Summary
Mast cells are hematopoietic cells that reside in virtually all vascularized tissues and that represent potential sources of a wide variety of biologically active secreted products, including diverse cytokines and growth factors. There is strong evidence for important non-redundant roles of mast cells in many types of innate or adaptive immune responses, including making important contributions to immediate and chronic IgE-associated allergic disorders and enhancing host resistance to certain venoms and parasites. However, mast cells have been proposed to influence many other biological processes, including responses to bacteria and virus, angiogenesis, wound healing, fibrosis, autoimmune and metabolic disorders, and cancer. The potential functions of mast cells in many of these settings is thought to reflect their ability to secrete, upon appropriate activation by a range of immune or non-immune stimuli, a broad spectrum of cytokines (including many chemokines) and growth factors, with potential autocrine, paracrine, local and systemic effects. In this review, we summarize the evidence indicating which cytokines and growth factors can be produced by various populations of rodent and human mast cells in response to particular immune or non-immune stimuli, and comment on the proven or potential roles of such mast cell products in health and disease.
Keywords: Chemokines, cytokines, growth factors, immunity, inflammation, mast cells
1 INTRODUCTION
Although mast cells (MCs) were described by Paul Ehrlich long ago 1, the appreciation that these cells represent a potential source of diverse cytokines, chemokines, and growth factors is a relatively recent development 2. Early work reported the ability of neoplastic MC lines to produce certain hematopoietic cytokines 3, and subsequent studies provided evidence that both in vitro-derived mouse MCs and purified mouse peritoneal MCs (PMCs) could produce and secrete TNF, both in response to LPS and after activation via the FcεRI 2, 4–6. While most of the TNF secreted by MCs appears to require induction of the corresponding mRNA upon MC activation, there is evidence that some TNF is physically associated with the secretory granules and is thereby ‘preformed’ and ready for more rapid release upon appropriate activation of the cells 2, 5, 6. Human MCs were identified as a potential source of TNF shortly after the finding was reported for mouse MCs 7, and evidence was presented that these cells also could contain preformed stores of the cytokine in their granules 7.
IL-4 was reported to be a potential product of mouse MC lines in 1987 8 and three groups subsequently reported the ability of various populations of in vitro-derived mouse mast cells or long term mouse MC lines to secrete IL-4 and several other cytokines in response to activation via the FcεRI 9–11 and the Burd et al. paper 11 also added a few chemokines to the growing list of cytokines which could be considered as potential products of mouse MCs.
As reviewed herein, the list of cytokines, chemokines, growth factors and mitogens which now have been identified as MC products is very long (Fig. 1, Tables 1 & 2). And while many of these were first identified, at the mRNA or protein level, in in vitro-derived mouse or human MCs, there is evidence that several of these can be considered to be at least potential products of native populations of mouse or human tissue MCs. However, while it can be relatively straightforward to generate evidence that MCs might represent a source of particular cytokines, chemokines, growth factors and mitogens, it is much more difficult to determine the biological importance of MCs as sources of such molecules, particularly in settings where multiple different immune cells and structural cells represent alternative potential sources of the same products.
Figure 1. Highly simplified overview of the diverse stimuli and potential consequences of mast cell activation and secretion of cytokines, chemokines and growth factors.
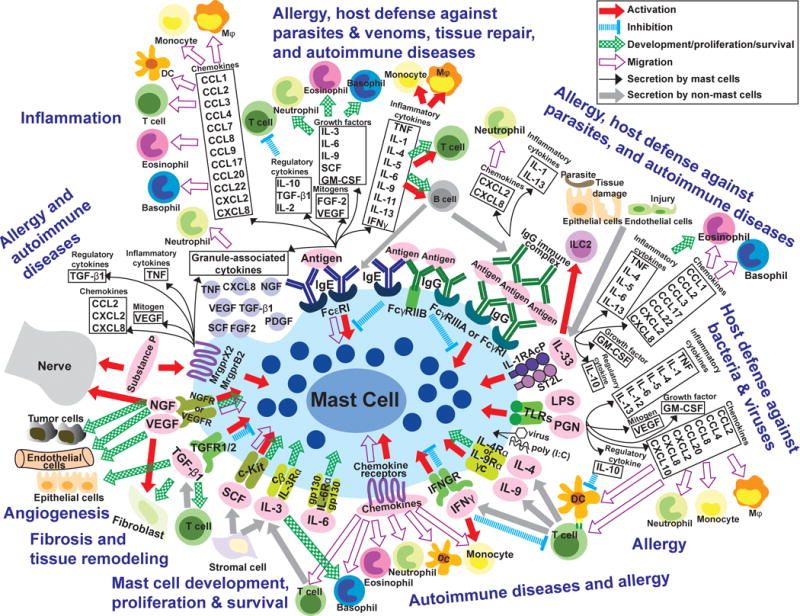
Mast cells (MCs) can be activated through various receptors when they are exposed to the corresponding ligands (e.g., in pink ovals). This can induce MC activation (red arrows), inhibition (blue dotted lines), or migration (purple open arrows), influence MC development/proliferation/survival (green patterned arrows), and/or induce MCs to secrete many cytokines, chemokines and growth factors (black arrows and related boxes). Grey arrows depict secretion of products from cells other than MCs. Depending on the type of stimulus/stimuli, as well as the type/phenotype of the MCs, such activated MCs also may secrete many other stored and/or newly synthesized mediators (not shown). In adaptive immune responses (e.g., elicited by parasites, animal venoms or allergens), MCs can be activated when IgE bound to surface FcεRI receptors is crosslinked by bi- or multivalent antigens, or when immune complexes (IgG-ICs) bind to FcγRs. In some settings, for example in mouse BMCMCs, co-ligation of FcεRI with inhibitory FcγRIIb receptors can down-regulate MC activation 349, 350. FcγRI is a high affinity receptor induced in human MCs by IFNγ stimulation in vitro 231 or in the IFNγ enriched environment of skin MCs in the setting of psoriasis 351. However, this FcγRI expression is observed in humans not in mice. Upon antibody/antigen-mediated stimulation, MCs can synthesize and secrete a panel of factors as indicated in the black boxes. In turn, those factors can influence other immune and non-immune (structural) cells and contribute to pathogenesis of various types of allergic reactions and perhaps autoimmune disorders, such as some forms of arthritis, as well as to host defense against venoms or parasites. Many of the immune and structural cells depicted are comprised of functionally distinct subtypes (e.g., T cells, DCs, macrophages, fibroblasts, nerves) and the effects of particular MC products on such cells may vary importantly depending on the target cell subtype (not shown). In some settings, such MC-derived products also may contribute to tissue repair and remodeling, both through effects on structural cells and by regulating aspects of the inflammatory/immune response. Antibody/antigen-mediated stimulation also can induce MCs to secrete preformed mediators such as histamine, serotonin (in rodents, primarily), proteoglycans, and proteases (not shown), as well as certain cytokines and growth factors which can be granule-associated (black boxes and the purple granules underneath), as well as many lipid mediators including cysteinyl leukotrienes and certain prostaglandins (not shown). IL-33, which is produced by endothelial/epithelial cells in sites of tissue damage, can stimulate MCs to secrete many factors (indicated in the black boxes) with diverse potential effects on other immune and non-immune cells that can contribute to the pathogenesis of allergies and to host defense. Products of pathogens such as LPS (lipopolysaccharide) and PGN (peptidoglycan), poly (I:C), and certain viruses can directly activate MCs through TLRs (toll-like receptors), resulting in the secretion of a variety of factors (as indicated in black boxes); depending on the setting, this could contribute to host defense and/or disease (e.g., there is a well-established clinical association between certain viral infections and exacerbations of asthma). During Th2 cell-associated immune responses, IL-4 or IL-9 from T cells or from immature cells in the MC lineage can activate MCs and promote their development/proliferation. IFNγ can deliver positive or negative signals to MCs, probably depending on species of animal, MC subpopulation, and setting (such as a disease or a particular beneficial host response). MCs can migrate in response to certain chemokines, but MCs also can be activated by chemokines. IL-3 and SCF (stem cell factor) are representatives of factors which support MC development, proliferation and/or survival (others include, depending on the MCs, IL-4, IL-6, IL-9, and NGF). IL-3 can have similar effects on basophils. NGF (nerve growth factor), VEGF (vascular endothelial growth factor), FGFs (fibroblast growth factors), and TGF-β1 (transforming growth factor type-β) can contribute to the development of fibrosis or angiogenesis, and there is some evidence indicating that these factors, like TNF (tumor necrosis factor), can be constitutively stored in the granules of some MCs. These factors also can influence MCs (as indicated with arrows). Substance P is a product of certain neurons that can potently activate some types of MCs, which in turn can secrete preformed mediators that may include granule-associated cytokines (as indicated in the black boxes). Bidirectional interactions between certain nerve cells and MCs have been studied extensively, and there is considerable interest in the potential importance of such nerve-MC interactions in health and disease. Finally, it should be kept in mind that proteases released from activated MCs can degrade TNF 61, IL-1β 352, IL-18 353, IL-33 209, 211, SCF 354, CCL5 and CCL11 355, CCL26 356, and likely other factors shown in the figure, and this may represent an important mechanism by which MCs can control the intensity and duration of the biological effects of such factors. Please see Tables 1 and 2 for additional information about how variation in MC subtype may influence the extent to which these cells can produce and/or respond to the factors shown in the figure.
Table 1.
Mast cell-derived Cytokines, Growth Factors & Mitogens
| Factor | Stimulus | Product (if not the protein) | Mast cell type(s) | References |
|---|---|---|---|---|
| TNF | Anti-IgE, IgE/Ag | Mouse BMCMCs, PMCs, cell lines | 2, 5, 6, 51 | |
| Constitutive | Human skin | 7 | ||
| Morphine sulfate | Human skin | 7 | ||
| Anti-IgE | Human skin | 21 | ||
| Anti-IgE | Human lung | 22 | ||
| Substance P | Human skin | 23 | ||
| Anti-IgE | Human skin | 27 | ||
| SCF | Human skin | 27 | ||
| A23187 | Human skin | 27 | ||
| Compound 48/80 | Human skin | 27 | ||
| Substance P | Human skin | 27 | ||
| UVB | Human skin | 26 | ||
| LPS | Mouse BMCMCs, rat PMCs | 357, 358 | ||
| PGN | Mouse BMCMCs | 57, 222 | ||
| LPS | Mouse BMCMCs | 57, 222 | ||
| LPS or PGN | IL-4 pretreated human CBMCs | 58 | ||
| IL-1β | IgE/Ag | Mouse BMCMCs, cell lines | 11 | |
| IgG receptor crosslinking: anti-FcγRII/III (2.4G2)+anti-rat F(ab’)2 | Mouse BMCMCs | 66 | ||
| IgE/Ag | Mouse BMCMCs | 66 | ||
| Ionophore | Mouse BMCMCs | 66 | ||
| Constitutive | Mouse in vivo | 70 | ||
| Constitutive | Mouse in vivo | 69 | ||
| LPS+ATP or R837 | Mouse BMCMCs | 67, 68 | ||
| LPS, PGN, Zymosan, PamCys | Human CBMCs | 222 | ||
| LPS, PGN | Mouse BMCMCs | 57 | ||
| IL-2 | IgE/Ag or PMA+ ionomycin | Mouse BMCMCs, PDMCs | 72 | |
| IL-33 | Mouse BMCMCs | 73 | ||
| IL-9 | Mouse lung MCs | 74 | ||
| IL-3 | IgE/Ag | Mouse BMCMCs | 10 | |
| IgE/anti-IgE | mRNA | Human BM-derived | 109 | |
| Constitutive | Human gastroduodenal | 111 | ||
| IL-4 | Constitutive | mRNA, bioactivity | Mouse cell lines | 8 |
| IgE/Ag | Mouse BMCMCs | 84 | ||
| Ionophore | mRNA is constitutive; protein is detected after stimulation | Mouse BMCMCs | 83 | |
| IgE/Ag or ionophore | Mouse cell lines | 9 | ||
| PGN | Mouse BMCMCs | 57 | ||
| LPS | mRNA | Mouse BMCMCs | 88 | |
| Constitutive | Human nasal, bronchial, skin | 89–92 | ||
| Anti-IgE | Human skin | 23 | ||
| IL-33 | Mouse PMCs | 85 | ||
| IL-5 | IgE/Ag or ionophore | mRNA | Mouse cell lines | 9 |
| IgE/Ag or ionophore | mRNA | Mouse BMCMCs, cell lines (Cl.MC/9, Cl.MC/C57.1) | 11 | |
| Anti-IgE | mRNA | Rat PMCs | 108 | |
| IgE/anti-IgE | Human bone marrow-derived | 109 | ||
| IgE/Ag or LPS | Mouse BMCMCs | 107 | ||
| IL-33 | mRNA | Mouse BMCMCs | 110 | |
| IL-33 | Human PBMCs, CBMCs | 191 | ||
| LPS or PGN | Human CBMCs | 58 | ||
| PGN | Mouse BMCMCs | 57 | ||
| Constitutive | Human airway | 90, 92 | ||
| Constitutive | Human gastroduodenal | 111 | ||
| IL-6 | IgE/Ag or ionophore | Mouse BMCMCs, cell lines | 11 | |
| IgE/Ag or ionophore | Mouse cell lines | 9 | ||
| Anti-IgE or LPS | Rat PMCs | 122 | ||
| Constitutive | Mouse BMCMCs | 359 | ||
| Constitutive | Human bronchial, nasal | 90, 93 | ||
| Constitutive | Mouse in vivo | 126 | ||
| Constitutive | Mouse in vivo | 127 | ||
| IL-33 | Human PBMCs, CBMCs | 191 | ||
| IL-9 | IgE/Ag | Mouse BMCMCs | 135 | |
| IgE/Ag or ionophore | Mouse BMCMCs | 137 | ||
| IL-1β | Mouse BMCMCs | 136 | ||
| IL-10 | LPS, lipid A +/− IgE/Ag | Mouse BMCMCs | 107 | |
| IL-33 | Human PBMCs, CBMCs | 191 | ||
| IL-11 | Anti-IgE | Human CBMCs | 179 | |
| IL-12 | LPS+IFNγ | Mouse BMCMCs | 182 | |
| LPS+SCF | Human PBMCs | 87 | ||
| Constitutive | mRNA | SCF derived-BMCMCs | 183 | |
| IL-13 | IgE/Ag or ionomycin or PMA | mRNA and bioactivity | Mouse BMCMCs, C1.MC/C57.1 cell line | 188 |
| LPS or PGN | Human CBMCs | 58 | ||
| LPS or PGN | Mouse BMCMCs | 57 | ||
| LPS or Lipid A | Mouse BMCMCs | 107 | ||
| IL-33 | Mouse BMCMCs | 73, 125, 192 | ||
| IL-33 | Human PBMCs, CBMCs | 191 | ||
| SCF | Mouse BMCMCs | 193 | ||
| IgG receptor crosslinking | Mouse BMCMCs | 360 | ||
| IL-1β | Human CBMCs | 190 | ||
| TSLP | Human CBMCs | 361 | ||
| IL-16 | Constitutive | Human BM-derived & lung | 199 | |
| IL-33 | IgE/Ag | Mouse BMCMCs | 110 | |
| PMA+ ionomycin | mRNA | Mouse BMCMCs | 208 | |
| EGF | Human thyroid | 214 | ||
| bFGF/FGF-2 | Constitutive | Human dermal | 215 | |
| Constitutive | Human cutaneous | 362 | ||
| Constitutive | Human lung and skin | 217 | ||
| Constitutive | Human thyroid | 214 | ||
| Constitutive | Rat PMCs | 34 | ||
| IL-17A | Human PBMCs | 219 | ||
| Constitutive | Mouse BMCMCs | 220 | ||
| Constitutive | Human skin | 218 | ||
| GM-CSF | IL-33 | Human PBMCs, CBMCs | 191 | |
| LPS, PGN, Zymosan, PamCys | Human CBMCs | 222 | ||
| IgE or IL-33 | Mouse BMCMCs | 41 | ||
| IgE/Ag | Mouse BMCMCs | 10 | ||
| IgE/Ag | mRNA | Mouse cell lines | 11 | |
| IgE/anti-IgE | Human BM-derived | 109 | ||
| Constitutive | Human gastroduodenal | 111 | ||
| P. aeruginosa | Human CBMCs | 363 | ||
| IFNγ | IL-12 | Rat PMCs | 184 | |
| Anti-IgE | mRNA | Rat PMCs | 108 | |
| IgE/Ag | mRNA | Mouse cell lines | 11 | |
| Ionophore | mRNA | Mouse cell lines | 9 | |
| Anti-IgE | mRNA | Rat PMCs | 108 | |
| Constitutive | Mouse in vivo | 126 | ||
| Constitutive | Mouse in vivo | 127 | ||
| NGF | Constitutive | Rat PMCs | 262 | |
| Constitutive | mRNA | Human CBMCs | 257 | |
| PDGF | Human thyroid | 214 | ||
| Coculture with cardiac myocytes or fibroblasts | Mouse BMCMCs | 267 | ||
| SCF | Constitutive | Human lung and skin | 275 | |
| Constitutive, IgE/anti-IgE, ionophore | Human skin, CBMCs, PBMCs | 277 | ||
| Anti-IgE | Human lung and skin | 278 | ||
| Constitutive | Human cardiac | 276 | ||
| Constitutive | Human mastocytosis in bone marrow | 279 | ||
| TGF-β1 | IgE/Ag | Mouse BMCMCs, cell lines, mouse PMCs | 51 | |
| Constitutive, phorbol ester | Dog mastocytoma | 288 | ||
| Compound 48/80 | Rat PMCs | 289 | ||
| N/A | Human CBMCs | 290 | ||
| IL-33 | Mouse BMCMCs | 73 | ||
| IL-9 | Mouse lung MCs | 74 | ||
| VEGF/VPF | Constitutive | Human skin | 318 | |
| Constitutive | Rat small intestine | 364 | ||
| IgE/Ag, PMA, A23187, SCF | BMCMCs | 317 | ||
| PMA | Mouse PMCs | 317 | ||
| Anti-IgE | Human CBMCs | 317 | ||
| Substance P, IL-1, Substance P+ IL-33, IL-1+ L-33 | Human CBMCs | 319 | ||
| Corticotropin-releasing hormone | Human CBMCs | 320 | ||
| Constitutive | Mouse in vivo | 336 | ||
| Constitutive | Human thyroid | 214 | ||
| Constitutive | Rat PMCs | 34 | ||
| Constitutive | Human, in laryngeal squamous cell carcinoma | 330 | ||
| Constitutive | Human, in malignant melanoma | 331 | ||
| Constitutive | mRNA | Human, in non-Hodgkin lymphoma | 332 | |
| IL-17A | Human PBMCs | 219 | ||
| Live S. aureus | Mouse PMCs | 321 |
BMCMCs: Mouse bone marrow-derived cultured mast cells (these are reported by many groups as “BMMCs” – referring to “bone marrow-derived mast cells”, but we prefer BMCMCs to refer to such cells as this is more specific and emphasizes that the cells have been derived in vitro.
CBMCs: Human umbilical cord blood-derived mast cells
PBMCs: Human peripheral blood-derived mast cells
PMCs: Peritoneal mast cells (from mice or rats, as noted)
PDMCs: Peritoneal-derived cultured mast cells.
Constitutive: There is evidence, such as from IHC or detection of mRNA, that the analyzed mast cells can constitutively express that factor under “baseline” conditions.
In the Product column: Detected product was the protein unless “mRNA” is listed, which indicates that the mRNA for that product was identified, but not yet the protein.
In the References column: Papers listed include first reports and/or those with key data.
Table 2.
Mast cell-derived Chemokines
| Factor | Stimulus | Product (if not the protein) | Mast cell type(s) | References |
|---|---|---|---|---|
| CCL1 | IgE/Ag | mRNA | Mouse cell lines | 11 |
| IL-33 | Human PBMCs or CBMCs | 191 | ||
| IgE/Ag or IL-33 | mRNA | Mouse BMCMCs | 365, 366 | |
| IgE/Ag | Mouse liver-derived | 367 | ||
| IgE/Ag | Human skin | 365 | ||
| CCL2 | IgE/Ag | mRNA | Mouse cell lines | 11 |
| IgE/Ag or IL-33 | Mouse BMCMCs, Human skin | 192 | ||
| PMA/ionophore, IgE/anti-IgE, IL-1β | Human CBMCs | 190 | ||
| IL-33 | Human PBMC or CBMCs | 191 | ||
| Poly(I:C) | Human CBMCs | 368 | ||
| CCL3 | IgE/Ag | mRNA | Mouse cell lines | 11 |
| IgE/Ag | Mouse liver-derived | 367 | ||
| IL-33 | Mouse BMCMCs | 192 | ||
| IgG receptor crosslinking | Mouse BMCMCs | 360 | ||
| Anti-IgE | Human CBMCs | 369 | ||
| CCL4 | IgE/Ag | mRNA | Mouse cell lines | 11 |
| In vivo | Mouse skin | 370 | ||
| Poly(I:C) | Human CBMCs | 368 | ||
| Dengue virus + Anti-dengue Ab | Human CBMCs | 371 | ||
| RSV | Human CBMCs | 372 | ||
| CCL5 | Human skin | 373 | ||
| Dengue virus + Anti-dengue Ab | Human CBMCs | 371 | ||
| RSV | Human CBMCs | 372 | ||
| CCL7 | IgE/Ag | Mouse BMCMCs | 374 | |
| Anti-FcεRIα Ab | mRNA | Human PBMCs | 375 | |
| CCL8 | LPS | mRNA | Human PBMCs | 233 |
| IgE/Ag or PMA/ionophore | mRNA | Mouse cell lines | 376 | |
| CCL9 | IgE/Ag | Mouse liver-derived | 367 | |
| CCL11 | Human skin | 373 | ||
| CCL17 | IL-33 | Human PBMCs or CBMCs | 191 | |
| Anti-FcεRIα Ab | mRNA | Human PBMCs | 375 | |
| mRNA | Human skin | 377 | ||
| CCL20 | Anti-FcεRIα Ab | mRNA | Human PBMCs | 375 |
| P. aeruginosa | Human CBMCs | 363 | ||
| CCL22 | IL-33 | Human PBMCs or CBMCs | 191 | |
| Anti-FcεRIα Ab | mRNA | Human PBMCs | 375 | |
| mRNA | Human skin | 377 | ||
| human CXCL2 | Anti-IgE | Human skin | 378 | |
| IgG immune complexes | Human synovium-derived | 379 | ||
| Anti-IgE, SCF, Substance P, Compound 48/80 or A23187 | Human skin | 27 | ||
| LPS+SCF | Human PBMCs | 87 | ||
| mouse CXCL8 | Anti-IgE | mRNA | Rat PMCs | 108 |
| IL-33 | Human PBMC or CBMC | 191 | ||
| Poly(I:C) | Human CBMCs | 368 | ||
| Substance P or ionomycin | Human CBMCs | 380 | ||
| CXCL10 | Poly(I:C) | Human CBMCs | 368 | |
| RSV | Human CBMCs | 372 |
BMCMCs: Mouse bone marrow-derived cultured mast cells (these are reported by many groups as “BMMCs” – referring to “bone marrow-derived mast cells”, but we prefer BMCMCs to refer to such cells as this is more specific and emphasizes that the cells have been derived in vitro.
