SUMMARY
Comprising the majority of leukocytes in humans, neutrophils are the first immune cells to respond to inflammatory or infectious etiologies and are crucial participants in the proper functioning of both innate and adaptive immune responses. From their initial appearance in the liver, thymus, and spleen at around the eighth week of human gestation to their generation in large numbers in the bone marrow at the end of term gestation, the differentiation of the pluripotent hematopoietic stem cell into a mature, segmented neutrophil is a highly controlled process where the transcriptional regulators C/EBP-α and C/EBP-ε play a vital role. Recent advances in neutrophil biology have clarified the life cycle of these cells and revealed striking differences between neonatal and adult neutrophils based on fetal maturation and environmental factors. Here we detail neutrophil ontogeny, granulopoiesis, and neutrophil homeostasis and highlight important differences between neonatal and adult neutrophil populations.
KEYWORDS: neutrophils, granulopoiesis, innate immunity, phagocytosis, chemokine, extracellular traps, cell death, apoptosis, degranulation, neonates, hematopoiesis
INTRODUCTION
The greatest percentage of hematopoiesis is committed to the production of neutrophils, with an estimated 60% of all leukocytes in the bone marrow comprising granulocyte precursors (1). Neutrophils play a key role in host defense against bacterial, viral, and fungal infections, but the nature of their cytotoxic contents dictates that appropriate developmental and clearance mechanisms be in place to protect the host against unintended inflammatory injury. Neutrophil homeostasis is maintained through a careful balance of granulopoiesis, bone marrow storage and release, and migration into vascular compartments and peripheral tissues. Despite their relatively short life span, these intriguing cells not only are vital for pathogen elimination during early infection but also link innate and adaptive immune responses to promote the resolution of inflammation and wound healing. In this review, neutrophil development is discussed, commencing at the earliest stages of embryogenesis. A detailed examination of granulopoiesis, mechanisms regulating neutrophil homeostasis, and specific differences between neonatal and adult neutrophil biology is also provided. Finally, the neutrophil's role in host immunity and factors directly influencing cell survival are addressed.
DEVELOPMENT
Hematopoiesis
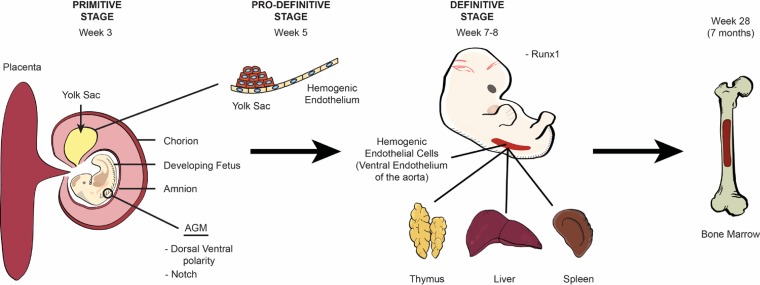
Fetal hematopoiesis, or the creation of all blood cells, is an evolutionarily conserved process that proceeds in three distinct stages during human development (2). The first, or primitive, stage originates from the mesoderm in the extraembryonic yolk sac around the third week of embryogenesis and gives rise to a transient primeval population primarily composed of large, nucleated erythroid cells with limited numbers of macrophages and megakaryocytes (3–5). The appearance of the hemogenic endothelium within the yolk sac around the fifth week of fetal growth marks the prodefinitive, or second, stage of blood cell development. This dedicated endothelium can produce a greater variety of transitory myeloid-lineage precursor cells, including macrophage, monocyte, and granulocyte progenitors, in addition to primitive erythrocytes and megakaryocytes (Fig. 1) (6–8). It is not until the third, or definitive, stage around the seventh to eighth week of gestation, however, that genuine pluripotent hematopoietic stem cells (HSCs) originate from specialized intraembryonic endothelial cells within the major arteries of the developing embryo, including the ventral wall of the descending aorta and vitelline and umbilical arteries, in a process that has been termed the endothelial-to-hematopoietic transition (EHT) (3, 4, 9–14).
FIG 1.
Ontogeny of hematopoiesis. The origin of human blood cells begins in the mesoderm of the extraembryonic yolk sac around the third week of embryogenesis and is known as the primitive stage. The emergence of blood cells from the hemogenic endothelium begins within the yolk sac in week 5 during the prodefinitive (second) stage and is regulated by dorsal-ventral polarity and Notch signaling. Blood cell production then transitions to the ventral wall of the aorta at around the seventh to eighth week during the definitive stage and is regulated by the transcription factor Runx1 in a process known as the endothelial-to-hematopoietic transition (EHT), where HSCs are first identified. After the eighth week of gestation, early blood cells seed the liver, thymus, and spleen until the seventh month of gestation, when hematopoiesis transitions to the bone marrow. After this time, the bone marrow becomes the sole site for platelet and red and white blood cell formation.
The development of the distal aorta from the lateral plate mesoderm and the emergence of HSCs are closely correlated via common KDR/vascular endothelial growth factor (VEGF) signaling pathways (15, 16). Dorsal-ventral polarity of the descending aorta (17) and Notch signaling (18–20) are essential for HSC and hematopoietic progenitor formation from the ventral wall of the distal aorta in an area known as the aorta-gonad-mesonephros (AGM) region (21, 22). In mice, Notch 1 and Notch 2 are primary regulators of transcription factors required to establish the arterial identity of the endothelium and specification of the hematogenic endothelium in the lateral plate mesoderm (18, 23–25). Having the more vital role, Notch 1 mutants display severely impaired hematopoiesis and are embryonic lethal (18, 19, 26), while Notch 2 knockouts exhibit no obvious hematopoietic defects (18, 26).
Developing HSCs in the AGM region become organized into intra-aortic hematopoietic clusters (IAHCs) by Runx1 (15), expressed by endothelial cells in the vitelline and umbilical arteries, the ventral aspect of the dorsal aorta, and some endothelial cells in the yolk sac (27, 28). Runx1 encodes the DNA-binding subunit of core-binding factor (CBF), which is required for the establishment of definitive but not primitive hematopoiesis (27, 29). Murine knockout models of Runx1 exhibit normal blood island development and progression through the yolk sac phase of hematopoiesis but die prior to definitive hematopoiesis (29). Runx1-null mice contain only primitive nucleated erythrocytes in liver tissues, with a complete lack of definitive erythroid, myeloid, and megakaryocytic cells (29). Although Notch signaling is crucial during the earliest stages of the EHT, HSCs become Notch independent during definitive hematopoiesis, at which time Notch is downregulated (18, 30).
Conversely, a more recent theory suggests that primordial germ cell (PGC) populations link the traditional second and third stages of fetal hematopoiesis (31). In this model, PGCs located in the AGM region of the developing fetus are destined to become either gametes if they express high levels of Oct3/4 or HSCs if they are weakly Oct3/4 positive and express distinct PGC markers (BLIMP-1, adaptor protein complex [AP], TG-1, and STELLA), coexpressed proteins (CD34, CD41, and FLK-1), and genes (Brachyury, Hox-B4, Scl/Tal-1, and GATA-2) (31). Presently, no human studies support this concept of definitive hematopoiesis, with limited favorable data available only for murine models.
Regardless of how these cells come to occupy the ventral wall of the descending aorta, self-renewing HSCs, containing granulocyte-macrophage (GM) progenitors, seed the liver, thymus, and spleen, where hematopoiesis continues until the seventh month of gestation (9, 32, 33). Data from Notta and colleagues and Baron et al. reveal that the fetal liver consists of oligopotent progenitors with myeloid-erythroid-megakaryocyte and erythroid-megakaryocyte activities (32, 34). These progenitors have an increased proliferation potential (33, 35) and comprise the primary blood cells of neonates with a gestational age (GA) of less than 32 weeks. After this time, hematopoiesis transitions to the bone marrow so that by the end of term gestation, the bone marrow remains the only site for platelet and red and white blood cell development (9, 32, 33). In contrast to the fetal liver, the bone marrow is dominated by unilineage progenitors with primary myeloid and erythroid potential (32). Recent in-depth reviews of hematopoiesis are offered by Nandakumar et al. (36), Ivanovs et al. (15), Ditadi et al. (37), and Palis (38).
Hemangioblasts.
Recent evidence presented by Kennedy and colleagues (39) suggests that the human hemogenic endothelium may constitute a mesoderm-derived lineage distinct from their suspected endothelial origins (15, 39). Hemangioblasts are KDR+ CD34− cells that emerge from the lateral plate mesoderm immediately prior to blood circulation (15, 40) and exhibit both hematopoietic and vascular potential (37). Representing the earliest stage of hematopoietic commitment, hemangioblasts are thought to promote hematopoiesis in the hemogenic endothelium of the yolk sac upon the initiation of heart contractility and blood flow (39). Additionally, these cells are believed to give rise to the dorsal aorta, hemogenic endothelium, and HSCs (41). Utilizing human embryonic stem cell cultures, Kennedy et al. identified two distinct types of human hemangioblasts, including those giving rise to primitive erythroid cells, macrophages, and endothelial cells and those that generated only primitive erythroid and endothelial cells (39). Because these cells are primarily studied ex vivo, it has been proposed that hemangioblasts exist in a state of competency that is never fulfilled during in vivo experimentation due to restrictions and constraints imposed by the microenvironment (3, 42). While in vivo studies in humans and higher vertebrates are lacking (3), some animal models have significantly contributed to our understanding of hemangioblasts and their contribution to embryonic hematopoiesis (3, 41). Current reviews on hemangioblasts are offered by Lacaud and Kouskoff (3) and Ciau-Uitz and Patient (41).
Differentiation of hematopoietic stem cells.
