Short abstract
Discovery and qualification of novel biomarkers with improved specificity and sensitivity for detection of xenobiotic-induced injuries is an area of active research across multiple sectors. However, the majority of efforts in this arena have used genetically limited rodent stocks that lack variability in xenobiotic responses inherent to genetically heterogeneous human populations. In this study, genetically diverse Diversity Outbred (DO) mice were used as a surrogate for human clinical populations to investigate performance of urinary kidney biomarkers against classical preclinical kidney injury biomarkers (blood urea nitrogen [BUN] and serum creatinine). In this study, cisplatin was used as a paradigm kidney toxicant, with female adult DO mice exposed to a single injection (5 mg/kg) of cisplatin or vehicle and necropsied 72 h post-exposure and 18 h following overnight urine collection. Interindividual variability in kidney toxicity was observed, with DO mice experiencing either no tubule cell necrosis or minimal-mild necrosis. A panel of urinary protein biomarkers and profiled miRNAs were assessed by receiver-operating characteristic curves as to their ability to distinguish non-responder versus responder animals, as defined by histopathological evidence of renal tubule cell necrosis. A surprising outcome of these studies was that BUN was elevated alongside of urinary miRNA and protein biomarkers in animals with grade 2 proximal tubular cell necrosis; but not elevated with grade 1 necrosis. These studies demonstrate a novel approach for using a rodent population to better assess sensitivity of candidate biomarkers, especially for proposed clinical applications.
Impact statement
Recent studies have indicated that several urinary proteins and miRNA species may be suitable as biomarkers for acute kidney injury. A major focus on biomarker qualification is demonstrating improved specificity and sensitivity over gold standard tests. In this study, a mouse population model, Diversity Outbred mice, was used to demonstrate that neither the urinary protein markers nor the miRNA species assayed in urine could reliably detect low severity kidney injury better than blood urea nitrogen. This study has implications for use of these biomarkers in the clinic, where interindividual heterogeneity is present within patient populations and for which the underlying tissue pathology may not be known.
Keywords: Biomarkers, cisplatin, Diversity Outbred, kidney, kidney injury molecule-1, miRNA
Introduction
There has been a great deal of investment into biomarker discovery to improve the detection of acute kidney injury (AKI) in both preclinical and clinical settings. AKI has been associated with high inpatient mortality, particularly in pediatric populations.1,2 A key challenge in mitigating AKI risks associated with both disease processes and therapeutic exposures is that the currently used diagnostic tests, increases in blood urea nitrogen (BUN), and serum creatinine (sCre) are not observed until there is a relatively high degree of underlying kidney tissue injury. Furthermore, interpretation of sCre levels suffers from a long latency to elevation (days to peak) and spurious increases due to autoregulation and decreased glomerular filtration due to non-renal causes (such as intravascular volume depletion, increased abdominal pressure, or reduced cardiac function).3
Urine is an attractive matrix biofluid for development of AKI detection assays owing to direct accessibility to the injured kidney. Urine sediment and urinary indices (such as fractional excretion of sodium and urine osmolality) are also relatively insensitive to detect AKI; thus, efforts have been made to evaluate urinary proteins as potential replacement biomarkers.4 In contrast to traditional serum markers that indicate a reduction in kidney function, measurement of kidney enzymes released into the urine can more directly indicate injury to underlying kidney tissue. In the preclinical therapeutic testing arena, several collaborative efforts toward biomarker qualification have yielded promising results. In 2007, a panel of seven urinary proteins was qualified for use in rat toxicology studies by the US FDA and the European Medicines Agency, through efforts spearheaded by the Predictive Safety Testing Consortium and its partner agencies. These biomarkers included kidney injury molecule-1 (KIM-1), albumin, β2-microglubulin, clusterin, cystatin C, total protein, and trefoil factor-3; some of which demonstrated improved sensitivity and specificity for detection of AKI over BUN and sCre by receiver-operating characteristic (ROC) curve analysis. During the review of these biomarkers, experts assembled by the FDA urged for evaluation of these biomarkers in broader contexts and highlighted the role of histopathological evaluations in assessing the value of novel biomarkers.5 Similarly, several groups, including ours in collaboration with the HESI Biomarkers of Nephrotoxicity committee, have investigated utility of urinary miRNAs leaked into the urine as adjunct biomarkers that inform site of injury to the renal nephron in conventional Sprague Dawley rats.6–8
Traditional biomarker discovery efforts typically take a reductionist approach, evaluating a set of candidate biomarkers and their differences in abundance between a subset of patients or within constrained model systems. Such systems science approaches seek to discern molecular changes that – for example – distinguish responders from non-responders, suggest disease course or progression, or can inform status of an organ system. However, the reality of the clinical experience is that patient populations are heterogeneous, with diverse ethnic origins, life stages, and co-morbidities. In addition, accurate measurement of biomarkers is dependent upon clearance half-life, stability, and standardization of assay methods. Thus, the constraints of system science may limit applicability to population health in that the value of a given biomarker may be highly context dependent.
A promising approach to evaluate novel biomarkers in a diverse biological space is to use a population-based approach in the biomarker discovery phase. Such a method would represent diversity inherent to human populations, while providing a defined model system that could be used to estimate population dynamics. Genetically heterogeneous and well-defined Diversity Outbred (DO) mice provide an attractive population-based model for biomarker discovery. DO mice are a heterogeneous stock derived from eight genetic co-founder strains.9 Each DO mouse is a unique individual with high levels of well-randomized polymorphisms and genetic heterozygosity, similar to that found in human populations. In the context of exposure to drugs and chemicals, DO mice have displayed a range in overall response, with a subset of exposed animals displaying a toxicity response and others being resistant to a variety of xenobiotic agents, including chemotherapeutics, herbal supplements, and inhaled benzene.10–12 In this study, we utilized cisplatin as a model compound known to cause AKI and exposed a population of DO mice. The dose selected was intentionally low, relative to prior cisplatin biomarker investigations, in order to observe exposed mice that had varying degrees of underlying renal injury. Using this population model, performance of urinary proteins and relative urinary miRNA abundance was evaluated against the gold standard AKI biomarker for preclinical studies, BUN.
