Short abstract
Various small molecules act as neurotransmitters and orchestrate neural communication. Growing evidence suggests that not only classical neurotransmitters but also several small molecules, including amino acid derivatives, modulate synaptic transmission. As conditions of acute and chronic pain alter neuronal excitability in the nucleus accumbens, we hypothesized that small molecules released in the nucleus accumbens might play important roles in modulating the pain sensation. However, it is not easy to identify possible pain modulators owing to the absence of a method for comprehensively measuring extracellular small molecules in the brain. In this study, through the use of an emerging metabolomics technique, namely ion chromatography coupled with high-resolution mass spectrometry, we simultaneously analyzed the dynamics of more than 60 small molecules in brain fluids collected by microdialysis, under both the application of pain stimuli and the administration of analgesics. We identified N-acetylaspartylglutamate as a potential pain modulator that is endogenously released in the nucleus accumbens. Infusion of N-acetylaspartylglutamate into the nucleus accumbens significantly attenuated the pain induced by the activation of sensory nerves through optical stimulation. These findings suggest that N-acetylaspartylglutamate released in the nucleus accumbens could modulate pain sensation.
Keywords: Pain, analgesia, morphine, nucleus accumbens, dopamine, optogenetics, imaging mass spectrometry, in vivo microdialysis, mass spectrometry, N-acetylaspartylglutamate
Introduction
Pain is a warning of potential tissue injury and functions to drive behavioral actions that result in protection of the body from further harm. Nociceptive signals engage broad neural networks in the brain including the thalamus; somatosensory, insula, and cingulate cortices; the hypothalamus; amygdala; periaqueductal gray as well as the ventral striatum.1,2
Various small molecules, including several amino acid derivatives and peptides, play roles in neural communication as not only classical neurotransmitters but also neuromodulators,3 and their synaptic concentrations alter the excitability of specific neural circuits. As chronic pain alters neuronal excitability in the nucleus accumbens (N.Acc.),2 we hypothesized that brain extracellular small molecules, including small peptides, as well as classical neurotransmitters play important roles in modulating the pain sensation. However, it is not easy to identify molecules having the potential to modulate pain owing to the absence of an analytical technique for comprehensively measuring brain extracellular metabolites.
In the present study, we applied a mass spectrometry (MS)-based metabolomics technique coupled with microdialysis to analyze extracellular metabolites released from cells in the brain and identified small molecules that are endogenously released in the N.Acc. for modulating pain and analgesia. This microdialysis/MS integration has also allowed monitoring of the changes in the extracellular metabolome during the application of pain stimuli or the administration of analgesics. Among various MS methods, we used a recently established ion chromatography (IC) system together with Fourier-transform high-resolution MS (HRMS). Owing to the high chromatographic separation performance of IC for hydrophilic metabolites4 and the high mass resolution of Orbitrap,5,6 this IC-HRMS approach allows the comprehensive detection of anionic metabolites in microdialyzed brain fluids, including nucleotides, organic acids, and small peptides.7,8 These studies allowed us to identify N-acetylaspartylglutamate (NAAG) as an endogenously released transmitter in the N.Acc. and to explore its relevance to modulation of nociception.
Materials and methods
Animals
Male C57BL/6J mice (8–12 weeks old) (Tokyo Laboratory Animals Science Co., Ltd., Tokyo, Japan) were housed up to six per cage and kept in a temperature-controlled room (24 ± 1°C). All mice were maintained under a 12-h light–dark cycle (light on at 8 a.m.), and behavioral tests were performed during the light phase. Food and water were available ad libitum. All experiments were performed in accordance with the Guiding Principles for the Care and Use of Laboratory Animals, Hoshi University, as adopted by the Committee on Animal Research of Hoshi University.
Drugs
Morphine hydrochloride (Daiichi-Sankyo Co., Ltd., Tokyo, Japan), NAAG (Sigma-Aldrich, MO, USA), and 2-phosphonomethyl-pentanedioic acid (PMPA; TOCRIS Bioscience, Bristol, UK) were used in this study. Morphine, NAAG, and PMPA were dissolved in saline (Otsuka normal saline, 0.9% NaCl; Otsuka Pharmaceutical Factory Inc., Tokushima, Japan).
Artificial activation of sensory neurons by channel rhodopsin-2
Virus vector
To achieve cell-type-specific channelrhodopsin-2 (ChR2) or enhanced green fluorescent protein (EGFP) expression, we used the adeno-associated virus serotype 6 (AAV6) vector with a human synapsin 1 promoter (AAV-hSyn-ChR2 (ET/TC)-enhanced yellow fluorescent protein (EYFP) (AAV6-ChR2) or AAV-hSyn-EGFP (AAV6-EGFP)). Both virus vectors were serotyped with AAV6 coat proteins and packaged by the viral vector core at Nagoya University. The final viral concentration was 1 × 1011 and 3 × 1012 copies/mL. Aliquots of this virus were stored at −80°C until use.
