Abstract
A growing body of literature has begun to explore social attention in infant siblings of children with autism spectrum disorder (ASD) with hopes of identifying early differences that are associated with later ASD or other aspects of development. The present study used eye-tracking to familiar (mother) and unfamiliar (stranger) faces in two groups of 6-month-old infants: infants with no family history of ASD (low-risk controls; LRC), and infants at high risk for ASD (HRA), by virtue of having an older sibling with ASD. HRA infants were further characterized based on autism classification at 24 months or older as HRA- (HRA without an ASD outcome) or HRA+ (HRA with an ASD outcome). For time scanning faces overall, HRA+ and LRC showed similar patterns of attention, and this was significantly greater than in HRA-. When examining duration of time spent on eyes and mouth, all infants spent more time on eyes than mouth, but HRA+ showed the greatest amount of time looking at these regions, followed by LRC, then HRA-. LRC showed a positive association between 6-month attention to eyes and 18-month social-communicative behavior, while HRA- showed a negative association between attention to eyes at 6 months and expressive language at 18 months (all correlations controlled for non-verbal IQ; HRA- correlations held with and without the inclusion of the small sample of HRA+). Differences found in face scanning at 6 months, as well as associations with social communication at 18 months, point to potential variation in the developmental significance of early social attention in children at low and high risk for ASD.
Keywords: high-risk infants, face processing, eye-tracking, infancy, autism spectrum disorder, social development, language development
The principles of developmental psychology remind us to consider human development as a cascading process, where each earlier stage has profound and lasting effects on later ones. Understanding the complexities of these processes has the potential to enrich not just our understanding of healthy, ‘typical’ development, but also our understanding of less typical pathways associated with developmental delay or disorder. It is possible that even a very small displacement in one stage of development can seriously impact subsequent stages, leading development to run off course. An example of this pattern of development may be found in infants at high risk for autism spectrum disorder (ASD), who early in life may appear to develop along a typical trajectory but then subtly could begin to deviate from this pattern, eventually resulting in the development of ASD or other developmental difficulties. In our previous work (Wagner, Luyster, Yim, Tager-Flusberg, & Nelson, 2013), we have reported on the extent to which early attention to faces (in 6, 9 and 12 month old infants) predicts social communication outcomes in toddlerhood. Here, we expand our previous work to explore associations between attention to faces at 6 months of age and 18-month social-communication and language skills in children at high risk for ASD.
Infants' preference for faces emerges shortly after birth (e.g., Johnson, Dziurawiec, Ellis & Morton, 1991; Valenza, Simion, Cassia & Umiltà, 1996), and they prefer their mother's face over the face of a stranger (e.g., Bushnell, 2001; Field, Cohen, Garcia & Greenberg, 1984; Pascalis, de Schonen, Morton, Deruelle, & Fabre-Grenet, 1995). Younger infants (e.g., those under 3-4 months of age) seem particularly attentive to eyes (Hunnius & Geuze, 2004), whereas older infants, who are actively engaged in language perception and development, show increased focus towards the mouth (Lewkowicz & Hansen-Tift, 2012; Oakes & Ellis, 2013; Tenenbaum, Shah, Sobel, Malle, & Morgan, 2013). Moreover, a small body of literature has suggested that the ways in which a young infant (i.e., approximately 6 months old) attends to faces is associated with later social abilities (Schietecatte, Roeyers & Warreyn, 2012; Wagner et al., 2013) and communication skills (Young, Merin, Rogers & Ozonoff, 2009; Elsabbagh et al., 2014). Collectively, these results highlight the importance of attention to faces as an ability that facilitates positive social communication development in the months and years to come.
There is a large and complex body of literature on attention to faces in children at elevated genetic risk (according to family history) for ASD. While about 20% of children at high risk for ASD (HRA, by virtue of having an older sibling with the diagnosis) end up receiving an ASD diagnosis themselves (HRA+), the majority (∼80%) do not (HRA-); however, the HRA-group shows considerable variability in development, with nearly half showing sub-clinical features similar to those observed in the condition and the other half (roughly) apparently typically developing (Landa & Garrett-Mayer, 2006; Landa, Holman, & Garrett-Mayer, 2007; Messinger et al., 2013; Ozonoff et al., 2011, 2014; Ozonoff et al., 2014; Zwaigenbaum et al., 2005). A growing area of research has been aimed at explaining this diversity in outcome, and much of it has invoked early attention to faces as potential variable of interest (e.g., see Jones, Gliga, Bedford, Charman, & Johnson, 2014 for a recent review).
A small set of recent studies has explored attention to faces in infants at risk for ASD using eye-tracking; stimuli have differed in important ways from one study to the next (e.g., familiar and unfamiliar, static and dynamic, affective and neutral). Results have been mixed, and the role of methodological variation is unclear. Chawarska, Macari and Shic (2013) showed infants a video of an actress speaking directly to the camera; they found that 6-month-olds later diagnosed with ASD (HRA+, n = 12) spent a smaller proportion of time gazing at an adult's face than high-risk children who did not receive a diagnosis (HRA-, n = 37). However, the authors did not find differences in gaze according to whether HRA- infants were exhibiting social communication deficits, suggesting that reduced attention to faces may be predictive of diagnostic outcome but not for the wider range of behavioral variability in high-risk infants. On the other hand, another study revealed that, when viewing an array of static images, 7-month-old infants at high risk (regardless of outcome group, with an overall sample size of 54) were equally likely to have their attention captured by a face (measured by the infant's first look) as a control group (Elsabbagh, Gliga, et al., 2013) and, in fact, spent proportionally more time looking at faces than a low-risk comparison sample (n = 50). Similarly, Nele et al. (Nele, Ellen, Petra, & Herbert, 2015) reported no difference in looking time to static faces (using a visual paired comparison) between 5-month-olds at high and low risk for ASD (sample sizes of 18 and 41, respectively); they also noted that both groups showed a preference for familiar (that is, mother's) faces than unfamiliar ones. In sum, reduced attention to faces in infants later diagnosed with ASD has emerged in a paradigm using dynamic stimuli; however, there is less evidence for this difference when presenting static images or when comparing high- and low-risk groups.
With regard to patterns of attention to particular facial regions, there is mixed evidence suggesting that infants at risk for ASD or later diagnosed with the disorder show atypical attention to the eyes versus the mouth region of a face (see Falck-Ytter, Bölte & Gredebäck, 2013 and Guillon, Hadjikhani, Baduel, & Rogé, 2014 for recent reviews). Chawarska et al. (2013) reported 6-month olds at low (n = 35) and high risk for ASD (regardless of outcome, n = 49) showed the same patterns of attention to the eyes versus mouth when viewing a video of an adult directly addressing the camera and using child-directed speech. Similarly, in a sample of 54 high-risk and 50 low-risk infants, Elsabbagh et al. (2014) similarly reported that the eye-mouth-index (capturing relative attention to eyes and mouth while infants viewed a video of peek-a-boo) at 7-months of age showed no association with outcome group at 36-months. However, work by Shic, Macari, and Chawarska (2014) revealed that 6-month olds in the HRA+ group (n = 12) reduced their attention to inner features (eye and mouth) but only when the face was talking (i.e., reciting a nursery rhyme) and not during a static face or a video of a smiling face; attention to inner features in the HRA- group (n = 45) did not vary according to whether children were showing sub-clinical features of ASD. Across these studies, all of which used videos presenting child-directed speech and activities, none found evidence for differential allotment of attention to faces in 6-month-olds based solely on risk status, and only Shic et al. (2014) reported a differential pattern in 6-month-old infants later diagnosed with ASD.
