Short abstract
Cancer treatment with doxorubicin (DOX) can induce cumulative dose-dependent cardiotoxicity. Currently, there are no specific biomarkers that can identify patients at risk during the initial doses of chemotherapy. The aim of this study was to examine plasma cytokines/chemokines and potential cardiovascular biomarkers for the prediction of DOX-induced cardiotoxicity. Plasma samples were collected before (T0), and after the first (T1) and the second (T2) cycles of DOX-based chemotherapy of 27 breast cancer patients, including five patients who presented with >10% decline of left ventricular ejection fraction (LVEF), five patients with LVEF decline of 5–10%, and 17 patients who maintained normal LVEF at the end of chemotherapy (240 mg/m2 cumulative dose of DOX from four cycles of treatment). Multiplex immunoassays were used to screen plasma samples for 40 distinct chemokines, nine matrix metalloproteinases, 33 potential markers of cardiovascular diseases, and the fourth-generation cardiac troponin T assay. The results showed that the patients with abnormal decline of LVEF (>10%) had lower levels of CXCL6 and sICAM-1 and higher levels of CCL23 and CCL27 at T0; higher levels of CCL23 and lower levels of CXCL5, CCL26, CXCL6, GM-CSF, CXCL1, IFN-γ, IL-2, IL-8, CXCL11, CXCL9, CCL17, and CCL25 at T1; and higher levels of MIF and CCL23 at T2 than the patients who maintained normal LVEF. Patients with LVEF decline of 5–10% had lower plasma levels of CXCL1, CCL3, GDF-15, and haptoglobin at T0; lower levels of IL-16, FABP3, and myoglobin at T1; and lower levels of myoglobin and CCL23 at T2 as compared to the patients who maintained normal LVEF. This pilot study identified potential biomarkers that may help predict which patients are vulnerable to DOX-induced cardiotoxicity although further validation is needed in a larger cohort of patients.
Impact statement
Drug-induced cardiotoxicity is one of the major concerns in drug development and clinical practice. It is critical to detect potential cardiotoxicity early before onset of symptomatic cardiac dysfunction or heart failure. Currently there are no qualified clinical biomarkers for the prediction of cardiotoxicity caused by cancer treatment such as doxorubicin (DOX). By using multiplex immunoassays, we identified proteins with significantly changed plasma levels in a group of breast cancer patients who were treated with DOX-based chemotherapy and produced cardiotoxicity. These proteins were associated with immune response and were identified before DOX treatment or at early doses of treatment, thus they could be potential predictive biomarkers of cardiotoxicity although further validation is required to warrant their clinical values.
Keywords: Cardiotoxicity, doxorubicin, predictive biomarkers, chemokines, breast cancer, multiplex immunoassays
Introduction
Doxorubicin (DOX) is one of the most effective and commonly used drugs for treatment of a wide range of cancers. However, a life-threatening side effect of DOX is cumulative dose-dependent cardiotoxicity. DOX-induced cardiotoxicity begins with the first dose with subclinical myocardial injury, followed by an early asymptomatic decline in left ventricular ejection fraction (LVEF) that can progress to left ventricular dysfunction (LVD) and symptomatic heart failure.1–3 Development of DOX-associated cardiotoxicity can range from days to years, though often times it is delayed, and the early stages of cardiotoxicity are asymptomatic.2 The life-threatening cardiotoxicity significantly limits the adequate dosing of this drug.
Left ventricular dysfunction is a frequent clinical manifestation of DOX-induced cardiotoxicity. Imaging tests (e.g. echocardiography, multigated acquisition or MUGA scan) are the most common tools for monitoring LVEF; however, they are costly and lack sensitivity for early detection of cardiotoxicity.4 Once imaging tests detect LVD, critical damage to myocardium has already taken place; therefore, patients largely lose the opportunity for complete recovery even after cardioprotection treatment. A few sensitive techniques are under study and validation.4 Blood biomarkers such as cardiac troponin T (cTnT) and I (cTnI) are sensitive biomarkers of early cardiac tissue damage, but their ability to predict cardiotoxicity is limited5,6 because cardiac troponins are released after tissue damage has occurred. Therefore, it would be extremely valuable to develop new predictive biomarkers to identify patients at risk for cardiotoxicity prior to the occurrence of overt cardiac tissue damage to prevent permanent cardiac damage.
Inflammation and immunological response play important roles in disease development and drug-induced toxicity. Previous genome-wide gene expression profiling of peripheral blood cells of breast cancer patients undergoing DOX-based chemotherapy showed that individual sensitivity to DOX cardiotoxicity appeared to be associated with differential expression of several genes implicated in inflammatory response and immune trafficking.7 The analysis and comparison of the genotype distribution of breast cancer patients who developed abnormal LVEF decline after DOX-based chemotherapy and patients who did not identified genetic variability in several human leukocyte antigen (HLA) genes as potential candidates for association with DOX cardiotoxicity.8 The later finding is consistent with reports showing the presence of susceptibility loci within the HLA gene region for several inflammatory and autoimmune diseases,9 between chemotherapy, and development of autoimmune and rheumatic features in cancer patients.10 Proteins in blood that represent the inflammation status or a drug-induced immunological response potentially could be early biomarkers of drug-induced cardiotoxicity. To investigate this hypothesis, plasma samples were collected from 27 breast cancer patients before the initiation of treatment and after the first and second cycles of a four-cycle DOX-based chemotherapy regime. Multiplex immunoassays for chemokines, matrix metalloproteinases (MMPs), and three panels of human cardiovascular disease (CVD) biomarkers were used to analyze samples to identify biomarkers that have a potential to predict the development of DOX-induced cardiotoxicity.
