Abstract
Background and Purpose
Falls commonly occur as weight is transferred laterally, and impaired reactive stepping responses are associated with falls after stroke. The purpose of this study was to examine differences in, and the determinants of medio-lateral (M-L) protective stepping strategies when pulled off balance towards the paretic and non-paretic sides.
Method
Eighteen individuals >6 months post-stroke were pulled in the M-L direction by a lateral waist-pull perturbation system. Step type (crossover, medial, lateral) and count were recorded, along with first step initiation time, length and clearance. Sensorimotor variables including hip adductor/abductor and ankle plantarflexor/dorsiflexor peak isokinetic torques, paretic foot plantar cutaneous sensation, and motor recovery were used to predict step type by discriminant function analyses (DFA).
Results
Regardless of pull direction, nearly 70% of trials required ≥2 recovery steps, with more frequent non-paretic leg first steps, 63.5%. The step type was significantly different for pull direction (p=0.005), with a greater percentage of lateral steps when pulled towards the non-paretic side (45.1%) compared to the paretic side (17.5%). The M-L step length of the lateral step was increased (p<0.001), with a reduced step clearance (p=0.05), when pulled towards the paretic side compared to a pull towards the non-paretic side. DFA revealed non-paretic and paretic side pulls could respectively classify step type 64% and 60% of the time, with foot cutaneous sensation discriminating for pull direction.
Discussion and Conclusions
Balance recovery initiated with the non-paretic leg occurred more frequently in response to medio-lateral perturbations, and paretic foot cutaneous sensation was an important predictor of the stepping response regardless of the pull direction. Video Abstract available for more insights from the authors (see Video, Supplementary Digital Content 1,
Keywords: stroke, postural balance, falls, sensation
Introduction
Following a stroke, residual sensorimotor deficits, such as spasticity, muscle weakness, sensory impairments, and poor muscle coordination, are common. These deficits impact ones’ ability to successfully recover balance, which is reflected in the fall rate, which has been reported between 14% and 65% while in the hospital1-3 and up to 73% in the first 6 months after being discharged into the community.4,5 Falls after a stroke are just as likely to be caused by self-induced movements as by externally induced perturbations (e.g. slip, trip, or push).6 Regardless of the manner that induces loss of balance, falls can be devastating, resulting in fractures,7 a fear of falling8 and activity limitations,9 which in turn, reduces mobility.
External mechanical perturbations are forces imposed on the body that cause imbalance either by disrupting the base of support (BOS) (translating support surface or rotations),10,11 or moving the center of mass (COM) relative to the actual or perceived stability limits of the BOS (pull or push at pelvis or trunk or lean and release).12-17 In contrast, self-induced perturbations are imposed by internally generated forces through voluntary movements. Typically, the responses to external perturbations involve rapid sensorimotor feedback mediated reactions, whereas feedforward responses also contribute to counteracting internally initiated perturbations. Both forms of balance involve sensory integration from somatosensory, visual or vestibular input to produce an appropriate response. Thus, after stroke, responding effectively to perturbations of balance may be especially challenging since sensory impairments are experienced by approximately 50% of stroke survivors.18
Falls can be prevented during everyday activities by using protective movements of the limbs such as stepping or grasping surfaces, which stabilize balance by adjusting the COM-BOS relationship. Effective balance recovery through stepping requires that appropriately timed, directed and scaled movements are matched with the changing position and motion characteristics of the COM to adjust the COM-BOS relationship to stabilize balance and prevent falling. When balance is recovered with a single protective step, a larger safety margin of balance stability occurs at first step landing compared to when multiple steps are taken.19 After a stroke, multiple steps are more commonly taken than single recovery steps when standing balance is perturbed anteriorly, indicating a less efficient recovery pattern.12,20,21 We know in older adults that multiple steps are a predictor of prospective falls.22 This observation may also have relevance for falls after stroke in particular among those who are of older age.
While many of the aforementioned studies perturbed standing balance in the anterior direction, information about balance function to an external perturbation in the medio-lateral (M-L) direction is more limited. Understanding M-L balance control after stroke is important since falls occur more frequently as weight is shifted laterally towards the paretic limb.7,9,23,24 A few studies that have evaluated the feet-in-place response to a lateral push perturbation at the hip in people with stroke found a diminished response from the hip abductor-adductor muscles and longer time to stabilize the pelvis.14,15,17 Impaired M-L control of balance after stroke is further supported by reduced biomechanical limits of stability on the paretic side,25 and weight bearing asymmetry towards the non-paretic limb.25-27 All of these factors can impact M-L control and the ability to generate an effective protective stepping.
Lateral perturbations of standing balance involve a unique biomechanical feature whereby the COM is initially moved sideways such that the leg on the side of the direction of imbalance receives increased loading force while the opposite leg is passively unloaded.28 The passive unloading assists with weight transfer allowing for a faster step initiation with the unloaded leg29 resulting in either a medially-directed or crossover step. A lateral step with the loaded leg in the direction of imbalance would take longer to initiate since the passively loaded leg would need to be actively unloaded to initiate a step.29,30 Based on the increased frequency of responses favoring the non-paretic limb in prior studies,12,21 we would expect differences in the step type to be further dependent on the direction of perturbation as well as the biomechanical advantages of using the passively unloaded leg. Thus, examining lateral challenges to balance directed towards both the paretic and non-paretic sides is important for understanding balance recovery and the effectiveness of the stepping strategies that are used.