Using a unique growth curves design and focusing on the preceding months of life (i.e., before 6 months), Jones and Klin (2013) presented infants with videos of a female adult actor directly addressing the camera and using child-directed speech; the authors reported that HRA+ infants (n = 11) showed declining attention to eyes between 2 and 6 months of age. In contrast, they found that patterns of change in the HRA- group differed according to whether infants had sub-clinical features of ASD: infants exhibiting these symptoms (n = 10) showed stable gaze to eyes between 2 and 6 months, while infants with no social communication deficits (n = 18) increased attention to eyes over the 4 month period, similar to the control group. Interestingly, in another longitudinal study, Rutherford, Walsh, and Lee (2015) reported that the LRC (n = 31) and HRA- (n = 21) groups both showed decreasing attention to eyes between 3 and 9 months, similar to the findings in Lewkowicz and Hansen-Tift (2012). However, the HRA+ group (n = 10) showed increasing attention to eyes during this period, in contrast to the findings from Jones and Klin (2013). It is important to note the contrast in stimuli: while Jones & Klin (2013) employed a video of a speaking actress, Rutherford et al. (2015) used a video of a blinking but silent face; the potential artifact of this methodological variation is unknown. Nevertheless, as with attention to faces, patterns of gaze to eyes vs. mouth seems to be a marker primarily for the ASD+ group (rather than for the at-risk group more broadly), and they appear to vary in important ways according to experimental or analytic conditions.
One final area of investigation is whether, as in typically-developing populations, early attention to faces predicts later social communication ability for infants at risk for ASD. Young, Merin, Rogers, and Ozonoff (2009) found that increased attention to mouths, relative to eyes, during a live interaction at 6-months was predictive of better expressive language at 36-months for the low-risk and high-risk groups. This finding was replicated with 7-month-olds (Elsabbagh et al., 2014), again with greater attention to mouths (while viewing peekaboo) predicting better expressive language at 36 months, though no predictive association was found with 36-month social skills. Interestingly, de Klerk and colleagues (2014) reported that for the high-risk group, the proportion of time spent looking at faces negatively predicted face recognition at 3 years, and this effect was not found in the low-risk infants (nor does it align with other studies of typically developing infants, see Wagner et al., 2013) and did not seem to be driven by those children manifesting overt ASD symptoms. Altogether, then, there seems to be evidence linking language development in the toddler and preschool years with differential patterns of attention to faces in infancy for high-risk infants; moreover, these associations closely resemble what is seen in typical, low-risk children. However, there is some indication that the link between early attention to faces and later social communicative abilities may differ for children at high risk and those at low genetic risk for ASD.
The current report is a follow-up to our previous work (Wagner et al., 2013) and extends our prior analyses to include children at high risk for ASD and a larger group of typically-developing infants. Our initial work was submitted to a special issue relating to the development of face processing, and at that time, because ASD outcome data was unavailable for our high-risk infant sample (who had not yet reached 24 months or older), Wagner et al. (2013) focused on low-risk infants only. Using data from 6 months and 18 months, the present manuscript addresses a similar set of questions, focusing on whether infant risk status (high or low risk for ASD) affects: 1) Preferences for facial region and identify; and 2) Associations between visual attention to faces in the first year and social communication skills in the second year.
Method
Participants
The initial sample consisted of 148 6-month-old infants: low-risk control infants (LRC) with a typically-developing older sibling and no family history of ASD (n = 69) and high-risk ASD infants (HRA) with an older sibling with ASD (n = 79). A set of infants were excluded from the sample due to insufficient eye-tracking data, looking less than 30% of the time images were on the screen during the relevant trials (26 LRC out of 69 total LRC: 38%; 19 HRA out of 79 total HRA: 24%) and an additional four infants (3 LRC; 1 HRA) were excluded due to technical errors in stimulus presentation or data export. Furthermore, to be included in the final sample, HRA infants were required to have been followed longitudinally in order to classify them based on ASD outcomes, so an additional 22 HRA were excluded for not having a lab visit at 24 months or older with a research reliable Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000).
Of the remaining 77 infants (40 LRC and 37 HRA), HRA infants were further subdivided into positive (HRA+) and negative (HRA-) ASD classification, with all infants in the HRA+ group meeting two criteria: 1) exceeding the ASD ADOS algorithm cutoff on their most recent visit at 24 or 36 months and 2) having received a clinical judgment of ASD by a staff clinician based on all available information. Following these criteria, the final sample consisted of 40 LRC (mean age = 194 days, SD = 10; 17 female), 29 HRA- (mean age = 193 days, SD = 10; 11 female), and 8 HRA+ (mean age = 194 days, SD = 6; 5 female). Included infants spent on average 64% of the time (SD = 18%) attending to the stimuli presented, with attention ranging from 31% to 96%. Table 1 illustrates ADOS scores for HRA- and HRA+. Project approval was obtained from the Institutional Review Boards of Boston Children's Hospital and Boston University and informed consent was obtained from the parent(s) of each infant participant.
Table 1. Autism Diagnostic Observation Schedule means (standard deviations in parentheses) for High-risk Autism group with and without a later Autism Spectrum Disorder diagnosis.
| High-risk Autism (No diagnosis) | High-risk Autism (With diagnosis) | Significance and Effect Size | |
|---|---|---|---|
|
|
|||
| Autism Diagnostic Observation Schedule - Module 1 | n = 11 | n = 3 | |
| Social Total | 2.64 (1.75) | 5.00 (3.00) | p = .096, d = 1.27 |
| Range | 0-6 | 2-8 | |
| Communication Total | 1.45 (1.13) | 1.67 (1.15) | p = .78, d = .21 |
| Range | 0-4 | 1-3 | |
| Social+Communication Total | 4.09 (2.30) | 6.67 (4.04) | p = .16, d = 1.04 |
| Range | 1-8 | 3-11 | |
| Autism Diagnostic Observation Schedule - Module 2 | n = 18 | n = 5 | |
| Social Total | 1.56 (1.46) | 5.80 (1.79) | p < .001, d = 2.9 |
| Range | 0-4 | 3-7 | |
| Communication Total | 1.89 (1.41) | 3.60 (2.07) | p = .041, d = 1.15 |
| Range | 0-4 | 1-6 | |
| Social+Communication Total | 3.44 (2.15) | 9.40 (3.78) | p < .001, d = 2.45 |
| Range | 0-7 | 4-13 | |
Note. Autism Diagnostic Observation Schedule (ADOS) data are from the most recent ADOS administration (either 24 or 36 months). Final sample included n=29 for High-risk Autism with no clinical diagnosis and n=8 for High-risk Autism with an Autism Spectrum Disorder diagnosis at the most recent lab visit.
