Abstract
Acute myocarditis is an inflammatory disease of the heart muscle that may progress to dilated cardiomyopathy and chronic heart failure. A number of factors including the sex hormone testosterone, components of innate immunity, and profibrotic cytokines have been identified in animal models as important pathogenic mechanisms that increase inflammation and susceptibility to chronic dilated cardiomyopathy. The clinical presentation of acute myocarditis is non-specific and mimics more common causes of heart failure and arrhythmias. Suspected myocarditis is currently confirmed using advanced non-invasive imaging and histopathologic examination of heart tissue. However, the diverse presentations of myocarditis and the lack of widely available, safe, and accurate non-invasive diagnostic tests remain major obstacles to early diagnosis and population based research. Recent advances in the understanding of disease pathogenesis described in this review should lead to more accurate diagnostic algorithms and non-invasive tests.
INTRODUCTION
Acute myocarditis is an inflammatory disease of the heart muscle that may be confirmed using imaging and/or histopathologic criteria. Patients often report a viral prodrome of fever, rash, myalgias, arthralgias, fatigue and respiratory or gastrointestinal symptoms; these non-specific complaints typically precede the cardiovascular symptoms by a few days to weeks. The clinical presentation is variable and symptoms vary from mild dyspnoea or chest pain that resolve spontaneously to arrhythmias and cardiogenic shock.1,2 If the epicardium is involved, a syndrome of myopericarditis may develop with characteristic chest pain and sometimes pericardial effusion. Acute necrotising eosinophilic myocarditis and giant cell myocarditis are two rare idiopathic disorders that often present with fulminant or acute heart failure, and are frequently associated with ventricular arrhythmias or heart block. The clinical diversity of myocarditis and the lack of widely available, safe, and accurate non-invasive diagnostic tests have made its true incidence difficult to determine. In one series myocarditis was identified as a cause of unexplained dilated cardiomyopathy (DCM) in 9.6% of cases.3 In a registry of 1866 young athletes who died suddenly, 6% with cardiovascular disease (CVD) had myocarditis.4 Between 4% and 20% of sudden cardiovascular deaths among young adults, military service members, and athletes are due to myocarditis.5 Thus, myocarditis leads to a significant minority of DCM cases and contributes to the global burden of chronic heart failure.
Myocarditis can result from a wide spectrum of infectious pathogens including viruses, bacteria, spirochetes, fungi, and protozoans, as well as being induced by cardiotoxins and hypersensitivity reactions (table 1). Although most cases of suspected myocarditis are not linked to a specific aetiology, viral infection has been identified as the most common cause of myocarditis in Western Europe and North America. The most frequently identified virus in endomyocardial biopsies from myocarditis patients in the 1950s through the 1990s was coxsackievirus B, but adenovirus and parvovirus B19 have now become more prevalent.6 Although cardiovirulent coxsackievirus B strains reproducibly cause myocarditis and DCM in susceptible animal models, many wild type viruses from the same family evidently do not lead to cardiomyopathy in most human cases. The factors responsible for individual variations in susceptibility are not fully known but likely include genetic predisposition, sex hormones and/or sex chromosomes, and environmental factors like poor nutrition and vitamin deficiency.2
Table 1.
Causes of myocarditis
| Viruses/disorders | Bacteria/disorders | Cardiotoxins | Hypersensitivity |
| Adenovirus* | Chlamydia | Ethanol* | Cephalosporins |
| Coxsackievirus B* | Cholera | Anthracycline drugs* | Clozapine |
| Cytomegalovirus* | Mycoplasma | Arsenic | Diuretics |
| Epstein–Barr virus | Neisseria | Carbon monoxide | Insect bites |
| Hepatitis C virus | Salmonella | Catecholamines | Lithium |
| Herpes simplex virus | Staphylococcus | Cocaine* | Snake bites |
| HIV* | Streptococcus | Heavy metals | Sulfonamides |
| Influenza virus | Tetanus | Copper | Tetanus toxoid |
| Mumps | Tuberculosis | Mercury | Tetracycline |
| Parvovirus B19 | Lead | ||
| Poliovirus | Spirochetal | Systemic disorders | |
| Rabies | Leptospirosis | Protozoa | Hypereosinophilia |
| Rubella | Lyme disease | Chagas disease | Kawasaki disease |
| Varicella zoster virus | Relapsing fever | Leishmaniasis | Sarcoidosis |
| Yellow fever | Syphilis | Malaria | Wegener granulomatosis |
Frequent cause of myocarditis.
PATHOGENESIS OF MYOCARDITIS
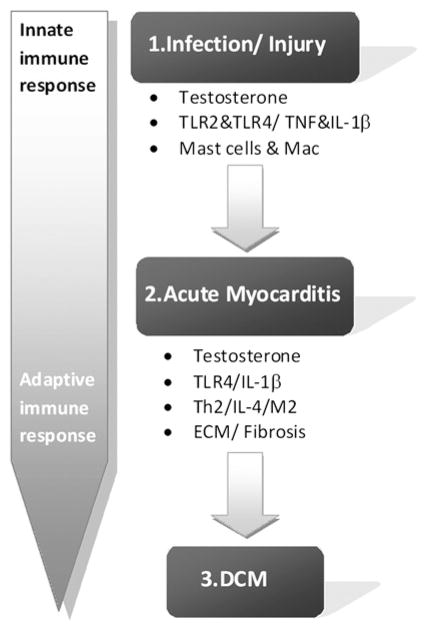
Most of the information about the pathogenesis of myocarditis and DCM comes from animal models rather than human studies. In animal models, the progression from acute myocarditis to chronic DCM can be simplified to a three-stage process: phase 1, cardiac injury and activation of the innate immune response; phase 2, acute myocarditis involving components of the innate and acquired immune response; and phase 3 recovery in resistant individuals versus progression to DCM in susceptible individuals (figure 1). A number of factors including sex hormones, particularly testosterone, components of innate immunity, and profibrotic cytokines have been identified in animal models as important pathogenic mechanisms that increase inflammation and susceptibility to chronic DCM (figure 1).