Materials and methods
Experimental animals
Female DO mice (approximately eight weeks of age) were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed in polycarbonate cages on a 12 h light–dark cycle. Mice were provided pelleted Harlan Teklad 22/5 pelleted rodent chow (Madison, WI) ad libitum, with the exception of during 18 h fasting periods (overnight) during urine collection; during this period, animals were individually housed in metabolism cages. Reverse osmosis water was available to rodents, ad libitum, at all times. All animal use was in accordance to the ‘Guide for the Care and Use of Laboratory Animals’ under a protocol approved by the UAMS Institutional Animal Care and Use Committee.
Drug administration, tissue, and biofluid collection
Following arrival, mice were acclimated at least two weeks prior to the initiation of experimentation. Animals were randomized by weight to treatment groups and testing cohorts as only 10 mouse metabolism cages were available to the research team at the time of the study (N = 10 mice/testing cohort; each cohort contained five vehicle- and five drug-treated mice for a total of nine cohorts). Dosing cohorts were staggered due to the caging limitations, with the entirety of the dosing occurring over a period of five weeks. On the first morning of dosing, study day 0, all mice received a bolus i.p. administration of 5 mg/kg cisplatin (50 mg/mL, Mylan Institutional LLC, Rockford, IL) dissolved in 0.9% saline (N = 45) in a volume of 5 mL/kg or vehicle (N = 45). Urine samples were stored overnight in ice-chilled, sterile containers for approximately 18 h prior to necropsy (between study days 2 and 3). A predose urine sample was collected seven days prior to dosing; however, these samples were not analyzed in the current study owing to biomarker concentrations in healthy animals assessed in the postdose urine samples that were generally below the lower limit of quantification. Urine samples aliquoted for downstream miRNA applications were centrifuged at 3000 rpm at 4°C for 5 min (min) and stored at −80°C until use. Necropsy occurred on study day 3 (72 h postdosing), at the same time of day as dosing on day 0. Mice were euthanized by cardiac exsanguination under CO2 anesthesia. Following humane euthanasia, representative tissues were collected and stored in formalin fixative for 48 h prior to histological processing.
Kidney and liver histopathology
Formalin-fixed and paraffin-embedded right kidney and a section of the left liver lobe were sectioned and stained with hematoxylin and eosin (H&E) according to standard methods. Microscopic examination of the H&E-stained slides was scored by JCS (EPL, Inc., RTP, NC). Microscopic findings were graded on a scale of 0–4, where 0 = none, 1 = minimal, 2 = mild, 3 = moderate, and 4 = marked changes.
Clinical chemistry analysis
Blood chemistry analysis was determined in heparinized blood (arterial) using a hand-held clinical chemistry analyzer, iSTAT™, and cartridges (CHEM8+) as described by the manufacturer (Vetscan®, Abaxis, USA). Analytes measured included sodium, potassium, chloride, total carbon dioxide, ionized calcium, glucose, urea nitrogen (BUN), creatinine (sCre), hematocrit, hemoglobin, and anion gap.
Urinary protein biomarker analysis
Isolated urine samples were utilized for the measurement of β2-microglobulin (B2M), interferon gamma-induced protein 10 (IP-10), KIM-1, renin, TIMP metalloprotease inhibitor 1 (TIMP-1), vascular endothelial growth factor (VEGF), clusterin, cystatin c, epidermal growth factor (EGF), neutrophil gelatinase-associated lipocalin (lipocalin-2/NGAL), and osteopontin (OPN) using Milliplex MAP Mouse Kidney Injury magnetic bead Panels 1 and 2 (MilliporeSigma, Billerica, MA). Fluorescence was quantified using a MILLPLEX® analyst V 5.1 (MilliporeSigma, Billerica, MA). Sample concentrations were normalized to total urine volume collected over 18 h. For urinary protein concentrations values that did not fall within the standard curve, the upper and lower limit of quantification values were utilized to extrapolate protein biomarker concentrations. All values are provided in Supplemental Table 1.
Urinary RNA isolation
Total RNA was extracted from 200 µL of all urine samples using the miRNeasy serum/plasma kit (Qiagen, Valencia, CA) in accordance with the manufacturer’s protocol with slight alterations as previously described. Briefly, 3.5 volumes of QIAzol Lysis reagent were added to urine samples. Following a 5 min incubation period, 30 pg of Arabidopsis thaliana miR-159a synthetic miRNA (UUUGGAUUGAAGGGAGCUCUA; Qiagen, Valencia, CA) was added to each sample. The spike-in miRNA was utilized to normalize for RNA isolation efficiency following quantitative real-time PCR (qRT-PCR). Samples that were incubated at room temperature (RT) for 3 min following addition of chloroform (140 µL); samples were then mixed and centrifuged (12,000 g at 4°C for 15 min) in prespun 5 PRIME Phase Lock Gel tubes (Fisher Scientific, Pittsburgh, PA). The upper aqueous layer of each sample was transferred to a new tube and 1.5 volumes of 100% ethanol were added and samples thoroughly mixed. Samples were spun in miRNeasy spin columns (8000 g for 15 s at RT) discarding flow-through. Buffer RWT (700 µL) was added and spun down (8000 g for 15 s at RT), discarding flow-through. Buffer RPE (500 µL) was twice added to columns and spun down (8000 g for 15 s at RT), discarding flow-through. Spin columns were centrifuged at full speed for 2 min and then transferred into clean tubes. Twelve microliters of RNase-free water was added to the membrane of the spin columns and centrifuged at full speed for 2 min. Eluate was collected and stored at −80°C until time of analysis.
MicroRNA microarray profiling
Three microliters of total RNA from each urine sample was reverse transcribed using the TaqMan MicroRNA Reverse Transcription Kit and the Megaplex Reverse Transcription Rodent Pools Set V 3.0 (Life Technologies, Foster City, CA). The resulting cDNA was preamplified using TaqMan PreAmp Master Mix and MegaPlex PreAmp Rodent Pools Set V 3.0 (Life Technologies, Foster City, CA) following the manufacturer’s instructions. The preamplified product was diluted 1:4 in 0.1X TE (pH 8.0). MiRNA profiling was conducted using TaqMan Rodent miRNA Set A v. 3.0 Arrays and Taqman Fast advanced Master Mix (Life Technologies, Foster City, CA) following the manufacturer’s specifications. A no template control was also run on microarray plates. qRT-PCR of these arrays was performed using an Applied Biosystems ViiA® 7 Real-Time PCR System (Life Technologies, Foster City, CA).
