling of many species. Trends in Ecology & Evolution. 2016;31:860–871. doi: 10.1016/j.tree.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Ford KR. Unpublished PhD University of Washington; Seattle, USA: 2014. Climate change impacts on the distribution and performance of plant species at Mount Rainier. [Google Scholar]
- Gaston KJ. The Structure and Dynamics of Species Ranges. Oxford University Press; Oxford: 2003. [Google Scholar]
- Gottfried M, Pauli H, Futschik A, et al. Continent-wide response of mountain vegetation to climate change. Nature Climate Change. 2012;2:111–115. doi: 10.1038/nclimate1329. [DOI] [Google Scholar]
- Gough L. Neighbor effects on germination, survival, and growth in two arctic tundra plant communities. Ecography. 2006;29:44–56. doi: 10.1111/j.2005.0906-7590.04096.x. [DOI] [Google Scholar]
- Graae BJ, Ejrnæs R, Lang SI, Meineri E, Ibarra PT, Bruun HH. Strong microsite control of seedling recruitment in tundra. Oecologia. 2011;166:565–576. doi: 10.1007/s00442-010-1878-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. Dieback of Nematolepis ovatifolia (Rutaceae), an endemic shrub in the alpine-subalpine heaths of the Snowy Mountains, is facilitated by climate change. Cunninghamia. 2016;16:1–9. doi: 10.7751/cunninghamia.2016.16.001. [DOI] [Google Scholar]
- Grime JP. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. The American Naturalist. 1977;111:1169–1194. [Google Scholar]
- Guisan A, Rahbek C. SESAM – a new framework integrating macroecological and species distribution models for predicting spatio-temporal patterns of species assemblages. Journal of Biogeography. 2011;38:1433–1444. doi: 10.1111/j.1365-2699.2011.02550.x. [DOI] [Google Scholar]
- Hampe A. Plants on the move: The role of seed dispersal and initial population establishment for climate-driven range expansions. Acta Oecologica. 2011;37:666–673. doi: 10.1016/j.actao.2011.05.001. [DOI] [Google Scholar]
- Hampe A, Jump AS. Climate relicts: past, present, future. Annual Review of Ecology, Evolution, and Systematics. 2011;42:313–333. doi: 10.1146/annurev-ecolsys-102710-145015. [DOI] [Google Scholar]
- Harsch MA, Phillips A, Zhou Y, Leung M-R, Rinnan DS, Kot M. Moving forward: insights and applications of moving-habitat models for climate change ecology. Journal of Ecology. 2017;105:1169–1181. doi: 10.1111/1365-2745.12724. [DOI] [Google Scholar]
- Hargreaves AL, Samis KE, Eckert CG. Are species’ range limits simply niche limits writ large? A review of transplant experiments beyond the range. The American Naturalist. 2014;183:157–173. doi: 10.1086/674525. [DOI] [PubMed] [Google Scholar]
- Henne PD, Elkin CM, Reineking B, Bugmann H, Tinner W. Did soil development limit spruce (Picea abies) expansion in the Central Alps during the Holocene? Testing a palaeobotanical hypothesis with a dynamic landscape model. Journal of Biogeography. 2011;38:933–949. doi: 10.1111/j.1365-2699.2010.02460.x. [DOI] [Google Scholar]
- HilleRisLambers J, Harsch MA, Ettinger AK, Ford KR, Theobald EJ. How will biotic interactions influence climate change–induced range shifts? Annals of the New York Academy of Sciences. 2013;1297:112–125. doi: 10.1111/nyas.12182. [DOI] [PubMed] [Google Scholar]
- Hillyer R, Silman MR. Changes in species interactions across a 2.5 km elevation gradient: effects on plant migration in response to climate change. Global Change Biology. 2010;16:3205–3214. doi: 10.1111/j.1365-2486.2010.02268.x. [DOI] [Google Scholar]
- Hülber K, Wessely J, Gattringer A, et al. Uncertainty in predicting range dynamics of endemic alpine plants under climate warming. Global Change Biology. 2016;22:2608–2619. doi: 10.1111/gcb.13232. [DOI] [PubMed] [Google Scholar]