CBMCs: Human umbilical cord blood-derived mast cells.
PBMCs: Human peripheral blood-derived mast cells.
PMCs: Peritoneal mast cells (from mice or rats, as noted).
In the Product column: Detected product was the protein unless “mRNA” is listed, which indicates that the mRNA for that product was identified, but not yet the protein.
In the References column: Papers listed include first reports and/or those with key data.
In this review, we describe the evidence that MCs represent potential sources of cytokines (including chemokines), growth factors and mitogens, and mention the potential or proven roles of these products, e.g., as “pro-inflammatory” or “regulatory” cytokines; or influencing MC development, survival and/or proliferation; or functioning as mitogens or chemokines. As noted in Tables 1 & 2, much of the evidence that MCs can secrete such products is derived from studies of in vitro-derived mouse or human MCs, or MCs purified from mouse body fluids or mouse or human tissues. Importantly, we note that in many instances it has not yet been confirmed whether, and under which circumstances, MCs can secrete such products in vivo. Some of the many considerations to be kept in mind when evaluating the evidence that MCs represent potentially important sources of particular cytokines, chemokines, growth factors or mitogens are listed in Table 3.
Table 3.
Principles of MC biology to keep in mind when assessing the importance of MCs as sources of cytokines, chemokines and growth factors*.
|
All of these factors may influence the MC’s ability to produce cytokines, chemokines and growth factors, and/or to produce and secrete proteases and other factors that can influence the structure and bioactivity of these molecules.
Immunohistochemistry (IHC) has often been used to identify cytokines and growth factors in MCs in tissue sections, and the identification of such immunoreactivity is of interest (e.g., it can be taken to represent one line of evidence that some of the products so identified can be stored in or associated with the MC’s cytoplasmic granules). However, this approach by itself can’t determine the importance of MCs as a source of these molecules, which typically also can be produced by many other cell types. Moreover, in many of these “non-MC” sources of these products, the cytokines or growth factors may be rapidly secreted and therefore provide little signal for detection of cell-associated product by IHC.
Accordingly, when it is available, we have commented on more direct lines of evidence that MC production of particular cytokines, chemokines, growth factors or mitogens has relevance for understanding the roles of MCs in vivo. We think that the reader will appreciate that while we are still far from understanding the importance of most of these products in contributing to critical roles of MCs in physiological or pathological processes, we now have many interesting possibilities to assess, as well as increasingly powerful tools to provide more definitive answers to these questions.
2 MAST CELL-DERIVED CYTOKINES, GROWTH FACTORS, AND MITOGENS
2.1 TNF (tumor necrosis factor)
History
TNF (tumor necrosis factor/cachectin) was first described by Carswell et al in 1975 12 as a factor found in the serum of bacillus Calmette-Guerin (BCG)-infected mice that induces tumor necrosis. This and several other studies showed that TNF can be released from macrophages upon endotoxin stimulation 13–15. Later, evidence was reported that some MC lines (C57.1, 2D4, t1C9, AI, RBL-2H3, PT18) 4, 16, IL-3-maintained bone marrow-derived cells (which were reported to be “natural cytotoxic cells”, but in retrospect almost certainly were MCs 17), IL-3-derived mouse bone marrow-derived MCs (BMCMCs) and purified rat or mouse PMCs 4, rat connective type MCs 18, and human bone marrow-derived “basophils/MCs” 19 also can have a bioactivity capable of lysing certain types of tumor cell lines, such as the sarcoma WEHI-164, and that one of the factors responsible for causing such cytotoxicity had properties similar to that of TNF.
Subsequently, Gordon and Galli 5 showed that freshly isolated mouse peritoneal MCs (PMCs) constitutively express preformed TNF that can be released rapidly and can mediate TNF bioactivity. Various MCs also can exhibit enhanced TNF gene expression upon IgE-dependent activation 5, 16, 20–23, as shown by increased levels of TNF mRNA in Northern blots 5, 20, 22. Furthermore, TNF mRNA expression and TNF production have been detected in a mouse mastocytoma cell line, MMC-1, after FcγR activation 24 as well as in an IL-3-dependent mouse mast cell line, CFTL12 25 and in human skin MCs 23 after stimulation with substance P.
2.1.2 Preformed TNF
The ability of some populations of MCs to contain preformed TNF, which can be released rapidly from the cells upon their appropriate activation, identifies MCs as one of the first potential sources of this cytokine during innate or adaptive immune responses. Early work provided evidence that the TNF released by MCs for the first ~10 minutes after IgE-dependent stimulation was derived from a preformed pool and that at later time points TNF is secreted from a newly synthesized pool 6, 20; findings consistent with this conclusion also were reported for human skin MCs after their exposure to UVB 26 or anti-IgE, substance P, stem cell factor (SCF), A23187, or compound 48/80 27. De novo TNF synthesis in MCs takes several hours and appears to require mitochondrial translocation near the sites of exocytosis 28.
Evidence supporting the conclusion that MCs represent a source of “early TNF” in vivo was obtained in studies of immune complex peritonitis in genetically MC-deficient KitW/W-v mice and the corresponding control (Kit+/+) mice. In this model, rapid TNF secretion from MCs was thought to help to initiate inflammation by recruiting neutrophils into peritoneum 29. Such rapidly released TNF can induce endothelial-leukocyte adhesion molecule 1 (ELAM-1), intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion protein 1 (VCAM-1) on vascular endothelial cells in vitro 7, 26, 30, which represents one MC-TNF-dependent mechanism for enhancing the adhesion and recruitment of neutrophils and other leukocytes to sites of MC activation. Indeed, helping to initiate local inflammation during innate and adaptive immune responses may be one of the most important functions of the TNF rapidly released from suitably stimulated MCs, and one of the major mechanisms by which MCs function as sentinels during such host responses.
The molecular mechanisms which affect the storage of TNF within MC cytoplasmic granules remain to be fully elucidated. However, there is evidence from work with rat basophil leukemia cells (RBL cells) and in vitro-derived mouse MCs that TNF travels from the endoplasmic reticulum (ER) to the cytoplasmic granules at least in part through N-linked glycosylation of TNF and via a mannose-6-phosphate receptor (MPR)-dependent pathway (RBL-2H3) 31. By contrast, human MCs (LAD2 MCs and the human MC leukemia cell line, HMC-1) appear to employ a different mechanism, which does not involve glycosylation of the TNF and therefore is carbohydrate independent, and that involves a pathway by which the newly formed TNF is transiently exposed to the extracellular space and then is followed by endocytosis 32.
2.1.3 Roles of MC-derived TNF
In principle, MC-derived TNF could contribute to any biological response that is influenced by that cytokine. However, attention has focused mainly on the role of MC-derived TNF in various inflammatory responses. An early idea was that the cytotoxicity mediated by MC-derived TNF might play a role in tumor regression. However, while it has long been known (since Ehrlich’s time 1) that numbers of MCs are increased in various types of tumors (reviewed in 33), the roles of MCs in such settings, let alone MC-derived TNF, are largely yet to be determined 33–35. MCs have the potential to secrete a wide spectrum of cytokines, growth factors and other mediators that can have positive or negative effects on tumors and their relationship to the microenvironment (e.g., see Table 1), and TNF is just one among many such products.
By contrast, several lines of evidence indicate that MC-derived TNF can contribute to leukocyte recruitment at sites of inflammation. Studies employing a neutralizing antibody to TNF indicated that this cytokine can promote leukocyte recruitment into sites of IgE- and MC-dependent passive cutaneous anaphylaxis in mice 36, 37. In certain delayed hypersensitivity responses elicited in the skin of mice, there is evidence that MC-derived TNF, as well as the MC-derived chemokine, macrophage inflammatory protein 2 (MIP-2), can contribute to neutrophil recruitment 38. In antigen-induced neutrophil infiltration into the airways of ovalbumin (OVA)-specific TCR-expressing OTII mice, there was evidence that MC-derived TNF can contribute to neutrophil recruitment in a Th17-cell dependent manner 39.
In mouse models of hapten-induced contact hypersensitivity, two groups provided evidence that MC-derived TNF can contribute to the migration of skin dendritic cells 40, 41 and airway dendritic cells 40 to the draining lymph nodes. Those findings, which indicated that MC-derived TNF can modulate DC function and thus adaptive immunity, were generated using adoptive transfer approaches to compare the function of TNF+/+ vs. TNF−/− MCs in vivo after their engraftment into the tissues of Kit mutant MC-deficient mice. Recently, studies employing Mcpt5-CreTNFfl/fl mice, in which TNF is specifically deleted in MCs under the control of the Mcpt5 promoter, have confirmed the findings obtained using MC-engrafted Kit mutant mice and identified CD8+ DCs as the main target cells of MC-derived TNF in this setting 42.
Notably, Kunder et al. 43 identified a previously unknown mechanism by which MC-derived TNF can help to initiate adaptive immune responses. Specifically, Kunder et al. 43 reported evidence that the TNF associated with exteriorized MC cytoplasmic granule structures can be transported in such granules via lymphatics, thus traveling from sites of local cutaneous inflammation, in this case induced by the injection of E. coli bacteria into the mouse footpad, to the draining lymph nodes. This provided a mechanism to explain the group’s prior observation that such MC activation by E. coli results in hypertrophy of the draining lymph nodes and the promotion of an adaptive immune response to the bacteria 44. Subsequently, Gaudenzio et al. 45 reported evidence that IgE-dependent MC activation in the mouse footpad also can result in the transport of exteriorized MC cytoplasmic granules to the draining lymph nodes and the induction of their enlargement. Finally, there is also a report that, in vitro, TNF derived from MCs upon IgE and antigen stimulation can enhance T cell activation by increasing their expression of OX40 (also known as tumor necrosis factor receptor superfamily, member 4 [TNFRSF4] and CD134) 46. These studies highlight the potentially diverse and non-mutually exclusive mechanisms by which MC-derived TNF can influence adaptive immunity, and we think it likely that additional mechanisms remain to be discovered.
MC-derived TNF can also influence non-immune cells. In a mouse model of oxazolone-induced contact hypersensitivity, there is evidence that MC-derived TNF can contribute to nerve elongation, perhaps via induction of nerve growth factor (NGF) production by keratinocytes 47. Close association between MCs and nerves is often observed in inflammatory skin lesions 48–50, and further studies are needed to elucidate the molecular mechanisms which underlie functional associations between MCs, MC-derived TNF (and other MC-derived mediators) and nerves in this and other settings.
In mice, there is evidence that, after MCs are activated with IgE and antigen in vivo or in vitro, MC-derived TNF and MC-derived TGF-β1 can increase type I collagen production in fibroblasts 51, 52. Fibrosis can occur as part of the tissue remodeling associated with allergic asthma and atopic dermatitis, and many other settings characterized by chronic inflammation. It will be of interest to determine in such settings the extent to which MCs represent important sources of TNF, TGF-β1, and other products that may drive or regulate various aspects of these complex tissue responses.
Given how many factors may potentially influence MC phenotype and function, including the cells’ ability to produce cytokines (Table 1), and how many other immune and non-immune cell types can participate in complex inflammatory or immune responses, through production of cytokines and many other mechanisms, it is not surprising that the importance of MCs as sources of particular cytokines may vary depending on the specific setting being analyzed. This is illustrated by the history of attempts to analyze the roles of MCs and MC-derived TNF in a commonly used mouse model of sepsis: cecal ligation and puncture (CLP). In work employing MC-engrafted genetically MC-deficient KitW/W-v mice, Echtenacher et al. 53 reported that MCs can contribute to enhanced survival during CLP and that administration of a neutralizing antibody (Ab) to TNF could diminish this effect. In a study employing MC-engrafted genetically MC-deficient KitW/W-v mice that was published back-to-back with the Echtenacher et al. 53 report, Malaviya et al. 54 provided additional evidence for a role for MC-derived TNF in enhancing survival in another model of bacterial infection in mice. Subsequent mechanistic work indicated that activation of MCs either by products of complement activation 55, 56 or via toll-like receptors (TLRs), particularly TLR4 57, can contribute to MC activation for TNF production during CLP and perhaps other forms of bacterial infection. The observation that IL-4-pretreated human cord blood MCs can produce TNF upon LPS or PGN (peptidoglycan) stimulation 58 highlighted the potential clinical relevance of the mouse studies.
Subsequent work employing MC-engrafted KitW/W-v mice confirmed that MCs can enhance survival during the model of CLP tested, and that repetitive administration of the Kit ligand and MC growth factor, stem cell factor (SCF) can also do so 59. However, SCF treatment also significantly enhanced survival after CLP in TNF-deficient mice, showing that this effect can occur independently of TNF, whether of MC or non-MC origin 59. Later work provided evidence that the role of MCs, and MC-derived TNF, can vary depending on both the severity of the CLP model being tested and mouse strain background 60.
Analysis of MC-engrafted KitW/W-v mice confirmed that engrafted MCs can enhance survival of KitW/W-v mice during a model of moderately severe CLP, but that was not true in mice subjected to a severe model of CLP 60. However, experiments employing MC-engrafted genetically MC-deficient KitW-sh/W-sh mice indicated that the beneficial role of MCs in this setting can occur independently of MC-derived TNF 60. By contrast, work in MC-engrafted KitW-sh/W-sh mice indicated that MC-derived TNF can increase mortality during severe CLP and also can enhance bacterial growth and hasten death after intraperitoneal inoculation of Salmonella typhimurium 60. Finally, Piliponsky, et al. 61 reported that MC-derived TNF can be degraded by mouse mast cell protease 4 (mMCP-4) in vitro and that the reduction of TNF levels by mMCP-4 in vivo can help to limit inflammation and promote survival in mice subjected to a moderately severe model of CLP.
Taken together, these findings support the hypothesis that, depending on the circumstances (including mouse strain background, the nature of the mutation resulting in the MC deficiency, and type and severity of the infection), MCs can have either no detectable effect or even opposite effects on survival during bacterial infections. As discussed in detail elsewhere 62–64, a caveat about these findings is that much of the work reviewed above was performed using mice that were MC-deficient because of mutations affecting c-kit structure or expression, and such mice have multiple phenotypic abnormalities in addition to their profound MC deficiency.
In summary, current evidence indicates that MC-derived TNF can contribute to the initiation and amplification of inflammation, particularly in its early stages, during certain innate and adaptive immune responses, and that TNF (particularly that associated with exteriorized MC cytoplasmic granules) also may contribute to the development of certain adaptive immune responses. However, MC-derived TNF may be a two-edged sword, which in some settings contributes more substantially to pathology than to host defense.
2.2 IL-1β
IL-1β is an important pro-inflammatory cytokine that can be involved in various inflammatory diseases. The IL-1 family is a target for treating inflammatory and autoimmune diseases and multiple molecules/biologics are currently being clinically investigated, some of which have demonstrated efficacy (reviewed in 65).
In vitro studies indicate that MCs can produce IL-1β upon stimulation via the FcεRI 11, 66, FcγRs 66, calcium ionophore 66, LPS and ATP (Adenosine 5′-triphosphate), or R837 67, 68. Moreover, there is evidence that MC-derived IL-1β can contribute to the development of various models of arthritis 69, 70, and skin inflammation 67, 68 in mice in vivo.
2.3 IL-2
IL-2 can have effects on many immune cells, and is especially important for Treg cell development and homeostasis 71. The critical sources of IL-2 in the skin have been unclear, but recent work indicates that MCs represent one source, along with T cells. Mouse peritoneal- or bone marrow-derived cultured MCs produce IL-2 upon activation with IgE and antigen in vitro 72. In a model of oxazolone-induced contact hypersensitivity (CHS), MC expansion occurred both at the site of pathology in the skin and in the spleen, and spleen MCs exhibited increased production of IL-2 72. Moreover, engraftment of wild type (WT) but not IL-2-deficient MCs into the skin of genetically MC-deficient KitW-sh/W-sh mice suppressed inflammation at sites of oxazolone-induced CHS, and, in the absence of MC-derived IL-2, the ratio of activated to Treg cells at the site of skin pathology was increased 72. This work indicates that, in these models, MC-derived IL-2 can contribute to the immune suppression of oxazolone-induced CHS.
MC IL-2 production also has been reported to contribute to the expansion of Treg T cells which contribute to immune suppression in a mouse model of IL-33-induced airway inflammation 73. By contrast, Moretti et al 74 recently reported evidence for a positive feedback loop involving MC IL-2 production that can contribute to lung pathology in a mouse model of cystic fibrosis. Specifically, they reported that IL-9 can induce enhanced production of IL-2 by lung MCs, which is associated with expansion of CD25+ group 2 innate lymphoid cells (ILC2s) and subsequent activation of Th9 T cells. It will be of interest to extend these findings, as well as other work which has suggested potential roles of MC-derived IL-2 in immune responses, using mice in which IL-2 is selectively ablated in MCs.
2.4 IL-3
IL-3 has been well characterized as a cytokine which supports MC and basophil differentiation, growth, survival, and expansion 75–79. IL-3 is dispensable in mice for MC and basophil production, in that IL-3-deficient mice have numbers of MCs and basophils similar to those in WT controls (at least when the mice have been maintained under standard conditions in specific pathogen-free colonies), but it is essential for normal expansion of numbers of blood basophils and intestinal and spleen MC populations during infections with certain parasites 78. At least certain MC populations can produce IL-3 upon IgE-mediated stimulation 9–11 and in some cases even when IgE is tested in the absence of specific antigen 80. Such MC production of IL-3 thus might constitute an autocrine signal for promoting MC survival and growth in vivo, and MC-derived IL-3 (together with other MC-derived cytokines with similar or overlapping effects) also might promote the recruitment, development, and survival of additional myeloid cells.
2.5 IL-4
IL-4 is the paradigmatic cytokine involved in type-2 immune responses and plays a critical role in the development of Th2 cells and subsequent allergic reactions. Mouse MC lines were first identified as a source of IL-4 in 1987 (first described in Brown et al 8, reviewed in 81, 82), and MCs can produce IL-4 upon IgE-mediated stimulation or in response to calcium ionophore 83, 84, IL-33 (in mouse MCs 85) or certain lectins (in human cord blood MCs 86). LPS or PGN didn’t induce IL-4 in certain human MCs in vitro 87 but LPS or PGN can induce the cytokine, at least at the mRNA level, in a strain-specific manner in mouse MCs 88 and PGN can induce secretion of IL-4 protein from mouse bone marrow-derived MCs 57.
IL-4 immunoreactive MCs can be detected using IHC in biopsies of patients with allergic rhinitis, asthma, or atopic dermatitis 89–92. Furthermore, the number of such IL-4 immunoreactive MCs can be increased in biopsies of allergic subjects compared to healthy controls 93. IL-4 immunoreactive MCs also were detected in human skin mast cells isolated from patients with atopic dermatitis 91 and after anti-IgE stimulation 23, but were not detected in the skin of healthy control subjects 27.
Later research provided several lines of evidence indicating that basophils can represent a more important source of IL-4 than MCs 94–96. Using IL-4 reporter mice, Gessner et al. 83 showed that MCs, basophils, and eosinophils can express constitutive IL-4 transcript, but the secretion of IL-4 is stimulus-dependent, findings which are consistent with those of earlier studies 8, 9, 97, 98. It was shown recently that ILC2s can produce some IL-4 in humans, but not in mice 99, 100. In addition to being a cytokine of potential MC origin, IL-4 is also known to influence MC function and differentiation/growth 101–104.
2.6 IL-5
IL-5 is a well-known type-2 cytokine with important effects on eosinophils 105, 106. Both mouse and human MCs can produce IL-5 upon IgE-mediated stimulation 11, 107–109 or upon their activation with IL-33 110, or with LPS or PGN 57, 58. MCs that are immunoreactive for IL-5 can be demonstrated in human duodenal 111, bronchial 93, and nasal biopsies 90. Like other type-2 cytokines, IL-5 can have priming effects on MCs 112. It has recently been recognized that ILC2 cells represent a potentially important source of IL-5, both in mice and humans 113–115. These interesting findings further complicate efforts to determine whether IL-5 derived from MCs has any important non-redundant functions in inflammation or immunity. Moreover, MCs potentially can influence immune responses involving ILCs because MCs can both be activated by IL-33 and can inactivate IL-33, a cytokine which also has important effects on ILC2 cells 113–116.
2.7 IL-6
IL-6 is a pleiotropic cytokine which is produced during a variety of inflammatory responses (reviewed in 117, 118), and which is considered a therapeutic target in certain autoimmune and inflammatory disorders 119–121. Many immune cells can produce IL-6, and MCs can produce IL-6 in response to IgE-dependent stimulation 9, 11, 122, LPS 122, substance P 123, IL-1 124, or IL-33 73, 125. Human airway MCs can exhibit IL-6 immunoreactivity by IHC, suggesting that MC-derived IL-6 might contribute to the pathogenesis of asthma or allergic rhinitis 90, 93. Although early studies in Kit mutant MC-deficient mice implicated MC–derived IL-6 (and IFNγ) in the promotion of mouse models of atherogenesis 126 and in diet-induced obesity and glucose intolerance 127, later work with a Kit-independent MC-deficient mouse strain (Cpa3Cre/+) detected no role for MCs in diet-induced or genetic (LepOb/Ob background) models of obesity 128. Such findings indicate that the interpretation of the results of the earlier studies may have been confounded by the use in these models of KitW-sh/W-sh mice, which have increased levels of neutrophils compared to the corresponding wild type mice, as well as other MC-independent phenotypic abnormalities 62, 63, 129. MCs not only represent a potentially important source of relatively large amounts of IL-6, but can in turn be influenced by this cytokine, e.g., IL-6 supports MC growth and is used in growth media to generate human MCs in vitro 130, 131.
