Abstract
Purpose of review
Antiphospholipid syndrome (APS) is a leading acquired cause of thrombosis and pregnancy loss. Upon diagnosis (which is not made until at least one morbid event has occurred), anticoagulant medications are typically prescribed in an attempt to prevent future events. This approach is not uniformly effective and does not prevent associated autoimmune and inflammatory complications. The goal of this review is to update clinicians and scientists on mechanistic and clinically-relevant studies from the past 18 months, which have especially focused on inflammatory aspects of APS pathophysiology.
Recent findings
How antiphospholipid antibodies leverage receptors and signaling pathways to activate cells are being increasingly defined. While established mediators of disease pathogenesis (like endothelial cells and the complement system) continue to receive intensive study, emerging concepts (such as the role of neutrophils) are also receiving increasing attention. In vivo animal studies and small clinical trials are demonstrating how repurposed medications (hydroxychloroquine, statins, rivaroxaban) may have clinical benefit in APS, with these concepts importantly supported by mechanistic data.
Summary
As anticoagulant medications are not uniformly effective and do not comprehensively target the underlying pathophysiology of APS, there is a continued need to reveal the inflammatory aspects of APS, which may be modulated by novel and repurposed therapies.
Keywords: Antiphospholipid syndrome, thrombosis, pregnancy loss, endothelial cells, neutrophils, complement
Introduction
Vascular complications, including thrombotic events, are among the leading causes of morbidity and mortality in lupus. Antiphospholipid antibodies (aPL), a major driver of thrombosis risk, are present in up to one-third of lupus patients. When aPL are associated with certain clinical complications (either thrombotic or obstetric), a diagnosis of antiphospholipid syndrome (APS) is assigned (Table 1) [1]. Beyond lupus-associated APS, approximately half of APS cases will be diagnosed as a standalone syndrome (i.e., primary APS) [2].
Table 1.
Classification Criteria for Antiphospholipid Syndrome [1]
APS is present if 1 of the clinical criteria and 1 of the laboratory criteria are met
| Clinical criteria | 1. Vascular thrombosis | ≥1 clinical episode of arterial, venous, or small-vessel thrombosis. |
| 2. Pregnancy morbidity |
|
|
| Laboratory criteria | The presence of antiphospholipid antibodies on ≥2 occasions ≥12 weeks apart:
|
|
APS is a leading acquired cause of thrombosis and pregnancy loss, with an estimated prevalence of 1 in 2,000 [3]. Framing this risk another way, aPL can be detected on the order of 10% of the time in the setting of certain events including pregnancy morbidity, stroke, myocardial infarction, and deep venous thrombosis (DVT) [4]. Emphasizing the systemic nature of APS, the diagnosis also portends risk for cytopenias (especially hemolytic anemia and thrombocytopenia), mitral and aortic valve lesions, seizure disorder, accelerated cognitive decline, and nephropathy in the form of thrombotic microangiopathy [5]. The approach to treatment is typically with anticoagulant drugs, which are not uniformly effective in preventing recurrent aPL-mediated thrombosis and pregnancy loss, and offer insufficient protection against the varied “non-criteria” manifestations of APS. Indeed, 44% of “triple-positive” APS patients (positive testing for anticardiolipin, anti-beta-2-glycoprotein I, and lupus anticoagulant) will develop recurrent thrombosis over a 10-year follow-up period (even with the majority being prescribed anticoagulants) [6]. Furthermore, at least 20% of obstetric APS patients have adverse outcomes in spite of therapy with aspirin and low-molecular-weight heparin [7].
Despite its high prevalence and potential for devastating morbidity, APS pathophysiology has yet to be fully defined. APS was historically viewed as a coagulation problem; however, clinical observations and basic science discoveries are increasingly highlighting a more multifaceted syndrome with an associated (and perhaps even central) inflammatory component [8]. Herein we will discuss recent discoveries over the past 18 months, which have continued to increase our understanding of APS pathophysiology. We will also discuss how this improved basic understanding may translate to new and repurposed therapeutics for APS (Table 2)
Table 2.
Summary of efficacy and mechanisms by which adjuvant therapeutics could potentially benefit APS patients
| Hydroxychloroquine | Statins | Rivaroxaban | ||
|---|---|---|---|---|
| Summary of efficacy: | ||||
| Thrombotic risk | Mouse models | Protects [9,10] | Protects [11] | |
| APS patients | No prospective studies in APS, but protects in post-operative setting [12] | No studies in APS, but protects in the general population [13] | Efficacy may be similar to warfarin (although further study is needed) [14] | |
| Obstetric events | Mouse models | Prevents fetal death and metabolic changes [15] | Prevents fetal death [16] | |
| APS patients | May prevent pregnancy loss [7,17] | May prevent fetal morbidity and mortality [18] | ||
| Potential anti-inflammatory mechanisms: | ||||
| Complement | Inhibits activation and deposition [15] | Decreases activation [19] | ||
| Type I IFN signature | Decreases [20] | Decreases [20] | ||
| NET release | Possibly inhibits [21] | |||
Cell activation and signaling pathways: new concepts
Understanding the cellular signaling pathways that mediate APS pathogenesis has remained somewhat elusive, at least partially the consequence of study heterogeneity. Studies have utilized different types of aPL (monoclonal vs. patient-derived; protein cofactor-dependent vs. -independent) and have focused on a variety of cellular targets (endothelial cells, platelets, monocytes, neutrophils, trophoblast cells, etc.).
Many (perhaps most) pathogenic antibodies in APS do not target phospholipids themselves, but rather phospholipid-binding protein cofactors. The best characterized of these cofactors is beta-2 glycoprotein I (β2GPI), a lipid-binding protein present at high levels in plasma [22,23], albeit with largely unknown endogenous function. The mechanistic schema is that anti-β2GPI antibodies potentiate thrombosis by engaging β2GPI protein that has been recruited to cell surfaces—and thereby promote cell activation [24–26]. The mechanisms by which anti-β2GPI antibodies activate cells have been recently reviewed [27], with roles especially suggested for the cell surface proteins annexin A2, apolipoprotein E receptor 2 (ApoER2), Toll-like receptor 2 (TLR2), and TLR4 [27].