At present, the initiating factors that determine whether a HSC will differentiate into a myeloid or lymphoid precursor cell remain poorly understood. Contrasting models have been used to describe the generation of blood cells from the common progenitor HSC. In the “classical model” or “hierarchical model,” so-called multilineage priming is functionally related to the cell's ability to determine its fate prior to single-lineage commitment and differentiation, after which its capacity to differentiate into any other cell type is lost (43–45). In this model, HSCs in the bone marrow give rise to either a common myeloid progenitor (CMP) or a common lymphoid progenitor (CLP). The CMP differentiates into either a granulocyte monocyte progenitor (GMP) or a megakaryocyte erythroid progenitor (MEP), while CLP precursors will become either T cells, B cells, or NK cells (36). In this classical/hierarchical model, all HSCs have equal multilineage differentiation potential (43). In contrast, the “alternative model” contends that common myeloid and lymphoid progenitor cells have mixed-lineage potential with transcriptional and functional heterogeneity (43). Cell fate is determined by the availability of survival and differentiation factors (46). Recent studies support this model by demonstrating that HSCs can directly differentiate into CMPs, MEPs, and megakaryocytes. HSCs can also differentiate into lymphoid-primed multipotent progenitors (LMPPs), which give rise to CLPs or GMPs but lack the potential to become megakaryocytes or erythrocytes (32, 36). Additionally, the absence of oligopotent intermediates that gradually become restricted to unilineage progenitors in the bone marrow cannot be reconciled under the classical/hierarchical model of HSC differentiation, making the alternative model more likely (32, 46). Detailed reviews of the alternative model of hematopoiesis are offered by Nandakumar et al. (36), Notta et al. (32), and Paul et al. (46).
Once destined to become a myeloid cell, the HSC enters a well-described, closely regulated process that results in the development of both megakaryocyte/erythroid and granulocyte/macrophage lineages from a pluripotent common myeloid progenitor cell (Fig. 2). The lineage path followed is dependent upon several transcription factors, including CCAAT/enhancer-binding proteins (C/EBPs), GATA-1, and PU.1 (47, 48). C/EBPs comprise a family of six transcription factors (C/EBP-α, -β, -γ, -δ, -ε, and -ζ), characterized by a conserved leucine zipper C-terminal domain next to a positively charged DNA-binding domain (49, 50). C/EBPs can modulate many biological processes, including cell differentiation, motility, growth arrest, proliferation, and cell death, in a variety of tissues, including bone marrow, adipose tissue, the central nervous system, and lung (51). C/EBP-α, -β, and -ε have important regulatory control over neutrophil development, and mutations in C/EBP-α and -β can result in a variety of lymphocytic and myeloid leukemias (52–54).
FIG 2.
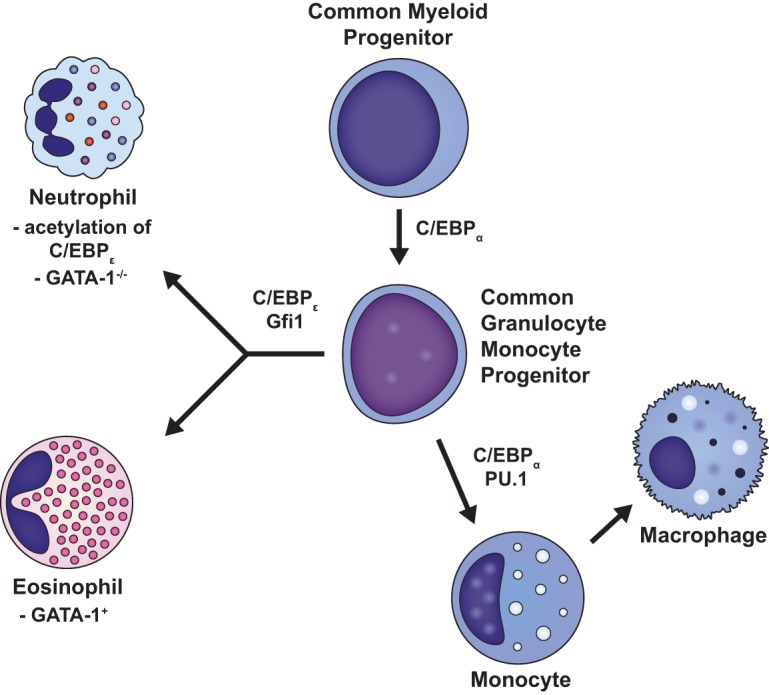
Differentiation of common myeloid progenitor cells. Once destined to become a myeloid cell, the HSC enters a well-described, closely regulated process that results in the development of both megakaryocyte/erythroid and granulocyte/macrophage lineages from a pluripotent common myeloid progenitor cell. Whereas C/EBP-α and PU.1 induce CMPs to differentiate into monocytes and macrophages, C/EBP-ε and Gfi-1 generate neutrophils and eosinophils. It is the acetylation of C/EBP-ε at specific lysines (K121 and K198) and the lack of expression of GATA-1, however, that cause early CMPs to ultimately differentiate into neutrophils instead of eosinophils.
C/EBP-α is an integral factor in the earliest stages of neutrophil development, with C/EBP-α-null mice exhibiting an early block in granulocyte differentiation (55, 56) while retaining monocyte-producing capabilities (57). By negatively regulating the expression of c-Myc, a helix-loop-helix leucine zipper protein that locates to the promoters of certain genes with important regulatory control of the cell cycle, C/EBP-α induces early myeloid precursors to enter CMP differentiation pathways (55, 58). Whereas C/EBP-α, PU.1, and Irf8 induce CMPs to differentiate into monocytes and macrophages, C/EBP-ε and Gfi-1 generate neutrophils and eosinophils (46, 48, 59, 60). It is the acetylation of C/EBP-ε at specific lysines (K121 and K198) and the lack of expression of GATA-1, however, that cause early CMPs to ultimately differentiate into neutrophils instead of eosinophils (48, 54, 61). Notably, knockout of C/EBP-ε does not prevent initial differentiation toward a neutrophil progenitor but rather blocks neutrophil maturation and differentiation out of the progenitor compartment (46). Neutrophil differentiation therefore begins at the myeloblast-to-promyelocyte developmental state and involves a complex interplay between transcription factors and modulators such as C/EBP-ε, PU.1, CCAAT displacement protein (CDP), Gfi-1, and retinoic acid receptor (RAR) (48, 61, 62). In humans, the transit time through the postmitotic pool is 4 to 6 days, after which neutrophils are available for release into the circulation (62–64). To exit the bone marrow, these cells must traverse the bone marrow endothelium through tight-fitting pores by a process of transcellular migration, whereby neutrophils pass through the cell bodies of the endothelium rather than through cell-cell junctions (63, 65).
Neutrophils first appear in the human clavicular marrow at 10 to 11 weeks postconception (66). By the end of the first trimester, neutrophil precursors can be detected in the peripheral blood, while mature cells appear by 14 to 16 weeks of gestation (67, 68). HSCs that generate neutrophils are situated in specialized niches in the trabecular regions of long bones near the endosteum, or the interface between the bone and bone marrow, in close proximity to bone-forming osteoblasts (35, 69–71). Perivascular cells and osteoblasts express the chemokine CXCL12, a ligand for the neutrophil cell membrane chemokine receptor CXCR4, which is important for the retention of neutrophils in the bone marrow (35, 71). Osteoblasts and perivascular cells also secrete proteins such as angiopoietin, thrombopoietin, and stem cell factor, which are necessary for the regulation, generation, and maintenance of HSCs (35, 71–73). As the neutrophil matures, the abundances of the chemokine CXCL2 and its cell membrane receptor, CXCR2, increase, while CXCR4 levels decrease, leading to the release of the neutrophil from the bone marrow as the neutrophil becomes less responsive to CXCL12 (60, 71). Conventional dendritic cells (cDCs) also participate in neutrophil homeostasis and regulate the distribution of neutrophils between the bone marrow, peripheral blood, and organs through the controlled production of the growth factor granulocyte colony-stimulating factor (G-CSF) and the chemokines CXCL1, CCL2, and CXCL10, although the exact mechanism by which this occurs remains unclear (71). The involvement of cDCs, however, has been verified in murine models, where their depletion leads to increased neutrophil counts, while cDC expansion causes neutropenia, or low numbers of circulating neutrophils, due to their loss within the bone marrow (71, 74).
Steady-state and emergency granulopoiesis.
Because neutrophils exhibit rapid homeostatic turnover, with an estimated 109 cells/kg of body weight leaving the bone marrow each day, a delicate balance between granulopoiesis, bone marrow storage and release, intravascular margination, and migration into peripheral tissues must exist, which depends upon and impacts the individual's health (74–76). Therefore, the regulation of neutrophil production has been described in terms of steady-state versus emergency granulopoiesis. Modulated by external stimuli at a molecular level, continual shifts occur between these two states, with the extent of each shift being dependent upon the type, strength, and duration of the activating factor(s) (77).
In steady-state granulopoiesis, the ingestion of apoptotic neutrophils by tissue macrophages activates the transcription of C/EBP-α and factors of the LXR family, thereby suppressing the production of proinflammatory cytokines and, in turn, lowering G-CSF levels (71, 75, 78–80). Conversely, following a microbial challenge, emergency granulopoiesis ensues and increases the release of both immature and mature neutrophil forms into the circulation. This process leads to clinical neutrophilia with a “left shift” or rise in the number of circulating neutrophils with an increased proportion of immature forms. This neutrophilia occurs in response to the presence of bacterial products, the induction of C/EBP-β, and increases in the levels of early inflammatory mediators such as interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), G-CSF, and granulocyte-macrophage colony-stimulating factor (GM-CSF) (71, 75, 79, 81). Notably, heightened C/EBP-β expression during infectious or inflammatory states can induce granulopoiesis in the absence of C/EBP-α, as C/EBP-β is the only member of the C/EBP family that is not downregulated in response to cytokine stimulation (77, 79). While C/EBP-β is not necessary for steady-state granulopoiesis, its role in emergency granulopoiesis is clear with attenuated granulopoiesis in Cebpb knockout mice under stress conditions (77, 79). C/EBP-α and C/EBP-β share many common target molecules associated with granulocytic differentiation (77, 82) but demonstrate contrasting regulation of the cell cycle (77). By strongly inhibiting the cell cycle through interactions with cell cycle regulators, C/EBP-α is important under steady-state conditions (55, 77, 83), while C/EBP-β is vital during emergency granulopoiesis because it demonstrates less inhibitory control over the cell cycle (77, 84).