2.8 IL-9
IL-9 is a pleiotropic cytokine, as reviewed in 132. IL-9 is produced by and can influence a variety of immune cells. In addition to the well-known IL-9 source, Th9 T cells 133, 134, MCs also can produce IL-9 upon stimulation with ionomycin or IgE/Ag, alone or combination with IL-1, IL-10 or SCF 135–137. It has recently been reported that a subpopulation of mucosal MCs (MMCs), perhaps representing immature stages in the MMC lineage, can produce large amounts of IL-9 that in turn may contribute to the pathology of IgE-mediated food allergy in a mouse model 138. Many reports indicate that IL-9 is involved in various examples of type 2 immunity, including host defenses against parasitic infections and the pathogenesis of allergic diseases. In such contexts, ILC2s also can represent critical producers of IL-9 139, 140.
MCs express the receptor for IL-9 141 and IL-9 stimulation can alter patterns of MC gene expression 142, 143, suggesting that IL-9 produced by MCs has the potential to exert autocrine effects on these cells. IL-9 can enhance the growth of mouse BMCMCs, either alone or synergistically with IL-3 144, and IL-9 also can enhance the growth of human MC progenitors 141.
In vivo, IL-9 transgenic mice exhibit expansion of MMCs, including those in airway and intestinal sites 145, and can exhibit enhanced expulsion of the nematode Trichuris muris 146, 147. IL-9 also promotes MC production of TGF-β1 and studies in transgenic mice indicate that IL-9 can increase numbers of MCs in models of allergic inflammation 148, 149. IL-9 overexpression in transgenic mice also can result in MC hyperplasia associated with airway inflammation and bronchial hyperresponsiveness 150 as well as intestinal mastocytosis which is thought to contribute to food allergy 151. By contrast, IL-9-deficient mice have impaired pulmonary mastocytosis and diminished goblet cell hyperplasia in a model of S. mansoni infection compared to wild type mice 152. It has been reported that IL-9 from Tregs can contribute to recruitment and/or proliferation of MCs in the development of skin allograft tolerance 153 and in Treg-induced immune suppression in models of nephritis 154.
2.9 IL-10
IL-10 is an anti-inflammatory and regulatory cytokine which can be secreted by many kinds of immune cells including Th1, Th2, Th17, Treg, and CD8+ T cells, B cells, dendritic cells, macrophages, NK cells, eosinophils, neutrophils, basophils and MCs, as well as non-immune cells including keratinocytes (reviewed in 155, 156). MCs can secrete IL-10 upon LPS or lipid A stimulation and its production can be synergistically enhanced with IgE crosslinking 107. In vitro-derived mouse BMCMCs also can secrete IL-10 via activation of FcγRIII 157.
There is substantial evidence that many immune responses, including allergic reactions, can be regulated by IL-10 secreted from Tregs (reviewed in 155, 158). However, it now appears that MC IL-10 production also can contribute to immune regulation, at least in certain model systems. Based in part on studies of KitW/W-v or KitW-sh/W-sh mice (that can be called “Kit-dependent MC-deficient mice”) which had been engrafted with MCs derived from WT or IL-10-deficient mice, Grimbaldeston et al. 157 reported that mast cell-derived IL-10 can limit the severity of severe cutaneous contact hypersensitivity (CHS) reactions. In this setting, in vivo and in vitro studies indicated that MC activation via IgG1 and FcγRIII may represent a more important mechanism for triggering MC IL-10 production than IgE crosslinking.
Later, Dudeck et al. 159, working with strains of MC-deficient mice that had normal c-kit, (i.e., “Kit-independent MC-deficient mice”) reported that, in their models of CHS, MCs promoted the intensity of the reactions rather than having a suppressive effect. The latter findings were in accord with prior work indicating that, in some settings, MCs 38, 159–162 and IgE 160 can have effects that amplify the local expression of CHS responses, and it was suggested that the disparate results reported by Grimbaldeston et al. 157 may have reflected the effects of some of the MC-independent abnormalities which were present in the Kit-dependent MC-deficient mice used in that study.
However, inspection of the figures in the two papers indicated that, in addition to using different strains of MC-deficient mice, the two groups were studying CHS responses of different severity and duration. This is important, in that Gimenez-Rivera et al. 163 recently reported additional evidence, derived from studies using a different model of CHS tested in both “Kit-dependent” and “Kit-independent” MC-deficient mice, that MCs can limit the features of this model CHS. Indeed, many different CHS models have been examined with various types of MC-deficient mice, and the results obtained could be interpreted to indicate that, depending on the circumstances, MCs can enhance, suppress, or have no detectable effects on the features of the tested model 164.
In part to address the “controversy” regarding the different conclusions of the studies by Grimbaldeston et al 157 and Dudeck et al 159, Reber et al 165 developed a new a fluorescent imaging approach that enables selective in vivo labeling (with sulforhodamine 101-coupled avidin [Av.SRho]) and tracking of MC secretory granules by real-time intravital 2-photon microscopy in living mice, and permits the identification of such MCs as a potential source of cytokines in different disease models (Figure 2). Specifically, Reber et al. 165 injected Av.SRho i.d. into ear pinnae of IL-10-GFP mice expressing a GFP tracker under the control of the Il10 promoter 166, to monitor simultaneously both MC secretory granules and activation of Il10 gene transcription.
Figure 2. Longitudinal imaging of mast cell (MC) degranulation and Il10 gene activation in a model of severe cutaneous contact hypersensitivity (CHS).
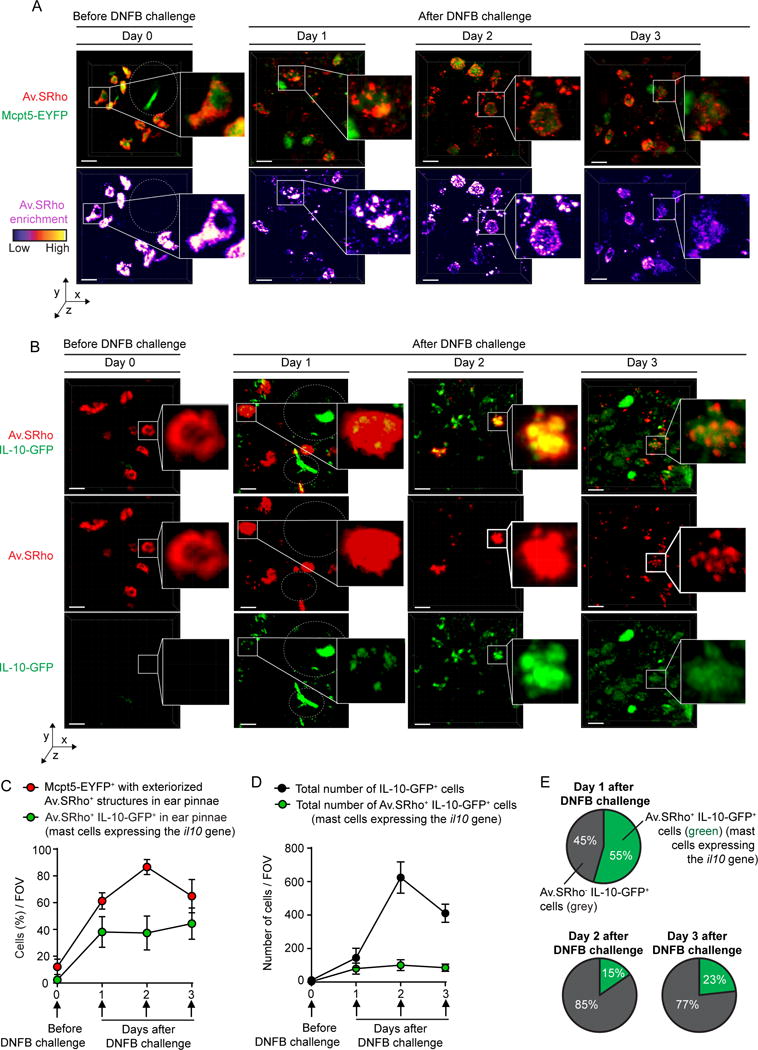
Sulforhodamine 101–coupled avidin (Av.SRho; 5 μg) was injected intradermally (i.d.) into the ear pinna of mice. One week later, the mice were treated as described in 165 to induce a severe 1-fluoro-2,4-dinitrobenzene–induced (DNFB-induced) contact hypersensitivity (CHS) reaction. (A) Longitudinal monitoring of the release of Av.SRho+ granules by dermal MCs at the site of CHS using intravital 2-photon microscopy. Representative 3D photographs of the ear pinna before DNFB challenge or at day 1, 2, or 3 after DNFB challenge. Upper panel: merged fluorescence of Av.SRho (red) and Mcpt5-EYFP (green). Lower panel: Av.SRho fluorescence (pseudocolor scale). Dashed white circles identify hair follicles. (B) Longitudinal monitoring of both the release of dermal MC Av.SRho+ granules and activation of Il10 gene transcription (IL-10-GFP, as detected by emission of GFP fluorescent signal) at the site of CHS using intravital 2-photon microscopy. Representative 3D photographs of the ear pinna before DNFB challenge or at day 1, 2, or 3 after DNFB challenge. Upper panel: merged fluorescence of Av.SRho (red) and IL-10-GFP (green). Middle panel: Av.SRho (red) fluorescence. Lower panel: IL-10-GFP (green) fluorescence. White lines identify the magnified areas and dashed white circles identify hair follicles. Scale bars: 20 μm. (C) Percentage of Mcpt5-EYFP+ cells with exteriorized Av.SRho+ structures (i.e., degranulated dermal MCs, red circles) and of Av.SRho+ IL-10-GFP+ cells (i.e., representing MCs expressing the Il10 gene, green circles) per field of view (FOV) in ear pinnae. (D) Total number of Av.SRho+ IL-10-GFP+ cells (MCs expressing the Il10 gene, green circles) per FOV in ear pinnae and total number of IL-10-GFP+ cells in ear pinnae (black circles). (E) Percentage of Av.SRho+ IL-10-GFP+ cells (i.e., representing MCs expressing the Il10 gene, green) and of Av.SRho–IL-10-GFP+ cells (i.e., representing other cell types expressing the Il10 gene, gray) among total IL-10-GFP+ cells in ear pinnae per FOV. Mean ± SEM; data (n = 3 per group) are pooled from the 3 independent experiments performed (each done with 1 mouse per group), each of which gave similar results. (This is Figure 3 from 165.)
Before hapten (DNFB) challenge (day 0), no Av.SRho+ dermal MCs were positive for GFP, suggesting that, at least under those baseline conditions, the Il10 gene was not substantially activated (Figure 2, B and C). However, a clear GFP signal (i.e., emission of green fluorescence detectable above the green autofluorescence of the dermis) was detected in ~40% of Av.SRho+ MCs as soon as 1 day after hapten (DNFB) challenge, a percentage that remained stable for the next 2 days (Figure 3, B and C). By quantifying the total number of IL-10-GFP+ cells at sites of CHS, and assessing how many of these cells were Av.SRho+ MCs, Reber et al 165 observed a progressive increase over time in the total number of IL-10-GFP+ cells, with the highest numbers 2 days after DNFB challenge (Figure 3, B and D), a finding which is consistent with previous reports describing the kinetics of infiltration of Treg cells at sites of CHS 167. IL-10-GFP+ Av.SRho+ dermal MCs represented up to ~55% of all detected IL-10-GFP+ cells at day 1, but only ~10%–20% at days 2 and 3 after DNFB challenge (Figure 3, B, D, and E).
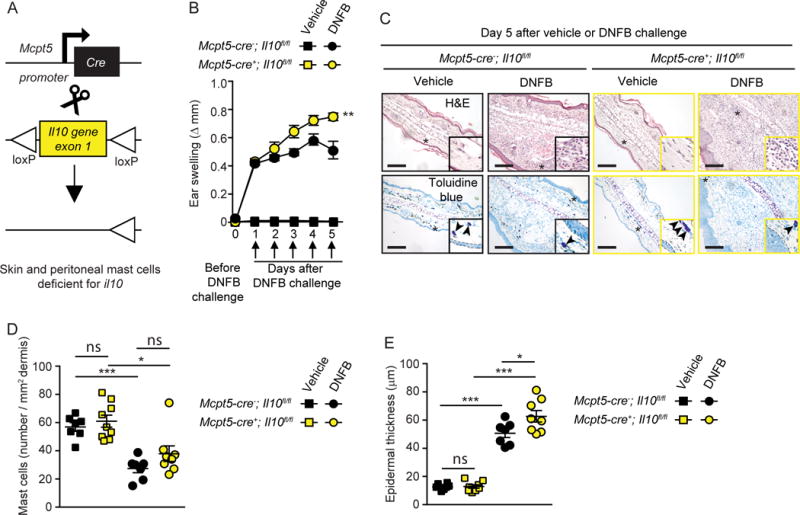
Figure 3. Mast cell (MC) production of IL-10 limits inflammation and epidermal hyperplasia in a model of severe cutaneous contact hypersensitivity (CHS).
Mice were treated as described in 165 to elicit a 1-fluoro-2,4-dinitrobenzene–induced (DNFB-induced) severe CHS reaction. (A) Breeding strategy to obtain Mcpt5-Cre+; Il10fl/fl (MC IL-10 deficient) mice. (B) Changes (Δ) in ear thickness over time after challenge with vehicle (squares) or DNFB (circles) in Mcpt5-Cre+; Il10fl/fl (MC IL-10 deficient, yellow) or Mcpt5-Cre–; Il10fl/fl (MC IL-10 sufficient, black) mice. (C) Photomicrographs of representative H&E (upper panel) and toluidine blue (lower panel) stained sections of ear pinnae of mice sacrificed 5 days after challenge. Asterisks indicate areas shown at higher magnification (×60) in insets, arrowheads indicate MCs, and dashed white lines in insets depict epidermis. (D) Number of MCs/mm2 dermis and (E) epidermal thickness 5 days after vehicle (squares) or DNFB (circles) challenge in Mcpt5-Cre+; Il10fl/fl (MC IL-10 deficient, yellow) or Mcpt5-Cre–; Il10fl/fl (MC IL-10 sufficient, black) mice. Scale bars: 200 μm. Mean ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001; (B) 2-way ANOVA; (D and E) 2-tailed, unpaired t test. Data (n = 6–12 mice per group) are pooled from the 3 independent experiments performed (each done with n = 2–4 mice per group), each of which gave similar results. (This is Figure 5 from 165.)
Taken together, these results indicate that dermal MCs are one of the first immune cells to produce IL-10 at sites of severe CHS, before the substantial infiltration of other IL-10–producing immune cells. By contrast, Reber et al 165 reported that, in a mild model of CHS, in which studies in Cpa3-Cre+; Mcl-1fl/fl (Kit-independent) MC-deficient mice 168 indicated that MCs promoted the development of inflammation and epidermal hyperplasia (see Supplemental Figure 3 in Reber et al 2017 165), intravital microscopy detected only minimal, if any, changes from baseline levels of MC Il10 gene expression (see Supplemental Figure 4 in Reber et al 2017 165).
Confirming the findings of Grimbaldeston et al. 157 in Kit-mutant mice, two types of Kit-independent MC–deficient mice, Cpa3-Cre+; Mcl-1fl/fl 168 and Mcpt5-Cre+; DTA 159 mice, exhibited significantly enhanced ear swelling and epidermal hyperplasia compared with the values in their respective littermate controls 165. However, while KitW-sh/W-sh mice exhibited an ~200% increase in ear swelling on day 5 of the reaction as compared with their littermate controls, this difference was less pronounced in Kit-independent MC-deficient mice at the same time point (~120% increase in Cpa3-Cre+; Mcl-1fl/fl mice and ~50% increase in Mcpt5-Cre+; DTA mice). Reber et al. 165 suggested that these findings are consistent with the conclusion that MCs can have effects that can substantially limit features of this model of severe CHS in each of the 3 examined mouse strains, but that additional phenotypic abnormalities in KitW-sh/W-sh mice beside their MC deficiency probably also contribute to the exacerbation of severe CHS responses in this strain.
To assess the potential role of MC-derived IL-10 in this model of severe CHS, Reber et al 165 tested mice in which the Il10 gene was floxed out specifically in connective tissue–type MCs by generating Mcpt5-Cre+; Il10fl/fl mice (Figure 3A). Dermal MCs were present in similar numbers in the ear pinnae of Mcpt5-Cre+; Il10fl/fl mice, in which connective tissue–type MCs are deficient for IL-10, and littermate control Mcpt5-Cre–; Il10fl/fl mice (Figure 3, C and D). However, Reber et al. 165 found that the Mcpt5-Cre+; Il10fl/fl mice exhibited significantly enhanced ear swelling and epidermal hyperplasia compared with the littermate control mice (Figure 3, B, C, and E). Notably, the enhancement of both the tissue swelling and the epidermal thickness associated with the reactions observed in Mcpt5-Cre+; Il10fl/fl mice was less pronounced than that observed in the Kit-independent MC-deficient mice, suggesting that MCs might help to limit these features of this acute model of severe CHS by both IL-10–dependent and IL-10–independent mechanisms 165.
In addition to having the potential to regulate the intensity of CHS, studies in mice in which IL-10 was specifically deleted in MCs indicate that MC-derived IL-10 can suppress the adaptive immune response and thereby result in enhanced persistence of bacteria in a mouse model of bladder infection of Escherichia coli 169. MC-derived IL-10 also can suppress germinal center formation by affecting T follicular helper (Tfh) cell function 170. Evidence derived from studies in Kit-dependent MC-deficient mice suggests that MC-derived IL-10 also can limit the cutaneous pathology associated with chronic UVB irradiation 157 and can suppress graft versus host disease (GVHD) in a mouse model independently of Treg 171. However, to our knowledge, the latter two findings have not yet been assessed in tests of Kit-independent MC-deficient mice.
The studies reviewed above indicate that MC-derived IL-10 indeed can contribute to the suppression of certain adaptive immune responses in mice, with beneficial consequences in the case of a model of severe CHS 165 but with detrimental effects in a model of bladder infection with E. coli 169. The findings of Reber et al 165 also support the conclusion that the same MC population, in this case mouse dermal MCs, can exhibit markedly different levels of Il10 gene expression, with upregulation of expression occurring rather rapidly in response to the induction of a severe CHS reaction. Clearly, further studies are needed to clarify the roles of MC-derived IL-10 in various immune responses.
2.10 IL-11
IL-11 is multifunctional cytokine that belongs to IL-6 cytokine family. Indeed, by structure, IL-11 is the cytokine that is most closely related to IL-6 and they share gp130 as a component of their receptors (reviewed in 172–174). Various functions are also shared among IL-6 cytokine family members, and IL-11, which can promote thrombopoiesis, is used to prevent the development of chemotherapy-induced thrombocytopenia 172, 174, 175. IL-11 can be produced by many kinds of cells including leukocytes, epithelial cells, and fibroblasts, and is thought to be involved in the pathogenesis of asthma, airway hyperresponsiveness, and lung inflammation 176–178. One report indicated that human umbilical cord blood-derived MCs can produce IL-11 in response to an IgE-mediated stimulus 179. However, the importance of MCs as a potential source of IL-11 remains to be determined.
2.11 IL-12
IL-12 is important for the induction of Th1 responses and for stimulating IFNγ production from Th1 cells and NK cells 180, 181. IL-12-deficient mice are severely susceptible to bacterial and viral infections, and IL-12 is important for mounting adequate cellular immune responses to intracellular pathogens. One of the causes of vulnerability to pathogens is impaired IL-12 production from various immune cells in response to pathogen-derived products such as LPS. Besides activating IL-12 production in dendritic cells and macrophages, LPS (but not IgE-mediated stimulation) can stimulate IL-12 production in MCs 87, 182. SCF-derived mouse BMCMCs express IL-12 mRNA but not IL-3-derived mouse BMCMC 183. Moreover, IL-12 can induce production of IFNγ in rat PMCs 184, raising the possibility that IL-12 might have autocrine effects on MCs.
2.12 IL-13
IL-13 is an important cytokine in type-2 immune responses, with functions that partially overlap with those of IL-4 185–187. Human and mouse MCs produce IL-13 upon stimulation with IgE and antigen 107, 137, 188, 189, PMA (phorbol 12-myristate 13-acetate) and ionomycin 188, 190, LPS or PGN 57, 58, 107, or IL-33 73, 125, 191, 192. Human MCs produce IL-13 upon IL-1β stimulation 190 and mouse MC IL-13 production by IgE/Ag stimulation can be enhanced in the presence of IL-1β 135. SCF can induce IL-13 production in mouse MCs 193.
IL-13 is also produced by many other cell types including T cells, basophils, eosinophils, and epithelial cells. A series of studies now suggest that ILC2-derived IL-13 plays a critical role in host defense to infections with certain parasites and in the pathogenesis of type-2 immune responses 185, 194, 195. Further research is needed to understand the importance of MC production of IL-13, especially in those in settings in which many other cell types also elaborate this product.
2.13 IL-16
IL-16 is a pro-inflammatory cytokine that can act as a chemoattractant for T cells, eosinophils, monocytes, dendritic cells, and MCs (reviewed in 196). In addition to functioning as a MC chemoattractant via its binding to CD9 197, IL-16 also can promote maturation and differentiation of human umbilical cord blood-derived MCs when administered together with SCF 198. Qi et al 198 also showed that IL-16-treated human cord blood-derived CD3−/CD4+/CD117+ cells, which contained cells the authors called “mast cells/basophils”, are less susceptible to HIV infection. It has been reported that IL-16 can be produced without any stimulation in human CBMCs 199 and that IL-16 mRNA can be detected constitutively in human intestinal MCs 200. IL-16 also has been detected by IHC in tryptase+ MCs present in bronchial biopsies from normal subjects as well as from patients with asthma 201.