Figure 1.
Pathogenic mechanisms in myocarditis and dilated cardiomyopathy (DCM). The incidence and severity of myocarditis and DCM is greater in men than women (and male and female mice). Studies in animal models of myocarditis show that testosterone drives the inflammation and remodelling that allows progression to DCM. Infection is a major source of cardiac injury that activates innate immune mechanisms like Toll-like receptor (TLR) 2 and TLR4 on mast cells and macrophages. Innate immunity is a dominant feature during acute myocarditis, where TLR2 and TLR4 levels remain elevated in the heart and induce proinflammatory cytokines like tumour necrosis factor (TNF) and interleukin 1β (IL1β). TNF and IL1β directly alter cardiac function and promote extracellular matrix remodelling, resulting in fibrosis and cardiac dilation. Cytokines and immune cells associated with an adaptive T helper (Th)2 type immune response, such as IL4 and alternatively activated macrophages (M2), appear to be necessary in animal models for the progression from myocarditis to DCM.
Phase 1: injury and activation of innate immunity
One of the hallmarks of myocarditis is injury to the myocardium. In animal models injury is induced by cardiotropic infections (ie, virus) that can damage cardiac tissue and/or by exposing animals to damaged self in the form of cardiac proteins or peptides (ie, cardiac myosin or troponins) in experimental autoimmune models. Damaged self proteins and infections (or adjuvants containing inactivated microbes) strongly induce the innate immune response, activating Toll-like receptors (TLRs) and the inflammasome, resulting in release of the proinflammatory cytokines tumour necrosis factor α (TNFα) and interleukin 1β (IL1β) (figure 1).7,8
A characteristic of the early innate immune response in mice that are susceptible to develop DCM (ie, BALB/c and A/J) is elevated levels of the proinflammatory cytokines TNFα and IL1β in the spleen and heart, and higher expression of TLR2 and TLR4 on innate immune cells like mast cells and macrophages.9–11 In humans and mice, TLR2 and TLR4 signalling has been demonstrated to be activated by damaged self proteins including cardiac myosin.12,13 TLR2 and TLR4 signalling increase TNF concentrations, and TLR2 acts synergistically with TLR4 to increase IL1β concentrations.14 Cardiac TLR4 mRNA expression has been found to be higher in patients with myocarditis than controls, and to correlate with viral RNA values in the heart.15 Myocarditis patients with active viral replication had higher concentrations of TLR4 that was associated with lower systolic function. Mice with defective TLR4 signalling have reduced acute experimental and viral myocarditis and reduced viral replication in the heart, indicating that TLR4 increases susceptibility to infection.16,17 For the most part, TLRs that protect the host from viral infections, such as TLR3, TLR7, TLR9 and their downstream adaptors MyD88 and TRIF, have been shown to reduce myocarditis and viral replication in animal models.13,18–20 Thus, data so far suggest that viral specific TLRs like TLR3 and TLR9 reduce acute myocarditis, while TLR2 and TLR4, which increase viral replication and the immune response to infection and damaged self, increase disease. Susceptibility in model systems to an elevated innate immune response is dependent on at least two factors: (1) male sex (eg, testosterone); and (2) genetic background (ie, T helper (Th)2 responding BALB/c mice) (figure 1).9,10,21,22
Phase 2: acute myocardial inflammation
Similar to findings in clinical biopsies of myocarditis patients, the primary infiltrate found in mouse models of acute myocarditis consists of macrophages and neutrophils with lower levels of T cells, B cells, mast cells, and dendritic cells.10,23,24 IL13 protects against experimental autoimmune myocarditis by regulating macrophage differentiation. Natural killer cells, CD8 T cells and γδT cells, needed for antiviral defence, are also important in the early cardiac cellular response in viral animal models.25–27 TLR4 and IL1β remain elevated on mast cells and macrophages in the heart of susceptible male BALB/c mice during acute myocarditis (figure 1).10,28 IL1β and IL17 are known to induce cardiac remodelling that leads to fibrosis, DCM, and heart failure following acute myocarditis in mice.11,29–32 Regulatory mechanisms like Tim-3+ CD4 T cells, alternatively activated macrophages (M2), and regulatory T cells (Treg) are elevated at the later stage of acute myocarditis, resulting in the disappearance of acute inflammation from the heart of susceptible and resistant mouse strains.10,27,33–35
Phase 3: progression to DCM
Several weeks later in susceptible strains of mice (ie, BALB/c males) a low level inflammation re-emerges that is associated with myocyte necrosis, fibrosis, and DCM.35,36 TNFα and IL1β are elevated in the heart during acute myocarditis in mice and humans and are known to induce myocyte hypertrophy, contractile dysfunction, and myocyte apoptosis, and contribute to extracellular matrix remodelling—a step critical in the progression from myocarditis to DCM.11,16,29,37 Although acute myocarditis is characterised by a predominantly Th1 and/or Th17 response,17,32,38–40 only mice that develop a Th2 response during acute myocarditis, like BALB/c and A/J mouse strains, go on to develop the chronic stage of myocarditis associated with low level inflammation, necrosis, fibrosis, and DCM (figure 1).20,30,35,41 Profibrotic cytokines like TNFα, IL1β, IL4, IL17, and transforming growth factor β1 (TGFβ1) are believed to initiate remodelling during acute myocarditis that results in the appearance of cardiac fibrosis/scar tissue several weeks or months later.11,20,30,32 However, controversy remains concerning whether the primary mechanisms that lead to chronic DCM involve viral persistence, autoimmune or autoinflammatory derived damage.8,36,42 Many researchers contend that DCM is due to persistent viral replication in the heart, and have shown that persistent virus can induce cardiac inflammation, fibrosis, and necrosis.8 Other studies have found that virus persists within the heart of susceptible and resistant strains of mice, yet only susceptible mice develop chronic myocarditis and DCM.43 Additionally, adjuvant induced models of autoimmune myocarditis do not require virus persistence to induce chronic disease.32,36,41 The ability of testosterone to amplify acute and chronic myocarditis without altering viral replication requires further investigation.10,22 A better understanding of the mechanisms involved in the progression of myocarditis to DCM in susceptible individuals will enable researchers and clinicians to develop more effective therapeutic strategies to prevent dilation and heart failure.