miRNA microarray data analysis
Sample cycle threshold (Ct) values for each miRNA RT-PCR reaction were determined using the Applied Biosystems ViiA™ 7 software v1.1 (Life Technologies, Foster City, CA). All curves that appeared to represent background noise were removed from further analyses. The miR-159a Ct values were utilized to normalize Ct values for RNA isolation efficiency, as previously described.13 Ct values were linearized (2−Ct) and then divided by urine volume collected over the 18 h collection window. Normalized Ct values were returned to log scale and imported into Partek Genomics Suite 6.16.0812 (Partek, Inc., St. Louis, MO). Two-way analysis of variance (ANOVA) analysis (for treatment and presence of tubule necrosis) was used to determine significantly altered miRNA. Significance was considered p < 0.05. Fold changes for significantly altered miRNAs were determined by calculating 2−ΔCt where ΔCt represented (individual cisplatin-treated normalized miRNA Ct value − average vehicle-treated normalized miRNA Ct value).
miRNA biomarker performance analysis
ROC curve analysis was employed to compare biomarker performance, whereby the area under the ROC curve (AUROC) was used to determine the accuracy of miRNA abundance for biomarker candidates in the prediction of cisplatin-related nephrotoxicity (as defined by presence of renal tubule necrosis pathology). AUROC represented biomarker performance according to the following scale: ≥0.90 (high), 0.80–0.90 (moderate), 0.70–0.80 (mild), and ≤0.60 (poor) as previously described. The statistical significance of the AUROC values was assessed by Mann–Whitney p values, which represent the probabilities of rejecting the null hypothesis that the AUROC curve is 0.5, indicating that there is a lack of predictive power.
Data analysis
Data are expressed as the mean ± standard deviation except where otherwise indicated. Data analysis was performed using GraphPad Prism 7 for Mac OS X v7.0b software (GraphPad Software, Inc., La Jolla, CA). Group differences between cisplatin and vehicle treatment were assessed by a Mann–Whitney test for analytes that were not normally distributed (as defined by p < 0.05 as determined by D’Agostino & Pearson normality test). For cross-pathology group comparisons, a one-way ANOVA was conducted with Sidak’s multiple comparisons post hoc tests to compare all pathology groups to each other. Student’s t-test was used for comparing two groups that were normally distributed. ROC curve analysis was performed with the 95% confidence interval calculated. ROC curve analysis was performed according to the method of Hanley and computing the p value by computing a z ratio, then determining the p value from the normal distribution (two-tailed).14 Significance was assessed as p < 0.05.
Data access
The full dataset, included raw and normalized values for each biomarker, is available as Supplemental Table 1.
Results
Conventional blood-based biomarkers of kidney injury
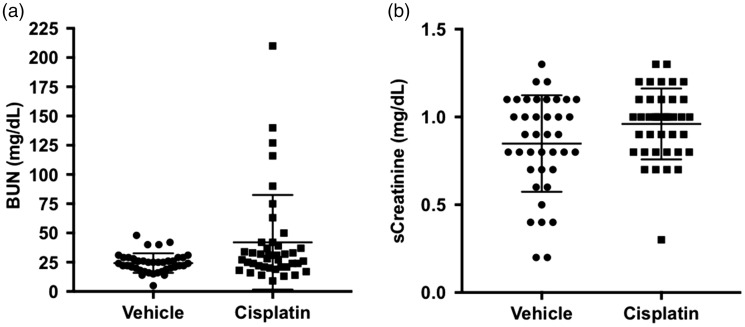
DO mice exposed to a low dose of cisplatin (5 mg/kg, i.p.) exhibited a dynamic renal injury response, in which both responder and non-responder mice were identified. Concentrations of BUN were significantly elevated in the cisplatin group (Figure 1(a); P = 0.0284). The average BUN in the vehicle control group was 24 ± 8 mg/dL (median: 24) and for the cisplatin-treated group was 42 ± 6 mg/dL (median: 28). Within the cisplatin treatment group, 20% (8/41) of the DO mice exhibited values greater than the maximal value of the vehicle treatment group (48 mg/dL); and values ranged from 50 to 210 mg/dL. sCre concentrations did not differ between treatment groups (Figure 1(b); p > 0.05).
Figure 1.
Conventional kidney injury biomarkers in DO mice. Concentrations of (a) BUN and (b) sCre are shown. Dots represent values for individual animals within each treatment group. Bars represent the mean ± SD. *p < 0.05. BUN: blood urea nitrogen.
Histopathology
Cisplatin preferentially causes renal injury in acute high-dose exposure scenarios. However, because the current study was focused on analysis of kidney injury biomarkers, a critical exercise is to rule out alternative injury sites that could contribute to miRNA leakage into biofluid compartments. Histopathology of target organs was evaluated on day 3 (72 h postdosing). The liver was chosen as a non-kidney control organ to assess site specificity of cisplatin toxicity, owing to high tissue exposure following i.p. administration and because aggressive, high-dose cisplatin chemotherapy has been associated with hepatotoxicity in the clinic.15 In the current study, no morphological cisplatin liver injury was observed in any DO mouse treated with cisplatin. There was a similar incidence between treatment and control groups of common minimal and spontaneous findings, including ‘inflammation, acute, focal,’ ‘necrosis, hepatocyte,’ and ‘vacuolation, hepatocyte, random’ (data not shown).
In the kidneys of vehicle and cisplatin treated mice, limited numbers of minimal and spontaneous findings were present, which included predominately ‘infiltrate, mononuclear cell.’ Mononuclear cell infiltrates of this nature are commonly observed in mice and were noted randomly distributed throughout the kidney sections. Aside from one animal (animal #2815), renal tubule degeneration, and/or necrosis were not evident in the vehicle control group (Figure 2(a)).
Figure 2.
Differential susceptibility to cisplatin-induced kidney tubule necrosis. Representative photomicrographs are shown for H&E-stained kidney tissue derived from vehicle-treated (a) and cisplatin-treated (b–d) DO mice. Severity grade of renal tubule necrosis is indicated by the color bars. Panels a and b demonstrate no tubule injury, panel c demonstrates minimal tubule necrosis (grade 1), and panel d demonstrates mild renal tubule necrosis (grade 2). Magnification is at 400X. (A color version of this figure is available in the online journal.)
