Abstract
Treatment of Crohn’s disease (CD) is intrinsically reliant on imaging techniques, due to the preponderance of small bowel disease and its transmural pattern of inflammation. Ultrasound (US), computed tomography (CT) and magnetic resonance imaging (MRI) are the most widely employed imaging methods and have excellent diagnostic accuracy in most instances. Some limitations persist, perhaps the most clinically relevant being the distinction between inflammatory and fibrotic strictures. In this regard, several methodologies have recently been tested in animal models and human patients, namely US strain elastography, shear wave elastography, contrast-enhanced US, magnetization transfer MRI and contrast dynamics in standard MRI. Technical advances in each of the imaging methods may expand their indications. The addition of oral contrast to abdominal US appears to substantially improve its diagnostic capabilities compared to standard US. Ionizing dose-reduction methods in CT can decrease concern about cumulative radiation exposure in CD patients and diffusion-weighted MRI may reduce the need for gadolinium contrast. Clinical indexes of disease activity and severity are also increasingly relying on imaging scores, such as the recently developed Lémann Index. In this review we summarize some of the recent advances in small bowel CD imaging and how they might affect clinical practice in the near future.
Keywords: Crohn disease, diagnostic imaging, fibrosis, inflammation, magnetic resonance imaging, positron-emission tomography, small intestine, ultrasonography
Introduction
Crohn’s disease (CD) is characterized by an immune-mediated inflammation of the digestive tube wall. Inflammatory changes can affect any segment of the digestive system, from mouth to anus, and inflammation can span all layers of the digestive tube wall (i.e. transmural inflammation). For these reasons, radiologic examinations are an integral part of its diagnosis and monitoring, in conjunction with endoscopic investigations. Whenever CD disease is confined to small bowel sections not reachable with colonoscopy and ileoscopy, small bowel capsule endoscopy and radiological exams are the most commonly used tools for diagnosis. However, cross-sectional imaging techniques have the important advantage over endoscopy of allowing assessment of transmural and extramural disease.
The intensity of inflammatory changes in imaging exams permits grading the severity of disease. In addition, all CD patients should be assessed at diagnosis with both lower digestive endoscopy and a cross-sectional imaging to evaluate disease extension.1 While the terminal ileum is the most frequently affected segment, imaging tools should permit good visualization of the entire small bowel to permit accurate evaluation of disease extent, as extensive disease and upper gastrointestinal involvement have prognostic and therapeutic implications. Moreover, CD patients will invariably require multiple imaging exams during the course of their disease, to monitor response to therapy or to diagnose and evaluate relapses, which raises concerns about cumulative radiation exposure in long-standing disease.
All phases of CD patient management are therefore highly dependent on imaging examinations. Historically, contrast barium studies used to be the standard. With the development and dissemination of cross-sectional techniques, which have higher diagnostic accuracy in several situations, these have become the norm.
Several limitations persist and recent advances in imaging techniques and interpretation might be paving the way for future CD management.
Current imaging techniques
Ultrasound (US), computed tomography (CT) and magnetic resonance imaging (MRI) are the most widespread imaging techniques used for small bowel CD evaluation.
Comparison studies between the three approaches have shown good diagnostic accuracy in the assessment of disease extent and severity and in the diagnosis of stricturing and penetrating complications.2,3 The decision between them is usually the result of local experience, availability, cost and their specific drawbacks and advantages according to the clinical scenario.
According to the European Crohn’s and Colitis Organization (ECCO), CT and MRI are the current standard for small bowel assessment. The main limitation of CT is radiation exposure. MRI presents a radiation-free but costly and more time-consuming alternative that is not as widely available. Both require luminal distension for adequate visualization of the small bowel, as well as intravenous contrast. Bowel distention can be achieved through orally administered contrast (enterography) or through a nasojejunal tube (enteroclysis). Diagnostic accuracy in CT and MRI is generally the same whichever the route of administration, with slightly higher specificity for enteroclysis.4,5 The oral route has better patient tolerance, is easier to administer and requires less radiation in the case of CT, making it the first-line choice in most instances. However, it may not achieve adequate distension of the jejunum; in established or suspected jejunal disease, enteroclysis may provide additional benefit.
US is a non-invasive, radiation-free and well-tolerated imaging alternative in some circumstances. The terminal ileum is usually well visualized, but the proximal ileum and jejunum may not be due to their deeper location and overlying bowel loops. Moreover, its diagnostic accuracy is highly operator-dependent when compared to CT or MRI.
Future
Distinction between fibrotic and inflammatory lesions
US, CT and MRI have several validated signs that are consistently associated with CD disease activity and severity. In particular, cross-sectional techniques have good diagnostic accuracy for detecting stricturing lesions, their number and location. However, imagiological distinction between inflammatory and fibrotic strictures remains elusive. Because the inflammatory process is transmural, endoscopic biopsies lack sensitivity in the detection of fibrosis. Differentiation between inflammatory and fibrotic stenoses remains a holy grail in CD bowel imaging, especially because of its clinical and therapeutic implications (a predominantly inflammatory stricture is expected to improve with anti-inflammatory medication while a marked fibrotic component usually entails an endoscopic or surgical solution). European Crohn’s and Colitis Organization guidelines on CD state that current imaging methods do not possess validated diagnostic accuracy for the distinction between inflammatory and fibrotic strictures.1 Nevertheless, several imaging methods have been studied in recent years with promising results that may change this panorama.
Ultrasound
US strain elastography (or US elasticity imaging) utilizes external compression of tissue and US measurement of its deformation to estimate its stiffness. This degree of compressibility is shown in real time as colors superimposed on grayscale US images, with different colors representing different degrees of stiffness. A numerical value can also be extracted for a region of interest by specialized software that calculates the ratio between the region of interest and a reference tissue (strain ratio).6 A few studies have tested its discriminatory power between normal, inflamed and fibrotic bowel in rat models, all of them with histopathological confirmation of these changes. In a rat model of chronic colonic inflammation achieved with repeated trinitrobenzenesulfonic acid (TNBS) enemas, Kim and colleagues showed statistically significant differences between normal control rats and chronically fibrotic intestine (p < 0.0005). Intestinal stiffness was also measured ex vivo and correlation between in vivo strain measurements and ex vivo elastography was strong (k = 0.67).7 A later study on the same rat model also included an acute inflammation model achieved with a single TNBS enema. Differences between acute inflammation and chronic fibrosis strain measurements achieved statistical significance but with a higher p value than the previous study (p = 0.037).8 Xu and colleagues expanded on this method by noting that tissue’s strain response to a dynamic range of pressures, rather than one single strain measurement with static bowel pressure, may improve discriminatory power between inflammation and fibrosis. They note that unlike normal bowel, which has a linear strain response to increasing applied strain, both inflamed and fibrotic tissue’s strain response is non-linear, and this non-linear curve’s slope differs between inflamed and fibrotic tissue. They proposed a quantitative parameter that characterizes this curve slope based on in vivo strain measurements, which showed highly significant (p = 0.003) differences between rat models of acute bowel inflammation and chronic bowel fibrosis.9
These US strain elastography findings have been translated to humans in 3 studies in a total of 40 CD patients scheduled for bowel resection. Stidham and colleagues submitted seven CD patients with stenosing disease to strain elastography, with stiffness measurements of strictured and adjacent normal bowel, before surgical resection. Strain measurements differed significantly between the two (p = 0.0008) and showed a strong inverse correlation with direct ex vivo elastography. All of the bowel strictures evaluated were confirmed to be predominantly fibrotic with histopathological examination.8 Baumgart and colleagues confirmed these findings in 10 CD patients undergoing bowel resection for symptomatic stenosis and in addition found a significant association between in vivo strain measurement and tissue collagen content, quantified by histochemistry.10 In another study on 23 CD patients elected for surgical resection, strain ratio correlated well with the histopathological fibrosis grade and could distinguish severe fibrosis with an area under receiving operating characteristic curve (AUC) of 0.917. The fact that interobserver agreement on bowel strain ratio was excellent between two experienced operators (intraclass correlation coefficient of 0.78) was also promising. More importantly, neither acute nor chronic inflammation score were predictors of strain ratio in a multivariate analysis, suggesting that strain elastography can identify chronic fibrotic tissue despite inflammatory changes.11
While these results are promising, it is important to note that only two studies compared fibrotic and inflamed bowel; in all the rest, fibrotic strictures were compared with normal bowel. In clinical practice, this is rarely a problem as currently available techniques such as traditional US, CT and MRI are already very accurate at identifying strictures, and the question lies in quantifying its fibrotic component.
