Short abstract
Current strategies to delineate the risk of serious drug-induced liver injury associated with drugs rely on assessment of serum biomarkers that have been utilized for many decades. In particular, serum alanine aminotransferase and total bilirubin levels are typically used to assess hepatic integrity and function, respectively. Parallel measurement of these biomarkers is utilized to identify patients with drug-induced hepatocellular jaundice (“Hy’s Law” cases) which carries at least a 10% risk of death or liver transplant. However, current guidelines regarding use of these biomarkers in clinical trials can put study subjects at risk for life-threatening drug-induced liver injury, or result in over estimation of risk that may halt development of safe drugs. In addition, pharmaceutical companies are increasingly being required to conduct large and expensive clinical trials to “defend” the safety of their new drug when results from smaller trials are inconclusive. Innovative approaches and some novel biomarkers are now being employed to maximize the value of traditional biochemical tests. DILIsym®, a product of the DILIsim Initiative, utilizes serial serum alanine aminotransferase values, along with serum biomarkers of apoptosis vs necrosis, to estimate percent hepatocyte loss and total bilirubin elevations resulting from loss of global liver function. The results from analyses conducted with DILIsym have been reported to the FDA to support the safety of entolimod and cimaglermin alfa after elevations in serum alanine aminotransferase and/or bilirubin halted clinical development. DILIsym can also be utilized to determine whether rises in serum conjugated and unconjugated bilirubin are consistent with mechanisms unrelated to toxicity (i.e. inhibition of bilirubin transport or metabolism). In silico modeling of traditional and novel drug-induced liver injury biomarker data obtained in clinical trials may be the most efficient and accurate way to define the liver safety profile of new drug candidates.
Impact statement
Blood tests used in clinical trials to detect and monitor drug-induced liver injury (DILI) have not changed in half a century. These tests have several shortcomings: their use has not completely prevented clinical trial participants from risk of life-threatening DILI, they can give false positive results that halt the development of safe drug candidates, and they can create liver safety “concerns” that require large additional clinical trials to accurately define DILI risk. This review highlights the use of in silico modeling to improve interpretation of the blood tests currently available to detect DILI risk in new drug candidates. This approach is increasingly being applied in clinical trials to more precisely assess the degree of hepatocellular injury and its functional impact. This new approach holds the promise of more accurately defining DILI risk in smaller clinical trials.
Keywords: Biomarkers, DILIsym, drug-induced liver injury, evaluation of drug-induced severe hepatoxicity, hepatoxicity, Hy’s Law
DILI remains a major adverse event limiting drug development. Detection of liver safety issues in a clinical trial can lead to terminating development of otherwise promising drugs, or to regulatory requirements for larger and longer clinical trials to more accurately assess liver safety. If approved, new drugs with liver safety concerns may be restricted for use in only specific patient subpopulations, may be accompanied by black box label warnings, and may require frequent blood test monitoring, all of which potentially reduce the value of the drug to public health. Despite the clear need for innovative solutions to address this problem, detection and monitoring of liver safety currently rely primarily on the same serum biomarkers that have been used for over half a century: serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and total bilirubin (TBIL). These biochemical tests aid in prediction of liver pathology when concurrent biopsy data are not available. Calculation of an “R value” [ALT value divided by ALP value when both are expressed as fold upper limit of normal (ULN)] provides evidence for a predominance of hepatocellular, mixed, or cholestatic injury. A case is considered hepatocellular when R ≥ 5, cholestatic when R ≤ 2 and mixed when 2 < R < 5.
The major concern for drug developers and regulators is hepatocellular injury (R ≥ 5). This is partly because with cholestatic or mixed injuries, the treated subject develops jaundice early during injury and becomes their own “color indicator” of the event; therefore, routine monitoring of liver blood tests is less important for early injury detection. More importantly, in cholestatic or mixed injuries, jaundice occurs before there is global and potentially life-threatening loss of liver function. When jaundice occurs during hepatocellular injury, potentially life-threatening liver injury has already occurred. Hepatocellular injury due to drugs will be the focus of this review.
The most sensitive and specific of the traditional biomarkers to detect hepatocellular injury is ALT which is present at high concentrations inside the cytoplasm of hepatocytes. AST is also present in hepatocytes, but is less liver-specific and will not be further discussed in this review. The normal slow turnover of hepatocytes (i.e. death and replacement) leads to release of ALT into circulation and this largely accounts for the range of serum levels easily detected in healthy people. A rise in serum ALT, particularly to high multiples of the ULN, is interpreted as an increase in the death of hepatocytes as occurs during hepatocellular DILI. Nonetheless, ALT has shortcomings that can complicate interpretation in the clinic. Although ALT is considered liver-specific when very large elevations are observed in circulation, ALT is also present in muscles. Muscle injury can result in the release of this enzyme into the blood; elevated levels of serum ALT are observed following strenuous exercise, grand mal seizures, or limb crush injuries, but in these cases, concomitant rises in serum creatinine phosphokinase (CPK), a muscle-specific enzyme, are helpful to identify the organ of origin.1,2 However, in treatment trials of hereditary muscle diseases such as Duchenne’s muscular dystrophy, interpretation of serum CPK levels is confounded.