2.14 IL-33
IL-33 is recognized as an important alarmin secreted by damaged or necrotic cells, particularly vascular endothelial and epithelial cells 202–205. IL-33 has been implicated in the activation of ILC2s in the settings of infections and allergic diseases 205. MCs constitutively express the IL-33 receptor ST2, therefore they can respond to IL-33. MCs can produce a variety of cytokines and chemokines upon IL-33 stimulation, including TNF 191, 192, IL-2 73, IL-4 85, IL-5 191, IL-6 191, IL-10 191, IL-8 206, IL-13 125, 191, 206, granulocyte-macrophage colony-stimulating factor (GM-CSF) 191, CXCL8 191, CCL1 191, CCL2 191, CCL17 191, and CCL22 191 (also see the sections on each of these products). Moreover, in vitro studies indicate that IL-33 can act on CD34+ cells to facilitate MC maturation and differentiation 191, both physiologically and in the setting of chronic myeloid leukemia 207. Recent evidence has identified mouse BMCMCs as a potential source of IL-33 110, 208, as well as a target of this cytokine. MCs also can be involved in the activation of IL-33 by converting full-length IL-33 into more active mature forms with either chymase or tryptase 209, 210. Finally, MC chymase (mMCP-4 or human chymase) can further degrade 17.5 kDa active IL-33 into a biologically inactive form 211, 212.
2.15 EGF (epidermal growth factor)
Epidermal growth factors stimulate proliferation and differentiation of various cells including fibroblasts, endothelial cells, and epithelial cells 213. Human MCs in the thyroid are EGF positive by IHC 214 and freshly isolated human dermal MCs are positive for heparin-binding EGF-like growth factor (HB-EGF) mRNA by RT-PCR 215.
2.16 FGF2 (fibroblast growth factor 2)/bFGF (basic fibroblast growth factor)
MC-derived FGF2 is considered to be a potential pro-angiogeneic factor 216. Immunoreactivity for FGF2 has been detected by IHC in MCs in human fibrotic lung tissue, rheumatoid synovia, and skin hemangiomas 217, in human thyroid MCs 214, and in rat PMCc 34, and MCs containing FGF2 in a granule-associated form with heparan sulfate were detected in human skin using a binding assay with biotinylated FGF2 218. Secretion of FGF2 has been reported for human dermal MCs and HMC-1 cells 215. There is a report that IL-17A can increase the secretion of FGF2 from human CD133+ progenitor derived cultured MCs 219. In addition to FGF2, other factors with mitogenic activity on fibroblasts, including FGF7 and FGF10 are also can be detected in human dermal MCs 215.
The importance of FGF2 production by MCs in vivo is not yet understood. However, Wroblewski et al. 220 have suggested that one reason VEGF-targeted therapy becomes less effective is that MCs re-activate angiogenesis in part by secreting FGF2.
2.17 GM-CSF
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a cytokine which facilitates the development of granulocytes and macrophage from precursors in bone marrow 221. MCs can produce GM-CSF upon IgE-mediated stimulation 10, 11, 109, 222, or after exposure to LPS 222, PGN 222, zymosan 222, or Pam3Cys 222. Mucosal mast cells in the airways of asthmatic patients can exhibit GM-CSF immunoreactivity 111, indicating that MC-derived GM-CSF might participate in the pathogenesis of allergic diseases, for example by promoting eosinophil survival. There is evidence that MC-derived GM-CSF can contribute, together with TNF, to the migration of graft-derived dendritic cells to lymph nodes by enhancing their survival and thereby contributing to the development of peripheral tolerance 41.
2.18 IFN-γ
IFN-γ is considered a paradigmatic Th1 cytokine 223. mRNA for IFN-γ can be upregulated upon IgE-mediated or ionophore stimulation of rat PMCs and certain mouse MC lines 9, 11, 108. Gupta et al. 184 later detected IFN-γ protein in rat PMCs after IL-12 stimulation, but not after IgE-mediated activation. We discuss above, in the section on IL-6, recent work 128 that has called into question the interpretation of prior work indicating that MC–derived IL-6 and IFN-γ may be important in the promotion of atherogenesis 126 or diet-induced obesity and glucose intolerance 127.
IFN-γ can influence MCs directly, since MCs can express the receptor for IFNγ 189, 224. Varied effects of IFN-γ on MCs have been reported, including both positive and negative effects. For example, IFN-γ can inhibit the growth and/or induce apoptosis in mouse BMCMCs 224, 225 and in human bone marrow-derived MCs 226. IFN-γ can inhibit serotonin release from mouse PMCs 227 and also can inhibit IL-4 mediated enhancement of serotonin/arachidonate release upon IgE and antigen stimulation 228. IFN-γ can inhibit MC-associated cytotoxicity by inhibiting TNF release from rat PMCs 229.
By contrast, other studies showed that IFNγ can promote the survival of, and histamine release from, human umbilical cord blood-derived MCs 230, or have no effect on the degranulation of human peripheral blood-derived MCs 231 or human MCs derived in vitro from intestinal MCs 232. Human (peripheral blood progenitor-derived) cultured mast cells can express functional Toll-like receptor 4 only when they have been preincubated with IFNγ. The profile of cytokines which these MCs can express in response to LPS is unique compared to other stimuli. For example, they can produce far more TNFγ 233. Studies using BMCMCs derived from wild type (WT) mice or IFNγR-deficient mice showed that IFN-γ can significantly increase the release of histamine, IL-6, and IL-13 by IgE+antigen-stimulated WT BMCMCs, whereas treatment of the cells with IFN-γ alone was without effect 189. The ability of IFN-γ to enhance dose-dependently the IgE+antigen-induced mast cell production of IL-13 is of particular interest, since IL-13 is thought to contribute to the development of asthma through such effects as promoting subepithelial fibrosis (in part by upregulating synthesis of arginase-1), increasing mucus secretion, and eliciting airway hyperresponsiveness (AHR) 234.
The varied results obtained in studies of effects of IFN-γ on MCs might reflect, at least in part, differences in the effects of IFN-γ on different populations of MCs. For example, IFNγ inhibited histamine and TNF release from rat PMCs, but had no detectable effect on rat intestinal MCs 235. Exposure of human MCs to IL-4, IL-5, and IFNγ during growth and differentiation generally downregulated MC numbers and function, but when these cytokines were administered to mature human peripheral blood-derived MCs, IFN-γ and IL-5 had no effects on degranulation and cell division, but IL-4 induced division and potentiated FcεRI-mediated degranulation 104. Furthermore, IFN-γ decreased proliferation, without affecting apoptosis, in human intestinal MCs cultured in the presence of optimal concentrations of SCF or SCF and IL-4 232. However, in the absence of growth factors or at suboptimal concentrations of SCF, IFN-γ promoted survival through inhibition of MC apoptosis 232.
Both mouse and human studies suggest that effects of IFN-γ on MCs may importantly influence multiple aspects of the pathology of certain forms of asthma, particularly those associated with high levels of neutrophil infiltration of the airways and certain forms of severe asthma 189, 236–238. However, in such settings, MCs are more likely to represent important targets of IFN-γ rather than critical sources of this cytokine.
2.19 NGF
NGF is a neurotropic polypeptide with effects which regulate the development, growth, survival, and function of central and peripheral neurons (reviewed in 239, 240). As mentioned in the section on TNF, close anatomical associations between MCs and nerves have long been recognized 48–50. NGF is one of the key factors to link these two cell types, and was the first mitogen to be identified as able to directly or indirectly promote MC development in vivo, in this case in neonatal but not adult rats 241. NGF can support rat PMC survival 242, the development by mouse BMCMCs of features of “connective tissue type MCs” 243, and the growth of rat PMCs 241, 244. Furthermore, NGF can induce degranulation of rat skin MCs 245 and PMCs 246–249 and can induce chemotaxis in rat PMCs 250. Moreover, correlations have been reported for numbers of MCs and levels of NGF mRNA levels in bronchial biopsies from patients with asthma 251, vernal keratoconjunctivitis 252, or systemic sclerosis 253.
However, there are reports indicating that NGF has few if any direct effects on some populations of human MCs 254, 255, but can influence human basophils 255, 256. On the other hand, it has been reported that the HMC-1 leukemic MC line and human CBMCs can express functional receptors for NGF 257, 258 and that NGF can support the development and differentiation of some types of human MCs (HMC-1 and CBMCs) 259, 260. Tam et al. 258 identified mRNA transcripts of full-length tyrosine kinase-containing trkA, trkB, and trkC neurotrophin receptor genes in HMC-1 cells and, by flow cytometry, HMC-1 cells exhibited expression of TrkA, TrkB, and TrkC receptor proteins containing full-length tyrosine kinase domains. Highly purified populations of human lung MCs expressed mRNAs for trkA, trkB and trkC, whereas preparations of human umbilical cord blood-derived MCs expressed mRNAs for trkA and trkC, but not trkB. Populations of the latter cells also exhibited significantly higher numbers of chymase-positive MCs after the addition of NGF to their culture medium for 3 weeks 258. HMC-1 cells expressed mRNAs for NGF, brain-derived neurotrophic factor (BDNF), and neurotrophin-3 (NT-3), the cognate ligands for TrkA, TrkB, and TrkC, whereas NGF and BDNF transcripts were detectable in human umbilical cord blood MCs 258.
Taken together, the findings of Nilsson et al. 257 and Tam et al. 258, and subsequent work 259, 261, indicate that at least some populations of human MCs can express functional TrkA receptors and suggest that NGF may be able to promote certain aspects of MC development and/or maturation in humans. These studies, and reports that rodent rat PMCs can contain and secrete NGF 262, indicate that MCs may represent a potential source of neurotrophins.
2.20 PDGF (platelet-derived growth factor)
PDGF is an important mitogen that can contribute to angiogenesis by facilitating the growth of blood vessels 263–265. By IHC, PDGF positive MCs are increased in the areas of thyroid tissue regeneration in patients with subacute thyroiditis 214, suggesting a role in tissue repair, as well as in Graves’ ophthalmopathy, an autoimmune inflammatory disease of the periorbital and orbital tissues 266. Mouse MCs also can produce PDGF after co-culture with cardiac myocytes or fibroblasts, and it has been suggested that such MC-derived PDGF can contribute to the pathogenesis of atrial fibrillation 267.
2.21 SCF (stem cell factor)
The KIT ligand, SCF is essential for normal MC differentiation, growth, and survival 268–271. Non-hematopoietic cells such as endothelial cells or fibroblasts are considered to be more important sources of SCF than are hematopoietic cells 272–274. However, human MCs have been reported to exhibit SCF immunoreactivity in their granules 275–279. SCF mRNA and/or protein has been reported in human skin and lung mast cells and human PBMCs and CBMCs 275, 277, 278. SCF production by MCs may have autocrine effects on MCs, and/or paracrine effects on other cell types, under physiological conditions or in settings of pathology, such as during some forms of mastocytosis 278, 280.
2.22 TGF-β1
Transforming growth factor type-β (TGF-β) has many biological activities, and is thought to be a particularly important contributor to fibrosis, angiogenesis, and tissue repair. In addition, TGF-β can influence T cells, including Th17 and Treg cells (reviewed in 281–285), as well as B cells, dendritic cells, NK cells, neutrophils, eosinophils, and MCs (reviewed in 192, 284–287).
MCs can be a source of TGF-β1 51, 288, and can secrete TGF-β1 upon IgE and antigen stimulation 51. In vitro evidence obtained from mice suggests that, along with MC-derived TNF, MC-derived TGF-β1 can enhance the production of type-I collagen by fibroblasts 51. Evidence from IL-9 blockade in mouse cystic fibrosis model suggests that TGF-β1 derived from MCs (and other cells) stimulated with IL-9 can contribute to the pathogenesis of cystic fibrosis 74.
Similar to TNF, TGF-β1 has been shown to be secreted rapidly by MCs 288, 289 and to be stored in MC cytoplasmic secretory granules together with chymase 1 289. Human cord blood-derived MCs constitutively express TGF-β1, but its expression is not upregulated after calcium ionophore stimulation 290.
Many reports indicate that TGF-β1 can suppress the functions of diverse immune cells, including MCs 192, 286, 291, and it has been proposed that MC-derived TGF-β1 can suppress MC functions in an autocrine 292 or paracrine manner. TGF-β1 can inhibit the release of multiple mediators upon IgE-mediated stimulation of MCs, including release of histamine and TNF in rat PMCs 292, IL-6 and TNF in mouse BMCMCs 192, 286, IL-6 in human skin-derived MCs 286, and β-hexosaminidase, TNF, GM-CSF, IL-13, and IL-6 in SCF cultured MCs derived from human skin 293. Co-exposure to TGF-β1 can also inhibit the IL-33-induced release of multiple mediators from mouse BMCMCs including TNF, MCP-1, IL-6, IL-13, and MIP-1α 192. There is evidence that TGF-β1 can have autocrine effects which inhibit the proliferation of mouse BMCMCs 294 and cultured mouse PMCs 294, 295. One mechanism by which TGF-β1 may suppress the IgE-dependent activation of some MC populations is its ability to reduce levels of expression of FcεRI on the MC surface 296.
In vivo administration of TGF-β1 can inhibit immediate and delayed type hypersensitivity reactions, although this might reflect indirect effects rather than actions specifically on MCs 297. On the other hand, there are reports that TGF-β1 either can enhance mediator production in certain types of MCs in vitro 298, 299 and in vivo 300 or have no effect in BMCMCs in vitro 294. For example, Ganeshan and Bryce 298 found that membrane-bound TGF-β1 on Tregs can promote IL-6 production from mouse BMCMCs, whereas, by contrast, Tregs can inhibit MC degranulation through OX40/OX40L 301.
Finally, the cytoplasmic granule-stored MC protease, chymase (from human skin 302 or stomach 303 or rat PMCs 289) can generate active TGF-β1 from its inactive latent form. In vivo studies with chymase inhibitors (in hamsters 303, rats 304 and in mice 305, 306), as well as work in mMCP4-deficient mice (which genetically lack the mouse chymase most like the human enzyme)307, have suggested possible direct or indirect effects of chymase in the pathogenesis of fibrotic diseases. However, the extent to which any such effects of chymase reflect its ability to activate latent TGF-β1 (derived from MCs or other sources) remains to be determined. Also, it seems likely that TGF-β1’s bioactivity, e.g., as an enhancer or suppressor of various MC functions, may depending on the particular types of MCs in that microenvironment, as well as other local factors that can influence the cytokine’s bioactivity or biodistribution.
2.23 VEGF (vascular endothelial growth factor)/VPF (vascular permeability factor)
Angiogenesis is critically important in normal development and tissue homeostasis and repair, and can contribute to diverse forms of pathology, e.g., tumor development and metastasis, psoriasis, rheumatoid arthritis, and wet macular degeneration 308, 309. Observational studies have implicated MCs in angiogenesis in various settings and one of the most important MC products which may contribute to such roles is thought to be VEGF 216.
The molecule now called VEGF was initially discovered as a component of a guinea pig tumor ascites that can markedly enhance cutaneous vascular permeability in vivo, the bioactivity which was the basis of its initial name, vascular permeability factor (VPF) 310–313. VPF later was found to be identical to VEGF, which was cloned and characterized in 1989 314. The initially described VPF/VEGF, now called VEGF-A, is one of five members of the VEGF family in mammals, that also includes placental growth factor (PGF), VEGF-B, VEGF-C and VEGF-D 315, 316. VEGF-A is a pro-angiogenetic factor which can enhance the angiogenesis process by promoting endothelial cell proliferation and migration, and, as its alternative name (VPF) indicates, VEGF-A also can potently enhance vascular permeability, with a molar potency roughly 1000 times that of histamine 311–313.
Many normal and neoplastic cell types can secrete VEGF, and two groups provided evidence that MCs should be added to that list 317, 318. Moreover, there is evidence that MCs can constitutively contain VEGF as a preformed, heparin-binding factor 34, 317, 318 and can secrete this protein after stimulation by diverse triggers, including IgE and antigen (this was the first evidence that secretion of VEGF could be induced in an antigen-specific way in any cell type), PMA, A23187, or SCF 317, substance P or IL-1 (with enhanced release when either agent was tested together with IL-33 319), corticotropin-releasing hormone 320, IL-17A 219, or live Staphylococcus aureus bacteria 321. It also has been shown that human CBMCs and purified lung MCs can constitutively express VEGF-A isoforms (VEGF-A121 and VEGF-A165 in CBMCs; VEGF-A121, VEGF-A165 and VEGF-A189 in purified human lung MCs), VEGF-B, VEGF-C and VEGF-D, and their receptors (VEGFR1 and VEGFR2) 322, 323, indicating the potential involvement of such MC-derived products in angiogenesis and lymphangiogenesis 324. VEGF can act as a chemoattractant for certain MCs in vitro 325 and in vivo 326, suggesting a mechanism by which VEGF can have autocrine or paracrine effects on this lineage.
Given the large number of other cell types that also can produce VEGF, it may be difficult to identify settings in which MCs represent important or non-redundant sources of this cytokine. IgE-associated disorders represent one potential setting of this kind, in that relatively few cells other than MCs express the FcεRI, and it has been reported that VEGF immunoreactive MCs are increased in the airways of asthmatic patients compared to controls 327–329.
There are also several reports that MCs can be immunoreactive for VEGF in certain tumors including laryngeal squamous cell carcinoma 330, malignant melanoma 331, and non-Hodgkin lymphoma 332, suggesting their possible involvement of MC-derived VEGF in tumor-associated angiogenesis. Evidence has been reported based on tests of several tumor models, using both Kit-dependent and Kit-independent MC-deficient mice, that MCs can contribute to tumor-related angiogenesis and other features of tumor progression 333–335. MCs are increased in the areas of thyroid tissue regeneration in patients with subacute thyroiditis, and such MCs can exhibit immunoreactivity for VEGF, bFGF, PDGF, TGF-β1 and EGF 214. In mice, several lines of evidence indicate that low-dose irradiation can promote tissue revascularization at least in part through MC production of VEGF 336. However, this point needs to be investigated further, ideally by employing mice in which VEGF production can be selectively ablated in MCs.
3 MAST CELL-DERIVED CHEMOKINES
Chemokines are cytokines which have chemotactic activities on various immune cells 337–339. Chemokines play important roles in the development and homeostasis of the immune system and in the pathogenesis of inflammatory responses, including those associated with diverse disorders including allergic and autoimmune diseases, infections, and cancer 338, 340–342. Beyond inducing chemotaxis, chemokines can also activate immune cells, including MCs, and play a critical role during HIV infection 343. Therefore chemokines are considered potential therapeutic targets in many diseases 344–346.
The first identification of MCs (in this case in vitro-derived mouse MCs and mouse MC lines) as a potential source of certain chemokines, specifically CCL1, CCL2, CCL3, and CCL4 mRNA, was in 1989 11. Since then, various populations of mouse or human MCs, most often representing in vitro-derived MCs 347, have been identified as potential sources of a now very long list of these molecules (Table 2). Given: 1) the vast number of biological responses in which chemokines are involved, 2) the large number of chemokines which MCs have at least the potential to produce, 3) the long list of stimuli (including IgE in the absence of known specific antigen 348) which can elicit chemokine production by MCs (Table 2), in some cases without inducing substantial MC degranulation, and 4) the large number of other cell types which also represent a potential source of these mediators, it will be challenging to attempt to identify situations in which MCs represent important non-redundant sources of these molecules.
In fact, there is relatively little work attempting to identify MCs as sources of chemokines in vivo and we are not aware of any published work reporting results of experiments in mice in which individual chemokines have been deleted selectively in MCs. Until experiments of that type are performed, MCs may be best regarded as potentially important sources of certain chemokines in health and disease, especially in those settings in which relatively selective MC activation occurs (e.g., by IgE and specific antigen); but in many settings in which chemokines have been implicated, MCs may not represent critical non-redundant producers of these mediators.
CONCLUSION
The long and growing list of cytokines, chemokines and growth factors which have been identified in analyses of various populations of rodent and human MCs offers great scope for the imagination concerning the potential roles of MCs as important sources of these products in health and disease. However, to date, all of the cytokines, chemokines and growth factors identified in MCs also can be produced by other (and often many other) cell types.
Moreover, as described herein, many of these potentially MC-derived products have been identified in analyses of in vitro-derived MCs, and it remains to be shown whether and to what extent, and it which settings, native populations of MCs in vivo represent important or even non-redundant sources of these molecules. Finally, as we have discussed, there appears to be substantial variation in the types of products which MCs can produce and in the signals that can elicit production of these molecules, based on animal species, type and anatomical location of MCs, stage of MC development, and current (and perhaps past) exposure of MCs to inflammatory or immune responses. Different MC populations also will vary in the extent to which they can produce and release mediators, such as their granule-stored proteases, which have the capacity to biochemically activate and/or degrade, or otherwise regulate levels of, cytokines, chemokines or growth factors of MC or non-MC origin.
In light of these considerations, defining the actual roles of MC-derived cytokines, chemokines and growth factors in health and disease will require the application of more definitive tools than those used in the past to identify MCs as potential sources of these products. The good news is that a large number of new approaches for analyzing the importance of MCs as sources of such products already have been described (Table 4). We anticipate that the thoughtful application of such new methods will enable us to identify many settings in which the production of particular cytokines and growth factors, by particular MC populations, makes a real difference in the initiation, amplification or regulation of biological responses that contribute importantly to the maintenance of health or to features of disease. Such work also may help to identify new therapeutic targets in at least some of the disorders in which MCs play an important role.
Table 4.
New approaches for analyzing MC production of cytokines, chemokines and growth factors.
|
Acknowledgments
We thank all of those, in the Galli laboratory and elsewhere, who have contributed to the large body of work reviewed herein. Stephen J. Galli is receiving funding from NIH grants U19 AI 104209, R01 AI132494, and R01 AR067145, as well as from the United States-Israel Binational Science Foundation (Grant 2013263).