Shear wave velocity US or shear wave elastography (SWE) is another ultrasonographic method for measuring tissue stiffness. Instead of direct pressure, it applies energy to the tissues through a pulse wave generated by the US probe. These vibrations displace tissue across multiple geometrical planes instead of only deforming it perpendicularly. They are propagated through tissues as a shear wave, which travels faster in denser and stiffer materials. Dillman and colleagues have tested this tool both in an animal model of CD and in ex vivo human bowel specimens from CD patients. In rat models of acute and chronic bowel inflammation (with histopathological phenotype confirmation), shear wave velocity demonstrated excellent discriminatory power between fibrotic and acutely inflamed bowel (AUC = 0.971).12 In 23 ex vivo human bowel specimens, it showed a moderate correlation with fibrosis score, while correlation with inflammation grade was weak. It could also differentiate areas of low and high histological fibrosis score with an AUC of 0.9.13 More recently, Lu and colleagues have applied both SWE and contrast-enhanced US (CEUS) to 105 CD patients, 15 of which later went on to have bowel resection. They hypothesized that the combination of the two methods would be relevant as SWE would measure the fibrotic component of the bowel while CEUS would serve as a measure of acute inflammation. SWE measurements were significantly correlated with bowel wall muscle hypertrophy but not fibrosis (the authors suggest this might be due to the specific histopathological score used). Interestingly, they could only find an association between CEUS and chronic inflammation (but not acute inflammation). These findings might be explained by the relatively long interval between US and surgery (median interval of 71 days) and the fact that one-third of the surgical patients were on immunosuppressive medication at the time of bowel imaging. Nonetheless, the study was a step toward demonstrating the applicability of SWE in clinical practice, as only 10 out of 105 patients were excluded due to high interquartile range of their individual measurements.14
CEUS is an ultrasonographic method that analyses tissue perfusion characteristics. It utilizes an intravenous contrast agent composed of microscopic gas bubbles that enhance the signal from blood cells and therefore increase vascular contrast. Unlike CT or MRI contrast, it carries no risk of nephrotoxicity as it is excreted through the lungs after only 15 min in circulation and it is non-ionizing. A specific software can be used to obtain quantitative parameters that characterize the perfusion kinetics in selected regions of interest. Quaia and colleagues found significant differences in two quantitative parameters obtained from CEUS measurements (percentage of maximal enhancement and area under the time-intensity curve) between fibrotic and inflammatory strictures. These parameters could differentiate between the two types of stenoses with AUC of 0.75 and 0.88, respectively. The study was limited by the fact that histological confirmation came from endoscopic biopsies and not surgical specimens and by the fact that the US operators were not blinded to histological results.15 In another study on 39 CD patients, 20 of which were scheduled for bowel resection and 19 of which were to receive medical treatment, CEUS was performed pre-treatment. Indications for surgery were bowel stenosis, refractory disease and one bowel perforation. Blood volume and blood flow as assessed by CEUS were significantly lower in the surgical group, suggesting that these changes may be related to increased fibrosis. The ratio between blood volume and wall thickness could predict surgical indication with an AUC of 0.92 (p < 0.001).16 Ripollés and colleagues correlated CEUS findings with histopathological examination in 25 CD patients undergoing bowel resection. They found that percentage of increase in contrast enhancement was significantly higher in inflammatory lesions and that it could predict bowel inflammation with an AUC of 0.84. They employed a US score (which included percentage of increase in contrast enhancement, among other ultrasonographic features) to classify bowel segments into predominantly fibrotic or predominantly inflammatory, with good agreement with histological classification (kappa = 0.632).17
All US methods have limitations inherent to the technique. They are operator-dependent, and disease location and patient body habitus may limit their sensitivity. The new ultrasonographic techniques are further limited by the fact that each commercial system uses different methods for analysis and different parameters for quantification, making reproducibility and generalization of results difficult. Table 1 summarizes the studies testing ultrasonographic tools for fibrosis distinction.
Table 1.
Distinguishing fibrosis from inflammation in Crohn’s disease with ultrasonographic imaging techniques.