Materials and methods
Patients and plasma samples
Twenty-seven women with breast cancer were enrolled for DOX-based chemotherapy at the University of Arkansas for Medical Sciences under an Institutional Review Board-approved protocol with written informed consent from each patient. The study was also approved by the U.S. Food and Drug Administration (FDA) Research Involving Human Subjects Committee. All patients were treated with a combination of 60 mg/m2 DOX and 600 mg/m2 cyclophosphamide every 2–3 weeks for four cycles (cumulative dose of DOX 240 mg/m2 and cumulative dose of cyclophosphamide 2400 mg/m2). Blood samples were collected into EDTA blood collection tubes immediately prior to the first (T0), the second (T1), and the third (T2) doses of DOX treatment. Blood samples were immediately processed into plasma by centrifugation. The plasma samples were deidentified and stored at −80°C until further analysis.
Measurement of cardiac function
Cardiac function of the patients was assessed by measuring LVEF. LVEF was measured by a MUGA scan before the first dose and after completion of the fourth cycle of DOX-based treatment. An asymptomatic reduction of the LVEF of >10% or LVEF <50%, or a reduction of LVEF >5% to LVEF < 55% with symptoms of heart failure was considered abnormal.11,12
Measurement of plasma cTnT concentrations
Patients’ plasma concentrations of cTnT were measured using the fourth-generation cTnT Short Turn Around Time immunoassay (Roche Diagnostics, Indianapolis, IN, USA). The assay was run on a Cobas analyzer (Roche Diagnostics, Indianapolis, IN, USA), following the manufacturer’s standard procedure. The lower detection limit of the assay was 0.010 ng/mL. All the samples collected at the three time points were tested.
Magnetic bead-based multiplex immunoassays
Two Bio-Plex and three Milli-Plex magnetic bead-based multiplex immunoassay panels were analyzed to measure concentrations of protein biomarkers in plasma samples collected from the breast cancer patients. The Bio-Plex Pro™ human chemokine 40-plex panel (Bio-Rad #171AK99MR2) includes 6Ckine/CCL21, BCA-1/CXCL13, CTACK/CCL27, ENA-78/CXCL5, eotaxin/CCL11, eotaxin-2/CCL24, eotaxin-3/CCL26, fractalkine/CX3CL1, GCP-2/CXCL6, GM-CSF, Gro-α/CXCL1, Gro-β/CXCL2, I-309/CCL1, IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8/CXCL8, IL-10, IL-16, IP-10/CXCL10, I-TAC/CXCL11, MCP-1/CCL2, MCP-2/CCL8, MCP-3/CCL7, MCP-4/CCL13, MDC/CCL22, MIF, MIG/CXCL9, MIP-1α/CCL3, MIP-1δ/CCL15, MIP-3α/CCL20, MIP-3β/CCL19, myeloid progenitor inhibitory factor 1 (MPIF-1)/CCL23, SCYB16/CXCL16, SDF-1α + β/CXCL12, TARC/CCL17, TECK/CCL25, and TNF-α. Bio-Plex Pro™ human MMP 9-Plex panel (Bio-Rad #171AM001M) is for quantification of nine MMPs, which includes MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-10, MMP-12, and MMP-13. The MILLIPLEX MAP human CVD magnetic bead panel 1 (EMDMillipore #HCVD1MAG-67K) includes BNP, NT-proBNP, CK-MB, CXCL6/GCP-2, CXCL16, endocan-1 (ESM-1), FABP3, FABP4, LIGHT, oncostatin M, placental growth factor, and troponin I. The MILLIPLEX MAP human CVD magnetic bead panel 2 (EMDMillipore #HCVD2MAG-67K) includes ADAMTS13, D-dimer, GDF-15, myoglobin, sICAM-1, MPO, P-selectin, lipocalin-2/NGAL, sVCAM-1, and SAA. The MILLIPLEX MAP human CVD (acute phase) magnetic bead panel 3 (EMDMillipore #HCVD3MAG-67K) includes alpha-1 acid glycoprotein, adipsin, α2-macroglobulin, CRP, fetuin A, fibrinogen, l-selectin, serum amyloid P, haptoglobin, platelet factor-4, and von Willebrand factor.
Plasma sample dilution and the assay were performed according to the manufacturer’s instructions using 96-well plates. The plates were washed with appropriate solutions from each panel using a Bio-Plex Pro™ II wash station (Bio-Rad Laboratories, Hercules, CA, USA). Samples were measured in duplicate and the plates were read using the Luminex® 200™ system (Bio-Rad Laboratories, Hercules, CA, USA). Duplicate measurements were averaged. Based on the measurements of seven standard concentrations provided by the manufacturer, a standard calibration curve was generated with five-parameter logistic regression using Bio-Plex Manager™ Software Version 4.0 (Bio-Rad Laboratories, Hercules, CA, USA). The standard curve of each protein was used to convert the optical density values of the samples into concentrations. Sample measurements were considered reliable if the standards for the calibration curve fell within 70–130% of recovery as recommended by the manufacturer and the sample concentrations were within the range of standard concentrations.