ApoER2 (also known as LDL receptor-related protein 8) is one receptor for β2GPI (and consequently β2GPI-dependent aPL) on monocytes, endothelial cells, and platelets. Indeed, in a 2011 study, Ramesh and colleagues demonstrated ApoER2−/− mice are relatively resistant to thrombosis when confronted with aPL [28]. More recently, it has been revealed that ApoER2 may play an important role in obstetric APS [29]. Specifically, Ulrich and colleagues demonstrated enhanced placental trophoblast cell proliferation and migration in vitro when aPL engage β2GPI/ApoER2 complexes on the trophoblast cell surface [29]. Extending these studies to an in vivo model of aPL-mediated pregnancy loss, they demonstrated protection in ApoER2−/− mice [29]. In another recent study, Mineo and colleagues developed a monoclonal antibody against β2GPI that prevents pathogenic aPL binding, thereby protecting against aPL-mediated cell activation [30]*. The antibody attenuated the association of β2GPI with ApoER2, thereby normalizing endothelial and trophoblast cell function in vitro, as well as preventing thrombosis and fetal loss in vivo [30]*. Although further study is clearly needed, the intersection of aPL, β2GPI, and ApoER2 warrants further investigation as a potential therapeutic target in patients.
Since neither β2GPI itself, nor some β2GPI “receptors” such as annexin A2, have a cytoplasmic domain to mediate signaling, there has been interest in additional partner proteins that may convey activating signals to the cytoplasm. On this front, particular attention has been given to the cell-surface TLRs, TLR2 and TLR4. In mouse models, TLR4 deletion protects against venous and arterial thrombosis in some [31–33], but not all [34]*, studies (it is worth pointing out that the latter study utilized cofactor-independent aPL). Studies of obstetric APS have also yielded mixed results with an older study demonstrating no role for TLR4 in an in vivo model of pregnancy loss [35]. In contrast, Azuma and colleagues recently suggested that, at least in vitro, TLR2 and TLR4 facilitate inflammatory cytokine production by trophoblast cells in response to anti-β2GPI antibodies [36].
Signaling pathways downstream of the aforementioned receptors, at least as they relate to APS pathogenesis, remain incompletely understood. Terrisse and colleagues recently investigated downstream signaling pathways by which aPL (especially IgG isolated from APS patients) activate platelets [37]*. The authors demonstrated that aPL potentiate ex vivo platelet activation through surface glycoprotein Ibα (the platelet receptor for von Willebrand factor) and TLR2, via a mechanism involving class IA phosphoinositide 3-kinase (PI3K) α and β isoforms [37]*. At least one downstream consequence of PI3K signaling is activation of the serine/threonine kinase Akt, a pathway that supports cell survival, proliferation, and migration [37]*. Indeed, PI3K inhibitors, which are being explored as potential drug targets in other contexts [38], are effective at preventing aPL-mediated platelet activation [37]*. Interestingly, another study has suggested that Akt activation is a downstream consequence of trophoblast cell activation by aPL [29].
Beyond the engagement of aPL with cell surfaces, a recent report by Wu and colleagues suggests an intriguing new mechanism by which aPL-activated endothelial cells may propagate this activation in paracrine fashion to other endothelial cells [39]*. Anti-β2GPI antibodies trigger the release of “extracellular vesicles” from endothelial cells, which the authors define as inclusive of both microparticles and exosomes [39]*. These vesicles then activate endothelial cells through a mechanism that is not dependent upon packaged cytokines such as IL-1, but rather single-stranded RNA that signals through TLR7 in the recipient cell [39]*. They also speculate that these vesicles may be a mechanism for delivery of specific and functionally-relevant micro-RNA, although this hypothesis requires further study.
The vessel wall: endothelial progenitors and interferons
Our group recently looked “upstream” of endothelial cells, asking whether a deficiency in reparative, circulating endothelial progenitors might contribute to defective maintenance and health of the endothelium over time. Indeed, a deficiency in the number and function of such progenitors is a well-recognized aspect of both lupus and rheumatoid arthritis [40]. We found that primary APS patients have a reduction in functional endothelial progenitors, which was interestingly not dependent upon patient IgG; rather, we discovered a type I IFN signature in the APS patients, abrogation of which could restore normal progenitor function [41]*. These findings were recently replicated by van den Hoogen and colleagues, who found that approximately 50% of primary APS patients have a type I IFN signature, which was less likely to be present in patients taking either hydroxychloroquine or statins [20]**. Interestingly, they also found that the IFN signature correlated with expansion of “intermediate” and “non-classical” monocytes (which have been previously linked to cardiovascular disease in lupus and rheumatoid arthritis) [20]**. How these monocytes intersect with endothelial progenitors [42], and whether there is a role for anti-interferon therapy in APS [43], are questions that deserve further consideration.
One potential consequence of endothelial cell (and progenitor) dysfunction is atherosclerosis, an accelerated version of which is a well-known complication of lupus [44], and which has also been reported in APS [45,46]. The recent work of Benagiano and colleagues has examined the role of TH1 specific inflammatory responses to β2GPI in established atherosclerotic lesions of primary APS patients. Their work demonstrated that plaque-derived, β2GPI-specific CD4+ T lymphocytes facilitate perforin- and Fas ligand-mediated cytotoxicity, pointing to a role for these autoreactive T cells in plaque destabilization (and potentially the arterial thrombotic events that are known to occur at higher frequency in APS) [47]**. They also demonstrated that β2GPI can induce proliferation of (and IFN-γ expression by) plaque-derived T cell clones [47]**. Furthermore, these T cells amplify monocyte responses, such as the production of tissue factor and matrix metalloproteinases, which can be inhibited with an anti-IFN-γ antibody [47]**.
Myeloid-lineage cells: neutrophil extracellular traps (NETs) and monocyte NOX2
The role of neutrophils in APS pathogenesis has only recently been investigated. This interest was precipitated by emerging descriptions of neutrophils as mediators of both pathologic clotting and autoimmune diseases [48,49]. In particular, NETs (extracellular chromatin-based structures released by activated neutrophils) have been described as triggers of autoimmunity and tissue damage, as well as important instigators of thrombosis [50].