(ii) AAV injection
To selectively express ChR2 or EGFP in sensory nerves, AAV6-ChR2 or AAV6-EGFP was microinjected into the sciatic nerve of C57BL/6J mice.9 Briefly, mice were anesthetized under isoflurane (3%, inhalation). Sterilized forceps and scissors were used to make a 2-cm incision at the level of the sciatic nerve. The sciatic nerve was exposed by cutting the connective tissue. AAV was microinjected through an internal cannula (Eicom Co., Kyoto, Japan) at 1 µL/min for 8 min (8 µL total volume) with a gas-tight syringe (10 µL; Eicom Co.) and an air pressure injector system (Micro-syringe Pump-Model ESP-32; Eicom Co.).
Measurement of the sensitivity for tactile stimulus
To quantify the change in sensitivity to a tactile stimulus induced by the optical activation of sensory nerves, paw withdrawal in response to a tactile stimulus during blue light stimulation (473 nm, continuous wave; CW) of the plantar surface was measured using von Frey filaments with a bending force of 0.16 g (Aesthesio®, DanMic Global, LLC, CA, USA). Each of the hind paws was tested individually. Paw withdrawal in response to a tactile stimulus was evaluated by scoring as follows: 0, no response; 1, a slow and slight withdrawal response; 2, a slow and prolonged flexion withdrawal response (sustained lifting of the paw) to the stimulus; 3, a quick withdrawal response away from the stimulus without flinching or licking; 4, an intense withdrawal response away from the stimulus with brisk flinching and/or licking. Paw movements associated with locomotion or weight shifting were not counted as a response. Before the behavioral responses to a tactile stimulus were tested, mice were habituated to their surroundings for 1 h.
Immunohistochemistry
Mice were deeply anesthetized with 3% isoflurane (inhalation) and transcardially perfusion fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Sciatic nerve and dorsal root ganglion (DRG) were post-fixed and cryoprotected in 20 to 30 (w/v)% sucrose. Samples were then embedded in optimal cutting temperature compound (Tissue Tek; Sakura Fine Technical, Tokyo, Japan), and frozen sections were cut on a cryostat (CM1860; Leica Microsystems, Heidelberg, Germany) (8 µm). The brain sections were incubated in appropriate blocking solution and then with primary antibodies (Table 1). Following washes, they were incubated with appropriate secondary antibody conjugated with Alexa 488 or 546. For myelin staining, samples were incubated with FluoroMyelin Red (1:300, #F34652, Thermo Fisher Scientific, MA, USA) for 20 min and then rinsed in phosphate-buffered saline. The sections were mounted with Dako fluorescent mounting medium (Dako, Glostrup, Denmark). Fluorescence of immunolabeling was detected using a light microscope (BX-61; Olympus, Tokyo, Japan) and photographed with a digital camera (MD695; Molecular Devices, CA, USA).
Table 1.
Details of antibodies used for immunohistochemistry.
| Target | Conjugation | Host | Source | Catalog No. | Dilution |
|---|---|---|---|---|---|
| GFP | None | Chicken | Abcam | Ab13970 | 1:1000 |
| Substance P | None | Rabbit | Immunostar | 20064 | 1:800 |
| Rabbit IgG | Alexa546 | Goat | Thermo Fisher Scientific | A-11010 | 1:10000 |
| Chicken IgY | Alexa488 | Goat | Thermo Fisher Scientific | A-11039 | 1:400 |
GFP: green fluorescent protein; IgG: immunoglobulin G; IgY: immunoglobulin Y.
MALDI-imaging
Two weeks after intrasciatic injection of AAV6-ChR2 or AAV6-EGFP, the mice expressing ChR2 or EGFP in sensory nerves were performed optical stimulation (473 nm, CW, 30 min) to the plantar surface of ipsilateral hind paw. Thirty minutes after optical stimulation, mice were sacrificed, and whole brain was flash frozen in solid CO2 and stored at −80°C.
Thin sections (8 µm thickness) were cut with a cryomicrotome (CM3050, Leica Microsystems) and thaw mounted on an indium thin oxide-coated glass slide (Bruker Daltonics, MA, USA) at −16°C. After the sectioning, deuterium-labeled dopamine (DA-d4) as an internal standard (IS) was sprayed onto the sections by using a robotic sprayer device (SunCollect system, SunChrom, Friedrichsdorf, Germany). The sections were manually spray coated with 2,4-diphenyl-pyranylium (DPP) (1.3 mg/mL in methanol) using an artistic airbrush (Procon Boy FWA Platinum 0.2-mm caliber airbrush, Mr. Hobby, Tokyo, Japan) to employ on-tissue derivatization of the monoamines.10 The sections were then automatically spray coated with 2,5-dihydroxybenzoic acid as a matrix (50 mg/mL, dissolved in 50% methanol) using the robotic sprayer. MALDI-IMS was performed using a linear ion trap MS with a MALDI source (MALDI LTQ XL, Thermo Fisher Scientific) equipped with a nitrogen laser (337 nm; 60 Hz). The laser energy and the raster step size were set at 32 µJ and 80 µm, respectively. During imaging measurements, signals of DPP-DA (m/z 368→232) and DPP-DA-d4 (m/z 372→232) were monitored with a precursor ion isolation width of 1.0 m/z units. The obtained spectral data were then transformed to image data using ImageQuest 1.0.1 software (Thermo Fisher Scientific). Note that the DA signal was normalized by the IS (DA-d4) signal simultaneously obtained from the same section.