Recently, evidence has emerged to suggest that pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) that detect conserved pathogen-associated molecular patterns (PAMPs), may unite the steady-state and emergency granulopoiesis pathways (71). This physiological response is proposed to occur through the direct or indirect activation of PRRs on hematopoietic stem and/or progenitor cells that subsequently stimulates the proliferation and differentiation of neutrophils (71, 75, 85, 86). Alternatively, proinflammatory chemokines such as keratinocyte chemoattractant (KC), macrophage inflammatory protein 2 (MIP-2), G-CSF, and TNF-α can elicit the activation of NADPH oxidase to enhance reactive oxygen species (ROS) production by bone marrow myeloid cells (87). ROS then act via paracrine mechanisms to trigger the oxidation and deactivation of phosphate and tensin homologue (PTEN) in resident myeloid cells, which leads to the upregulation of PtIns(3,4,5)P3 signaling, increased G-CSF production, and induction of emergency granulopoiesis (87).
Granulopoiesis
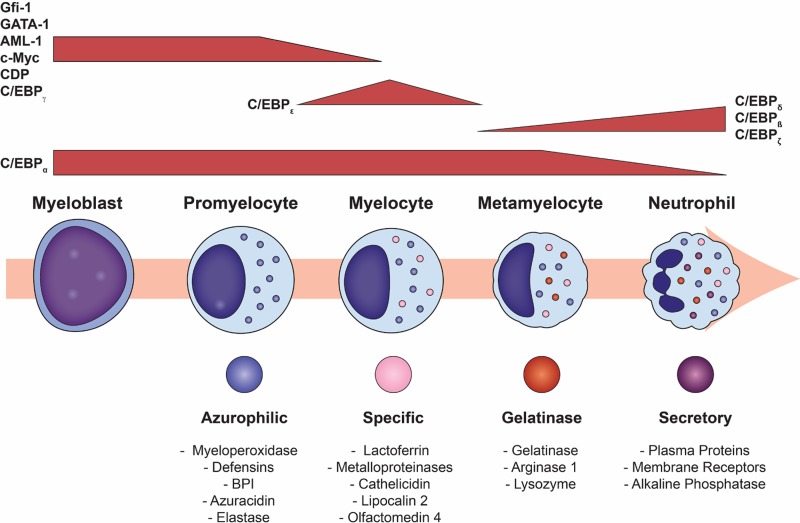
Granulopoiesis, or the formation of granules within the developing neutrophil, begins between the myeloblast and promyelocyte stages of development and proceeds over the subsequent 4 to 6 days (1, 63, 88, 89). Neutrophil granulopoiesis can be divided into two stages, with the first comprising neutrophil lineage determination and the second committed granulopoiesis (90). While early neutrophil precursors, including myeloblasts, promyelocytes, and young myelocytes, retain proliferation capabilities, cells become committed to the neutrophil lineage during the transition between myelocytes and metamyelocytes, after which cell division ceases (90). Characterized by the stepwise emergence of neutrophil granules and secretory vesicles during cell maturation, granulopoiesis begins with the appearance of azurophilic (primary) granules in myeloblasts and promyelocytes, which contain large, round nuclei. This stage is followed by the production of specific granules in myelocytes and metamyelocytes, during which the round nucleus morphs into a kidney-shaped structure. Gelatinase granules are formed next during the transition of metamyelocytes into band neutrophils, where the nucleus assumes a band-like shape. Neutrophil granulopoiesis is concluded with the development of ficolin-1 granules and secretory vesicles in segmented cells, where the neutrophil acquires a characteristic segmented nucleus (1, 88, 90–92).
The myeloblast is the first recognizable cell of the neutrophil lineage to differentiate from the HSC (90). In myeloblasts, low concentrations of PU.1 and large amounts of C/EBP-α favor granulocytic rather than monocytic differentiation (93). Runx1, Gfi-1, and C/EBP-ε constitute the other major transcription factors that regulate neutrophil granulopoiesis (90). In murine models, fetal knockout of Runx1 results in the loss of myeloid hematopoiesis (94), while the deletion of this gene in adult mice leads to primary monopoiesis at the expense of granulopoiesis (95). Gfi-1 deletion blocks neutrophil maturation at the promyelocyte stage, with cells demonstrating developmental failures of gelatinase granules and secretory vesicles (59, 90, 96). C/EBP-ε controls the promyelocyte-to-myelocyte transition in granulocytes and is essential for the formation of specific and gelatinase granules (97, 98). Inhibition of C/EBP-ε does not affect initial neutrophil commitment but impairs terminal differentiation and maturation (23).
The depletion of the natural inhibitor of neutrophil elastase, known as secretory leukocyte protease inhibitor (SLPI), also impairs neutrophil granulopoiesis and results in the clinical condition known as severe congenital neutropenia (SCN), also known as Kostmann syndrome. SCN is characterized by recurrent invasive bacterial and fungal infections as well as an increased risk for the development of myelodysplastic syndrome and acute myelogenous leukemia (99). The inhibition or loss of SLPI blocks G-CSF phosphorylation of STAT5, extracellular signal-regulated kinase 1/2 (ERK1/2), and lymphoid enhancer-binding factor 1 (LEF-1) in bone marrow myeloid progenitors, leading to the downregulation of LEF-1 target genes that are vital for neutrophil proliferation and survival, such as c-Myc, survivin, and cyclin D1 (99, 100). LEF-1 also induces C/EBP-α, which binds to the SLPI promoter to enhance SLPI gene expression. The inhibition of LEF-1, therefore, results in markedly diminished granulocytic differentiation without disrupting monocytopoiesis and erythropoiesis, via reduced C/EBP-α and increased c-Myc levels (99).
In summary, C/EBP-α, a differentiation factor, is critical for the transition of CMPs to GMPs by its suppression of the c-Myc gene (55). Hence, neutrophils will not develop and differentiate if C/EBP-α is deleted (101). Conversely, c-Myc is an important proliferation factor, the inhibition of which results in the absence of all hematopoietic lineages except megakaryocytes (102). A failure to suppress the c-Myc gene in the appropriate developmental state will, therefore, result in myeloid leukemias (55).
Neutrophil granules and secretory vesicles.
Historically, neutrophils were thought to contain three distinct types of granules, known as azurophilic (primary), specific (secondary), and gelatinase (tertiary) granules, as well as secretory vesicles (Fig. 3) (1, 88, 91, 92). Rørvig and colleagues, however, recently described a fourth type of neutrophil granule, the ficolin-1-rich granule, which originates during the transition from myelocytes to metamyelocytes but is packaged into granules of segmented forms between the beginning of the development of gelatinase granules and that of secretory vesicles (103). Descriptions of the different neutrophil granules and secretory vesicles are offered here, and several excellent in-depth reviews by Faurschou and Borregaard (88), Pham (91), and Cowland and Borregaard (90) are available.
FIG 3.
Granulopoiesis and associated transcription factors. Terminal neutrophil maturation is characterized by the sequential formation of the three different neutrophil granules and secretory vesicles as well as nuclear segmentation. Granulopoiesis begins with the development of azurophilic granules in myeloblasts and early promyelocytes and ends after the creation of secretory vesicles in mature, segmented cells. Neutrophil granule formation is hierarchical and dependent upon the timing of constituent protein biosynthesis, while exocytosis occurs in the reverse but ordered sequence. Gene expressions of GATA-1, C/EBP-ζ, AML-1, and c-Myc are imperative for azurophilic granule formation. The creation of specific granules occurs in conjunction with declining AML-1, c-Myc, and CDP concentrations. Reductions in the levels of CDP relieve its repression of C/EBP-ε genes, such as gp91phox, allowing the C/EBP-ε-induced transcription of both C/EBP-δ and specific granule proteins. Once the neutrophil matures into a metamyelocyte, it can no longer proliferate, marking the beginning of terminal neutrophil differentiation. This change results from the inhibition of proliferative genes, AML-1, C/EBP-γ, and CDP, and the emergence of antiproliferative factors such as C/EBP-δ and C/EBP-ζ. The transcription factor C/EBP-ε becomes downregulated as gene expressions for C/EBP-β, C/EBP-δ, and C/EBP-ζ are enhanced to form gelatinase granules.
(i) Azurophilic granules.
Azurophilic granules demonstrate considerable heterogeneity in their size and shape, which is directly regulated by granular protein synthesis and packaging (104–106). Even though azurophilic (primary) granules are packed with acidic hydrolases and microbicidal proteins, they are generally defined by their high content of myeloperoxidase (MPO), which accounts for 5% of the neutrophil's total dry weight (107, 108). While the gene expression of GATA-1 and C/EBP-ζ is involved in azurophilic granule production (93), transcription factors that induce proliferation but block differentiation, such as acute myeloid leukemia 1 (AML-1) and c-Myc, are imperative for azurophilic granule formation (109, 110). In particular, AML-1 and c-Myc control the expression of MPO and elastase (109, 110) as well as the cell membrane receptors for IL-6 and G-CSF (93, 111). Several membrane proteins have been identified in azurophilic granules, including CD63 (112), CD68 (112), presenilin1 (113), stomatin (114), and vacuolar-type H+-ATPase (88, 115). Azurophilic granules also contain a variety of proteins, a few of which are outlined below.
MPO is typically released into phagolysosomes formed by the fusion of azurophilic granules and phagosomes containing engulfed microorganisms. Phagolysosomes are vital neutrophil organelles, as they provide small, confined spaces for toxic oxidative reactions, such as the respiratory burst, designed to kill pathogens while protecting host tissue against harmful metabolites (116, 117). NADPH oxidase, localized on the phagolysosome membrane, mediates the production of ROS. By expediting the conversion of oxygen (O2) to superoxide (O2·−), NADPH oxidase also facilitates the transfer of electrons formed during this reaction to the extracellular space. Superoxide dismutase then enables the conversion of O2·− to hydroxydioxylic acid (HO2) and hydrogen peroxide (H2O2), both of which are weakly bactericidal and contribute considerably to phagolysosome acidification (116). Excessive cytosolic acidification and plasma membrane depolarization during these reactions are prevented by the voltage-gated proton channel Hv1/VOSP, which extrudes protons that accumulate during NADPH oxidase activity (89, 90, 118, 119). Myeloperoxidase then catalyzes the oxidation reaction between H2O2 and chloride (Cl−) to form hypochlorous acid (HOCl) (120), hydroxyl radicals (·OH), and chloramines, all of which are potent oxidants that further contribute to the neutrophil's microbicidal capabilities (121). Inactivating mutations of NADPH oxidase result in a devastating clinical condition known as chronic granulomatous disease (CGD). Characterized by recurrent bacterial and fungal infections, patients with CGD also develop detrimental granulomas from the neutrophils' inability to completely kill and eliminate pathogens (122).