With this background in mind [51], our group recently identified increased levels of cell-free DNA and NETs in the circulation of primary APS patients, as compared with healthy controls [52]**. When APS neutrophils were cultured in vitro, they demonstrated an enhanced propensity to spontaneously release NETs [52]**. Mechanistically, anti-β2GPI IgG appears to be at least one factor in patient blood that supports NET release, with the mechanism dependent upon both TLR4 and formation of reactive oxygen species [52]**. Furthermore, the prothrombotic potential of aPL-mediated NETs was demonstrated in a thrombin generation assay, with this potential abrogated by treatment with deoxyribonuclease (DNase) [52]**. In parallel to our work, van den Hoogen and colleagues reported increased levels of circulating “low-density granulocytes” or LDGs in patients with primary APS [53]. This pro-inflammatory subset of neutrophils has been well characterized in SLE and other autoimmune disorders, where they are reported to release NETs in exaggerated fashion [54]. Whether LDGs are important sources of NETs in APS awaits further study [55].
The in vivo relevance of NETs was recently confirmed by our group in a mouse model of APS. In this model, IgG from triple-positive APS patients potentiated venous thrombosis in mice that had been subjected to flow restriction in the inferior vena cava by a standard surgical stenosis [56]*. As compared with control mice, mice treated with APS IgG were twice as likely to develop macroscopic thrombi in response to flow restriction. Mechanistically, APS thrombi were enriched for NETs, while patient IgG could be detected on the surface of circulating neutrophils [56]*. Furthermore, APS IgG-mediated thrombosis could be reversed by either neutrophil depletion or administration of systemic DNase [56]*. Around the same time, Manukyan and colleagues published an elegant study demonstrating that cofactor-independent aPL could similarly potentiate thrombosis in an inferior vena cava flow-restriction model [34]*. Their interesting work found a major role for leukocyte activation in thrombus formation, which could be abrogated by deletion of NOX2 (the catalytic subunit of NADPH oxidase) from bone marrow-derived cells. While the authors’ primary interest was in monocyte NOX2 and its role in tissue factor expression, there is also a well-accepted role for neutrophil NOX2 in NET formation [57]. Further studies may assess the role of these cofactor-independent antiphospholipid antibodies in inducing NET release in vitro and in vivo.
Complement: at the intersection of coagulation and inflammation in APS
Animal models of APS have supported a role for complement activation in both thrombotic events and pregnancy loss [58,59]. Studies in APS patients have demonstrated smoldering activity of the complement cascade [60–62], while a recent case report revealed deposition of β2GPI protein, IgG, and complement components C1q, C4, C3, and C5b-9 at the endothelial surface of an occluded artery in an APS patient [63]. Furthermore, this patient, who had suffered recurrent arterial occlusions, was successfully revascularized while under treatment with eculizumab, a terminal complement inhibitor [63].
In lupus, antibodies to C1q (a complex that initiates the complement cascade in response to immune complexes) amplify complement activation and strongly correlate with certain clinical manifestations such as proliferative nephritis [64]. Oku and colleagues recently investigated these antibodies in primary APS patients, demonstrating that 36% of patients had detectable anti-C1q (compared to 55% of lupus patients) [65]. Interestingly titers of anti-C1q were significantly higher in patients with refractory APS [65].
Rivaroxaban, a direct factor Xa inhibitor, has recently been proposed as an alternative agent to vitamin K antagonists in APS. The first randomized, prospective study investigating use of rivaroxaban in APS (RAPS trial) was recently published. In patients with a history of venous thromboembolism (who had already demonstrated stable disease on warfarin), both warfarin and rivaroxaban prevented new thrombotic events for 210 days in every study patient [14]**. Bleeding events and overall adverse events were also similar between the groups [14]**. While a full recounting of this important trial is beyond the scope of this brief review, we would refer you to a detailed comment on the topic [66]. Related to our discussion of the complement pathway, a post-hoc analysis of the RAPS trial revealed that, prior to randomization, APS patients had significantly higher markers of complement activation as compared with normal controls [19]*. While patients in the warfarin group showed stable elevation of these markers over time, patients randomized to rivaroxaban demonstrated decreased C3a, C5a, and soluble C5b-9 (all markers of classical pathway activation) [19]*. In contrast, the alternative pathway marker, Bb, was unchanged with rivaroxaban treatment [19]*. Whether direct oral anticoagulants have additional anti-inflammatory properties is a topic that certainly warrants further study.
Repurposing medications: statins and hydroxychloroquine as adjuvant therapies in APS?
HMG-CoA reductase inhibitors (or statins) have long been recognized to have pleotropic anti-inflammatory effects supportive of vascular health, including reductions in inflammation, oxidative stress, and coagulation [67]. Clinically, statins appear to reduce the risk of venous thromboembolism in the general population [13]. In mouse models of APS, statins mitigate aPL-mediated thrombotic events and fetal death [11,16]. Furthermore, when administered to APS patients, statins decrease both prothrombotic and proinflammatory biomarkers [68].
The standard of care for managing pregnancy complications in APS is the administration of low-dose aspirin and low-molecular-weight heparin (the latter at either prophylactic or therapeutic doses, depending on the patient’s thrombosis history) [69,70]. However, as detailed in recent review articles [69,70], pregnancy complications in APS are often not based in frank placental thrombosis, but rather spiral artery vasculopathy, as well as acute and chronic inflammation—with increased infiltration of inflammatory cells and deposition of complement in the placentae of women with APS [71–73]. Lefkou and colleagues recently investigated the use of pravastatin in refractory obstetric APS [18]**. In their clinical trial, 21 patients with refractory obstetric APS (emergence of preeclampsia and/or intrauterine growth restriction [IUGR] despite treatment with low-dose aspirin and low-molecular-weight heparin) were randomized either to continue standard therapy or to receive pravastatin 20 mg/day at the onset of preeclampsia/IUGR [18]**. There was a remarkable therapeutic benefit, with all the patients receiving pravastatin delivering healthy infants at 34–38 weeks [18]**. In contrast, the 10 patients who remained on standard therapy had three stillbirths at 25–26 weeks, and seven pre-term Cesarean sections (resulting in two fetal deaths) [18]**.