- Jackson ST, Sax DF. Balancing biodiversity in a changing environment: extinction debt, immigration credit and species turnover. Trends in Ecology & Evolution. 2010;25:153–160. doi: 10.1016/j.tree.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Jones NT, Gilbert B. Biotic forcing: the push–pull of plant ranges. Plant Ecology. 2016;217:1331–1344. doi: 10.1007/s11258-016-0603-z. [DOI] [Google Scholar]
- Jost L. Partitioning diversity into independent alpha and beta components. Ecology. 2007;88:2427–2439. doi: 10.1890/06-1736.1. [DOI] [PubMed] [Google Scholar]
- Jump AS, Mátyás C, Peñuelas J. The altitude-for-latitude disparity in the range retractions of woody species. Trends in Ecology & Evolution. 2009;24:694–701. doi: 10.1016/j.tree.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Kaarlejärvi E, Eskelinen A, Olofsson J. Herbivory prevents positive responses of lowland plants to warmer and more fertile conditions at high altitudes. Functional Ecology. 2013;27:1244–1253. doi: 10.1111/1365-2435.12113. [DOI] [Google Scholar]
- Körner C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems. Springer; Heidelberg: 2003. [Google Scholar]
- Körner C. Mountain biodiversity, its causes and function. Ambio. 2004;13:11–17. [PubMed] [Google Scholar]
- Kraft NJB, Godoy O, Levine JM. Plant functional traits and the multidimensional nature of species coexistence. Proceedings of the National Academy of Sciences. 2015;112:797–802. doi: 10.1073/pnas.1413650112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroiss SJ, HilleRisLambers J. Recruitment limitation of long-lived conifers: implications for climate change responses. Ecology. 2014;96:1286–1297. doi: 10.1890/14-0595.1. [DOI] [PubMed] [Google Scholar]
- Kudo G, Amagai Y, Hoshino B, Kaneko M. Invasion of dwarf bamboo into alpine snow-meadows in northern Japan: pattern of expansion and impact on species diversity. Ecology and Evolution. 2011;1:85–96. doi: 10.1002/ece3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulonen A. Faster, taller, more - patterns and drivers of floristic change on European mountain summits. Unpublished PhD University of Bergen; 2017. [Google Scholar]
- Lankau RA, Zhu K, Ordonez A. Mycorrhizal strategies of tree species correlate with trailing range edge responses to current and past climate change. Ecology. 2015;96:1451–1458. doi: 10.1890/14-2419.1. [DOI] [Google Scholar]
- le Roux PC, McGeoch MA. Rapid range expansion and community reorganization in response to warming. Global Change Biology. 2008;14:2950–2962. doi: 10.1111/j.1365-2486.2008.01687.x. [DOI] [Google Scholar]
- le Roux PC, Shaw JD, Chown SL. Ontogenetic shifts in plant interactions vary with environmental severity and affect population structure. New Phytologist. 2013;200:241–250. doi: 10.1111/nph.12349. [DOI] [PubMed] [Google Scholar]
- le Roux PC, Virtanen R, Heikkinen RK, Luoto M. Biotic interactions affect the elevational ranges of high-latitude plant species. Ecography. 2012;35:1048–1056. doi: 10.1111/j.1600-0587.2012.07534.x. [DOI] [Google Scholar]
- Lembrechts J, Pauchard A, Lenoir J, et al. Disturbance is the key to plant invasions in cold environments. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:14061–14066. doi: 10.1073/pnas.1608980113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembrechts JJ, Milbau A, Nijs I. Trade-off between competition and facilitation defines gap colonisation in mountains. AoB Plants. 2015;7:plv128. doi: 10.1093/aobpla/plv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir J, Gégout JC, Marquet PA, de Ruffray P, Brisse H. A significant upward shift in plant species optimum elevation during the 20th century. Science. 2008;320:1768–1771. doi: 10.1126/science.1156831. [DOI] [PubMed] [Google Scholar]