Furthermore, drugs can cause serum ALT elevations, sometimes even high elevations, that do not lead to liver impairment and resolve with continued treatment on drug. These instances of “benign” ALT elevations, or “transaminitis,” are well-documented in the literature and are believed to result from hepatocyte injury or death that ceases despite continued drug exposure due to “adaptation.”3 For instance, significant serum ALT elevations were frequently noted in patients receiving tacrine in a large clinical trial in Alzheimer’s disease patients.4 Asymptomatic ALT alterations were observed in 49% of patients, with elevations exceeding 20× ULN in 40 patients (2%). Yet, some patients who experienced tacrine-related elevations in serum ALT > 20× ULN were able to continue uninterrupted treatment with the drug with complete resolution of the biochemical abnormalities.5 Moreover, life-threatening liver injuries have not been reported among patients receiving treatment with tacrine either in the preapproval clinical trials or after the drug was marketed.6
Similarly, heparins and cholestyramine are also well-known to cause relatively frequent ALT elevations, but these drugs are not associated with a serious DILI liability.7,8 Finally, ALT elevations are surprisingly frequent in healthy volunteers receiving recurrent therapeutic doses of acetaminophen (APAP).9 Although overdose of APAP is the most common cause of drug-induced liver failure, clinically important liver injury among patients taking this drug as directed must be extraordinarily rare.
The above examples illustrate that drugs can cause frequent and even high elevations in serum ALT, yet have no or low potential for causing serious liver injury when taken correctly.
The FDA approach to assess liver safety in clinical trials
Recognizing the phenomenon that drugs can cause transient ALT elevations that do not indicate potential for severe liver injury, the Food and Drug Administration (FDA) now recommends continuing treatment with a new drug candidate when a clinical trial patient experiences a moderate rise in serum ALT, even if the event is clearly related to the study drug. The most current regulatory guidelines recommend that subjects with ALT elevations < 8× ULN remain on study drug with frequent monitoring for symptoms of liver injury and/or a rise in serum TBIL indicating functional impairment to the liver.10 The prognostic importance of a rise in serum TBIL in a patient with DILI was first appreciated by Dr. Hyman (“Hy”) Zimmerman. He observed that when hepatocellular DILI was accompanied by jaundice, the patient had at least a 10% chance of going on to liver failure.11 Moreover, this observation seemed to apply to virtually any drug capable of causing hepatocellular jaundice. This was an important observation for clinicians because patients with hepatocellular jaundice from viral hepatitis will only very rarely develop liver failure. The FDA guidance document built on Dr. Zimmerman’s observation by defining a “Hy’s Law case” as a patient with hepatocellular DILI simultaneously experiencing rises in serum ALT > 3× ULN and TBIL > 2× ULN. Hepatocellular injury was originally defined as a concomitant serum ALP rise < 2× ULN but now is typically defined by the initial R value (i.e. R ≥ 5).12 Large DILI registries have corroborated Dr. Zimmerman’s prediction, reporting that approximately 10% of DILI patients meeting Hy’s Law Case criteria go on to a liver-related death or require a liver transplant.13–15
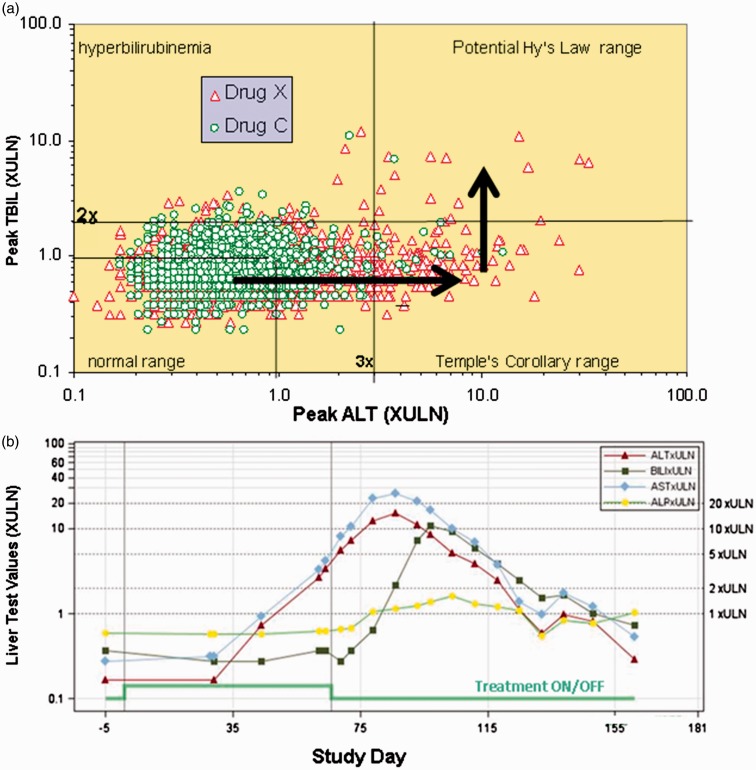
A substantial advancement in assessing liver safety in clinical trials was the development of “eDISH” (evaluation of Drug-Induced Serious Hepatotoxicity) software by John Senior and Ted Guo at the FDA.16 This is based on a graphical log/log display of the peak serum ALT levels and peak serum TBIL levels observed in each subject in a clinical trial. Horizontal and vertical lines are inserted to indicate 3× ULN for ALT and 2× ULN for TBIL (Figure 1(a)).17 The upper right quadrant of the eDISH plot, in which both ALT and TBIL are elevated above these thresholds, identifies potential Hy’s Law cases. The lower right quadrant identifies subjects with ALT elevations >3× ULN and is called the “Temple’s Corollary” quadrant after Bob Temple who noted that all drugs capable of causing Hy’s Law cases also cause more frequent elevations in serum ALT > 3× ULN in the absence of appreciable elevations in serum TBIL. With these drugs, there is characteristically a much larger population of treated patients appearing in the Temple’s Corollary quadrant than in the Hy’s Law quadrant. This is because these isolated events of serum ALT elevation generally resolve due to either discontinuation of study drug, or more often, due to adaptation with continued study drug treatment. Software is now available that permits examination of the eDISH plot in near real-time during a clinical trial. Concern is raised if a clinical trial subject drifts into the Temple’s Corollary quadrant (i.e. has a rise in serum ALT > 3× ULN but not a concurrent rise in serum TBIL > 2× ULN). The subject can be followed to see if they fall back into the left lower quadrant or experience a rise in serum TBIL and therefore drift into the potential Hy’s Law quadrant. In a “true” Hy’s Law case, the rise in serum TBIL should follow the rise in serum ALT (Figure 1(b)). This is because in a hepatocellular injury, the global functional impairment that results in a serum TBIL rise should occur only after fairly massive hepatocyte death (i.e. release of ALT). The presence of a single Hy’s Law case in clinical trials is considered very concerning. The finding of two Hy’s Law cases is considered highly predictive of acute liver failure potential and few development programs outside of oncology can survive this finding.10
Figure 1.
