Abstract
Objective
The goal of this systematic review was to examine the evidence for the use of the neuromodulating agents, amitriptyline, gabapentin, pregabalin, and baclofen, in the management of chronic, idiopathic cough patients.
Data Sources
Online databases, including PubMed, Embase, Cochrane Review, and Web of Science, and publications cited in bibliographies were used.
Review Methods
Literature was searched by the 2 authors with a priori criteria for study selection.
Results
Eight relevant articles were identified, including 2 randomized controlled trials, 2 prospective cohort or case-series designs with consecutive patients, 1 retrospective case series of consecutive patients, 1 retrospective case series whose consecutive status was not known, and 2 case reports of 6 and 2 patients, respectively. Improvements in cough-specific quality of life were noted in the randomized controlled trials. Cough severity was reduced in studies that measured this outcome measure. In the remaining studies, cough symptoms were less after neuromodulator treatment.
Conclusion
Benefit from neuromodulator treatment with amitriptyline, gabapentin, pregabalin, and baclofen in chronic, idiopathic cough patients was demonstrated. Further investigations using objective and subjective outcome measures are needed as well as studies exploring optimal dose, length of treatment, and relapse rates posttreatment.
Keywords: chronic cough, idiopathic cough, neuromodulator, treatment, amitriptyline, gabapentin, pregabalin, baclofen
Chronic cough has significant public health implications. The cross-sectional prevalence of cough in a community population sample was 17.9%.1 In National Ambulatory Medical Care Survey data, cough was the most common patient-reported symptom prompting a physician visit.2 In 2010, over-the-counter costs for cough and cold remedies were $4 billion.3 Patients may have associated physical problems such as syncope, urinary incontinence, chest paint, sleep disturbance, and impaired quality of life (QOL), with 53% of chronic cough patients having depressive symptoms.4–7
Although a sequential, algorithmic approach can improve a chronic cough, some patients’ cough persists.8 Among specialty cough clinics, between 12% and 42% of patients have a refractory, idiopathic cough despite evaluation and treatment trials related to reflux, pulmonary disease, sinonasal disease, and stopping angiotensin-converting enzyme inhibitors (ACEI), among other common pathology.9,10 The role of laryngeal irritability and the concept of idiopathic, chronic cough as a sensory neuropathy provide new potential treatment options for this challenging clinical problem.11 Some patients have evidence of a neuropathy involving the recurrent or superior laryngeal nerve on laryngoscopy or laryngeal electromyography.12,13 Similar to patients with other neuralgias, chronic cough patients have been reported to have a trigger phenomenon where particular stimuli can induce coughing episodes.14 Consequently, the use of neuromodulating agents, such as amitriptyline, gabapentin, baclofen, and pregabalin, often used in the management of chronic, neuropathic pain, may provide some benefit in chronic cough patients.11–14 This is further supported by data showing that in healthy volunteers, baclofen raised the cough threshold during exposure to irritants such as capsaicin.15
Systematically examining the evidence regarding the use of neuromodulating medications in the treatment of idiopathic chronic cough patients may provide insights about their utility and a better understanding of the literature regarding this emerging management strategy. Current systematic reviews have focused on acute cough, reflux treatment in cough without primary lung disease, and pharmacologic and nonpharmacologic interventions in respiratory and nonrespiratory disease, to name a few.16–18 Thus, this systematic review was conducted to assess the literature concerning the use of amitriptyline, gabapentin, baclofen, and pregabalin in the management of idiopathic, chronic cough.
Methods
An online search strategy completed on April 1, 2012, was developed in conjunction with an information specialist at the Duke University Medical Center Library and University of Minnesota Medical Center Library. The PubMed, Exerpta Medica Database (Embase), Web of Science, and Cochrane Review databases were searched to identify potentially relevant articles using the following combinations: (cough or bronchitis or respiratory tract infections or idiopathic cough or postviral cough or irritable larynx or pharyngeal diseases or laryngeal diseases or postviral vagal neuropathy) and (elavil or amitriptyline or neurontin or gabapentin or lyrica or pregabalin or dibenzocycloheptenes or cyclohexanecarboxylic acids or gamma-aminobutryic acid). The terms dibenzocycloheptenes, cyclohexanecarboxylic acid, gamma-aminobutryic acid, or dimethylamines were 1 level higher in the MeSH tree and were incorporated to broaden the search and aid in identifying relevant articles.