Hydroxychloroquine (which is nowadays prescribed to essentially all patients with lupus) was utilized in the 1970s to reduce the risk of venous-thromboembolism in post-operative patients [12]. In the 1990s, hydroxychloroquine was demonstrated to protect against aPL-mediated thrombosis in mice [9]. Furthermore, there have been hints of a reduction in thrombosis risk in lupus patients taking hydroxychloroquine, as compared to those who are not [74,75]. Mechanistically, a recent study demonstrated that hydroxychloroquine inhibits the translocation of monocyte NOX2 to the endosome in response to stimulants such as TNFα, IL-1β, and aPL [10]**. This was accompanied by mitigation of aPL-induced, NOX2-mediated thrombus formation in vivo [10]**. As the related drug chloroquine has been shown to antagonize NET release [21], further studies should continue to explore the intersection of hydroxychloroquine, activated monocytes/neutrophils, and APS.
Given its excellent safety profile in pregnancy [76], and its nearly standard-of-care application in lupus pregnancies, hydroxychloroquine has been increasingly considered as adjuvant therapy in APS pregnancies. Indeed, recent retrospective studies have suggested a beneficial effect of hydroxychloroquine in APS pregnancies [7,17]. In a mouse model of obstetric APS, Bertolaccini and colleagues recently demonstrated that hydroxychloroquine prevents fetal death and placental metabolic changes [15]*. Going further, they demonstrated that labeled aPL especially localize to the placenta and the developing fetal brain, and that hydroxychloroquine mitigates complement deposition at both sites (which correlated with lower levels of C3a and C5a in blood) [15]*. Intriguingly, C3a and C5a were also reduced in the blood of APS patients after 6 months of hydroxychloroquine treatment [15]*.
Conclusion
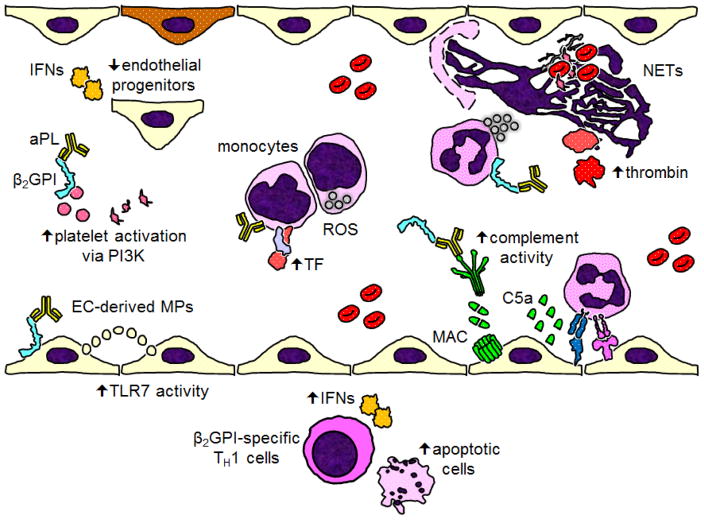
Since its description in the 1980s, APS has been managed primarily with anticoagulant medications. These medications are not universally protective against subsequent thrombotic events and pregnancy loss, and have little proven track record in treating “non-criteria” manifestations of APS such as cytopenias and cardiac valvular disease. Basic science studies continue to refine the signaling pathways, activated cells, and non-cellular effectors critical for APS pathogenesis (Figure 1). In addition to a search for novel therapeutics, established medications such as rivaroxaban, statins, and hydroxychloroquine are receiving increasing interest as adjuvant therapies. In the near future, we hope to see more well-designed clinical trials with both mechanistic and clinical endpoints.
Figure 1.
Recent mechanistic insights into the pathophysiology of antiphospholipid antibodies (aPL) and APS. Starting at the bottom of the figure and moving roughly clockwise: In the vessel wall of atherosclerotic plaques, beta-2 glycoprotein I (β2GPI)-specific TH1 cells trigger cell death and release interferons (IFNs). Endothelial cells (ECs) release vesicles (like microparticles) that activate TLR7 in other ECs by delivery of single-stranded RNA. aPL-mediated platelet activation relies on phosphoinositide 3-kinase (PI3K). Type I IFNs reduce the function of restorative circulating endothelial progenitors, which may lead to the accrual of endothelial damage over time. Cofactor-independent aPL activate monocytes via endosomal reactive oxygen species (ROS), resulting in increased expression of tissue factor (TF). In response to aPL, neutrophils release neutrophil extracellular traps (NETs), which help facilitate thrombin activation. Complement activation, especially through the classical pathway, leads to the assembly of the membrane attack complex (MAC) on the endothelial surface, while also facilitating the recruitment and activation of inflammatory cells.
Key points.
Current standard-of-care therapy for APS does not explicitly target inflammatory aspects of APS pathophysiology.
A better understanding of inter- and intra-cellular signaling pathways in APS has revealed potential drug targets (i.e., interferons, phosphoinositide 3-kinase, etc.).
In addition to the well-established cellular mediators of APS pathogenesis (endothelial cells, platelets, etc.), there is emerging interest in the contribution of myeloid-lineage cells to APS pathogenesis. The role of neutrophil extracellular trap release, in particular, warrants further study.
Complement activation and deposition continue to be recognized for their role in APS pathogenesis. Activity of this pathway may be mitigated by several medications including rivaroxaban and hydroxychloroquine.
Adjuvant therapeutics including statins and hydroxychloroquine have the potential to improve APS pregnancy outcomes, based upon animal studies and small clinical trials.