In vivo microdialysis and sample collection
Mice were anesthetized by the inhalation of 3% isoflurane and placed in a small-animal stereotaxic instrument (RWD Life Science, CA, USA) for surgical implantation of a microdialysis guide cannula (CXG-6; Eicom Co.). The microdialysis guide cannula with dummy probes (CXD-6; Eicom Co.) was implanted into the N.Acc. (AP, +1.4 mm; ML, +1.5 mm; DV, −3.1 mm; angle, 10°). More than six days after implantation of the guide cannula, the dummy cannula was replaced by a microdialysis probe (CX-I-6-01; Eicom Co.). The next day, the probe was continuously perfused with artificial cerebrospinal fluid (0.9 mM MgCl2, 147.0 mM NaCl, 4.0 mM KCl, and 1.2 mM CaCl2) at a flow rate of 1 µL min−1 by a syringe pump (ESP-32; Eicom Co.) for at least 1 h before sample collection. Outflow fractions were collected every 30 min (30 min/fraction). After more than 2 baseline fractions were collected, mice were subjected to optical stimulation (activation of sensory nerves) or morphine injection (10 mg/kg, s.c.). Dialysis samples were stored at −80°C until use.
Qualification and quantification of metabolites by internal and external standards
We used both IS (added to the dialysates) and external standard (ES) compounds to determine m/z value and specific retention time in IC for all metabolites examined. The detailed method is as follows:
IS compounds
We used 2-morpholinoethanesulfonic acid as IS for anionic metabolites. The IS compound is not present in the brain fluids; thus, it serves as ideal standard. Loss of endogenous metabolites during sample preparation was corrected by calculating the recovery rate (%) for each sample measurement.
ES compounds
Before sample analysis, we measured the mixture of authentic compounds of target metabolites in ultrapure water to determine both m/z value and retention time of all metabolites examined.
IC tandem MS for anionic metabolites
For the analysis of small molecules released in the N.Acc induced by pain or the administration of analgesics, anionic metabolites were measured using an Orbitrap-type MS (Q-Exactive Focus; Thermo Fisher Scientific) connected to a high-performance IC system (ICS-5000+; Thermo Fisher Scientific) that enables highly selective and sensitive metabolite quantification due to IC separation and the Fourier-transform MS principle.4 The IC was equipped with an anion electrolytic suppressor (Thermo Scientific Dionex AERS 500; Thermo Fisher Scientific) to convert the potassium hydroxide gradient into pure water before the sample entered the mass spectrometer. Separation was performed using a Thermo Scientific Dionex IonPac AS11-HC, with a 4-µm particle-size column. The IC flow rate of 0.25 mL/min was supplemented post-column with 0.18 mL/min makeup flow of MeOH. The potassium hydroxide gradient conditions for IC separation were as follows: from 1 mM to 100 mM (0–40 min), 100 mM (40–50 min), and 1 mM (50.1–60 min), at a column temperature of 30°C. The Q-Exactive Focus mass spectrometer was operated under an ESI negative mode for all detections. Full mass scan (m/z 70 − 900) was used at a resolution of 70,000. The automatic gain control target was set at 3 × 106 ions, and the maximum ion injection time was 100 ms. Source ionization parameters were optimized with a spray voltage of 3 kV, and other parameters were as follows: transfer temperature of 320°C, S-Lens level of 50, heater temperature of 300°C, Sheath gas at 36, and Aux gas at 10.
The hierarchical clustering algorithm in Eisen’s software11 was applied to investigate the metabolite fluctuations before, during, and after optogenetically induced pain as well as morphine-induced analgesia.
Sustained infusion of NAAG or PMPA into the N.Acc
Mice were anesthetized by the inhalation of 3% isoflurane and placed in a small-animal stereotaxic instrument for the surgical implantation of a microdialysis probe (D-I-6-01; Eicom Co.). The microdialysis probe was directly implanted into the N.Acc. (AP, +1.4 mm; ML, ±1.5 mm; DV, −3.6 mm; angle, 10°). The next day, NAAG or PMPA was injected into the N.Acc. at a flow rate of 0.5 µL/min by a syringe pump (ESP-64; Eicom Co.).
Statistics
The data are presented as the mean ± SEM or SD. We chose the sample size based on similar publications in the field. The statistical significance of differences between groups was assessed by Student’s t-test (unpaired, two-tailed) or one-way analysis of variance followed by the Bonferroni multiple comparisons test. All statistical analyses were performed with GraphPad Prism 5.0 (GraphPad Software). A p value of < 0.05 was considered to reflect significance.