Use of the eDISH software during drug development. The eDISH plot shown (a) displays the peak serum ALT and peak serum TBIL values in multiples of upper limit of normal (XULN) obtained in a Phase 3 clinical trial of drug X (red triangles). Patients treated with the non-hepatotoxic comparator, drug "C," are shown as green circles. It can be observed that drug X treatment is associated with more elevations in serum ALT > 3× ULN relative to comparator. Moreover, there is an imbalance in patients treated with drug X who experience elevations in serum ALT > 3× ULN and serum TBIL > 2× ULN and therefore qualify as potential Hy’s Law cases. Typically, most patients who experience serum ALT elevations have resolution to baseline even with continued treatment on study drug. Current guidelines recommend continued cautious treatment in subjects experiencing serum ALT elevations < 8× ULN to see if they will progress to global liver dysfunction, indicated by a rise in serum TBIL (following the arrows shown). However, such patients may be at risk for liver failure despite discontinuing treatment (see text). By mouse clicking on each dot, information on the corresponding subject can be interrogated, including a graph of liver chemistries as a function of time. The example shown (b) is a true Hy’s Law case from an actual phase 3 clinical trial. As is typical, the serum transaminases rise to very high values (>10× ULN) followed by a rise in serum TBIL. In this case, the treatment with study drug was stopped prior to the rise in serum TBIL but the patient’s liver injury progressed to hepatocellular jaundice and, although this subject recovered, there was at least a 10% chance of liver failure. (A color version of this figure is available in the online journal.) Graphs have not been previously published and were provided courtesy of Dr. John Senior.
Continuing clinical trial subjects who develop moderate serum ALT elevations on study drug treatment may, by distinguishing benign from malignant liver effects, prevent the unnecessary abandonment of safe drugs and facilitate approval of drugs that might not otherwise become commercially available. However, this practice also puts patients at serious risk because, following the current DILI management guidelines, rare patients have progressed to liver failure in clinical trials.18 The reality is that with a Hy’s Law case, the combined use of ALT and TBIL is not a biomarker of the potential for serious liver injury as much as it is an indicator that serious and potentially life-threatening liver injury is already present in that subject.
It should be emphasized that a patient appearing in the Hy’s Law quadrant is only potentially a Hy’s Law case. The peak serum ALT and peak serum TBIL may not have been concomitant, the injury may not have been hepatocellular, and there may be another more likely cause for the biochemical abnormalities (e.g. passage of a gall stone, viral hepatitis, etc.). It is therefore important that, by simply mouse clicking on the points on the eDISH plot, key clinical data from each subject can be interrogated, including a graphic display of serial liver chemistries obtained during the trial, as is illustrated in Figure 1(b).