The inclusion criteria included articles in English, involving adults with cough of unknown etiology of at least 6 weeks’ duration, and the use of the neuromodulating agents of interest in cough treatment. Articles involving patients younger than 18 years, animal studies, acute cough less than 6 weeks’ duration, cough due to other conditions (such as reflux disease, sinonasal pathology, allergy, pulmonary diseases, ACEIs), and other treatment modalities were excluded. All resulting abstracts were assessed by at least 1 reviewer to eliminate duplicate publications and unrelated topics. All relevant article bibliographies were examined to identify additional publications. Authors were contacted when needed to obtain full-text publications or publications in English.
A data abstraction form was developed a priori to facilitate consistent data extraction. The reference, country of origin, medications studied, medication regimen, diagnoses included, definition of chronic cough, study design, type of study outcome, number and type of participants, study setting, follow-up data, study inclusion and exclusion criteria, definition of treatment success, and medication side effects were noted for each study. All relevant studies were independently evaluated by 2 reviewers and consensus reached for study inclusion. Study validity was assessed qualitatively by describing the study design, including consecutive status of patients for nonrandomized controlled studies, type of study outcome, whether study outcome was chosen a priori, and inclusion/exclusion criteria for patient enrollment.
Because of the small numbers of studies, variability in the quality of studies, and inconsistent reporting of results, formal meta-analysis was not conducted. A qualitative analysis was performed. In studies in which patients with symptoms other than cough were included, data for the individuals with chronic cough were extracted.12,13,19 Patients with associated symptoms of throat clearing, chronic laryngitis, dysphonia, laryngeal spasm, globus, dysphagia, and pain were included if they also presented with an idiopathic chronic cough. Mean follow-up time was presented for all subjects included in a given study if it was not possible to determine mean follow-up time only for the subjects of relevance to this review.
Results
Forty-six potentially relevant studies were identified by the search strategy (Figure 1). Thirty-eight were excluded because they were review articles without new data, used healthy volunteers, did not involve the use of one of the neuromodulating agents of interest, were letters to the editor without new data, and/or included multiple concurrent treatment modalities in conjunction with a neuromodulator. In particular, 2 articles were excluded because they involved the use of neuromodulators in patients with chronic cough and postviral vagal neuropathy (of which some patients had a cough as part of their symptom complex) but also included other treatment modalities, making it difficult to assess the specific impact of the neuromodulating agent.20,21 Although an English translation of an Italian article involving baclofen in 2 patients with a chronic cough was obtained, it was excluded as each patient had pulmonary disease that may have rendered his or her cough not idiopathic.22
Figure 1.
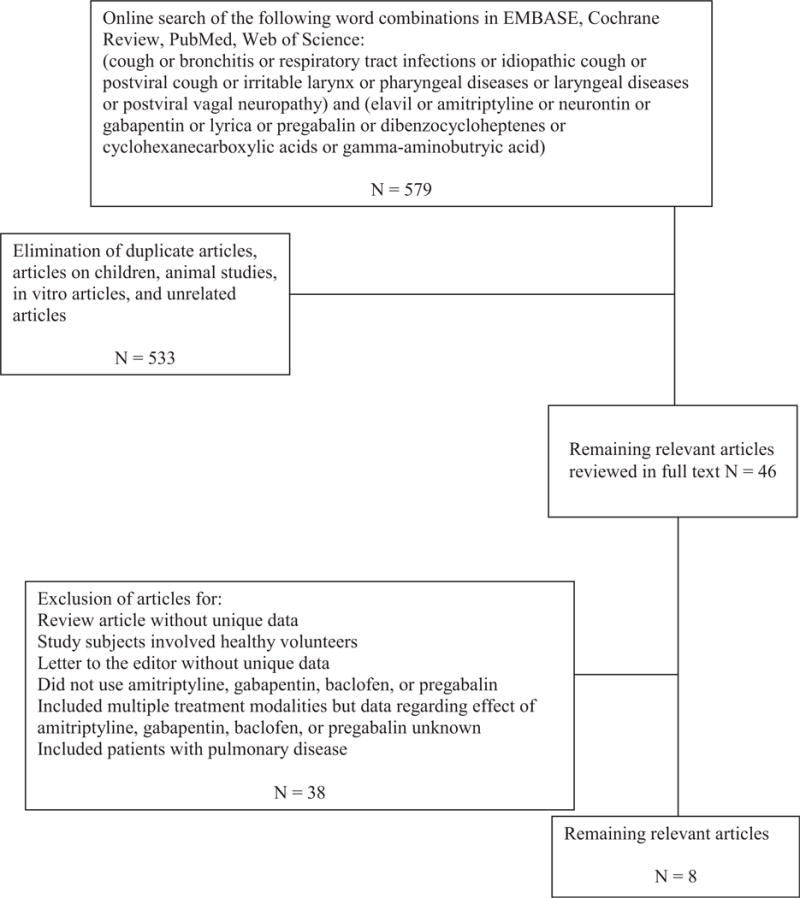
Flowchart for study selection.