Stimuli
Color photographs of emotionally neutral female faces were employed as stimuli. One of the faces was the infant's mother; the second was a featurally similar stranger, matched to mother according to racial/ethnic background and other salient aspects (e.g., glasses). Images were cropped and re-sized for uniformity and inserted into stimulus presentation software (i.e., Clearview or Tobii Studio) for display on the eye-tracking monitor.
Apparatus
Images were presented on a 17″ TFT Tobii T60 monitor using Clearview or Tobii Studio software (Tobii Technology AB; www.tobii.com) running off of a PC computer. The eye-tracking monitor recorded gaze position of both eyes at 60Hz based on the reflection of near-infrared light from the cornea and pupil.
Procedure
Infants were seated on their caregiver's lap in a darkened room approximately 60cm from the eye-tracking monitor. Before the testing session began, a 5-point calibration procedure was used to confirm that the infant and monitor positions allowed for satisfactory gaze tracking. Following successful calibration, a modified visual paired comparison (VPC) paradigm was administered. Because the mother's face was used as one of the stimuli, a familiarization phase was not incorporated into the session. The presentation included four 10-second trials, each of which showed the mother's face and a stranger's face side-by-side, for a total of 40 seconds of presentation. The positions of the faces were counter-balanced across trials, so that each face was on the right and left side for an equal amount of time.
Social Communication Measure at 18 Months
The Communication and Symbolic Behavior Scales Developmental Profile (CSBS-DP, Wetherby, Allen, Cleary, Kublin, & Goldstein, 2002) is a norm-referenced measure used to capture the early communicative competence of young children; it includes 45 questions covering seven domains of social communication and symbolic development: emotion and eye gaze, communication, gestures, sounds, words, understanding, and object use. Scoring yields three composite scores: Social (comprised of the Emotion and Eye Gaze, Communication, and Gestures clusters), Speech (comprised of the Sounds and Words clusters) and Symbolic (comprised of the Understanding and Object Use clusters). An overall Total score, which captures performance across the three composites, is also obtained. Each raw score is assigned a standard score and percentile rank according to previously established norms (Wetherby et al., 2002).
Cognitive Assessment at 18 Months
The Mullen Scales of Early Learning (MSEL; Mullen, 1995) evaluates cognitive functioning for children from birth to 68 months of age. Standardized domain scores (T-scores: M = 50, SD = 10) are calculated for five subtests (gross motor, fine motor, visual reception, receptive language, and expressive language). Non-verbal developmental quotient (NVDQ) is generated from fine motor and visual reception domain scores.
Data Analysis
Infant eye-tracking at 6 months
Following the completion of the experiment, ten overlapping areas of interest (AOIs) were defined: left image, right image, mother's image, stranger's image, mother's face, stranger's face, mother's eyes, stranger's eyes, mother's mouth, and stranger's mouth (see Figure 1). Left and right AOIs were used for analysis of side bias exceeding 85% to the left or right, but no additional infants showed this bias. Gaze data were exported using a 100 millisecond fixation filter and a 20 pixel fixation radius. The resulting text file was then run through a custom-made Python script (Python Programming Language; www.python.org) that summed duration of gaze within each of the pre-defined AOIs.
Figure 1.

Sample stimuli for mother vs. stranger visual paired comparison test with areas of interest outlined. For each infant, areas of interest included left image, right image, mother image, stranger image, mother's face, stranger's face, mother's eyes, stranger's eyes, mother's mouth, and stranger's mouth.
In an effort to capitalize on infants' initial response to the viewing of their mother next to a stranger, while still counterbalancing on which side each image appeared, the present analyses focused on the first two 10 second trials presented to infants, consistent with analyses reported by Wagner et al. (2013). Variables of interest for mother and stranger included: 1) Total time on face, 2) Total time on eyes and mouth, and 3) Proportion of time on eyes and mouth (calculated out of total time spent on face).. Past work by Merin and colleagues (Merin et al., 2007; Young et al., 2009) using dynamic face stimuli with infants focused analyses of visual attention on an eye-mouth index (EMI), calculated as total time on eyes divided by total time on eyes and mouth combined. Merin et al. (2007) show EMI values ranging widely, with some infants showing strong preferences for the mouth (EMI of roughly 15% across the study) and others showing strong preferences for the eyes (EMI of roughly 80% across the study). When preliminary calculation of the EMI was done for the present study, there was little to no attention to the mouth, resulting in a mean EMI of 95% (SD = 10%; range: 54%-100%), and only 14% of infants (11 out of 77) showing an EMI below 90%. With this limited variability in EMI (likely due to the use of static images), the EMI was not used in subsequent analyses.
CSBS-DP at 18 months
When infants were 18 months, parents were asked to complete the CSBS-DP Caregiver Questionnaire (Wetherby et al., 2002) as a measure of children's social and communicative development. The present analyses focused on the percentile ranks for the Social composite score and the Total score. CSBS-DP scores were unavailable for a subset of children due to failure to return the completed questionnaire (12 LRC, 6 HRA-, 2 HRA+).
MSEL at 18 months
The MSEL was administered by an experimenter during the lab visit at 18 months. The present analyses examined language ability with the expressive and receptive language domain scores, and examined non-verbal cognitive ability with the NVDQ. MSEL scores were unavailable for 10 children who missed their 18-month lab visit (8 LRC, 2 HRA+).
Results
Eye-tracking at 6 Months
Eye-tracking results focused on three sets of analyses using group as a between-subjects variable: 1) duration of time on the face AOI; 2) duration of time on the eyes and mouth AOIs, and 3) proportion of time on the eyes and mouth AOIs out of time on the face AOI. All analyses were conducted using SPSS statistical software. A preliminary repeated-measures ANOVA was run to examine the between-subjects effect of presentation software (Clearview versus Tobii Studio) for each of the analyses outlined above. There was no main effect of presentation in any case, so all subsequent analyses collapsed across the presentation software variable.
Duration of Time on Face
A 2 (Identity: mother, stranger) × 3 (Group: LRC, HRA-, HRA+) repeated-measures ANOVA using identity as the within-subjects factor and group as the between-subjects factor found a main effect of group for looking to the face, F(2,74) = 7.182, p = .001, ηp2 = .163. HRA+ (M = 7159 ms, SD = 1739) and LRC infants (M = 6289 ms, SD = 1670) showed similar attention to faces, t(46) = 1.34, p = .19, d = .53, but both groups showed greater attention to faces as compared to HRA- (M = 5088 ms, SD = 1520; ts > 3.05, ps < .005, ds > .75). No other main effects or interactions were significant (Fs < .42, ps > .65).