Acknowledgments
Financial support and sponsorship: JSK was supported by NIH K08AR066569 and career development awards from the Burroughs Wellcome Fund, the Rheumatology Research Foundation, and the Arthritis National Research Foundation.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, PGDEG, Koike T, Meroni PL, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 2.Bertolaccini ML, Amengual O, Andreoli L, Atsumi T, Chighizola CB, Forastiero R, de Groot P, Lakos G, Lambert M, Meroni P, et al. 14th International Congress on Antiphospholipid Antibodies Task Force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmun Rev. 2014;13:917–930. doi: 10.1016/j.autrev.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Gomez-Puerta JA, Cervera R. Diagnosis and classification of the antiphospholipid syndrome. J Autoimmun. 2014;48–49:20–25. doi: 10.1016/j.jaut.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Andreoli L, Chighizola CB, Banzato A, Pons-Estel GJ, Ramire de Jesus G, Erkan D. Estimated frequency of antiphospholipid antibodies in patients with pregnancy morbidity, stroke, myocardial infarction, and deep vein thrombosis: a critical review of the literature. Arthritis Care Res (Hoboken) 2013;65:1869–1873. doi: 10.1002/acr.22066. [DOI] [PubMed] [Google Scholar]
- 5.Abreu MM, Danowski A, Wahl DG, Amigo MC, Tektonidou M, Pacheco MS, Fleming N, Domingues V, Sciascia S, Lyra JO, et al. The relevance of “non-criteria” clinical manifestations of antiphospholipid syndrome: 14th International Congress on Antiphospholipid Antibodies Technical Task Force Report on Antiphospholipid Syndrome Clinical Features. Autoimmun Rev. 2015;14:401–414. doi: 10.1016/j.autrev.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Pengo V, Ruffatti A, Legnani C, Gresele P, Barcellona D, Erba N, Testa S, Marongiu F, Bison E, Denas G, et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J Thromb Haemost. 2010;8:237–242. doi: 10.1111/j.1538-7836.2009.03674.x. [DOI] [PubMed] [Google Scholar]
- 7.Mekinian A, Costedoat-Chalumeau N, Masseau A, Tincani A, De Caroli S, Alijotas-Reig J, Ruffatti A, Ambrozic A, Botta A, Le Guern V, et al. Obstetrical APS: is there a place for hydroxychloroquine to improve the pregnancy outcome? Autoimmun Rev. 2015;14:23–29. doi: 10.1016/j.autrev.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 8.de Groot PG, Urbanus RT. Antiphospholipid Syndrome--Not a Noninflammatory Disease. Semin Thromb Hemost. 2015;41:607–614. doi: 10.1055/s-0035-1556725. [DOI] [PubMed] [Google Scholar]
- 9.Edwards MH, Pierangeli S, Liu X, Barker JH, Anderson G, Harris EN. Hydroxychloroquine reverses thrombogenic properties of antiphospholipid antibodies in mice. Circulation. 1997;96:4380–4384. doi: 10.1161/01.cir.96.12.4380. [DOI] [PubMed] [Google Scholar]
- 10**.Muller-Calleja N, Manukyan D, Canisius A, Strand D, Lackner KJ. Hydroxychloroquine inhibits proinflammatory signalling pathways by targeting endosomal NADPH oxidase. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2016-210012. This study demonstrates a new mechanism by which hydroxychloroquine may inhibit aPL-mediated thrombosis, namely the inhibition of endosomal NADPH oxidase. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara DE, Liu X, Espinola RG, Meroni PL, Abukhalaf I, Harris EN, Pierangeli SS. Inhibition of the thrombogenic and inflammatory properties of antiphospholipid antibodies by fluvastatin in an in vivo animal model. Arthritis Rheum. 2003;48:3272–3279. doi: 10.1002/art.11449. [DOI] [PubMed] [Google Scholar]
- 12.Carter AE, Eban R. Prevention of postoperative deep venous thrombosis in legs by orally administered hydroxychloroquine sulphate. Br Med J. 1974;3:94–95. doi: 10.1136/bmj.3.5923.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glynn RJ, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851–1861. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14**.Cohen H, Hunt BJ, Efthymiou M, Arachchillage DR, Mackie IJ, Clawson S, Sylvestre Y, Machin SJ, Bertolaccini ML, Ruiz-Castellano M, et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol. 2016;3:e426–436. doi: 10.1016/S2352-3026(16)30079-5. This is the first randomized controlled study investigating a direct oral anticoagulant in APS patients. In a cohort of patients with history of venous thromboembolism who already had stable disease on warfarin, both rivaroxaban and warfarin prevented thrombosis over a 6-month period in all patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Bertolaccini ML, Contento G, Lennen R, Sanna G, Blower PJ, Ma MT, Sunassee K, Girardi G. Complement inhibition by hydroxychloroquine prevents placental and fetal brain abnormalities in antiphospholipid syndrome. J Autoimmun. 2016;75:30–38. doi: 10.1016/j.jaut.2016.04.008. This study provides additional mechanistic insights into how hydroxychloroquine may function as adjuvant therapy in obstetric APS, namely by inhibition of complement activation and deposition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redecha P, Franzke CW, Ruf W, Mackman N, Girardi G. Neutrophil activation by the tissue factor/Factor VIIa/PAR2 axis mediates fetal death in a mouse model of antiphospholipid syndrome. J Clin Invest. 2008;118:3453–3461. doi: 10.1172/JCI36089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mekinian A, Lazzaroni MG, Kuzenko A, Alijotas-Reig J, Ruffatti A, Levy P, Canti V, Bremme K, Bezanahary H, Bertero T, et al. The efficacy of hydroxychloroquine for obstetrical outcome in anti-phospholipid syndrome: Data from a European multicenter retrospective study. Autoimmun Rev. 2015;14:498–502. doi: 10.1016/j.autrev.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 18**.Lefkou E, Mamopoulos A, Dagklis T, Vosnakis C, Rousso D, Girardi G. Pravastatin improves pregnancy outcomes in obstetric antiphospholipid syndrome refractory to antithrombotic therapy. J Clin Invest. 2016;126:2933–2940. doi: 10.1172/JCI86957. This intriguing small clinical trial highlights the potential role of statins in treatment of obstetric APS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19*.Arachchillage DR, Mackie IJ, Efthymiou M, Chitolie A, Hunt BJ, Isenberg DA, Khamashta M, Machin SJ, Cohen H. Rivaroxaban limits complement activation compared with warfarin in antiphospholipid syndrome patients with venous thromboembolism. J Thromb Haemost. 2016;14:2177–2186. doi: 10.1111/jth.13475. This study interestingly links coagulation and complement pathways, demonstrating that the factor Xa inhibitor rivaroxaban suppresses smoldering complement pathway activation in APS patients. [DOI] [PubMed] [Google Scholar]