DIAGNOSIS OF MYOCARDITIS
Endomyocardial biopsy
The gold standard for the diagnosis of myocarditis is the endomyocardial biopsy (EMB). However, most clinical settings have limited ability to perform EMB and the additive value of EMB, over clinical evaluation and non-invasive imaging, to refine prognosis and guide treatment in most cases of acute myocarditis is not established. The 2007 American Heart Association/American College of Cardiology Foundation/European Society of Cardiology (AHA/ACCF/ESC) scientific statement on the role of EMB in CVD limited the class I indications to only two relatively uncommon clinical scenarios.44 EMB should be performed in that small subset of adults who present with a sudden onset of severe heart failure requiring inotropic or mechanical circulatory support within 2 weeks of a viral illness, because they frequently can be bridged to recovery if they have lymphocytic myocarditis and survive the initial illness. Also patients who present with fulminant or acute DCM with sustained or symptomatic ventricular tachycardia, high degree heart block, or who fail to respond to standard heart failure treatment should be biopsied for possible giant cell myocarditis. When performed by experienced clinicians, EMB has a complication rate of <1% for both left and right ventricular biopsy.45
According to the Dallas criteria acute myocarditis is defined as histologic evidence of lymphocytic infiltrates in association with myocyte necrosis, while borderline myocarditis is characterised by inflammatory infiltrates without evidence of myocyte necrosis.46 The diagnosis of myocarditis using the Dallas criteria is limited due to potential inter-observer variability in interpreting histology sections.47 Although four to six biopsy samples are routinely obtained (using a Stanford-Caves bioptome), a postmortem analysis of proven myocarditis cases found that more than 17 samples are needed to diagnose myocarditis accurately in >80% of cases.48 More recent diagnostic criteria that are more sensitive define inflammation by a focal or diffuse mononuclear infiltrate (ie, CD3 stained T lymphocytes and/or CD68 stained macrophages) at >14 cells/mm.2 49 Immunohistological signs of inflammation also correlate with risk of death or transplantation over the following 10 years in patients with suspected myocarditis.50
Recent studies to refine diagnosis, predict prognosis, and guide therapy have used EMB as a research tool. For example, Heidecker et al recently used mRNA transcriptome profiles to distinguish myocarditis from non-inflammatory DCM.51 Smith et al used cells from EMB specimens to create ‘cardiosphere derived cells’ that promote cardiac regeneration.52 This technology is currently being used in the CADUCEUS trial for treatment of recent myocardial infarction.
Serum biomarkers
Serum biomarkers provide valuable information for diagnosis of CVDs including myocarditis. For a biomarker to be clinically useful it should fulfil several criteria: (1) biomarker levels should be able to be accurately assessed using widely available and cost efficient methods; (2) biomarkers should provide additional information from the tests already conducted such asMRI; and (3) biomarker information should aid in medical decision making.53 A growing list of enzymes, hormones, markers of cardiac stress (eg, troponins) or necrosis (eg, Fas and Fas ligand), and cytokines (eg, IL10) have been examined as possible biomarkers for disease.
Troponins I and T
The release of cardiac troponins (I and T) from cardiomyocytes signals cell damage or death. Unfortunately, most studies suggest that a single value of troponin used for the diagnosis of suspected myocarditis has a low sensitivity.53 Lauer et al reported that 28 of 80 patients (35%) with clinically suspected myocarditis had elevated cardiac troponin T values.54 If a cut-off of >0.1 ng/ml was used, the sensitivity for troponin T to detect myocarditis was 53% and the specificity 94%.54 Smith et al evaluated the sensitivity of troponin I to detect myocarditis in a subgroup of patients from the Multicenter Myocarditis Treatment Trial, and found that the sensitivity was 34% and the specificity 89%.55 However, a gradual rise in troponin concentrations over more than 24 h, with a peak a day or more after the initial rise, may help distinguish myocarditis from acute ischaemic injury.
BNP and NT-proBNP
B-type natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP) are important indicators of cardiovascular stress and have the advantage of being able to distinguish acute from chronic heart failure. In contrast to troponins that are released due to cell wall compromise, BNP is synthesised in healthy cardiac myocytes from its precursor NT-proBNP.56 The prohormone BNP is only released to the circulation when the ventricles become dilated, hypertrophic or during other conditions that induce wall distension and stretching, and by neurohormonal activation. BNP has been found to be elevated in the serum of patients with myocarditis and DCM.53,57,58 Overall, BNP and NT-proBNP are biomarkers with a high sensitivity and specificity in predicting heart failure in a number of cardiovascular conditions including myocarditis and DCM.
