Abstract
Objective:
This study was conducted to evaluate the efficacy and tolerability of the oral intake of promerim in the elimination of acute pain and discomfort associated with knee osteoarthritis (OA).
Methods:
Single-center, 1-month, prospective, observational clinical trial. A total of 92 patients not older than 70 years were included. Patients were offered to use 720-mg promerim for the first 15 days after admission after breakfast and then 360 mg for the second 15 days. All patients were analyzed with the visual analog scale (VAS) for pain, which ranges from 0 to 10, and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score before the start of treatment and 1 month after the start. Statistical analysis was performed by SPSS 15.0 software. An α level of P < .05 was assumed to be statistically significant.
Results:
This study comprised 92 patients (69 women and 23 men) with a mean age of 51.5 (range: 40-69) years. Before treatment, the mean VAS score was 5.6 ± 1.1, and after treatment, the mean VAS score was 2.6 ± 1.7. Treatment with promerim consistently showed a significant decrease in the VAS score (P < .001). The mean WOMAC score of the patients was 46.4 ± 8.2 before treatment. After treatment, the mean WOMAC score was 72.1 ± 14.4. Treatment with promerim consistently showed a significant increase in the WOMAC score (P < .001).
Conclusions:
The results of this single-center, open-label clinical study demonstrate that promerim is a viable natural treatment option for treating knee OA. We recommend that 720-mg promerim taken once daily for the first 15 days after admission and 360 mg taken once daily for the next 15 days significantly and rapidly reduced composite pain and stiffness in the knee OA within 1 month.
Keywords: Promerim, knee joint, osteoarthritis, gonarthrosis, hydrolyzed collagen
Introduction
Osteoarthritis (OA) is the single most important cause of locomotor disability in Western societies and is a major issue in health care systems.1,2 It is a progressive, chronic condition leading to pain and loss of function that dramatically reduces the quality of life of patients and their ability to work.
Osteoarthritis affects approximately 27 million adults in the United States, and one-third of the population more than 65 years of age is diagnosed with OA.3 Osteoarthritis is a degenerative joint disease in which interleukin-1β plays a major role in the inflammatory process. Collagen hydrolysates (CHs) are peptidic mixtures that are often used as nutraceuticals for OA. Administration of CH was an optional treatment of OA.
The main clinical manifestations of OA are inflammation, pain, and bone resorption. Chronic inflammation and bone loss are closely related to the onset of pathophysiological events. Traditional treatment usually comprises the single or combined use of analgesics, nonsteroidal anti-inflammatory drugs (NSAIDs), or cyclooxygenase 2–specific NSAIDs. Traditional treatments for OA include the management of disease-related symptoms (pain, inflammation, and discomfort). Steroid and hyaluronic acid injections have also been used for treating OA with some success. Randomized controlled trials have demonstrated limited efficacy for most of these treatments.4–7 Many patients have started using complementary and alternative medications such as dietary supplements to avoid cardiac risks and gastrointestinal problems associated with long-term traditional OA treatments.8–13 Glucosamine, chondroitin, and methyl sulfonyl methane are commonly used as dietary supplements for treating OA-associated joint pain, either singly or in combination.
Promerim is a new dietary supplement that contains hydrolyzed fish collagen and has been shown to be clinically effective for treating joint and connective tissue diseases, particularly pain and stiffness associated with OA, in various studies on humans. This study was conducted to evaluate the efficacy and tolerability of the oral intake of promerim in the elimination of acute pain and discomfort associated with knee OA.
Materials and Methods
This prospective, single-center, open-label study was conducted in accordance with the International Conference on Harmonization guideline for the principles of Good Clinical Practice (ICH E6) and the Declaration of Helsinki. A total of 92 patients (69 women and 23 men) were included. Data collection and treatment took place at a single institution. This study was a 1-month, prospective, observational clinical trial.