Statistical analyses
Statistical tests were performed to assess the significance between group differences. Protein concentration values were log transformed, and then a two-tailed unpaired t-test with unequal variances (Welch’s t-test) was used to compare between the patient groups with different cardiac function at the same time point or between time points within the same group. The concentration of each protein in each group (normal, intermediate, and abnormal) at each time point (T0, T1, and T2) was expressed as mean±SD, and p < 0.05 was viewed as a statistically significant difference. Statistical significance was annotated on the figures or in the figure legends. Statistical analyses and graphics were done using the program R (https://www.r-project.org/) with packages ggplot2.13
Results
Cardiotoxicity in breast cancer patients
Twenty-seven breast cancer patients who underwent DOX treatment were enrolled under an IRB-approved protocol for the identification of potential predictive biomarkers of cardiotoxicity. For this purpose, an asymptomatic reduction of the LVEF of >10% or below <50%, or a reduction of LVEF >5% to LVEF < 55% with symptoms of heart failure after completion of four cycles of DOX treatment was defined as abnormal cardiac function, i.e. cardiotoxicity. Cardiac function was considered normal when the reduction of LVEF was <5% after the DOX treatment was completed. Decline in LVEF > 4%14 or >5%15 was suggested as predictors of subclinical cardiotoxicity of DOX. Since the decrease of cardiac function is under a progressive course, an LVEF reduction of 5–10% was considered to be subcardiotoxicity or at the intermediate stage. This group would facilitate biomarker discovery by examining the trends in protein concentration changes. Based on the degree of LVEF reduction after completion of DOX treatment, the breast cancer patients were categorized into three groups: normal, intermediate, and abnormal groups (Table 1). Seventeen patients were within the normal group, five patients were in the intermediate group with an average reduction of LVEF of 6.4%, and five patients showed signs of cardiotoxicity (an LVEF reduction of over 10%) with an average LVEF reduction of 13.2%. In addition, metastasis was found in one patient in the normal group and one patient in the abnormal group (Table 1).
Table 1.
Demographic data of breast cancer patients and cardiac function outcomes after DOX-based treatment.
| Patient group | Patient # | Ethnicity | Age range | Average age | Number of patients with metastasis | LVEF (%) decrease |
|---|---|---|---|---|---|---|
| Normal | 17 | AA (5), CC (12) | 43–68 | 53 | 1 | <5 |
| Intermediate | 5 | AA (2), CC(3) | 35–64 | 48 | 0 | 5–8 |
| Abnormal | 5 | CC (5) | 51–60 | 58 | 1 | >10 |
AA: African-American; CC: Caucasian; DOX: doxorubicin; LVEF: left ventricular ejection fraction.
Comparison of baseline plasma levels of biomarkers between patient groups before DOX treatment
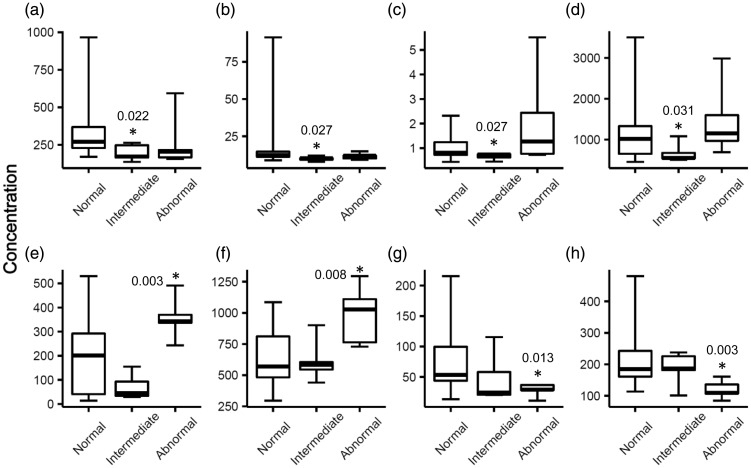
To examine if there were molecular differences in the baseline levels of plasma proteins between the three patient groups, plasma samples were evaluated using multiplex immunoassays for five biomarker panels that measure concentrations of chemokines, MMPs, and CVD biomarkers before DOX treatment (T0). Four proteins demonstrated statistically significantly (p < 0.05) lower levels of expression in the intermediate group than the normal group: CXCL1 (196±55 versus 340±203 pg/mL, −1.7x), CCL3 (10 ±1.5 versus 17±19 pg/mL, −1.7x), GDF-15 (0.66±0.13 versus 1.01±0.48 ng/mL, −1.5x), and haptoglobin (669±237 versus 1181±741 µg/mL, −1.8x) (Figure 1). Four proteins demonstrated statistically significant abundance differences between the abnormal and normal groups: CCL23 (357±89 versus 201±168 pg/mL, +1.8x), CCL27 (985±239 versus 643±240 pg/mL, +1.5x), as well as CXCL6 (28±11 versus 74±48 pg/mL, −2.6x) and sICAM-1 (120±29 versus 222±98 ng/mL, −1.9x) (Figure 1).
Figure 1.
Proteins that had differential plasma levels in the intermediate or abnormal patient group as compared to the normal group before DOX treatment (T0). An asterisk denotes the protein had a statistically significant different level in that group as compared to the normal group. P-values were calculated by Welch’s t-test and indicated by the asterisks. Edges of boxes denote 25th and 75th percentiles, lines are median concentrations, and error bars are minimum and maximum concentrations. (a) CXCL1 (pg/mL), (b) CCL3 (pg/mL), (c) GDF-15 (ng/mL), (d) haptoglobin (µg/mL), (e) CCL23 (pg/mL), (f) CCL27 (pg/mL), (g) CXCL6 (pg/mL), and (h) sICAM-1 (ng/mL). GDF-15: growth/differentiation factor 15; sICAM-1: soluble intercellular adhesion molecule 1.