| Author | Population | Technique | Reference | Results |
|---|---|---|---|---|
| Kim and colleagues7 | Animal model (rats, TNBS enemas) 5 controls 6 chronic fibrosis |
Strain elastography | Direct ex vivo measurement Histopathology (surgical specimens) |
Significant strain differences between proximal normal bowel and distal fibrotic bowel (p < 0.0005). Strong correlation between in vivo and ex vivo strain measurements (k = 0.67, p < 0.0005). |
| Stidham and colleagues8 | Animal model (rats, TNBS enemas) 3 controls 5 acute inflammation 6 chronic fibrosis |
Strain elastography | Direct ex vivo measurement Histopathology (surgical specimens) |
Significant strain differences between acute inflammation and chronic fibrosis (p = 0.037). |
| Xu and colleagues9 | Animal model (rats, TNBS enemas) 4 acute inflammation 4 chronic fibrosis |
Strain elastography (over a dynamic range of applied pressures) | Direct ex vivo measurement Histopathology (surgical specimens) |
Highly significant non-linear parameter difference between acutely inflamed and fibrotic tissues (p = 0.0029). |
| Stidham and colleagues8 | 7 CD patients scheduled for bowel resection | Strain elastography | Direct ex vivo measurement Histopathology (surgical specimens) |
Significant strain differences between stenotic and adjacent normal bowel (p = 0.0008). Strong correlation between in vivo and ex vivo strain measurements (k = −0.81). |
| Baumgart and colleagues10 | 10 CD patients scheduled for bowel resection | Strain elastography | Direct ex vivo measurement Histopathology (surgical specimens) |
Significant strain differences between strictured and normal bowel (p < 0.001). Strain measurements significantly associated with collagen content (p < 0.001). |
| Fraquelli and colleagues11 | 23 CD patients scheduled for bowel resection 20 CD patients with no surgical indication |
Strain elastography | Histopathology (surgical specimens) | Strain ratio significantly associated with histologic fibrosis score (p = 0.005). Good discriminatory ability of strain measurement for severe fibrosis (AUC = 0.917, CI 0.788–1.000). Fibrosis, but not inflammation, was an independent determinant of strain measurement in multivariate analysis. |
| Dillman and colleagues12 | Animal model (rats, TNBS enemas) 3 controls 6 acute inflammation 7 chronic fibrosis |
SWE (ARFI-derived) |
Histopathology (surgical specimens) | Significant SWE between acute inflammation and chronic fibrosis (p = 0.0009). Good discriminatory ability of SWE to distinguish acute inflammation from chronic fibrosis (AUC = 0.971 in post-hoc analysis). |
| Dillman and colleagues13 | 17 bowel segments from 12 CD patients 11 high fibrosis 6 low fibrosis |
SWE (ex vivo) (ARFI-derived) |
Histopathology (surgical specimens) | Significant difference in shear wave speed between high and low fibrosis score (p = 0.004). Good discriminatory ability between high and low fibrosis score (AUC = 0.91, CI 0.67–0.99). No significant difference in shear wave speed between high and low inflammation score |
| Lu and colleagues14 | 95 ileal/ileocolonic CD patients (15 of which had bowel resection) |
Shear wave elastography | Histopathology (surgical specimens) | Significant difference in SWE between patients who required and did not require surgery (p < 0.01). Significant correlation between muscle hypertrophy and SWE (p < 0.02). No significant correlation between SWE and fibrosis or inflammation. |
| Lu and colleagues14 | 95 ileal/ileocolonic CD patients (15 of which had bowel resection) |
Contrast-enhanced ultrasound | Histopathology (surgical specimens) | Moderate negative correlation between CEUS peak enhancement and fibrosis (r = −0.59, p = 0.02). Moderate negative correlation between CEUS peak enhancement and chronic inflammation (r = 0.6, p = 0.03). No significant correlation between CEUS peak enhancement and acute inflammation. |
| Quaia and colleagues15 | 28 CD patients 12 inflammatory strictures 16 fibrotic strictures |
Contrast-enhanced ultrasound | Histopathology (endoscopic biopsies) | Percentage of maximal enhancement and area under the time-intensity curve significantly lower in fibrotic versus inflammatory strictures (p = 0.002 and p = 0.001; AUC of 0.75 and 0.88, respectively). |
| Nylund and colleagues16 | 39 CD patients 20 surgical treatment 19 medical treatment |
Contrast-enhanced ultrasound | Surgical indication | Significantly higher blood volume and blood flow in the medical group versus surgery group (p < 0.001 and p = 0.002). Ratio of blood volume to bowel wall thickness could predict need for surgery with an AUC of 0.92 (p < 0.001). |
| Ripollés and colleagues17 | 28 bowel segments from 25 CD patients | Contrast-enhanced ultrasound | Histopathology (surgical specimens) | Percentage of increase in contrast enhancement significantly higher in inflammation versus fibrosis (p = 0.03). Good predictive ability of percentage of increase in contrast enhancement for inflammation (AUC of 0.844, p = 0.002). |
ARFI, acoustic radiation force impulse; AUC, area under the receiver operating characteristic curve; CD, Crohn’s disease; SWE, shear wave elastography; TNBS, trinitrobenzenesulfonic acid.
MRI
While MRI is a very accurate tool to assess disease activity, MRI signs associated with fibrosis have been inconsistent in the literature. Table 2 is a summary of recent studies using MRI signs for the distinction between bowel wall fibrosis and inflammation. Punwani and colleagues performed MRI enterography on 18 CD patients before intestinal resection. Forty-nine segments of interest were identified and MRI findings correlated with histological examination after imaging and histopathological matching. There was no association between fibrosis and wall thickness, wall signal intensity or degree of mural enhancement at 30 s or 70 s post-contrast administration. A layered pattern of contrast enhancement was most commonly associated with histological fibrosis while a homogeneous pattern was usually associated with absence of fibrosis.18 Zappa and colleagues in 2011 found an association between fibrosis score and wall thickness, but no correlation between fibrosis score and MRI pattern or degree of wall enhancement, in 53 CD patients who underwent bowel resection.19
Table 2.
Distinguishing fibrosis from inflammation in Crohn’s disease with magnetic resonance imaging techniques.
| Author | Population | Technique | Reference | Results |
|---|---|---|---|---|
| Punwani and colleagues18 | 49 intestinal segments from 18 CD patients scheduled for bowel resection | MRI | Histopathology (surgical specimens) | No association between fibrosis and wall thickness or wall signal intensity. No association between fibrosis and degree of mural enhancement at 30 or 70 seconds post-contrast administration. Layered pattern of enhancement most commonly associated with fibrosis. Homogeneous pattern of enhancement most commonly associated with absence of fibrosis. |
| Zappa and colleagues19 | 53 CD patients scheduled for bowel resection | MRI | Histopathology (surgical specimens) | Significant association between fibrosis and wall thickness (p = 0.002 and p = 0.004 in T2- and T1-weighted sequences, respectively). No association between fibrosis and pattern of wall enhancement. |
| Rimola and colleagues20 | 44 intestinal segments from 41 CD patients scheduled for bowel resection | MRI (delayed gadolinium enhancement) | Histopathology (surgical specimens) | Percentage of enhancement gain between 7 seconds and 7 min was significantly associated with severe fibrosis in multivariate analysis (OR 1.4, CI 1.2–2). Homogeneous pattern of enhancement was significantly associated with severe fibrosis in multivariate analysis (OR 475, CI 9.2–6.8 × 105). Good discriminatory ability between severe fibrosis and lower grades of fibrosis with percentage of enhancement gain (AUC of 0.93, CI 0.86–1). With a cutoff of 24%, sensitivity and specificity were 0.94 and 0.89, respectively. |
| Adler and colleagues21 | CD animal model (rats, PG-PS injection) 13 controls 25 chronic inflammation |
Magnetization transfer MRI | Histopathology (surgical specimens) | Magnetization transfer ratio significantly higher in chronic inflammation versus controls (p < 0.001). Magnetization ratio increased over time in the chronic inflammation group. Good correlation between magnetization transfer ratio and tissue collagen (r = 0.74, p = 0.0003). Good correlation between magnetization transfer ratio and fibrosis score (r = 0.61, p = 0.0001). Magnetization ratio had good discriminatory ability for fibrosis (AUC of 0.88 for collagen content and 0.67 for fibrosis score). |
| Pazahr and colleagues22 | 50 intestinal segments from 31 CD patients | Magnetization transfer MRI | Standard MRI findings | Significant difference in MT ratio between fibrotic and unaffected bowel segments (p < 0.0001). AUC of 0.98 for identification of fibrotic segments. |
| Dillman and colleagues23 | CD animal model (rats, TNBS enemas) 10 acute inflammation 10 chronic fibrosis |
Magnetization transfer MRI | Histopathology (surgical specimens) | MT ratio significantly higher in chronic fibrosis versus acute inflammation (p < 0.001). Weak correlation between MT ratio and collagen content (r = 0.35). Ratio between T2-weighted sequence wall signal intensity and MT ratio had good discriminatory power for fibrotic bowel (AUC of 0.97). |
AUC, area under the receiver operating characteristic curve; CD, Crohn’s disease; MRI, magnetic resonance imaging; MT, magnetization transfer; PG-PS, peptidoglycan-polysaccharide; TNBS, trinitrobenzene sulfonic acid.