MPO is a cationic glycoprotein that can also bind to the surface of neutrophils (123) and platelets (124) via electrostatic carbohydrate-dependent mechanisms, thereby triggering proinflammatory functional activities (125). The priming and activation of neutrophils by inflammatory mediators, such as Toll-like receptor (TLR) ligands (126), GM-CSF (127), TNF-α, and Ig/Fc receptor-mediated signaling (128), can liberate MPO extracellularly by both degranulation and cell death pathways, including apoptosis and necrosis (108). Once in the extracellular space, MPO can bind to the plasma membrane via CD11b/CD18 receptors, provoking the degranulation of azurophilic and specific granule substances such as lactoferrin, lysozyme, and elastase in a dose-dependent manner via the induction of tyrosine kinase, phosphatidylinositol 3-kinase (PI3K), and calcium signaling pathways (125, 129).
Additionally, azurophilic granules contain serprocidins (serine proteases), including proteinase 3, cathepsin G, elastase, and neutrophil serine protease 4 (NSP4) (130), which display proteolytic enzymatic activity against extracellular matrix components such as elastin, fibronectin, laminin, type IV collagen, and vitronectin (88, 131). Excluding NSP4, the serprocidins are potent antimicrobial substances that can also induce the activation of endothelial cells, macrophages, lymphocytes, and platelets (88, 131). Specific functions of elastase include antimicrobial activity against Gram-negative bacteria (132), whereas cathepsin G is known to target Staphylococcus aureus (133). Both elastase and cathepsin G have potent microbicidal activity against fungal organisms (133, 134). The deletion of ELA2, which codes for neutrophil elastase, is implicated in cyclic neutropenia and congenital neutropenia, although the mechanisms involved remain unknown (91). Conversely, the inhibition of NSP4 did not result in a significant loss of neutrophil function (130). Serprocidins are generally synthesized as zymogens, or inactive proteins, and require two separate processing steps to become activated: (i) cleavage of the signal peptide by cathepsin C, which is imperative for both the activation of enzymatic activity and the optimization of storage within the azurophilic granule (135), and (ii) carboxy-terminal processing that facilitates interactions with adaptor protein 3, enabling proper trafficking to their granular compartment (91, 136).
Other vital microbicidal peptides in the azurophilic granule include α-defensin, azurocidin, and bactericidal/permeability-increasing protein (BPI). α-Defensin, making up at least 5% of the protein content of neutrophils (137), has antimicrobial activity against bacteria, enveloped viruses, fungi, and protozoa through the creation of multimeric transmembrane pores in the microbial outer membrane (88, 137–140). Following extracellular exocytosis, α-defensins also induce the chemotaxis of monocytes (141), CD4+ T helper cells, and CD8+ cytotoxic T cells (142). Azurocidin is an inactive serine protease homologue with broad microbicidal capabilities. Azurocidin can increase vascular permeability during neutrophil extravasation (143) and is an effective chemoattractant for monocytes, fibroblasts, and T cells (144). BPI is a potent antimicrobial substance that specifically targets the obliteration of Gram-negative bacteria at nanomolar concentrations (88, 145, 146). BPI has a high affinity for the lipid A portion of lipopolysaccharide (LPS), thereby neutralizing its proinflammatory properties (145, 147). By acting as an opsonin, BPI also enhances the phagocytosis and intracellular killing of Gram-negative bacterium (147, 148).
(ii) Specific granules.
Specific (secondary) granules are rich in antibiotic substances that participate in neutrophil microbicidal activities either upon mobilization with the phagosome or through release into the extracellular milieu. Lactoferrin, a primary specific granule protein, has direct bacteriostatic and bactericidal activities against viruses, Gram-positive bacteria, Gram-negative bacilli, and fungi (149). By sequestering iron in biological fluids, lactoferrin disrupts and destabilizes microbial cell membranes (150). Additionally, lactoferrin can impair the production of ROS (151, 152) and sequester LPS and CD14, thereby preventing the activation of the proinflammatory pathway and tissue damage (153). Lactoferrin also modulates adaptive immune responses by accelerating the maturation of T-cell precursors into competent CD4+ T helper cells (154) and enhances the differentiation of immature B cells into antigen-presenting cells (155). Following neutrophil activation, lactoferrin released from specific granules enhances the activation of cathepsin G and serine proteases, thereby promoting innate immune responses during acute inflammation (156, 157).
Specific granule proteins also include the following. (i) Neutrophil gelatinase-associated lipocalin (NGAL) exhibits antimicrobial properties and works in coordination with lactoferrin by binding siderophores, i.e., iron chelators generated by microorganisms, when the availability of iron is limiting their growth (158). Due to its ability to bind N-formylmethionine-leucyl-phenylanine (FLMP) in vitro, NGAL is also thought to bind small lipophilic inflammatory mediators such as platelet-activating factor, leukotriene B4, and LPS (159). (ii) Resistin, a proinflammatory cytokine that also localizes to the neutrophil's cell membrane, limits the accumulation of neutrophils at inflamed sites by inhibiting their chemotactic capabilities through the dose-dependent induction of NF-κB activity. Resistin is also a chemoattractant for CD4+ T helper cells and dampens proinflammatory neutrophil responses by reducing the cell's oxidative bursts (160–162). (iii) Olfactomedin-4 (OLFM-4) inhibits cathepsin C-mediated protease activities and in doing so attenuates neutrophil killing of S. aureus and Escherichia coli (163). OLFM-4 is unique among neutrophil granule proteins, as it is recovered from only 20 to 25% of mature adult neutrophils (164). (iv) Signal-regulatory protein alpha (SIRPα) is a cell surface glycoprotein with inhibitory properties. SIRPα is rapidly mobilized to the neutrophil cell surface and is also responsible for regulating neutrophil accumulation at sites of inflammation. While SIRPα is primarily located in specific granules, SIRPα is also found in gelatinase granules and secretory vesicles (165).
The creation of specific granules occurs in conjunction with declining AML-1 and c-Myc concentrations, which halts the expression of azurophilic granule protein genes. Additionally, reductions in active CDP levels cause its repression of C/EBP-ε genes, such as gp91phox, to be relieved, thereby allowing the C/EBP-ε-induced transcription of both C/EBP-δ and specific granule proteins (93, 166). Congenital absence of specific granules is a rare disorder that is characterized by atypical neutrophil structure and function as well as frequent and severe bacterial infections (167).
(iii) Gelatinase granules.
Gelatinase (tertiary) granules are mobilized when the neutrophil establishes primary rolling contact with the activated endothelium (168). These granules contain matrix-degrading enzymes, such as gelatinase, and membrane receptors including CD11b/CD18, CD67, CD177, fMLF-R, SCAMP, and VAMP2 (90), which are important in the earliest phases of the neutrophil inflammatory responses and extravasation into inflamed tissues. Arginase 1, a key gelatinase protein, metabolizes arginine, thereby reducing its availability as a substrate for nitric oxide (NO) synthase (NOS). This reaction leads to the diminished synthesis of NO, which is generally associated with endothelial dysfunction. By promoting the production of ornithine, arginase 1 also diminishes proinflammatory immune responses and fosters tissue regeneration (169).
Gelatinase granules form during the transition of metamyelocytes to band neutrophils. Once the neutrophil matures into a metamyelocyte, it can no longer proliferate, marking the beginning of terminal neutrophil differentiation. This change results from the inhibition of proliferative genes, AML-1, C/EBP-γ, and CDP, and the emergence of antiproliferative factors such as C/EBP-δ and C/EBP-ζ (93). Additionally, the transcription factor C/EBP-ε, which was vital for specific granule formation, becomes downregulated as gene expressions for C/EBP-β, C/EBP-δ, and C/EBP-ζ are enhanced (93).
(iv) Ficolin-1-rich granules.
Discovered by Rørvig and colleagues by employing a four-layer Percoll gradient in conjunction with transcriptomic and proteomic data, ficolin-1-rich granules are similar to secretory vesicles in protein content and function (103). Ficolin-1 is synthesized in maturing neutrophils during the transition from myelocytes to metamyelocytes, but it localizes in highly mobilized granules that form in segmented cells during the very late stages of terminal granulopoiesis (103). This unusual delay in protein packaging most likely indicates that the travel time through the endoplasmic reticulum and Golgi compartment varies depending on the protein (103). Like secretory granules, ficolin-1 granules are easily exocytosed from the neutrophil following minimal stimulation and primarily contain human serum albumin, CR1, vanin-2 (VVN2), LFA-1, actin, and several cytoskeleton-binding proteins. Components of these granules are primarily involved in neutrophil locomotion, firm adhesion, and transendothelial migration (103, 170).
(v) Secretory vesicles.
Not considered to be true neutrophil granules, secretory vesicles constitute an important reservoir of membrane-associated receptors, including CD10, CD11b/CD18, CD15, CD16, CD35, MMP-25, SCAMP, VAMP2, NRAMP2, LFA-1, and MAC-1, as well as actin, actin-binding proteins, and alkaline phosphatase that are required at the earliest phases of neutrophil-mediated inflammatory responses (90, 103). Secretory vesicles are significantly smaller than neutrophil granules and are the most exocytosed cell organelles in the neutrophil. Located throughout the cytoplasm of the cell, these organelles contain cell membrane receptors that are vital for the neutrophil to establish firm contact with the activated vascular endothelium, complete diapedesis into inflamed tissue, and undergo chemotaxis-directed migration within the inflamed tissues to locate and eradicate the offending pathogen (103). Secretory vesicles and ficolin-1-rich granules are formed in segmented neutrophils, after which the cell becomes a fully matured polymorphonuclear (PMN) cell (90).
Targeting-by-timing model.