Markers of inflammation
Non-specific serum markers of inflammation, including erythrocyte sedimentation rate, C reactive protein, and leucocyte count, are often elevated in myocarditis patients but seldom used for diagnosis. Elevated serum values of Fas and Fas ligand on initial presentation are associated with increased mortality in patients with acute myocarditis.59 Fas ligand is a membrane protein that belongs to the TNF family that induces apoptotic cell death when it binds to Fas and is converted to soluble Fas by matrix metalloproteinases (eg, MMP7). Fas has been shown to be notably increased on cardiac myocytes during acute viral myocarditis in mice, and myocardial inflammation is significantly decreased in Fas deficient mice.60 Fas is believed to cause cell death and myocardial necrosis indirectly by activating CD8+ T and NK (natural killer) cell mediated killing.60
Increased serum concentrations of the cytokines TNFα, IL1β, and IL10 have also been found to predict an increased risk of death in myocarditis patients.61,62 Serum concentrations of IL10 and TNFα were significantly higher in myocarditis patients compared to those patients with acute myocardial infarction, with IL10 values in particular predicting poor survival.61 IL1β and TNFα are thought of as prototypical proinflammatory cytokines. These two cytokines work synergistically to depress myocardial contractility, and their levels in sera have been shown to relate directly to New York Heart Association (NYHA) heart failure class and to predict patient mortality.62 In a separate study, cardiac TNF mRNA expression was found to be elevated more often in myocarditis patients when viral genomes were also detected, and greater mRNA values of TNF and its receptor TNFRI correlated with impaired cardiac function.63
Autoantibodies
Patients with myocarditis often develop autoantibodies against cardiac myosin or the β-adrenergic receptor. Anti-myosin antibodies are associated with left ventricular systolic dysfunction and diastolic stiffness in patients with chronic myocarditis.64 Anti-β1 receptor antibodies have been associated with greater risk of death or heart transplantation.65 Approximately 59% of myocarditis patients in one study were found to be positive for heart specific antibodies by immunofluorescence.66 In rodent models of myocarditis, anti-cardiac myosin antibodies have been shown to form immune complexes in cardiac tissue leading to apoptosis through β1-adrenoreceptor signalling.67
Viral serology
The diagnostic value of viral serology is limited by the fact that most viral infections believed to be involved in the pathogenesis of myocarditis are highly prevalent in the general population.68 The interpretation of viral antibody titres is complicated by confounders such as reactivation, reinfection, and/or cross-reactivity. Additionally, antibody levels vary over the time course of disease with the precise time of infection of individuals being largely unknown. IgM antibodies against parvovirus B19 are only detectable for a short period of around 2–10 weeks after acute infection, and so levels are likely to have resolved in many patients close to the time that they develop signs or symptoms of myocarditis.69
A positive viral serology does not indicate myocarditis, suggesting that assessing the presence of virus is not a particularly good diagnostic tool. Only five out of 124 (4%) patients with suspected myocarditis had serological evidence of infection with the same virus that was detected by reverse transcriptase-polymerase chain reaction (RT-PCR) in EMBs.70 The sensitivity for serological and EMB detection was 9% and the specificity was 77%, indicating that virus serology is not helpful for the diagnosis of myocarditis.70 Considering that immunohistological signs of inflammation rather than detection of viral genome were related to poor outcome in a large study of patients with suspected myocarditis,50 EMBs appear to be a better diagnostic tool than serologic testing for virus.
Electrocardiography
Electrocardiography (ECG) has a low sensitivity for diagnosing myocarditis. Non-specific ECG changes in myocarditis patients include sinus tachycardia, ST and T wave abnormalities, and ST elevations mimicking an acute myocardial infarction and occasionally atrial or ventricular conduction delays, as well as supra-ventricular and ventricular arrhythmias.71 Widened QRS and the presence of a Q wave are associated with higher rates of cardiac death or heart transplantation.72 None of these ECG changes correlate with myocarditis and cannot replace the ability of EMB to detect inflammation in patients with myocarditis.73
Echocardiography
There are no specific echocardiographic features of myocarditis and it is used mainly to exclude other causes of heart failure such as valvular, congenital, or pericardial CVD.2 Impaired right ventricular function is a strong predictor of death or the need for cardiac transplantation and has been reported in 23% of patients with biopsy proven myocarditis.74 Patients with fulminant myocarditis tend to present with normal cardiac chamber dimensions and thickened walls compared with patients with less acute myocarditis who have greater left ventricular dilation and normal wall thickness.75 Thus, echocardiography has value in classifying patients with acute myocarditis and may provide prognostic information.
Cardiac MRI
Cardiac MRI (CMR) is useful to distinguish ischaemic from nonischaemic cardiomyopathy in the setting of acute DCM.76 Criteria suggested by an expert panel recommended that both T1 and T2 weighted imaging be used to obtain optimal sensitivity and specificity when myocarditis is suspected (table 2).77 When two or more of these ‘Lake Louise’ criteria are positive, acute myocardial inflammation can be predicted with a diagnostic accuracy of 78%. CMR in acute myocarditis may demonstrate regional rather than global involvement that evolves into inferolateral myocardial delayed enhancement, with sparing of the subendocardium.78,79 T1 weighted sequences obtained about 10 min after gadolinium injection (delayed) are perhaps the most reproducible, but not the most specific, for acute myocarditis.79 The combined application of CMR and EMB may yield diagnostic synergy (95%) and overcome some limitations of CMR or EMB as individually applied techniques.80
Table 2.
Overview of the diagnostic accuracy of several combinations of tissue criteria
| Validation | Sens | Spec | Acc | PPV | NPV | |
|---|---|---|---|---|---|---|
| T2 + LGE | ||||||
| Abdel-Aty et al, 2005 | Clinical | 40% | 100% | 69% | 100% | 61% |
| Gutberlet et al, 2008 | Histology | 17% | 91% | 48% | 73% | 44% |
| Pooled data (n=130) | 25% | 95% | 56% | 86% | 50% | |
| T2 and/or LGE | ||||||
| Abdel-Aty et al, 2005 | Clinical | 88% | 74% | 81% | 100% | 85% |
| Gutberlet et al, 2008 | Histology | 50% | 57% | 52% | 80% | 25% |
| Pooled data (n=130) | 60% | 66% | 62% | 79% | 43% | |
| Any one of three | ||||||
| Abdel-Aty et al, 2005 | Clinical | 100% | 48% | 75% | 68% | 100% |
| Gutberlet et al, 2008 | Histology | 81% | 49% | 67% | 68% | 65% |
| Pooled data (n=130) | 88% | 48% | 70% | 68% | 76% | |
| Any two of three | ||||||
| Abdel-Aty et al, 2005 | Clinical | 76% | 96% | 85% | 95% | 79% |
| Gutberlet et al, 2008 | Histology | 63% | 89% | 73% | 88% | 63% |
| Pooled data (n=130) | 67% | 91% | 78% | 91% | 69% | |
Table modified from Hundley et al.77
Acc, accuracy; LGE, late gadolinium enhancement; NPV, negative predictive value; PPV, positive predictive value; Sens, sensitivity; Spec, specificity.