- 20**.van den Hoogen LL, Fritsch-Stork RD, Versnel MA, Derksen RH, van Roon JA, Radstake TR. Monocyte type I interferon signature in antiphospholipid syndrome is related to proinflammatory monocyte subsets, hydroxychloroquine and statin use. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2016-210485. This study also reports a type I IFN signature in primary APS patients, and finds that the signature is less likely to be present in patients taking either hydroxychloroquine or statins. The authors also describe an expansion of “non-classical” monocytes in the blood of primary APS patients; these cells have been linked to cardiovascular damage in other autoimmune diseases. [DOI] [PubMed] [Google Scholar]
- 21.Smith CK, Vivekanandan-Giri A, Tang C, Knight JS, Mathew A, Padilla RL, Gillespie BW, Carmona-Rivera C, Liu X, Subramanian V, et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. 2014;66:2532–2544. doi: 10.1002/art.38703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conti F, Sorice M, Circella A, Alessandri C, Pittoni V, Caronti B, Calderaro C, Griggi T, Misasi R, Valesini G. Beta-2-glycoprotein I expression on monocytes is increased in anti-phospholipid antibody syndrome and correlates with tissue factor expression. Clin Exp Immunol. 2003;132:509–516. doi: 10.1046/j.1365-2249.2003.02180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caronti B, Calderaro C, Alessandri C, Conti F, Tinghino R, Palladini G, Valesini G. Beta2-glycoprotein I (beta2-GPI) mRNA is expressed by several cell types involved in anti-phospholipid syndrome-related tissue damage. Clin Exp Immunol. 1999;115:214–219. doi: 10.1046/j.1365-2249.1999.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma K, Simantov R, Zhang JC, Silverstein R, Hajjar KA, McCrae KR. High affinity binding of beta 2-glycoprotein I to human endothelial cells is mediated by annexin II. J Biol Chem. 2000;275:15541–15548. doi: 10.1074/jbc.275.20.15541. [DOI] [PubMed] [Google Scholar]
- 25.Allen KL, Fonseca FV, Betapudi V, Willard B, Zhang J, McCrae KR. A novel pathway for human endothelial cell activation by antiphospholipid/anti-beta2 glycoprotein I antibodies. Blood. 2012;119:884–893. doi: 10.1182/blood-2011-03-344671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorice M, Longo A, Capozzi A, Garofalo T, Misasi R, Alessandri C, Conti F, Buttari B, Rigano R, Ortona E, et al. Anti-beta2-glycoprotein I antibodies induce monocyte release of tumor necrosis factor alpha and tissue factor by signal transduction pathways involving lipid rafts. Arthritis Rheum. 2007;56:2687–2697. doi: 10.1002/art.22802. [DOI] [PubMed] [Google Scholar]
- 27.Muller-Calleja N, Lackner KJ. Mechanisms of Cellular Activation in the Antiphospholipid Syndrome. Semin Thromb Hemost. 2017 doi: 10.1055/s-0036-1597290. [DOI] [PubMed] [Google Scholar]
- 28.Ramesh S, Morrell CN, Tarango C, Thomas GD, Yuhanna IS, Girardi G, Herz J, Urbanus RT, de Groot PG, Thorpe PE, et al. Antiphospholipid antibodies promote leukocyte-endothelial cell adhesion and thrombosis in mice by antagonizing eNOS via beta2GPI and apoER2. J Clin Invest. 2011;121:120–131. doi: 10.1172/JCI39828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulrich V, Gelber SE, Vukelic M, Sacharidou A, Herz J, Urbanus RT, de Groot PG, Natale DR, Harihara A, Redecha P, et al. ApoE Receptor 2 Mediation of Trophoblast Dysfunction and Pregnancy Complications Induced by Antiphospholipid Antibodies in Mice. Arthritis Rheumatol. 2016;68:730–739. doi: 10.1002/art.39453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Mineo C, Lanier L, Jung E, Sengupta S, Ulrich V, Sacharidou A, Tarango C, Osunbunmi O, Shen YM, Salmon JE, et al. Identification of a Monoclonal Antibody That Attenuates Antiphospholipid Syndrome-Related Pregnancy Complications and Thrombosis. PLoS One. 2016;11:e0158757. doi: 10.1371/journal.pone.0158757. The authors describe a novel monoclonal antibody that targets β2GPI and thereby prevents aPL-mediated cell activation. The study further extends our mechanistic understanding of the association between β2GPI and ApoER2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierangeli SS, Vega-Ostertag ME, Raschi E, Liu X, Romay-Penabad Z, De Micheli V, Galli M, Moia M, Tincani A, Borghi MO, et al. Toll-like receptor and antiphospholipid mediated thrombosis: in vivo studies. Ann Rheum Dis. 2007;66:1327–1333. doi: 10.1136/ard.2006.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie H, Kong X, Zhou H, Xie Y, Sheng L, Wang T, Xia L, Yan J. TLR4 is involved in the pathogenic effects observed in a murine model of antiphospholipid syndrome. Clin Immunol. 2015;160:198–210. doi: 10.1016/j.clim.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Laplante P, Fuentes R, Salem D, Subang R, Gillis MA, Hachem A, Farhat N, Qureshi ST, Fletcher CA, Roubey RA, et al. Antiphospholipid antibody-mediated effects in an arterial model of thrombosis are dependent on Toll-like receptor 4. Lupus. 2016;25:162–176. doi: 10.1177/0961203315603146. [DOI] [PubMed] [Google Scholar]
- 34*.Manukyan D, Muller-Calleja N, Jackel S, Luchmann K, Monnikes R, Kiouptsi K, Reinhardt C, Jurk K, Walter U, Lackner KJ. Cofactor-independent human antiphospholipid antibodies induce venous thrombosis in mice. J Thromb Haemost. 2016;14:1011–1020. doi: 10.1111/jth.13263. This work supports a role for “cofactor-independent” aPL in APS pathogenesis. These aPL mediate thrombosis through a mechanism that relies upon the catalytic subunit of NADPH oxidase, NOX2. [DOI] [PubMed] [Google Scholar]