Although identification of Hy’s Law cases is the current gold standard liver safety signal, they are typically very rare events even during treatment with drugs known to cause acute liver failure. Therefore, when serum ALT elevations are observed in a clinical trial, particularly high or frequent elevations, the FDA may request large additional clinical trials, sometimes solely to assess liver safety. A recent such example is the FDA’s decision regarding the antibiotic solithromycin. The new drug application (NDA) database for this drug did not contain any Hy’s Law cases, but serum ALT elevations > 3× ULN were more frequent during treatment with solithromcyin than with a comparator (moxifloxacin). There was also concern regarding the structural similarity of solithromycin to another antibiotic, telithromycin, known to have a serious liver safety liability.19 The FDA noted that according to the “rule of three,” the available data in the 900 patients treated only excluded the ability of solithromycin to cause a Hy’s Law case in about 1 in 300 treated patients and, assuming a 10% estimate of liver failure among Hy’s Law Cases, excluded a liver failure risk of only 1 in 3000 treated patients.20 The FDA requested an additional clinical trial with 9000 patients prior to considering marketing approval.19
In silico modeling to improve interpretation of DILI biomarkers
Relying solely on peak values of serum ALT and/or TBIL can occasionally lead to erroneous conclusions regarding liver safety. It has recently been shown that mechanistic, mathematical modeling can improve interpretation of traditional biomarkers. One recent example, which was included in correspondence with the FDA, was interpretation of serum ALT elevations caused by Entolimod, a biologic agent shown to reduce mortality from radiation in monkeys. Because large human efficacy trials would require an unthinkable disaster, the monkey data were considered adequate to establish benefit according to the “animal rule.” However, the safety of the drug had to be established in a clinical trial. When this was conducted in healthy volunteers, a small subset of subjects experienced very high serum ALT elevations, including >1000 U/L (∼20× ULN) in one individual.21 However, it was noted that in every subject affected, the serum ALT rose almost immediately to the peak value and then fell at more or less the published half-life of serum ALT (about two days) suggesting that the injury to the liver was of very short duration. A public–private partnership (the DILIsim Initiative, https://www.dilisym.com/) has been developing software (DILIsym®) with the goal of better predicting liver safety liability in new drug candidates. Included in the DILIsym model is the relationship between death of hepatocytes and levels of serum ALT based on published estimates of the mean content of serum ALT per hepatocyte and the mean published half-life of serum ALT. Based on the greatest AUC of serum ALT vs. time curve observed in the Entolimod clinical study, it was estimated that hepatocyte loss was just 3.5% of the total hepatocytes present in the liver.21 Additional modeling based on published hepatocyte regeneration data indicated that this hepatocyte loss would be essentially restored within three weeks.
The modelers went further by incorporating ranges in variables in the model, including the range of published values for the half-life of serum ALT and estimates of hepatocyte content of ALT, creating heterogeneity in over 300 simulated individuals.21 In this simulated population, the hepatocyte loss predicted for a peak serum ALT elevation of 1001–1100 U/L (corresponding to the maximum observed ALT elevation in this study) ranged from 2.6 to 4.9% loss. Regeneration time, also based on variation in published hepatocyte regeneration rates, ranged from two to nine weeks. To put this into context, living adult to adult liver transplants can result in over 50% loss of liver parenchyma in the donor.22 Taken together, modeling with DILIsym provided evidence that the ALT elevations produced by Entolimod, although well above typical protocol-driven criteria for treatment discontinuation, were in fact likely to represent minor liver injury with little heath risk to the study participants.
In continuing the clinical trial of Entolimod, the usual practice would be to set a high but arbitrary peak level of serum ALT (such as 2000 U/L) that would halt the trial. Because the serum ALT vs. time values had a nearly identical pattern in each affected subject, it may be possible to use the mechanistic modeling to set more rational ALT stopping criteria based on a data driven values of hepatocyte loss known to be associated with morbidity.
It is important to note that these modeled estimates of hepatocyte loss based on serum ALT levels are specific to Entolimod; the almost immediate rise to a peak ALT value and the subsequent rapid fall is an unusual DILI presentation. The rate of rise and fall in serum ALT observed in clinical trials during DILI events is usually quite different from what was observed with Entolimod, but is nonetheless often characteristic of the specific investigational drug. This means that modeled estimates of hepatocyte loss vs. peak serum ALT can be estimated for peak values higher than those actually observed in clinical trials.
In silico modeling of Hy’s law cases
Computational modeling can also be useful in certain instances to improve interpretation of subjects who satisfy the current Hy’s Law case criteria.
A recent example of this concerns Cimaglermin alfa, a recombinant version of a naturally occurring glial growth factor 2 that is being developed to treat patients with heart failure. In a phase I clinical trial, two drug-treated patients fulfilled all the criteria for Hy’s Law cases, suspending further development of the compound. However, these biomarker alterations were not typical of Hy’s Law cases (e.g. case shown in Figure 1(b)). In both subjects, the peak serum ALT values observed, although greater than 3× ULN, were relatively modest. In addition, the rise in serum TBIL did not clearly follow the rise in serum ALT but rose in parallel with it. As with Entolimod, DILIsym modeling was utilized to predict the percent hepatocyte loss from the AUC of ALT values over time, with an added sophistication. Total keratin 18 (K18) and caspase cleaved K18 (ccK18) were also measured in the serial serum samples and the “Apoptotic Index” (ratio of ccK18 to K18) was calculated. This calculation suggested that the predominant mode of hepatocyte death was apoptosis rather than necrosis in this study. ALT is partially digested during apoptosis and DILIsym considers this when estimating hepatocyte loss. In this case, the estimated hepatocyte loss ranged from 6.6 to 12.4% for the two potential Hy’s Law cases.23 This modeling was also included in correspondence with the FDA.