More recently, Rimola and colleagues analyzed bowel perfusion dynamics with MRI. Delayed gadolinium contrast enhancement due to fibrotic changes has already been described for cholangiocarcinoma and myocardial infarction scars.24,25 It is speculated that in fibrotic structures, because of denser tissue and a reduced number of vessels, diffusion of intravenous contrast into the extravascular space is slower and the gadolinium enhancement peak occurs later that in inflamed tissues; however, this had not been previously studied in the bowel. This group found that both an increase in bowel wall enhancement between early and late phases of gadolinium administration and homogeneous pattern of delayed wall enhancement are associated with histopathological fibrosis score. Due to the subjectivity in classifying patterns of wall enhancement, they propose percentage of enhancement increase between early (70 s) and delayed (7 min) phases to differentiate between minimal and marked fibrotic changes. With a cutoff of >24%, they could diagnose marked fibrosis with a specificity of 0.89 and a sensitivity of 0.94.20 After replication and validation this could be an easy way to implement a marker of fibrotic wall changes.20
Magnetization transfer (MT) MRI is a form of MRI that generates contrast through the interaction between protons in free water and those in large immobile macromolecules, such as collagen. The MT signal is stronger with increasing amounts of macromolecules (including collagen) and should theoretically be able to differentiate highly fibrotic tissues. Adler and colleagues studied this hypothesis on a rat model of CD. Twenty-five Lewis rats were injected subserosally at laparotomy with peptidoglycan-polysaccharide, while 13 controls were injected with albumin. All rats developed an acute (<24 h) intestinal inflammatory reaction, while only test animals went on to develop late-phase (>14 days) bowel fibrosis. In the test group, MT ratio increased over time in serial weekly MRI scans, consistent with progressive bowel fibrosis; there was no significant change in MT ratio over time in the control animals. In the late-phase exams, MT ratios were significantly higher in rats with fibrotic bowel versus controls. MT ratio correlated well with collagen tissue content (r = 0.74, p = 0.0003) and with histological fibrosis grade (r = 0.61, p = 0.0001). MT ratio could detect a collagen content over 500 densitometric units and a fibrosis score ⩾2 with AUC of 0.88 and 0.67, respectively.21 In 2015, Dillman and colleagues corroborated these findings in a different rat model of CD. MT ratio was significantly different between acute inflammation and chronic fibrosis rat groups although, surprisingly, the correlation between MT ratio and bowel collagen content was only weak. They proposed, in addition, that the best discriminator between purely inflammatory strictures and those with fibrous components is the ratio between T2 wall signal intensity (which measures inflammatory activity) and MT ratio (which detects fibrosis), with an area under the ROC curve of 0.97.23 Pazahr and colleagues have tested this technique in CD patients with good results. They found a good discriminatory power of MT ratio for identification of fibrotic segments, although the gold standard used was standard MRI and there was no histopathological correlation.22 Nevertheless, the feasibility of MT imaging of the small bowel in human patients and in a clinical setting was proved. MT sequences are possible in most MRI machines and can be acquired during fast imaging techniques, making them easy to implement in clinical practice.
In the future, we expect that one or several of these methods will be validated and become widespread in stratifying patients according to fibrosis degree, guiding clinical decision between medical and endoscopic/surgical therapies.
Lémann Index and the concept of global bowel damage
CD classification is rapidly evolving, due to the perception that current classification systems give insufficient information on a particular patient’s disease extent, severity and prognosis.26 Most CD classifications of disease severity are primarily symptom-based and are a better measure of disease activity at a point in time than a general measure of a patient’s disease severity, making them an insufficient basis for clinical and therapeutic decisions. The same is true for clinical trials, where there is a shift toward more objective measures of inflammation as outcomes, such as endoscopy, histology, inflammatory biomarkers and imaging exams.27 One novel concept that heavily relies on imaging is the Lémann Index (LI), which proposes measuring a particular individual’s cumulative digestive tract damage at a point in time. It assesses the whole bowel from mouth to anus, per segment, analyzes and grades each stricturing and penetrating lesion according to severity, and includes history of organ resection. LI is meant to be a longitudinal instrument and to measure a particular patient’s cumulative structural damage independently of disease duration, extent or current inflammatory activity.
Its clinical utility was highlighted in a 2016 retrospective study that aimed to follow changes in LI over time, in a group of 363 CD patients followed for 5 years. LI was calculated at two different time points of follow up, at least 3 months apart (median interval, 42 months) based on endoscopic and imaging exams. LI decreased in 17% of patients and remained stable in 35%. In almost 50% of the patients there was an increase in LI during follow up. This group had a significantly greater proportion on perianal and penetrating phenotypes, more frequent surgical interventions (both previous to and during follow up), was more often medicated with steroids and biologics, and had a greater rate of hospital visits and admissions.28 A small 2015 pilot study also investigated the progression of bowel damage as assessed by the LI in a cohort of 30 CD patients starting anti-TNF monotherapy (infliximab or adalimumab) and followed for at least 12 months. Inclusion required clinical remission (defined as Harvey–Bradshaw Index <5) after induction and at 12 months. Based on ROC curve analysis and using a blinded clinician’s judgment as a reference standard, LI cutoff for bowel damage progression was established as a change of >0.3. Mean LI decreased over follow up and the majority of the cohort (83%) did not experience bowel damage progression until the last follow-up point. The minority of patients with LI progression was more likely to require surgery in the following 12 months, demonstrating that changes in this score over time are of prognostic value.29 Further evidence of the LI’s prognostic value comes from a prospective observational study on 142 CD patients followed from diagnosis. Baseline bowel damage (defined as presence of stricture, fistula or abscess at diagnosis) and baseline LI were independent predictors of surgery (hazard ratio of 3.21 and 1.11, respectively) and hospitalizations (hazard ratios of 1.88 and 1.08, respectively) during follow up.30 A more recent study compared LI progression over time according to the type of therapy. A total of 104 CD patients treated with either anti-TNF, azathioprine or mesalazine monotherapy were enrolled at diagnosis. The LI was calculated at two time points of clinical and biochemical remission (Harvey–Bradshaw Index <5 and C-reactive protein <7 mg/L). Median LI remained stable in the anti-TNF group but significantly increased in the azathioprine and mesalazine groups, even when adjusted for disease duration. The proportion of patients that experienced LI increase was significantly higher in the azathioprine and mesalazine groups.31 In a smaller prospective cohort of 41 CD patients in clinical remission (defined as a CD Activity Index <220), the authors did not a find a significant change in LI, despite variability in current anti-inflammatory therapy, blood inflammatory biomarkers and Lewis score. However, the follow-up period was only 1 year – it would be relevant to try to replicate these findings in a larger cohort, with subgroup analysis and a lengthier follow up.32
It appears that despite therapy and symptom-based clinical remission, structural bowel damage may still accumulate as disease progresses, and this is translated as an increase in LI over time. Anti-TNF treated patients have the lowest risk of progression compared with other therapies and some of the structural damage can be reversed. Furthermore, baseline LI and its progression over time can predict a more severe disease course.