Results
Optical stimulation of ChR2-expressing sensory nerves induces pain-like behavior followed by N.Acc.-specific DA reduction
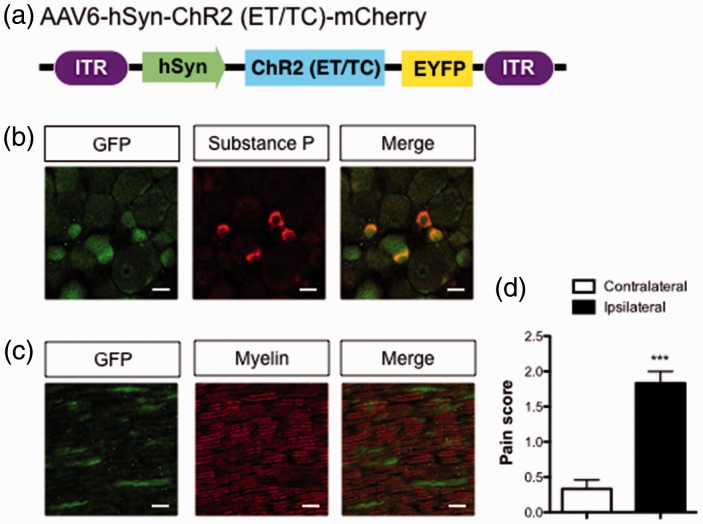
In this study, we sought to identify brain extracellular molecules that are dynamically altered by the application of noxious stimulation or by the administration of analgesics. For this purpose, we needed to create an animal model in which nociceptive stimulation/relief could be induced within desired time windows, as well as without the use of anesthetics. Therefore, we used an optogenetic approach to induce acute nociception.9 We generated mice expressing ChR2 in sensory nerves with the injection of retrograde AAV: AAV6-hSyn-ChR2 (ET/TC)-EGFP (Figure 1(a)). In this model, we could apply temporally controlled nociceptive stimulation by optogenetic neuronal activation.
Figure 1.
Effects of the optical activation of sensory nerves on the pain threshold. (a) Construction of the AAV. (b) Expression of ChR2 (ET/TC)-EYFP and substance P in the lumbar DRG (ipsilateral side). Scale bar = 20 µm. (c) Expressions of ChR2 (ET/TC)-EYFP and myelin in the sciatic nerve. Scale bar = 20 µm. (d) von Frey thresholds in response to optical stimulation of the plantar surface (n = 8, t(14) = 7.183). Data are presented as mean ± SEM. ***p < 0.001; by two-tailed Student’s t-test. AAV: adeno-associated virus; EYFP: enhanced yellow fluorescent protein; GFP: green fluorescent protein; hSyn: human synapsin; DRG: dorsal root ganglion.
Immunohistochemical analysis showed that ChR2-EYFP was expressed in cells containing substance P, a marker of nociceptive c-fiber neurons in the lumbar DRG (Figure 1(b)). Additionally, ChR2-expressing axons of sciatic nerves were not found in myelinated A-fibers (Figure 1(c)). Exposure of the plantar surface of the ipsilateral hind paw with 473 nm blue light induced avoidance behaviors and reduced sensory thresholds consistent with the activation of nociceptors (Figure 1(d)).
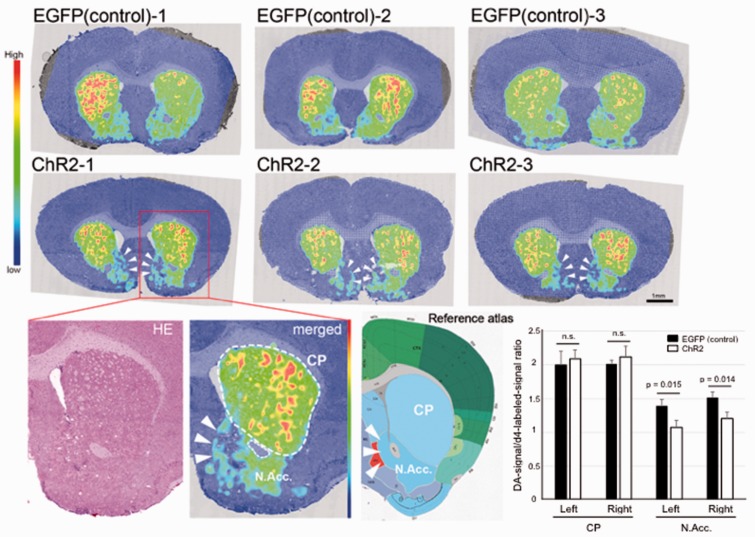
Having generated the algesthesia-inducible mouse, we then evaluated a biochemical effect of the pain in the N.Acc.-induced by optogenetic approach. Since DA in N.Acc. plays critical role for both nociceptive as well as analgesia inputs,12,13 to demonstrate the efficacy of the optogenetic stimulation-induced pain, we assessed local DA concentration changes between ChR2-expressing noxious-stimulated and EGFP-expressing control animals. For this purpose, we employed an imaging MS allowing in situ visualization of DA concentration (Figure 2). Strikingly, we found that tissue content of DA was specifically reduced in the N.Acc. (Figure 2, arrowheads), but not caudoputamen, demonstrating this noxious stimulation affects the N.Acc. DA metabolism.