Renal injury responses to cisplatin exposure were variable across exposed DO mice. Forty-one percent (18/44) of cisplatin-treated animals exhibited no detectable cellular necrosis in response to exposure (Table 1; Figure 2(b)). Cisplatin-induced renal injury was recognized as ‘degeneration, tubule and/or necrosis, tubule’ and graded for severity. Most cases represented minimal severities where degeneration and/or necrosis were limited to small focal or multifocal areas. Minimal also represented a finding that involved only a few individual tubule cells. Degeneration was characterized by slight cytoplasmic basophilia and minimally enlarged tubule cells. Necrosis was characterized by increased cytoplasmic eosinophilia, nuclear pyknosis, or karyorrhexis with some epithelial cell sloughing. Tubule necrosis ranged in severity across cisplatin treated animals from minimal which affected single tubules (grade 1; Figure 2(c); Animal #2871) to mild which affected several tubules (grade 2; Figure 2(d); Animal #2891). Mild cases of tubule necrosis included a more diffuse injury of tubule segments and the presence of tubule granular cast formation (intraluminal necrotic cell debris).
Table 1.
Incidence of histopathological lesions in the kidney by degree of severity.
| Treatment group | Vehicle | Cisplatin |
|---|---|---|
| Number examined | 45 | 44 |
| No degeneration/necrosis present | 44 | 13 |
| Degeneration (tubule) | 1* | 18 |
| Minimal (grade 1) | 1 | 15 |
| Mild (grade 2) | – | 3 |
| Necrosis (tubule) | – | 26 |
| Minimal (grade 1) | – | 17 |
| Mild (grade 2) | – | 9 |
Incidence (numbers of animals) that sustained each lesion for a given severity grade is indicated in the table. N = 45 vehicle-treated mice and N = 44 cisplatin-treated mice.
*Indicates finding occurred in vehicle group and is unrelated to cisplatin exposure.
There were interindividual differences in susceptibility observed within the DO mouse population. Of the 44 cisplatin treated mice for which renal pathology was available, 41% (18/44) had findings of tubule degeneration and 59% (26/44) had findings of tubule necrosis. In contrast, 13 cisplatin treated mice (30%) exhibited kidneys without findings of either tubule necrosis nor degeneration (Table 1).
Urine protein biomarkers
Concentrations of urinary protein biomarkers were assessed in urine collected over 18 h prior to necropsy. In order to evaluate performance of these biomarkers, values for the cisplatin-treated group were grouped by no findings of tubule necrosis, minimal (grade 1) tubule necrosis, or mild (grade 2) tubule necrosis. There were no significant differences in urinary protein biomarker abundance in cisplatin-treated individuals that experienced either no necrosis or minimal levels of necrosis of the renal tubule cells.
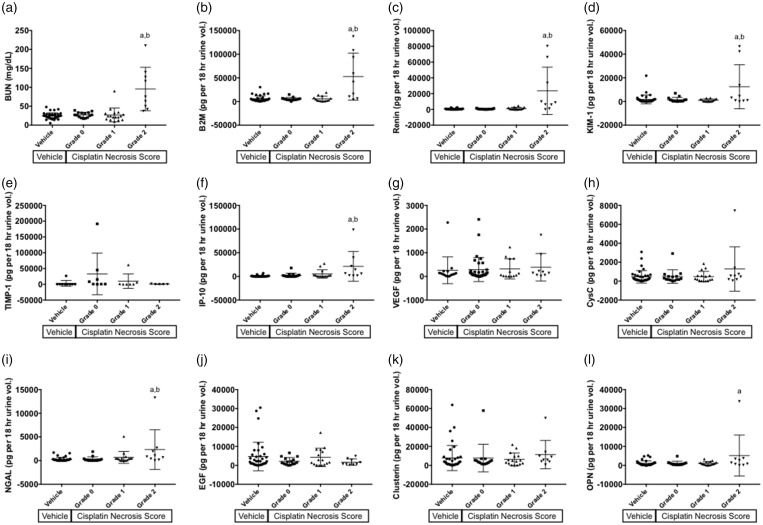
There were significant increases in urinary abundance in the cisplatin-treated animals experiencing mild tubule necrosis for the markers B2M, renin, KIM-1, IP-10, and OPN (p < 0.05; Figure 3). The abundance of these markers was also significantly elevated when compared to cisplatin-treated animals without evidence of tubule injury, with the exception of OPN (p < 0.05). BUN displayed a similar pattern, with marked elevations in the blood of animals that sustained tubule necrosis (p < 0.05); however, there was a lower degree of overlap in the values of the mild necrosis group with the groups sustaining less injury, as compared to the urinary biomarkers (Figure 3(a)).
Figure 3.
BUN and urinary protein biomarkers separated by necrosis score. (a) Concentrations of BUN are shown for DO mice and are separated by severity score for renal tubule necrosis. Similarly, abundance of urinary proteins normalized to urine volume collected over 18 h is shown in (b–l). Analytes shown include (b) B2M, (c) renin, (d) KIM-1, (e) TIMP-1, (f) IP-10, (g) VEGF, (h) cystatin C, (i) NGAL, (j) EGF, (k) clusterin, and (l) OPN. Bars indicate ± SD. a: p < 0.05 for group comparison of cisplatin grade 2 and vehicle control, b: p < 0.05 for group comparison of cisplatin grade 2 and cisplatin grade 0. BUN: blood urea nitrogen; B2M: β2-microglubulin; EGF: epidermal growth factor; IP-10: interferon gamma-induced protein 10; KIM-1: kidney injury molecule-1, albumin; NGAL: neutrophil gelatinase-associated lipocalin; OPN: osteopontin; TIMP-1: TIMP metalloprotease inhibitor 1; VEGF: vascular endothelial growth factor.