Eight relevant articles were included for further analysis (Table 1). Two articles were randomized controlled trials (RCTs). The RCT involving gabapentin was placebo controlled, and study participants and research staff were blinded (investigators assessing the outcome were not blinded).23 The other prospective RCT compared amitriptyline with codeine, but whether clinicians or patients were blinded could not be ascertained.24 Two studies were a prospective cohort or case series design of consecutive patients.13,14 One study was a retrospective case series of consecutive patients.19 Whether consecutive patients were evaluated in the other retrospective case series is not clear.12 The final studies were a case series of 6 patients whose consecutive status was not known and a case report of 2 patients who underwent a placebo crossover treatment algorithm.25,26
Table 1.
Study Type, Intervention, Sample Size, and Patient Age Range of Included Studies
| Author | Study Type | Intervention | N | Patient Age, y |
|---|---|---|---|---|
| Bastian et al14 | Prospective cohort, consecutive patients | 10 mg amitriptyline daily for 21 days | 8 male, 4 female | Median 52; range, 20–75 |
| Norris and Schweinfurth12 | Retrospective case series, consecutive unknown | 25–100 mg amitriptyline, failures treated with gabapentin or pregabalin, unknown duration of treatment | 1 male, 7 female | Mean 67.3; range, 52–77; median 71.5 |
| Lee and Woo13 | Prospective case series, consecutive | Gabapentin 100–900 mg daily over 4 weeks, nonresponders stop at 4 weeks, responders continue dose for 3 months and then taper, intolerant carbamazepine 100 mg tid | 9 male, 17 female | Mean (SD) 51.2 (17.0); range, 14–80; median 50.5 |
| Halum et al19 | Retrospective cohort, consecutive | Pregabalin 75–150 mg bid | 2 male, 3 female | Mean (SD) 51.6 (17.1); range, 34–76; median 52 |
| Jeyakumar et al24 | Randomized controlled trial; randomized by chart numbers and presence of nasal allergies | Amitriptyline 10 mg daily vs codeine/guaifenesin 10 mL every 6 hours for 10 days | Amitriptyline: 8 male, 7 female; codeine: 5 male, 8 female | Amitriptyline: median 54.6 Codeine: 49.7 |
| Ryan et al23 | Randomized, double-blinded, placebo-controlled trial. Note that patients and research staff were blinded; investigators assessing outcomes were not blinded. Block randomization, sex stratified |
Gabapentin (300-1800 mg daily) vs placebo for 10 weeks. Included 1 week taper on, 8 weeks treatment, 1 week taper off | Gabapentin: 12 male, 20 female; placebo: 10 male, 20 female | Gabapentin: mean (SD) 62.7 (14.0) Placebo: mean (SD) 60.9 (12.9) |
| Mintz and Lee25 | Case series, consecutive status unknown | Gabapentin 100 mg bid to 1600 mg daily dose | 6 females | 59; range, 34–77 |
| Dicpinigaitis and Rauf26 | Case report with placebo crossover treatment, consecutive status unknown | Baclofen 10 mg tid | 2 female | 37, 69 |
Two studies were supported by funding from national research agency grants.19,23 The remaining studies did not comment on financial disclosures, which could reflect the absence of specific funding for the studies or variable journal publishing guidelines.
All studies included patients with cough of at least 6 weeks’ duration (Table 2). Included patients had a negative evaluation for reflux, pulmonary disease, and allergy/postnasal drip in 5 studies,12,14,19,23,24 ACEI use in 3 studies,12,14,24 tobacco use in 4 studies,14,23,24,26 and psychiatric disease in 2 studies.14,19 In the study by Lee and Woo,13 most patients had a previous workup, including reflux treatment, modified barium swallow, computed tomography, magnetic resonance imaging, and pH monitoring, but it was not known whether these had been systematically performed across all patients. In the report by Mintz and Lee,25 not all patients had evaluations for postnasal drip but did have prior reflux treatment trials and investigations for pulmonary disease in some fashion, and they were not on an ACEI. One of the 2 patients in Dicpingaitis and Rauf26 had nonspecific interstitial changes on radiographic studies of the chest but was included as treatment trials with prednisone and albuterol had failed.