In the setting of myocarditis mimicking a suspected acute myocardial infraction with normal coronary arteries, CMR may demonstrate extensive TI enhancement in delayed images after gadolinium injection.81 Over the course of several months, the enhancement may decrease in intensity and distribution, with normalisation of left ventricular function.
FUTURE DIRECTIONS
The use of gene expression array technologies and proteomics will be valuable for identifying integrated biomarkers as well as pathogenic mechanisms that can be explored to improve interventions and therapy.51 A better understanding of the role of sex hormones on disease pathogenesis is needed. There is also a need for integrated biomarkers that will identify myocarditis at an earlier stage and predict development of chronic DCM. To improve our understanding of the pathogenesis of disease more translational studies between the clinic and animal researchers is required.
CONCLUSIONS
In the past decade, the diagnosis and prognosis of acute myocarditis has improved by using a combination of imaging and newer immunohistological staining techniques. Researchers using animal models of myocarditis have gained a better understanding of the pathogenesis of disease, particularly the importance of the sex hormone testosterone and innate immune mechanisms in driving the disease. There continues to be a need for better tools to assess the early stage of myocarditis/DCM and biomarkers that will more accurately identify individuals susceptible to develop DCM and heart failure.
Acknowledgments
Funding Dr Fairweather is supported by funding from the National Institutes of Health (R01 HL087033). Dr Cooper is supported by funding from the National Institutes of Health (R01 HL56267, Co-PI).
Footnotes
Contributors We certify that this manuscript has not been published or is being considered for publication elsewhere.
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Cooper LT., Jr Myocarditis. N Engl J Med. 2009;360:1526–38. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blauwet L, Cooper LT. Myocarditis. Prog Cardiovasc Dis. 2010;52:274–88. doi: 10.1016/j.pcad.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hahn EA, Hartz VL, Moon TE, et al. The Myocarditis Treatment Trial: design, methods and patients enrollment. Eur Heart J. 1995;16(Suppl O):162–7. doi: 10.1093/eurheartj/16.suppl_o.162. [DOI] [PubMed] [Google Scholar]
- 4.Maron BJ, Doerer JJ, Haas TS, et al. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation. 2009;119:1085–92. doi: 10.1161/CIRCULATIONAHA.108.804617. [DOI] [PubMed] [Google Scholar]
- 5.Gupta S, Markham DW, Drazner MH, et al. Fulminant myocarditis. Nat Clin Pract Cardiovasc Med. 2008;5:693–706. doi: 10.1038/ncpcardio1331. [DOI] [PubMed] [Google Scholar]
- 6.Kuhl U, Pauschinger M, Seeberg B, et al. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation. 2005;112:1965–70. doi: 10.1161/CIRCULATIONAHA.105.548156. [DOI] [PubMed] [Google Scholar]
- 7.Cihakova D, Sharma RB, Fairweather D, et al. Animal models for autoimmune myocarditis and autoimmune thyroiditis. Methods Mol Med. 2004;102:175–93. doi: 10.1385/1-59259-805-6:175. [DOI] [PubMed] [Google Scholar]
- 8.Yajima T, Knowlton KU. Viral myocarditis from the perspective of the virus. Circulation. 2009;119:2615–24. doi: 10.1161/CIRCULATIONAHA.108.766022. [DOI] [PubMed] [Google Scholar]
- 9.Fairweather D, Frisancho-Kiss S, Gatewood S, et al. Mast cells and innate cytokines are associated with susceptibility to autoimmune heart disease following coxsackievirus B3 infection. Autoimmunity. 2004;37:131–45. doi: 10.1080/0891693042000196200. [DOI] [PubMed] [Google Scholar]
- 10.Frisancho-Kiss S, Davis SE, Nyland JF, et al. Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol. 2007;178:6710–14. doi: 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]
- 11.Lane JR, Neumann DA, Lafond-Walker A, et al. Interleukin 1 or tumor necrosis factor can promote caxsackievirus B-3-induced myocarditis in resistant B10. A mice. J Exp Med. 1992;175:1123–9. doi: 10.1084/jem.175.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang P, Cox CJ, Alvarez KM, et al. Cutting edge: cardiac myosin activates innate immune responses through TLRs. J Immunol. 2009;183:27–31. doi: 10.4049/jimmunol.0800861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res. 2011;108:1133–45. doi: 10.1161/CIRCRESAHA.110.226936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vallejo JG. Role of Toll-like receptors in cardiovascular diseases. Clin Sci (Lond) 2011;121:1–10. doi: 10.1042/CS20100539. [DOI] [PubMed] [Google Scholar]
- 15.Satoh M, Nakamura M, Akatsu T, et al. Expression of Toll-like receptor 4 is associated with enteroviral replication in human myocarditis. Clin Sci (Lond) 2003;104:577–84. doi: 10.1042/CS20020263. [DOI] [PubMed] [Google Scholar]
- 16.Fairweather D, Yusung S, Frisancho S, et al. IL-12Rβ1 and TLR4 increase IL-1β and IL-18-associated myocarditis and coxsackievirus replication. J Immunol. 2003;170:4731–7. doi: 10.4049/jimmunol.170.9.4731. [DOI] [PubMed] [Google Scholar]