Urine miRNA biomarkers
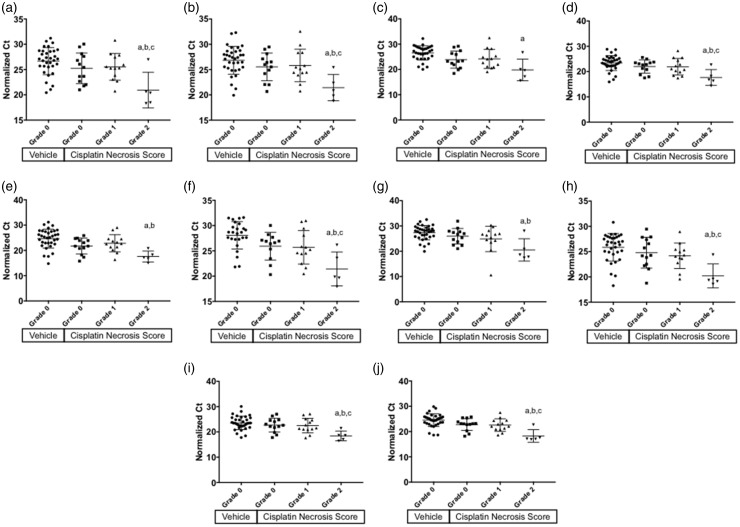
miRNA abundance in the urine was measured over an 18 h collection period prior to necropsy. To correct for dilution effects, Ct values were normalized to the total volume of urine collected. A complete list of tested miRNAs and ANOVA output is available in Supplemental Table 2. Of the 335 unique miRNAs assayed on the array platform, 10 miRNA species were significantly affected by treatment and differed in abundance based on presence of tubule necrosis (FDR corrected p < 0.05; Supplemental Table 2). When plotted by tubule necrosis score (grade 0, 1, or 2), all were significantly increased in the urine of mice experiencing Grade 2 tubule necrosis with respect to the vehicle control group (p < 0.05; Figure 4). For each of the 10 urinary miRNA biomarker candidates, there were no significant increases present in the animals experiencing grade 1 necrosis (p > 0.05), nor were the miRNA increases associated with cisplatin exposure alone, owing to a lack of increase in the cisplatin-treated animals without necrosis (grade 0, p > 0.05; Figure 4).
Figure 4.
Relative urinary abundance of miRNA separated by necrosis score. Normalized Ct values are shown for miRNA biomarker candidates identified by ANOVA analysis. Owing to the nature of Ct data, lower values indicate a higher abundance on a logarithmic scale. Ct values of urinary miRNA is shown for (a) miR-130a, (b) miR-151–3p, (c) miR-218, (d) miR-320, (e) miR-680, (f) miR-138, (g) miR-152, (h) miR-221, (i) miR-328, and (j) miR-685. Bars indicate mean ± SD. a: p < 0.05 for group comparison of cisplatin grade 2 and vehicle control, b: p < 0.05 for group comparison of cisplatin grade 2 and cisplatin grade 0, c: p < 0.05 for group comparison of cisplatin grade 2 and cisplatin grade 1. Ct: cycle threshold.
Biomarker performance analysis
ROC curve analysis was performed for each of the miRNA biomarkers, the urinary protein biomarkers, and conventional blood-based markers (BUN and sCre). ROC analysis was used to evaluate the performance of miRNA urinary abundance to diagnose underlying kidney injury, using the presence (grade 1 or 2) of tubule necrosis as the gold standard for diagnosis. The derived summary measure of accuracy, the AUROC, was calculated for each biomarker. The AUROC can be interpreted as the probability that a randomly chosen affected subject is rated as more likely to be affected than a randomly chosen non-affected subject. The interpretation is based on nonparametric Mann–Whitney U statistics that are used in calculating AUROC, in which an AUROC of 0.5 indicates a chance level of correct classification and an AUROC of 0 indicates complete failure of the test to classify subjects by response. Typically, biomarker classifications are considered ‘good’ with AUROC values greater than 0.7 and ‘excellent’ with AUROC values greater than 0.9. In the present study, candidate miRNA biomarker AUROC values were all significant, but this is perhaps not surprising given that presence of necrosis was used as a factor in the ANOVA analysis that was used to identify these species as potential biomarker candidates (Figure 5). AUROC values for miRNA candidates ranged from 0.71 to 0.82, with the highest values associated with miR-138, miR-218, and miR-685 (p < 0.0005; Table 2).
Figure 5.
ROC curves are shown for candidate urinary miRNA biomarkers of renal tubule necrosis. Comparative ROC curve analysis was performed to determine the ability of candidate miRNA biomarkers to predict histopathological kidney injury observed at time of necropsy in DO mice (grade 1 or 2 tubule necrosis). Significance was determined using the Mann–Whitney test. AUROC and ROC curve fit p values are indicated in Table 2. ROC curves of urinary miRNA are shown for (a) miR-130a, (b) miR-151-3p, (c) miR-218, (d) miR-320, (e) miR-680, (f) miR-138, (g) miR-152, (h) miR-221, (i) miR-328, and (j) miR-685. On the y-axis, sensitivity % indicates the true positive prediction rate while on the x-axis, 100%−specificity% indicates the false positive prediction rate. ROC: receiver-operating characteristic.
Table 2.
ROC curve analysis of blood and urinary biomarkers.
| Biomarker | AUROC | Std. error | 95% confidence interval | P value |
|---|---|---|---|---|
| Blood/conventional | ||||
| BUN | 0.6368 | 0.07728 | 0.4853–0.7882 | 0.0486 |
| sCre | 0.5692 | 0.06723 | 0.4375–0.701 | 0.3385 |
| Urinary protein | ||||
| IP-10 | 0.8015 | 0.0567 | 0.6903–0.9126 | <0.0001 |
| Renin | 0.7334 | 0.07199 | 0.5923–0.8745 | 0.0013 |
| NGAL | 0.6962 | 0.0711 | 0.5569–0.8356 | 0.0054 |
| B2M | 0.6251 | 0.07968 | 0.4689–0.7813 | 0.0847 |
| Clusterin | 0.6062 | 0.07064 | 0.4677–0.7446 | 0.1333 |
| Osteopontin | 0.5827 | 0.07393 | 0.4378–0.7276 | 0.2422 |
| KIM-1 | 0.5771 | 0.07733 | 0.4256–0.7287 | 0.2879 |
| Cystatin C | 0.5683 | 0.07173 | 0.4277–0.7089 | 0.3326 |
| TIMP-1* | 0.5833 | 0.1099 | 0.3679–0.7988 | 0.4516 |
| VEGF | 0.5236 | 0.07896 | 0.3689–0.6784 | 0.7506 |
| EGF | 0.5087 | 0.07218 | 0.3672–0.6502 | 0.902 |
| Urinary miRNA | ||||
| miR-685 | 0.8234 | 0.05461 | 0.7163–0.9304 | 0.0001 |
| miR-138 | 0.8108 | 0.06258 | 0.6882–0.9335 | 0.0004 |
| miR-218 | 0.7891 | 0.05807 | 0.6752–0.9029 | 0.0007 |
| miR-221 | 0.769 | 0.06628 | 0.6391–0.8989 | 0.0014 |
| miR-680 | 0.7649 | 0.06285 | 0.6418–0.8881 | 0.0017 |
| miR-320 | 0.7595 | 0.06793 | 0.6264–0.8927 | 0.0021 |
| miR-130a | 0.7486 | 0.06136 | 0.6284–0.8689 | 0.0032 |
| miR-151-3p | 0.7473 | 0.07087 | 0.6084–0.8862 | 0.0034 |
| miR-328 | 0.7351 | 0.07534 | 0.5874–0.8827 | 0.0054 |
| miR-152 | 0.7133 | 0.06969 | 0.5767–0.8499 | 0.0115 |
AUROC: area under the ROC; BUN: blood urea nitrogen; B2M: β2-microglubulin; EGF: epidermal growth factor; IP-10: interferon gamma-induced protein 10; KIM-1: kidney injury molecule-1 (KIM-1), albumin; NGAL: neutrophil gelatinase-associated lipocalin; ROC: receiver-operating characteristic; TIMP-1: TIMP metalloprotease inhibitor 1; VEGF: vascular endothelial growth factor.