Footnotes
PROFESSOR STEPHEN GALLI (Orcid ID : 0000-0001-5736-5340)
CONFLICT OF INTEREST
The authors have no conflict of interest.
References
- 1.Ehrlich P. Thesis. Leipzig University; Leipzig: 1878. Beiträge zur Theorie und Praxis der histologiscen Färbung. [Google Scholar]
- 2.Gordon JR, Burd PR, Galli SJ. Mast cells as a source of multifunctional cytokines. Immunol Today. 1990;11:458–464. doi: 10.1016/0167-5699(90)90176-a. [DOI] [PubMed] [Google Scholar]
- 3.Chung SW, Wong PM, Shen-Ong G, et al. Production of granulocyte-macrophage colony-stimulating factor by Abelson virus-induced tumorigenic mast cell lines. Blood. 1986;68:1074–1081. [PubMed] [Google Scholar]
- 4.Young JD, Liu CC, Butler G, Cohn ZA, Galli SJ. Identification, purification, and characterization of a mast cell-associated cytolytic factor related to tumor necrosis factor. Proc Natl Acad Sci U S A. 1987;84:9175–9179. doi: 10.1073/pnas.84.24.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 6.Gordon JR, Galli SJ. Release of both preformed and newly synthesized tumor necrosis factor alpha (TNF-alpha)/cachectin by mouse mast cells stimulated via the Fc epsilon RI. A mechanism for the sustained action of mast cell-derived TNF-alpha during IgE-dependent biological responses. J Exp Med. 1991;174:103–107. doi: 10.1084/jem.174.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh LJ, Trinchieri G, Waldorf HA, Whitaker D, Murphy GF. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci U S A. 1991;88:4220–4224. doi: 10.1073/pnas.88.10.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown MA, Pierce JH, Watson CJ, et al. B cell stimulatory factor-1/interleukin-4 mRNA is expressed by normal and transformed mast cells. Cell. 1987;50:809–818. doi: 10.1016/0092-8674(87)90339-4. [DOI] [PubMed] [Google Scholar]
- 9.Plaut M, Pierce JH, Watson CJ, et al. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989;339:64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- 10.Wodnar-Filipowicz A, Heusser CH, Moroni C. Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature. 1989;339:150–152. doi: 10.1038/339150a0. [DOI] [PubMed] [Google Scholar]
- 11.Burd PR, Rogers HW, Gordon JR, et al. Interleukin 3-dependent and -independent mast cells stimulated with IgE and antigen express multiple cytokines. J Exp Med. 1989;170:245–257. doi: 10.1084/jem.170.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carswell EA, Old LJ, Kassel RL, et al. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Old LJ. Tumor necrosis factor (TNF) Science. 1985;230:630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- 14.Goeddel DV, Aggarwal BB, Gray PW, et al. Tumor necrosis factors: gene structure and biological activities. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):597–609. doi: 10.1101/sqb.1986.051.01.072. [DOI] [PubMed] [Google Scholar]
- 15.Beutler B, Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- 16.Okuno T, Takagaki Y, Pluznik DH, Djeu JY. Natural cytotoxic (NC) cell activity in basophilic cells: release of NC-specific cytotoxic factor by IgE receptor triggering. J Immunol. 1986;136:4652–4658. [PubMed] [Google Scholar]
- 17.Jadus MR, Schmunk G, Djeu JY, Parkman R. Morphology and lytic mechanisms of interleukin 3-dependent natural cytotoxic cells: tumor necrosis factor as a possible mediator. J Immunol. 1986;137:2774–2783. [PubMed] [Google Scholar]
- 18.Tharp MD, Kasper C, Thiele D, et al. Studies of connective tissue mast cell-mediated cytotoxicity. J Invest Dermatol. 1989;93:423–428. [PubMed] [Google Scholar]
- 19.Steffen M, Abboud M, Potter GK, Yung YP, Moore MA. Presence of tumour necrosis factor or a related factor in human basophil/mast cells. Immunology. 1989;66:445–450. [PMC free article] [PubMed] [Google Scholar]
- 20.Galli SJ, Gordon JR, Wershil BK. Cytokine production by mast cells and basophils. Curr Opin Immunol. 1991;3:865–872. doi: 10.1016/s0952-7915(05)80005-6. [DOI] [PubMed] [Google Scholar]
- 21.Benyon RC, Bissonnette EY, Befus AD. Tumor necrosis factor-alpha dependent cytotoxicity of human skin mast cells is enhanced by anti-IgE antibodies. J Immunol. 1991;147:2253–2258. [PubMed] [Google Scholar]
- 22.Ohkawara Y, Yamauchi K, Tanno Y, et al. Human lung mast cells and pulmonary macrophages produce tumor necrosis factor-alpha in sensitized lung tissue after IgE receptor triggering. Am J Respir Cell Mol Biol. 1992;7:385–392. doi: 10.1165/ajrcmb/7.4.385. [DOI] [PubMed] [Google Scholar]
- 23.Okayama Y, Ono Y, Nakazawa T, Church MK, Mori M. Human skin mast cells produce TNF-alpha by substance P. Int Arch Allergy Immunol. 1998;117(Suppl 1):48–51. doi: 10.1159/000053571. [DOI] [PubMed] [Google Scholar]
- 24.Latour S, Bonnerot C, Fridman WH, Daeron M. Induction of tumor necrosis factor-alpha production by mast cells via Fc gamma R. Role of the Fc gamma RIII gamma subunit. J Immunol. 1992;149:2155–2162. [PubMed] [Google Scholar]
- 25.Ansel JC, Brown JR, Payan DG, Brown MA. Substance P selectively activates TNF-alpha gene expression in murine mast cells. J Immunol. 1993;150:4478–4485. [PubMed] [Google Scholar]
- 26.Walsh LJ. Ultraviolet B irradiation of skin induces mast cell degranulation and release of tumour necrosis factor-alpha. Immunol Cell Biol. 1995;73:226–233. doi: 10.1038/icb.1995.37. [DOI] [PubMed] [Google Scholar]
- 27.Gibbs BF, Wierecky J, Welker P, et al. Human skin mast cells rapidly release preformed and newly generated TNF-alpha and IL-8 following stimulation with anti-IgE and other secretagogues. Exp Dermatol. 2001;10:312–320. doi: 10.1034/j.1600-0625.2001.100503.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhang B, Alysandratos KD, Angelidou A, et al. Human mast cell degranulation and preformed TNF secretion require mitochondrial translocation to exocytosis sites: relevance to atopic dermatitis. J Allergy Clin Immunol. 2011;127:1522–1531.e1528. doi: 10.1016/j.jaci.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Ramos BF, Jakschik BA. Neutrophil recruitment by tumor necrosis factor from mast cells in immune complex peritonitis. Science. 1992;258:1957–1959. doi: 10.1126/science.1470922. [DOI] [PubMed] [Google Scholar]
- 30.Groves RW, Allen MH, Ross EL, Barker JN, MacDonald DM. Tumour necrosis factor alpha is pro-inflammatory in normal human skin and modulates cutaneous adhesion molecule expression. Br J Dermatol. 1995;132:345–352. doi: 10.1111/j.1365-2133.1995.tb08666.x. [DOI] [PubMed] [Google Scholar]
- 31.Olszewski MB, Trzaska D, Knol EF, Adamczewska V, Dastych J. Efficient sorting of TNF-alpha to rodent mast cell granules is dependent on N-linked glycosylation. Eur J Immunol. 2006;36:997–1008. doi: 10.1002/eji.200535323. [DOI] [PubMed] [Google Scholar]
- 32.Olszewski MB, Groot AJ, Dastych J, Knol EF. TNF trafficking to human mast cell granules: mature chain-dependent endocytosis. J Immunol. 2007;178:5701–5709. doi: 10.4049/jimmunol.178.9.5701. [DOI] [PubMed] [Google Scholar]
- 33.Varricchi G, Galdiero MR, Loffredo S, et al. Are Mast Cells MASTers in Cancer? Front Immunol. 2017;8:424. doi: 10.3389/fimmu.2017.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ribatti D, Crivellato E, Candussio L, et al. Mast cells and their secretory granules are angiogenic in the chick embryo chorioallantoic membrane. Clin Exp Allergy. 2001;31:602–608. doi: 10.1046/j.1365-2222.2001.00986.x. [DOI] [PubMed] [Google Scholar]
- 35.Marichal T, Tsai M, Galli SJ. Mast cells: potential positive and negative roles in tumor biology. Cancer Immunol Res. 2013;1:269–279. doi: 10.1158/2326-6066.CIR-13-0119. [DOI] [PubMed] [Google Scholar]
- 36.Wershil BK, Wang ZS, Gordon JR, Galli SJ. Recruitment of neutrophils during IgE-dependent cutaneous late phase reactions in the mouse is mast cell-dependent. Partial inhibition of the reaction with antiserum against tumor necrosis factor-alpha. J Clin Invest. 1991;87:446–453. doi: 10.1172/JCI115016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biedermann T, Kneilling M, Mailhammer R, et al. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J Exp Med. 2000;192:1441–1452. doi: 10.1084/jem.192.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakae S, Suto H, Berry GJ, Galli SJ. Mast cell-derived TNF can promote Th17 cell-dependent neutrophil recruitment in ovalbumin-challenged OTII mice. Blood. 2007;109:3640–3648. doi: 10.1182/blood-2006-09-046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suto H, Nakae S, Kakurai M, et al. Mast cell-associated TNF promotes dendritic cell migration. J Immunol. 2006;176:4102–4112. doi: 10.4049/jimmunol.176.7.4102. [DOI] [PubMed] [Google Scholar]
- 41.de Vries VC, Pino-Lagos K, Nowak EC, et al. Mast cells condition dendritic cells to mediate allograft tolerance. Immunity. 2011;35:550–561. doi: 10.1016/j.immuni.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dudeck J, Ghouse SM, Lehmann CH, et al. Mast-Cell-Derived TNF Amplifies CD8(+) Dendritic Cell Functionality and CD8(+) T Cell Priming. Cell Rep. 2015;13:399–411. doi: 10.1016/j.celrep.2015.08.078. [DOI] [PubMed] [Google Scholar]
- 43.Kunder CA, St John AL, Li G, et al. Mast cell-derived particles deliver peripheral signals to remote lymph nodes. J Exp Med. 2009;206:2455–2467. doi: 10.1084/jem.20090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLachlan JB, Hart JP, Pizzo SV, et al. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat Immunol. 2003;4:1199–1205. doi: 10.1038/ni1005. [DOI] [PubMed] [Google Scholar]
- 45.Gaudenzio N, Sibilano R, Marichal T, et al. Different activation signals induce distinct mast cell degranulation strategies. J Clin Invest. 2016;126:3981–3998. doi: 10.1172/JCI85538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakae S, Suto H, Iikura M, et al. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol. 2006;176:2238–2248. doi: 10.4049/jimmunol.176.4.2238. [DOI] [PubMed] [Google Scholar]
- 47.Kakurai M, Monteforte R, Suto H, et al. Mast cell-derived tumor necrosis factor can promote nerve fiber elongation in the skin during contact hypersensitivity in mice. Am J Pathol. 2006;169:1713–1721. doi: 10.2353/ajpath.2006.060602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naukkarinen A, Harvima IT, Aalto ML, Harvima RJ, Horsmanheimo M. Quantitative analysis of contact sites between mast cells and sensory nerves in cutaneous psoriasis and lichen planus based on a histochemical double staining technique. Arch Dermatol Res. 1991;283:433–437. doi: 10.1007/BF00371778. [DOI] [PubMed] [Google Scholar]
- 49.Hagforsen E, Nordlind K, Michaelsson G. Skin nerve fibres and their contacts with mast cells in patients with palmoplantar pustulosis. Arch Dermatol Res. 2000;292:269–274. doi: 10.1007/s004030000132. [DOI] [PubMed] [Google Scholar]
- 50.Bauer O, Razin E. Mast Cell-Nerve Interactions. News Physiol Sci. 2000;15:213–218. doi: 10.1152/physiologyonline.2000.15.5.213. [DOI] [PubMed] [Google Scholar]
- 51.Gordon JR, Galli SJ. Promotion of mouse fibroblast collagen gene expression by mast cells stimulated via the Fc epsilon RI. Role for mast cell-derived transforming growth factor beta and tumor necrosis factor alpha. J Exp Med. 1994;180:2027–2037. doi: 10.1084/jem.180.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kendall JC, Li XH, Galli SJ, Gordon JR. Promotion of mouse fibroblast proliferation by IgE-dependent activation of mouse mast cells: role for mast cell tumor necrosis factor-alpha and transforming growth factor-beta 1. J Allergy Clin Immunol. 1997;99:113–123. doi: 10.1016/s0091-6749(97)70308-7. [DOI] [PubMed] [Google Scholar]
- 53.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 54.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 55.Prodeus AP, Zhou X, Maurer M, Galli SJ, Carroll MC. Impaired mast cell-dependent natural immunity in complement C3-deficient mice. Nature. 1997;390:172–175. doi: 10.1038/36586. [DOI] [PubMed] [Google Scholar]
- 56.Gommerman JL, Oh DY, Zhou X, et al. A role for CD21/CD35 and CD19 in responses to acute septic peritonitis: a potential mechanism for mast cell activation. J Immunol. 2000;165:6915–6921. doi: 10.4049/jimmunol.165.12.6915. [DOI] [PubMed] [Google Scholar]
- 57.Supajatura V, Ushio H, Nakao A, et al. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest. 2002;109:1351–1359. doi: 10.1172/JCI14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Varadaradjalou S, Feger F, Thieblemont N, et al. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human mast cells. Eur J Immunol. 2003;33:899–906. doi: 10.1002/eji.200323830. [DOI] [PubMed] [Google Scholar]
- 59.Maurer M, Echtenacher B, Hultner L, et al. The c-kit ligand, stem cell factor, can enhance innate immunity through effects on mast cells. J Exp Med. 1998;188:2343–2348. doi: 10.1084/jem.188.12.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piliponsky AM, Chen CC, Grimbaldeston MA, et al. Mast cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-KitW-sh/W-sh mice. Am J Pathol. 2010;176:926–938. doi: 10.2353/ajpath.2010.090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piliponsky AM, Chen CC, Rios EJ, et al. The chymase mouse mast cell protease 4 degrades TNF, limits inflammation, and promotes survival in a model of sepsis. Am J Pathol. 2012;181:875–886. doi: 10.1016/j.ajpath.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodewald HR, Feyerabend TB. Widespread immunological functions of mast cells: fact or fiction? Immunity. 2012;37:13–24. doi: 10.1016/j.immuni.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 63.Reber LL, Marichal T, Galli SJ. New models for analyzing mast cell functions in vivo. Trends Immunol. 2012;33:613–625. doi: 10.1016/j.it.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galli SJ, Tsai M, Marichal T, et al. Approaches for analyzing the roles of mast cells and their proteases in vivo. Adv Immunol. 2015;126:45–127. doi: 10.1016/bs.ai.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Striz I. Cytokines of the IL-1 family: recognized targets in chronic inflammation underrated in organ transplantations. Clin Sci (Lond) 2017;131:2241–2256. doi: 10.1042/CS20170098. [DOI] [PubMed] [Google Scholar]
- 66.Guma M, Kashiwakura J, Crain B, et al. JNK1 controls mast cell degranulation and IL-1{beta} production in inflammatory arthritis. Proc Natl Acad Sci U S A. 2010;107:22122–22127. doi: 10.1073/pnas.1016401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakamura Y, Franchi L, Kambe N, et al. Critical role for mast cells in interleukin-1beta-driven skin inflammation associated with an activating mutation in the nlrp3 protein. Immunity. 2012;37:85–95. doi: 10.1016/j.immuni.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakamura Y, Kambe N, Saito M, et al. Mast cells mediate neutrophil recruitment and vascular leakage through the NLRP3 inflammasome in histamine-independent urticaria. J Exp Med. 2009;206:1037–1046. doi: 10.1084/jem.20082179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nigrovic PA, Binstadt BA, Monach PA, et al. Mast cells contribute to initiation of autoantibody-mediated arthritis via IL-1. Proc Natl Acad Sci U S A. 2007;104:2325–2330. doi: 10.1073/pnas.0610852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reber LL, Marichal T, Sokolove J, et al. Contribution of mast cell-derived interleukin-1beta to uric acid crystal-induced acute arthritis in mice. Arthritis Rheumatol. 2014;66:2881–2891. doi: 10.1002/art.38747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 72.Hershko AY, Suzuki R, Charles N, et al. Mast cell interleukin-2 production contributes to suppression of chronic allergic dermatitis. Immunity. 2011;35:562–571. doi: 10.1016/j.immuni.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morita H, Arae K, Unno H, et al. An Interleukin-33-Mast Cell-Interleukin-2 Axis Suppresses Papain-Induced Allergic Inflammation by Promoting Regulatory T Cell Numbers. Immunity. 2015;43:175–186. doi: 10.1016/j.immuni.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moretti S, Renga G, Oikonomou V, et al. A mast cell-ILC2-Th9 pathway promotes lung inflammation in cystic fibrosis. Nat Commun. 2017;8:14017. doi: 10.1038/ncomms14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ihle JN, Keller J, Oroszlan S, et al. Biologic properties of homogeneous interleukin 3. I. Demonstration of WEHI-3 growth factor activity, mast cell growth factor activity, p cell-stimulating factor activity, colony-stimulating factor activity, and histamine-producing cell-stimulating factor activity. J Immunol. 1983;131:282–287. [PubMed] [Google Scholar]
- 76.Razin E, Ihle JN, Seldin D, et al. Interleukin 3: A differentiation and growth factor for the mouse mast cell that contains chondroitin sulfate E proteoglycan. J Immunol. 1984;132:1479–1486. [PubMed] [Google Scholar]
- 77.Kirshenbaum AS, Goff JP, Dreskin SC, et al. IL-3-dependent growth of basophil-like cells and mastlike cells from human bone marrow. J Immunol. 1989;142:2424–2429. [PubMed] [Google Scholar]
- 78.Lantz CS, Boesiger J, Song CH, et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 79.Mukai K, BenBarak MJ, Tachibana M, et al. Critical role of P1-Runx1 in mouse basophil development. Blood. 2012;120:76–85. doi: 10.1182/blood-2011-12-399113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kohno M, Yamasaki S, Tybulewicz VL, Saito T. Rapid and large amount of autocrine IL-3 production is responsible for mast cell survival by IgE in the absence of antigen. Blood. 2005;105:2059–2065. doi: 10.1182/blood-2004-07-2639. [DOI] [PubMed] [Google Scholar]
- 81.Williams CM, Galli SJ. The diverse potential effector and immunoregulatory roles of mast cells in allergic disease. J Allergy Clin Immunol. 2000;105:847–859. doi: 10.1067/mai.2000.106485. [DOI] [PubMed] [Google Scholar]
- 82.McLeod JJ, Baker B, Ryan JJ. Mast cell production and response to IL-4 and IL-13. Cytokine. 2015;75:57–61. doi: 10.1016/j.cyto.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174:1063–1072. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 84.MacNeil AJ, Yang YJ, Lin TJ. MAPK kinase 3 specifically regulates Fc epsilonRI-mediated IL-4 production by mast cells. J Immunol. 2011;187:3374–3382. doi: 10.4049/jimmunol.1003126. [DOI] [PubMed] [Google Scholar]
- 85.Komai-Koma M, Brombacher F, Pushparaj PN, et al. Interleukin-33 amplifies IgE synthesis and triggers mast cell degranulation via interleukin-4 in naive mice. Allergy. 2012;67:1118–1126. doi: 10.1111/j.1398-9995.2012.02859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoffmann HJ, Dahl C, Schiotz PO, Berglund L, Dahl R. Lectins interact differentially with purified human eosinophils, cultured cord blood-derived mast cells and the myeloid leukaemic cell line AML14.3D10: induction of interleukin-4 secretion is conserved among granulocytes, but is not proportional to agglutination or lectin-glycoprotein interaction. Clin Exp Allergy. 2003;33:930–935. doi: 10.1046/j.1365-2222.2003.01625.x. [DOI] [PubMed] [Google Scholar]
- 87.Kirshenbaum AS, Swindle E, Kulka M, Wu Y, Metcalfe DD. Effect of lipopolysaccharide (LPS) and peptidoglycan (PGN) on human mast cell numbers, cytokine production, and protease composition. BMC Immunol. 2008;9:45. doi: 10.1186/1471-2172-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gregory GD, Raju SS, Winandy S, Brown MA. Mast cell IL-4 expression is regulated by Ikaros and influences encephalitogenic Th1 responses in EAE. J Clin Invest. 2006;116:1327–1336. doi: 10.1172/JCI27227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bradding P, Feather IH, Howarth PH, et al. Interleukin 4 is localized to and released by human mast cells. J Exp Med. 1992;176:1381–1386. doi: 10.1084/jem.176.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bradding P, Feather IH, Wilson S, et al. Immunolocalization of cytokines in the nasal mucosa of normal and perennial rhinitic subjects. The mast cell as a source of IL-4, IL-5, and IL-6 in human allergic mucosal inflammation. J Immunol. 1993;151:3853–3865. [PubMed] [Google Scholar]
- 91.Horsmanheimo L, Harvima IT, Jarvikallio A, et al. Mast cells are one major source of interleukin-4 in atopic dermatitis. Br J Dermatol. 1994;131:348–353. doi: 10.1111/j.1365-2133.1994.tb08522.x. [DOI] [PubMed] [Google Scholar]
- 92.Bradding P, Feather IH, Wilson S, Holgate ST, Howarth PH. Cytokine immunoreactivity in seasonal rhinitis: regulation by a topical corticosteroid. Am J Respir Crit Care Med. 1995;151:1900–1906. doi: 10.1164/ajrccm.151.6.7767538. [DOI] [PubMed] [Google Scholar]
- 93.Bradding P, Roberts JA, Britten KM, et al. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol. 1994;10:471–480. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- 94.Min B, Prout M, Hu-Li J, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mitre E, Taylor RT, Kubofcik J, Nutman TB. Parasite antigen-driven basophils are a major source of IL-4 in human filarial infections. J Immunol. 2004;172:2439–2445. doi: 10.4049/jimmunol.172.4.2439. [DOI] [PubMed] [Google Scholar]