The purpose of this study was to characterize the stepping response induced by a lateral perturbation generated through a motorized waist-pull system to the paretic and non-paretic sides in chronic stroke. Additionally, we determined whether impairments in selected sensory and motor functions including the plantar cutaneous sensation of the paretic foot, motor recovery, hip abduction and adduction torque, and ankle dorsiflexion and plantarflexion torque, could predict the protective step that was used.
Methods
Eighteen community-dwelling adults with hemiparesis (12 left; 6 right) participated. Participation in the study included individuals who were more than 6 months post-stroke, ≥50 years of age, able to walk 10m with/without an assistive device, stand unsupported for 5 minutes and have no medical conditions significantly impacting their ability to walk beyond the effects of the stroke. All participants gave informed consent to participate, and the study was approved by the University of Maryland Institutional Review Board.
Participants wore a safety harness and received 24 randomly applied lateral perturbations at 4 different intensities in two directions (paretic, non-paretic) (2 directions×4 intensities×3 trials) with a motorized waist-pull system. The lateral perturbation was applied through an adjustable waist belt, aligned in the frontal plane so that the waist-pulls were applied in the M-L direction. The acceleration was fixed at 720 cm/s2, and the velocity (v) and displacement (d) were Level 1 v=18.0 cm/s; d=8.6 cm, Level 2 v=27.0 cm/s d=12.1 cm, Level 3 v=36.0 cm/s d=15.7 cm, and Level 4 v=45cm/s d=19.3 cm. The selection of waist-pull magnitudes was based on our previous studies of stepping responses in younger and older adults and those with stroke. The range of perturbations to balance was based on displacement-velocity-acceleration combinations where steps were reliably likely to occur (level 1), and steps always occurred (levels 2-4) with or without multiple steps.12,31 The direction and timing of each trial were randomly presented to minimize anticipation and learning effects. The system has been previously described32 and used in prior studies of older adults22,28,33 and individuals post-stroke.12
Participants stood on a force platform (Advanced Mechanical Technology Inc., Watertown, MA, USA) using a comfortable self-selected position since the same standardized foot placement was not possible due to increased external rotation of the paretic limb in some participants. For each participant, we ensured the same initial position on the force platform for each trial by tracing the outline of the feet. Shoes were worn during the testing protocol, and those individuals with ankle foot arthroses kept them on during the testing. An investigator monitored the vertical ground reaction forces that were depicted on the screen to ensure an approximate symmetrical weight bearing at the start of each trial. All participants were able to shift their weight to their paretic limb when given the verbal instruction to, “evenly distribute your weight between their two legs.” Participants were instructed to, “respond naturally and if necessary prevent yourself from falling.” A reflective marker was affixed on the lateral malleoli and recorded for 7s per trial at a sampling rate of 120Hz, using a 10-camera motion analysis system (Vicon, Oxford, UK).
Peak isokinetic joint torques of the non-paretic and paretic side were measured in 5 trials at 30°/s using the Biodex System Pro4 (Biodex Medical Systems, NY, USA) for ankle dorsiflexion and plantarflexion and hip abduction and adduction. The Chedoke McMaster Stroke Assessment Impairment Inventory for the leg and foot was used to assess motor recovery.34 The cutaneous sensation was evaluated on the plantar aspect of the foot bilaterally with a series of Semmes-Weinstein monofilaments, ranging from 1.65-6.65, with the lowest value representing normal cutaneous sensation.35 All participants had intact sensation of the non-paretic limb. Thus only the plantar sensation of the paretic foot was used in the analyses. Other clinical outcome measures of balance and mobility, Community Balance and Mobility Scale and Timed Up and Go Test (TUG) were used to characterize the functional level of the group. The TUG can be utilized as a predictor of fall risk in older persons with stroke,36 and the Community Balance and Mobility Scale incorporates high-level balance and mobility tasks required by individuals living in the community37 and is validated in people with stroke.38
Data analysis
Step count and step type were determined for each trial. First step type was categorized as, 1) lateral step, whereby the passively loaded leg moved in the direction of the waist-pull, 2) crossover step, when the passively unloaded leg moved beyond (front or back) the loaded leg in the direction of the waist-pull, and 3) medial step, whereby the passively unloaded leg moved towards the loaded leg but not beyond (Figure 1).30 Matlab customized programs were used to calculate spatiotemporal parameters of the first step of step initiation onset time, M-L step length and step clearance (maximum vertical displacement) indicated by displacement of the ankle marker. Step initiation onset time was calculated as the time between the perturbation onset and first step lift off indicated by the force platform. The M-L step length and step clearance were normalized to the person’s height. Peak isokinetic torque was defined as a deficit ratio relative to the non-paretic leg (paretic peak torque/non-paretic torque).
Figure 1.