- 17.Nishikubo K, Imanaka-Yoshida K, Tamaki S, et al. Th1-type immune responses by Toll-like receptor 4 signaling are required for the development of myocarditis in mice with BCG-induced myocarditis. J Autoimmun. 2007;29:146–53. doi: 10.1016/j.jaut.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Riad A, Westermann D, Zietsch C, et al. TRIF is a critical survival factor in viral cardiomyopathy. J Immunol. 2011;186:2561–70. doi: 10.4049/jimmunol.1002029. [DOI] [PubMed] [Google Scholar]
- 19.Riad A, Westermann D, Escher F, et al. Myeloid differentiation factor-88 contributes to TLR9-mediated modulation of acute coxsackievirus B3-induced myocarditis in vivo. Am J Physiol Heart Circ Physiol. 2010;298:H2024–31. doi: 10.1152/ajpheart.01188.2009. [DOI] [PubMed] [Google Scholar]
- 20.Abston ED, Coronado MJ, Bucek A, et al. Th2 regulation of viral myocarditis in mice: different roles for TLR3 vs. TRIF progression chronic disease. Clin Dev Immunol. 2012;2012:129486. doi: 10.1155/2012/129486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herskowitz A, Wolfgram LJ, Rose NR, et al. Coxsackievirus B3 murine myocarditis: a pathologic spectrum of myocarditis in genetically defined inbred strains. J Am Coll Cardiol. 1987;9:1311–19. doi: 10.1016/s0735-1097(87)80471-0. [DOI] [PubMed] [Google Scholar]
- 22.Onyimba JA, Coronado MJ, Garton AE, et al. The innate immune response to coxsackievirus B3 predicts progression to cardiovascular disease and heart failure in male mice. Biol Sex Differ. 2011;2:2. doi: 10.1186/2042-6410-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huber SA, Job LP. Cellular immune mechanisms in Coxsackievirus group B, type 3 induced myocarditis in Balb/C mice. Adv Exp Med Biol. 1983;161:491–508. doi: 10.1007/978-1-4684-4472-8_29. [DOI] [PubMed] [Google Scholar]
- 24.Cihakova D, Barin JG, Afanasyeva M, et al. Interleukin-13 protects against experimental autoimmune myocarditis by regulating macrophage differentiation. Am J Pathol. 2008;172:1195–208. doi: 10.2353/ajpath.2008.070207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fairweather D, Kaya Z, Shellam GR, et al. From infection to autoimmunity. J Autoimmun. 2001;16:175–86. doi: 10.1006/jaut.2000.0492. [DOI] [PubMed] [Google Scholar]
- 26.Huber S, Shi C, Budd RC. Gammadelta T cells promote a Th1 response during coxsackievirus B3 infection in vivo: role of Fas and Fas ligand. J Virol. 2002;76:6487–94. doi: 10.1128/JVI.76.13.6487-6494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huber SA. γδ T lymphocytes kill T regulatory cells through CD1d. Immunology. 2010;131:202–9. doi: 10.1111/j.1365-2567.2010.03292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frisancho-Kiss S, Coronado MJ, Frisancho JA, et al. Gonadectomy of male BALB/c mice increases Tim-3(+) alternatively activated M2 macrophages, Tim-3(+) T cells, Th2 cells and Treg in the heart during acute coxsackievirus-induced myocarditis. Brain Behav immun. 2009;23:649–57. doi: 10.1016/j.bbi.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blyszczuk P, Kania G, Dieterle T, et al. Myeloid differentiation factor-88/interleukin-1 signaling controls cardiac fibrosis and heart failure progression in inflammatory dilated cardiomyopathy. Circ Res. 2009;105:912–20. doi: 10.1161/CIRCRESAHA.109.199802. [DOI] [PubMed] [Google Scholar]
- 30.Fairweather D, Frisancho-Kiss S, Yusung SA, et al. Interferon-gamma protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-beta 1, interleukin-1 beta, and interleukin-4 in the heart. Am J Pathol. 2004;165:1883–94. doi: 10.1016/s0002-9440(10)63241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fairweather D, Frisancho-Kiss S, Njoku DB, et al. Complement receptor 1 and 2 deficiency increases coxsackievirus B3-induced myocarditis, dilated cardiomyopathy, and heart failure by increasing macrophages, IL-1beta, and immune complex deposition in the heart. J Immunol. 2006;176:3516–24. doi: 10.4049/jimmunol.176.6.3516. [DOI] [PubMed] [Google Scholar]
- 32.Baldeviano G, Barin J, Talor M, et al. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ Res. 2010;106:1646–55. doi: 10.1161/CIRCRESAHA.109.213157. [DOI] [PubMed] [Google Scholar]
- 33.Frisancho-Kiss S, Nyland JF, Davis SE, et al. Cutting Edge: T cell Ig mucin-3 reduces inflammatory heart disease by increasing CTLA-4 during innate immunity. J Immunol. 2006;176:6411–15. doi: 10.4049/jimmunol.176.11.6411. [DOI] [PubMed] [Google Scholar]
- 34.Fairweather D, Cihakova D. Alternatively activated macrophages in infection and autoimmunity. J Autoimmun. 2009;33:222–30. doi: 10.1016/j.jaut.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fairweather D, Rose NR. Coxsackievirus-induced myocarditis in mice: a model of autoimmune disease for studying immunotoxicity. Methods. 2007;41:118–22. doi: 10.1016/j.ymeth.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cihakova D, Rose N. Pathogenesis of myocarditis and dilated cardiomyopathy. Adv Immunol. 2009;99:95–114. doi: 10.1016/S0065-2776(08)00604-4. [DOI] [PubMed] [Google Scholar]