Figure 2.
Visualization of dopamine concentration changes by optogenetic stimulation in the mouse brain sections of noxious-stimulated mice. Imaging mass spectrometry visualized distribution of dopamine in coronal brain sections those containing N.Acc., from ChR2-expressing noxious-stimulated (middle) and EGFP-expressing control (upper) mice. Representative dopamine image of noxious-stimulated brain in high magnification and its corresponding reference atlas (Allen Brain Atlas, http://www.brain-map.org/) were also shown. Note that endogenous dopamine signal was normalized by an internal standard (deuterium-labeled dopamine signal homogeneously spayed by a robotic sprayer device, see also Methods section). The normalized signal intensities from caudoputamen (CP) and N.Acc. regions were quantified (lower right). Data are presented as mean ± SD. EGFP: enhanced green fluorescent protein; N.Acc.: nucleus accumbens.
Identification of small molecules released in the N.Acc. at concentrations oppositely respond to pain stimuli and analgesia
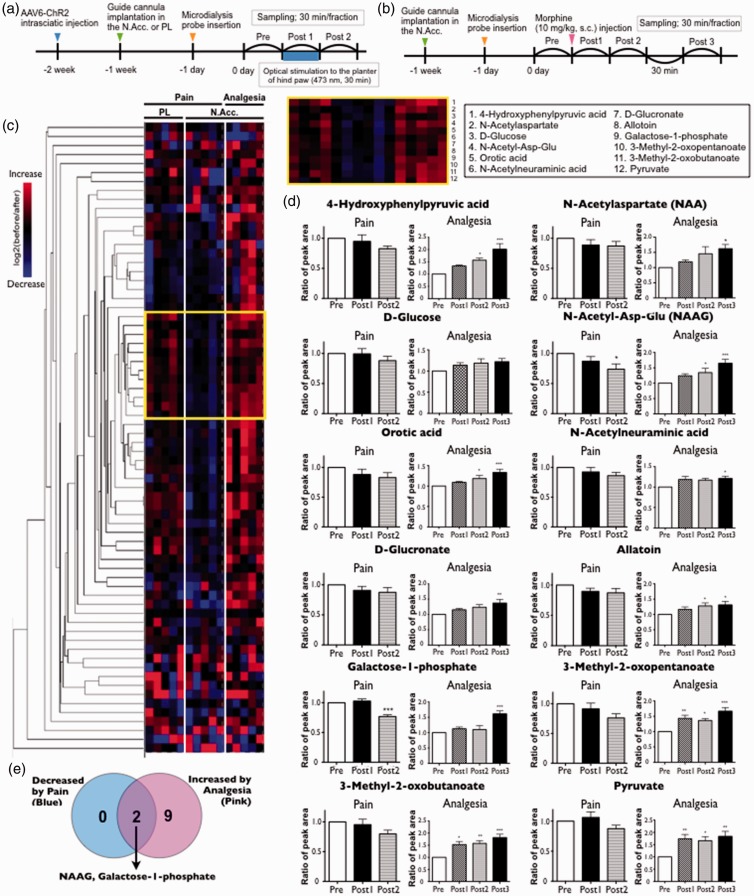
We collected dialysates in the N.Acc. before, during, and after the application of optogenetically induced pain and morphine-induced analgesia and have presented the experimental timeline in Figure 3(a) and (b), respectively. For pain analysis, dialysates were additionally collected in the prelimbic cortex (PL) to refine specific responders in the N.Acc. region.
Figure 3.
Profiling of dynamic changes of small molecules in the cerebral fluids of the N.Acc. in response to the optical activation of sensory nerves (pain stimuli) or systemic morphine injection (analgesia) by IC-HRMS analysis. (a, b) Experimental timeline of microdialysis experiment for mice with optogenetically induced pain (a) and morphine-induced analgesia (b). (c) Hierarchical clustering revealed a group of molecules shows up-regulation by pain stimuli in N.Acc., but not PL, and reduction by analgesia (yellow square). The clustering analysis was performed using log2 fold change values (pre/post). Red indicates increase and blue indicates decrease compared to the pre. (d) Time-course concentration changes of the clustered metabolites into the yellow square shown in (c). (e) The Venn diagram shows the number of decreased molecules by pain (blue) and increased molecules by analgesia (pink). Only NAAG and galactose-1-phosphate were identified as those significantly reduced by pain as well as elevated by analgesia. Data are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001; by one-way ANOVA with Bonferroni post hoc analysis. N.Acc.: nucleus accumbens; IC-HRMS: ion chromatography coupled with high-resolution mass spectrometry; PL: prelimbic cortex; ANOVA: analysis of variance; AAV: adeno-associated virus; NAAG: N-acetylaspartylglutamate.