Table 2.
Diseases Excluded, Follow-up, and Cough Duration of Included Studies
| Author | Diseases Excluded | Follow-up | Duration of Cough |
|---|---|---|---|
| Bastian et al14 | Smoking ACEI Postnasal drip Pulmonary disease Reflux Psychogenic cough |
20 days after completing 21-day trial | Median duration 24 months |
| Norris and Schweinfurth12 | Laryngeal cancer ACEI Postnasal drip Pulmonary disease Reflux |
Mean 20.4 months; range, 2–61 months | 8 weeks |
| Lee and Woo13 | Prior workup included (not systematic or uniform across patients): Modified barium swallow CT MRI pH testing |
Mean follow-up unknown | Median 7.5 months; range, 1.5–240 months |
| Halum et al19 | Pulmonary disease Allergy Psychiatric disease Reflux, cricopharyngeal dysfunction |
Mean 6.8 months; range, 5–20 months for all enrolled in study | Median 11 months; range, 4–36 months |
| Jeyakumar et al24 | Reflux Smoking Pulmonary disease ACEI Postnasal drip |
10-day trial | All patients amitriptyline: months; codeine: 6 months; median 15.1 11.4 months |
| Ryan et al23 | Smoking Pulmonary disease or infection (including asthma, productive cough) Reflux Postnasal drip ACEI Pregnant/breastfeeding Impaired liver function |
Primary outcome assessed after 8 weeks of treatment; additional assessment 4 weeks after drug stopped | Gabapentin: median 36 months; interquartile range, 18–150 Placebo: median 48 months; interquartile range, 18–156 |
| Mintz and Lee25 | Not systematic or uniform across patients: Reflux, asthma, postnasal drip exclusion mentioned in the abstract; manuscript mentions prior workup, including modified barium swallow study, CT, MRI, and pH monitoring |
12 months | Median 7.5 months; range, 1.5–240 months; mean (SD) 31.4, (57.4) |
| Dicpinigaitis and Rauf26 | Smoking 1 patient failed oral inhalers, oral steroids, antihistamine/decongestants, ranitidine 1 patient failed oral steroid and albuterol, had interstitial infiltrates that were nonspecific |
14 days after end of 28-day treatment period (14 days of baclofen and 14 days of placebo) | 1-year and 10-year history of cough |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitors; CT, computed tomography; MRI, magnetic resonance imaging.
Various outcome measures were used to determine the clinical response to neuromodulating agents (Table 3). The RCTs employed both cough-specific QOL instruments as well as patient self-report, and Ryan et al23 also incorporated an objective cough monitor to evaluation cough frequency. Two studies categorized treatment response based on self-reported percent improvement.14,24 Halum et al19 employed a self-report cough severity rating on a 6-point Likert scale. Two other studies noted binary improvement or not based on patient self-report to assess outcome, with Mintz and Lee25 using clinician reports to assess treatment response.12,13 Two studies also assessed objective outcome using the capsaicin cough challenge, in which patients’ cough threshold in response to the chemical tussive capsaicin was assessed.23,26 Whether the outcome variables were chosen a priori was not clear in 2 studies13,25 but appeared to be decided a priori in the remaining 6 studies.12–14,19,23,24
Table 3.