- 37.Cain BS, Meldrum DR, Dinarello CA, et al. Tumor necrosis factor-alpha and interleukin-1beta synergistically depress human myocardial function. Crit Care Med. 1999;27:1309–18. doi: 10.1097/00003246-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 38.Noutsias M, Rohde M, Goldner K, et al. Expression of functional T-cell markers and T-cell receptor Vbeta repertoire in endomyocardial biopsies from patients presenting with acute myocarditis and dilated cardiomyopathy. Eur J Heart Fail. 2011;13:611–18. doi: 10.1093/eurjhf/hfr014. [DOI] [PubMed] [Google Scholar]
- 39.Huber SA. CD1d expression on hemopoietic cells promotes CD4+ Th1 response in coxsackievirus B3 induced myocarditis. Virology. 2006;352:226–36. doi: 10.1016/j.virol.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 40.Yuan J, Cao AL, Yu M, et al. Th17 cells facilitate the humoral immune response in patients with acute viral myocarditis. J clin immunol. 2010;30:226–34. doi: 10.1007/s10875-009-9355-z. [DOI] [PubMed] [Google Scholar]
- 41.Afanasyeva M, Wang Y, Kaya Z, et al. Experimental autoimmune myocarditis in A/J mice is an interleukin-4-dependent disease with a Th2 phenotype. Am J Pathol. 2001;159:193–203. doi: 10.1016/S0002-9440(10)61685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nussinovitch U, Shoenfeld Y. Autoimmunity and heart diseases: pathogenesis and diagnostic criteria. Arch Immunol Ther Exp (Warsz) 2009;57:95–104. doi: 10.1007/s00005-009-0013-1. [DOI] [PubMed] [Google Scholar]
- 43.Lenzo JC, Fairweather D, Cull V, et al. Characterisation of murine cytomegalovirus myocarditis: cellular infiltration of the heart and virus persistence. J Mol Cell Cardiol. 2002;34:629–40. doi: 10.1006/jmcc.2002.2003. [DOI] [PubMed] [Google Scholar]
- 44.Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American heart association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116:2216–33. doi: 10.1161/CIRCULATIONAHA.107.186093. [DOI] [PubMed] [Google Scholar]
- 45.Yilmaz A, Kindermann I, Kindermann M, et al. Comparative evaluation of left and right ventricular endomyocardial biopsy: differences in complication rate and diagnostic performance. Circulation. 2010;122:900–9. doi: 10.1161/CIRCULATIONAHA.109.924167. [DOI] [PubMed] [Google Scholar]
- 46.Aretz HT, Billingham ME, Edwards WD, et al. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1:3–14. [PubMed] [Google Scholar]
- 47.Baughman KL. Diagnosis of myocarditis: death of Dallas criteria. Circulation. 2006;113:593–5. doi: 10.1161/CIRCULATIONAHA.105.589663. [DOI] [PubMed] [Google Scholar]
- 48.Hauck AJ, Kearney DL, Edwards WD. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc. 1989;64:1235–45. doi: 10.1016/s0025-6196(12)61286-5. [DOI] [PubMed] [Google Scholar]
- 49.Wojnicz R, Nowalany-Kozielska E, Wodniecki J, et al. Immunohistological diagnosis of myocarditis. Potential role of sarcolemmal induction of the MHC and ICAM-1 in the detection of autoimmune mediated myocyte injury. Eur Heart J. 1998;19:1564–72. doi: 10.1053/euhj.1998.1085. [DOI] [PubMed] [Google Scholar]
- 50.Kindermann I, Kindermann M, Kandolf R, et al. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118:639–48. doi: 10.1161/CIRCULATIONAHA.108.769489. [DOI] [PubMed] [Google Scholar]
- 51.Heidecker B, Kittleson M, Kasper EK, et al. Transcriptomic biomarkers for the accurate diagnosis of myocarditis. Circulation. 2011;123:1174–84. doi: 10.1161/CIRCULATIONAHA.110.002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimen. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 53.Miller WL, Hartman KA, Burritt MF, et al. Troponin, B-type natriuretic peptides and outcomes in severe heart failure: differences between ischemic and dilated cardiomyopathies. Clin Cardiol. 2007;30:245–50. doi: 10.1002/clc.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lauer B, Niederau C, Kühl U, et al. Cardiac troponin T in patients with clinically suspected myocarditis. J Am Coll Cardiol. 1997;30:1354–9. doi: 10.1016/s0735-1097(97)00317-3. [DOI] [PubMed] [Google Scholar]
- 55.Smith SC, Ladenson JH, Mason JW, et al. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation. 1997;95:163–8. [PubMed] [Google Scholar]
- 56.Chen WC, Tran KD, Maisel AS. Biomarkers in heart failure. Heart. 2010;96:314–20. doi: 10.1136/hrt.2008.151639. [DOI] [PubMed] [Google Scholar]
- 57.Grabowski M, Karpinski G, Filipiak JK, et al. Diagnostic value of BNP in suspected perimyocarditis–a preliminary report (In Polish) Kardiol Pol. 2004;61:451–8. discussion 459–60. [PubMed] [Google Scholar]
- 58.Ogawa T, Veinot JP, Kuroski de Bold ML, et al. Angiotensin II receptor antagonism reverts the selective cardiac BNP upregulation and secretion observed in myocarditis. Am J Physiol Heart Circ Physiol. 2008;294:H2596–603. doi: 10.1152/ajpheart.00215.2008. [DOI] [PubMed] [Google Scholar]
- 59.Fuse K, Kodama M, Okura Y, et al. Predictors of disease course in patients with acute myocarditis. Circulation. 2000;102:2829–35. doi: 10.1161/01.cir.102.23.2829. [DOI] [PubMed] [Google Scholar]