Traditionally, neutrophil granules have been subdivided based on the presence or absence of myeloperoxidase. Peroxidase-positive granules were known as azurophilic granules, while peroxidase-negative ones were known as specific granules. In reality, neutrophil granules are heterogeneous, with an overlap of contents due to a process described by the targeting-by-timing model, whereby granule protein production occurs in a continuum during all stages of neutrophil development and proteins are simply packed into granules as they are produced (60, 171). In this model, the timing of protein synthesis is dependent only upon cell maturity, such that azurophilic granule proteins are synthesized only at the promyelocyte developmental stage, specific and ficolin-1 granule proteins are synthesized at the myelocyte stage, and gelatinase granule proteins are synthesized at the metamyelocyte and band stages of neutrophil maturation, after which granule formation concludes and secretory vesicles form (88, 103, 171–173). It is becoming clear, however, that additional factors, such as the acetylation of the transcription factor C/EBP-ε, are also important for granule production and neutrophil differentiation. Neutrophils from C/EBP-ε-deficient mice lack both secondary and tertiary granules in an autosomal recessive immunodeficiency disorder known as neutrophil-specific granule deficiency (SGD). Neutrophils associated with SGD exhibit bilobed nuclei, an abnormal respiratory burst, impaired chemotaxis, and diminished bactericidal activity (48, 61, 174–176). Hence, without C/EBP-ε, neutrophils cannot differentiate beyond the myelocyte stage of neutrophil maturation (175). Notably, C/EBP-ε mRNA levels increase with maturation from myeloblasts, with peak levels being observed in myelocytes/metamyelocytes, after which the cells stop proliferating and gene expression declines (177). Conversely, the C/EBP-ε protein is detected only in myelocytes/metamyelocytes. Larsen and colleagues discovered that the microRNA miRNA-130a regulates C/EBP-ε protein expression in granulocytic precursors and that the overexpression of miRNA-130a downregulates C/EBP-ε protein as well as lactoferrin, cathelicidin antimicrobial peptide, and lipocalin-2 gene expressions, giving rise to cells with an immature phenotype, as seen in Cebpe−/− murine models (177). C/EBP-ε is also vital in regulating cell cycle protein expression by inducing the gene expression of p27kip1 and concomitantly inhibiting cyclin-dependent kinase 4 (CDK4), CDK6, cyclin A, and cyclin D (178), thus ensuring a robust and irreversible exit from cell proliferation by the metamyelocyte stage of neutrophil development (90).
Neutrophil granule protein trafficking and sorting.
Until recently, it was assumed that direct sorting of granule proteins did not occur and that trafficking was merely a function of the timing of protein production. New findings are challenging this dogma, however, including the discovery of the anionic proteoglycan serglycin, which is essential for the proper shuttling and packaging of the cationic azurophilic granule proteins myeloperoxidase (179), defensin, and elastase (180–182). Additional support is offered by the complex co- and posttranslational processing of granule proteins, which facilitates their recognition by multisubunit adaptor protein complexes (APs), particularly AP1, AP3, and AP4, and the monomeric Golgi-localized γ-adaptin ear homology ARF (GGA) binding protein. Together, these complexes are important for the organization and trafficking of granule proteins from the trans-Golgi network to their respective neutrophil granule compartments in a manner similar to that exhibited by lysosomal sorting in other cell types (60, 91). Likewise, the synthesis of inactive granule enzymes, or zymogens, expedites their safe and accurate transfer within the neutrophil to their final destination, where the proteolytic cleavage of their prodomains triggers their activation (60). Missorting of proteins between granules can also occur, leading to an alteration in the cell's function. This generally results from the premature activation and degradation of granule substances by catalytically active azurophilic proteases, making them biologically active (173).
Degranulation and exocytosis of neutrophil granules.
Exocytosis of neutrophil granules occurs in a hierarchical fashion and is inversely related to granule production (88, 173). Consequently, minimal cellular stimulation or activation is sufficient to provoke the release of secretory vesicles, while increasing stimulus strength is associated with the release of ficolin-1-rich, gelatinase, specific, and, finally, azurophilic granules (88, 103, 173, 183, 184). Azurophilic granules are unique among the neutrophil granules not only because they require a very powerful agonist to promote the degranulation of their contents but also because they primarily mobilize to phagosomes, although a small amount of their content is exocytosed extracellularly (185, 186). Moreover, recently identified variations in soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes explain these differences: whereas all neutrophil granules contain syntaxin 4 and SNARE complexes, specific and gelatinase granules have SNARE complexes with high concentrations of VAMP1, VAMP2, and 23-kDa synaptosome-associated protein (SNAP-23), while azurophilic granules have increased levels of VAMP1 and VAMP7 (186, 187). Additionally, gelatinase granules are also regulated by syntaxin 3, which controls the degranulation of cytokines, including IL-1α, IL-1β, IL-12b, and CCL4, during early inflammatory responses (188, 189).
SNARE-interacting Sec1/Munc18 (SM) family members, particularly MUNC18-2 and MUNC18-3, also play essential roles in selective vesicular trafficking in neutrophils (189). MUNC18-2 and MUNC18-3 are generally located near their respective SNARE-binding partners syntaxin 3 and syntaxin 4 (189). MUNC18-2 is preferentially associated with the regulation of azurophilic granular exocytosis, while MUNC18-3 regulates extracellular membrane fusion and the degranulation of specific and gelatinase granules (189).
Finally, MUNC13-4 is a RAB27A effector and key coordinator of azurophilic granular exocytosis (190, 191). As such, MUNC13-4 regulates phagosomal maturation, which is crucial for intracellular bacterial killing, and controls the intracellular and extracellular production of ROS through the induction of p22phox. Although RAB27A is a master organizer of vesicular trafficking, RAB27A-deficient neutrophils retained normal phagosomal maturation, including the normal delivery of azurophilic and specific granule proteins to the phagosomal membrane, while the inhibition of MUNC13-4 led to impaired phagosomal maturation and defective shuttling of azurophilic granule components (190). Notably, RAB27A deficiency causes Griscelli syndrome type 2 (192), an immunodeficiency with partial albinism, whereas MUNC13-4 deficiency leads to familial hemophagocytic lymphohistiocytosis type 3 (FHL3) (190, 193).
In summary, multiple mechanisms regulate neutrophil granular exocytosis. Specificity in exocytosis pathways provides important insights into neutrophil biology. Whereas specific, gelatinase, and ficolin-1-rich granules specialize in early inflammatory responses involving cell adhesion, diapedesis, and microorganism killing, azurophilic granules contain a wide array of damaging lytic enzymes and proteins with bactericidal activity that can be detrimental to surrounding tissue if secreted in an unregulated fashion (186).
Special Considerations in Neonates
Neutrophils from term healthy newborns and adults contain equal concentrations of the azurophilic granule proteins MPO and α-defensin, while the level of BPI is decreased 3-fold in unstimulated term neonatal neutrophils compared to adult controls (147). In term infants with early-onset sepsis (EOS), however, plasma levels of BPI can rise to concentrations similar to those in older children and adults with sepsis (194, 195) and/or pneumonia (196, 197). Moreover, BPI mobilization exhibits an age-dependent maturational effect (196). Differential bacterial susceptibility to these factors may explain why Escherichia coli is the leading cause of EOS in preterm infants, while group B Streptococcus remains the leading cause of EOS in term neonates (198).
Lactoferrin concentrations in term neonatal neutrophils are half of adult concentrations, while even lower quantities are found in neutrophils from preterm infants (199). Exposure to labor, however, appears to heighten lactoferrin gene expression in term, healthy newborns (160). Likewise, OLFM-4 is upregulated 3-fold in term neonatal neutrophils, irrespective of labor exposure, compared to adults, although protein levels have not been measured (160). Because OLFM-4 attenuates neutrophil bactericidal activities and host immunity against Gram-positive and Gram-negative bacteria (200, 201), increased quantities in neonatal cells may also contribute to the heightened risk of bacterial sepsis in this vulnerable patient population (160).
The neonatal NADPH oxidase system demonstrates differences in kinetic activity between neonates who are exposed and those who are not exposed to labor, with labor exposure enhancing its activity (202–204). Healthy neonatal and adult neutrophils exhibit similar bactericidal activities against Staphylococcus aureus, E. coli, Serratia marcescens, Pseudomonas species, and group A and B streptococci, while neutrophils from stressed preterm and term infants demonstrated significantly decreased bactericidal activities against both Gram-positive and -negative bacteria (202, 205). Neutrophils from neonates with perinatal distress also exhibited respiratory burst suppression (202, 206–208). In preterm infants, the respiratory burst normalized to adult cellular function by 2 months of age, while ill infants receiving intensive care continued to demonstrate deficiencies by 2 months of age (202, 209). Degranulation capabilities are similar between term neonatal and adult neutrophils, while those from preterm infants have considerable impairments in the release of BPI, elastase, and lactoferrin compared to either term neonatal or adult cells (196, 210).
NEUTROPHIL DISTRIBUTION
Neutrophils reside in three different groups, or pools, known as the proliferative, circulating, and marginating pools, with numbers of neutrophils in each pool being influenced by the individual's state of health and the maturational development of the cell. Homeostasis between pools is closely regulated by conventional dendritic cells through the controlled production of G-CSF, CXCL1, CCL2, and CXCL10, which influences their migration from the bone marrow to their recruitment site and local survival in peripheral organs, although the exact mechanisms are unclear (74). Recently, the kidneys have also been implicated in neutrophil homeostasis through the production of the unique glycoprotein Tamm-Horsfall protein (THP) (211). Deficiency of THP, commonly associated with advanced chronic kidney disease, increases renal concentrations of IL-17 and the secretion of IL-23 by the proximal tubular epithelium, resulting in enhanced granulopoiesis and systemic neutrophilia via the IL-23/IL-17 axis (211).
Proliferative Pool
The proliferative, or mitotic, pool is comprised of early neutrophil precursors, including myeloblasts, promyelocytes, and myelocytes, which are located in the bone marrow and maintain the ability to multiply to replenish neutrophil numbers (212, 213). Based on extrapolation using data from rodent models, it has been estimated that the proliferative pool in human adults contains between 4 × 109 and 5 × 109 cells/kg of body weight (212, 214). In contrast, term human newborns have a significantly smaller mitotic pool, estimated to be only 10% of adult values, with almost 75% of these cells residing in an active cell cycle, resulting in significant cell turnover (215–217). Term newborns also have considerable reductions in their absolute neutrophil cell mass per gram of body weight, which is projected to be only one-quarter of that of adults, while preterm infants exhibit even lower numbers (∼20% of adult levels). Given their limited ability to recruit or produce substantial numbers of neutrophils during sepsis episodes, this vulnerable newborn patient population routinely develops neutropenia when confronted with a pathogenic challenge, thereby increasing sepsis-associated morbidity and mortality risks (35, 205, 215–218). In contrast, adults have high numbers of quiescent progenitors that can be rapidly recruited into the cell cycle during times of sepsis, triggering a surge in numbers of neutrophils available to combat the infection (35, 215, 217). In addition, adults have an ample supply of mature and near-mature granulocytes in the marrow that can be mobilized in early inflammatory responses, with reserves that are about 20 times higher than those found in circulation (214). Low numbers of neonatal neutrophils are observed for a relatively short period, however, with values rising over the first few weeks of life and reaching adult values by about 4 weeks of age (205, 215).