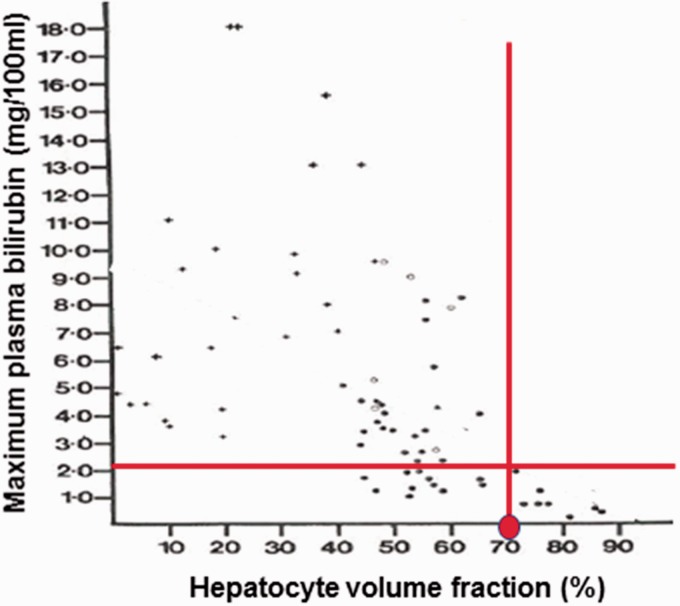
Hepatectomy studies, including observations in autologous liver transplantation in patients, suggest that at least 60% of the liver must be removed before serum bilirubin would rise >2× ULN.22 However, hepatectomy may not be an appropriate model for DILI. This is because during a DILI event, the liver’s functional capacity, including the uptake, processing, and secretion of bile might be reduced due to drug-induced stress in addition to overt death of hepatocytes. To address this issue, DILIsym modeled data obtained from a liver biopsy study conducted in acetaminophen overdose patients.21,24 In this study, the percent hepatocyte lost was estimated from histologic examination of liver biopsies and correlated with the peak serum TBIL observed in the corresponding patient. As shown in Figure 2, it appears that a rise in serum TBIL during acetaminophen-induced DILI occurs when the percent hepatocyte loss is greater than 30%. With this estimate, the 6.6–12.4% loss of hepatocytes estimated in the potential Hy’s Law cases would not account for the observed rise in serum TBIL.23
Figure 2.
Liver biopsy study in patients experiencing DILI due to acetaminophen overdose. In this study, patients (n = 76) experiencing acetaminophen-induced DILI underwent liver biopsy and the percent of intact hepatocytes was assessed and correlated with the peak serum TBIL values observed in the patients. It can be observed (red lines) that elevations in serum TBIL > 2× ULN (∼2.2 mg/dl) correlated with a loss of approximately 30% of hepatocytes (i.e. 70% remaining). Figure reprinted with approval.24 (A color version of this figure is available in the online journal.)
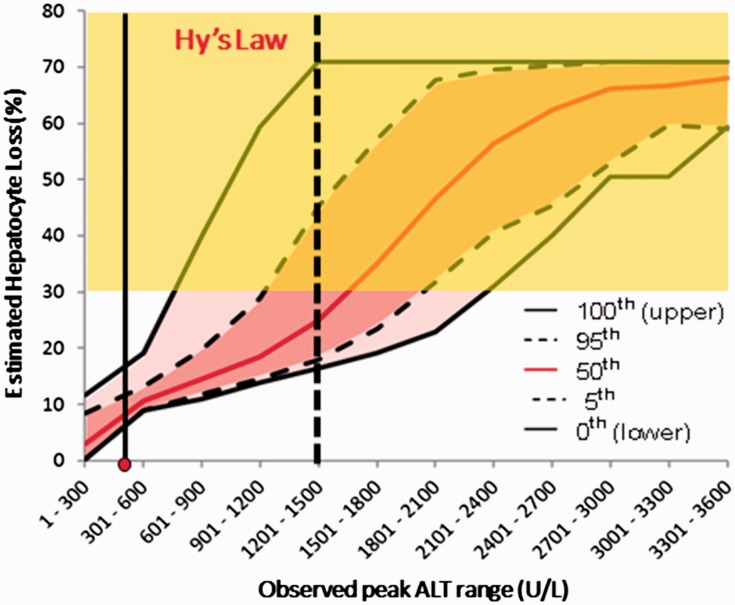
It should be noted that although the timing, rates of rise, and fall in serum ALT observed in the Cimaglermin alfa clinical trials were very different from what was observed with Entolimod, like Entolimod, these characteristics were similar among the Cimaglermin alfa-treated subjects experiencing these abnormalities. It was therefore possible to use the modeling to predict what peak serum ALT values would correspond to 30% hepatocyte loss and thereby account for a rise in serum TBIL (Figure 3). Furthermore, because of the variation introduced in the simulated populations, it is possible to estimate for any peak serum ALT value the probability that a given patient could have manifested a rise in serum bilirubin sufficient to qualify as a Hy’s Law case. This raises the possibility that it may not be necessary to actually observe a Hy’s Law case in a clinical trial to estimate the risk of acute liver failure from the study drug. If the serial serum ALT values observed in a given patient are modeled and reveal that 25% of simulated patients with those values experienced a concomitant rise in serum TBIL > 2× ULN, it may be possible to use the modeling to estimate the risk of acute liver failure without ever actually observing a Hy’s Law case in the clinical trial.
Figure 3.