The major drawbacks of the LI derive from its complexity. It requires multiple examinations for complete structural evaluation (upper and lower endoscopy and cross-sectional abdominal and pelvic imaging) and is time-consuming. Nevertheless, it aggregates important information on disease severity and prognosis and may soon gain a place in clinical practice and as a clinical trial outcome.
Technical advances
Oral contrast ultrasound
The overall role of US in small bowel CD assessment may expand through the introduction of oral contrast. Small intestine contrast ultrasonography (SICUS) expands on traditional US by extending bowel loops through the ingestion of a contrast solution. It has consistently shown excellent diagnostic accuracy for the assessment of CD lesions,33,34 disease extension and location,33–36 transmural complications37,38 and post-operative recurrence,39–41 using either small bowel enteroclysis, CT/MRI enterography, endoscopy or intraoperative findings as a reference standard. When directly compared to standard US, it improves detection of jejunal and ileal disease, strictures, fistulae and abscesses, and has better correlation with other imaging techniques.37 The diagnostic capabilities of SICUS have been extensively reviewed in a recent consensus by Calabrese and colleagues.42 Even though this technique has been studied and used for over a decade and despite the excellent results, it has not gained much traction outside of a few centers. Some limitations include the longer examination time and interobserver variability. Nevertheless, when considering some of the drawbacks of other cross-sectional techniques (namely ionizing radiation for CT and the cost, duration and availability of MRI), there is a rationale for the expansion of SICUS’s role in certain centers and populations.
Low-dose CT
Radiation dose is a particular concern in CD patients because they are often diagnosed at a young age and usually require multiple imaging studies throughout the disease course to assess relapses and monitor response to therapy. While there are non-ionizing imaging alternatives, such as US and MRI, CT is frequently the most readily available option, especially in the emergency setting. Decreasing X-ray tube voltage is the simplest method for decreasing CD radiation dose. However, this has the potential to increase image noise and reduce image quality, especially in patients with higher body mass index and in the abdominal region. One possible solution is automatic exposure control, in which voltage reduction can be achieved automatically, according to patient body shape and on multiple spatial planes. This method has been shown to reduce mean radiation exposure by 15% in the abdomen, with no deterioration of image quality.43 In addition, most CT machine vendors offer novel iterative reconstruction methods that permit radiation dose reduction with no decline in image resolution. These have already been tested for CT enterography with good image quality and diagnostic results when compared with standard reconstruction methods.43,44 Radiation dose reduction in standard abdominal CT is also very relevant for the CD patient population, as they represent a much greater proportion of their cumulative radiation dose than CT enterography,45 probably as a result of emergent examinations.
Diffusion-weighted imaging MRI
Diffusion-weighted imaging (DWI) is a specialized MRI technique that creates contrast through the motion of water and other small molecules within tissue, mostly used in the nervous system and in oncology. One of the advantages of DWI over MRI enterography is the possibility to avoid intravenous contrast. Gadolinium contrast, while generally safer than CT contrast in terms of anaphylactoid reactions, still carries a small risk of nephrotoxicity and of nephrogenic systemic fibrosis. In addition, deposition of gadolinium in the deep nuclei of the brain after multiple contrast-enhanced MRI exams has been described, although its adverse effects remain unknown.46 Another useful gain is the use of the apparent diffusion coefficient (ADC) as a quantitative inflammation score for a particular small bowel region. Evidence is accumulating on the utility of DWI in detecting active inflammation (Table 3). In general, it seems to be more sensitive but less specific than standard MRI enterography.47,48 Kim and colleagues have tested the usefulness of adding DWI sequences to conventional MRI enterography, using ileocolonoscopy as a reference standard. A total of 44 CD patients underwent MRI with DWI sequence acquisition and ileocolonoscopy less than one week apart. MRI and DWI images were independently analyzed by two readers, on two separate occasions at least 1 month apart to prevent recall bias. Active disease was considered present if either one of the techniques was regarded as positive by the readers. The addition of DWI images increased sensitivity (83% versus 62%), but at the cost of decreased specificity (60% versus 94%) compared to MRI alone. Most of the added sensitivity resulted from patients with mild inflammatory lesions (aphtae, erythema or edema) of the colorectum, which would be readily apparent in colonoscopy, casting doubt on the added benefit of DWI.47 In contrast, a 2016 non-inferiority study comparing DWI without intravenous contrast to standard gadolinium-enhanced MRI enterography obtained excellent results, with agreement between DWI and MRI in 92% of the bowel segments evaluated. Using ileocolonoscopy as a reference standard, there was no significant difference in sensitivity and specificity for the diagnosis of terminal ileum inflammation between the two imaging methods.49 A French group has proposed a DWI score based on linear regression and using the MaRIA score as the dependent variable.50 This DWI-MaRIA or Clermont score has been externally validated in a cohort of 130 CD patients (848 bowel segments) and showed excellent correlation with the MaRIA score for ileal disease.51 In this same cohort, an ADC cutoff of 1.9 × 10−3 mm2/s could diagnose active ileal disease with a sensitivity and specificity of 86% and 82%, respectively. A direct comparison between the MaRIA and Clermont scores showed good accuracy for both, with better operational characteristics for the MaRIA score in the diagnosis of disease activity (AUC of 0.92 versus 0.84) as well as the detection of mucosal ulcerations (AUC of 0.90 versus 0.6).52
Table 3.
Assessing Crohn’s disease activity with diffusion-weighted magnetic resonance imaging.
| Author | Population | Reference standard | Results |
|---|---|---|---|
| Klang and colleagues53 | 52 CD patients | MRI enterography Video capsule enterography |
Good discriminatory power of ADC for mucosal ulceration in patients with elevated fecal calprotectin (AUC = 0.819 or 0.832, according to reader). |
| Pendsé and colleagues48 | 98 CD patients 69 with MRE 29 with endoscopy |
MRI enterography Fecal calprotectin Histopathology (endoscopic biopsies) |
Significant difference in fecal calprotectin and in MEGS between patients with normal and abnormal DWI signal (qualitative evaluation) (p < 0.0001). DWI on its own has poor specificity (54%) for bowel inflammation. Quantitative DWI not significantly different between active and inactive disease (reference standard MEGS or histopathology). |
| Stanescu-Siegmund and colleagues54 | 96 CD patients (208 bowel segments) 42 controls |
MRI enterography/enteroclysis | Significant lower ADC values in inflammation compared with normal bowel wall (p < 0.001). Good discriminatory power of ADC for inflamed bowel (AUC = 0.998). |
| Kopylov and colleagues55 | 78 CD patients in remission | Video capsule enterography | Moderate correlation between Clermont score and Lewis score (r = 0.53, p = 0.001). Good discriminatory ability of Clermont score for detection of moderate-to-severe inflammation (AUC = 0.91, p = 0.0001). |
| Seo and colleagues49 | 44 CD patients (171 bowel segments) | MRI enterography Ileocolonoscopy |
Excellent agreement between DWI and CE-MRI enterography (91.8%) and between DWI and ileoscopy (95%) for the identification of bowel inflammation. Good correlation between DWI and CE-MRI enterography (0.937, p < 0.001) and ileoscopy (0.860, p < 0.001). DWI and CE-MRI enterography did not differ significantly regarding the sensitivity and specificity for the diagnosis of terminal ileal inflammation (p > 0.999). |
| Buisson and colleagues56 | 44 CD patients (194 bowel segments, of which 36 were ileal) | Ileocolonoscopy | Moderate inverse correlation of segmental ADC with endoscopic indices of activity in the ileum (r = −0.56 for CDEIS, r = −0.55 for SES-CD). ADC values significantly lower in areas of deep ulceration (p = 0.001) and could discriminate them with an AUC of 0.84. |
| Kim and colleagues47 | 44 CD patients (58 bowel segments) | Ileocolonoscopy | MRE + DWI increased sensitivity (83% versus 62%) but decreased specificity (60% versus 94%) compared to MRE alone. |
| Hordonneau and colleagues51 | 130 CD patients (848 bowel segments) | MRI enterography (MaRIA score) | Good sensitivity (86%) and specificity (82%) of ADC cutoff 1.9 × 10−3 mm2/s for differentiating active disease. Excellent correlation between MaRIA score and Clermont score (r = 0.99) in distal ileum. |
| Tielbeek and colleagues57 | 20 CD patients scheduled for bowel resection (50 bowel segments) | Histopathology (surgical specimens) | No significant correlation between ADC and histological inflammatory score. Significantly lower ADC in fibrotic segments (p = 0.023). |
| Buisson and colleagues50 | 31 CD patients | MRI enterography (MaRIA score) | Strong inverse correlation between ADC and MaRIA score (r = −0.77, p = 0.0001). Good discriminatory power of ADC for active disease (AUC 0.96). Good interobserver agreement (k = 0.69). |
ADC, abnormal diffusion coefficient; AUC, area under the receiver operating characteristic curve; CD, Crohn’s disease; CE-MRI, contrast-enhanced magnetic resonance imaging; DWI, diffusion-weighted imaging; MaRIA, magnetic resonance index of activity; MEGS, magnetic resonance enterography global score; MRE, magnetic resonance imaging.