Within each biomarker category, markers are sorted by AUROC value from highest to lowest.
*Several values for TIMP-1 in both the vehicle- and cisplatin-treated mice were below the lower limit of quantification; therefore, these data should be interpreted with caution.
Overall, miRNA candidates performed better in distinguishing affected (grade 1 or 2 necrosis) versus non-affected individuals (grade 0 necrosis) than the urinary proteins assayed in this study. Of the urinary proteins, IP-10, renin, and NGAL were all significant in the ROC analysis (P < 0.01; Table 2). Of these, IP-10 and renin demonstrated the best performance with AUROC values of 0.80 and 0.73, respectively (Table 2).
In contrast, conventional biomarkers BUN and sCre demonstrated a poorer performance to distinguish subjects with tubule necrosis from those without. BUN was barely significant (p = 0.0486) and had a marginal AUROC value of 0.64. sCre was not useful in distinguishing subject response under the cisplatin exposure conditions in the present study (AUROC 0.60, p = 0.3385).
Discussion
In genetically sensitive DO mice, cisplatin caused minimal-to-mild necrosis of tubule cells in the presence of a degeneration (including cellular swelling and vacuolation). Because degeneration is not typically associated with leakage of cellular contents, the analysis was primarily focused on a subset of animals that had experienced necrosis. In the current study, neither the classical (BUN) nor novel biomarkers tested (urinary miRNA or proteins) was significantly elevated in cisplatin treated mice until mild (grade 2) tubule necrosis was apparent in the kidney. This finding reflects a serious limitation in the ability of cellular leakage markers to reflect early phase minimal necrosis that may precede more serious injury. From the standpoint of improving early detection of renal injury, there appears to be little advantage to using the measured miRNA or urinary proteins for broad diagnosis of renal injury compared to the gold standard biomarker BUN.
Interestingly, when severity grade was ignored and ROC analysis was employed to determine biomarker ability to classify mice as those sustaining renal injury (grade 1 or 2 necrosis) versus those that had none, several markers appeared to have a reasonable degree of accuracy in distinguishing the groups. Considering that higher AUROC values are considered a measure of greater accuracy of the positive distribution model, it appeared that the relative urinary miRNA abundance of miR-685, miR-138, miR-218 and the urinary protein abundance of IP-10 were the top performing tests. However, the predictive value of these markers is limited by inability to detect very early stage necrosis prior to elevations in BUN.
AUROC is widely used as a method to compare predictive accuracy of diagnostic tests using distributional models derived from case–control data. It should be noted that inherent limitations in AUROC assessment from a statistical standpoint are several and have been elucidated elsewhere.16 In addition, the ROC curve analysis for each biomarker tested in this study is not necessarily independent because each biomarker represented a separate diagnostic task that was performed on the same set of individual mice. The current study, using a population of DO mice, demonstrated variability in adverse outcomes across the population (responders of various severity and non-responders to the drug exposure), which is ideal for assessing accuracy of specificity of each biomarker.17 However, the biomarker levels themselves have some limitations for cross-comparison because of differences in dynamic range of the values measured in mice (low: BUN, sCre, miRNA to high: urinary proteins).
The biomarkers that were elevated alongside of BUN during grade 2 necrosis are informative owing to potential connections to toxicity mode of action and regional site of injury within the renal nephron. Measuring these markers may provide added value by enabling additional interpretation of the underlying pathology (so-called liquid biopsy), particularly in cases where tissue pathology is contraindicated or unavailable. KIM-1 is a type 1 transmembrane glycoprotein localized to the proximal tubule of the kidney. KIM-1 is markedly upregulated in the kidney in rat and increased in urine in mice upon ischemic injury, yet is virtually undetectable in renal tubules in healthy kidney tissue.18,19 Thus, KIM-1 has utility for diagnosis of renal tubule injury, as occurred in sensitive DO mice exposed to cisplatin. NGAL is a protein in the lipocalin family that is secreted by a variety of body tissues. In the kidney, NGAL is synthesized in the distal nephron and is secreted by the thick ascending loop of Henle and collecting ducts. It has previously shown to be a useful biomarker of acute renal injury following cardiac surgery.20 A limitation of this study in fully comparing novel urinary protein biomarker levels to rat and human studies is the lack of albumin and trefoil factor 3 (TFF3) measurements in the current study. Each of these markers has been previously shown to have promising utility in detecting early kidney injury associated with renal tubular injury (albumin and TFF3)21 and with glomerular cell injury (albumin)6 in rats. Measurement of these two potentially sensitive biomarkers represents an important future direction for qualification in genetically diverse rodent and human patient populations.miR-138 and miR-218 were previously shown to be elevated in the urine of male Wistar rats exposed to gentamicin, an agent that also causes renal tubule cell necrosis.22 In those studies, it is notable that neither BUN nor sCre was concurrently elevated in the blood when the urinary increases in miRNA were observed, suggesting that these miRNAs may provide additional utility for sensitively detecting renal tubule cell necrosis. In the prior rat studies, low group sizes (N = 6) precluded using severity of injury as a factor for the analysis and tubule cell necrosis ranged from minimal (grade 1) to marked (grade 4), depending on the tested dose. Therefore, it is unknown whether urinary elevations in miR-138 and miR-218 are truly sensitive enough for detecting mild tubule necrosis. It is more likely that these markers provide specificity to detecting tubule necrosis versus injury that occurs elsewhere in the nephron. This assumption is supported by a lack of observance in urinary elevations in rat studies that investigated glomerular cell injury with doxorubicin.6 It should be noted that miR-221, which was significantly elevated in DO mice with grade 2 tubule necrosis, has been shown in humans to have promise as a circulating blood-based biomarker of lupus nephritis.23 Further work is needed on miRNA biomarker candidates identified in the present study in order to qualify them as specific biomarkers for tubule cell necrosis. So doing would provide additional context for measuring these biomarkers in concert with BUN and sCre to assess underlying renal pathology.