Modeled percent hepatocyte loss vs. variation in peak serum ALT for cimaglermin alfa. The relationship between peak serum ALT and estimated range of hepatocyte loss was determined using DILIsym® in a simulated population that incorporates variability in factors relevant to ALT dynamics and hepatocyte regeneration. The pink shaded regions indicate confidence intervals for percent hepatocyte loss observed in the simulated subjects for given peak serum ALT values. The 30% hepatocyte loss that would result in a rise in serum TBIL > 2× ULN was estimated from Figure 2 and results in the “Hy’s Law” (yellow) shading. Because the pattern of rise and fall in serum ALT was characteristic for this drug, elevations much higher than observed could be modeled. In this clinical trial data set, the maximum peak serum ALT observed (shown as the red dot on the x-axis) could not alone account for the rise in serum TBIL > 2× ULN that was observed in this subject. The conclusion was that, although all qualifications for a Hy’s Law case were met in this subject and a second subject with a lower peak serum ALT value, they should not be considered Hy’s Law cases. Furthermore, the figure suggests the ability of a drug to cause liver failure might be estimated from the probability that a given peak serum ALT might have resulted in sufficient hepatocyte loss to cause a rise in serum bilirubin > 2× ULN, even if this was not actually observed. For example, a subject with a peak serum ALT value of between 1201 and 1500 (vertical dotted black line) is predicted to have a > 50% chance of not exhibiting a rise in serum TBIL > 2× ULN. It is proposed that such a patient should be considered a Hy’s Law case although they may not have met the currently accepted the biochemical criteria. It should be noted that DILIsym simulation results are highly dependent upon time course of ALT elevations which was similar across affected subjects treated with cimaglermin alfa, but can differ markedly from one drug to another. (A color version of this figure is available in the online journal.)
In silico modeling of hyperbilirubinemia
In the Cimaglermin alfa trial, if percent loss of hepatocytes did not account for the rise in serum TBIL observed in the two potential Hy’s Law cases, what did? It is well known that causes unrelated to hepatocellular injury can increase serum levels of TBIL. Bilirubin is a breakdown product of red blood cells and hemolytic anemia can increase circulatory levels of unconjugated bilirubin.25 Following release into circulation, unconjugated bilirubin is actively taken up by hepatocytes via organic anion transporting polypeptide (OATP) 1B1 and 1B3 or by passive diffusion.26 Within hepatocytes, UDP glucuronosyltransferase (UGT) 1A1 catalyzes glucuronidation of unconjugated bilirubin to create conjugated (also called “direct”) bilirubin. Conjugated bilirubin is, in turn, either secreted into bile primarily by multidrug resistance-associated protein (MRP)2 or returned to circulation primarily by the efflux transporter MRP3. Conjugated bilirubin can then be taken up by downstream hepatocytes via OATP1B1/1B3. Alteration to any one of these processes can result in elevations in serum TBIL levels as evidenced in individuals with genetic mutations affecting bilirubin transport or conjugation (e.g. Rotor Syndrome, Gilbert’s Syndrome, Crigler–Najjar Syndome, and Dubin–Johnson Syndrome).27 Drugs can also selectively inhibit bilirubin metabolism and transport resulting in elevated TBIL levels without causing hepatocellular injury.28
DILIsym has recently added a submodel incorporating genetic and non-genetic variation in each process that may result in elevations in unconjugated, conjugated, and total serum bilirubin.29 To validate this model, changes in unconjugated and conjugated bilirubin following administration of indinavir and nelfinavir were interrogated. Both compounds are antivirals used for the treatment of human immunodeficiency virus and both are known to inhibit UGT1A1 and OATP1B1; however, only indinavir is demonstrated to provoke hyperbilirubinemia in the clinic. Modeling of indinavir administration in a simulated baseline human produced a rise in TBIL that was consistent with clinical data and reasonably recapitulated the magnitude of change. Simulation of nelfinavir administration in a baseline human did not result in elevated levels of TBIL, also consistent with clinical data. Additionally, simulations demonstrated that TBIL elevations in response to indinavir treatment were more substantial in simulated patients with Gilbert’s Syndrome, again in agreement with clinical observations. Individuals with Gilbert’s Syndrome have approximately 30–50% of normal UGT1A1 function and presentation with elevated baseline levels of unconjugated bilirubin is common.27 The effects of an experimental chemokine receptor antagonist (CKA) were also explored utilizing this DILIsym submodel. This compound is known to inhibit multiple transporters involved in bilirubin hepatic circulation, producing only mild ALT elevations in the absence of TBIL changes in humans.30 In agreement with these clinical observations, a human simulated population administered CKA did not develop clinically relevant TBIL elevations.
In the case of Cimaglermin alfa, recent studies in cultured human hepatocytes confirm that the rise in serum TBIL observed is not due to overt toxicity but may be related to altered regulation of genes involved in bilirubin homeostasis.31
The future
While the work performed with DILIsym clearly demonstrates that traditional biomarker data can be utilized in innovative ways to more accurately assess liver safety of new drug candidates, ongoing work is being conducted to identify novel biomarkers that may further improve DILI risk assessment. These biomarkers may offer multiple advantages over traditional tests including: increased liver specificity, mechanistic insightfulness, and improved sensitivity. A detailed discussion of these biomarkers is out of scope of the current review and we have recently reviewed these developments elsewhere.32 Some of these new biomarkers are under review by regulatory agencies and several have already received letters of support to encourage further exploration in preclinical and clinical settings.33–35 However, it is unlikely that new biomarkers will replace older clinical tests any time soon; instead it seems more realistic that simultaneous measurement of traditional and select newer biomarkers, in a panel approach, will give the most complete assessment of a compound’s DILI risk. Already, release and clearance kinetics for candidate DILI biomarkers is being incorporated into DILIsym to improve their application to clinical trials and to improve their interpretation. For instance, as described earlier, measurement of mechanistic candidate biomarkers K18 and ccK18, and calculation of the Apoptotic Index, refined DILIsym modeling of Cimaglermin alfa DILI by suggesting that apoptosis was the primary mode of cell death. The model also supports the incorporation of additional candidate biomarkers including microRNA-122 (miR-122), glutamate dehydrogenase (GLDH), and high mobility group box 1 (HMGB1) which can be useful in confirming liver as the source of injury (miR-122 and GLDH) or identifying an innate immune response (HMGB1). While the utility of these newer biomarkers in the model is still being explored, we believe an approach that incorporates both traditional and the newer biomarkers with in silico modeling is the path forward and will provide the best assessment of DILI liabilities of a new compounds in clinical trials. Ultimately, we believe this combination of modeling and biomarker utilization will spare pharmaceutical companies the necessity of conducting large and costly clinical trials without reducing the sensitivity to detect liver safety concerns.