At present, the role of DWI in CD imaging is not completely established. Nonetheless, it presents a valuable option when there is a relative contraindication for gadolinium contrast, such as in moderate-to-severe kidney disease. Figures 1 and 2 show DWI and standard MRI images from two different CD patients.
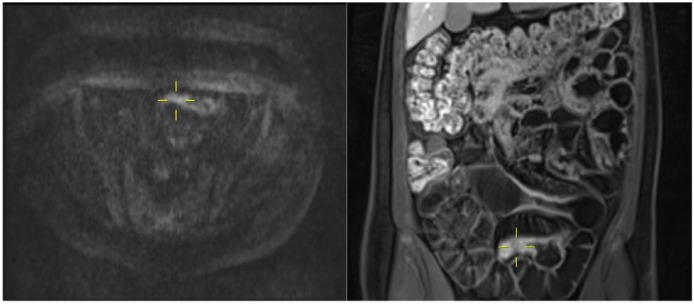
Figure 1.
MR enterography images of a terminal ileitis in a 22-year-old man with CD. Short segment of terminal ileum (*) shows thickening on coronal (a) and axial (c) T2-weighted images, slight hyper-enhancement on coronal T1-weighted image (b) and diffusion restriction in axial DWI (b value = 800) (d). Please note the evident absence of restriction of the other bowel loops.
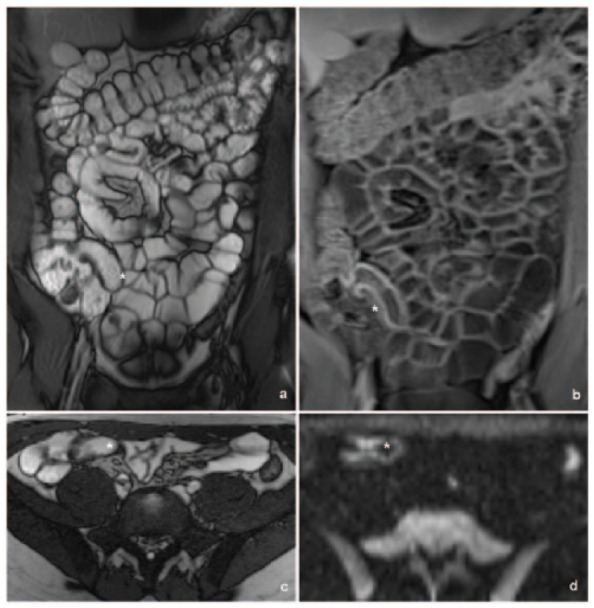
Figure 2.
Axial DWI and coronal contrast-enhanced MR enterography images demonstrate a concordant interpretation of small bowel inflammation in a 19-year-old man with CD. A pelvic ileal segment (yellow cross) shows moderate diffusion restriction and concordantly shows mural thickening and hyper-enhancement on the T1-weighted image.
Conclusion
CD is intrinsically dependent on imaging tools because of the preponderance of disease activity in the small bowel, which is currently difficult to assess endoscopically. On the one hand, endoscopic examination of the small bowel is invasive and time-consuming, in the case of enteroscopy, or lacks motion control and targeting of lesions, in the case of capsule enteroscopy. On the other hand, endoscopic methods are insufficient in CD as they are incapable of completely evaluating transmural disease and complications.
US, CT and MRI have good diagnostic accuracy in most situations, but some limitations persist. Perhaps the most relevant of all is the distinction between inflammatory and fibrotic strictures. Currently there are no validated imaging tools for this differentiation but several are under active investigation, namely US elastography, CEUS and MRI. We expect that one or several will gather increasing evidence and become widespread in clinical practice.
CT and MRI have excellent diagnostic accuracy but concerns about adverse effects may limit their use, especially in patients with the need for repeated examinations. Improvements in CT acquisition and reconstruction techniques permit a reduction in ionizing dose per exam and are particularly relevant in this population. Gastroenterologists should be aware of the radiation doses administered in their centers and radiology departments should strive to implement these dose-reduction methods whenever feasible. Similarly, concerns about gadolinium contrast side effects exist and in this regard advances in DWI are important. While DWI has not shown superiority to standard MRI, at least one study reports its non-inferiority, making it a useful alternative when there is a risk with contrasted exams. Also in this respect, the excellent results of oral contrast US are promising as it may reduce the need for CT and MRI in some instances. It remains to be tested outside of a few centers and it would be interesting to discover whether the already-published results are reproducible.
CD classification systems are rapidly evolving to incorporate more objective markers such as endoscopic findings, biomarkers and imaging results. The LI is a novel scoring system for cumulative bowel damage that relies in part on cross-sectional imaging. It is burdensome and time-consuming but incorporates important prognostic information. There is currently a need for further studies that validate its place in clinical practice.
Acknowledgments
We would like to thank Dr. Rui Cunha for the magnetic resonance imaging figures in this article.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Fernando Magro  https://orcid.org/0000-0003-2634-9668
https://orcid.org/0000-0003-2634-9668
Contributor Information
Inês Pita, Department of Gastroenterology, Portuguese Institute of Oncology, Porto, Portugal.
Fernando Magro, Department of Pharmacology and Therapeutics, Faculty of Medicine, University of Porto, Alameda Prof. Hernâni Monteiro, 4200-319 Porto, Portugal MedInUP, Centre for Drug Discovery and Innovative Medicines, University of Porto, Porto, Portugal Gastroenterology Department, Centro Hospitalar São João, Porto, Portugal.