- 96.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–277. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 97.Weiss DL, Hural J, Tara D, et al. Nuclear factor of activated T cells is associated with a mast cell interleukin 4 transcription complex. Mol Cell Biol. 1996;16:228–235. doi: 10.1128/mcb.16.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Solymar DC, Agarwal S, Bassing CH, Alt FW, Rao A. A 3’ enhancer in the IL-4 gene regulates cytokine production by Th2 cells and mast cells. Immunity. 2002;17:41–50. doi: 10.1016/s1074-7613(02)00334-5. [DOI] [PubMed] [Google Scholar]
- 99.Doherty TA, Khorram N, Lund S, et al. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132:205–213. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pelly VS, Kannan Y, Coomes SM, et al. IL-4-producing ILC2s are required for the differentiation of TH2 cells following Heligmosomoides polygyrus infection. Mucosal Immunol. 2016;9:1407–1417. doi: 10.1038/mi.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yanagida M, Fukamachi H, Ohgami K, et al. Effects of T-helper 2-type cytokines, interleukin-3 (IL-3), IL-4, IL-5, and IL-6 on the survival of cultured human mast cells. Blood. 1995;86:3705–3714. [PubMed] [Google Scholar]
- 102.Toru H, Ra C, Nonoyama S, et al. Induction of the high-affinity IgE receptor (Fc epsilon RI) on human mast cells by IL-4. Int Immunol. 1996;8:1367–1373. doi: 10.1093/intimm/8.9.1367. [DOI] [PubMed] [Google Scholar]
- 103.Yamaguchi M, Sayama K, Yano K, et al. IgE enhances Fc epsilon receptor I expression and IgE-dependent release of histamine and lipid mediators from human umbilical cord blood-derived mast cells: synergistic effect of IL-4 and IgE on human mast cell Fc epsilon receptor I expression and mediator release. J Immunol. 1999;162:5455–5465. [PubMed] [Google Scholar]
- 104.Kulka M, Metcalfe DD. High-resolution tracking of cell division demonstrates differential effects of TH1 and TH2 cytokines on SCF-dependent human mast cell production in vitro: correlation with apoptosis and Kit expression. Blood. 2005;105:592–599. doi: 10.1182/blood-2004-07-2838. [DOI] [PubMed] [Google Scholar]
- 105.Takatsu K. Interleukin-5 and IL-5 receptor in health and diseases. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:463–485. doi: 10.2183/pjab.87.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Varricchi G, Bagnasco D, Borriello F, Heffler E, Canonica GW. Interleukin-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: evidence and unmet needs. Curr Opin Allergy Clin Immunol. 2016;16:186–200. doi: 10.1097/ACI.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Masuda A, Yoshikai Y, Aiba K, Matsuguchi T. Th2 cytokine production from mast cells is directly induced by lipopolysaccharide and distinctly regulated by c-Jun N-terminal kinase and p38 pathways. J Immunol. 2002;169:3801–3810. doi: 10.4049/jimmunol.169.7.3801. [DOI] [PubMed] [Google Scholar]
- 108.Williams CM, Coleman JW. Induced expression of mRNA for IL-5, IL-6, TNF-alpha, MIP-2 and IFN-gamma in immunologically activated rat peritoneal mast cells: inhibition by dexamethasone and cyclosporin A. Immunology. 1995;86:244–249. [PMC free article] [PubMed] [Google Scholar]
- 109.Bressler RB, Lesko J, Jones ML, et al. Production of IL-5 and granulocyte-macrophage colony-stimulating factor by naive human mast cells activated by high-affinity IgE receptor ligation. J Allergy Clin Immunol. 1997;99:508–514. doi: 10.1016/s0091-6749(97)70078-2. [DOI] [PubMed] [Google Scholar]
- 110.Hsu CL, Neilsen CV, Bryce PJ. IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PLoS One. 2010;5:e11944. doi: 10.1371/journal.pone.0011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wallaert B, Desreumaux P, Copin MC, et al. Immunoreactivity for interleukin 3 and 5 and granulocyte/macrophage colony-stimulating factor of intestinal mucosa in bronchial asthma. J Exp Med. 1995;182:1897–1904. doi: 10.1084/jem.182.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ochi H, De Jesus NH, Hsieh FH, Austen KF, Boyce JA. IL-4 and -5 prime human mast cells for different profiles of IgE-dependent cytokine production. Proc Natl Acad Sci U S A. 2000;97:10509–10513. doi: 10.1073/pnas.180318697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McKenzie AN. Type-2 innate lymphoid cells in asthma and allergy. Ann Am Thorac Soc. 2014;11(Suppl 5):S263–270. doi: 10.1513/AnnalsATS.201403-097AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim BS, Artis D. Group 2 innate lymphoid cells in health and disease. Cold Spring Harb Perspect Biol. 2015;7 doi: 10.1101/cshperspect.a016337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ealey KN, Moro K, Koyasu S. Are ILC2s Jekyll and Hyde in airway inflammation? Immunol Rev. 2017;278:207–218. doi: 10.1111/imr.12547. [DOI] [PubMed] [Google Scholar]
- 116.Huang Y, Paul WE. Inflammatory group 2 innate lymphoid cells. Int Immunol. 2016;28:23–28. doi: 10.1093/intimm/dxv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 118.Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood. 1995;86:1243–1254. [PubMed] [Google Scholar]
- 119.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 120.Tanaka T, Kishimoto T. Targeting interleukin-6: all the way to treat autoimmune and inflammatory diseases. Int J Biol Sci. 2012;8:1227–1236. doi: 10.7150/ijbs.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22:347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 122.Leal-Berumen I, Conlon P, Marshall JS. IL-6 production by rat peritoneal mast cells is not necessarily preceded by histamine release and can be induced by bacterial lipopolysaccharide. J Immunol. 1994;152:5468–5476. [PubMed] [Google Scholar]
- 123.Azzolina A, Bongiovanni A, Lampiasi N. Substance P induces TNF-alpha and IL-6 production through NF kappa B in peritoneal mast cells. Biochim Biophys Acta. 2003;1643:75–83. doi: 10.1016/j.bbamcr.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 124.Kandere-Grzybowska K, Letourneau R, Kempuraj D, et al. IL-1 induces vesicular secretion of IL-6 without degranulation from human mast cells. J Immunol. 2003;171:4830–4836. doi: 10.4049/jimmunol.171.9.4830. [DOI] [PubMed] [Google Scholar]
- 125.Ho LH, Ohno T, Oboki K, et al. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcepsilonRI signals. J Leukoc Biol. 2007;82:1481–1490. doi: 10.1189/jlb.0407200. [DOI] [PubMed] [Google Scholar]
- 126.Sun J, Sukhova GK, Wolters PJ, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 127.Liu J, Divoux A, Sun J, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gutierrez DA, Muralidhar S, Feyerabend TB, Herzig S, Rodewald HR. Hematopoietic Kit Deficiency, rather than Lack of Mast Cells, Protects Mice from Obesity and Insulin Resistance. Cell Metab. 2015;21:678–691. doi: 10.1016/j.cmet.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 129.Feyerabend TB, Gutierrez DA, Rodewald HR. Of Mouse Models of Mast Cell Deficiency and Metabolic Syndrome. Cell Metab. 2016;24:1–2. doi: 10.1016/j.cmet.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 130.Saito H, Ebisawa M, Tachimoto H, et al. Selective growth of human mast cells induced by Steel factor, IL-6, and prostaglandin E2 from cord blood mononuclear cells. J Immunol. 1996;157:343–350. [PubMed] [Google Scholar]
- 131.Desai A, Jung MY, Olivera A, et al. IL-6 promotes an increase in human mast cell numbers and reactivity through suppression of suppressor of cytokine signaling 3. J Allergy Clin Immunol. 2016;137:1863–1871 e1866. doi: 10.1016/j.jaci.2015.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rojas-Zuleta WG, Sanchez E. IL-9: Function, Sources, and Detection. Methods Mol Biol. 2017;1585:21–35. doi: 10.1007/978-1-4939-6877-0_2. [DOI] [PubMed] [Google Scholar]
- 133.Kaplan MH, Hufford MM, Olson MR. The development and in vivo function of T helper 9 cells. Nat Rev Immunol. 2015;15:295–307. doi: 10.1038/nri3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schmitt E, Bopp T. Discovery and initial characterization of Th9 cells: the early years. Semin Immunopathol. 2017;39:5–10. doi: 10.1007/s00281-016-0610-0. [DOI] [PubMed] [Google Scholar]
- 135.Stassen M, Arnold M, Hultner L, et al. Murine bone marrow-derived mast cells as potent producers of IL-9: costimulatory function of IL-10 and kit ligand in the presence of IL-1. J Immunol. 2000;164:5549–5555. doi: 10.4049/jimmunol.164.11.5549. [DOI] [PubMed] [Google Scholar]
- 136.Hultner L, Kolsch S, Stassen M, et al. In activated mast cells, IL-1 up-regulates the production of several Th2-related cytokines including IL-9. J Immunol. 2000;164:5556–5563. doi: 10.4049/jimmunol.164.11.5556. [DOI] [PubMed] [Google Scholar]
- 137.Stassen M, Muller C, Arnold M, et al. IL-9 and IL-13 production by activated mast cells is strongly enhanced in the presence of lipopolysaccharide: NF-kappa B is decisively involved in the expression of IL-9. J Immunol. 2001;166:4391–4398. doi: 10.4049/jimmunol.166.7.4391. [DOI] [PubMed] [Google Scholar]
- 138.Chen CY, Lee JB, Liu B, et al. Induction of Interleukin-9-Producing Mucosal Mast Cells Promotes Susceptibility to IgE-Mediated Experimental Food Allergy. Immunity. 2015;43:788–802. doi: 10.1016/j.immuni.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Barlow JL, McKenzie AN. Type-2 innate lymphoid cells in human allergic disease. Curr Opin Allergy Clin Immunol. 2014;14:397–403. doi: 10.1097/ACI.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 141.Matsuzawa S, Sakashita K, Kinoshita T, et al. IL-9 enhances the growth of human mast cell progenitors under stimulation with stem cell factor. J Immunol. 2003;170:3461–3467. doi: 10.4049/jimmunol.170.7.3461. [DOI] [PubMed] [Google Scholar]
- 142.Lora JM, Al-Garawi A, Pickard MD, et al. FcepsilonRI-dependent gene expression in human mast cells is differentially controlled by T helper type 2 cytokines. J Allergy Clin Immunol. 2003;112:1119–1126. doi: 10.1016/j.jaci.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 143.Wiener Z, Falus A, Toth S. IL-9 increases the expression of several cytokines in activated mast cells, while the IL-9-induced IL-9 production is inhibited in mast cells of histamine-free transgenic mice. Cytokine. 2004;26:122–130. doi: 10.1016/j.cyto.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 144.Hultner L, Druez C, Moeller J, et al. Mast cell growth-enhancing activity (MEA) is structurally related and functionally identical to the novel mouse T cell growth factor P40/TCGFIII (interleukin 9) Eur J Immunol. 1990;20:1413–1416. doi: 10.1002/eji.1830200632. [DOI] [PubMed] [Google Scholar]
- 145.Godfraind C, Louahed J, Faulkner H, et al. Intraepithelial infiltration by mast cells with both connective tissue-type and mucosal-type characteristics in gut, trachea, and kidneys of IL-9 transgenic mice. J Immunol. 1998;160:3989–3996. [PubMed] [Google Scholar]
- 146.Faulkner H, Humphreys N, Renauld JC, Van Snick J, Grencis R. Interleukin-9 is involved in host protective immunity to intestinal nematode infection. Eur J Immunol. 1997;27:2536–2540. doi: 10.1002/eji.1830271011. [DOI] [PubMed] [Google Scholar]
- 147.Faulkner H, Renauld JC, Van Snick J, Grencis RK. Interleukin-9 enhances resistance to the intestinal nematode Trichuris muris. Infect Immun. 1998;66:3832–3840. doi: 10.1128/iai.66.8.3832-3840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kearley J, Erjefalt JS, Andersson C, et al. IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways. Am J Respir Crit Care Med. 2011;183:865–875. doi: 10.1164/rccm.200909-1462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sehra S, Yao W, Nguyen ET, et al. TH9 cells are required for tissue mast cell accumulation during allergic inflammation. J Allergy Clin Immunol. 2015;136:433–440.e431. doi: 10.1016/j.jaci.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Temann UA, Geba GP, Rankin JA, Flavell RA. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. J Exp Med. 1998;188:1307–1320. doi: 10.1084/jem.188.7.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Forbes EE, Groschwitz K, Abonia JP, et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Townsend JM, Fallon GP, Matthews JD, et al. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 2000;13:573–583. doi: 10.1016/s1074-7613(00)00056-x. [DOI] [PubMed] [Google Scholar]
- 153.Lu LF, Lind EF, Gondek DC, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 154.Eller K, Wolf D, Huber JM, et al. IL-9 production by regulatory T cells recruits mast cells that are essential for regulatory T cell-induced immune suppression. J Immunol. 2011;186:83–91. doi: 10.4049/jimmunol.1001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 156.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 157.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 158.Hawrylowicz CM, O’Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5:271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 159.Dudeck A, Dudeck J, Scholten J, et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 2011;34:973–984. doi: 10.1016/j.immuni.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 160.Bryce PJ, Miller ML, Miyajima I, et al. Immune sensitization in the skin is enhanced by antigen-independent effects of IgE. Immunity. 2004;20:381–392. doi: 10.1016/s1074-7613(04)00080-9. [DOI] [PubMed] [Google Scholar]
- 161.Norman MU, Hwang J, Hulliger S, et al. Mast cells regulate the magnitude and the cytokine microenvironment of the contact hypersensitivity response. Am J Pathol. 2008;172:1638–1649. doi: 10.2353/ajpath.2008.070559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Feyerabend TB, Weiser A, Tietz A, et al. Cre-mediated cell ablation contests mast cell contribution in models of antibody- and T cell-mediated autoimmunity. Immunity. 2011;35:832–844. doi: 10.1016/j.immuni.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 163.Gimenez-Rivera VA, Siebenhaar F, Zimmermann C, et al. Mast Cells Limit the Exacerbation of Chronic Allergic Contact Dermatitis in Response to Repeated Allergen Exposure. J Immunol. 2016;197:4240–4246. doi: 10.4049/jimmunol.1600236. [DOI] [PubMed] [Google Scholar]
- 164.Morita H, Saito H, Matsumoto K, Nakae S. Regulatory roles of mast cells in immune responses. Semin Immunopathol. 2016;38:623–629. doi: 10.1007/s00281-016-0566-0. [DOI] [PubMed] [Google Scholar]
- 165.Reber LL, Sibilano R, Starkl P, et al. Imaging protective mast cells in living mice during severe contact hypersensitivity. JCI Insight. 2017;2 doi: 10.1172/jci.insight.92900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Madan R, Demircik F, Surianarayanan S, et al. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol. 2009;183:2312–2320. doi: 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lehtimaki S, Savinko T, Lahl K, et al. The temporal and spatial dynamics of Foxp3+ Treg cell-mediated suppression during contact hypersensitivity responses in a murine model. J Invest Dermatol. 2012;132:2744–2751. doi: 10.1038/jid.2012.212. [DOI] [PubMed] [Google Scholar]
- 168.Lilla JN, Chen CC, Mukai K, et al. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood. 2011;118:6930–6938. doi: 10.1182/blood-2011-03-343962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chan CY, St John AL, Abraham SN. Mast cell interleukin-10 drives localized tolerance in chronic bladder infection. Immunity. 2013;38:349–359. doi: 10.1016/j.immuni.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chacon-Salinas R, Limon-Flores AY, Chavez-Blanco AD, Gonzalez-Estrada A, Ullrich SE. Mast cell-derived IL-10 suppresses germinal center formation by affecting T follicular helper cell function. J Immunol. 2011;186:25–31. doi: 10.4049/jimmunol.1001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Leveson-Gower DB, Sega EI, Kalesnikoff J, et al. Mast cells suppress murine GVHD in a mechanism independent of CD4+CD25+ regulatory T cells. Blood. 2013;122:3659–3665. doi: 10.1182/blood-2013-08-519157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 173.Putoczki TL, Dobson RC, Griffin MD. The structure of human interleukin-11 reveals receptor-binding site features and structural differences from interleukin-6. Acta Crystallogr D Biol Crystallogr. 2014;70:2277–2285. doi: 10.1107/S1399004714012267. [DOI] [PubMed] [Google Scholar]
- 174.Negahdaripour M, Nezafat N, Ghasemi Y. A panoramic review and in silico analysis of IL-11 structure and function. Cytokine Growth Factor Rev. 2016;32:41–61. doi: 10.1016/j.cytogfr.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 175.Nandurkar HH, Robb L, Begley CG. The role of IL-II in hematopoiesis as revealed by a targeted mutation of its receptor. Stem Cells. 1998;16(Suppl 2):53–65. doi: 10.1002/stem.5530160708. [DOI] [PubMed] [Google Scholar]
- 176.Minshall E, Chakir J, Laviolette M, et al. IL-11 expression is increased in severe asthma: association with epithelial cells and eosinophils. J Allergy Clin Immunol. 2000;105:232–238. doi: 10.1016/s0091-6749(00)90070-8. [DOI] [PubMed] [Google Scholar]
- 177.Zheng T, Zhu Z, Wang J, Homer RJ, Elias JA. IL-11: insights in asthma from overexpression transgenic modeling. J Allergy Clin Immunol. 2001;108:489–496. doi: 10.1067/mai.2001.118510. [DOI] [PubMed] [Google Scholar]
- 178.Silver JS, Hunter CA. gp130 at the nexus of inflammation, autoimmunity, and cancer. J Leukoc Biol. 2010;88:1145–1156. doi: 10.1189/jlb.0410217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sayama K, Diehn M, Matsuda K, et al. Transcriptional response of human mast cells stimulated via the Fc(epsilon)RI and identification of mast cells as a source of IL-11. BMC Immunol. 2002;3:5. doi: 10.1186/1471-2172-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gately MK, Renzetti LM, Magram J, et al. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 181.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 182.Nakano N, Nishiyama C, Kanada S, et al. Involvement of mast cells in IL-12/23 p40 production is essential for survival from polymicrobial infections. Blood. 2007;109:4846–4855. doi: 10.1182/blood-2006-09-045641. [DOI] [PubMed] [Google Scholar]
- 183.Smith TJ, Ducharme LA, Weis JH. Preferential expression of interleukin-12 or interleukin-4 by murine bone marrow mast cells derived in mast cell growth factor or interleukin-3. Eur J Immunol. 1994;24:822–826. doi: 10.1002/eji.1830240408. [DOI] [PubMed] [Google Scholar]
- 184.Gupta AA, Leal-Berumen I, Croitoru K, Marshall JS. Rat peritoneal mast cells produce IFN-gamma following IL-12 treatment but not in response to IgE-mediated activation. J Immunol. 1996;157:2123–2128. [PubMed] [Google Scholar]
- 185.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 186.Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol. 2015;15:271–282. doi: 10.1038/nri3831. [DOI] [PubMed] [Google Scholar]
- 187.Bagnasco D, Ferrando M, Varricchi G, Passalacqua G, Canonica GW. A Critical Evaluation of Anti-IL-13 and Anti-IL-4 Strategies in Severe Asthma. Int Arch Allergy Immunol. 2016;170:122–131. doi: 10.1159/000447692. [DOI] [PubMed] [Google Scholar]
- 188.Burd PR, Thompson WC, Max EE, Mills FC. Activated mast cells produce interleukin 13. J Exp Med. 1995;181:1373–1380. doi: 10.1084/jem.181.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yu M, Eckart MR, Morgan AA, et al. Identification of an IFN-gamma/mast cell axis in a mouse model of chronic asthma. J Clin Invest. 2011;121:3133–3143. doi: 10.1172/JCI43598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lee SA, Fitzgerald SM, Huang SK, et al. Molecular regulation of interleukin-13 and monocyte chemoattractant protein-1 expression in human mast cells by interleukin-1beta. Am J Respir Cell Mol Biol. 2004;31:283–291. doi: 10.1165/rcmb.2004-0089OC. [DOI] [PubMed] [Google Scholar]
- 191.Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007;179:2051–2054. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- 192.Ndaw VS, Abebayehu D, Spence AJ, et al. TGF-beta1 Suppresses IL-33-Induced Mast Cell Function. J Immunol. 2017;199:866–873. doi: 10.4049/jimmunol.1601983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Li B, Berman J, Tang JT, Lin TJ. The early growth response factor-1 is involved in stem cell factor (SCF)-induced interleukin 13 production by mast cells, but is dispensable for SCF-dependent mast cell growth. J Biol Chem. 2007;282:22573–22581. doi: 10.1074/jbc.M610859200. [DOI] [PubMed] [Google Scholar]
- 194.Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14:536–542. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 195.Ebbo M, Crinier A, Vely F, Vivier E. Innate lymphoid cells: major players in inflammatory diseases. Nat Rev Immunol. 2017 doi: 10.1038/nri.2017.86. [DOI] [PubMed] [Google Scholar]
- 196.Cruikshank WW, Kornfeld H, Center DM. Interleukin-16. J Leukoc Biol. 2000;67:757–766. doi: 10.1002/jlb.67.6.757. [DOI] [PubMed] [Google Scholar]