Authors’ contributions
RJC co-wrote this minireview. PBW co-wrote this minireview.
Declaration of Conflicting Interests
RJC declares no conflicts of interest. PBW chairs the scientific advisory committee for the DILIsim Initiative and has a financial interest in Simulations Plus which acquired DILIsym Services Inc. He also served as a consultant for Cleveland Biolabs, Accorda, and Cempra whose drugs are discussed in this review.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Pettersson J, Hindorf U, Persson P, Bengtsson T, Malmqvist U, Werkstrom V, Ekelund M. Muscular exercise can cause highly pathological liver function tests in healthy men. Br J Clin Pharmacol 2008; 65:253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathwani RA, Pais S, Reynolds TB, Kaplowitz N. Serum alanine aminotransferase in skeletal muscle diseases. Hepatology 2005; 41:380–2 [DOI] [PubMed] [Google Scholar]
- 3.Dara L, Liu ZX, Kaplowitz N. Mechanisms of adaptation and progression in idiosyncratic drug induced liver injury, clinical implications. Liver Int 2016; 36:158–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watkins PB, Zimmerman HJ, Knapp MJ, Gracon SI, Lewis KW. Hepatotoxic effects of tacrine administration in patients with Alzheimer's disease. JAMA 1994; 271:992–8 [PubMed] [Google Scholar]
- 5.Watkins PB. Biomarkers for the diagnosis and management of drug-induced liver injury. Semin Liver Dis 2009; 29:393–9 [DOI] [PubMed] [Google Scholar]
- 6.Gracon SI, Knapp MJ, Berghoff WG, Pierce M, DeJong R, Lobbestael SJ, Symons J, Dombey SL, Luscombe FA, Kraemer D. Safety of tacrine: clinical trials, treatment IND, and postmarketing experience. Alzheimer Dis Assoc Disord 1998; 12:93–101 [DOI] [PubMed] [Google Scholar]
- 7.Dukes GE, Jr., Sanders SW, Russo J, Jr., Swenson E, Burnakis TG, Saffle JR, Warden GD. Transaminase elevations in patients receiving bovine or porcine heparin. Ann Intern Med 1984; 100:646–50 [DOI] [PubMed] [Google Scholar]
- 8.Singhal R, Harrill AH, Menguy-Vacheron F, Jayyosi Z, Benzerdjeb H, Watkins PB. Benign elevations in serum aminotransferases and biomarkers of hepatotoxicity in healthy volunteers treated with cholestyramine. BMC Pharmacol Toxicol 2014; 15:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watkins PB, Kaplowitz N, Slattery JT, Colonese CR, Colucci SV, Stewart PW, Harris SC. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA 2006; 296:87–93 [DOI] [PubMed] [Google Scholar]
- 10.FDA. Guidance for industry. Drug-induced liver injury: premarketing clinical evaluation, www.fda.gov/downloads/Drugs/…/guidances/UCM174090.pdf (2009, accessed 7 October 2017).