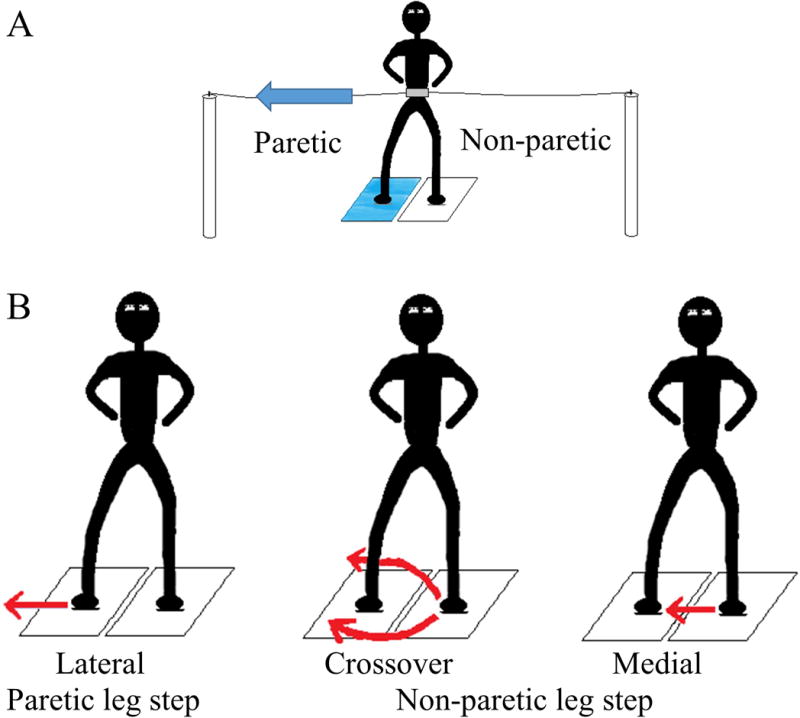
The different types of first step responses to lateral waist-pull perturbations. Panel A, illustrates the passively loaded right (paretic) leg (blue platform) and passively unloaded left (non-paretic) leg (white platform) from a lateral waist-pull perturbation towards the right (paretic side); Panel B, is the initiation of the first step with the passively loaded right (paretic) leg (lateral step) or the passively unloaded left (non-paretic) leg that will either cross in front of or behind the right (paretic) leg (crossover) or move toward but not beyond the right (paretic) leg (medial step).
Statistical Analyses
Descriptive statistics (means and standard deviations) are presented by pull direction (paretic, non-paretic). Mann-Whitney test was used to compare the differences in step type and step count and first step characteristics of step initiation onset time, and first step M-L length and clearance of a pull towards paretic and non-paretic. Nonparametric one-way ANOVA (Kruskal-Wallis), using SPSS for Windows v22.0 (IBM Company, Chicago, IL), was used to examine differences in step type (lateral, crossover, medial) for step initiation time. Significant differences were examined further with the Mann-Whitney U test, and a Bonferroni adjustment was used to correct for multiple comparisons with an adjusted P value (P≤0.025). To determine whether sensorimotor variables could predict the first step type, a multivariate discriminant function analysis (DFA) was performed to identify the sensorimotor variables that were associated with the step type (lateral, crossover, medial) for a paretic and non-paretic side pull. DFA is a procedure used to determine if a set of variables can predict group membership.39 Canonical correlation measures the ratio of the discriminant equation and is used to compare the importance of each variable. A high correlation indicates a function that discriminates well for step type. The Wilks’ lambda measures the variables that contribute significance in discriminant function, and a lower value means the variable contributes more to the discriminant function. Cross-validation, classification identified the accuracy of the model using the leave-one-out method by which each case is classified by discriminant functions derived from the other cases.
Results
The characteristics of the participants are presented in Table 1. A pull towards the paretic side resulted in participants needing assistance to recover balance in 12% of the trials, and 2% of trials when pulled towards the non-paretic side. Some of these trials resulted in no steps. Therefore 8% (pull towards paretic side) and 2% (pull towards non-paretic side) of all trials are included in the analyses where assistance was needed.
Table 1.
Demographic Characteristics, Cutaneous sensation, Motor recovery and Torque values, expressed as Mean ± SD
| Variables | Mean ± SD (N=18) |
|
|---|---|---|
| Age (years) | 61.4 ± 8.0 | |
| Gender | 10 females/8 males | |
| Paresis | 12 left/6 right | |
| Time post-stroke (years) | 10.2 ± 10.4 | |
| Timed Up and Go Test (seconds) | 15.0 ± 12.0 | |
| Community Balance and Mobility Scale (/96) | 29.9 ± 13.3 | |
| Cutaneous sensation plantar aspect of foot, force (g) range, (median) | 2.83–6.65 (4.31) | |
| Chedoke McMaster Stroke Assessment, leg + foot (/14) | 7.7 ± 2.5 | |
| Peak Isokinetic Torque Values measured at 30°/s (Nm) | Paretic | Non-paretic |
| Ankle Dorsiflexion (N=17) | 11.6 ± 10.7 | 23.6 ± 10.0 |
| Ankle Plantarflexion (N=17) | 23.8 ± 23.1 | 48.3 ± 24.1 |
| Hip Abduction (N=18) | 41.8 ± 26.2 | 60.7 ± 27.6 |
| Hip Adductor (N=18) | 43.5 ± 24.2 | 51.6 ± 25.7 |
A pull towards the paretic side resulted in a step in 80.1% of trials and 74.1% for pulls towards the non-paretic side. When combining all the waist-pull trials regardless of the pull direction, 36.5% of the steps were initiated with the paretic leg and 63.5% with the non-paretic leg. There was no significant difference in step count (p=0.8), between a pull towards the non-paretic side and paretic side. More than one recovery step was used in 69.7% (pull towards the paretic side) and 66.9% (pull towards the non-paretic side) of the trials (Figure 2A). The first step type used was significantly different for pull direction (p=0.005) (Figure 2B). A pull towards the paretic side resulted in fewer lateral steps with the paretic leg (18.8%) compared to the lateral steps performed with the non-paretic leg when pulled towards the non-paretic side (45.1%). A crossover step with the paretic leg was used less frequently for a pull towards the non-paretic side (5.2%) compared to a pull towards the paretic side (non-paretic crossover step) (28.8%).