References
- 1. Gomollon F, Dignass A, Annese V, et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: part 1. Diagnosis and medical management. J Crohns Colitis 2017; 11: 3–25. [DOI] [PubMed] [Google Scholar]
- 2. Horsthuis K, Bipat S, Bennink RJ, et al. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology 2008; 247: 64–79. [DOI] [PubMed] [Google Scholar]
- 3. Panes J, Bouzas R, Chaparro M, et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther 2011; 34: 125–145. [DOI] [PubMed] [Google Scholar]
- 4. Minordi LM, Vecchioli A, Mirk P, et al. CT enterography with polyethylene glycol solution vs CT enteroclysis in small bowel disease. Br J Radiol 2011; 84: 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Negaard A, Paulsen V, Sandvik L, et al. A prospective randomized comparison between two MRI studies of the small bowel in Crohn’s disease, the oral contrast method and MR enteroclysis. Eur Radiol 2007; 17: 2294–2301. [DOI] [PubMed] [Google Scholar]
- 6. Giannetti A, Matergi M, Biscontri M, et al. Real-time elastography in Crohn’s disease: feasibility in daily clinical practice. J Ultrasound 2017; 20: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim K, Johnson LA, Jia C, et al. Noninvasive ultrasound elasticity imaging (UEI) of Crohn’s disease: animal model. Ultrasound Med Biol 2008; 34: 902–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stidham RW, Xu J, Johnson LA, et al. Ultrasound elasticity imaging for detecting intestinal fibrosis and inflammation in rats and humans with Crohn’s disease. Gastroenterology 2011; 141: 819–826.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu J, Tripathy S, Rubin JM, et al. A new nonlinear parameter in the developed strain-to-applied strain of the soft tissues and its application in ultrasound elasticity imaging. Ultrasound Med Biol 2012; 38: 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baumgart DC, Muller HP, Grittner U, et al. US-based real-time elastography for the detection of fibrotic gut tissue in patients with stricturing Crohn disease. Radiology 2015; 275: 889–899. [DOI] [PubMed] [Google Scholar]
- 11. Fraquelli M, Branchi F, Cribiu FM, et al. The role of ultrasound elasticity imaging in predicting ileal fibrosis in Crohn’s disease patients. Inflamm Bowel Dis 2015; 21: 2605–2612. [DOI] [PubMed] [Google Scholar]
- 12. Dillman JR, Stidham RW, Higgins PD, et al. US elastography-derived shear wave velocity helps distinguish acutely inflamed from fibrotic bowel in a Crohn disease animal model. Radiology 2013; 267: 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dillman JR, Stidham RW, Higgins PD, et al. Ultrasound shear wave elastography helps discriminate low-grade from high-grade bowel wall fibrosis in ex vivo human intestinal specimens. J Ultrasound Med 2014; 33: 2115–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu C, Gui X, Chen W, et al. Ultrasound shear wave elastography and contrast enhancement: effective biomarkers in Crohn’s disease strictures. Inflamm Bowel Dis 2017; 23: 421–430. [DOI] [PubMed] [Google Scholar]
- 15. Quaia E, De Paoli L, Stocca T, et al. The value of small bowel wall contrast enhancement after sulfur hexafluoride-filled microbubble injection to differentiate inflammatory from fibrotic strictures in patients with Crohn’s disease. Ultrasound Med Biol 2012; 38: 1324–1332. [DOI] [PubMed] [Google Scholar]
- 16. Nylund K, Jirik R, Mezl M, et al. Quantitative contrast-enhanced ultrasound comparison between inflammatory and fibrotic lesions in patients with Crohn’s disease. Ultrasound Med Biol 2013; 39: 1197–1206. [DOI] [PubMed] [Google Scholar]
- 17. Ripolles T, Rausell N, Paredes JM, et al. Effectiveness of contrast-enhanced ultrasound for characterisation of intestinal inflammation in Crohn’s disease: a comparison with surgical histopathology analysis. J Crohns Colitis 2013; 7: 120–128. [DOI] [PubMed] [Google Scholar]
- 18. Punwani S, Rodriguez-Justo M, Bainbridge A, et al. Mural inflammation in Crohn disease: location-matched histologic validation of MR imaging features. Radiology 2009; 252: 712–720. [DOI] [PubMed] [Google Scholar]
- 19. Zappa M, Stefanescu C, Cazals-Hatem D, et al. Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn’s disease? A retrospective comparison with surgical pathologic analysis. Inflamm Bowel Dis 2011; 17: 984–993. [DOI] [PubMed] [Google Scholar]
- 20. Rimola J, Planell N, Rodriguez S, et al. Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol 2015; 110: 432–440. [DOI] [PubMed] [Google Scholar]
- 21. Adler J, Swanson SD, Schmiedlin-Ren P, et al. Magnetization transfer helps detect intestinal fibrosis in an animal model of Crohn disease. Radiology 2011; 259: 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pazahr S, Blume I, Frei P, et al. Magnetization transfer for the assessment of bowel fibrosis in patients with Crohn’s disease: initial experience. MAGMA 2013; 26: 291–301. [DOI] [PubMed] [Google Scholar]
- 23. Dillman JR, Swanson SD, Johnson LA, et al. Comparison of noncontrast MRI magnetization transfer and T2-weighted signal intensity ratios for detection of bowel wall fibrosis in a Crohn’s disease animal model. J Magn Reson Imaging 2015; 42: 801–810. [DOI] [PubMed] [Google Scholar]
- 24. Ordovas KG, Higgins CB. Delayed contrast enhancement on MR images of myocardium: past, present, future. Radiology 2011; 261: 358–374. [DOI] [PubMed] [Google Scholar]
- 25. Rimola J, Forner A, Reig M, et al. Cholangiocarcinoma in cirrhosis: absence of contrast washout in delayed phases by magnetic resonance imaging avoids misdiagnosis of hepatocellular carcinoma. Hepatology 2009; 50: 791–798. [DOI] [PubMed] [Google Scholar]
- 26. Peyrin-Biroulet L, Panes J, Sandborn WJ, et al. Defining disease severity in inflammatory bowel diseases: current and future directions. Clin Gastroenterol Hepatol 2016; 14: 348–354.e17. [DOI] [PubMed] [Google Scholar]
- 27. Ma C, Panaccione R, Fedorak RN, et al. Development of a core outcome set for clinical trials in inflammatory bowel disease: study protocol for a systematic review of the literature and identification of a core outcome set using a Delphi survey. BMJ Open 2017; 7: e016146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bhagya Rao B, Koutroubakis IE, Ramos Rivers C, et al. Delineation of Crohn’s disease trajectories using change in Lemann Index: a natural history study. J Clin Gastroenterol 2016; 50: 476–482. [DOI] [PubMed] [Google Scholar]
- 29. Fiorino G, Bonifacio C, Allocca M, et al. Bowel damage as assessed by the Lemann Index is reversible on anti-TNF therapy for Crohn’s disease. J Crohns Colitis 2015; 9: 633–639. [DOI] [PubMed] [Google Scholar]