Effects of the microbiome.
The establishment of a healthy neonatal microbiome after birth is also vital for maintaining neutrophil homeostasis and proper cellular functioning. This is because host microorganisms induce the production of IL-17 by group 3 innate lymphoid cells in the intestine, thereby increasing G-CSF and, hence, neutrophil production in a TLR-4- and Myd88-dependent manner (219). Interventions that hinder the natural development of the newborn's microbiota, such as exposure to intrapartum or postpartum antibiotics or cesarean section, may place the infant at an increased risk for adverse outcomes, including (i) necrotizing enterocolitis, a potentially lethal overgrowth of bacterial pathogens within the intestinal wall leading to bowel necrosis with possible perforation; (ii) late-onset sepsis; (iii) prolonged length of stay in the neonatal intensive care unit (NICU); and (iv) even death (220–222). Moreover, the size of the bone marrow myeloid pool correlates strongly with the complexity of the intestinal microbiota in adult mice, with delayed microbial clearance in germfree mice after systemic challenge with apathogenic bacteria (223).
TLR signaling by very low concentrations of microbial antigens and TLR ligands (or by concentrations below the threshold required for the induction of an adaptive immune response) is thought to set the bone marrow cell pool size during steady-state granulopoiesis (223). The coevolution of mammals and their microbiota has led to the reliance on microbiota-derived signals to provide tonic stimulation to the systemic innate immune system to maintain vigilance against infection (223). In murine models, this symbiotic relationship has likewise amplified the number of aged circulating neutrophils, which possess heightened levels of integrin αMβ2 (MAC-1). Hence, these aged neutrophils are more proficient at combating pathogens under acute inflammatory conditions through enhanced chemotaxis, transmigration, and production of neutrophil extracellular traps (NETs) (224). Additionally, TLR detection of PAMPs from invading microorganisms stimulates emergency granulopoiesis and the proliferation and differentiation of neutrophils via early inflammatory mediators such as IL-1β, TNF-α, G-CSF, and GM-CSF, causing an acute and drastic rise in the number of circulating neutrophils available to combat the ensuing infection (71, 75, 79, 85, 86).
Circulating and Marginating Pools
Neutrophils located outside the proliferative pool exist in equilibrium between the circulating and marginating pools (or postmitotic pool) and include more mature neutrophils or metamyelocytes, band forms, and segmented neutrophils (205, 212, 215). The circulating pool represents free-flowing neutrophils in the blood, while the marginating pool comprises those cells not retrieved by routine blood sampling (212). The marginating pool was first identified in the 1960s when healthy adult volunteers were transfused with ex vivo-radiolabeled autologous neutrophils. In these experiments, >50% of the neutrophils disappeared from circulation immediately after transfusion, but more than 60% were retrieved following adrenalin dosing, and close to 87% could be captured after the subjects exercised several hours after the transfusion (71, 212, 225–227).
The primary source of marginating neutrophils has previously eluded investigators, but recent studies suggest that it may be the lungs and pulmonary vasculature. Not only do mature neutrophils differ between the pulmonary bed and the bone marrow (228), but pulmonary vascular cells also express CXCL12, the ligand for CXCR4 (71). When CXCR4 is inhibited by the drug plerixafor, marginating neutrophils from the pulmonary bed are recruited back into the circulation without concomitant mobilization from the bone marrow (71, 229). However, other studies involving radiolabeled autologous neutrophils could not exclude the liver, spleen, and bone marrow as other potential sources of marginating cells due to the rapid accumulation of neutrophils in these organs following transfusion experiments (212, 230).
Special Considerations in Neonates
Neonates who are born small for gestational age (SGA), or have a birth weight in the <10th percentile, have an increased incidence of neutropenia (absolute neutrophil count of <1,000 neutrophils/ml) compared to that in non-SGA infants (231). This neutropenia generally persists for the first week of life and is associated with thrombocytopenia in more than 60% of neonates (231). SGA neutropenia is caused by in utero growth restriction and is directly correlated with the number of circulating nucleated red blood cells (231). Reduced concentrations of granulocyte-macrophage progenitors, diminished bone marrow neutrophil proliferative and storage pools, and the absence of evidence for excessive margination suggest that diminished neutrophil production is the primary mechanism underlying this phenomenon (231, 232). Neutropenic SGA infants have an increased risk of being diagnosed with late-onset sepsis, or infection after 72 h of life, as well as a 4-fold increased risk of developing necrotizing enterocolitis (231). Intriguingly, the highest frequency of neonatal neutropenia is observed in extremely-low-birth-weight (ELBW) infants, or those weighing <1,000 g at birth, for unknown reasons. Unlike older-gestational-age neonates, neutropenic ELBW infants do not experience an increased risk of neonatal sepsis and experience similar rates of mortality in the NICU (202, 233).
After birth, numbers of neonatal neutrophils increase in the circulation to levels never again encountered in their lifetime while healthy (160). In newborns with a GA of ≥28 weeks, this increase will occur in the first 6 to 12 h of life, with peak levels of around 25,000 to 28,000 cells/μl. In neonates with a GA of <28 weeks, however, a more gradual but dramatic increase is experienced, with neutrophil counts of up to 40,000 cells/μl being achieved at around 24 h of age (234). Neutrophil levels will then gradually decline over the subsequent 72 h to reach normal adult levels, independent of gestational age (234). Neutrophils involved in this surge are most likely accrued from the marginating pool in response to increases in levels of stress-associated hormones such as epinephrine, norepinephrine, and cortisol, but the source and exact mechanisms involved remain elusive.
Neonates also have an abundance of circulating immature granulocytes compared to those in adults (12% versus 5%, respectively), including promyelocytes, myelocytes, and metamyelocytes (160). These early neutrophil precursors lack vital early proinflammatory proteins and receptors due to the absent or incomplete development of their gelatinase and/or secretory granules (160, 234–236). Because of this, newborns may be more vulnerable to infection while balancing the essential need for protection against an acute inflammatory response as they become colonized with their microbiome postpartum. Further investigation is necessary to determine the significance of these findings. Detailed review articles concerning neonatal neutrophils are offered by Lawrence et al. (202), Urlichs and Speer (205), and Carr (215).
CONCLUSION
Representing the most abundant leukocytes in humans, neutrophils are critical first responders to inflammatory or infectious challenges and are essential elements in the proper execution of innate and adaptive immune responses. Clinical disorders such as chronic granulomatous disease (CGD), severe congenital neutropenia (SCN), and leukocyte adhesive deficiency (LAD) emphasize the vital role of neutrophils in host defense against infectious disease. From the early stages of embryogenesis, when they first appear in the liver, thymus, and spleen during the definitive stage of hematopoiesis, to their continual production in large quantities from the bone marrow throughout postnatal life, the differentiation of a pluripotent HSC into a mature, segmented neutrophil is a highly orchestrated process in which the transcriptional regulator C/EBP-α serves a central function.
Neutrophil steady-state homeostasis, activation, and antimicrobial properties depend on complex processes that not only are regulated by growth factors, cytokines, and chemokines but also are closely controlled by the host's microbiota. Our evolving understanding of this host-microbiome symbiosis may provide innovative therapies to treat infectious disease while emphasizing the importance of antibiotic stewardship in the treatment of neonatal, pediatric, and adult infectious disease. Understanding these interactions can inspire novel preventative and therapeutic approaches for infectious disease and inflammatory disorders.