Figure 2.
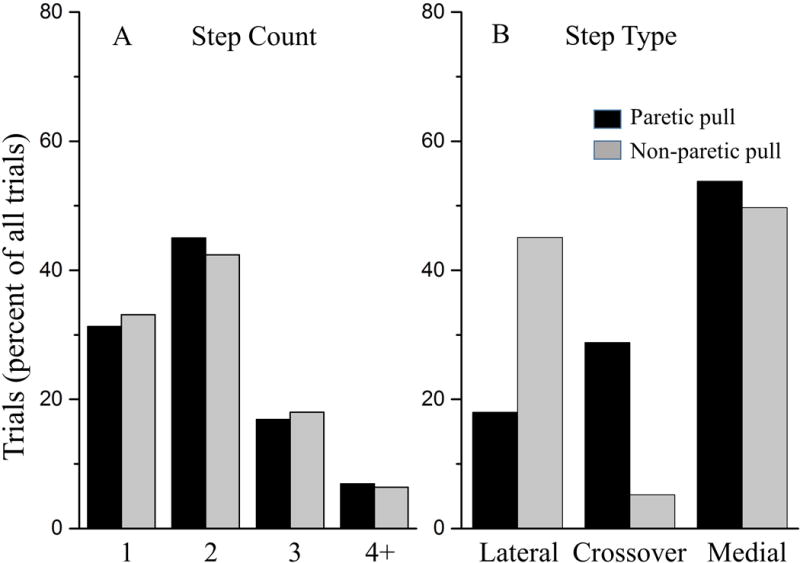
The number of trials (as a percentage of all trials) by direction of lateral waist-pull perturbation for A) step count and B) first step type. The black bars represent the trials when pulled towards the paretic side, and the gray bars represent the trials when pulled towards the non-paretic side.
Step initiation onset time, step length and clearance by step type for pulls towards the non-paretic and paretic sides are illustrated in Figure 3. There was no significant difference in step initiation time between a pull towards the paretic and non-paretic side for the lateral (p=0.2), crossover (p=0.1) or medial step (p=0.06). However, the lateral steps of both pull directions took longer to initiate compared to the crossover (non-paretic P=0.003; paretic P=0.009) and medial step (non-paretic P<0.001; paretic P<0.02). A lateral step when pulled towards the paretic side of the paretic leg had a greater M-L step length (P<0.001) and lower step clearance (P=0.05) than a non-paretic step when pulled towards the non-paretic side. There were no differences between the direction of perturbation for crossover step length or clearance. When pulled towards the non-paretic side, a medial step of the paretic leg resulted in a smaller M-L step length (P<0.001) compared to a medial step of the non-paretic leg when pulled towards the paretic side.
Figure 3.
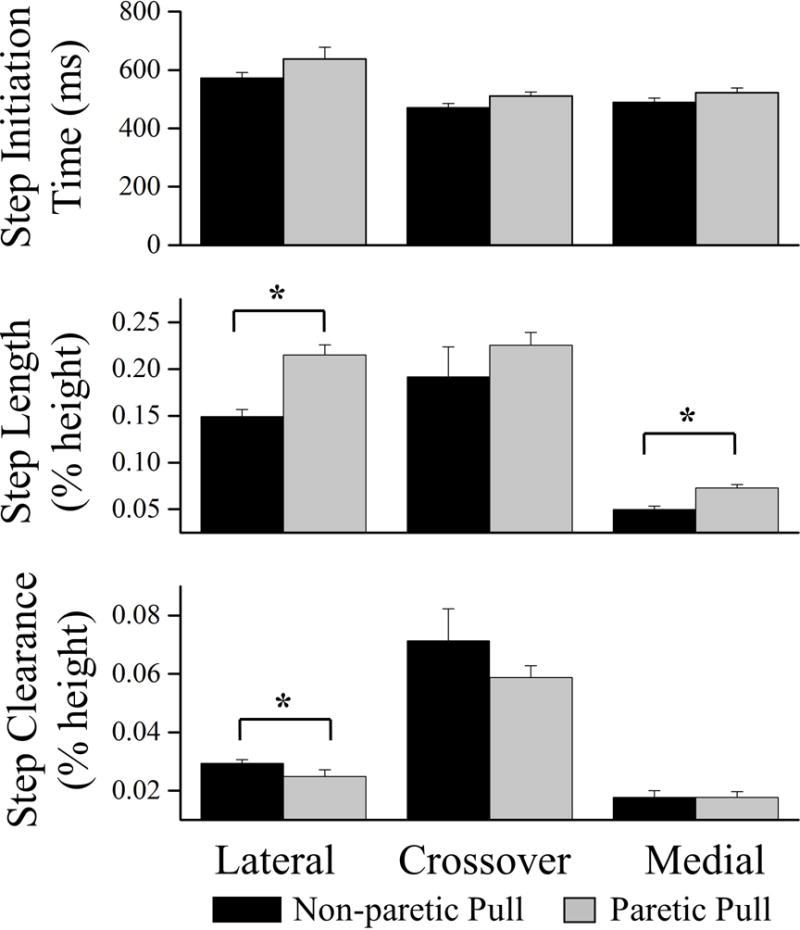
The step initiation time, medio-lateral step length and first step clearance for a lateral, crossover and medial step when pulled towards the non-paretic side (black) and paretic side (light gray). *denotes significant group differences P ≤ 0.05
The DFA for pulls towards the non-paretic side revealed that hip abductor torque, ankle dorsiflexor torque and paretic foot cutaneous sensation significantly discriminated between lateral steps of the non-paretic leg, and crossover and medial steps of the paretic leg (Wilks lambda=0.67; P<0.001; canonical correlation=0.47). The canonical correlation coefficients were 0.81 for paretic hip abductor torque, 0.49 for paretic ankle dorsiflexor torque and 0.63 for the plantar cutaneous sensation of the paretic foot, indicating that paretic hip abductor torque was the most important of these variables in discriminating between the step types. From the DFA, these three variables correctly predicted the step type of the cases as either a lateral, crossover or medial step for 64% of the trials.