Duration of Time on Eyes and Mouth
Time spent on eyes and mouth was examined with a 2 (Identity: mother, stranger) × 2 (Region: eyes, mouth) × 3 (Group: LRC, HRA-, HRA+) repeated-measures ANOVA, with identity and region as the within-subjects factors and group as the between-subjects factor, and revealed several significant findings. A main effect of region was found, F(1,74) = 281.55, p < .001, ηp2 = .79, with significantly more time spent on the eyes (M = 4225 ms, SD = 1856) than the mouth (M = 164 ms, SD = 369). Infants also showed a main effect of group, F(2,74) = 7.07, p = .002, ηp2 = .16 (see Figure 2), with HRA+ spending more time on the eyes and mouth (M = 3044 ms, SD = 880) than LRC (M = 2285 ms, SD = 860, t(46) = 2.27, p = .028, d = .9) and HRA-(M = 1837 ms, SD = 782, t(35) = 3.77, p = .001, d = 1.55). LRC also significantly differed from HRA- (t(67) = 2.22, p = .03, d = .55). Additionally, a significant interaction between region and group was found, F(2,74) = 5.13, p = .008, ηp2 = .12. For the eye region, HRA+ showed greater attention (M = 6039 ms, SD = 1724) than both LRC (M = 4296 ms, SD = 1864, t(46) = 2.44, p = .018, d = .97) and HRA- (M = 3627 ms, SD = 1567, t(35) = 3.78, p = .001, d = 1.55), but LRC and HRA- showed no group difference, t(67) = 1.57, p = .12, d = .39. For the mouth region, LRC showed greater attention (M = 274 ms, SD = 481) than HRA- (M = 46 ms, SD = 94, t(67) = 2.52, p = .014, d = .62), but neither group differed from HRA+ (M = 49 ms, SD = 95, ts < 1.31, ps > .19, ds < .53). No other main effects or interactions were significant (Fs < .68, ps > .51).
Figure 2.
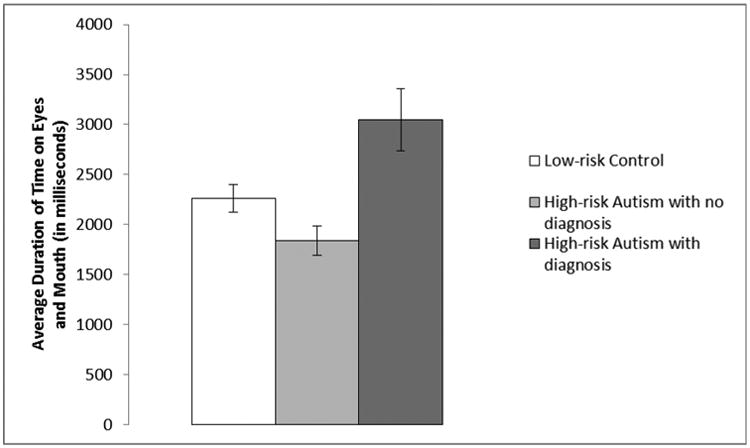
Duration of time spent on the eyes and mouth for Low-risk Controls (LRC; n = 40), High-risk Autism with no diagnosis (HRA-; n = 29), and High-risk Autism with an autism spectrum disorder diagnosis (HRA+; n = 8). HRA+ showed significantly greater attention to the eyes and mouth than LRC (p = .028) and HRA- (p = .001). LRC also showed greater time on eyes and mouth than HRA- (p = .03). Error bars are +/- standard error to the mean.
Proportion of Time on Eyes and Mouth
Parallel to the analysis above, the proportion of time spent on eyes and mouth out of total time on the face was examined with a 2 (Identity: mother, stranger) × 2 (Region: eyes, mouth) × 3 (Group: LRC, HRA-, HRA+) repeated-measures ANOVA (identity and region as the within-subjects factors; group as the between-subjects factor). A main effect of region was once again found, F(1,74) = 487.70, p < .001, ηp2 = .87, with a significantly greater proportion of time spent on the eyes (M = 71%, SD = 20%) than the mouth (M = 2%, SD = 5%). No other main effects or interactions were significant (Fs < 2.30, ps > .11).
Eye-tracking at 6 Months and Social and Language Abilities at 18 Months
A final set of analyses was run in order to examine the relations between visual attention to faces as measured by the eye-tracking task at 6 months and 18-month social-communicative behavior (measured via CSBS-DP) and language skill (measured via MSEL). The face scanning measures at 6 months included 1) average time on faces, 2) average time on eyes, and 3) proportion of time on eyes. Similar results were expected for time on faces and time on eyes, as infants spent 71% of their time on the eyes when scanning the face (LRC: M = .69, SD = .22; HRA-: M = .70, SD = .87; HRA+: M = .85, SD = .07), but both measures were included, as ANOVAs revealed different patterns of group differences for the two variables. Average time on the mouth and proportion of time on the mouth were not included in the correlational analyses, as infants were near floor for these measures (Duration: M = 330 ms, SD = 738; Proportion: M = 2%, SD = 5%), with 48 out of 77 infants (LRC = 22; HRA- = 22; HRA+ = 6) showing no time spent in this AOI. The CSBS-DP measures at 18 months included percentile rank for the Social composite score and for the Total score. MSEL language measures at 18 months included domain scores for expressive language (EL) and receptive language (RL). Partial correlations were run controlling for MSEL NVDQ at 18 months. Each of the eye-tracking measures was compared to the two CSBS-DP measures and the two MSEL language measures.
Correlations were run separately for groups. LRC infants showed significant positive associations between duration of time to the eyes, and more generally the faces, and CSBS social scores (both associations: r(24) = .49, p = .011; see Figure 3), showing that increased overall attention to the eyes and face at 6 months (though not relative attention as measured through proportion of time on eyes) related to better social functioning a year later. LRC showed no significant associations between eye-tracking at 6 months and 18-month CSBS-DP total scores, MSEL RL, or MSEL EL.
Figure 3.
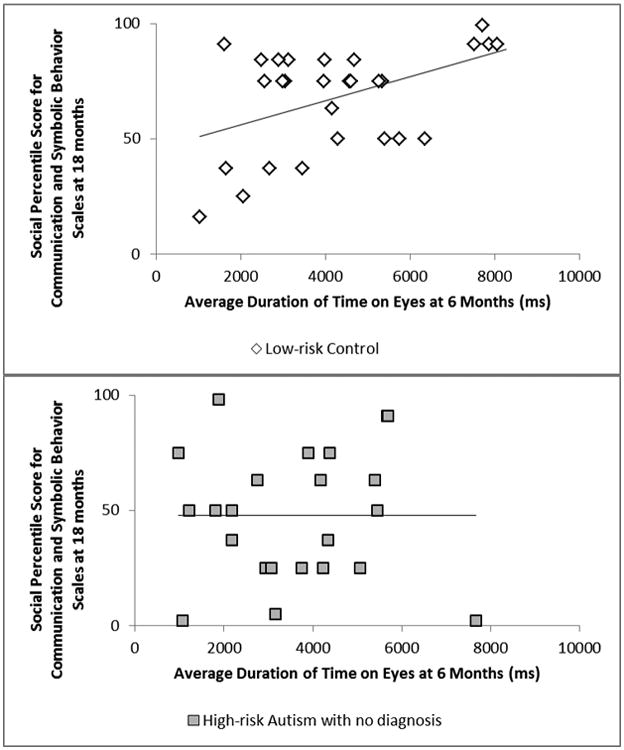
Associations between duration of time spent on eyes at 6 months and Communication and Symbolic Behavior Scales (CSBS) social scores at 18 months for Low-risk Controls (LRC) and High-risk Autism with no diagnosis (HRA-) after partialling out Mullen Scales of Early Learning nonverbal developmental quotient. In LRC, a significant positive association was found (p = .011), with greater attention to eyes relating to better CSBS social scores a year later. No significant relation between these variables was found for HRA- (p = .99).