While the study was not designed to investigate the specific mechanisms underlying differential susceptibility to cisplatin-induced kidney injury in exposed DO mice, there are potential parallels in clinical populations that may be informative. Cisplatin has been shown to accumulate within the mitochondria of renal cells, causing generation of reactive oxygen species and mitochondrial impairment. Once inside the cell, cisplatin forms intrastrand 1,2-DNA cross-links that impair DNA synthesis and replication, leading to cell cycle arrest.24 Because significant interindividual variability in the chemotherapeutic efficacy of cisplatin occurs among patients, contributions of human genetic polymorphisms have been investigated, particularly for glutathione S-transferases that bioactivate cisplatin (GSTM1 and GSST1),25 organic cation transporters (OCT1 and OCT226), and DNA repair gene (ERCC127). However, few studies have investigated the genetic drivers of cisplatin-induced nephrotoxicity, and those that have done so have been conducted in small patient populations. The current study in DO mice is also underpowered28 to conduct genome-wide association analyses that might inform genetic contributions to susceptibility and, thus, potential mechanisms; although, additional studies toward this aim are under consideration because larger populations of DO mice have been used successfully to investigate polymorphisms that contribute to chemotherapeutic-induced neutropenia.12
A potential explanation for increased susceptibility in individual DO mice may be differences in pharmacokinetics of cisplatin; for example, in recent study, it was found that decreased expression of efflux transporter Mate1 contributed to an increased risk of cisplatin-induced nephrotoxicity in C57BL/6 mice29 and in Mate1 knockout mice.30 Although it should be noted that variation in efficiency or abundance of additional cisplatin uptake (Oct231) and efflux transporters (Mrp232) could potentially play a role in modulating underlying tissue exposure to the drug, as has been shown in previous studies. There may additionally be a role for variation in pharmacodynamic processes to contribute to variation in toxicity outcome across DO mice. Also unknown is whether there are differences in clearance rates of urinary biomarkers measured in the study, which may vary by individual. A time course study, particularly focused on biomarker quantification at earlier time points may inform whether there is early leakage of miRNA or protein biomarkers that where cleared prior to the day 2–3 collection that occurred in the study. A caveat is there may be limited utility for clinical use of a given biomarker if its appearance in urine is transient and not sustained in measurable concentrations while injury is ongoing. Further work toward understanding biomarker clearance rates and investigation of underlying mechanisms driving susceptibility may be informative toward identifying personalized prescribing strategies that are protective of patients who are candidates for cisplatin chemotherapy.
This is the first study to demonstrate value in using a mouse population approach to evaluate biomarker performance in the context of xenobiotic-induced injury. We have shown that employing a broader biological context provided added value by allowing for discernment of specific biomarker changes that are firmly anchored to pathology severity score. As such, this approach has promise for clinical biomarker discovery and qualification. While a necessary drawback is the inherent species differences in metabolism (as well as other factors), a mouse population approach allows for phenotypic anchoring to underlying pathology, which is only rarely available in clinical investigations. Thus, mouse population approaches provide a complementary step to clinical investigations in evaluating performance and predictive capacity of novel biomarkers.
Supplementary Material
Acknowledgments
The authors express their gratitude to Drs Alex Merrick and Stephen Ferguson for critical review of the manuscript. The authors sincerely thank Drs Gordon Flake and Ron Herbert, along with the NIEHS Cellular and Molecular Pathology Branch for supporting pathological assessments.
Authors’ contribution
AHH wrote the manuscript, designed the studies, and conducted the primary data analysis. HL and JT performed the animal experiments, collected biospecimens, and generated the clinical chemistry data. JT developed study protocols for biofluid and tissue collection. HL conducted the urinary protein and urinary miRNA assays and performed data normalization. JCS conducted pathology assessment, generated histopathology photomicrographs, and performed critical review of the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
These studies were supported by the NIEHS Cellular and Molecular Pathology Branch pathology services contract to EPL, Inc. (HHSN273200800005C), the Arkansas Biosciences Institute, the University of Arkansas for Medical Sciences (UAMS) Translational Research Institute grant UL1TR000039 (NIH), UAMS COBRE Center for Studies of Host Response to Cancer Therapy (NIH 1P20GM109005–01A1), and by the UAMS Systems Pharmacology and Toxicology training grant that supported JT (NIH, T32 GM106999). The content of this article is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
References
- 1.Sutherland SM, Ji J, Sheikhi FH, Widen E, Tian L, Alexander SR, Ling XB. AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol 2013; 8:1661–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL, AWARE Investigators. Epidemiology of acute kidney injury in critically Ill children and young adults. N Engl J Med 2017; 376:11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberg JH, Parikh CR. Biomarkers for diagnosis and prognosis of AKI in children: one size does not fit all. Clin J Am Soc Nephrol 2017;12:1551-7. [DOI] [PMC free article] [PubMed]
- 4.Ferguson MA, Vaidya VS, Bonventre JV. Biomarkers of nephrotoxic acute kidney injury. Toxicology 2008; 245:182–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blank M, D, Felice A, Goodsaid F, Harlow P, Hausner E, Jacobson-Kram D, Taylor W, Thompson A, Throckmorton D, Xiao S. Review of qualification data for biomarkers of nephrotoxicity submitted by the predictive safety testing consortium: Center for Drug Evaluation and Research, US Food and Drug Administration, https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DrugDevelopmentTools QualificationProgram/UCM382536.pdf (2009, accessed 15 July 2017)