- Lenoir J, Hattab T, Pierre G. Climatic microrefugia under anthropogenic climate change: implications for species redistribution. Ecography. 2017;40:253–266. doi: 10.1111/ecog.02788. [DOI] [Google Scholar]
- Lenoir J, Svenning J-C. Encyclopedia of Biodiversity. Academic Press, Incorporated; 2013. Latitudinal and elevational range shifts under contemporary climate change; pp. 599–611. [DOI] [Google Scholar]
- Loehle C. Height growth rate tradeoffs determine northern and southern range limits for trees. Journal of Biogeography. 1998;25:735–742. doi: 10.1046/j.1365-2699.1998.2540735.x. [DOI] [Google Scholar]
- MacArthur RH. Geographic Ecology: Patterns in the Distribution of Species. Harper & Row; New York: 1972. [Google Scholar]
- Matteodo M, Wipf S, Stöckli V, Rixen C, Vittoz P. Elevation gradient of successful plant traits for colonizing alpine summits under climate change. Environmental Research Letters. 2013;8 doi: 10.1088/1748-9326/8/2/024043. 024043. [DOI] [Google Scholar]
- Maher EL, Germino MJ. Microsite differentiation among conifer species during seedling establishment at alpine treeline. Ecoscience. 2006;13:334–341. doi: 10.2980/i1195-6860-13-3-334.1. [DOI] [Google Scholar]
- Mayor JR, Sanders NJ, Classen AT, et al. Elevation alters ecosystem properties across temperate treelines globally. Nature. 2017;542:91–95. doi: 10.1038/nature21027. [DOI] [PubMed] [Google Scholar]
- Michalet R, Schöb C, Lortie CJ, Brooker RW, Callaway RM. Partitioning net interactions among plants along altitudinal gradients to study community responses to climate change. Functional Ecology. 2014;28:75–86. doi: 10.1111/1365-2435.12136. [DOI] [Google Scholar]
- Milbau A, Shevtsova A, Osler N, Mooshammer M, Graae BJ. Plant community type and small-scale disturbances, but not altitude, influence the invasibility in subarctic ecosystems. New Phytologist. 2013;197:1002–1011. doi: 10.1111/nph.12054. [DOI] [PubMed] [Google Scholar]
- Moen J. Positive versus negative interactions in a high alpine block field: Germination of Oxyria digyna seeds in a Ranunculus glacialis community. Arctic and Alpine Research. 1993;25:201–206. doi: 10.2307/1551814. [DOI] [Google Scholar]
- Molau U, Larsson E-L. Seed rain and seed bank along an alpine altitudinal gradient in Swedish Lapland. Canadian Journal of Botany. 2000;78:728–747. doi: 10.1139/b00-049. [DOI] [Google Scholar]
- Morales-Castilla I, Matias MG, Gravel D, Araújo MB. Inferring biotic interactions from proxies. Trends in Ecology & Evolution. 2015;30:347–356. doi: 10.1016/j.tree.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Mountain Research Initiative EDW Working Group. Elevation-dependent warming in mountain regions of the world. Nature Climate Change. 2015;5:424–430. doi: 10.1038/nclimate2563. [DOI] [Google Scholar]
- Müller E, Cooper EJ, Alsos IG. Germinability of arctic plants is high in perceived optimal conditions but low in the field. Botany. 2011;89:337–348. doi: 10.1139/b11-022. [DOI] [Google Scholar]
- Muller-Landau HC, Wright SJ, Calderón O, Hubbell SP, Foster RB. Assessing recruitment limitation: concepts, methods and case-studies from a tropical forest. In: Levey DJ, Silva WR, Galetti M, editors. Seed Dispersal and Frugivory: Ecology, Evolution and Conservation. CABI Publishing; Wallingford, UK: 2002. pp. 35–53. [DOI] [Google Scholar]