We subsequently performed IC-HRMS in the negative ion detection mode and qualitatively identified more than 60 endogenous molecules in the dialysates. We could simultaneously trace their extracellular concentrations. Owing to the large dataset, we employed hierarchical clustering, a multivariate analysis, which provided an overview of molecular fluctuations during the two perturbations (Figure 3).
As presented in Figure 3(c), we found a group of molecules that exhibited N.Acc.-specific reductions in response to pain stimuli as well as increases during analgesia. Focusing on this cluster, 12 metabolites, including amino acid derivatives (1 and 2), hexose and its metabolites (3, 6, 7, 9, and 12), a small peptide (4), a nucleotide precursor and decomposed product (5 and 8), and branched chain AA decomposed products (10 and 11), were identified. Finally, after statistical analysis (Figure 3(d)), we found that the extracellular concentrations of NAAG and galactose-1-phosphate in the N.Acc. significantly responded to pain and analgesia in opposite manners (reduced by pain and elevated by analgesia; Figure 3(e)).
Infusion of NAAG in the N.Acc. modulates the pain threshold
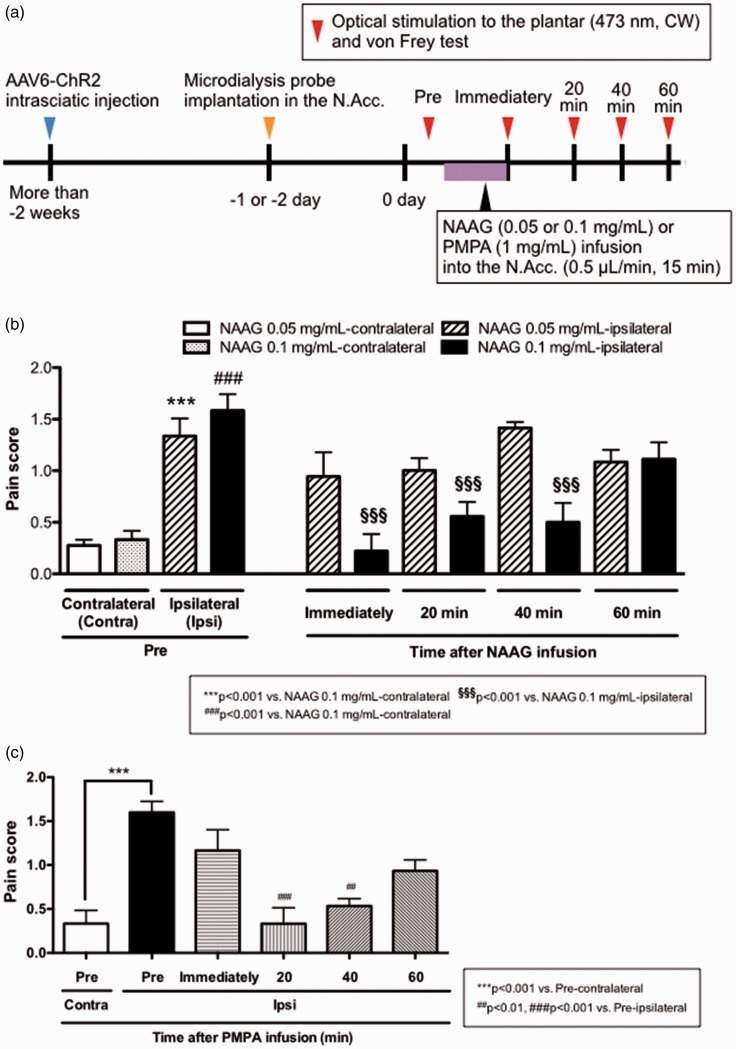
After identifying NAAG and galactose-1-phosphate as candidates for endogenous pain modulation in the N.Acc., we proceeded to assess NAAG bioactivity for modulation of the pain sensation. NAAG is one of the most abundant neuro co-transmitters in the mammalian central nervous system and is known to be released from synaptic terminals.14–16 On the other hand, galactose-1-phosphate is an intermediate in the intraconversion of glucose and galactose, and there is limited information on its physiological role in the intercellular space. Therefore, we focused on NAAG and investigated the pain-modulating effects of the sustained infusion of NAAG into the N.Acc. (Figure 4(a)).
Figure 4.
Effect of NAAG in the N.Acc. on optical activation of sensory nerves-mediated sensitivity to mechanical stimuli. (a) Experimental timeline. (b, c) Effect of sustained infusion of NAAG (0.05 or 0.1 mg/mL, n = 6) (b) or PMPA (1 mg/mL, n = 5) (c) into the N.Acc. on pain score in response to optical activation of sensory nerves and von Frey filament. Data are mean ± SEM. ***p < 0.001, ##p < 0.01, ###p < 0.001, §§§p < 0.001; by one-way ANOVA with Bonferroni post hoc analysis. NAAG: N-acetylaspartylglutamate; N.Acc.: nucleus accumbens; ANOVA: analysis of variance; CW: continuous wave; PMPA: 2-phosphonomethyl-pentanedioic acid; AAV6: adeno-associated virus serotype 6.