- 6.Church RJ, McDuffie JE, Sonee M, Otieno M, Ma JY, Liu XJ, Watkins PB, Harrill AH. MicroRNA-34c-3p is an early predictive biomarker for doxorubicin-induced glomerular injury progression in male Sprague-Dawley rats. Toxicol Res 2014; 3:384–94 [Google Scholar]
- 7.Nassirpour R, Homer BL, Mathur S, Li Y, Li Z, Brown T, Carraher D, Warneke J, Bailey S, Percival K, O’Neil SP, Whiteley LO. Identification of promising urinary MicroRNA biomarkers in two rat models of glomerular injury. Toxicol Sci 2015; 148:35–47 [DOI] [PubMed] [Google Scholar]
- 8.Pavkovic M, Riefke B, Frisk AL, Groticke I, Ellinger-Ziegelbauer H. Glomerulonephritis-induced changes in urinary and kidney MicroRNA profiles in rats. Toxicol Sci 2015; 145:348–59 [DOI] [PubMed] [Google Scholar]
- 9.Svenson KL, Gatti DM, Valdar W, Welsh CE, Cheng R, Chesler EJ, Palmer AA, McMillan L, Churchill GA. High-resolution genetic mapping using the mouse diversity outbred population. Genetics 2012; 190:437–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.French JE, Gatti DM, Morgan DL, Kissling GE, Shockley KR, Knudsen GA, Shepard KG, Price HC, King D, Witt KL, Pedersen LC, Munger SC, Svenson KL, Churchill GA. Diversity outbred mice identify population-based exposure thresholds and genetic factors that influence benzene-induced genotoxicity. Environ Health Perspect 2015; 123:237–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Church RJ, Gatti DM, Urban TJ, Long N, Yang X, Shi Q, Eaddy JS, Mosedale M, Ballard S, Churchill GA, Navarro V, Watkins PB, Threadgill DW, Harrill AH. Sensitivity to hepatotoxicity due to epigallocatechin gallate is affected by genetic background in diversity outbred mice. Food Chem Toxicol 2015; 76:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gatti DM, Weber SN, Goodwin NC, Lammert F, Churchill GA. Genetic background influences susceptibility to chemotherapy-induced hematotoxicity. Pharmacogenomics J. Epub ahead of print 13 June 2017. doi: 10.1038/tpj.2017.23. [DOI] [PMC free article] [PubMed]
- 13.Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods 2010; 50:298–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982; 143:29–36 [DOI] [PubMed] [Google Scholar]
- 15.Waseem M, Bhardwaj M, Tabassum H, Raisuddin S, Parvez S. Cisplatin hepatotoxicity mediated by mitochondrial stress. Drug Chem Toxicol 2015; 38:452–9 [DOI] [PubMed] [Google Scholar]
- 16.Lobo JM, Jiménez-Valverde A, Real R. AUC: a misleading measure of the performance of predictive distribution models. Global Ecol Biogeogr 2008; 17:145–51 [Google Scholar]
- 17.Hajian-Tilaki K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Caspian J Intern Med 2013; 4:627–35 [PMC free article] [PubMed] [Google Scholar]
- 18.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem 1998; 273:4135–42 [DOI] [PubMed] [Google Scholar]
- 19.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 2002; 62:237–44 [DOI] [PubMed] [Google Scholar]
- 20.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005; 365:1231–8 [DOI] [PubMed] [Google Scholar]
- 21.Yu Y, Jin H, Holder D, Ozer JS, Villarreal S, Shughrue P, Shi S, Figueroa DJ, Clouse H, Su M, Muniappa N, Troth SP, Bailey W, Seng J, Aslamkhan AG, Thudium D, Sistare FD, Gerhold DL. Urinary biomarkers trefoil factor 3 and albumin enable early detection of kidney tubular injury. Nat Biotechnol 2010; 28:470–7 [DOI] [PubMed] [Google Scholar]
- 22.Zhou X, Qu Z, Zhu C, Lin Z, Huo Y, Wang X, Wang J, Li B. Identification of urinary microRNA biomarkers for detection of gentamicin-induced acute kidney injury in rats. Regul Toxicol Pharmacol 2016; 78:78–84 [DOI] [PubMed] [Google Scholar]
- 23.Navarro-Quiroz E, Pacheco-Lugo L, Lorenzi H, Diaz-Olmos Y, Almendrales L, Rico E, Navarro R, Espana-Puccini P, Iglesias A, Egea E, Aroca G. High-throughput sequencing reveals circulating miRNAs as potential biomarkers of kidney damage in patients with systemic lupus erythematosus. PLoS One 2016; 11:e0166202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fichtinger-Schepman AM, van der Veer JL, den Hartog JH, Lohman PH, Reedijk J. Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: formation, identification, and quantitation. Biochemistry 1985; 24:707–13 [DOI] [PubMed] [Google Scholar]
- 25.Oldenburg J, Kraggerud SM, Cvancarova M, Lothe RA, Fossa SD. Cisplatin-induced long-term hearing impairment is associated with specific glutathione s-transferase genotypes in testicular cancer survivors. JCO 2007; 25:708–14 [DOI] [PubMed] [Google Scholar]
- 26.Ciarimboli G, Ludwig T, Lang D, Pavenstadt H, Koepsell H, Piechota HJ, Haier J, Jaehde U, Zisowsky J, Schlatter E. Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol 2005; 167:1477–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tzvetkov MV, Behrens G, O’Brien VP, Hohloch K, Brockmoller J., Benohr P. Pharmacogenetic analyses of cisplatin-induced nephrotoxicity indicate a renoprotective effect of ERCC1 polymorphisms. Pharmacogenomics 2011; 12:1417–27 [DOI] [PubMed] [Google Scholar]
- 28.Gatti DM, Svenson KL, Shabalin A, Wu LY, Valdar W, Simecek P, Goodwin N, Cheng R, Pomp D, Palmer A, Chesler EJ, Broman KW, Churchill GA. Quantitative trait locus mapping methods for diversity outbred mice. G3 (Bethesda) 2014; 4:1623–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen J, Zeng M, Shu Y, Guo D, Sun Y, Guo Z, Wang Y, Liu Z, Zhou H, Zhang W. Aging increases the susceptibility of cisplatin-induced nephrotoxicity. Age (Dordr) 2015; 37:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura T, Yonezawa A, Hashimoto S, Katsura T, Inui K. Disruption of multidrug and toxin extrusion MATE1 potentiates cisplatin-induced nephrotoxicity. Biochem Pharmacol 2010; 80:1762–7 [DOI] [PubMed] [Google Scholar]
- 31.Franke RM, Kosloske AM, Lancaster CS, Filipski KK, Hu C, Zolk O, Mathijssen RH, Sparreboom A. Influence of Oct1/Oct2-deficiency on cisplatin-induced changes in urinary N-acetyl-beta-d-glucosaminidase. Clin Cancer Res 2010; 16:4198–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wen X, Buckley B, McCandlish E, Goedken MJ, Syed S, Pelis R, Manautou JE, Aleksunes LM. Transgenic expression of the human MRP2 transporter reduces cisplatin accumulation and nephrotoxicity in Mrp2-null mice. Am J Pathol 2014; 184:1299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.