REFERENCES
- 1.Kennedy AD, DeLeo FR. 2009. Neutrophil apoptosis and the resolution of infection. Immunol Res 43:25–61. doi: 10.1007/s12026-008-8049-6. [DOI] [PubMed] [Google Scholar]
- 2.Medvinsky A, Rybtsov S, Taoudi S. 2011. Embryonic origin of the adult hematopoietic system: advances and questions. Development 138:1017–1031. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- 3.Lacaud G, Kouskoff V. 2017. Hemangioblast, hemogenic endothelium, and primitive versus definitive hematopoiesis. Exp Hematol 49:19–24. doi: 10.1016/j.exphem.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Tavian M, Peault B. 2005. Embryonic development of the human hematopoietic system. Int J Dev Biol 49:243–250. doi: 10.1387/ijdb.041957mt. [DOI] [PubMed] [Google Scholar]
- 5.Tober J, Koniski A, McGrath KE, Vemishetti R, Emerson R, de Mesy-Bentley KK, Waugh R, Palis J. 2007. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood 109:1433–1441. doi: 10.1182/blood-2006-06-031898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGrath KE, Frame JM, Palis J. 2015. Early hematopoiesis and macrophage development. Semin Immunol 27:379–387. doi: 10.1016/j.smim.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palis J, Robertson S, Kennedy M, Wall C, Keller G. 1999. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 126:5073–5084. [DOI] [PubMed] [Google Scholar]
- 8.Bertrand JY, Jalil A, Klaine M, Jung S, Cumano A, Godin I. 2005. Three pathways to mature macrophages in the early mouse yolk sac. Blood 106:3004–3011. doi: 10.1182/blood-2005-02-0461. [DOI] [PubMed] [Google Scholar]
- 9.Ciau-Uitz A, Monteiro R, Kirmizitas A, Patient R. 2014. Developmental hematopoiesis: ontogeny, genetic programming and conservation. Exp Hematol 42:669–683. doi: 10.1016/j.exphem.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Lam EY, Hall CJ, Crosier PS, Crosier KE, Flores MV. 2010. Live imaging of Runx1 expression in the dorsal aorta tracks the emergence of blood progenitors from endothelial cells. Blood 116:909–914. doi: 10.1182/blood-2010-01-264382. [DOI] [PubMed] [Google Scholar]
- 11.Kissa K, Herbomel P. 2010. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 12.Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. 2010. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orkin SH, Zon LI. 2008. Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dzierzak E, Speck NA. 2008. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol 9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivanovs A, Rybtsov S, Ng ES, Stanley EG, Elefanty AG, Medvinsky A. 2017. Human haematopoietic stem cell development: from the embryo to the dish. Development 144:2323–2337. doi: 10.1242/dev.134866. [DOI] [PubMed] [Google Scholar]
- 16.Ciau-Uitz A, Patient R, Medvinsky A. 2016. Ontogeny of the haematopoietic system, p 1–14. In Ratcliffe MJH. (ed), Encyclopedia of immunobiology, vol 1 Academic Press, Oxford, United Kingdom. [Google Scholar]
- 17.Souilhol C, Gonneau C, Lendinez JG, Batsivari A, Rybtsov S, Wilson H, Morgado-Palacin L, Hills D, Taoudi S, Antonchuk J, Zhao S, Medvinsky A. 2016. Inductive interactions mediated by interplay of asymmetric signalling underlie development of adult haematopoietic stem cells. Nat Commun 7:10784. doi: 10.1038/ncomms10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Souilhol C, Lendinez JG, Rybtsov S, Murphy F, Wilson H, Hills D, Batsivari A, Binagui-Casas A, McGarvey AC, MacDonald HR, Kageyama R, Siebel C, Zhao S, Medvinsky A. 2016. Developing HSCs become Notch independent by the end of maturation in the AGM region. Blood 128:1567–1577. doi: 10.1182/blood-2016-03-708164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robert-Moreno A, Espinosa L, de la Pompa JL, Bigas A. 2005. RBPjkappa-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells. Development 132:1117–1126. doi: 10.1242/dev.01660. [DOI] [PubMed] [Google Scholar]
- 20.Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI. 2005. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev 19:2331–2342. doi: 10.1101/gad.1337005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGarvey AC, Rybtsov S, Souilhol C, Tamagno S, Rice R, Hills D, Godwin D, Rice D, Tomlinson SR, Medvinsky A. 2017. A molecular roadmap of the AGM region reveals BMPER as a novel regulator of HSC maturation. J Exp Med 214:3731–3751. doi: 10.1084/jem.20162012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taoudi S, Medvinsky A. 2007. Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta. Proc Natl Acad Sci U S A 104:9399–9403. doi: 10.1073/pnas.0700984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clements WK, Kim AD, Ong KG, Moore JC, Lawson ND, Traver D. 2011. A somitic Wnt16/Notch pathway specifies haematopoietic stem cells. Nature 474:220–224. doi: 10.1038/nature10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim AD, Melick CH, Clements WK, Stachura DL, Distel M, Panáková D, MacRae C, Mork LA, Crump JG, Traver D. 2014. Discrete Notch signaling requirements in the specification of hematopoietic stem cells. EMBO J 33:2363–2373. doi: 10.15252/embj.201488784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi I, Kobayashi-Sun J, Kim AD, Pouget C, Fujita N, Suda T, Traver D. 2014. Jam1a-Jam2a interactions regulate haematopoietic stem cell fate through Notch signalling. Nature 512:319–323. doi: 10.1038/nature13623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumano K, Chiba S, Kunisato A, Sata M, Saito T, Nakagami-Yamaguchi E, Yamaguchi T, Masuda S, Shimizu K, Takahashi T, Ogawa S, Hamada Y, Hirai H. 2003. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity 18:699–711. doi: 10.1016/S1074-7613(03)00117-1. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Chen MJ, Stacy T, Speck NA. 2006. Runx1 function in hematopoiesis is required in cells that express Tek. Blood 107:106–110. doi: 10.1182/blood-2005-05-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.North T, Gu TL, Stacy T, Wang Q, Howard L, Binder M, Marín-Padilla M, Speck NA. 1999. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development 126:2563–2575. [DOI] [PubMed] [Google Scholar]
- 29.Lacaud G, Gore L, Kennedy M, Kouskoff V, Kingsley P, Hogan C, Carlsson L, Speck N, Palis J, Keller G. 2002. Runx1 is essential for hematopoietic commitment at the hemangioblast stage of development in vitro. Blood 100:458–466. doi: 10.1182/blood-2001-12-0321. [DOI] [PubMed] [Google Scholar]
- 30.Maillard I, Koch U, Dumortier A, Shestova O, Xu L, Sai H, Pross SE, Aster JC, Bhandoola A, Radtke F, Pear WS. 2008. Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell 2:356–366. doi: 10.1016/j.stem.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scaldaferri ML, Klinger FG, Farini D, Di Carlo A, Carsetti R, Giorda E, De Felici M. 2015. Hematopoietic activity in putative mouse primordial germ cell populations. Mech Dev 136:53–63. doi: 10.1016/j.mod.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Notta F, Zandi S, Takayama N, Dobson S, Gan OI, Wilson G, Kaufmann KB, McLeod J, Laurenti E, Dunant CF, McPherson JD, Stein LD, Dror Y, Dick JE. 2016. Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science 351:aab2116. doi: 10.1126/science.aab2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haneline LS, Marshall KP, Clapp DW. 1996. The highest concentration of primitive hematopoietic progenitor cells in cord blood is found in extremely premature infants. Pediatr Res 39:820–825. doi: 10.1203/00006450-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Baron MH, Isern J, Fraser ST. 2012. The embryonic origins of erythropoiesis in mammals. Blood 119:4828–4837. doi: 10.1182/blood-2012-01-153486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luis TC, Killmann NM, Staal FJ. 2012. Signal transduction pathways regulating hematopoietic stem cell biology: introduction to a series of spotlight reviews. Leukemia 26:86–90. doi: 10.1038/leu.2011.260. [DOI] [PubMed] [Google Scholar]
- 36.Nandakumar SK, Ulirsch JC, Sankaran VG. 2016. Advances in understanding erythropoiesis: evolving perspectives. Br J Haematol 173:206–218. doi: 10.1111/bjh.13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ditadi A, Sturgeon CM, Keller G. 2017. A view of human haematopoietic development from the petri dish. Nat Rev Mol Cell Biol 18:56–67. doi: 10.1038/nrm.2016.127. [DOI] [PubMed] [Google Scholar]
- 38.Palis J. 2016. Hematopoietic stem cell-independent hematopoiesis: emergence of erythroid, megakaryocyte, and myeloid potential in the mammalian embryo. FEBS Lett 590:3965–3974. doi: 10.1002/1873-3468.12459. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy M, D'Souza SL, Lynch-Kattman M, Schwantz S, Keller G. 2007. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood 109:2679–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cortés F, Debacker C, Péault B, Labastie MC. 1999. Differential expression of KDR/VEGFR-2 and CD34 during mesoderm development of the early human embryo. Mech Dev 83:161–164. [DOI] [PubMed] [Google Scholar]
- 41.Ciau-Uitz A, Patient R. 2016. The embryonic origins and genetic programming of emerging haematopoietic stem cells. FEBS Lett 590:4002–4015. doi: 10.1002/1873-3468.12363. [DOI] [PubMed] [Google Scholar]
- 42.Amaya E. 2013. The hemangioblast: a state of competence. Blood 122:3853–3854. doi: 10.1182/blood-2013-10-533075. [DOI] [PubMed] [Google Scholar]
- 43.Cvejic A. 2016. Mechanisms of fate decision and lineage commitment during haematopoiesis. Immunol Cell Biol 94:230–235. doi: 10.1038/icb.2015.96. [DOI] [PubMed] [Google Scholar]
- 44.Akashi K, He X, Chen J, Iwasaki H, Niu C, Steenhard B, Zhang J, Haug J, Li L. 2003. Transcriptional accessibility for genes of multiple tissues and hematopoietic lineages is hierarchically controlled during early hematopoiesis. Blood 101:383–389. doi: 10.1182/blood-2002-06-1780. [DOI] [PubMed] [Google Scholar]
- 45.Miyamoto T, Iwasaki H, Reizis B, Ye M, Graf T, Weissman IL, Akashi K. 2002. Myeloid or lymphoid promiscuity as a critical step in hematopoietic lineage commitment. Dev Cell 3:137–147. doi: 10.1016/S1534-5807(02)00201-0. [DOI] [PubMed] [Google Scholar]
- 46.Paul F, Arkin Y, Giladi A, Jaitin DA, Kenigsberg E, Keren-Shaul H, Winter D, Lara-Astiaso D, Gury M, Weiner A, David E, Cohen N, Lauridsen FK, Haas S, Schlitzer A, Mildner A, Ginhoux F, Jung S, Trumpp A, Porse BT, Tanay A, Amit I. 2015. Transcriptional heterogeneity and lineage commitment in myeloid progenitors. Cell 163:1663–1677. doi: 10.1016/j.cell.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 47.Doulatov S, Notta F, Eppert K, Nguyen LT, Ohashi PS, Dick JE. 2010. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat Immunol 11:585–593. doi: 10.1038/ni.1889. [DOI] [PubMed] [Google Scholar]