Outcome Measure, Results, and Side Effects of Included Studies
| Author | Outcome | Results | Side Effects |
|---|---|---|---|
| Bastian et al14 | Patient self-report on 0% to 100% scale | 10 of 12 patients had ≥50% response; 6 of 8 patients ≥50% response >20 days off amitriptyline. One patient had no benefit with amitriptyline but did with subsequent gabapentin. | None noted |
| Norris and Schweinfurth12 | Patient self-report (yes or no) | 6 of 8 improved | Dry mouth in 30% of all enrolled patients |
| Lee and Woo13 | Symptom response (unclear if clinician or patient report), yes or no | 69% improved. Variable responses depending on presence of neuropathy upon laryngeal electromyography | Dizziness or somnolence in 18% of all enrolled patients |
| Halum et al19 | Troublesome cough, patient self-report on 6-point Likert scale | 3 of 5 improved cough severity | Sedation in 80%, of whom half tolerated, half discontinued medication |
| Jeyakumar et al24 | Patient self-report % reduction in cough frequency and severity; cough quality-of-life (QOL) questionnaire | 13 of 15 in amitriptyline group had 50% improvement as compared with 1 of 13 in codeine/guaifenesin group; improved QOL scores associated with amitriptyline | No mention of adverse effects |
| Ryan et al23 | Primary end point: cough-specific quality of life Secondary end points: cough severity, capsaicin cough reflux sensitivity, cough frequency using objective cough monitor, urge-to-cough score, laryngeal dysfunction score |
Improved cough-specific QOL scores, cough severity, cough frequency compared with placebo; no effect on capsaicin cough reflex sensitivity. Effects not sustained after treatment cessation | Adverse events reported in 31% of gabapentin group: confusion, dizziness, dry mouth, fatigue, and/or nausea; blurred vision, headache, and memory loss reported in only 1 patient each; adverse events reported in 10% of placebo group |
| Mintz and Lee25 | Clinician assessment | 3 of 6 complete resolution, 1 10% to 15% improved, 1 probably improved, 1 decreased frequency and intensity of cough | Fatigue in 17%, drowsiness for 1 week in 17% |
| Dicpinigaitis and Rauf26 | Capsaicin cough challenge, subject diary (frequency and severity of cough) | Both patients had decreased cough frequency and severity and increased cough threshold | No patients reported adverse reactions |
Neuromodulator treatment improved cough symptoms (Table 3). In the RCTs involving amitriptyline and gabapentin, improvements were seen in cough-specific QOL as well as a reduction in cough severity.23,24 Capsaicin cough reflex sensitivity did not change significantly with neuromodulator treatment in the RCT involving gabapentin, but in the baclofen report, capsaicin cough reflux sensitivity increased by 3 and 5, doubling concentrations in 2 patients.23 Halum et al19 also demonstrated an improvement in patients’ cough severity ratings. When percent improvement in cough symptoms was used as an outcome measure, between 86.7% and 83.3% of patients experienced at least a 50% response.14,24 Using a binary rating of improvement, rates of treatment response ranged from 69% to 80%, and treatment response was greater in patients with some evidence of motor or sensory neuropathy.12,13 In the report using clinician-assessed response, 50% of patients had complete resolution and the remaining 50% some degree of improvement in cough.25 Three studies also examined the time to symptom improvement and found a maximum benefit after a median of 5 days (range, 1–10 days), mean time of 2 months (range, 1–5 months), and treatment effect within 4 weeks.12,14,23 Although Bastian et al14 observed improved cough at least 20 days off amitriptyline in 75% of patients, Ryan et al23 did not note a sustained treatment response after discontinuing gabapentin.
Side effects may result from neuromodulator treatment of cough (Table 3). Reported side effects included dry mouth, sedation, and dizziness.12,13,19,25 Rates of patient-reported side effects in chronic cough patients ranged from 80% to 0% with varying degrees of severity.12–14,19,25 In 1 report, there was no specific mention of a patient query for adverse effects.24 The RCT involving gabapentin found side effects in 31% of patients taking gabapentin vs 10% in the placebo group, including blurred vision, confusion, dizziness, dry mouth, fatigue, headache, memory loss, and nausea or stomach pain.23
Discussion
Because of the significant health care burden associated with cough, examining treatment modalities, especially for patients with chronic, idiopathic cough, is important. Various changes occur that may promote a cough, including plasticity of the cough pathways due to alterations in nerve transmitter release, the excitability of neurons, or the structure of neurons as well as tissue remodeling in the airway mucosa.27 The use of neuromodulators, such as amitriptyline, gabapentin, baclofen, and pregabalin, has recently been identified as a promising treatment modality for chronic idiopathic cough patients. This systematic review examined the data in the current literature regarding this management strategy.
There was considerable variation in the type and quality of articles describing treatment of idiopathic, chronic cough using the neuromodulators of interest. Although 2 RCTs were identified, issues regarding sample size calculation could not be fully assessed.23,24 The remaining studies included non-controlled prospective series of consecutive patients, a non-controlled retrospective analysis of consecutive patients, and a retrospective case series and 2 case reports for which the consecutive status is not known.12–14,19,25,26 Although all included studies involved patients with cough of at least 6 weeks who had other etiologies ruled out, articles varied in the prior investigations and prior treatment trials of studied patients. Variability regarding how particular diagnoses, such as reflux or asthma, were ruled out may have introduced some heterogeneity.