- 60.Seko Y, Kayagaki N, Seino K, et al. Role of Fas/FasL pathway in the activation of infiltrating cells in murine acute myocarditis caused by Coxsackievirus B3. J Am Coll Cardiol. 2002;39:1399–403. doi: 10.1016/s0735-1097(02)01776-x. [DOI] [PubMed] [Google Scholar]
- 61.Nishii M, Inomata T, Takehana H, et al. Serum levels of interleukin-10 on admission as a prognostic predictor of human fulminant myocarditis. J Am Coll Cardiol. 2004;44:1292–7. doi: 10.1016/j.jacc.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 62.Anker SD, von Haehling S. Inflammatory mediators in chronic heart failure: an overview. Heart. 2004;90:464–70. doi: 10.1136/hrt.2002.007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calabrese F, Carturan E, Chimenti C, et al. Overexpression of tumor necrosis factor (TNF) alpha and TNFalpha receptor I in human viral myocarditis: clinicopathologic correlations. Mod Pathol. 2004;17:1108–18. doi: 10.1038/modpathol.3800158. [DOI] [PubMed] [Google Scholar]
- 64.Lauer B, Schannwell M, Kühl U, et al. Antimyosin autoantibodies are associated with deterioration of systolic and. diastolic left ventricular function in patients with chronic myocarditis. J Am Coll Cardiol. 2000;35:11–18. doi: 10.1016/s0735-1097(99)00485-4. [DOI] [PubMed] [Google Scholar]
- 65.Störk S, Boivin V, Horf R, et al. Stimulating autoantibodies directed against the cardiac beta1-adrenergic receptor predict increased mortality in idiopathic cardiomyopathy. Am Heart J. 2006;152:697–704. doi: 10.1016/j.ahj.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 66.Neumann DA, Burek CL, Baughman KL, et al. Circulating heart-reactive antibodies in patients with myocarditis or cardiomyopathy. J Am Coll Cardiol. 1990;16:839–46. doi: 10.1016/s0735-1097(10)80331-6. [DOI] [PubMed] [Google Scholar]
- 67.Li Y, Heuser JS, Cunningham LC, et al. Mimicry and antibody-mediated cell signaling in autoimmune myocarditis. J Immunol. 2006;177:8234–40. doi: 10.4049/jimmunol.177.11.8234. [DOI] [PubMed] [Google Scholar]
- 68.Röhrer C, Gärtner B, Sauerbrei A, et al. Seroprevalence of parvovirus B19 in the German population. Epidemiol Infect. 2008;136:1564–75. doi: 10.1017/S0950268807009958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Modrow S, Dorsch S. Antibody responses in parvovirus B19 infected patients. Pathol Biol (Paris) 2002;50:326–31. doi: 10.1016/s0369-8114(02)00302-4. [DOI] [PubMed] [Google Scholar]
- 70.Mahfoud F, Gartner B, Kindermann M, et al. Virus serology in patients with suspected myocarditis: utility or futility? Eur Heart J. 2011;32:897–903. doi: 10.1093/eurheartj/ehq493. [DOI] [PubMed] [Google Scholar]
- 71.Morgera T, Di Lenarda A, Dreas L, et al. Electrocardiography of myocarditis revisited: clinical and prognostic significance of electrocardiographic changes. Am Heart J. 1992;124:455–67. doi: 10.1016/0002-8703(92)90613-z. [DOI] [PubMed] [Google Scholar]
- 72.Nakashima H, Honda Y, Katayama T. Serial electrocardiographic findings in acute myocarditis. Intern Med. 1994;33:659–66. doi: 10.2169/internalmedicine.33.659. [DOI] [PubMed] [Google Scholar]
- 73.Magnani JW, Dec GW. Myocarditis: current trends in diagnosis and treatment. Circulation. 2006;113:876–90. doi: 10.1161/CIRCULATIONAHA.105.584532. [DOI] [PubMed] [Google Scholar]
- 74.Mendes LA, Dec GW, Picard MH, et al. Right ventricular dysfunction: an independent predictor of adverse outcome in patients with myocarditis. Am Heart J. 1994;128:301–7. doi: 10.1016/0002-8703(94)90483-9. [DOI] [PubMed] [Google Scholar]
- 75.Felker GM, Boehmer JP, Hruban RH, et al. Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol. 2000;36:227–32. doi: 10.1016/s0735-1097(00)00690-2. [DOI] [PubMed] [Google Scholar]
- 76.Hunold P, Schlosser T, Vogt FM, et al. Myocardial late enhancement in contrast-enhanced cardiac MRI: distinction between infarction scar and non-infarction-related disease. AJR Am J Roentgenol. 2005;184:1420–6. doi: 10.2214/ajr.184.5.01841420. [DOI] [PubMed] [Google Scholar]
- 77.Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol. 2009;53:1475–87. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goitein O, Matetzky S, Beinart R, et al. Acute myocarditis: noninvasive evaluation with cardiac MRI and transthoracic echocardiography. AJR Am J Roentgenol. 2009;192:254–8. doi: 10.2214/AJR.08.1281. [DOI] [PubMed] [Google Scholar]
- 79.Hundley WG, Bluemke DA, Finn JP, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 Expert Consensus Document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on expert Consensus Documents. Circulation. 2010;121:2462–508. doi: 10.1161/CIR.0b013e3181d44a8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baccouche H, Mahrholdt H, Meinhardt G, et al. Diagnostic synergy of non-invasive cardiovascular magnetic resonance and invasive endomyocardial biopsy in troponin-positive patients without coronary artery disease. Eur Heart J. 2009;30:2869–79. doi: 10.1093/eurheartj/ehp328. [DOI] [PubMed] [Google Scholar]
- 81.Monney PA, Sekhri N, Burchell T, et al. Acute myocarditis presenting as acute coronary syndrome: role of early cardiac magnetic resonance in its diagnosis. Heart. 2011;97:1312–18. doi: 10.1136/hrt.2010.204818. [DOI] [PubMed] [Google Scholar]