- Naoe S, Tayasu I, Sakai Y, et al. Mountain-climbing bears protect cherry species from global warming through vertical seed dispersal. Current Biology. 2016;26:R315–R316. doi: 10.1016/j.cub.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Neuschulz EL, Merges D, Bollmann K, Gugerli F, Böhning-Gaese K. Biotic interactions and seed deposition rather than abiotic factors determine recruitment at elevational range limits of an alpine tree. Journal of Ecology. doi: 10.1111/1365-2745.12818. (in press) [DOI] [Google Scholar]
- Niu S, Wan S. Warming changes plant competitive hierarchy in a temperate steppe in northern China. Journal of Plant Ecology. 2008;1:103–110. doi: 10.1093/jpe/rtn003. [DOI] [Google Scholar]
- Nuñez MA, Horton TR, Simberloff D. Lack of belowground mutualisms hinders Pinaceae invasions. Ecology. 2009;90:2352–2359. doi: 10.1890/08-2139.1. [DOI] [PubMed] [Google Scholar]
- Olsen SL, Töpper JP, Skarpaas O, Vandvik V, Klanderud K. From facilitation to competition: temperature-driven shift in dominant plant interactions affects population dynamics in seminatural grasslands. Global Change Biology. 2016;22:1915–1926. doi: 10.1111/gcb.13241. [DOI] [PubMed] [Google Scholar]
- Ouburg NJ, Eriksson O. Toward a metapopulation concept for plants. In: Hanski I, Gaggiotti OE, editors. Ecology, Genetics and Evolution of Metapopulations. Elsevier; San Diego, California, U.S.A: 2004. pp. 447–469. [Google Scholar]
- Pagel J, Schurr FM. Forecasting species ranges by statistical estimation of ecological niches and spatial population dynamics. Global Ecology and Biogeography. 2012;21:293–304. doi: 10.1111/j.1466-8238.2011.00663.x. [DOI] [Google Scholar]
- Parolo G, Rossi G. Upward migration of vascular plants following a climate warming trend in the Alps. Basic and Applied Ecology. 2008;9:100–107. doi: 10.1016/j.baae.2007.01.005. [DOI] [Google Scholar]
- Pauchard A, Milbau A, Albihn A, et al. Non-native and native organisms moving into high elevation and high latitude ecosystems in an era of climate change: new challenges for ecology and conservation. Biological Invasions. 2016;18:345–353. doi: 10.1007/s10530-015-1025-x. [DOI] [Google Scholar]
- Pauli H, Gottfried M, Dullinger S, et al. Recent plant diversity changes on Europe’s mountain summits. Science. 2012;336:353–355. doi: 10.1126/science.1219033. [DOI] [PubMed] [Google Scholar]
- Pellissier L, Bråthen KA, Vittoz P, et al. Thermal niches are more conserved at cold than warm limits in arctic-alpine plant species. Global Ecology and Biogeography. 2013;22:933–941. doi: 10.1111/geb.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellissier L, Fiedler K, Ndribe C, Dubuis A, Pradervand J-N, Guisan A, Rasmann S. Shifts in species richness, herbivore specialization, and plant resistance along elevation gradients. Ecology and Evolution. 2012;2:1818–1825. doi: 10.1002/ece3.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering CM, Mount A, Wichmann MC, Bullock JM. Estimating human-mediated dispersal of seeds within an Australian protected area. Biological Invasions. 2011;13:1869–1880. doi: 10.1007/s10530-011-0006-y. [DOI] [Google Scholar]
- Plassmann G, Kohler Y, Badura M, Walzer C. Alpine Nature 2030. Creating [Ecological] Connectivity for Generations to Come. Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety (BMUB); Berlin, Germany: 2016. [Google Scholar]
- Prock S, Körner C. A cross-continental comparison of phenology, leaf dynamics and dry matter allocation in arctic and temperate zone herbaceous plants from contrasting altitudes. Ecological Bulletins. 1996;45:93–103. [Google Scholar]