Patients
All patients presented to the orthopedic outpatient clinic with a bilateral knee pain score of ≥4 points in the visual analog scale (VAS) on the day of examination. To evaluate pain severity, analgesic and anti-inflammatory medications were discontinued 3 weeks before the start of treatment. The washout period was 3 weeks starting from the day of inclusion until the first medication was administered. Patients were included if they had radiologically verified bilateral grade 2 to 3 OA of the knee according to the Kellgren-Lawrence classification.14 All patients were dissatisfied with previous attempts at conservative treatment including NSAIDs.
No patient dropped out or underwent surgery while they were enrolled. Patients with secondary arthritis, grade IV OA, systemic or inflammatory joint diseases, a history of crystal arthropathy, clinically relevant hematologic or abnormal clinical chemistry values, bone cancer, metastasis or tumor-like lesions in immediate proximity to the treated joint, joint instability, a history of intra-articular corticosteroid injection within the previous 6 months, a history of diabetes mellitus, and a recent history of trauma to the knee were excluded. Patients who used systemic corticosteroids and who had a VAS difference of more than 2 points between their knees were also excluded.
All participants signed the free and informed consent term of the study.
Outcome measurements
All patients were analyzed with the VAS for pain, which ranges from 0 (no pain) to 10 (most severe pain), and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score before the start of treatment (pretreatment) and 1 month after the start (posttreatment).
Medication
Patients were offered to use 720-mg promerim for the first 15 days after admission after breakfast and then 360 mg for the second 15 days. They were recommended not to use NSAIDs or apply local ice for a week after treatment. In addition, an exercise program was implemented for patients and they were recommended to perform normal daily activities whenever they were able to.
A surgeon who was unaware of the study design performed the clinical evaluation. Patients underwent a clinical evaluation before and after treatment. Possible complications and side effects were evaluated during each visit.
Statistical analysis
Statistical analysis was performed by SPSS 15.0 software (IBM Corp., Chicago, IL, USA). Descriptive statistics were expressed as mean, standard deviation, minimum, and maximum for numerical variables and in numbers and percentages for categorical variables. Differences in numerical variables in dependent groups were analyzed using the Wilcoxon signed rank test as the data were not normally distributed. An α level of P < .05 was assumed to be statistically significant.
Results
This study comprised 92 patients (69 women and 23 men) with a mean age of 51.5 (range: 40-69) years; their general characteristics are demonstrated in Table 1. These patients were highly symptomatic. Before treatment, the mean VAS score was 5.6 ± 1.1, and after treatment, the mean VAS score was 2.6 ± 1.7. The change in the VAS score over time before and after treatment was statistically significant. Treatment with promerim consistently showed a significant decrease in the VAS score (P < .001; Table 2, Figure 1).
Table 1.
General characteristics.
| Mean ± SD (min-max) | ||
|---|---|---|
| Age | 51.5 ± 7.1 (40-69) | |
| Sex | No. (%) | |
| Male | 23 (25) | |
| Female | 69 (75) |
Table 2.
VAS-WOMAC evaluation before and after the treatment.
| Mean ± SD (min-max) | P value | ||
|---|---|---|---|
| VAS | Pre | 5.6 ± 1.1 (4-8) | <.001 |
| Post | 2.6 ± 1.7 (0-6) | ||
| Difference | −3.0 ± 1.7 (95% CI: 2.7-3.4) | ||
| WOMAC-PRE | Pre | 46.4 ± 8.2 (21.2-66.4) | <.001 |
| Post | 72.1 ± 14.4 (47-97.7) | ||
| Difference | 25.7 ± 14.1 (28.7-22.8) |
Abbreviations: VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Figure 1.