- 197.Qi JC, Wang J, Mandadi S, et al. Human and mouse mast cells use the tetraspanin CD9 as an alternate interleukin-16 receptor. Blood. 2006;107:135–142. doi: 10.1182/blood-2005-03-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Qi JC, Stevens RL, Wadley R, et al. IL-16 regulation of human mast cells/basophils and their susceptibility to HIV-1. J Immunol. 2002;168:4127–4134. doi: 10.4049/jimmunol.168.8.4127. [DOI] [PubMed] [Google Scholar]
- 199.Rumsaeng V, Cruikshank WW, Foster B, et al. Human mast cells produce the CD4+ T lymphocyte chemoattractant factor, IL-16. J Immunol. 1997;159:2904–2910. [PubMed] [Google Scholar]
- 200.Lorentz A, Schwengberg S, Sellge G, Manns MP, Bischoff SC. Human intestinal mast cells are capable of producing different cytokine profiles: role of IgE receptor cross-linking and IL-4. J Immunol. 2000;164:43–48. doi: 10.4049/jimmunol.164.1.43. [DOI] [PubMed] [Google Scholar]
- 201.Laberge S, Pinsonneault S, Ernst P, et al. Phenotype of IL-16-producing cells in bronchial mucosa: evidence for the human eosinophil and mast cell as cellular sources of IL-16 in asthma. Int Arch Allergy Immunol. 1999;119:120–125. doi: 10.1159/000024186. [DOI] [PubMed] [Google Scholar]
- 202.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Oboki K, Ohno T, Kajiwara N, Saito H, Nakae S. IL-33 and IL-33 receptors in host defense and diseases. Allergol Int. 2010;59:143–160. doi: 10.2332/allergolint.10-RAI-0186. [DOI] [PubMed] [Google Scholar]
- 204.Cayrol C, Girard JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol. 2014;31:31–37. doi: 10.1016/j.coi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 205.Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16:676–689. doi: 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- 206.Iikura M, Suto H, Kajiwara N, et al. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007;87:971–978. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 207.Levescot A, Flamant S, Basbous S, et al. BCR-ABL-induced deregulation of the IL-33/ST2 pathway in CD34+ progenitors from chronic myeloid leukemia patients. Cancer Res. 2014;74:2669–2676. doi: 10.1158/0008-5472.CAN-13-2797. [DOI] [PubMed] [Google Scholar]
- 208.Ohno T, Oboki K, Kajiwara N, et al. Caspase-1, caspase-8, and calpain are dispensable for IL-33 release by macrophages. J Immunol. 2009;183:7890–7897. doi: 10.4049/jimmunol.0802449. [DOI] [PubMed] [Google Scholar]
- 209.Lefrancais E, Duval A, Mirey E, et al. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci U S A. 2014;111:15502–15507. doi: 10.1073/pnas.1410700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Morita H, Nakae S, Saito H, Matsumoto K. IL-33 in clinical practice: Size matters? J Allergy Clin Immunol. 2017;140:381–383. doi: 10.1016/j.jaci.2017.03.042. [DOI] [PubMed] [Google Scholar]
- 211.Waern I, Lundequist A, Pejler G, Wernersson S. Mast cell chymase modulates IL-33 levels and controls allergic sensitization in dust-mite induced airway inflammation. Mucosal Immunol. 2013;6:911–920. doi: 10.1038/mi.2012.129. [DOI] [PubMed] [Google Scholar]
- 212.Roy A, Ganesh G, Sippola H, et al. Mast cell chymase degrades the alarmins heat shock protein 70, biglycan, HMGB1, and interleukin-33 (IL-33) and limits danger-induced inflammation. J Biol Chem. 2014;289:237–250. doi: 10.1074/jbc.M112.435156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Singh B, Carpenter G, Coffey RJ. EGF receptor ligands: recent advances. F1000Res. 2016:5. doi: 10.12688/f1000research.9025.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Toda S, Tokuda Y, Koike N, et al. Growth factor-expressing mast cells accumulate at the thyroid tissue-regenerative site of subacute thyroiditis. Thyroid. 2000;10:381–386. doi: 10.1089/thy.2000.10.381. [DOI] [PubMed] [Google Scholar]
- 215.Artuc M, Steckelings UM, Henz BM. Mast cell-fibroblast interactions: human mast cells as source and inducers of fibroblast and epithelial growth factors. J Invest Dermatol. 2002;118:391–395. doi: 10.1046/j.0022-202x.2001.01705.x. [DOI] [PubMed] [Google Scholar]
- 216.Norrby K. Mast cells and angiogenesis. Apmis. 2002;110:355–371. doi: 10.1034/j.1600-0463.2002.100501.x. [DOI] [PubMed] [Google Scholar]
- 217.Qu Z, Liebler JM, Powers MR, et al. Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous hemangioma. Am J Pathol. 1995;147:564–573. [PMC free article] [PubMed] [Google Scholar]
- 218.Friedl A, Chang Z, Tierney A, Rapraeger AC. Differential binding of fibroblast growth factor-2 and -7 to basement membrane heparan sulfate: comparison of normal and abnormal human tissues. Am J Pathol. 1997;150:1443–1455. [PMC free article] [PubMed] [Google Scholar]
- 219.Roos AB, Mori M, Gura HK, et al. Increased IL-17RA and IL-17RC in End-Stage COPD and the Contribution to Mast Cell Secretion of FGF-2 and VEGF. Respir Res. 2017;18:48. doi: 10.1186/s12931-017-0534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wroblewski M, Bauer R, Cubas Cordova M, et al. Mast cells decrease efficacy of anti-angiogenic therapy by secreting matrix-degrading granzyme B. Nat Commun. 2017;8:269. doi: 10.1038/s41467-017-00327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986;67:257–267. [PubMed] [Google Scholar]
- 222.McCurdy JD, Olynych TJ, Maher LH, Marshall JS. Cutting edge: distinct Toll-like receptor 2 activators selectively induce different classes of mediator production from human mast cells. J Immunol. 2003;170:1625–1629. doi: 10.4049/jimmunol.170.4.1625. [DOI] [PubMed] [Google Scholar]
- 223.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 224.Nafziger J, Arock M, Guillosson JJ, Wietzerbin J. Specific high-affinity receptors for interferon-gamma on mouse bone marrow-derived mast cells: inhibitory effect of interferon-gamma on mast cell precursors. Eur J Immunol. 1990;20:113–117. doi: 10.1002/eji.1830200117. [DOI] [PubMed] [Google Scholar]
- 225.Mann-Chandler MN, Kashyap M, Wright HV, et al. IFN-gamma induces apoptosis in developing mast cells. J Immunol. 2005;175:3000–3005. doi: 10.4049/jimmunol.175.5.3000. [DOI] [PubMed] [Google Scholar]
- 226.Kirshenbaum AS, Worobec AS, Davis TA, et al. Inhibition of human mast cell growth and differentiation by interferon gamma-1b. Exp Hematol. 1998;26:245–251. [PubMed] [Google Scholar]
- 227.Coleman JW, Buckley MG, Holliday MR, Morris AG. Interferon-gamma inhibits serotonin release from mouse peritoneal mast cells. Eur J Immunol. 1991;21:2559–2564. doi: 10.1002/eji.1830211037. [DOI] [PubMed] [Google Scholar]
- 228.Holliday MR, Banks EM, Dearman RJ, Kimber I, Coleman JW. Interactions of IFN-gamma with IL-3 and IL-4 in the regulation of serotonin and arachidonate release from mouse peritoneal mast cells. Immunology. 1994;82:70–74. [PMC free article] [PubMed] [Google Scholar]
- 229.Bissonnette EY, Befus AD. Inhibition of mast cell-mediated cytotoxicity by IFN-alpha/beta and -gamma. J Immunol. 1990;145:3385–3390. [PubMed] [Google Scholar]
- 230.Yanagida M, Fukamachi H, Takei M, et al. Interferon-gamma promotes the survival and Fc epsilon RI-mediated histamine release in cultured human mast cells. Immunology. 1996;89:547–552. doi: 10.1046/j.1365-2567.1996.d01-768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Okayama Y, Kirshenbaum AS, Metcalfe DD. Expression of a functional high-affinity IgG receptor, Fc gamma RI, on human mast cells: Up-regulation by IFN-gamma. J Immunol. 2000;164:4332–4339. doi: 10.4049/jimmunol.164.8.4332. [DOI] [PubMed] [Google Scholar]
- 232.Sellge G, Barkowsky M, Kramer S, et al. Interferon-gamma regulates growth and controls Fcgamma receptor expression and activation in human intestinal mast cells. BMC Immunol. 2014;15:27. doi: 10.1186/1471-2172-15-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Okumura S, Kashiwakura J, Tomita H, et al. Identification of specific gene expression profiles in human mast cells mediated by Toll-like receptor 4 and FcepsilonRI. Blood. 2003;102:2547–2554. doi: 10.1182/blood-2002-12-3929. [DOI] [PubMed] [Google Scholar]
- 234.Lewis CC, Aronow B, Hutton J, et al. Unique and overlapping gene expression patterns driven by IL-4 and IL-13 in the mouse lung. J Allergy Clin Immunol. 2009;123:795–804.e798. doi: 10.1016/j.jaci.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Bissonnette EY, Chin B, Befus AD. Interferons differentially regulate histamine and TNF-alpha in rat intestinal mucosal mast cells. Immunology. 1995;86:12–17. [PMC free article] [PubMed] [Google Scholar]
- 236.Raundhal M, Morse C, Khare A, et al. High IFN-gamma and low SLPI mark severe asthma in mice and humans. J Clin Invest. 2015;125:3037–3050. doi: 10.1172/JCI80911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ray A, Raundhal M, Oriss TB, Ray P, Wenzel SE. Current concepts of severe asthma. J Clin Invest. 2016;126:2394–2403. doi: 10.1172/JCI84144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gauthier M, Chakraborty K, Oriss TB, et al. Severe asthma in humans and mouse model suggests a CXCL10 signature underlies corticosteroid-resistant Th1 bias. JCI Insight. 2017;2 doi: 10.1172/jci.insight.94580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Levi-Montalcini R, Angeletti PU. Essential role of the nerve growth factor in the survival and maintenance of dissociated sensory and sympathetic embryonic nerve cells in vitro. Dev Biol. 1963;6:653–659. doi: 10.1016/0012-1606(63)90149-0. [DOI] [PubMed] [Google Scholar]
- 240.Yankner BA, Shooter EM. The biology and mechanism of action of nerve growth factor. Annu Rev Biochem. 1982;51:845–868. doi: 10.1146/annurev.bi.51.070182.004213. [DOI] [PubMed] [Google Scholar]
- 241.Aloe L, Levi-Montalcini R. Mast cells increase in tissues of neonatal rats injected with the nerve growth factor. Brain Res. 1977;133:358–366. doi: 10.1016/0006-8993(77)90772-7. [DOI] [PubMed] [Google Scholar]
- 242.Horigome K, Bullock ED, Johnson EM., Jr Effects of nerve growth factor on rat peritoneal mast cells. Survival promotion and immediate-early gene induction. J Biol Chem. 1994;269:2695–2702. [PubMed] [Google Scholar]
- 243.Matsuda H, Kannan Y, Ushio H, et al. Nerve growth factor induces development of connective tissue-type mast cells in vitro from murine bone marrow cells. J Exp Med. 1991;174:7–14. doi: 10.1084/jem.174.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Aloe L. The effect of nerve growth factor and its antibody on mast cells in vivo. J Neuroimmunol. 1988;18:1–12. doi: 10.1016/0165-5728(88)90129-4. [DOI] [PubMed] [Google Scholar]
- 245.Sugiyama K, Suzuki Y, Furuta H. Histamine-release induced by 7S nerve-growth factor of mouse submandibular salivary glands. Arch Oral Biol. 1985;30:93–95. doi: 10.1016/0003-9969(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 246.Bruni A, Bigon E, Boarato E, et al. Interaction between nerve growth factor and lysophosphatidylserine on rat peritoneal mast cells. FEBS Lett. 1982;138:190–192. doi: 10.1016/0014-5793(82)80438-9. [DOI] [PubMed] [Google Scholar]
- 247.Pearce FL, Thompson HL. Some characteristics of histamine secretion from rat peritoneal mast cells stimulated with nerve growth factor. J Physiol. 1986;372:379–393. doi: 10.1113/jphysiol.1986.sp016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Mazurek N, Weskamp G, Erne P, Otten U. Nerve growth factor induces mast cell degranulation without changing intracellular calcium levels. FEBS Lett. 1986;198:315–320. doi: 10.1016/0014-5793(86)80428-8. [DOI] [PubMed] [Google Scholar]
- 249.Kawamoto K, Aoki J, Tanaka A, et al. Nerve growth factor activates mast cells through the collaborative interaction with lysophosphatidylserine expressed on the membrane surface of activated platelets. J Immunol. 2002;168:6412–6419. doi: 10.4049/jimmunol.168.12.6412. [DOI] [PubMed] [Google Scholar]
- 250.Sawada J, Itakura A, Tanaka A, Furusaka T, Matsuda H. Nerve growth factor functions as a chemoattractant for mast cells through both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling pathways. Blood. 2000;95:2052–2058. [PubMed] [Google Scholar]
- 251.Kassel O, de Blay F, Duvernelle C, et al. Local increase in the number of mast cells and expression of nerve growth factor in the bronchus of asthmatic patients after repeated inhalation of allergen at low-dose. Clin Exp Allergy. 2001;31:1432–1440. doi: 10.1046/j.1365-2222.2001.01177.x. [DOI] [PubMed] [Google Scholar]
- 252.Lambiase A, Bonini S, Bonini S, et al. Increased plasma levels of nerve growth factor in vernal keratoconjunctivitis and relationship to conjunctival mast cells. Invest Ophthalmol Vis Sci. 1995;36:2127–2132. [PubMed] [Google Scholar]
- 253.Tuveri MA, Passiu G, Mathieu A, Aloe L. Nerve growth factor and mast cell distribution in the skin of patients with systemic sclerosis. Clin Exp Rheumatol. 1993;11:319–322. [PubMed] [Google Scholar]
- 254.Valent P, Spanblochl E, Sperr WR, et al. Induction of differentiation of human mast cells from bone marrow and peripheral blood mononuclear cells by recombinant human stem cell factor/kit-ligand in long-term culture. Blood. 1992;80:2237–2245. [PubMed] [Google Scholar]
- 255.Bischoff SC, Dahinden CA. Effect of nerve growth factor on the release of inflammatory mediators by mature human basophils. Blood. 1992;79:2662–2669. [PubMed] [Google Scholar]
- 256.Tsuda T, Wong D, Dolovich J, et al. Synergistic effects of nerve growth factor and granulocyte-macrophage colony-stimulating factor on human basophilic cell differentiation. Blood. 1991;77:971–979. [PubMed] [Google Scholar]
- 257.Nilsson G, Forsberg-Nilsson K, Xiang Z, et al. Human mast cells express functional TrkA and are a source of nerve growth factor. Eur J Immunol. 1997;27:2295–2301. doi: 10.1002/eji.1830270925. [DOI] [PubMed] [Google Scholar]
- 258.Tam SY, Tsai M, Yamaguchi M, et al. Expression of functional TrkA receptor tyrosine kinase in the HMC-1 human mast cell line and in human mast cells. Blood. 1997;90:1807–1820. [PubMed] [Google Scholar]
- 259.Welker P, Grabbe J, Grutzkau A, Henz BM. Effects of nerve growth factor (NGF) and other fibroblast-derived growth factors on immature human mast cells (HMC-1) Immunology. 1998;94:310–317. [PMC free article] [PubMed] [Google Scholar]
- 260.Welker P, Grabbe J, Gibbs B, Zuberbier T, Henz BM. Nerve growth factor-beta induces mast-cell marker expression during in vitro culture of human umbilical cord blood cells. Immunology. 2000;99:418–426. doi: 10.1046/j.1365-2567.2000.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kanbe N, Kurosawa M, Miyachi Y, et al. Nerve growth factor prevents apoptosis of cord blood-derived human cultured mast cells synergistically with stem cell factor. Clin Exp Allergy. 2000;30:1113–1120. doi: 10.1046/j.1365-2222.2000.00866.x. [DOI] [PubMed] [Google Scholar]
- 262.Leon A, Buriani A, Dal Toso R, et al. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci U S A. 1994;91:3739–3743. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 264.Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annu Rev Pathol. 2013;8:241–276. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Gacche RN, Meshram RJ. Angiogenic factors as potential drug target: efficacy and limitations of anti-angiogenic therapy. Biochim Biophys Acta. 2014;1846:161–179. doi: 10.1016/j.bbcan.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 266.van Steensel L, Hooijkaas H, Paridaens D, et al. PDGF enhances orbital fibroblast responses to TSHR stimulating autoantibodies in Graves’ ophthalmopathy patients. J Clin Endocrinol Metab. 2012;97:E944–953. doi: 10.1210/jc.2012-1020. [DOI] [PubMed] [Google Scholar]
- 267.Liao CH, Akazawa H, Tamagawa M, et al. Cardiac mast cells cause atrial fibrillation through PDGF-A-mediated fibrosis in pressure-overloaded mouse hearts. J Clin Invest. 2010;120:242–253. doi: 10.1172/JCI39942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Huang E, Nocka K, Beier DR, et al. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990;63:225–233. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- 269.Zsebo KM, Williams DA, Geissler EN, et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63:213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]
- 270.Galli SJ, Tsai M, Gordon JR, Geissler EN, Wershil BK. Analyzing mast cell development and function using mice carrying mutations at W/c-kit or Sl/MGF (SCF) loci. Ann N Y Acad Sci. 1992;664:69–88. doi: 10.1111/j.1749-6632.1992.tb39750.x. [DOI] [PubMed] [Google Scholar]
- 271.Galli SJ, Tsai M, Wershil BK, Tam SY, Costa JJ. Regulation of mouse and human mast cell development, survival and function by stem cell factor, the ligand for the c-kit receptor. Int Arch Allergy Immunol. 1995;107:51–53. doi: 10.1159/000236928. [DOI] [PubMed] [Google Scholar]
- 272.Heinrich MC, Dooley DC, Freed AC, et al. Constitutive expression of steel factor gene by human stromal cells. Blood. 1993;82:771–783. [PubMed] [Google Scholar]
- 273.Linenberger ML, Jacobson FW, Bennett LG, et al. Stem cell factor production by human marrow stromal fibroblasts. Exp Hematol. 1995;23:1104–1114. [PubMed] [Google Scholar]
- 274.Ashman LK. The biology of stem cell factor and its receptor C-kit. Int J Biochem Cell Biol. 1999;31:1037–1051. doi: 10.1016/s1357-2725(99)00076-x. [DOI] [PubMed] [Google Scholar]
- 275.Zhang S, Anderson DF, Bradding P, et al. Human mast cells express stem cell factor. J Pathol. 1998;186:59–66. doi: 10.1002/(SICI)1096-9896(199809)186:1<59::AID-PATH140>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 276.Patella V, Marino I, Arbustini E, et al. Stem cell factor in mast cells and increased mast cell density in idiopathic and ischemic cardiomyopathy. Circulation. 1998;97:971–978. doi: 10.1161/01.cir.97.10.971. [DOI] [PubMed] [Google Scholar]
- 277.Welker P, Grabbe J, Gibbs B, Zuberbier T, Henz BM. Human mast cells produce and differentially express both soluble and membrane-bound stem cell factor. Scand J Immunol. 1999;49:495–500. doi: 10.1046/j.1365-3083.1999.00519.x. [DOI] [PubMed] [Google Scholar]
- 278.de Paulis A, Minopoli G, Arbustini E, et al. Stem cell factor is localized in, released from, and cleaved by human mast cells. J Immunol. 1999;163:2799–2808. [PubMed] [Google Scholar]
- 279.Akin C, Jaffe ES, Raffeld M, et al. An immunohistochemical study of the bone marrow lesions of systemic mastocytosis: expression of stem cell factor by lesional mast cells. Am J Clin Pathol. 2002;118:242–247. doi: 10.1309/71KH-4JE4-E0J1-7THH. [DOI] [PubMed] [Google Scholar]
- 280.Amagai Y, Tanaka A, Matsuda A, et al. Stem cell factor contributes to tumorigenesis of mast cells via an autocrine/paracrine mechanism. J Leukoc Biol. 2013;93:245–250. doi: 10.1189/jlb.0512245. [DOI] [PubMed] [Google Scholar]
- 281.Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 283.Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 284.Travis MA, Sheppard D. TGF-beta activation and function in immunity. Annu Rev Immunol. 2014;32:51–82. doi: 10.1146/annurev-immunol-032713-120257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Sanjabi S, Oh SA, Li MO. Regulation of the Immune Response by TGF-beta: From Conception to Autoimmunity and Infection. Cold Spring Harb Perspect Biol. 2017;9 doi: 10.1101/cshperspect.a022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Fernando J, Faber TW, Pullen NA, et al. Genotype-dependent effects of TGF-beta1 on mast cell function: targeting the Stat5 pathway. J Immunol. 2013;191:4505–4513. doi: 10.4049/jimmunol.1202723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kelly A, Houston SA, Sherwood E, Casulli J, Travis MA. Regulation of Innate and Adaptive Immunity by TGFbeta. Adv Immunol. 2017;134:137–233. doi: 10.1016/bs.ai.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 288.Pennington DW, Lopez AR, Thomas PS, Peck C, Gold WM. Dog mastocytoma cells produce transforming growth factor beta 1. J Clin Invest. 1992;90:35–41. doi: 10.1172/JCI115853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Lindstedt KA, Wang Y, Shiota N, et al. Activation of paracrine TGF-beta1 signaling upon stimulation and degranulation of rat serosal mast cells: a novel function for chymase. Faseb j. 2001;15:1377–1388. doi: 10.1096/fj.00-0273com. [DOI] [PubMed] [Google Scholar]
- 290.Kanbe N, Kurosawa M, Nagata H, Saitoh H, Miyachi Y. Cord blood-derived human cultured mast cells produce transforming growth factor beta1. Clin Exp Allergy. 1999;29:105–113. doi: 10.1046/j.1365-2222.1999.00459.x. [DOI] [PubMed] [Google Scholar]
- 291.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 292.Bissonnette EY, Enciso JA, Befus AD. TGF-beta1 inhibits the release of histamine and tumor necrosis factor-alpha from mast cells through an autocrine pathway. Am J Respir Cell Mol Biol. 1997;16:275–282. doi: 10.1165/ajrcmb.16.3.9070612. [DOI] [PubMed] [Google Scholar]