- Pyšek P, Jarošík V, Pergl J, et al. The global invasion success of Central European plants is related to distribution characteristics in their native range and species traits. Diversity and Distributions. 2009;15:891–903. doi: 10.1111/j.1472-4642.2009.00602.x. [DOI] [Google Scholar]
- Randin CF, Engler R, Normand S, et al. Climate change and plant distribution: local models predict high-elevation persistence. Global Change Biology. 2009;15:1557–1569. doi: 10.1111/j.1365-2486.2008.01766.x. [DOI] [Google Scholar]
- Rasmann S, Pellissier L, Defossez E, Jactel H, Kunstler G. Climate-driven change in plant–insect interactions along elevation gradients. Functional Ecology. 2014;28:46–54. doi: 10.1111/1365-2435.12135. [DOI] [Google Scholar]
- Scherrer D, Körner C. Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. Journal of Biogeography. 2011;38:406–416. doi: 10.1111/j.1365-2699.2010.02407.x. [DOI] [Google Scholar]
- Schurr FM, Pagel J, Cabral JS, et al. How to understand species’ niches and range dynamics: a demographic research agenda for biogeography. Journal of Biogeography. 2012;39:2146–2162. doi: 10.1111/j.1365-2699.2012.02737.x. [DOI] [Google Scholar]
- Sheth SN, Angert AL. The evolution of environmental tolerance and range size: A comparison of geographically restricted and widespread Mimulus. Evolution. 2014;68:2917–2931. doi: 10.1111/evo.12494. [DOI] [PubMed] [Google Scholar]
- Shevtsova A, Graae BJ, Jochum T, Milbau ANN, Kockelbergh F, Beyens L, Nijs I. Critical periods for impact of climate warming on early seedling establishment in subarctic tundra. Global Change Biology. 2009;15:2662–2680. doi: 10.1111/j.1365-2486.2009.01947.x. [DOI] [Google Scholar]
- Slatyer RA, Hirst M, Sexton JP. Niche breadth predicts geographical range size: a general ecological pattern. Ecology Letters. 2013;16:1104–1114. doi: 10.1111/ele.12140. [DOI] [PubMed] [Google Scholar]
- Speed JDM, Austrheim G, Hester AJ, Mysterud A. Experimental evidence for herbivore limitation of the treeline. Ecology. 2010;91:3414–3420. doi: 10.1890/09-2300.1. [DOI] [PubMed] [Google Scholar]
- Spillmann JH, Holderegger R. Die Alpenpflanzen des Tössberglandes. Einhundert Jahre nach Gustav Hegi. Haupt; Bern: 2008. [Google Scholar]
- Sundqvist MK, Sanders NJ, Wardle DA. Community and ecosystem responses to elevational gradients: Processes, mechanisms, and insights for global change. Annual Review of Ecology, Evolution, and Systematics. 2013;44:261–280. doi: 10.1146/annurev-ecolsys-110512-135750. [DOI] [Google Scholar]
- Svenning J-C, Gravel D, Holt RD, et al. The influence of interspecific interactions on species range expansion rates. Ecography. 2014;37:1198–1209. doi: 10.1111/j.1600-0587.2013.00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenning J-C, Sandel B. Disequilibrium vegetation dynamics under future climate change. American Journal of Botany. 2013;100:1266–1286. doi: 10.3732/ajb.1200469. [DOI] [PubMed] [Google Scholar]
- Tackenberg O. Modeling long-distance dispersal of plant diaspores by wind. Ecological Monographs. 2003;73:173–189. doi: 10.1890/0012-9615(2003)073[0173:MLDOPD]2.0.CO;2. [DOI] [Google Scholar]
- Theurillat J-P, Felber F, Geissler P, et al. Sensitivity of plant and soil ecosystems of the Alps to climate change. In: Cebon P, Dahinden U, Davis H, Imboden DM, Jaeger CC, editors. Views From The Alps: Regional Perspectives On Climate Change. The MIT Press; Cambridge, Massachusetts: 1998. pp. 225–308. [Google Scholar]
- Thiel-Egenter C, Alvarez N, Holderegger R, et al. Break zones in the distributions of alleles and species in alpine plants. Journal of Biogeography. 2011;38:772–782. doi: 10.1111/j.1365-2699.2010.02441.x. [DOI] [Google Scholar]