- 35.Girardi G, Mackman N. Tissue factor in antiphospholipid antibody-induced pregnancy loss: a pro-inflammatory molecule. Lupus. 2008;17:931–936. doi: 10.1177/0961203308094994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azuma H, Yamamoto T, Chishima F. Effects of anti-beta2-GPI antibodies on cytokine production in normal first-trimester trophoblast cells. J Obstet Gynaecol Res. 2016;42:769–775. doi: 10.1111/jog.12993. [DOI] [PubMed] [Google Scholar]
- 37*.Terrisse AD, Laurent PA, Garcia C, Gratacap MP, Vanhaesebroeck B, Sie P, Payrastre B. The class I phosphoinositide 3-kinases alpha and beta control antiphospholipid antibodies-induced platelet activation. Thromb Haemost. 2016;115:1138–1146. doi: 10.1160/TH15-08-0661. This study provides important clues to ApoER2-mediated intracellular signaling in platelets, with a particular focus on PI3K-related pathways. [DOI] [PubMed] [Google Scholar]
- 38.Nylander S, Kull B, Bjorkman JA, Ulvinge JC, Oakes N, Emanuelsson BM, Andersson M, Skarby T, Inghardt T, Fjellstrom O, et al. Human target validation of phosphoinositide 3-kinase (PI3K)beta: effects on platelets and insulin sensitivity, using AZD6482 a novel PI3Kbeta inhibitor. J Thromb Haemost. 2012;10:2127–2136. doi: 10.1111/j.1538-7836.2012.04898.x. [DOI] [PubMed] [Google Scholar]
- 39*.Wu M, Barnard J, Kundu S, McCrae KR. A novel pathway of cellular activation mediated by antiphospholipid antibody-induced extracellular vesicles. J Thromb Haemost. 2015;13:1928–1940. doi: 10.1111/jth.13072. This work demonstrates a new mechanism by which aPL activate endothelial cells, namely paracrine signaling via vesicle-delivered single-stranded RNA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westerweel PE, Verhaar MC. Endothelial progenitor cell dysfunction in rheumatic disease. Nat Rev Rheumatol. 2009;5:332–340. doi: 10.1038/nrrheum.2009.81. [DOI] [PubMed] [Google Scholar]
- 41*.Grenn RC, Yalavarthi S, Gandhi AA, Kazzaz NM, Nunez-Alvarez C, Hernandez-Ramirez D, Cabral AR, McCune WJ, Bockenstedt PL, Knight JS. Endothelial progenitor dysfunction associates with a type I interferon signature in primary antiphospholipid syndrome. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2016-209442. This work demonstrates for the first time a type I IFN signature in primary APS patients, which promotes dysfunction of circulating endothelial progenitors. Mechanistically, this could contribute to accrual of endothelial damage over time. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yalavarthi S, Grenn RC, Knight JS. Response to: ‘Monocyte type I interferon signature in antiphospholipid syndrome is related to pro-inflammatory monocyte subsets, hydroxychloroquine and statin use’ by van den Hoogen et al. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2016-210529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furie R, Khamashta M, Merrill JT, Werth VP, Kalunian K, Brohawn P, Illei GG, Drappa J, Wang L, Yoo S, et al. Anifrolumab, an Anti-Interferon-alpha Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017;69:376–386. doi: 10.1002/art.39962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knight JS, Kaplan MJ. Cardiovascular disease in lupus: insights and updates. Curr Opin Rheumatol. 2013;25:597–605. doi: 10.1097/BOR.0b013e328363eba3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denas G, Jose SP, Bracco A, Zoppellaro G, Pengo V. Antiphospholipid syndrome and the heart: a case series and literature review. Autoimmun Rev. 2015;14:214–222. doi: 10.1016/j.autrev.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Kazzaz NM, Wilson AM, Kado R, Barnes GD, Knight JS. A 37-year-old man with primary antiphospholipid syndrome presenting with respiratory distress and worsening toe ischemia. Arthritis Care Res (Hoboken) 2016 doi: 10.1002/acr.23168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47**.Benagiano M, Gerosa M, Romagnoli J, Mahler M, Borghi MO, Grassi A, Della Bella C, Emmi G, Amedei A, Silvestri E, et al. beta2 Glycoprotein I Recognition Drives Th1 Inflammation in Atherosclerotic Plaques of Patients with Primary Antiphospholipid Syndrome. J Immunol. 2017 doi: 10.4049/jimmunol.1600305. This interesting study examines the inflammatory milieu of atherosclerotic lesions in APS, with a special focus on TH1 cell-mediated inflammation and interferon signaling. [DOI] [PubMed] [Google Scholar]
- 48.Grayson PC, Kaplan MJ. At the Bench: Neutrophil extracellular traps (NETs) highlight novel aspects of innate immune system involvement in autoimmune diseases. J Leukoc Biol. 2016;99:253–264. doi: 10.1189/jlb.5BT0615-247R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:1777–1783. doi: 10.1161/ATVBAHA.111.242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rao AN, Kazzaz NM, Knight JS. Do neutrophil extracellular traps contribute to the heightened risk of thrombosis in inflammatory diseases? World J Cardiol. 2015;7:829–842. doi: 10.4330/wjc.v7.i12.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leffler J, Stojanovich L, Shoenfeld Y, Bogdanovic G, Hesselstrand R, Blom AM. Degradation of neutrophil extracellular traps is decreased in patients with antiphospholipid syndrome. Clin Exp Rheumatol. 2014;32:66–70. [PubMed] [Google Scholar]
- 52**.Yalavarthi S, Gould TJ, Rao AN, Mazza LF, Morris AE, Nunez-Alvarez C, Hernandez-Ramirez D, Bockenstedt PL, Liaw PC, Cabral AR, et al. Release of Neutrophil Extracellular Traps by Neutrophils Stimulated With Antiphospholipid Antibodies: A Newly Identified Mechanism of Thrombosis in the Antiphospholipid Syndrome. Arthritis Rheumatol. 2015;67:2990–3003. doi: 10.1002/art.39247. This study demonstrates the ability of aPL to trigger NET release in vitro, through a pathway that involves TLR4 and reactive oxygen species. Furthermore, these aPL-triggered NETs promote thrombin generation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van den Hoogen LL, Fritsch-Stork RD, van Roon JA, Radstake TR. Low-Density Granulocytes Are Increased in Antiphospholipid Syndrome and Are Associated With Anti-beta2 -Glycoprotein I Antibodies: Comment on the Article by Yalavarthi et al. Arthritis Rheumatol. 2016;68:1320–1321. doi: 10.1002/art.39576. [DOI] [PubMed] [Google Scholar]