Comparison of plasma levels of biomarkers between patient groups after the first cycle of DOX treatment
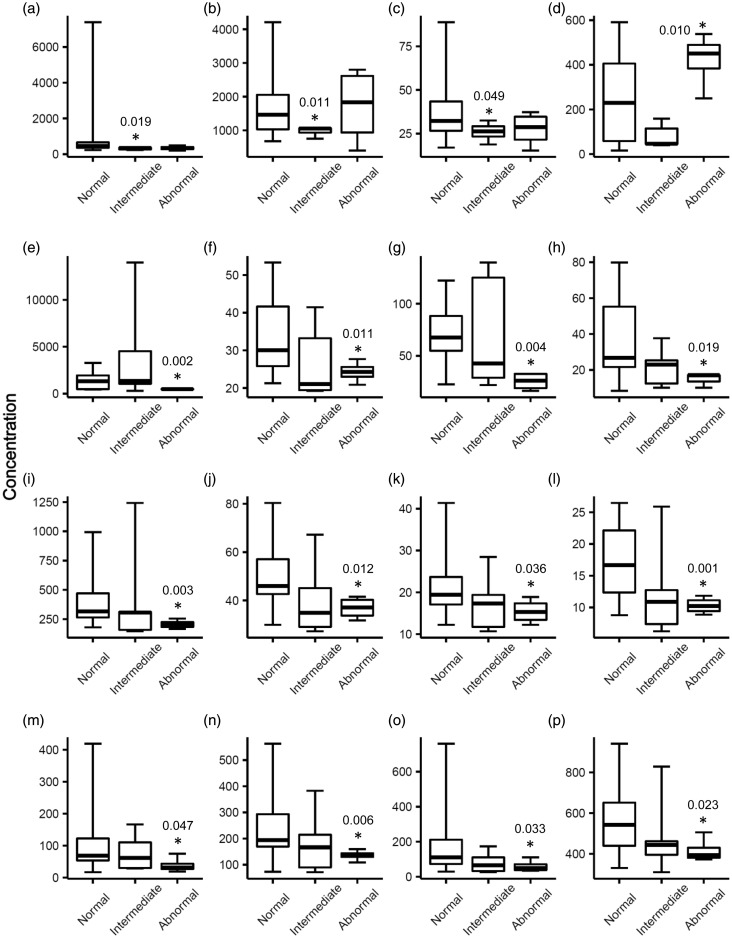
After the first dose and right before the second dose of DOX treatment (T1), biomarker panel analyses identified three proteins with significant abundance decreases in plasma (p < 0.05) in the intermediate group: IL-16 (315±62 pg/mL, −3.2x), FABP3 (977±137 pg/mL, −1.8x), and myoglobin (26±5 ng/mL, −1.5x) as compared to the normal group (Figure 2). Thirteen proteins demonstrated significant abundance differences between the abnormal and normal groups. CCL23 (422±123 pg/mL, +1.7x) was the only protein to demonstrate an increase in the abnormal group. CXCL5 (485±54 pg/mL, −2.8x), CCL26 (24±3 pg/mL, −1.4x), CXCL6 (25±9 pg/mL, −2.7x), GM-CSF (15±4 pg/mL, −2.5x), CXCL1 (206±38 pg/mL, −1.9x), IFN-γ (37±5 pg/mL, −1.4x), IL-2 (15±3 pg/mL, −1.4x), IL-8 (10±1 pg/mL, −1.7x), CXCL11 (39±24 pg/mL, −3.0x), CXCL9 (136±21 pg/mL, −1.9x), CCL17 (60±35 pg/mL, −3.0x), and CCL25 (416±61 pg/mL, −1.4x) had decreased plasma levels in the abnormal group (Figure 2).
Figure 2.
Proteins that had differential plasma levels in the intermediate or abnormal patient group as compared to the normal group after the first cycle of DOX treatment (T1). An asterisk denotes the protein had a statistically significant different level in that group as compared to the normal group. P-values were calculated by Welch’s t-test and indicated by the asterisks. Edges of boxes denote 25th and 75th percentiles, lines are median concentrations, and error bars are minimum and maximum concentrations. (a) IL-16 (pg/mL), (b) FABP3 (pg/mL), (c) myoglobin (ng/mL), (d) CCL23 (pg/mL), (e) CXCL5 (pg/mL), (f) CCL26 (pg/mL), (g) CXCL6 (pg/mL), (h) GM-CSF (pg/mL), (i) CXCL1 (pg/mL), (j) IFN-γ (pg/mL), (k) IL-2 (pg/mL), (l) IL-8 (pg/mL), (m) CXCL11 (pg/mL), (n) CXCL9 (pg/mL), (o) CCL17 (pg/mL), and (p) CCL25 (pg/mL). FABP3: fatty acid-binding protein 3; GM-CSF: granulocyte-macrophage colony-stimulating factor; IFN-γ: interferon gamma; IL-2: interleukin 2; IL-8: interleukin 8; IL-16: interleukin 16.