Despite the use of different outcome measures, benefit from amitriptyline, gabapentin, baclofen, and pregabalin was demonstrated. Patients experienced improvements in cough-specific QOL and cough severity measures.19,23,24,26 In addition, more than two-thirds of patients had some treatment response, and more than 80% had at least a 50% reduction in cough symptoms.12–14,24 However, some questions remain, such as the optimal dose, length of treatment, time to maximum benefit, and symptom relapse rates after treatment. Although Bastian et al14 found that 6 of 8 patients had a treatment response more than 20 days off amitriptyline treatment, Ryan et al23 did not observe a sustained response 4 weeks after cessation of gabapentin. Both Norris and Schweinfurth12 and Lee and Woo13 found higher treatment response in patients with evidence of a motor or sensory neuropathy based on laryngeal examination and/or laryngeal electromyography (EMG). Several reports included patients with additional symptoms attributable to laryngeal irritability, such as globus, throat clearing, and laryngospasm.12,13,19 Further studies examining the role of laryngeal neuropathy and the influence of additional symptoms of laryngeal irritability on treatment response may help identify patient subsets likely to benefit from neuromodulator treatment of their cough. Comparison studies of the treatment response from amitriptyline, gabapentin, baclofen, and pregabalin are also needed.
Although the current literature shows promise of these neuromodulators in the treatment of cough, further investigation is warranted. In considering future trials, Birring28 has identified important issues that should be addressed. Cough should be the main symptom, and because cough often persists despite treatment for reflux and rhinitis, patients with these comorbidities as well as those with idiopathic cough could be suitable subjects.28 Since cough reflux sensitivity is affected by age and sex, control groups should be matched for these variables.29 The potential side effects from neuromodulators may make blinding difficult. Thus, comparing to codeine might be more advantageous than placebo and would allow an assessment of treatment response and side effects between treatment arms.28 Last, a combination of outcome measures could be used to measure various cough characteristics. Cough monitors, such as the Leicester cough monitor, objectively measure the cough frequency.30 The cough reflex sensitivity to inhaled tussive agents objectively measures the reflex cough, but correlation to cough symptoms is poor.31,32 Cough symptom scales record patients’ self-report of cough severity. Various cough-specific QOL measures are also available to evaluate the physical and psychosocial impact of cough.33
Continued investigation into the mechanism of action of chronic cough and its treatment is essential. One family of cough receptors, the transient receptor potential (TRP) nociceptors, appears upregulated in patients with chronic cough and may mediate the hypersensitive cough response in such patients.34 In animal models, blocking this receptor inhibited the cough reflux from capsaicin and citric acid.35 The TRP receptor may be a useful target for future therapeutic trials.
Certain methodological limitations must be addressed. The evaluation of diseases for exclusion varied between studies and, in some cases, potentially among study participants. Another concern is the potential for publication bias in which studies that did not find a benefit from amitriptyline, gabapentin, baclofen, or pregabalin were not published. Although amitriptyline, gabapentin, baclofen, and pregabalin are different drugs with various and potentially unknown mechanisms of action, they are interventions that have been used, similar to the treatment of chronic pain, in managing chronic, idiopathic cough. Consequently, these neuromodulating agents were selected as the subject of this systematic review of chronic cough, a condition with significant public health implications. The variation in the study design, medication, dosing, and outcomes did not allow for a formal meta-analysis. This systematic review demonstrated apparent benefit in the use of neuromodulating agents for patients with chronic cough, and further studies are needed.
Conclusion
Although the type and quality of study varied, benefit from amitriptyline, gabapentin, baclofen, and pregabalin was noted in patients with chronic, idiopathic cough. Future investigations should explore comparative evaluations of different dosing and medication protocols, incorporate control groups as well as objective and subjective validated outcome measures, and examine treatment duration and symptom relapse after treatment cessation.
Acknowledgments
The authors thank Judith Stanke, University of Minnesota Medical Center Library, and Brandi Tuttle, Duke University Medical Center Library, for their assistance in designing and executing the search strategy.
Sponsorships: This study was supported in part by the American Academy of Otolaryngology–Head & Neck Surgery Cochrane Travel Grant to the 19th Cochrane Colloquium.