For high-risk infants, the first set of correlations combined HRA- and HRA+ infants together, as the group of HRA+ infants was too small to be in its own analysis. This composite HRA group showed significant negative associations between time spent on the eyes, and more generally the face, and MSEL EL scores (both associations: r(25) = -.53, p = .005), showing that increased time to the eyes and face related to worse expressive language scores a year later in the high-risk group overall. This was marginally true for proportion of time on eyes as well, r(25) = -.33, p = .092. To determine if these findings were true for HRA- infants and not driven by HRA+ infants, the same set of correlations was run for HRA- infants alone. HRA- again showed significant negative associations between duration of time scanning the eyes and face at 6 months and MSEL expressive language at 18 months (eyes: r(20) = -.56, p = .007; face; r(20) = -.51, p = .015; see Figure 4). Analyses with HRA- (and those with the combined HRA- and HRA+ group) showed no significant associations between eye-tracking at 6 months and CSBS-DP scores or MSEL RL at 18 months (see Table 2 for CSBS-DP and MSEL scores for all groups).
Figure 4.
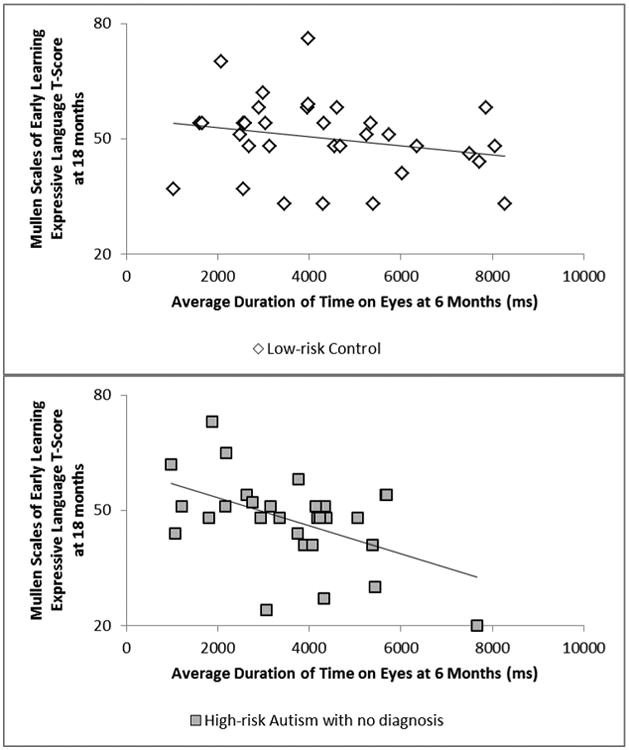
Associations between duration of time spent on eyes at 6 months and Mullen Scales of Early Learning (MSEL) expressive language T scores at 18 months for Low-risk Controls (LRC) and High-risk Autism with no diagnosis (HRA-) after partialling out MSEL nonverbal developmental quotient. In HRA-, a significant negative association was found (p = .007), with greater attention to eyes relating to worse MSEL expressive language scores a year later. No significant relation between these variables was found for LRC (p = .96).
Table 2. Communication and Symbolic Behavior Scales and Mullen Scales of Early Learning means (standard deviations in parentheses) for Low-risk Controls and High-risk Autism with and without a later Autism Spectrum Disorder (ASD) diagnosis.
| Low-risk Controls (LRC) | High-risk Autism with no diagnosis (HRA-) | High-risk Autism, with ASD diagnosis (HRA+) | Group Differences and Effect Size | |
|---|---|---|---|---|
| Communication and Symbolic Behavior Scales at 18 months | n = 28 | n = 23 | n = 6 | |
| Social Percentile | 67.79 (22.37) | 47.91 (28.64) | 48.83 (38.52) | LRC > HRA-, d = .8 |
| Range | 16-99 | 2-98 | 2-98 | |
| Total Percentile | 67.04 (28.27) | 44.70 (32.13) | 37.50 (33.76) | LRC > HRA-, d = .76 |
| Range | 18-99 | 3-98 | 3-89 | LRC > HRA+, d = 1.04 |
| Mullen Scales of Early Learning at 18 months | n = 32 | n = 29 | n = 6 | |
| Receptive Language T-score | 55.91 (16.43) | 47.07 (16.32) | 34.50 (12.65) | LRC > HRA-, d = .55 |
| Range | 26-77 | 20-72 | 20-53 | LRC > HRA+, d = 1.38 |
| Expressive Language T-score | 50.03 (10.33) | 47.41 (11.45) | 37.50 (10.13) | LRC > HRA+, d = 1.25 |
| Range | 33-76 | 20-73 | 24-51 | |
| Non-verbal Developmental Quotient | 108.50 (12.77) | 106.38 (11.44) | 101.75 (12.91) | none |
| Range | 89-139 | 81-125 | 81-114 |
Note: Communication and Symbolic Behavior Scales at 18 months missing for 12 Low-risk Controls, 6 High-risk Autism with no diagnosis, and 2 High-risk Autism with an ASD diagnosis; Mullen Scales of Early Learning at 18 months missing for 8 Low-risk Controls and 2 High-risk Autism with an ASD diagnosis (see Method for more detail). Group differences significant at p < .05.
Discussion
The present study examined scanning of familiar and unfamiliar faces at 6 months and relations with social ability and language development at 18 months. Extending prior work by Wagner et al. (2013) that focused only on typically-developing infants (referred to here as low-risk controls or LRC), this work added a group of infants at high risk for ASD (HRA), divided into a group with an ASD outcome by 36 months (HRA+) and a group which did not (HRA-); Results revealed several significant differences in scanning between LRC and HRA infants. First, HRA+ and LRC spent significantly more time scanning the face than HRA- infants. Second, while all groups showed greater attention to the eye region than the mouth region, HRA+ showed greatest attention to these regions, followed by LRC, then HRA-. There were, however, no group differences in relative attention to the eye and mouth regions as calculated as proportions of time to these regions out of total time on the face. For associations between 6-month scanning of faces and 18-month social and language abilities (controlling for non-verbal IQ), LRC infants with greater overall attention to faces/eyes at 6 months showed better social ability at 18 months, while HRA- infants with greater overall attention to faces/eyes at 6 months showed worse expressive language ability at 18 months. These correlations were significant when the HRA+ group was included as well, but importantly were not driven by this positive outcome group. Taken together, this work suggests early differences in scanning that could relate to ASD outcome and the broader endophenotype. The results also indicate that the associations between early scanning and later social-communication skills differ according to whether a child is at genetic risk for ASD.