- 30. Fiorino G, Morin M, Bonovas S, et al. Prevalence of bowel damage assessed by cross-sectional imaging in early Crohn’s disease and its impact on disease outcome. J Crohns Colitis 2017; 11: 274–280. [DOI] [PubMed] [Google Scholar]
- 31. Bodini G, Giannini EG, De Maria C, et al. Anti-TNF therapy is able to stabilize bowel damage progression in patients with Crohn’s disease: a study performed using the Lemann Index. Dig Liver Dis 2017; 49: 175–180. [DOI] [PubMed] [Google Scholar]
- 32. Amitai MM, Zarchin M, Lahat A, et al. Structural bowel damage in quiescent Crohn’s disease. Dig Liver Dis 2017; 49: 490–494. [DOI] [PubMed] [Google Scholar]
- 33. Calabrese E, La Seta F, Buccellato A, et al. Crohn’s disease: a comparative prospective study of transabdominal ultrasonography, small intestine contrast ultrasonography, and small bowel enema. Inflamm Bowel Dis 2005; 11: 139–145. [DOI] [PubMed] [Google Scholar]
- 34. Pallotta N, Civitelli F, Di Nardo G, et al. Small intestine contrast ultrasonography in pediatric Crohn’s disease. J Pediatr 2013; 163: 778–784.e1. [DOI] [PubMed] [Google Scholar]
- 35. Pallotta N, Tomei E, Viscido A, et al. Small intestine contrast ultrasonography: an alternative to radiology in the assessment of small bowel disease. Inflamm Bowel Dis 2005; 11: 146–153. [DOI] [PubMed] [Google Scholar]
- 36. Parente F, Greco S, Molteni M, et al. Oral contrast enhanced bowel ultrasonography in the assessment of small intestine Crohn’s disease: a prospective comparison with conventional ultrasound, x ray studies, and ileocolonoscopy. Gut 2004; 53: 1652–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pallotta N, Vincoli G, Montesani C, et al. Small intestine contrast ultrasonography (SICUS) for the detection of small bowel complications in Crohn’s disease: a prospective comparative study versus intraoperative findings. Inflamm Bowel Dis 2012; 18: 74–84. [DOI] [PubMed] [Google Scholar]
- 38. Onali S, Calabrese E, Petruzziello C, et al. Small intestine contrast ultrasonography vs computed tomography enteroclysis for assessing ileal Crohn’s disease. World J Gastroenterol 2012; 18: 6088–6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Biancone L, Calabrese E, Petruzziello C, et al. Wireless capsule endoscopy and small intestine contrast ultrasonography in recurrence of Crohn’s disease. Inflamm Bowel Dis 2007; 13: 1256–1265. [DOI] [PubMed] [Google Scholar]
- 40. Castiglione F, Bucci L, Pesce G, et al. Oral contrast-enhanced sonography for the diagnosis and grading of postsurgical recurrence of Crohn’s disease. Inflamm Bowel Dis 2008; 14: 1240–1245. [DOI] [PubMed] [Google Scholar]
- 41. Pallotta N, Giovannone M, Pezzotti P, et al. Ultrasonographic detection and assessment of the severity of Crohn’s disease recurrence after ileal resection. BMC Gastroenterol 2010; 10: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Calabrese E, Maaser C, Zorzi F, et al. Bowel ultrasonography in the management of Crohn’s disease: a review with recommendations of an international panel of experts. Inflamm Bowel Dis 2016; 22: 1168–1183. [DOI] [PubMed] [Google Scholar]
- 43. Greess H, Wolf H, Baum U, et al. Dose reduction in computed tomography by attenuation-based on-line modulation of tube current: evaluation of six anatomical regions. Eur Radiol 2000; 10: 391–394. [DOI] [PubMed] [Google Scholar]
- 44. Fletcher JG, Hara AK, Fidler JL, et al. Observer performance for adaptive, image-based denoising and filtered back projection compared to scanner-based iterative reconstruction for lower dose CT enterography. Abdom Imaging 2015; 40: 1050–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Magro F, Coelho R, Guimaraes LS, et al. Ionizing radiation exposure is still increasing in Crohn’s disease: who should be blamed? Scand J Gastroenterol 2015; 50: 1214–1225. [DOI] [PubMed] [Google Scholar]
- 46. Gulani V, Calamante F, Shellock FG, et al. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol 2017; 16: 564–570. [DOI] [PubMed] [Google Scholar]
- 47. Kim KJ, Lee Y, Park SH, et al. Diffusion-weighted MR enterography for evaluating Crohn’s disease: how does it add diagnostically to conventional MR enterography? Inflamm Bowel Dis 2015; 21: 101–109. [DOI] [PubMed] [Google Scholar]
- 48. Pendse DA, Makanyanga JC, Plumb AA, et al. Diffusion-weighted imaging for evaluating inflammatory activity in Crohn’s disease: comparison with histopathology, conventional MRI activity scores, and faecal calprotectin. Abdom Radiol 2017; 42: 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Seo N, Park SH, Kim KJ, et al. MR enterography for the evaluation of small-bowel inflammation in Crohn disease by using diffusion-weighted imaging without intravenous contrast material: a prospective noninferiority study. Radiology 2016; 278: 762–772. [DOI] [PubMed] [Google Scholar]
- 50. Buisson A, Joubert A, Montoriol PF, et al. Diffusion-weighted magnetic resonance imaging for detecting and assessing ileal inflammation in Crohn’s disease. Aliment Pharmacol Ther 2013; 37: 537–545. [DOI] [PubMed] [Google Scholar]
- 51. Hordonneau C, Buisson A, Scanzi J, et al. Diffusion-weighted magnetic resonance imaging in ileocolonic Crohn’s disease: validation of quantitative index of activity. Am J Gastroenterol 2014; 109: 89–98. [DOI] [PubMed] [Google Scholar]
- 52. Rimola J, Alvarez-Cofino A, Perez-Jeldres T, et al. Comparison of three magnetic resonance enterography indices for grading activity in Crohn’s disease. J Gastroenterol 2017; 52: 585–593. [DOI] [PubMed] [Google Scholar]
- 53. Klang E, Kopylov U, Eliakim R, et al. Diffusion-weighted imaging in quiescent Crohn’s disease: correlation with inflammatory biomarkers and video capsule endoscopy. Clin Radiol 2017; 72: 798.e7–798.e13. [DOI] [PubMed] [Google Scholar]
- 54. Stanescu-Siegmund N, Nimsch Y, Wunderlich AP, et al. Quantification of inflammatory activity in patients with Crohn’s disease using diffusion weighted imaging (DWI) in MR enteroclysis and MR enterography. Acta Radiol 2017; 58: 264–271. [DOI] [PubMed] [Google Scholar]
- 55. Kopylov U, Klang E, Yablecovitch D, et al. Magnetic resonance enterography versus capsule endoscopy activity indices for quantification of small bowel inflammation in Crohn’s disease. Therap Adv Gastroenterol 2016; 9: 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Buisson A, Hordonneau C, Goutte M, et al. Diffusion-weighted magnetic resonance imaging is effective to detect ileocolonic ulcerations in Crohn’s disease. Aliment Pharmacol Ther 2015; 42: 452–460. [DOI] [PubMed] [Google Scholar]
- 57. Tielbeek JA, Ziech ML, Li Z, et al. Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn’s disease assessment with histopathology of surgical specimens. Eur Radiol 2014; 24: 619–629. [DOI] [PubMed] [Google Scholar]