Comparison of plasma levels of biomarkers between patients groups after the second cycle of DOX treatment
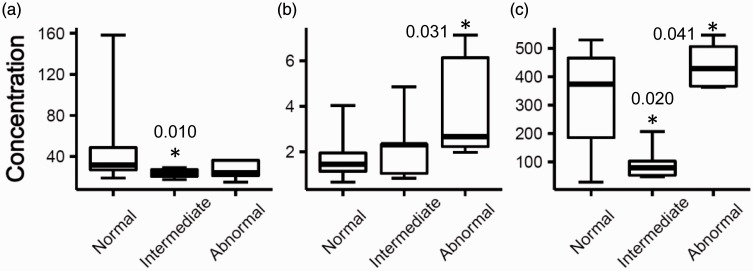
Just prior to the third cycle of DOX treatment (T2), two proteins demonstrated significant abundance decreases in plasma (p < 0.05) in the intermediate group: myoglobin (24±5 ng/mL, −2.0x) and CCL23 (98±65 pg/mL, −3.3x), as compared to the normal group (Figure 3). Two proteins demonstrated abundance increases in the abnormal group: MIF (4.0±2.4 ng/mL, +2.3x) and CCL23 (442±83 pg/mL, +1.4x), as compared to the normal group (Figure 3).
Figure 3.
Proteins that had differential plasma levels in the intermediate or abnormal patient group as compared to the normal group after the second cycle of DOX treatment (T2). An asterisk denotes the protein had a statistically significant different level in that group as compared to the normal group. P-values were calculated by Welch’s t-test and indicated by the asterisks. Edges of boxes denote 25th and 75th percentiles, lines are median concentrations, and error bars are minimum and maximum concentrations. (a) Myoglobin (ng/mL), (b) MIF (ng/mL), and (c) CCL23 (pg/mL). MIF: macrophage migration inhibitory factor.
Discussion
This early phase discovery study was designed to identify potential biomarkers that may aid in the identification of patients at risk of DOX-related cardiotoxicity. Currently there are no validated biomarkers that could be used to predict the development of drug-induced cardiotoxicity. CVD biomarkers such as cTnT and cTnI have been qualified for use in non-clinical cardiac safety assessment studies;16 however, their efficacy in predicting drug-induced clinical cardiotoxicity has not been validated. DOX has been used to treat many types of cancer for decades and has been notorious for causing dose-cumulative cardiotoxicity. Under an IRB-approved protocol, 27 breast cancer patients who were scheduled to undergo DOX treatment were recruited in this pilot biomarker study. Blood samples were collected and processed to plasma before (T0), after the first cycle (T1) and second cycle (T2) of DOX treatment. While the clinical endpoint evaluation of cardiac function was after the completion of four cycles of DOX treatment, the time points for blood collection represent the early time points at which abnormal cardiac function by imaging is usually not observed. It should be noted that blood collection in this study was 2–3 weeks after the first dose and right before the second dose for T1 samples, and it was 2–3 weeks after the second dose and right before the third dose for T2 samples. Sample collection was a part of the routine clinical procedures with DOX treatment, thus any biomarkers identified could be practically feasible for clinical implementation. For all the three time points, cTnT levels were below the lower detection limit of the Roche’s fourth-generation cTnT assay for all the samples tested in a Cobas clinical analyzer; thus, the results further confirmed cTnT could not be a predictive biomarker for the defined time points in this study.
There could be pre-existing differences at the baseline levels of circulation proteins, which could be potential biomarkers for cardiotoxicity prediction. Analysis of five multiplex immunoassay panels (chemokines, MMPs, and three CVD panels) for the plasma samples collected before DOX treatment (T0) identified four proteins with lower abundance in the intermediate patient group while four other proteins had differential levels in the abnormal group as compared to the normal group. These data suggest measurement of protein baseline levels could identify promising predictive biomarkers of cardiotoxicity. These biomarkers would be screened prior to initiation of DOX treatment to prevent cardiotoxicity although further clinical validation is required in a large cohort of patients.
As discussed earlier, T1 and T2 represent the early time points after DOX treatment. Biomarkers discovered at these time points are useful for the prediction of cardiotoxicity after completion of the chemotherapy. Multiplex immunoassay analysis revealed that three proteins in the intermediate group and 13 proteins in the abnormal group had abundance levels different from that in the normal group at T1. Three proteins showed differential levels at T2 in either the intermediate or the abnormal group or both groups. The results suggest these proteins are promising predictive biomarkers at the two time points.
It was found that the plasma levels of CCL23 were higher in the abnormal group at all the three time points as compared to the normal group with respective fold changes of 1.8, 1.7, and 1.4 at T0, T1, and T2. The concentration of this protein trended toward an increase over treatment time in both groups although the increase was not statistically significant. CCL23 is a relatively new chemokine and is also known as macrophage inflammatory protein 3 and MPIF-1. It has inhibitory activity on hematopoietic progenitor cells.17 Studies have demonstrated that increased CCL23 is associated with coronary atherosclerosis.18 It was also found that levels of CCL23 increased in patients with severe chronic kidney disease, which was correlated with kidney function.19 However, CCL23 as a biomarker of cardiotoxicity has not been reported. It remains to be elucidated whether CCL23 contributes to the development of drug-induced cardiotoxicity.