- 11.Temple R. Hy's law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf 2006; 15:241–3 [DOI] [PubMed] [Google Scholar]
- 12.Robles-Diaz M, Lucena MI, Kaplowitz N, Stephens C, Medina-Caliz I, Gonzalez-Jimenez A, Ulzurrun E, Gonzalez AF, Fernandez MC, Romero-Gomez M, Jimenez-Perez M, Bruguera M, Prieto M, Bessone F, Hernandez N, Arrese M, Andrade RJ. Use of Hy's law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterology 2014; 147:109–18 e5 [DOI] [PubMed] [Google Scholar]
- 13.Andrade RJ, Lucena MI, Fernandez MC, Pelaez G, Pachkoria K, Garcia-Ruiz E, Garcia-Munoz B, Gonzalez-Grande R, Pizarro A, Duran JA, Jimenez M, Rodrigo L, Romero GM, Navarro JM, Planas R, Costa J, Borras A, Soler A, Salmeron J, Martin VR. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology 2005; 129:512–21 [DOI] [PubMed] [Google Scholar]
- 14.Bjornsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology 2005; 42:481–9 [DOI] [PubMed] [Google Scholar]
- 15.Fontana RJ, Hayashi PH, Gu J, Reddy KR, Barnhart H, Watkins PB, Serrano J, Lee WM, Chalasani N, Stolz A, Davern T, Talwakar JA. Idiosyncratic drug-induced liver injury is associated with substantial morbidity and mortality within 6 months from onset. Gastroenterology 2014; 147:96–108 e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watkins PB, Desai M, Berkowitz SD, Peters G, Horsmans Y, Larrey D, Maddrey W. Evaluation of drug-induced serious hepatotoxicity (eDISH): application of this data organization approach to phase III clinical trials of rivaroxaban after total hip or knee replacement surgery. Drug Saf 2011; 34:243–52 [DOI] [PubMed] [Google Scholar]
- 17.Merz M, Lee KR, Kullak-Ublick GA, Brueckner A, Watkins PB. Methodology to assess clinical liver safety data. Drug Saf 2014; 37(Suppl 1):S33–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watkins PB. Drug safety sciences and the bottleneck in drug development. Clin Pharmacol Ther 2011; 89:788–90 [DOI] [PubMed] [Google Scholar]
- 19.Owens B. Solithromycin rejection chills antibiotic sector. Nat Biotechnol 2017; 35:187–8 [DOI] [PubMed] [Google Scholar]
- 20.Hanley JA, Lippman-Hand A. If nothing goes wrong, is everything all right? Interpreting zero numerators. JAMA 1983; 249:1743–5 [PubMed] [Google Scholar]
- 21.Howell BA, Siler SQ, Shoda LK, Yang Y, Woodhead JL, Watkins PB. A mechanistic model of drug-induced liver injury AIDS the interpretation of elevated liver transaminase levels in a phase I clinical trial. CPT Pharmacometrics Syst Pharmacol 2014; 3:e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashikura Y, Makuuchi M, Kawasaki S, Matsunami H, Ikegami T, Nakazawa Y, Kiyosawa K, Ichida T. Successful living-related partial liver transplantation to an adult patient. Lancet 1994; 343:1233–4 [DOI] [PubMed] [Google Scholar]
- 23.Longo DM, Generaux GT, Howell BA, Siler SQ, Antoine DJ, Button D, Caggiano A, Eisen A, Iaci J, Stanulis R, Parry T, Mosedale M, Watkins PB. Refining liver safety risk assessment: application of mechanistic modeling and serum biomarkers to cimaglermin alfa (GGF2) clinical trials. Clin Pharmacol Ther. Epub ahead of print 17 April 2017. DOI: 10.1002/cpt.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Portmann B, Talbot IC, Day DW, Davidson AR, Murray-Lyon IM, Williams R. Histopathological changes in the liver following a paracetamol overdose: correlation with clinical and biochemical parameters. J Pathol 1975; 117:169–81 [DOI] [PubMed] [Google Scholar]
- 25.Barcellini W, Fattizzo B. Clinical applications of hemolytic markers in the differential diagnosis and management of hemolytic anemia. Dis Mark 2015; 2015:635670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keppler D. The roles of MRP2, MRP3, OATP1B1, and OATP1B3 in conjugated hyperbilirubinemia. Drug Metab Dispos 2014; 42:561–5 [DOI] [PubMed] [Google Scholar]
- 27.Erlinger S, Arias IM, Dhumeaux D. Inherited disorders of bilirubin transport and conjugation: new insights into molecular mechanisms and consequences. Gastroenterology 2014; 146:1625–38 [DOI] [PubMed] [Google Scholar]
- 28.Zucker SD, Qin X, Rouster SD, Yu F, Green RM, Keshavan P, Feinberg J, Sherman KE. Mechanism of indinavir-induced hyperbilirubinemia. Proc Natl Acad Sci U S A 2001; 98:12671–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang K, Battista C, Woodhead JL, Stahl SH, Mettetal JT, Watkins PB, Siler SQ, Howell BA. Systems pharmacology modeling of drug-induced hyperbilirubinemia: differentiating hepatotoxicity and inhibition of enzymes/transporters. Clin Pharmacol Ther 2017;101:501–9 [DOI] [PMC free article] [PubMed]
- 30.Ulloa JL, Stahl S, Yates J, Woodhouse N, Kenna JG, Jones HB, Waterton JC, Hockings PD. Assessment of gadoxetate DCE-MRI as a biomarker of hepatobiliary transporter inhibition. NMR Biomed 2013; 26:1258–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosedale M, Eaddy JS, Trask OJ, Holman NS, Wolf KK, LeCluyse EL, Ware BR, Khetani SR, Lu J, Brock WJ, Roth SE, Watkins PB. miR-122 Release in exosomes precedes overt tolvaptan-induced necrosis in a primary human hepatocyte micropatterned coculture model. Toxicological Sci. Epub ahead of print 28 September 2017 DOI: 10.1093/toxsci/kfx206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Church RJ, Watkins PB. The transformation in biomarker detection and management of drug-induced liver injury. Liver Int 2017;37: 1582–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.EMA. EMA Letter of support for drug-induced liver injury (DILI) biomarker, www.ema.europa.eu/docs/en_GB/document_library/Other/2016/09/WC500213479.pdf (accessed 15 June 2017).
- 34.FDA. U.S. Food & Drug Administration Letter of Support Initiative,www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/UCM517355.pdf (accessed 15 June 2017).
- 35.Current Biomarker Qualification Submissions, www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/BiomarkerQualificationProgram/ucm535881.htm (accessed 24 January 2017)