In examining differential responses to familiar and unfamiliar faces in the present study, results showed no differences in attention to mother versus stranger. While many studies with infants have found strong visual preferences for their mother's face when compared to that of a stranger soon after birth (e.g., Bushnell, 2001; Field et al., 1984; Pascalis et al., 1995), other work has found that this difference becomes less robust by the time infants reach 3 to 5 months (Bartrip, Morton, & de Schonen, 2001), and that by 6 months, this preference can be affected by the use of featurally-similar strangers as comparison stimuli, as were used in the present work (e.g., de Haan & Nelson, 1997). Bartrip et al. (2001) looked at differences in attention to mother and stranger across the first five months of development. This work found that the preference for mother is strong in 1- to 2-month-olds, but by 3 months, preferences had shifted, showing either no difference between conditions, or in some cases, preferences shifting towards increased looking to the stranger. Recent work by Nele et al. (2015) used a VPC to examine responses to mother and stranger in 5-month-olds at low and high risk for ASD and found that across 14 5-s trials, both groups showed a preference for mother over stranger, attending to mother's face 56% of the time and stranger 44% of the time. In Wagner et al. (2013), when looking at the VPC across 6-, 9-, and 12-month-old typically-developing infants, more time was spent on mother than stranger, amounting to roughly 53% of time on mother and 47% of time on stranger. Together, past work shows that by 5 to 6 months, though preferences for mother's face have been found, these preferences are not far above 50%, as compared to the more robust findings seen soon after birth where infants often show preferences closer to 65% for mother as compared to 35% for stranger (e.g., Bartrip et al., 2001; Pascalis et al., 1995). Interestingly, recent work with HRA and LRC has found that neural responses (e.g., event-related potentials) show differentiation between mother and stranger in both HRA and LRC beyond 6 months (e.g., Key & Stone, 2012; Luyster, Powell, Tager-Flusberg, & Nelson, 2014), suggesting that neural measures could be more sensitive for detecting such differences as infants get older (for further discussion, see de Haan & Nelson, 1997).
When examining scanning patterns across the three groups of infants at 6 months, greatest attention to faces was found for HRA+ and LRC as compared to HRA-. As in prior studies, especially those using static face images as we did, infants showed overall greater attention to the eye region as compared to the mouth region (e.g., Haith, Bergman, & Moore, 1977; Maurer & Salapatek, 1976; Wagner et al., 2013); however, greater attention to eyes was seen in HRA+ as compared to both LRC and HRA-. For attention to the mouth, HRA+ were no different from either group, but LRC showed greater attention to the mouth than HRA-. Overall then, HRA+ showed greater attention to faces and eyes than HRA-, LRC showed greater attention to faces and mouth than HRA-, and HRA+ and LRC showed similar attention to faces and mouth, but HRA+ showed increased attention to the eyes compared to LRC. While past work has found more general differences between face scanning in children with ASD and controls (e.g., Jones, Carr, & Klin, 2008) as well as in unaffected first-degree relatives of individuals with ASD and controls (e.g., Dalton et al., 2007), the literature with high-risk infants has typically reported no group differences in attention to faces for HRA (e.g., Key & Stone, 2012; Merin, Young, Ozonoff, & Rogers, 2007; Nele et al., 2015; Young et al., 2009). With findings of decreased attention to the face and mouth regions in HRA- as compared to LRC in the current study, this work is among the first to show that differential scanning of faces can relate to broader endophenotypes in behaviorally unaffected high-risk infants by 6 months.
Although the HRA+ sample was small, moderate effect sizes were observed for group differences in attention, increasing the statistical validity of the present findings. Evidence for HRA+ showing greater attention to the eye region as compared to both LRC and HRA- raises questions of how increased attention in this positive outcome group could be a marker of poorer functioning. At least one other study has identified a pattern similar to the present study, with increased social attention in HRA+. Work by Rozga et al. (2011) examined gaze during mother-infant interactions at 6 months in HRA+, HRA-, and LRC and found a trend towards greater attention to the mother's face for the HRA+ group. Several other studies have also found differences in attention related to ASD outcome, but the direction of the effect has typically been in the opposite direction, with HRA+ showing reduced attention to social stimuli (Chawarska et al., 2013; Shic et al., 2014). For example, Shic et al. (2014) found that 6-month-old HRA+ infants viewing complex dynamic scenes with an actress talking showed decreased attention to inner features of the face (eyes, nose, mouth) and increased attention to outer features of the face (skin, hair, body; though also see Elsabbagh et al., 2014, who found no differences in scanning of dynamic social scenes in 7-month-old HRA+). In a static neutral unfamiliar face condition, the condition closest to the present study, Shic et al. (2014) found no difference in percentage of time on inner face features or eye-to-mouth ratio between groups, though there was no report of absolute measures of attention to faces and facial regions; notably, in the current study, group differences were similarly non-existent when percentage variables were examined.
In the larger context, the finding of increased attention to faces/eyes in high-risk infants who later develop ASD can also be discussed in terms of two broader research areas. First, a large body of work studying visual attention in typically-developing infants has found strong evidence that increased attention is negatively related to cognitive ability, both concurrently and predictively, and this has been found for infant measures of longer average fixation duration during a task (e.g., Colombo, Mitchell, & Horowitz, 1988; Colombo, Mitchell, Coldren, & Freeseman, 1991) and longer time to reach habituation criterion (e.g., Rose, Slater, & Perry, 1986; for a recent meta-analysis, see Kavšek, 2004). These findings have been discussed in terms of ‘short lookers’ being more efficient processors of information, in contrast to ‘long lookers’ who might need additional viewing times to successfully manage the information presented. Overall then, work on typically-developing infants has found relations between increased looking times during attentional measures and worse developmental outcomes, in line with the present finding in infants at high risk for ASD who receive a positive diagnosis. Work by Elsabbagh, Gliga, et al., 2013 looked at visual attention in 7-month-old high-risk infants during a ‘face popout’ task (a visual array including several objects and one face) and found that HRA show increased face engagement (increased focus on the face within the array) as compared to LRC, potentially showing inefficient processing of faces within the array for this high-risk group. More recently, follow-up work by de Klerk et al. (2014) found that the HRA infants with higher face engagement at 7 months on this same task showed worse face recognition at 3 years old, suggesting a mechanism similar to that discussed in typical development whereby increased attention to faces at very young ages in high-risk infants is associated with decreased social functioning.