In addition, CCL27 and MIF had increased levels in the abnormal patient group at T0 and T2 with fold changes of 1.5 (p = 0.008) and 2.3 (p = 0.031), respectively. CCL27 is a small cytokine associated with homing of memory T lymphocytes to the inflammation sites.20 Its roles in T cell-mediated skin inflammation requires CCL27’s interaction with its receptor CCR10.21 Elevated CCL27 was reported in psoriasis, eczema, and urticarial.22,23 Although it has not been reported that CCL27 plays inflammatory roles in DOX-induced cardiotoxicity, elevated levels of CCL27 before DOX treatment could be a predictive biomarker. MIF is a proinflammatory cytokine involved in innate immune response and a mediator of acute and chronic inflammatory responses.24 It plays a role in the maintenance of cardiac homeostasis under stress conditions. Previous studies found that MIF was elevated in myocardial infarction, atherosclerosis, rheumatoid arthritis, sepsis, cancer, portopulmonary hypertension diseases,25–30 and in critically ill patients.31 Recent studies suggested that MIF could play cardioprotective roles against DOX-induced cardiomyopathy,32,33 probably through attenuating loss of autophagy and ATP availability in the heart.33 An increase of MIF may be an early protective response to potential cardiac injury that leads to the later development of irreversible cardiotoxicity. Higher plasma levels of MIF in the abnormal patient group after second cycles of DOX treatment suggest this protein could be a predictive biomarker of cardiotoxicity.
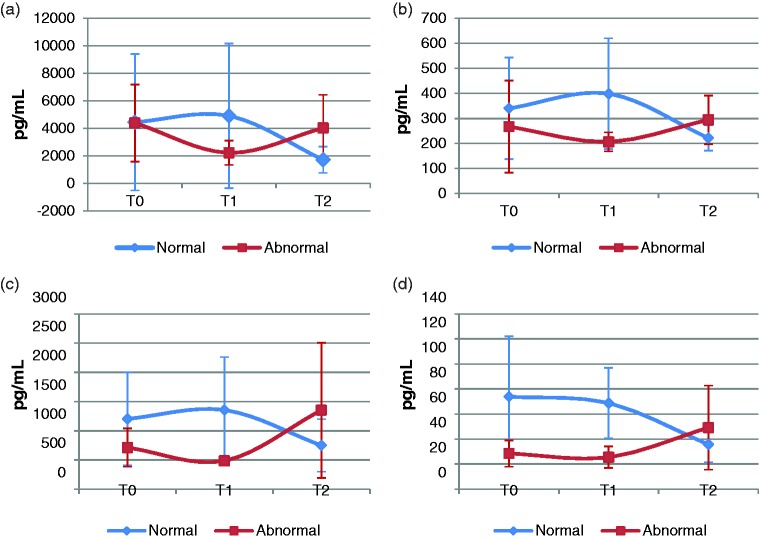
Except for CCL23, CCL27, and MIF, the other chemokines that were differentially changed had lower abundance in the abnormal group. While the majority of these chemokines are considered to be inflammatory or proinflammatory in general,34 the actual role of a chemokine depends on a variety of factors including specific disease state, injury, pathophysiology, etc. Studies have demonstrated that some cytokines/chemokines function as both proinflammatory and anti-inflammatory mediators.35,36 The exact biological function is dependent on the local concentration of a given cytokine, the stage of disease, and its combination with other cytokines.35 It requires further investigation to determine whether DOX treatment (as an antibiotic) enhances inflammation or not. Interestingly, it was found that many of the differentially expressed chemokines (e.g. MIF, CXCL1, CXCL5, and CXCL6) tended to decrease at T1 and increase in abundance at T2 in the abnormal group while they tended to remain steady or slightly increase in abundance at T1 and decrease at T2 in the normal group (Figure 4, Supplemental Tables S1 and S2). A few of the chemokines such as CXCL1 and CXCL6 showed a statistically significant decrease over time in the normal group (Figure 4, Supplemental Table S1). The differential dynamics of these immune response proteins with DOX treatment over time between the normal and abnormal patient groups account for the lower plasma abundance of 12 chemokines in the abnormal group at T1. Studies indicated that CXCL1 up-regulation was one of the cardioprotective effects of adding human amniotic fluid stem cell conditioned medium to mouse neonatal ventricular cardiomyocytes subjected to DOX treatment37; thus, lower levels of CXCL1 observed in this study could increase susceptibility of cardiomyocytes to DOX-induced damage.
Figure 4.
Plasma concentration changes of MIF, CXCL1, CXCL5, and CXCL6 over DOX treatment time course in the normal and abnormal patient groups. Shown are mean concentrations. Error bars are standard deviation. (a) MIF, (b) CXCL1, (c) CXCL5, and (d) CXCL6. MIF: macrophage migration inhibitory factor; T0: before DOX treatment; T1: after the first cycle of DOX treatment; T2: after the second cycle of DOX treatment. (A color version of this figure is available in the online journal.)
The results from this study indicate that inflammation and immunity play important roles in the early subclinical response to DOX-based chemotherapy. It may be possible that the cardiomyocytes of the immunologically sensitive patients are more susceptible to DOX-induced damage. More clinical and experimental studies are required to address these questions. This pilot study identified significant differences of the chemokine profiles or ‘immune signatures’ between the cardiotoxicity group and the normal group at each time point. These immune signatures are implicated in inflammatory response and immune trafficking and are associated with the increased individual sensitivity to DOX cardiotoxic effects in breast cancer patients, thus could be promising predictive biomarkers of cardiotoxicity. Although there were no statistically significant age differences between three patient groups in this study, expanded studies with consideration of other confounding factors such as previous treatment, cancer type, and stage in a large cohort of patients are required to further validate the findings of this initial discovery.