- 54.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, Rubin CJ, Zhao W, Olsen SH, Klinker M, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yalavarthi S, Knight JS. Reply. Arthritis Rheumatol. 2016;68:1321–1322. doi: 10.1002/art.39579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56*.Meng H, Yalavarthi S, Kanthi Y, Mazza LF, Elfline MA, Luke CE, Pinsky DJ, Henke PK, Knight JS. In vivo role of neutrophil extracellular traps in antiphospholipid antibody-mediated venous thrombosis. Arthritis Rheumatol. 2016 doi: 10.1002/art.39938. This work demonstrates the potential in vivo relevance of NETs in APS. NETs are present in exaggerated fashion in APS thrombi, while thrombosis is prevented by NET-disrupting treatments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singel KL, Segal BH. NOX2-dependent regulation of inflammation. Clin Sci (Lond) 2016;130:479–490. doi: 10.1042/CS20150660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salmon JE, Girardi G. Antiphospholipid antibodies and pregnancy loss: a disorder of inflammation. J Reprod Immunol. 2008;77:51–56. doi: 10.1016/j.jri.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pierangeli SS, Girardi G, Vega-Ostertag M, Liu X, Espinola RG, Salmon J. Requirement of activation of complement C3 and C5 for antiphospholipid antibody-mediated thrombophilia. Arthritis Rheum. 2005;52:2120–2124. doi: 10.1002/art.21157. [DOI] [PubMed] [Google Scholar]
- 60.Devreese KM, Hoylaerts MF. Is there an association between complement activation and antiphospholipid antibody-related thrombosis? Thromb Haemost. 2010;104:1279–1281. doi: 10.1160/TH10-06-0410. [DOI] [PubMed] [Google Scholar]
- 61.Sarmiento E, Dale J, Arraya M, Gallego A, Lanio N, Navarro J, Carbone J. CD8+DR+ T-Cells and C3 Complement Serum Concentration as Potential Biomarkers in Thrombotic Antiphospholipid Syndrome. Autoimmune Dis. 2014;2014:868652. doi: 10.1155/2014/868652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davis WD, Brey RL. Antiphospholipid antibodies and complement activation in patients with cerebral ischemia. Clin Exp Rheumatol. 1992;10:455–460. [PubMed] [Google Scholar]
- 63.Meroni PL, Macor P, Durigutto P, De Maso L, Gerosa M, Ferraresso M, Borghi MO, Mollnes TE, Tedesco F. Complement activation in antiphospholipid syndrome and its inhibition to prevent rethrombosis after arterial surgery. Blood. 2016;127:365–367. doi: 10.1182/blood-2015-09-672139. [DOI] [PubMed] [Google Scholar]
- 64.Stojan G, Petri M. Anti-C1q in systemic lupus erythematosus. Lupus. 2016;25:873–877. doi: 10.1177/0961203316645205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oku K, Amengual O, Hisada R, Ohmura K, Nakagawa I, Watanabe T, Bohgaki T, Horita T, Yasuda S, Atsumi T. Autoantibodies against a complement component 1 q subcomponent contribute to complement activation and recurrent thrombosis/pregnancy morbidity in anti-phospholipid syndrome. Rheumatology (Oxford) 2016;55:1403–1411. doi: 10.1093/rheumatology/kew196. [DOI] [PubMed] [Google Scholar]
- 66.Dufrost V, Risse J, Zuily S, Wahl D. Direct Oral Anticoagulants Use in Antiphospholipid Syndrome: Are These Drugs an Effective and Safe Alternative to Warfarin? A Systematic Review of the Literature. Curr Rheumatol Rep. 2016;18:74. doi: 10.1007/s11926-016-0623-7. [DOI] [PubMed] [Google Scholar]
- 67.Girardi G. Can statins prevent pregnancy complications? J Reprod Immunol. 2014;101–102:161–167. doi: 10.1016/j.jri.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 68.Erkan D, Willis R, Murthy VL, Basra G, Vega J, Ruiz-Limon P, Carrera AL, Papalardo E, Martinez-Martinez LA, Gonzalez EB, et al. A prospective open-label pilot study of fluvastatin on proinflammatory and prothrombotic biomarkers in antiphospholipid antibody positive patients. Ann Rheum Dis. 2014;73:1176–1180. doi: 10.1136/annrheumdis-2013-203622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schreiber K, Hunt BJ. Pregnancy and Antiphospholipid Syndrome. Semin Thromb Hemost. 2016;42:780–788. doi: 10.1055/s-0036-1592336. [DOI] [PubMed] [Google Scholar]
- 70.Meroni PL. Prevention & treatment of obstetrical complications in APS: Is hydroxychloroquine the Holy Grail we are looking for? J Autoimmun. 2016;75:1–5. doi: 10.1016/j.jaut.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 71.Stone S, Pijnenborg R, Vercruysse L, Poston R, Khamashta MA, Hunt BJ, Poston L. The placental bed in pregnancies complicated by primary antiphospholipid syndrome. Placenta. 2006;27:457–467. doi: 10.1016/j.placenta.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 72.Shamonki JM, Salmon JE, Hyjek E, Baergen RN. Excessive complement activation is associated with placental injury in patients with antiphospholipid antibodies. Am J Obstet Gynecol. 2007;196:167e161–165. doi: 10.1016/j.ajog.2006.10.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marder W, Knight JS, Kaplan MJ, Somers EC, Zhang X, O’Dell AA, Padmanabhan V, Lieberman RW. Placental histology and neutrophil extracellular traps in lupus and pre-eclampsia pregnancies. Lupus Sci Med. 2016;3:e000134. doi: 10.1136/lupus-2015-000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jung H, Bobba R, Su J, Shariati-Sarabi Z, Gladman DD, Urowitz M, Lou W, Fortin PR. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis Rheum. 2010;62:863–868. doi: 10.1002/art.27289. [DOI] [PubMed] [Google Scholar]
- 75.Tektonidou MG, Laskari K, Panagiotakos DB, Moutsopoulos HM. Risk factors for thrombosis and primary thrombosis prevention in patients with systemic lupus erythematosus with or without antiphospholipid antibodies. Arthritis Rheum. 2009;61:29–36. doi: 10.1002/art.24232. [DOI] [PubMed] [Google Scholar]
- 76.Kaplan YC, Ozsarfati J, Nickel C, Koren G. Reproductive outcomes following hydroxychloroquine use for autoimmune diseases: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;81:835–848. doi: 10.1111/bcp.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]