For a pull towards the paretic side, the ankle plantarflexor torque and plantar cutaneous sensation of the paretic foot discriminated between the step types (Wilks lambda=0.67; P<0.001; Canonical correlation=0.56). The canonical correlation coefficient was 0.80 for ankle plantarflexor torque and 0.52 for the plantar cutaneous sensation of the paretic foot, with ankle plantarflexor torque being the most important. From the DFA, these variables predicted the first step type in 60% of the trials. Table 2 indicates the mean and standard deviation by step type for the variables identified as important discriminators of step type. The cross validation for a pull towards the paretic side and the non-paretic side was the same, indicating an accurate classification with the discriminant function.
Table 2.
Mean value and standard deviation (unless specified) for sensorimotor measures by step type for all trials for the paretic and non-paretic side waist-pull perturbations.
| Non-paretic Pull | Paretic Pull | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | Lateral (n=67) | Crossover (n=4) | Medial (n=82) | Lateral (n=28) | Crossover(n=42) | Medial (n=74) |
|
| ||||||
| Stepping Leg | Non-paretic | Paretic | Paretic | Paretic | Non-paretic | Non-paretic |
| Sensation, force (g) 25th – 75th Percentile (Median) | 4.31–4.56† (4.31) | 2.83–4.31 (4.31) | 2.83–4.31 (4.31) | 2.83–4.31*† (2.83) | 2.83–4.31‡ (2.83) | 4.31–4.56 (4.31) |
| Ankle Dorsiflexion Torque | 0.60±0.54† | 0.53±0.53 | 0.37±0.43 | 0.77±0.64† | 0.44±0.53 | 0.42±0.39 |
| Ankle Plantarflexion Torque | 0.37±0.44 | 0.33±0.33 | 0.35±0.04 | 0.77±0.48*† | 0.31±0.27‡ | 0.24±0.33 |
| Hip Abduction Torque | 0.65±0.14 | 0.38±0.36 | 0.64±0.24 | 0.75±0.20*† | 0.61±0.19 | 0.65±0.16 |
P≤0.025 between lateral and crossover,
P≤0.025 between lateral and medial,
P≤0.025 between crossover and medial
Discussion
This study investigated protective stepping characteristics induced by lateral balance perturbations and the impact of selected sensorimotor deficits on stepping performance in individuals with chronic stroke. The main finding was that participants were less likely to step laterally with the paretic leg when perturbed towards the paretic side, especially when the cutaneous sensation of the paretic foot was not intact. Steps taken laterally with the paretic limb had an increased M-L length and decreased floor clearance height compared with lateral steps taken with the non-paretic limb.
Previous studies in generally healthy older adults have shown that multiple M-L balance recovery steps are a potent predictor of prospective falls.22,33 In this study, regardless of the direction of the pull, almost 70% of all trials resulted in multiple steps. These findings would indicate that participants had difficulty with recovering their balance when challenged to either their paretic or non-paretic side. Multiple steps are indicative of a less biomechanically stable first step requiring that additional steps be taken to stop the movement of the center of mass.45,46 Whether or not the multiple step behavior is an indicator of increased fall risk after stroke may be important for understanding balance recovery and designing interventions to prevent falls. Further investigation is needed to determine whether multiple steps to recover M-L balance is predictive of falls in people with chronic stroke.
Overall, the non-paretic limb was used more frequently to recover balance especially when pulled towards the paretic side. The decreased use of the paretic limb has been reported in other studies examining anterior perturbations.12,20,21 Efficient use of the paretic limb is necessary for balance recovery especially when the direction of lateral challenge limits the use of one limb through imposed changes in limb loading (e.g. non-paretic leg). Thus, recovery of balance due to biomechanical constraints may require responding with the other limb (e.g. paretic leg). In this study, the characteristics of the first step illustrate both the challenges and compensations necessary to step with the paretic leg when pulled towards the paretic side. The leg used for the first step may indicate better muscle force and power production capacity of that side or an inability to respond with the paretic limb based on diminished cutaneous sensation or reduced muscle strength. In older adults, deficits in these muscles can be used to discriminate fallers from non-fallers.22 Thus, interventions focused on paretic limb stepping appears to be important for enhancing balance recovery.