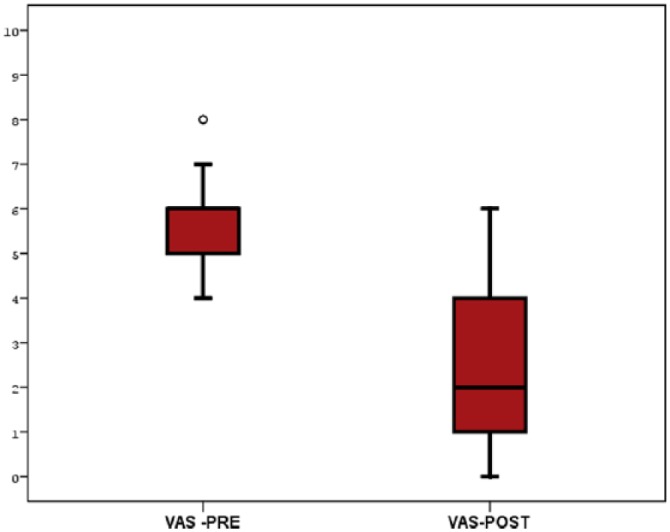
VAS changing before and after the treatment. VAS indicates visual analog scale.
The mean WOMAC score of the patients was 46.4 ± 8.2 before treatment. After treatment, the mean WOMAC score was 72.1 ± 14.4. The change in the WOMAC score of the patients over time before and after treatment was statistically significant. Treatment with promerim consistently showed a significant increase in the WOMAC score (P < .001; Table 2, Figure 2).
Figure 2.
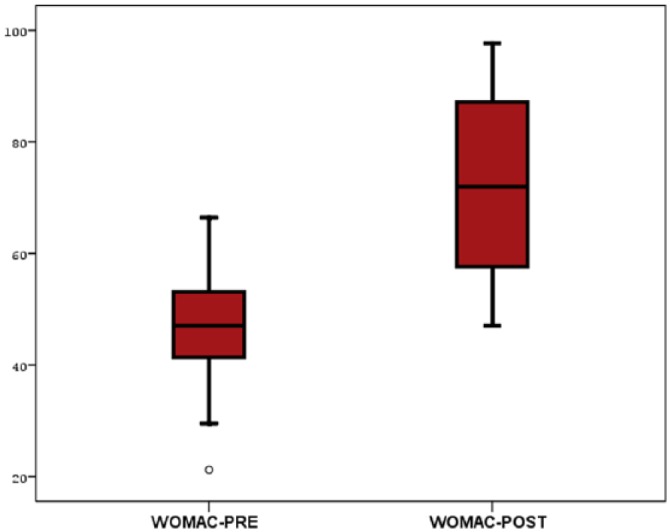
WOMAC changing before and after the treatment. WOMAC indicates Western Ontario and McMaster Universities Osteoarthritis Index.
Discussion
Osteoarthritis affects the cartilage, which is the tough elastic structure covering the extremities of bones. Knee OA is a major cause of pain and disability in older adults.15 Pain control is one of the main goals in treating knee OA. The structure of the cartilage progressively alters with age: small fragments detach, releasing foreign bodies within the joint that become sources of inflammation. The management of knee OA begins with conservative treatment such as physical therapy, exercise, weight loss, and medications. Surgical intervention can be indicated for patients with advanced OA.16,17
New nutritional supplements have gained considerable interest over the past 20 years for relieving symptoms and potentially creating structural changes to slow the process of OA. Collagen hydrolysate is a food ingredient that has the potential to improve joint comfort and function. Collagen itself is a natural component of the diet and is found in animal products such as meat and fish. However, the absorption of orally ingested collagen that has not been hydrolyzed is poor. Collagen contains unique amino acids found in no other protein. The use of CH therefore provides amino acids specific to the collagen network, which could help maintain the structure and function of joint cartilage, thus improving joint comfort in a safe and effective manner. Studies have demonstrated that some metabolite peptides act as bioactive peptides and functional molecules in particular tissues. It has also been shown that CH contains bioactive peptides that affect cartilage homeostasis. Growing evidence suggests that specific minerals play an important role in the health of joints.18–20
Minerals such as magnesium, copper, manganese, selenium, and zinc have been shown to have anti-inflammatory effects in animal and human studies. In a rat model of OA, magnesium deficiency in the diet was indicated to lead to severe cartilage damage.21 Few minerals such as boron and manganese have been shown to reduce the symptoms and pathogenesis of OA.22
Glucosamine and chondroitin are widely marketed dietary supplements that are used either singly or in combination for treating OA-associated joint pain. The role of the 2 dietary supplements in OA symptoms was investigated in 2 extensive clinical trials conducted in humans. The 6-month Glucosamine/Chondroitin Arthritis Intervention Trial supported by the National Institutes of Health included 1583 patients and showed no significant improvement in the WOMAC score of the overall patient population with both the single and combined use of glucosamine and chondroitin.23 A meta-analyis showed that some supplements with a limited number of studies and participants suggested large treatment effects, whereas widely used supplements such as glucosamine and chondroitin were either ineffective or showed small and arguably clinically unimportant treatment effects. Supplements had no clinically important effects on pain and function at medium-term and long-term follow-ups.24