Interestingly, infusion of NAAG into the N.Acc. significantly attenuated pain induced by the optical activation of sensory nerves (Figure 4(b)). In the mammalian brain, NAAG is degraded to N-acetylaspartate (NAA) and glutamate by glutamate carboxypeptidase II (GCP-II).17 To confirm the effect of NAAG on the pain threshold and exclude the effect of its decomposed products, i.e. glutamate and NAA, we investigated the effect of infusion of a GCP-II inhibitor (PMPA) on the pain threshold. The infusion of PMPA into the N.Acc. attenuated the pain induced by the optical activation of sensory nerves as well as NAAG (Figure 4(c)), demonstrating that intact NAAG bioactivity could reduce acute pain.
Discussion
We performed a comprehensive analysis of small molecules that are released in the N.Acc. in response to activation of nociceptors or following administration of morphine. Our goal was to identify, and begin to functionally characterize, potential neurotransmitter molecules relevant to pain in this brain region. Consequently, we detected over 60 metabolites in the N.Acc. and identified NAAG as a potential neurotransmitter relevant to pain and its modulation.
NAAG is considered to be a neurotransmitter that is mainly synthesized from NAA and glutamate in neurons17 and is degraded to NAA and glutamate by NAAG peptidase (also known as GCP-II) on astrocytes or neurons.18 NAAG has been suggested to act as an agonist for group II metabotropic glutamate receptors, especially mGluR3.19 Furthermore, NAA in the dorsolateral prefrontal cortex and thalamus has been shown to be reduced under chronic pain.20,21 Since NAAG derivatives in the brain may be reduced under chronic pain, we hypothesized that an increase of NAAG in the brain might produce analgesia. Consistent with this idea, increasing the level of NAAG by the inhibition of NAAG peptidase in the lateral ventricle, periaqueductal gray, rostral ventromedial medulla, or locus coeruleus has been shown to produce analgesia.22–24
Nevertheless, it was still unclear whether NAAG acted as a pain modulator in the N.Acc. Therefore, we established an animal model of pain using optogenetics and investigated the pain-controlling effects of NAAG and PMPA. We found that the infusion of either NAAG or PMPA into the N.Acc. reduced nociceptive behaviors induced by the optical activation of sensory nerves.
It is worth to note that in our pain model, N.Acc.-specific DA reduction was visualized by imaging MS (Figure 2, arrow heads).10 Since it is reported that injection of NAAG as well as NAAG peptidase inhibitors lowered DA release in the N.Acc.,25,26 decreased extracellular NAAG concentration observed might accelerate a temporal DA release and its metabolism and finally lead to shortage of DA content in the N.Acc. of our pain model. Although further studies are needed, these results suggest a linked regulation of NAAG and DA signaling in N.Acc., which might play an important role in the pain sensation. Furthermore, since the analgesic effect of NAAG in other central regions is considered to be associated with the activation of mGluR3,23,24 NAAG released in the N.Acc. may act on mGluR3 and produce analgesia.
In summary, using in vivo microdialysis coupled with an IC-HRMS technique, we found that NAAG is an endogenous pain modulator released in the N.Acc. Infusion of either NAAG or PMPA into the N.Acc. dramatically reduced pain induced by optical activation of sensory nerves. Our results suggest that NAAG released in the N.Acc. could control the sensation of pain and may be a new target of drug discovery for predicting the ability to modulate pain.
Acknowledgment
We thank Y Iwayama and H Ogata for their help with the experiments.
Author Contributions
MW carried out the experiments, interpreted the results, and wrote the manuscript. YS carried out and analyzed the mass spectrometry experiments, interpreted the results, and wrote the manuscript. ES carried out the imaging mass spectrometry experiments. Michiko Narita, TK, and NU contributed to carrying out the experiments. EN and FP contributed to writing the manuscript. AY contributed to generation of the AAV6-hSyn-ChR2 (ET/TC)-EYFP, and NK supervised the project. Minoru Narita supervised and conceived the project and contributed to writing the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Minoru Narita was supported by MEXT-Supported Program for the Strategic Research Foundation at Private Universities No.S1411019 and JSPS Grant-in-Aid for Scientific Research (B) No. 26293346. This work was also supported by JSPS Grant-in-Aid for Young Scientists (A) No. 16748651 (to YS).