In older adults, crossover steps are used more frequently to recover lateral balance, while younger adults favor lateral steps.28 Lateral steps increase the BOS and commonly result in one-step, whereas crossover steps are riskier due to inter-limb collisions and multiple steps. The step type used suggests a greater risk of falling in older adults, since, fallers more frequently take medial steps than non-fallers.40 One plausible reason for favoring medial stepping is diminished somatosensation. In a study examining healthy individuals,41 with sensation intact, a lateral step was mainly used to recover balance from a supporting surface translation. After hypothermic anesthesia disrupted the sensation of the plantar aspect of the feet, medial or crossover steps were more commonly used. The cutaneous afferents from the plantar surface mechanoreceptors of the feet provide significant sensory input to control standing balance.42 Hence, the increased likelihood of using reactive sensorimotor mechanisms in responding to externally applied perturbations would mean a greater reliance on sensory feedback systems. The impaired cutaneous sensation of the feet may result in detection errors of the center of pressure beneath the feet in relation to the position and motion of body’s COM and the stability margin of the BOS.43 In our study, a lateral step of the paretic leg was initiated with comparatively mild deficits in paretic foot cutaneous sensation, and a crossover or medial step was used when the cutaneous sensation of the paretic foot was impaired. The reverse was true when pulled towards the non-paretic side. Non-paretic lateral steps occurred with greater cutaneous sensory impairments of the paretic limb, which may indicate an unwillingness or inability to use the paretic limb. Thus, the functional status of the responding leg, rather than the type of step, may be a more important factor to consider in balance recovery in those with stroke. Overall, decreased sensory information from the paretic foot appeared to be a significant contributor to dynamic balance deficits as it has an impact on the step type used for balance recovery after stroke.
In forward stepping, bipedal body weight support is usually transferred to the impending single stance leg before a step is initiated. For lateral perturbations, the passive changes in limb loading due to the sideways perturbation assists with the weight transfer and allows for a faster step initiation with the unloaded leg.33,44 This observation was corroborated by the faster onset times for the crossover and medial steps, which were earlier than the lateral step onset time regardless of the pull direction. Many individuals took advantage of the passively unloaded leg to initiate a faster step. However, these strategies were used more frequent when pulled towards the paretic side than the non-paretic side as indicated by the fewer paretic lateral steps. Changes in the capacity to generate the required hip abductor torque at the required rate for an initial lateral step may be impaired by paresis or sensory changes found after stroke.
This study has several limitations, including a small sample size and the results would only apply to community-dwelling ambulatory individuals with chronic stroke. The replication of falls in the laboratory environment differs from naturally occurring circumstances since falls are unexpected. Participants were aware of an impending perturbation, although velocity-displacement-acceleration was not known. Responses are in a feedback manner, which is similar to many naturally occurring events involving external perturbations.
Summary
Effectively responding to lateral challenges to standing balance in people with chronic stroke is difficult, requiring multiple steps commonly initiated with the non-paretic leg. The step type appears to be partly determined by the level of paretic foot cutaneous sensation. The unwillingness or limited ability to step with the paretic leg that was evident when pulled towards the paretic side. Individuals unable to use both legs to initiate a protective step may have a higher risk for falls. Thus, interventions targeting increasing paretic limb use for maintaining balance may be necessary for reducing falls.
Supplementary Material
Supplementary Digital Content 1. Video of the abstract. wmv.
Acknowledgments
Source of Funding: VLG is currently receiving a grant (14CRP19880025) from American Heart Association, a Pepper Pilot grant (P30-AG028747) from the National Institute on Aging (NIA) Claude D. Pepper Older Americans Independence Center, and a grant (H133F140027) from the National Institute on Disability, Independent Living, and Rehabilitation Research. MWR currently receiving a grant (H133P100014) from the National Institute on Disability, Independent Living, and Rehabilitation Research.
Footnotes
Conflicts of Interest: For the remaining authors none were declared.
Presented Society for Neuroscience, Chicago, 2015: The influence of sensorimotor deficits on unexpected lateral perturbations after stroke.