A second area of research to consider in relation to the present findings with HRA+ infants relates to literature focused on disrupted attentional mechanisms in high-risk infants who later develop ASD. Work has found that difficulties disengaging attention in HRA are among the most prominent markers of a later ASD diagnosis (Zwaigenbaum et al., 2005; Elsabbagh, Fernandes, et al., 2013), and these problems in modulating attention could result in increased looking, as found in HRA+ in the present study. A recent review paper by Keehn and colleagues (Keehn, Müller, & Townsend, 2013) puts forth a novel framework through which the emergence of ASD relates to early difficulties disengaging attention that then lead to disrupted regulation of arousal responses. For example, in a situation where a stimulus is over-arousing (and therefore potentially aversive, as might be the case when viewing faces with direct eye contact), an adaptive response might be to disengage from that stimulus to regulate arousal levels; however, as Keehn et al. (2013) discuss, persistent difficulties shifting attention from socially-relevant stimuli early in development could result in over-arousal in response to social information, and later consequences could be a lack of engagement with these types of situations in the future (see Keehn et al., 2013 for further discussion). Relatedly, recent work has found that greater arousal responses in high-risk infants, as measured through pupil size during the viewing of emotional faces, was associated with worse social-communicative outcomes 9 months later (Wagner, Luyster, Tager-Flusberg, & Nelson, 2016), further suggesting the importance of studying how attention and arousal mechanisms might interact in the development of ASD and related endophenotypes.
When examining how early attention to faces might relate to later social and language abilities, the current study found that at 6 months, increased attention to faces and eyes predicted better social competence at 18 months in LRC, consistent with past work (e.g., Schietecatte et al., 2012) and extending our prior results with an increased sample (Wagner et al., 2013). This finding highlights a mechanism by which increased attention to a caregiver's informative facial regions can have positive consequences for development. Because of insufficient sample size, correlations were not calculated for the HRA+ sample; however, for HRA- infants (as well as the combined group of HRA+ and HRA-), a significant negative relation was found between overall attention to faces and eyes and expressive language ability at 18 months. Studies by Young et al. (2009) and Elsabbagh et al. (2014) found that increased attention to mouths relative to eyes (corresponding to a lower calculated EMI) during dynamic stimuli at 6- to 7-months predicted better expressive language abilities in HRA toddlers, and our present findings are consistent with these prior ones. The present study was unable to utilize EMI analyses, as reduced attention to the mouth resulted in limited variability in the EMI, likely a result of the use of static stimuli. The present findings are still highly related to the work of Young et al. (2009) and Elsabbagh et al. (2014), however, as their findings showed increased attention to the mouth was related to better EL at 24 and 36 months, and the present work showed that increased attention to core areas other than the mouth was related to worse EL at 18 months.
Several limitations and areas for future work should be noted. First, as noted above, the sample of HRA+ infants was small, and despite highly significant differences and moderate effects sizes, future work with larger samples will allow for further exploration of how early visual attention in infants later diagnosed with ASD might differ from LRC and unaffected infant siblings (though see related HRA+ findings with similar sample size in Rozga et al., 2011). Additionally, the use of static stimuli in the present study limited the amount of attention paid to the mouth region, so future studies should further explore how differences in attention to static and dynamic stimuli can be related to later social and language outcomes in LRC and HRA.
In summary, the present work found consistent group differences in attention to faces and eyes between HRA+ and HRA-, with HRA+ showing greater attention in these regions, while low-risk infants looked similar to HRA+ for faces and similar to HRA- for eyes alone. This provides further evidence that eye-tracking can reveal group differences in social attention for high-risk infants later diagnosed with ASD; further, unlike prior work, differences can also be seen between LRC and HRA-, with HRA- showing decreased attention for faces as well as decreased attention to eyes and mouth overall. Additionally, in low-risk infants, early attention to the face and eyes is positively related to social behavior at 18 months, while in high-risk infants who do not have ASD, early attention to the face and eyes is negatively related to expressive language ability at 18 months. These differential trajectories as they relate to attention to social information provide an early window into mechanisms of development that might differ based on the broader endophenotype in unaffected infant siblings of children with ASD, and future work should continue to explore early markers of variability among unaffected siblings as well as those diagnosed with ASD. As work with high-risk infants evolves, researchers should continue to combine information across a variety of measures, including measures of attention, social development, neural and physiological responses, and genetic markers, as this approach will allow for a richer picture of both typical and atypical developmental trajectories and could ultimately contribute to a more cohesive approach to screening infants for later developmental difficulties.
References
- Bartrip J, Morton J, de Schonen S. Responses to mother's face in 3-week to 5-month-old infants. British Journal of Developmental Psychology. 2001;19:219–232. [Google Scholar]
- Bushnell IWR. Mother's face recognition in newborn infants: Learning and memory. Infant and Child Development. 2001;10(1-2):67–74. [Google Scholar]
- Chawarska K, Macari S, Shic F. Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biological Psychiatry. 2013;74(3):195–203. doi: 10.1016/j.biopsych.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo J, Mitchell DW, Horowitz FD. Infant visual attention in the paired-comparison paradigm: Test-retest and attention-performance relations. Child Development. 1988;59:1198–1210. doi: 10.1111/j.1467-8624.1988.tb01489.x. [DOI] [PubMed] [Google Scholar]
- Colombo J, Mitchell DW, Coldren JT, Freeseman LJ. Individual differences n infant visual attention: Are short lookers faster processors or feature processors? Child Development. 1991;62:1247–1257. [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Alexander AL, Davidson RJ. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biological Psychiatry. 2007;61:512–520. doi: 10.1016/j.biopsych.2006.05.019. [DOI] [PubMed] [Google Scholar]
- de Haan M, Nelson CA. Recognition of the mother's face by six-month-old infants: A neurobehavioral study. Child Development. 1997;68(2):187–210. [PubMed] [Google Scholar]
- de Klerk CC, Gliga T, Charman T, Johnson MH. Face engagement during infancy predicts later face recognition ability in younger siblings of children with autism. Developmental Science. 2014;17(4):596–611. doi: 10.1111/desc.12141. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Fernandes J, Webb SJ, Dawson G, Charman T, Johnson MH BASIS Team. Disengagement of visual attention in infancy is associated with emerging autism in toddlerhood. Biological Psychiatry. 2013;74:189–194. doi: 10.1016/j.biopsych.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Gliga T, Pickles A, Hudry K, Charman T, Johnson MH BASIS Team. The development of face orienting mechanisms in infants at-risk for autism. Behavioural Brain Research. 2013;251:147–154. doi: 10.1016/j.bbr.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Bedford R, Senju A, Charman T, Pickles A, Johnson MH. What you see is what you get: Contextual modulation of face scanning in typical and atypical development. Social Cognitive and Affective Neuroscience. 2014;9(4):538–543. doi: 10.1093/scan/nst012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck-Ytter T, Bölte S, Gredebäck G. Eye tracking in early autism research. Journal of Neurodevelopmental Disorders. 2013;5(28):1–13. doi: 10.1186/1866-1955-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field TM, Cohen D, Garcia R, Greenberg R. Mother-stranger face discrimination by the newborn. Infant Behavior and Development. 1984;7:19–25. [Google Scholar]