- Thuiller W, Lavorel S, Araújo MB, Sykes MT, Prentice IC. Climate change threats to plant diversity in Europe. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8245–8250. doi: 10.1073/pnas.0409902102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomisto H. A diversity of beta diversities: straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography. 2010;33:2–22. doi: 10.1111/j.1600-0587.2009.05880.x. [DOI] [Google Scholar]
- Turnbull LA, Rees M, Crawley MJ. Seed mass and the competition/colonization trade-off: a sowing experiment. Journal of Ecology. 1999;87:899–912. doi: 10.1046/j.1365-2745.1999.00405.x. [DOI] [Google Scholar]
- Urban MC, Bocedi G, Hendry AP, et al. Improving the forecast for biodiversity under climate change. Science. 2016;353 doi: 10.1126/science.aad8466. aad8466. [DOI] [PubMed] [Google Scholar]
- Urban MC, Tewksbury JJ, Sheldon KS. On a collision course: competition and dispersal differences create no-analogue communities and cause extinctions during climate change. Proceedings of the Royal Society B: Biological Sciences. 2012;279:2072–2080. doi: 10.1098/rspb.2011.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venn SE, Morgan JW. Patterns in alpine seedling emergence and establishment across a stress gradient of mountain summits in south-eastern Australia. Plant Ecology & Diversity. 2009;2:5–16. doi: 10.1080/17550870802691356. [DOI] [Google Scholar]
- Venn SE, Morgan JW, Green PT. Do facilitative interactions with neighboring plants assist the growth of seedlings at high altitudes in alpine Australia? Arctic, Antarctic, and Alpine Research. 2009;41:381–387. doi: 10.1657/1938-4246-41.3.381. [DOI] [Google Scholar]
- Vetaas OR. Realized and potential climate niches: a comparison of four Rhododendron tree species. Journal of Biogeography. 2002;29:545–554. doi: 10.1046/j.1365-2699.2002.00694.x. [DOI] [Google Scholar]
- Viana DS, Santamaría L, Figuerola J. Migratory birds as global dispersal vectors. Trends in Ecology & Evolution. 2016;31:763–775. doi: 10.1016/j.tree.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Vittoz P, Randin C, Dutoit A, Bonnet F, Hegg O. Low impact of climate change on subalpine grasslands in the Swiss Northern Alps. Global Change Biology. 2009;15:209–220. doi: 10.1111/j.1365-2486.2008.01707.x. [DOI] [Google Scholar]
- Williams JL, Kendall BE, Levine JM. Rapid evolution accelerates plant population spread in fragmented experimental landscapes. Science. 2016;353:482. doi: 10.1126/science.aaf6268. [DOI] [PubMed] [Google Scholar]
- Williams JW, Jackson ST. Novel climates, no-analog communities, and ecological surprises. Frontiers in Ecology and the Environment. 2007;5:475–482. doi: 10.1890/070037. [DOI] [Google Scholar]
- Wipf S, Stöckli V, Herz K, Rixen C. The oldest monitoring site of the Alps revisited: accelerated increase in plant species richness on Piz Linard summit since 1835. Plant Ecology & Diversity. 2013;6:447–455. doi: 10.1080/17550874.2013.764943. [DOI] [Google Scholar]
- Wisz MS, Pottier J, Kissling WD, et al. The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biological Reviews. 2013;88:15–30. doi: 10.1111/j.1469-185X.2012.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong S, Xu J, Dege E, Wu Z, He H. Effective seed distribution pattern of an upward shift species in alpine tundra of Changbai Mountains. Chinese Geographical Science. 2016;26:48–58. doi: 10.1007/s11769-015-0775-9. [DOI] [Google Scholar]
- Zurell D, Thuiller W, Pagel J, et al. Benchmarking novel approaches for modelling species range dynamics. Global Change Biology. 2016;22:2651–2664. doi: 10.1111/gcb.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.