References
- 1.Davenport RJ, Dennis MS, Wellwood I, Warlow CP. Complications after acute stroke. Stroke. 1996;27:415–420. doi: 10.1161/01.str.27.3.415. [DOI] [PubMed] [Google Scholar]
- 2.Nyberg L, Gustafson Y. Patient falls in stroke rehabilitation. A challenge to rehabilitation strategies. Stroke. 1995;26:838–842. doi: 10.1161/01.str.26.5.838. [DOI] [PubMed] [Google Scholar]
- 3.Teasell R, McRae M, Foley N, Bhardwaj A. The incidence and consequences of falls in stroke patients during inpatient rehabilitation: factors associated with high risk. Arch Phys Med Rehabil. 2002;83:329–333. doi: 10.1053/apmr.2002.29623. [DOI] [PubMed] [Google Scholar]
- 4.Forster A, Young J. Incidence and consequences of falls due to stroke: a systematic inquiry. BMJ. 1995;311:83–86. doi: 10.1136/bmj.311.6997.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macintosh M, Goodacre S, Carter A. Organisational influences on the activity of chest pain units during the ESCAPE trial: a case study. Emerg Med J. 2010;27:672–676. doi: 10.1136/emj.2009.073908. [DOI] [PubMed] [Google Scholar]
- 6.Mansfield A, Inness EL, Wong JS, Fraser JE, McIlroy WE. Is impaired control of reactive stepping related to falls during inpatient stroke rehabilitation? Neurorehabil Neural Repair. 2013;27:526–533. doi: 10.1177/1545968313478486. [DOI] [PubMed] [Google Scholar]
- 7.Andersson AG, Seiger A, Appelros P. Hip fractures in persons with stroke. Stroke Res Treat. 2013;2013:954279. doi: 10.1155/2013/954279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe Y. Fear of falling among stroke survivors after discharge from inpatient rehabilitation. Int J Rehabil Res. 2005;28:149–152. doi: 10.1097/00004356-200506000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Mackintosh SF, Hill KD, Dodd KJ, Goldie PA, Culham EG. Balance score and a history of falls in hospital predict recurrent falls in the 6 months following stroke rehabilitation. Arch Phys Med Rehabil. 2006;87:1583–1589. doi: 10.1016/j.apmr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Marigold DS, Eng JJ. Altered timing of postural reflexes contributes to falling in persons with chronic stroke. Exp Brain Res. 2006;171:459–468. doi: 10.1007/s00221-005-0293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marigold DS, Eng JJ, Timothy Inglis J. Modulation of ankle muscle postural reflexes in stroke: influence of weight-bearing load. Clin Neurophysiol. 2004;115:2789–2797. doi: 10.1016/j.clinph.2004.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez KM, Mille ML, Zhang Y, Rogers MW. Stepping in persons poststroke: comparison of voluntary and perturbation-induced responses. Arch Phys Med Rehabil. 2013;94:2425–2432. doi: 10.1016/j.apmr.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 13.Inness EL, Mansfield A, Lakhani B, Bayley M, McIlroy WE. Impaired reactive stepping among patients ready for discharge from inpatient stroke rehabilitation. Phys Ther. 2014;94:1755–1764. doi: 10.2522/ptj.20130603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inness EL, Mansfield A, Bayley M, McIlroy WE. Reactive stepping after stroke: determinants of time to foot off in the paretic and nonparetic limb. J Neurol Phys Ther. 2016;40:196–202. doi: 10.1097/NPT.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 15.Holt RR, Simpson D, Jenner JR, Kirker SG, Wing AM. Ground reaction force after a sideways push as a measure of balance in recovery from stroke. Clin Rehabil. 2000;14:88–95. doi: 10.1191/026921500668655351. [DOI] [PubMed] [Google Scholar]
- 16.Kirker SG, Simpson DS, Jenner JR, Wing AM. Stepping before standing: hip muscle function in stepping and standing balance after stroke. J Neurol Neurosurg Psychiatry. 2000;68:458–464. doi: 10.1136/jnnp.68.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wing AM, Goodrich S, Virji-Babul N, Jenner JR, Clapp S. Balance evaluation in hemiparetic stroke patients using lateral forces applied to the hip. Arch Phys Med Rehabil. 1993;74:292–299. [PubMed] [Google Scholar]
- 18.Carey LM. Somatosensory loss after stroke. Crit Rev Phys Rehab Med. 1995;7:51–91. [Google Scholar]
- 19.Carty CP, Cronin NJ, Lichtwark GA, Mills PM, Barrett RS. Mechanisms of adaptation from a multiple to a single step recovery strategy following repeated exposure to forward loss of balance in older adults. PLoS One. 2012;7:e33591. doi: 10.1371/journal.pone.0033591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansfield A, Inness EL, Komar J, Biasin L, Brunton K, Lakhani B, et al. Training rapid stepping responses in an individual with stroke. Phys Ther. 2011;91:958–969. doi: 10.2522/ptj.20100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lakhani B, Mansfield A, Inness EL, McIlroy WE. Compensatory stepping responses in individuals with stroke: a pilot study. Physiother Theory Pract. 2011;27:299–309. doi: 10.3109/09593985.2010.501848. [DOI] [PubMed] [Google Scholar]
- 22.Hilliard MJ, Martinez KM, Janssen I, Edwards B, Mille ML, Zhang Y, et al. Lateral balance factors predict future falls in community-living older adults. Arch Phys Med Rehabil. 2008;89:1708–1713. doi: 10.1016/j.apmr.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu CH, Liou TH, Hsiao PL, Lin YC, Chang KH. Contribution of ischemic stroke to hip fracture risk and the influence of gender difference. Arch Phys Med Rehabil. 2011;92:1987–1991. doi: 10.1016/j.apmr.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Weerdesteyn V, de Niet M, van Duijnhoven HJ, Geurts AC. Falls in individuals with stroke. J Rehabil Res Dev. 2008;45:1195–1213. [PubMed] [Google Scholar]