- Guillon Q, Hadjikhani N, Baduel S, Rogé B. Visual social attention in autism spectrum disorder: insights from eye tracking studies. Neuroscience & Biobehavioral Reviews. 2014;42:279–297. doi: 10.1016/j.neubiorev.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Haith MM, Bergman T, Moore MJ. Eye contact and face scanning in early infancy. Science. 1977;198(4319):853–855. doi: 10.1126/science.918670. [DOI] [PubMed] [Google Scholar]
- Hunnius S, Geuze RH. Developmental changes in visual scanning of dynamic faces and abstract stimuli in infants: A longitudinal study. Infancy. 2004;6(2):231–255. doi: 10.1207/s15327078in0602_5. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns' preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40:1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- Jones EJH, Gliga T, Bedford R, Charman T, Johnson MH. Developmental pathways to autism: A review of prospective studies of infants at risk. Neuroscience and Biobehavioral Reviews. 2014;39:1–33. doi: 10.1016/j.neubiorev.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Archives of General Psychiatry. 2008;65(8):946–954. doi: 10.1001/archpsyc.65.8.946. [DOI] [PubMed] [Google Scholar]
- Jones W, Klin A. Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature. 2013;504(7480):427–431. doi: 10.1038/nature12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavšek M. Predicting later IQ from infant visual habituation and dishabituation: A meta-analysis. Journal of Applied Developmental Psychology. 2004;25:369–393. [Google Scholar]
- Keehn B, Müller RA, Townsend J. Atypical attentional networks and the emergence of autism. Neuroscience and Biobehavioral Reviews. 2013;37:164–183. doi: 10.1016/j.neubiorev.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key AP, Stone WL. Processing of novel and familiar faces in infants at average and high risk for autism. Developmental Cognitive Neuroscience. 2012;2(2):244–255. doi: 10.1016/j.dcn.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. Journal of Child Psychology and Psychiatry. 2006;47(6):629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Holman KC, Garrett-Mayer E. Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Archives of General Psychiatry. 2007;64(7):853–864. doi: 10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- Lewkowicz DJ, Hansen-Tift AM. Infants deploy selective attention to the mouth of a talking face when learning speech. Proceedings of the National Academy of Sciences. 2012;109(5):1431–1436. doi: 10.1073/pnas.1114783109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EHJ, Leventhal BL, DiLavore P, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Luyster RJ, Powell C, Tager-Flusberg H, Nelson CA. Neural measures of social attention across the first years of life: characterizing typical development and markers of autism risk. Developmental Cognitive Neuroscience. 2014;8:131–143. doi: 10.1016/j.dcn.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer D, Salapatek P. Developmental changes in the scanning of faces by young infants. Child Development. 1976;47:523–527. [PubMed] [Google Scholar]
- Merin N, Young GS, Ozonoff S, Rogers SJ. Visual fixation patterns during reciprocal social interaction distinguish a subgroup of 6-month-old infants at-risk for autism from comparison infants. Journal of Autism and Developmental Disorders. 2007;37(1):108–121. doi: 10.1007/s10803-006-0342-4. [DOI] [PubMed] [Google Scholar]
- Messinger D, Young GS, Ozonoff S, Dobkins K, Carter A, Zwaigenbaum L, Sigman M. Beyond autism: A baby siblings research consortium study of high-risk children at three years of age. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52(3):300–308. doi: 10.1016/j.jaac.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E. Mullen scales of early learning. Circle Pines, MN: American Guidance Service Inc; 1995. [Google Scholar]
- Nele D, Ellen D, Petra W, Herbert R. Social information processing in infants at risk for ASD at 5 months of age: The influence of a familiar face and direct gaze on attention allocation. Research in Autism Spectrum Disorders. 2015;17:95–105. [Google Scholar]
- Oakes LM, Ellis AE. An eye-tracking investigation of developmental changes in infants' exploration of upright and inverted human faces. Infancy. 2013;18:134–148. doi: 10.1111/j.1532-7078.2011.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, Stone WL. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics. 2011;128(3):e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Belding A, Hill M, Hill A, Hutman T, Iosif AM. The broader autism phenotype in infancy: when does it emerge? Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53(4):398–407. doi: 10.1016/j.jaac.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascalis O, de Schonen S, Morton J, Deruelle C, Fabre-Grenet M. Mother's face recognition by neonates: A replication and an extension. Infant Behavior and Development. 1995;18(1):79–85. [Google Scholar]
- Rose D, Slater A, Perry H. Prediction of intelligence in childhood from habituation in early infancy. Intelligence. 1986;10:251–263. [Google Scholar]
- Rozga A, Hutman T, Young GS, Rogers SJ, Ozonoff S, Dapretto M, Sigman M. Behavioral profiles of affected and unaffected siblings of children with autism: Contribution of measures of mother–infant interaction and nonverbal communication. Journal of Autism and Developmental Disorders. 2011;41:287–301. doi: 10.1007/s10803-010-1051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford MD, Walsh JA, Lee V. Brief report: Infants developing with ASD show a unique developmental pattern of facial feature scanning. Journal of Autism and Developmental Disorders. 2015;45:2618–2623. doi: 10.1007/s10803-015-2396-7. [DOI] [PubMed] [Google Scholar]
- Schietecatte I, Roeyers H, Warreyn P. Can infants' orientation to social stimuli predict later joint attention skills? British Journal of Developmental Psychology. 2012;30(2):267–282. doi: 10.1111/j.2044-835X.2011.02039.x. [DOI] [PubMed] [Google Scholar]
- Shic F, Macari S, Chawarska K. Speech disturbs face scanning in 6-month-old infants who develop autism spectrum disorder. Biological Psychiatry. 2014;75(3):231–237. doi: 10.1016/j.biopsych.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenbaum EJ, Shah RJ, Sobel DM, Malle BF, Morgan JL. Increased focus on the mouth among infants in the first year of life: A longitudinal eye-tracking study. Infancy. 2013;18(4):534–553. doi: 10.1111/j.1532-7078.2012.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenza E, Simion F, Cassia VM, Umiltà C. Face preference at birth. Journal of Experimental Psychology: Human Perception and Performance. 1996;22(4):892. doi: 10.1037//0096-1523.22.4.892. [DOI] [PubMed] [Google Scholar]
- Wagner JB, Luyster RJ, Tager-Flusberg H, Nelson CA. Greater pupil size in response to emotional faces as an early marker of social communicative difficulties in infants at high risk for autism. Infancy. 2016;21(5):560–581. doi: 10.1111/infa.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JB, Luyster RJ, Yim JY, Tager-Flusberg H, Nelson CA. The role of early visual attention in social development. International Journal of Behavioral Development. 2013;37(2):118–124. doi: 10.1177/0165025412468064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherby AM, Allen L, Cleary J, Kublin K, Goldstein H. Validity and reliability of the communication and symbolic behavior scales developmental profile with very young children. Journal of Speech and Hearing Research. 2002;45(6):1202–1218. doi: 10.1044/1092-4388(2002/097). [DOI] [PubMed] [Google Scholar]
- Young GS, Merin N, Rogers SJ, Ozonoff S. Gaze behavior and affect at 6 months: Predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Developmental Science. 2009;12(5):798–814. doi: 10.1111/j.1467-7687.2009.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23(2):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]