Clinical studies have suggested a role for CH in the management of OA based on the postulate that hydrolyzed collagen with its abundant amino acids plays a role in the synthesis of cartilage matrix. Fish has become an interesting source of CH because of religious reason and there is no risk from mad cow disease. Fish CH had no effect on cartilage metabolism in physiological condition but it had adverse effect on cartilage in pathologic condition.25 In a study, it was reported that the declaration of a CH as a safe and effective nutraceutical requires a thorough examination of its pleiotropic effects in the OA.26 This study was conducted to evaluate the efficacy and safety of promerim, which is a hydrolyzed collagen, as an option for the treatment of acute pain in knee OA. This study demonstrated that promerim is an effective and safe agent for treating knee pain in OA. Promerim also has the benefit of preventing side effects associated with the long-term use of other OA treatments such as NSAIDs. The WOMAC scores of the patients increased rapidly in the short term (1 month). This increase is much higher than the response shown in studies on glucose and chondroitin. It was also shown that the increase was statistically significant.
One-third of the population who are 65 years and older are diagnosed with OA, and the proportion is likely to increase as the general population ages. Therefore, it is important to provide effective and safe options for treating OA. This study offered scientific evidence supporting the efficacy and safety of promerim in the acute treatment of pain in OA and limitations in activity.
The safety profile of promerim was found to be excellent as there were no reports of adverse events or serious adverse events associated with our treatment. This is very important in a disease such as knee OA that requires long-term treatment. Analgesics and NSAIDs normally used to treat such conditions lead to gastric and cardiovascular complications that can considerably increase mortality in an elderly population.
Our study demonstrated that promerim is effective and safe for treating acute pain and stiffness associated with knee OA and results in the use of less analgesic medications.
Conclusions
It is important for patients to have treatment options that are both safe and effective in managing chronic diseases such as knee OA. The results of this single-center, open-label clinical study demonstrate that promerim is a viable natural treatment option for treating knee OA. In this clinical study, 720-mg promerim taken once daily for the first 15 days after admission and 360 mg taken once daily for the next 15 days significantly and rapidly reduced composite pain and stiffness (within 1 month).
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Each author made significant individual contributions to this manuscript. BEK and YO drafted the manuscript. BEK, EB, and GA administered the therapy, followed patients, and gathered clinical data. MK and OTE evaluated the data from the statistical analysis. BEK and YO performed the literature search, reviewed the manuscript, and contributed to the intellectual concept of the study.