- 25.Au-Yeung SS. Does weight-shifting exercise improve postural symmetry in sitting in people with hemiplegia? Brain Inj. 2003;17:789–797. doi: 10.1080/0269905031000088487. [DOI] [PubMed] [Google Scholar]
- 26.Roerdink M, Geurts AC, de Haart M, Beek PJ. On the relative contribution of the paretic leg to the control of posture after stroke. Neurorehabil Neural Repair. 2009;23:267–274. doi: 10.1177/1545968308323928. [DOI] [PubMed] [Google Scholar]
- 27.Gray VL, Juren LM, Ivanova TD, Garland SJ. Retraining postural responses with exercises emphasizing speed poststroke. Phys Ther. 2012;92:924–934. doi: 10.2522/ptj.20110432. [DOI] [PubMed] [Google Scholar]
- 28.Mille ML, Johnson ME, Martinez KM, Rogers MW. Age-dependent differences in lateral balance recovery through protective stepping. Clin Biomech (Bristol, Avon) 2005;20:607–616. doi: 10.1016/j.clinbiomech.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Maki BE, McIlroy WE, Perry SD. Influence of lateral destabilization on compensatory stepping responses. J Biomech. 1996;29:343–353. doi: 10.1016/0021-9290(95)00053-4. [DOI] [PubMed] [Google Scholar]
- 30.Yungher DA, Morgia J, Bair WN, Inacio M, Beamer BA, Prettyman MG, et al. Short-term changes in protective stepping for lateral balance recovery in older adults. Clin Biomech (Bristol, Avon) 2012;27:151–157. doi: 10.1016/j.clinbiomech.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mille ML, Rogers MW, Martinez K, Hedman LD, Johnson ME, Lord SR, et al. Thresholds for inducing protective stepping responses to external perturbations of human standing. J Neurophysiol. 2003;90:666–674. doi: 10.1152/jn.00974.2002. [DOI] [PubMed] [Google Scholar]
- 32.Pidcoe PE, Rogers MW. A closed-loop stepper motor waist-pull system for inducing protective stepping in humans. J Biomech. 1998;31:377–381. doi: 10.1016/s0021-9290(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 33.Mille ML, Johnson-Hilliard M, Martinez KM, Zhang Y, Edwards BJ, Rogers MW. One step, two steps, three steps more … Directional vulnerability to falls in community-dwelling older people. J Gerontol A Biol Sci Med Sci. 2013;68:1540–1548. doi: 10.1093/gerona/glt062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gowland C, Stratford P, Ward M, Moreland J, Torresin W, Van Hullenaar S, et al. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993;24:58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- 35.Roll R, Kavounoudias A, Roll JP. Cutaneous afferents from human plantar sole contribute to body posture awareness. Neuroreport. 2002;13:1957–1961. doi: 10.1097/00001756-200210280-00025. [DOI] [PubMed] [Google Scholar]
- 36.Andersson AG, Kamwendo K, Seiger A, Appelros P. How to identify potential fallers in a stroke unit: validity indexes of 4 test methods. J Rehabil Med. 2006;38:186–191. doi: 10.1080/16501970500478023. [DOI] [PubMed] [Google Scholar]
- 37.Pollock C, Eng J, Garland S. Clinical measurement of walking balance in people post stroke: a systematic review. Clin Rehabil. 2011;25:693–708. doi: 10.1177/0269215510397394. [DOI] [PubMed] [Google Scholar]
- 38.Knorr S, Brouwer B, Garland SJ. Validity of the Community Balance and Mobility Scale in community-dwelling persons after stroke. Arch Phys Med Rehabil. 2010;91:890–896. doi: 10.1016/j.apmr.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Tabachnick BG, Fidell LS. Using multivariate statistics. 5th. Boston: Pearson/Allyn & Bacon; 2007. [Google Scholar]
- 40.Bair WN, Prettyman MG, Beamer BA, Rogers MW. Kinematic and behavioral analyses of protective stepping strategies and risk for falls among community living older adults. Clin Biomech (Bristol, Avon) 2016;36:74–82. doi: 10.1016/j.clinbiomech.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perry SD, McIlroy WE, Maki BE. The role of plantar cutaneous mechanoreceptors in the control of compensatory stepping reactions evoked by unpredictable, multi-directional perturbation. Brain Res. 2000;877:401–406. doi: 10.1016/s0006-8993(00)02712-8. [DOI] [PubMed] [Google Scholar]
- 42.Magnusson M, Enbom H, Johansson R, Pyykko I. Significance of pressor input from the human feet in anterior-posterior postural control. The effect of hypothermia on vibration-induced body-sway. Acta Otolaryngol. 1990;110:182–188. doi: 10.3109/00016489009122535. [DOI] [PubMed] [Google Scholar]
- 43.Fujimoto M, Bair WN, Rogers MW. Center of pressure control for balance maintenance during lateral waist-pull perturbations in older adults. J Biomech. 2015;48:963–968. doi: 10.1016/j.jbiomech.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maki BE, Edmondstone MA, McIlroy WE. Age-related differences in laterally directed compensatory stepping behavior. J Gerontol A Biol Sci Med Sci. 2000;55:M270–277. doi: 10.1093/gerona/55.5.m270. [DOI] [PubMed] [Google Scholar]
- 45.Salot P, Patel P, Bhatt T. Reactive Balance in Individuals With Chronic Stroke: Biomechanical Factors Related to Perturbation-Induced Backward Falling. Phys Ther. 2016;96:338–347. doi: 10.2522/ptj.20150197. [DOI] [PubMed] [Google Scholar]
- 46.Peterson DS, Huisinga JM, Spain RI, Horak FB. Characterization of Compensatory Stepping in People With Multiple Sclerosis. Arch Phys Med Rehabil. 2016;97:513–521. doi: 10.1016/j.apmr.2015.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Digital Content 1. Video of the abstract. wmv.