Supplementary Material
Acknowledgments
JRD would like to acknowledge NCTR/FDA for postdoctoral support through the Oak Ridge Institute for Science and Education (ORISE). The information in this article is not a formal dissemination of information by FDA and does not represent agency position or policy.
Authors’ contributions
LRY and VKT participated in the design of the study. IM and VKT collected blood samples. LRY conducted the experiments. ZC and LRY analyzed data. RDB, JRD, IM, SK, JYW, JPFB, JL, JTL, and LRY interpreted the data. LRY wrote the main manuscript. All authors reviewed and edited the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This work was supported with funds from the National Center for Toxicological Research, U.S. Food and Drug Administration (NCTR/FDA) to LRY (E0756201); and in part with grants from the Arkansas Breast Cancer Research Programs and NIH/NIA Claude Pepper Center (P30AG028718) to VKT.
References
- 1.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med 1998; 339:900–5 [DOI] [PubMed] [Google Scholar]
- 2.Floyd JD, Nguyen DT, Lobins RL, Bashir Q, Doll DC, Perry MC. Cardiotoxicity of cancer therapy. J Clin Oncol 2005; 23:7685–96 [DOI] [PubMed] [Google Scholar]
- 3.Yeh ET. Cardiotoxicity induced by chemotherapy and antibody therapy. Annu Rev Med 2006; 57:485–98 [DOI] [PubMed] [Google Scholar]
- 4.Cardinale D, Bacchiani G, Beggiato M, Colombo A, Cipolla CM. Strategies to prevent and treat cardiovascular risk in cancer patients. Semin Oncol 2013; 40:186–98 [DOI] [PubMed] [Google Scholar]
- 5.Herman EH, Lipshultz SE, Rifai N, Zhang J, Papoian T, Yu ZX, Takeda K, Ferrans VJ. Use of cardiac troponin T levels as an indicator of doxorubicin-induced cardiotoxicity. Cancer Res 1998; 58:195–7 [PubMed] [Google Scholar]
- 6.Monsuez JJ. Detection and prevention of cardiac complications of cancer chemotherapy. Arch Cardiovasc Dis 2012; 105:593–604 [DOI] [PubMed] [Google Scholar]
- 7.Todorova VK, Makhoul I, Siegel ER, Wei J, Stone A, Carter W, Beggs ML, Owen A, Klimberg VS. Biomarkers for presymptomatic doxorubicin-induced cardiotoxicity in breast cancer patients. PLoS One 2016; 11:e0160224. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Todorova VK, Makhoul I, Dhakal I, Wei J, Stone A, Carter W, Owen A, Klimberg VS. Polymorphic variations associated with doxorubicin-induced cardiotoxicity in breast cancer patients. Oncol Res 2017; 25:1223–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernando MM, Stevens CR, Walsh EC, De Jager PL, Goyette P, Plenge RM, Vyse TJ, Rioux JD. Defining the role of the MHC in autoimmunity: a review and pooled analysis. PLoS Genet 2008; 4:e1000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abu-Shakra M, Buskila D, Ehrenfeld M, Conrad K, Shoenfeld Y. Cancer and autoimmunity: autoimmune and rheumatic features in patients with malignancies. Ann Rheum Dis 2001; 60:433–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mookadam F, Sharma A, Lee HR, Northfelt DW. Intersection of cardiology and oncology clinical practices. Front Oncol 2014; 4:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seidman AD, Fornier MN, Esteva FJ, Tan L, Kaptain S, Bach A, Panageas KS, Arroyo C, Valero V, Currie V, Gilewski T, Theodoulou M, Moynahan ME, Moasser M, Sklarin N, Dickler M, D'andrea G, Cristofanilli M, Rivera E, Hortobagyi GN, Norton L, Hudis CA. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. JCO 2001; 19:2587–95 [DOI] [PubMed] [Google Scholar]
- 13.Wickham H. ggplot2: elegant graphics for data analysis. 1st ed New York: Springer-Verlag, 2009 [Google Scholar]
- 14.Nousiainen T, Jantunen E, Vanninen E, Hartikainen J. Early decline in left ventricular ejection fraction predicts doxorubicin cardiotoxicity in lymphoma patients. Br J Cancer 2002; 86:1697–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fatima N, Zaman MU, Hashmi A, Kamal S, Hameed A. Assessing adriamycin-induced early cardiotoxicity by estimating left ventricular ejection fraction using technetium-99m multiple-gated acquisition scan and echocardiography. Nucl Med Commun 2011; 32:381–5 [DOI] [PubMed] [Google Scholar]
- 16.Hausner EA, Hicks KA, Leighton JK, Szarfman A, Thompson AM, Harlow P. Qualification of cardiac troponins for nonclinical use: a regulatory perspective. Regul Toxicol Pharmacol 2013; 67:108–14 [DOI] [PubMed] [Google Scholar]