Funding source: None.
Footnotes
Author Contributions
Seth M. Cohen, conception, design, data analysis, writing, final approval; Stephanie Misono, conception, design, data analysis, writing, final approval.
Disclosures
Competing interests: None.
References
- 1.Barbee RA, Halonen M, Kalktenborn WT, Burrows B. A longitudinal study of respiratory symptoms in a community population sample. Chest. 1991;99:20–26. doi: 10.1378/chest.99.1.20. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Ambulatory Medical Care Survey: 2009 summary tables. www.cdc.gov/nchs/data/ahcd/namcs_summary/2009_namcs_web_tables.pdf. Accessed September 5, 2012.
- 3.Consumer Healthcare Products Association. OTC sales by category—2008–2011. http://chpa-info.org/pressroom/Sales_Category.aspx. Accessed September 5, 2012.
- 4.Brignall K, Jayaraman B, Birring SS. Quality of life and psychosocial aspects of cough. Lung. 2008;371:1364–1374. doi: 10.1007/s00408-007-9034-x. [DOI] [PubMed] [Google Scholar]
- 5.Raj AA, Birring SS. Clinical assessment of chronic cough severity. Pulm Pharmacol Ther. 2007;20:334–337. doi: 10.1016/j.pupt.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Dicpinigaitis PV, Tso R, Banauch G. Prevalence of depressive symptoms among patients with chronic cough. Chest. 2006;130:1839–1843. doi: 10.1378/chest.130.6.1839. [DOI] [PubMed] [Google Scholar]
- 7.McGarvey LPA, Carton C, Gamble LA, et al. Prevalence of psychomorbidity among patients with chronic cough. Cough. 2006;2:4. doi: 10.1186/1745-9974-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pratter MR, Bartter T, Akers S, DuBois J. An algorithmic approach to chronic cough. Ann Intern Med. 1993;119:977–983. doi: 10.7326/0003-4819-119-10-199311150-00003. [DOI] [PubMed] [Google Scholar]
- 9.Haque RA, Usmani OS, Barnes PJ. Chronic idiopathic cough: a discrete clinical entity? Chest. 2005;127:1710–1713. doi: 10.1378/chest.127.5.1710. [DOI] [PubMed] [Google Scholar]
- 10.Kardos P. Proposals for a rationale and for rational diagnosis of cough. Pneumologie. 2000;54:110–115. doi: 10.1055/s-2000-11064. [DOI] [PubMed] [Google Scholar]
- 11.Gibson PG, Ryan NM. Cough pharmacotherapy: current and future status. Expert Opin Pharmacother. 2011;12:1745–1755. doi: 10.1517/14656566.2011.576249. [DOI] [PubMed] [Google Scholar]
- 12.Norris BK, Schweinfurth JM. Management of recurrent laryngeal sensory neuropathic symptoms. Ann Otol Rhinol Laryngol. 2010;119:188–191. doi: 10.1177/000348941011900307. [DOI] [PubMed] [Google Scholar]
- 13.Lee B, Woo P. Chronic cough as a sign of laryngeal sensory neuropathy: diagnosis and treatment. Ann Otol Rhinol Laryngol. 2005;114:253–257. doi: 10.1177/000348940511400401. [DOI] [PubMed] [Google Scholar]
- 14.Bastian RW, Vaidya AM, Delsupehe KG. Sensory neuropathic cough: a common and treatable cause of chronic cough. Otolaryngol Head Neck Surg. 2006;135:17–21. doi: 10.1016/j.otohns.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Dicpinigaitis PB, Dobkin JB. Antitussive effect of the GABA-agonist baclofen. Chest. 1997;111:996–999. doi: 10.1378/chest.111.4.996. [DOI] [PubMed] [Google Scholar]
- 16.Smith SM, Schroeder L, Fahey T. Over the counter medications for acute cough in children and adults in ambulatory settings. Cochrane Database Syst Rev. 2012;8:CD001831. doi: 10.1002/14651858.CD001831.pub3. [DOI] [PubMed] [Google Scholar]
- 17.Chang AB, Lasserson TJ, Gaffney J, Connor FL, Garske LA. Gastro-esophageal reflux treatment for prolonged non-specific cough in children and adults. Cochrane Database Syst Rev. 2011;1:CD004823. doi: 10.1002/14651858.CD004823.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molassiotis A, Bryan G, Caress A, Bailey C, Smith J. Pharamcological and non-pharmacological interventions for cough in adults with respiratory and non-respiratory diseases: a systematic review of the literature. Respir Med. 2010;104:934–944. doi: 10.1016/j.rmed.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Halum SL, Sycamore DL, McRae BR. A new treatment option for laryngeal sensory neuropathy. Laryngoscope. 2009;119:1844–1847. doi: 10.1002/lary.20553. [DOI] [PubMed] [Google Scholar]