References
- 1. Gupta S, Hawker GA, Laporte A, et al. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology (Oxford). 2005;44:1531–1537. [DOI] [PubMed] [Google Scholar]
- 2. Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States, part II. Arthritis Rheum. 2008;58:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Case JP, Baliunas AJ, Block JA. Lack of efficacy of acetaminophen in treating symptomatic knee osteoarthritis. Arch Intern Med. 2003;163:169–178. [DOI] [PubMed] [Google Scholar]
- 5. Towheed TE, Maxwell L, Judd MG, et al. Acetaminophen for osteoarthritis [published online ahead of print January 25, 2006]. Cochrane Database Syst Rev. doi: 10.1002/14651858.CD004257.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geba GP, Weaver AL, Polis AB, et al. Efficacy of rofecoxib, celecoxib, and acetaminophen in osteoarthritis of the knee. J Am Med Assoc. 2002;287:64–71. [DOI] [PubMed] [Google Scholar]
- 7. Altman RD. Ibuprofen, acetaminophen and placebo in osteoarthritis of the knee: a six-day double-blind study. Arthritis Rheum. 1999;42:S403. [Google Scholar]
- 8. Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–1102. [DOI] [PubMed] [Google Scholar]
- 9. Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. [DOI] [PubMed] [Google Scholar]
- 10. Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1081–1091. [DOI] [PubMed] [Google Scholar]
- 11. Singh G, Wu O, Langhorne P, Madhok R. Risk of acute myocardial infarction with nonselective non-steroidal anti-inflammatory drugs: a meta-analysis. Arthritis Res Ther. 2006;8:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deeks JD, Smith LA, Bradley MD. Efficacy, tolerability, and upper gastrointestinal safety of celecoxib for treatment of osteoarthritis and rheumatoid arthritis: systematic review of randomised controlled trials. Br Med J. 2002;325:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laine L. Nonsteroidal anti-inflammatory drug gastropathy. Gastrointest Endosc Clin N Am. 1996;6:489–504. [PubMed] [Google Scholar]
- 14. Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hadler NM. Osteoarthritis as a public health problem. Clin Rheum Dis. 1985;11:175–185. [PubMed] [Google Scholar]
- 16. Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48:370–377. [DOI] [PubMed] [Google Scholar]
- 17. Recommendations for the medical management of osteoarthritis of the hip knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000;43:1905–1915. [DOI] [PubMed] [Google Scholar]
- 18. Adam M. Therapy of osteoarthrosis what effect do gelatin preparations have? (Therapie Der Osteoarthrose-Welche Wirkung Haben Gelantineparaprate?). Therapiewoche. 1991;41:2456–2461. [Google Scholar]
- 19. Seeligmuller K, Happel HK. Can a mixture of gelatin and L-cystine stimulate proteoglycan synthesis? Therapiewoche. 1989;39:3153–3157. [Google Scholar]
- 20. Krug E. On supportive therapy for osteo- and chondropathies. Ztschr F Erfahrungsheilkunde. 1989;11:930–938. [Google Scholar]
- 21. Shakibaei M, Kociok K, Forster C, et al. Comparative evaluation of ultrastructural changes in articular cartilage of ofloxacin-treated and magnesium-deficient immature rats. Toxicol Pathol. 1996;24:580–587. [DOI] [PubMed] [Google Scholar]
- 22. Gaby AR. Natural treatments for osteoarthritis. Altern Med Rev. 1999;4:330–341. [PubMed] [Google Scholar]
- 23. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795–808. [DOI] [PubMed] [Google Scholar]
- 24. Liu X, Machado GC, Eyles JP, Ravi V, Hunter DJ. Dietary supplements for treating osteoarthritis: a systematic review and meta-analysis [published online ahead of print October 10, 2017]. Br J Sports Med. doi: 10.1136/bjsports-2016-097333. [DOI] [PubMed] [Google Scholar]
- 25. Boonmaleerat K, Wanachewin O, Phitak T, Pothacharoen P, Kongtawelert P. Fish collagen hydrolysates modulate cartilage metabolism [published online ahead of print August 22, 2017]. Cell Biochem Biophys. doi: 10.1007/s12013-017-0817-2. [DOI] [PubMed] [Google Scholar]
- 26. Schadow S, Simons VS, Lochnit G, et al. Metabolic response of human osteoarthritic cartilage to biochemically characterized collagen hydrolysates [published online ahead of print January 20, 2017]. Int J Mol Sci. doi: 10.3390/ijms18010207. [DOI] [PMC free article] [PubMed] [Google Scholar]