References
- 1.Apkarian AV, Bushnell MC, Treede RD, et al. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005; 9: 463–484. [DOI] [PubMed] [Google Scholar]
- 2.Baliki MN, Geha PY, Fields HL, et al. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron 2010; 66: 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kupfermann I. Functional studies of cotransmission. Physiol Rev 1991; 71: 683–732. [DOI] [PubMed] [Google Scholar]
- 4.Hu S, Wang J, Ji EH, et al. Targeted metabolomic analysis of head and neck cancer cells using high performance ion chromatography coupled with a Q Exactive HF mass spectrometer. Anal Chem 2015; 87: 6371–6379. [DOI] [PubMed] [Google Scholar]
- 5.Makarov A, Denisov E, Lange O, et al. Dynamic range of mass accuracy in LTQ Orbitrap hybrid mass spectrometer. J Am Soc Mass Spectrom 2006; 17: 977–982. [DOI] [PubMed] [Google Scholar]
- 6.Werner E, Heilier JF, Ducruix C, et al. Mass spectrometry for the identification of the discriminating signals from metabolomics: current status and future trends. J Chromatogr B Analyt Technol Biomed Life Sci 2008; 871: 143–163. [DOI] [PubMed] [Google Scholar]
- 7.Miyazawa H, Yamaguchi Y, Sugiura Y, et al. Rewiring of embryonic glucose metabolism via suppression of PFK-1 and aldolase during mouse chorioallantoic branching. Development 2017; 144: 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyajima M, Zhang B, Sugiura Y, et al. Metabolic shift induced by systemic activation of T cells in PD-1-deficient mice perturbs brain monoamines and emotional behavior. Nat Immunol 2017; 18: 1342–1352. [DOI] [PubMed]
- 9.Iyer SM, Montgomery KL, Towne C, et al. Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat Biotechnol 2014; 32: 274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shariatgorji M, Nilsson A, Goodwin RJA, et al. Direct targeted quantitative molecular imaging of neurotransmitters in brain tissue sections. Neuron 2014; 84: 697–707. [DOI] [PubMed] [Google Scholar]
- 11.Eisen MB, Spellman PT, Brown PO, et al. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998; 95: 14863–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altier N andStewart J.. The role of dopamine in the nucleus accumbens in analgesia. Life Sci 1999; 65: 2269–2287. [DOI] [PubMed] [Google Scholar]
- 13.Navratilova E, Xie JY, Okun A, et al. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc Natl Acad Sci U S A. 2012; 109: 20709–20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coyle JT. The nagging question of the function of N-acetylaspartylglutamate. Neurobiol Dis 1997; 4: 231–238. [DOI] [PubMed] [Google Scholar]
- 15.Neale JH Bzdega T andWroblewska B.. N-acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J Neurochem 2000; 75: 443–452. [DOI] [PubMed] [Google Scholar]
- 16.Curatolo A, D Arcangelo P, Lino A, et al. Distribution of N-acetyl-asparatic and N-acetyl-aspartyl-glutamic acids in nervous tissue. J Neurochem 1965; 12: 339–342. [DOI] [PubMed] [Google Scholar]
- 17.Becker I, Lodder J, Gieselmann V, et al. Molecular characterization of N-acetylaspartylglutamate synthetase. J Biol Chem 2010; 285: 29156–29164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neale JH, Olszewski RT, Gehl LM, et al. The neurotransmitter N-acetylaspartylglutamate in models of pain, ALS, diabetic neuropathy, CNS injury and schizophrenia. Trends Pharmacol Sci 2005; 26: 477–484. [DOI] [PubMed] [Google Scholar]
- 19.Wroblewska B, Wroblewski JT, Pshenichkin S, et al. N-acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. J Neurochem 1997; 69: 174–181. [DOI] [PubMed] [Google Scholar]
- 20.Fukui S, Matsuno M, Inubushi T, et al. N-acetylaspartate concentrations in the thalami of neuropathic pain patients and healthy comparison subjects measured with (1)H-MRS. Magn Reson Imaging 2006; 24: 75–79. [DOI] [PubMed] [Google Scholar]
- 21.Grachev ID Fredrickson BE andApkarian AV.. Abnormal brain chemistry in chronic back pain: an in vivo proton magnetic resonance spectroscopy study. Pain 2000; 89: 7–18. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto T, Kozikowski A, Zhou J, et al. Intracerebroventricular administration of N-acetylaspartylglutamate (NAAG) peptidase inhibitors is analgesic in inflammatory pain. Mol Pain 2008; 4: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada T, Zuo D, Yamamoto T, et al. NAAG peptidase inhibition in the periaqueductal gray and rostral ventromedial medulla reduces flinching in the formalin model of inflammation. Mol Pain 2012; 8: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nonaka T, Yamada T, Ishimura T, et al. A role for the locus coeruleus in the analgesic efficacy of N-acetylaspartylglutamate peptidase (GCPII) inhibitors ZJ43 and 2-PMPA. Mol Pain 2017; 13: 1744806917697008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyamoto Y, Ishikawa Y, Iegaki N, et al. Overexpression of Shati/Nat8l, an N-acetyltransferase, in the nucleus accumbens attenuates the response to methamphetamine via activation of group II mGluRs in mice. Int J Neuropsychopharm 2014; 17: 1283–1294. [DOI] [PubMed] [Google Scholar]
- 26.Xi ZX, Li X, Peng XQ, et al. Inhibition of NAALADase by 2-PMPA attenuates cocaine-induced relapse in rats: a NAAG-mGluR2/3-mediated mechanism. J Neurochem 2010; 112: 564–576. [DOI] [PMC free article] [PubMed] [Google Scholar]