- 293.Zhao W, Gomez G, Yu SH, Ryan JJ, Schwartz LB. TGF-beta1 attenuates mediator release and de novo Kit expression by human skin mast cells through a Smad-dependent pathway. J Immunol. 2008;181:7263–7272. doi: 10.4049/jimmunol.181.10.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Broide DH, Wasserman SI, Alvaro-Gracia J, Zvaifler NJ, Firestein GS. Transforming growth factor-beta 1 selectively inhibits IL-3-dependent mast cell proliferation without affecting mast cell function or differentiation. J Immunol. 1989;143:1591–1597. [PubMed] [Google Scholar]
- 295.Toyota N, Hashimoto Y, Matsuo S, Iizuka H. Transforming growth factor beta 1 inhibits IL-3- and IL-4-dependent mouse connective tissue-type mast cell proliferation. Arch Dermatol Res. 1995;287:198–201. doi: 10.1007/BF01262332. [DOI] [PubMed] [Google Scholar]
- 296.Gomez G, Ramirez CD, Rivera J, et al. TGF-beta 1 inhibits mast cell Fc epsilon RI expression. J Immunol. 2005;174:5987–5993. doi: 10.4049/jimmunol.174.10.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Meade R, Askenase PW, Geba GP, et al. Transforming growth factor-beta 1 inhibits murine immediate and delayed type hypersensitivity. J Immunol. 1992;149:521–528. [PubMed] [Google Scholar]
- 298.Ganeshan K, Bryce PJ. Regulatory T cells enhance mast cell production of IL-6 via surface-bound TGF-beta. J Immunol. 2012;188:594–603. doi: 10.4049/jimmunol.1102389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Miller HR, Wright SH, Knight PA, Thornton EM. A novel function for transforming growth factor-beta1: upregulation of the expression and the IgE-independent extracellular release of a mucosal mast cell granule-specific beta-chymase, mouse mast cell protease-1. Blood. 1999;93:3473–3486. [PubMed] [Google Scholar]
- 300.Kim HM, Lee YM. Role of TGF-beta 1 on the IgE-dependent anaphylaxis reaction. J Immunol. 1999;162:4960–4965. [PubMed] [Google Scholar]
- 301.Gri G, Piconese S, Frossi B, et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Taipale J, Lohi J, Saarinen J, Kovanen PT, Keski-Oja J. Human mast cell chymase and leukocyte elastase release latent transforming growth factor-beta 1 from the extracellular matrix of cultured human epithelial and endothelial cells. J Biol Chem. 1995;270:4689–4696. doi: 10.1074/jbc.270.9.4689. [DOI] [PubMed] [Google Scholar]
- 303.Takai S, Sakonjo H, Fukuda K, et al. A novel chymase inhibitor, 2-(5-formylamino-6-oxo-2-phenyl-1,6-dihydropyrimidine-1-yl)-N-[[,4-dioxo-1-phenyl -7-(2-pyridyloxy)]2-heptyl]acetamide (NK3201), suppressed intimal hyperplasia after balloon injury. J Pharmacol Exp Ther. 2003;304:841–844. doi: 10.1124/jpet.102.042580. [DOI] [PubMed] [Google Scholar]
- 304.Okamoto Y, Takai S, Miyazaki M. Effect of chymase-dependent transforming growth factor beta on peritoneal adhesion formation in a rat model. Surg Today. 2004;34:865–867. doi: 10.1007/s00595-004-2836-z. [DOI] [PubMed] [Google Scholar]
- 305.Tomimori Y, Muto T, Saito K, et al. Involvement of mast cell chymase in bleomycin-induced pulmonary fibrosis in mice. Eur J Pharmacol. 2003;478:179–185. doi: 10.1016/j.ejphar.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 306.Takato H, Yasui M, Ichikawa Y, et al. The specific chymase inhibitor TY-51469 suppresses the accumulation of neutrophils in the lung and reduces silica-induced pulmonary fibrosis in mice. Exp Lung Res. 2011;37:101–108. doi: 10.3109/01902148.2010.520815. [DOI] [PubMed] [Google Scholar]
- 307.Beghdadi W, Madjene LC, Claver J, et al. Mast cell chymase protects against renal fibrosis in murine unilateral ureteral obstruction. Kidney Int. 2013;84:317–326. doi: 10.1038/ki.2013.98. [DOI] [PubMed] [Google Scholar]
- 308.Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001;22:201–207. doi: 10.1016/s0165-6147(00)01676-x. [DOI] [PubMed] [Google Scholar]
- 309.Nagy JA, Dvorak AM, Dvorak HF. Vascular hyperpermeability, angiogenesis, and stroma generation. Cold Spring Harb Perspect Med. 2012;2:a006544. doi: 10.1101/cshperspect.a006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Dvorak HF, Dvorak AM, Manseau EJ, Wiberg L, Churchill WH. Fibrin gel investment associated with line 1 and line 10 solid tumor growth, angiogenesis, and fibroplasia in guinea pigs. Role of cellular immunity, myofibroblasts, microvascular damage, and infarction in line 1 tumor regression. J Natl Cancer Inst. 1979;62:1459–1472. [PubMed] [Google Scholar]
- 311.Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 312.Senger DR, Perruzzi CA, Feder J, Dvorak HF. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 1986;46:5629–5632. [PubMed] [Google Scholar]
- 313.Senger DR, Connolly DT, Van de Water L, Feder J, Dvorak HF. Purification and NH2-terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor. Cancer Res. 1990;50:1774–1778. [PubMed] [Google Scholar]
- 314.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 315.Matsumoto T, Claesson-Welsh L. VEGF receptor signal transduction. Sci STKE. 2001;2001:re21. doi: 10.1126/stke.2001.112.re21. [DOI] [PubMed] [Google Scholar]
- 316.Holmes DI, Zachary I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol. 2005;6:209. doi: 10.1186/gb-2005-6-2-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Boesiger J, Tsai M, Maurer M, et al. Mast cells can secrete vascular permeability factor/vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of Fc epsilon RI expression. J Exp Med. 1998;188:1135–1145. doi: 10.1084/jem.188.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Grutzkau A, Kruger-Krasagakes S, Baumeister H, et al. Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: implications for the biological significance of VEGF206. Mol Biol Cell. 1998;9:875–884. doi: 10.1091/mbc.9.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Theoharides TC, Zhang B, Kempuraj D, et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci U S A. 2010;107:4448–4453. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Cao J, Papadopoulou N, Kempuraj D, et al. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:7665–7675. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- 321.Johnzon CF, Ronnberg E, Guss B, Pejler G. Live Staphylococcus aureus Induces Expression and Release of Vascular Endothelial Growth Factor in Terminally Differentiated Mouse Mast Cells. Front Immunol. 2016;7:247. doi: 10.3389/fimmu.2016.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Abdel-Majid RM, Marshall JS. Prostaglandin E2 induces degranulation-independent production of vascular endothelial growth factor by human mast cells. J Immunol. 2004;172:1227–1236. doi: 10.4049/jimmunol.172.2.1227. [DOI] [PubMed] [Google Scholar]
- 323.Detoraki A, Staiano RI, Granata F, et al. Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. J Allergy Clin Immunol. 2009;123:1142–1149. 1149 e1141–1145. doi: 10.1016/j.jaci.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 324.Loffredo S, Staiano RI, Granata F, Genovese A, Marone G. Immune cells as a source and target of angiogenic and lymphangiogenic factors. Chem Immunol Allergy. 2014;99:15–36. doi: 10.1159/000353316. [DOI] [PubMed] [Google Scholar]
- 325.Gruber BL, Marchese MJ, Kew R. Angiogenic factors stimulate mast-cell migration. Blood. 1995;86:2488–2493. [PubMed] [Google Scholar]
- 326.Detmar M, Brown LF, Schon MP, et al. Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol. 1998;111:1–6. doi: 10.1046/j.1523-1747.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- 327.Chetta A, Zanini A, Foresi A, et al. Vascular endothelial growth factor up-regulation and bronchial wall remodelling in asthma. Clin Exp Allergy. 2005;35:1437–1442. doi: 10.1111/j.1365-2222.2005.02360.x. [DOI] [PubMed] [Google Scholar]
- 328.Zanini A, Chetta A, Saetta M, et al. Chymase-positive mast cells play a role in the vascular component of airway remodeling in asthma. J Allergy Clin Immunol. 2007;120:329–333. doi: 10.1016/j.jaci.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 329.Lee KS, Kim SR, Park SJ, et al. Mast cells can mediate vascular permeability through regulation of the PI3K-HIF-1alpha-VEGF axis. Am J Respir Crit Care Med. 2008;178:787–797. doi: 10.1164/rccm.200801-008OC. [DOI] [PubMed] [Google Scholar]
- 330.Sawatsubashi M, Yamada T, Fukushima N, et al. Association of vascular endothelial growth factor and mast cells with angiogenesis in laryngeal squamous cell carcinoma. Virchows Arch. 2000;436:243–248. doi: 10.1007/s004280050037. [DOI] [PubMed] [Google Scholar]
- 331.Toth-Jakatics R, Jimi S, Takebayashi S, Kawamoto N. Cutaneous malignant melanoma: correlation between neovascularization and peritumor accumulation of mast cells overexpressing vascular endothelial growth factor. Hum Pathol. 2000;31:955–960. doi: 10.1053/hupa.2000.16658. [DOI] [PubMed] [Google Scholar]
- 332.Fukushima N, Satoh T, Sano M, Tokunaga O. Angiogenesis and mast cells in non-Hodgkin’s lymphoma: a strong correlation in angioimmunoblastic T-cell lymphoma. Leuk Lymphoma. 2001;42:709–720. doi: 10.3109/10428190109099333. [DOI] [PubMed] [Google Scholar]
- 333.Coussens LM, Raymond WW, Bergers G, et al. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Soucek L, Lawlor ER, Soto D, et al. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 335.Rabenhorst A, Schlaak M, Heukamp LC, et al. Mast cells play a protumorigenic role in primary cutaneous lymphoma. Blood. 2012;120:2042–2054. doi: 10.1182/blood-2012-03-415638. [DOI] [PubMed] [Google Scholar]
- 336.Heissig B, Rafii S, Akiyama H, et al. Low-dose irradiation promotes tissue revascularization through VEGF release from mast cells and MMP-9-mediated progenitor cell mobilization. J Exp Med. 2005;202:739–750. doi: 10.1084/jem.20050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 338.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 339.Schulz O, Hammerschmidt SI, Moschovakis GL, Forster R. Chemokines and Chemokine Receptors in Lymphoid Tissue Dynamics. Annu Rev Immunol. 2016;34:203–242. doi: 10.1146/annurev-immunol-041015-055649. [DOI] [PubMed] [Google Scholar]
- 340.Sallusto F, Baggiolini M. Chemokines and leukocyte traffic. Nat Immunol. 2008;9:949–952. doi: 10.1038/ni.f.214. [DOI] [PubMed] [Google Scholar]
- 341.Bordon Y. A breathtaking chemokine. Nat Rev Immunol. 2010;10:810. doi: 10.1038/nri2897. [DOI] [PubMed] [Google Scholar]
- 342.Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17:559–572. doi: 10.1038/nri.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Ansari AW, Heiken H, Moenkemeyer M, Schmidt RE. Dichotomous effects of C-C chemokines in HIV-1 pathogenesis. Immunol Lett. 2007;110:1–5. doi: 10.1016/j.imlet.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 344.Proudfoot AE. Chemokine receptors: multifaceted therapeutic targets. Nat Rev Immunol. 2002;2:106–115. doi: 10.1038/nri722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol. 2006;6:907–918. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]
- 346.Schall TJ, Proudfoot AE. Overcoming hurdles in developing successful drugs targeting chemokine receptors. Nat Rev Immunol. 2011;11:355–363. doi: 10.1038/nri2972. [DOI] [PubMed] [Google Scholar]
- 347.Nakajima T, Inagaki N, Tanaka H, et al. Marked increase in CC chemokine gene expression in both human and mouse mast cell transcriptomes following Fcepsilon receptor I cross-linking: an interspecies comparison. Blood. 2002;100:3861–3868. doi: 10.1182/blood-2002-02-0602. Epub 2002 Aug 3861. [DOI] [PubMed] [Google Scholar]
- 348.Matsuda K, Piliponsky AM, Iikura M, et al. Monomeric IgE enhances human mast cell chemokine production: IL-4 augments and dexamethasone suppresses the response. J Allergy Clin Immunol. 2005;116:1357–1363. doi: 10.1016/j.jaci.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 349.Daeron M, Latour S, Malbec O, et al. The same tyrosine-based inhibition motif, in the intracytoplasmic domain of Fc gamma RIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity. 1995;3:635–646. doi: 10.1016/1074-7613(95)90134-5. [DOI] [PubMed] [Google Scholar]
- 350.Daeron M, Malbec O, Latour S, Arock M, Fridman WH. Regulation of high-affinity IgE receptor-mediated mast cell activation by murine low-affinity IgG receptors. J Clin Invest. 1995;95:577–585. doi: 10.1172/JCI117701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Tkaczyk C, Okayama Y, Woolhiser MR, et al. Activation of human mast cells through the high affinity IgG receptor. Mol Immunol. 2002;38:1289–1293. doi: 10.1016/s0161-5890(02)00077-9. [DOI] [PubMed] [Google Scholar]
- 352.Mizutani H, Schechter N, Lazarus G, Black RA, Kupper TS. Rapid and specific conversion of precursor interleukin 1 beta (IL-1 beta) to an active IL-1 species by human mast cell chymase. J Exp Med. 1991;174:821–825. doi: 10.1084/jem.174.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Omoto Y, Tokime K, Yamanaka K, et al. Human mast cell chymase cleaves pro-IL-18 and generates a novel and biologically active IL-18 fragment. J Immunol. 2006;177:8315–8319. doi: 10.4049/jimmunol.177.12.8315. [DOI] [PubMed] [Google Scholar]
- 354.Longley BJ, Tyrrell L, Ma Y, et al. Chymase cleavage of stem cell factor yields a bioactive, soluble product. Proc Natl Acad Sci U S A. 1997;94:9017–9021. doi: 10.1073/pnas.94.17.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Pang L, Nie M, Corbett L, Sutcliffe A, Knox AJ. Mast cell beta-tryptase selectively cleaves eotaxin and RANTES and abrogates their eosinophil chemotactic activities. J Immunol. 2006;176:3788–3795. doi: 10.4049/jimmunol.176.6.3788. [DOI] [PubMed] [Google Scholar]
- 356.Gela A, Kasetty G, Jovic S, et al. Eotaxin-3 (CCL26) exerts innate host defense activities that are modulated by mast cell proteases. Allergy. 2015;70:161–170. doi: 10.1111/all.12542. [DOI] [PubMed] [Google Scholar]
- 357.Marshall JS, Leal-Berumen I, Nielsen L, Glibetic M, Jordana M. Interleukin (IL)-10 inhibits long-term IL-6 production but not preformed mediator release from rat peritoneal mast cells. J Clin Invest. 1996;97:1122–1128. doi: 10.1172/JCI118506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Vosskuhl K, Greten TF, Manns MP, Korangy F, Wedemeyer J. Lipopolysaccharide-mediated mast cell activation induces IFN-gamma secretion by NK cells. J Immunol. 2010;185:119–125. doi: 10.4049/jimmunol.0902406. [DOI] [PubMed] [Google Scholar]
- 359.Hultner L, Szots H, Welle M, et al. Mouse bone marrow-derived IL-3-dependent mast cells and autonomous sublines produce IL-6. Immunology. 1989;67:408–413. [PMC free article] [PubMed] [Google Scholar]
- 360.Falanga YT, Chaimowitz NS, Charles N, et al. Lyn but not Fyn kinase controls IgG-mediated systemic anaphylaxis. J Immunol. 2012;188:4360–4368. doi: 10.4049/jimmunol.1003223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Allakhverdi Z, Comeau MR, Jessup HK, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Reed JA, Albino AP, McNutt NS. Human cutaneous mast cells express basic fibroblast growth factor. Lab Invest. 1995;72:215–222. [PubMed] [Google Scholar]
- 363.Lin TJ, Maher LH, Gomi K, et al. Selective early production of CCL20, or macrophage inflammatory protein 3alpha, by human mast cells in response to Pseudomonas aeruginosa. Infect Immun. 2003;71:365–373. doi: 10.1128/IAI.71.1.365-373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Fan L, Iseki S. Immunohistochemical localization of vascular endothelial growth factor in the globule leukocyte/mucosal mast cell of the rat respiratory and digestive tracts. Histochem Cell Biol. 1999;111:13–21. doi: 10.1007/s004180050328. [DOI] [PubMed] [Google Scholar]
- 365.Gombert M, Dieu-Nosjean MC, Winterberg F, et al. CCL1-CCR8 interactions: an axis mediating the recruitment of T cells and Langerhans-type dendritic cells to sites of atopic skin inflammation. J Immunol. 2005;174:5082–5091. doi: 10.4049/jimmunol.174.8.5082. [DOI] [PubMed] [Google Scholar]
- 366.Chhiba KD, Hsu CL, Berdnikovs S, Bryce PJ. Transcriptional Heterogeneity of Mast Cells and Basophils upon Activation. J Immunol. 2017;198:4868–4878. doi: 10.4049/jimmunol.1601825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Wu Z, Macneil AJ, Junkins R, et al. Mast cell FcepsilonRI-induced early growth response 2 regulates CC chemokine ligand 1-dependent CD4+ T cell migration. J Immunol. 2013;190:4500–4507. doi: 10.4049/jimmunol.1203158. [DOI] [PubMed] [Google Scholar]
- 368.Burke SM, Issekutz TB, Mohan K, et al. Human mast cell activation with virus-associated stimuli leads to the selective chemotaxis of natural killer cells by a CXCL8-dependent mechanism. Blood. 2008;111:5467–5476. doi: 10.1182/blood-2007-10-118547. [DOI] [PubMed] [Google Scholar]
- 369.Yano K, Yamaguchi M, de Mora F, et al. Production of macrophage inflammatory protein-1alpha by human mast cells: increased anti-IgE-dependent secretion after IgE-dependent enhancement of mast cell IgE-binding ability. Lab Invest. 1997;77:185–193. [PubMed] [Google Scholar]
- 370.Wang HW, Tedla N, Lloyd AR, Wakefield D, McNeil PH. Mast cell activation and migration to lymph nodes during induction of an immune response in mice. J Clin Invest. 1998;102:1617–1626. doi: 10.1172/JCI3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.King CA, Anderson R, Marshall JS. Dengue virus selectively induces human mast cell chemokine production. J Virol. 2002;76:8408–8419. doi: 10.1128/JVI.76.16.8408-8419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Al-Afif A, Alyazidi R, Oldford SA, et al. Respiratory syncytial virus infection of primary human mast cells induces the selective production of type I interferons, CXCL10, and CCL4. J Allergy Clin Immunol. 2015;136:1346–1354.e1341. doi: 10.1016/j.jaci.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 373.Kimura Y, Pawankar R, Aoki M, Niimi Y, Kawana S. Mast cells and T cells in Kimura’s disease express increased levels of interleukin-4, interleukin-5, eotaxin and RANTES. Clin Exp Allergy. 2002;32:1787–1793. doi: 10.1046/j.1365-2222.2002.01552.x. [DOI] [PubMed] [Google Scholar]
- 374.Kuo CH, Collins AM, Boettner DR, Yang Y, Ono SJ. Role of CCL7 in Type I Hypersensitivity Reactions in Murine Experimental Allergic Conjunctivitis. J Immunol. 2017;198:645–656. doi: 10.4049/jimmunol.1502416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Wakahara S, Fujii Y, Nakao T, et al. Gene expression profiles for Fc epsilon RI, cytokines and chemokines upon Fc epsilon RI activation in human cultured mast cells derived from peripheral blood. Cytokine. 2001;16:143–152. doi: 10.1006/cyto.2001.0958. [DOI] [PubMed] [Google Scholar]
- 376.Kulmburg PA, Huber NE, Scheer BJ, Wrann M, Baumruker T. Immunoglobulin E plus antigen challenge induces a novel intercrine/chemokine in mouse mast cells. J Exp Med. 1992;176:1773–1778. doi: 10.1084/jem.176.6.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Ying S, O’Connor B, Ratoff J, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 378.Moller A, Lippert U, Lessmann D, et al. Human mast cells produce IL-8. J Immunol. 1993;151:3261–3266. [PubMed] [Google Scholar]
- 379.Kashiwakura J, Yanagisawa M, Lee H, et al. Interleukin-33 synergistically enhances immune complex-induced tumor necrosis factor alpha and interleukin-8 production in cultured human synovium-derived mast cells. Int Arch Allergy Immunol. 2013;161(Suppl 2):32–36. doi: 10.1159/000350424. [DOI] [PubMed] [Google Scholar]
- 380.Castellani ML, Ciampoli C, Felaco M, et al. Neuropeptide substance P induces mRNA expression and secretion of CXCL8 chemokine, and HDC in human umbilical cord blood mast cells. Clin Invest Med. 2008;31:E362–372. doi: 10.25011/cim.v31i6.4923. [DOI] [PubMed] [Google Scholar]
- 381.Galli SJ, Kalesnikoff J, Grimbaldeston MA, et al. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 382.Crivellato E, Ribatti D. The fundamental contribution of William Bate Hardy to shape the concept of mast cell heterogeneity. Br J Haematol. 2010;150:152–157. doi: 10.1111/j.1365-2141.2009.07938.x. [DOI] [PubMed] [Google Scholar]
- 383.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12:1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Peschke K, Dudeck A, Rabenhorst A, Hartmann K, Roers A. Cre/loxP-based mouse models of mast cell deficiency and mast cell-specific gene inactivation. Methods Mol Biol. 2015;1220:403–421. doi: 10.1007/978-1-4939-1568-2_25. [DOI] [PubMed] [Google Scholar]
- 385.Threadgill DW, Churchill GA. Ten years of the Collaborative Cross. Genetics. 2012;190:291–294. doi: 10.1534/genetics.111.138032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Ghazalpour A, Rau CD, Farber CR, et al. Hybrid mouse diversity panel: a panel of inbred mouse strains suitable for analysis of complex genetic traits. Mamm Genome. 2012;23:680–692. doi: 10.1007/s00335-012-9411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Bogue MA, Churchill GA, Chesler EJ. Collaborative Cross and Diversity Outbred data resources in the Mouse Phenome Database. Mamm Genome. 2015;26:511–520. doi: 10.1007/s00335-015-9595-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