- 20.Amin MR, Koufman JA. Vagal neuropathy after upper respiratory infection: a viral etiology? Am J Otolaryngol. 2001;22:251–256. doi: 10.1053/ajot.2001.24823. [DOI] [PubMed] [Google Scholar]
- 21.Morrison RJ, Schindler JS. Evaluation and treatment of the patient with chronic cough referred to the otolaryngologist. Laryngoscope. 2011;121(suppl 5):S256. [Google Scholar]
- 22.Agostinis P, Bardus P, Di Piazza V. Treatment of recalcitrant cough with baclofen. Italian J Med. 2009;3:172–174. [Google Scholar]
- 23.Ryan CM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomized, double-blind, placebo-controlled trial. Lancet. 2012;380:1583–1589. doi: 10.1016/S0140-6736(12)60776-4. [DOI] [PubMed] [Google Scholar]
- 24.Jeyakumar A, Brickman TM, Haben M. Effectiveness of amitriptyline versus cough suppressants in the treatment of chronic cough resulting from postviral vagal neuropathy. Laryngoscope. 2006;116:2108–2112. doi: 10.1097/01.mlg.0000244377.60334.e3. [DOI] [PubMed] [Google Scholar]
- 25.Mintz S, Lee JK. Gabapentin in the treatment of intractable idiopathic chronic cough: case reports. Am J Med. 2006;119:e13–e15. doi: 10.1016/j.amjmed.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 26.Dicpinigaitis PV, Rauf K. Treatment of chronic, refractory cough with baclofen. Respiration. 1998;65:86–88. doi: 10.1159/000029232. [DOI] [PubMed] [Google Scholar]
- 27.Bonham AC, Sekizawa S, Chen CT, Joad JP. Plasticity of brainstem mechanisms of cough. Respir Physiol Neurobiol. 2006;152:312–319. doi: 10.1016/j.resp.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Birring SS. Developing antitussives: the ideal clinical trial. Pulm Pharmacol Ther. 2009;22:155–158. doi: 10.1016/j.pupt.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Prudon B, Birring SS, Vara DD, Hall AP, Thompson JP, Pavord ID. Cough and glottis-stop reflex sensitivity in health and disease. Eur Respir J. 2007;29:1256–1276. doi: 10.1378/chest.127.2.550. [DOI] [PubMed] [Google Scholar]
- 30.Birring SS, Fleming T, Matos S, Raj AA, Evans DH, Pavord ID. The Leicester cough monitor: preliminary validation of an automated cough detection system in chronic cough. Eur Respir J. 2008;31:1013–1018. doi: 10.1183/09031936.00057407. [DOI] [PubMed] [Google Scholar]
- 31.Dicpinigaitis PV, Alva RV. Safety of capsaicin cough challenge testing. Chest. 2005;128:196–202. doi: 10.1378/chest.128.1.196. [DOI] [PubMed] [Google Scholar]
- 32.Birring SS, Matos S, Patel RB, Prudon B, Evans DH, Pavord ID. Cough frequency, cough sensitivity and health status in patients with chronic cough. Respir Med. 2006;10:1105–1109. doi: 10.1016/j.rmed.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 33.Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MD, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ) Thorax. 2003;58:339–343. doi: 10.1136/thorax.58.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groneberg DA, Niimi A, Dinh QT, et al. Increased expression of transient receptor potential vanilloid-1 in airway nerves in chronic cough. Am J Respir Crit Care Med. 2004;12:1276–1280. doi: 10.1164/rccm.200402-174OC. [DOI] [PubMed] [Google Scholar]
- 35.Lalloo UG, Fox AJ, Belvisi MG, Chung KF, Barnes PJ. Capsazepine inhibits cough induced by capsaicin and citric acid but not by hypertonic saline in guinea pigs. J Appl Physiol. 1995;79:1082–1087. doi: 10.1152/jappl.1995.79.4.1082. [DOI] [PubMed] [Google Scholar]