- 17.Patel VP, Kreider BL, Li Y, Li H, Leung K, Salcedo T, Nardelli B, Pippalla V, Gentz S, Thotakura R, Parmelee D, Gentz R, Garotta G. Molecular and functional characterization of two novel human C-C chemokines as inhibitors of two distinct classes of myeloid progenitors. J Exp Med 1997; 185:1163–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castillo L, Rohatgi A, Ayers CR, Owens AW, Das SR, Khera A, McGuire DK, de Lemos JA. Associations of four circulating chemokines with multiple atherosclerosis phenotypes in a large population-based sample: results from the Dallas heart study. J Interferon Cytokine Res 2010; 30:339–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pawlak K, Mysliwiec M, Pawlak D. Effect of diabetes and oxidative stress on plasma CCL23 levels in patients with severe chronic kidney disease. Pol Arch Med Wewn 2014; 124:459–66 [DOI] [PubMed] [Google Scholar]
- 20.Morales J, Homey B, Vicari AP, Hudak S, Oldham E, Hedrick J, Orozco R, Copeland NG, Jenkins NA, McEvoy LM, Zlotnik A. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc Natl Acad Sci USA 1999; 96:14470–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Homey B, Alenius H, Muller A, Soto H, Bowman EP, Yuan W, McEvoy L, Lauerma AI, Assmann T, Bunemann E, Lehto M, Wolff H, Yen D, Marxhausen H, To W, Sedgwick J, Ruzicka T, Lehmann P, Zlotnik A. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med 2002; 8:157–65 [DOI] [PubMed] [Google Scholar]
- 22.Garzorz-Stark N, Krause L, Lauffer F, Atenhan A, Thomas J, Stark SP, Franz R, Weidinger S, Balato A, Mueller NS, Theis FJ, Ring J, Schmidt-Weber CB, Biedermann T, Eyerich S, Eyerich K. A novel molecular disease classifier for psoriasis and eczema. Exp Dermatol 2016; 25:767–74 [DOI] [PubMed] [Google Scholar]
- 23.Lu T, Jiao X, Si M, He P, Zou J, Zhang S, Zeng K. The correlation of serums CCL11, CCL17, CCL26, and CCL27 and disease severity in patients with urticaria. Dis Markers 2016; 2016:1381760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morand EF. New therapeutic target in inflammatory disease: macrophage migration inhibitory factor. Intern Med J 2005; 35:419–26 [DOI] [PubMed] [Google Scholar]
- 25.Rassaf T, Weber C, Bernhagen J. Macrophage migration inhibitory factor in myocardial ischaemia/reperfusion injury. Cardiovasc Res 2014; 102:321–8 [DOI] [PubMed] [Google Scholar]
- 26.Zernecke A, Bernhagen J, Weber C. Macrophage migration inhibitory factor in cardiovascular disease. Circulation 2008; 117:1594–602 [DOI] [PubMed] [Google Scholar]
- 27.Donn RP, Shelley E, Ollier WE, Thomson W. A novel 5′-flanking region polymorphism of macrophage migration inhibitory factor is associated with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum 2001; 44:1782–5 [DOI] [PubMed] [Google Scholar]
- 28.Chuang CC, Wang ST, Chen WC, Chen CC, Hor LI, Chuang AY. Increases in serum macrophage migration inhibitory factor in patients with severe sepsis predict early mortality. Shock 2007; 27:503–6 [DOI] [PubMed] [Google Scholar]
- 29.He XX, Yang J, Ding YW, Liu W, Shen QY, Xia HH. Increased epithelial and serum expression of macrophage migration inhibitory factor (MIF) in gastric cancer: potential role of MIF in gastric carcinogenesis. Gut 2006; 55:797–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DuBrock HM, Rodriguez-Lopez JM, LeVarge BL, Curry MP, VanderLaan PA, Zsengeller ZK, Pernicone E, Preston IR, Yu PB, Nikolic I, Xu D, Thadhani RI, Channick RN, Ananth Karumanchi S. Macrophage migration inhibitory factor as a novel biomarker of portopulmonary hypertension. Pulm Circ 2016; 6:498–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pohl J, Hendgen-Cotta UB, Stock P, Luedike P, Rassaf T. Elevated MIF-2 levels predict mortality in critically ill patients. J Crit Care 2017; 40:52–7 [DOI] [PubMed] [Google Scholar]
- 32.Xu X, Bucala R, Ren J. Macrophage migration inhibitory factor deficiency augments doxorubicin-induced cardiomyopathy. J Am Heart Assoc 2013; 2:e000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu X, Pang J, Chen Y, Bucala R, Zhang Y, Ren J. Macrophage Migration Inhibitory Factor (MIF) deficiency exacerbates aging-induced cardiac remodeling and dysfunction despite improved inflammation: role of autophagy regulation. Sci Rep 2016; 6:22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Y, Zhou Y, Iribarren P, Wang J. Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol Immunol 2004; 1:95–104 [PubMed] [Google Scholar]
- 35.Shachar I, Karin N. The dual roles of inflammatory cytokines and chemokines in the regulation of autoimmune diseases and their clinical implications. J Leukoc Biol 2013; 93:51–61 [DOI] [PubMed] [Google Scholar]
- 36.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 2011; 1813:878–88 [DOI] [PubMed] [Google Scholar]
- 37.Lazzarini E, Balbi C, Altieri P, Pfeffer U, Gambini E, Canepa M, Varesio L, Bosco MC, Coviello D, Pompilio G, Brunelli C, Cancedda R, Ameri P, Bollini S. The human amniotic fluid stem cell secretome effectively counteracts doxorubicin-induced cardiotoxicity. Sci Rep 2016; 6:29994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.