- 48.Bartles M, Govers AM, Fleskens V, Lourenço AR, Pals CE, Vervoort SJ, van Gent R, Brenkman AB, Bierings MB, Ackerman SJ, van Loosdregt J, Coffer PJ. 2015. Acetylation of C/EBPε is a prerequisite for terminal neutrophil differentiation. Blood 125:1782–1792. doi: 10.1182/blood-2013-12-543850. [DOI] [PubMed] [Google Scholar]
- 49.Hattori T, Ohoka N, Inoue Y, Hayashi H, Onozaki K. 2003. C/EBP family transcription factors are degraded by the proteasome but stabilized by forming dimer. Oncogene 22:1273–1280. doi: 10.1038/sj.onc.1206204. [DOI] [PubMed] [Google Scholar]
- 50.Ohlsson E, Schuster MB, Hasemann M, Porse BT. 2016. The multifaceted functions of C/EBPα in normal and malignant haematopoiesis. Leukemia 30:767–775. doi: 10.1038/leu.2015.324. [DOI] [PubMed] [Google Scholar]
- 51.Balamurugan K, Sterneck E. 2013. The many faces of C/EBPδ and their relevance for inflammation and cancer. Int J Biol Sci 9:917–933. doi: 10.7150/ijbs.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cirilli M, Bereshchenko O, Ermakova O, Nerlov C. 14 October 2016. Insights into specificity, redundancy and new cellular functions of C/EBPa and C/EBPb transcription factors through interactome network analysis. Biochim Biophys Acta doi: 10.1016/j.bbagen.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Gade P, Kimball AS, DiNardo AC, Gangwal P, Ross DD, Boswell HS, Keay SK, Kalvakolanu DV. 2016. Death-associated protein kinase-1 expression and autophagy in chronic lymphocytic leukemia are dependent on activating transcription factor-6 and CCAAT/enhancer-binding protein-β. J Biol Chem 291:22030–22042. doi: 10.1074/jbc.M116.725796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bararia D, Kwok HS, Welner RS, Numata A, Sárosi MB, Yang H, Wee S, Tschuri S, Ray D, Weigert O, Levantini E, Ebralidze AK, Gunaratne J, Tenen DG. 2016. Acetylation of C/EBPα inhibits its granulopoietic function. Nat Commun 7:10968. doi: 10.1038/ncomms10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johansen LM, Iwama A, Lodie TA, Sasaki K, Felsher DW, Golub TR, Tenen DG. 2001. c-Myc is a critical target for c/EBPalpha in granulopoiesis. Mol Cell Biol 21:3789–3806. doi: 10.1128/MCB.21.11.3789-3806.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang QF, Friedman AD. 2002. CCAAT/enhancer-binding proteins are required for granulopoiesis independent of their induction of the granulocyte colony-stimulating factor receptor. Blood 99:2776–2785. doi: 10.1182/blood.V99.8.2776. [DOI] [PubMed] [Google Scholar]
- 57.Ma O, Hong S, Guo H, Ghiaur G, Friedman AD. 2014. Granulopoiesis requires increased C/EBPα compared to monopoiesis, correlated with elevated Cebpa in immature G-CSF receptor versus M-CSF receptor expressing cells. PLoS One 9:e95784. doi: 10.1371/journal.pone.0095784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Obaya AJ, Mateyak MK, Sedivy JM. 1999. Mysterious liaisons: the relationship between c-Myc and the cell cycle. Oncogene 18:2934–2941. doi: 10.1038/sj.onc.1202749. [DOI] [PubMed] [Google Scholar]
- 59.Liu Q, Dong F. 2012. Gfi-1 inhibits the expression of eosinophil major basic protein (MBP) during G-CSF-induced neutrophilic differentiation. Int J Hematol 95:640–647. doi: 10.1007/s12185-012-1078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheshachalam A, Srivastava N, Mitchell T, Lacy P, Eitzen G. 2014. Granule protein processing and regulated secretion in neutrophils. Front Immunol 5:448. doi: 10.3389/fimmu.2014.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fiedler K, Brunner C. 2012. The role of transcription factors in the guidance of granulopoiesis. Am J Blood Res 2:57–65. [PMC free article] [PubMed] [Google Scholar]
- 62.Athens JW. 1963. Blood: leukocytes. Annu Rev Physiol 25:195–212. doi: 10.1146/annurev.ph.25.030163.001211. [DOI] [PubMed] [Google Scholar]
- 63.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. 2010. Neutrophil kinetics in health and disease. Trends Immunol 31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dancey JT, Deubelbeiss KA, Harker LA, Finch CA. 1976. Neutrophil kinetics in man. J Clin Invest 58:705–715. doi: 10.1172/JCI108517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burdon PC, Martin C, Rankin SM. 2008. Migration across the sinusoidal endothelium regulates neutrophil mobilization in response to ELR + CXC chemokines. Br J Haematol 142:100–108. doi: 10.1111/j.1365-2141.2008.07018.x. [DOI] [PubMed] [Google Scholar]
- 66.Slayton WB, Li Y, Calhoun DA, Juul SE, Iturraspe J, Braylan RC, Christensen RD. 1998. The first-appearance of neutrophils in the human fetal bone marrow cavity. Early Hum Dev 53:129–144. doi: 10.1016/S0378-3782(98)00049-8. [DOI] [PubMed] [Google Scholar]
- 67.Remington JS, Klein JO, Wilson CB, Nizet V, Maldonado YA. 2011. Infectious diseases of the fetus and newborn infant, 7th ed Elsevier Saunders Publishing, Philadelphia, PA. [Google Scholar]
- 68.Laver J, Duncan E, Abboud M, Gasparetto C, Sahdev I, Warren D, Bussel J, Auld P, O'Reilly RJ, Moore MA. 1990. High levels of granulocyte and granulocyte-macrophage colony-stimulating factors in cord blood of normal full-term neonates. J Pediatr 116:627–632. doi: 10.1016/S0022-3476(05)81617-8. [DOI] [PubMed] [Google Scholar]
- 69.Gong JK. 1978. Endosteal marrow: a rich source of hematopoietic stem cells. Science 199:1443–1445. doi: 10.1126/science.75570. [DOI] [PubMed] [Google Scholar]
- 70.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. 2003. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 71.Nauseef WM, Borregaard N. 2014 Neutrophils at work. Nat Immunol 15:602–611. doi: 10.1038/ni.2921. [DOI] [PubMed] [Google Scholar]
- 72.Ding L, Saunders TL, Enikolopov G, Morrison SJ. 2012. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Glatman Zaretsky A, Engiles JB, Hunter CA. 2014. Infection-induced changes in hematopoiesis. J Immunol 192:27–33. doi: 10.4049/jimmunol.1302061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiao J, Dragomir AC, Kocabayoglu P, Rahman AH, Chow A, Hashimoto D, Leboeuf M, Kraus T, Moran T, Carrasco-Avino G, Friedman SL, Merad M, Aloman C. 2014. Central role of conventional dendritic cells in regulation of bone marrow release and survival of neutrophils. J Immunol 192:3374–3382. doi: 10.4049/jimmunol.1300237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Manz MG, Boettcher S. 2014. Emergency granulopoiesis. Nat Rev Immunol 14:302–314. doi: 10.1038/nri3660. [DOI] [PubMed] [Google Scholar]
- 76.Strydom N, Rankin SM. 2013. Regulation of circulating neutrophil numbers under homeostasis and in disease. J Innate Immun 5:304–314. doi: 10.1159/000350282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hirai H, Yokota A, Tamura A, Sato A, Maekawa T. 2015. Non-steady-state hematopoiesis regulated by the C/EBPβ transcription factor. Cancer Sci 106:797–802. doi: 10.1111/cas.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.A-Gonzalez N, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, Deniz J, Ramirez C, Díaz M, Gallardo G, de Galarreta CR, Salazar J, Lopez F, Edwards P, Parks J, Andujar M, Tontonoz P, Castrillo A. 2009. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor. Immunity 31:245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hirai H, Zhang P, Dayaram T, Hetherington CJ, Mizuno S, Imanishi J, Akashi K, Tenen DG. 2006. C/EBPbeta is required for ‘emergency’ granulopoiesis. Nat Immunol 7:732–739. doi: 10.1038/ni1354. [DOI] [PubMed] [Google Scholar]
- 80.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. 2005. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 81.Panopoulos AD, Watowich SS. 2008. Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and ‘emergency’ hematopoiesis. Cytokine 42:277–288. doi: 10.1016/j.cyto.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jones LC, Lin ML, Chen SS, Krug U, Hofmann WK, Lee S, Lee YH, Koeffler HP. 2002. Expression of C/EBPbeta from the C/ebpalpha gene locus is sufficient for normal hematopoiesis in vivo. Blood 99:2032–2036. doi: 10.1182/blood.V99.6.2032. [DOI] [PubMed] [Google Scholar]
- 83.Wang H, Iakova P, Wilde M, Welm A, Goode T, Roesler WJ, Timchenko NA. 2001. C/EBPalpha arrests cell proliferation through direct inhibition of Cdk2 and Cdk4. Mol Cell 8:817–828. doi: 10.1016/S1097-2765(01)00366-5. [DOI] [PubMed] [Google Scholar]
- 84.Sebastian T, Johnson PF. 2006. Stop and go: anti-proliferative and mitogenic functions of the transcription factor C/EBPbeta. Cell Cycle 5:953–957. doi: 10.4161/cc.5.9.2733. [DOI] [PubMed] [Google Scholar]
- 85.Bugl S, Wirths S, Radsak MP, Schild H, Stein P, André MC, Müller MR, Malenke E, Wiesner T, Märklin M, Frick JS, Handgretinger R, Rammensee HG, Kanz L, Kopp HG. 2013. Steady-state neutrophil homeostasis is dependent on TLR4/TRIF signaling. Blood 121:723–733. doi: 10.1182/blood-2012-05-429589. [DOI] [PubMed] [Google Scholar]
- 86.Burberry A, Zeng MY, Ding L, Wicks I, Inohara N, Morrison SJ, Núñez G. 2014. Infection mobilizes hematopoietic stem cells through cooperative NOD-like receptor and Toll-like receptor signaling. Cell Host Microbe 15:779–791. doi: 10.1016/j.chom.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kwak HJ, Liu P, Bajrami B, Xu Y, Park SY, Nombela-Arrieta C, Mondal S, Sun Y, Zhu H, Chai L, Silberstein LE, Cheng T, Luo HR. 2015. Myeloid cell-derived reactive oxygen species externally regulate the proliferation of myeloid progenitors in emergency granulopoiesis. Immunity 42:159–171. doi: 10.1016/j.immuni.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Faurschou M, Borregaard N. 2003. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect 5:1317–1327. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 89.Bainton DF, Ullyot JL, Farquhar MG. 1971. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med 134:907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cowland JB, Borregaard N. 2016. Granulopoiesis and granules of human neutrophils. Immunol Rev 273:11–28. doi: 10.1111/imr.12440. [DOI] [PubMed] [Google Scholar]
- 91.Pham CT. 2006. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol 6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 92.Borregaard N, Cowland JB. 1997. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89:3503–3521. [PubMed] [Google Scholar]
- 93.Bjerregaard MD, Jurlander J, Klausen P, Borregaard N, Cowland JB. 2003. The in vivo profile of transcription factors during neutrophil differentiation in human bone marrow. Blood 101:4322–4332. doi: 10.1182/blood-2002-03-0835. [DOI] [PubMed] [Google Scholar]
- 94.Growney JD, Shigematsu H, Li Z, Lee BH, Adelsperger J, Rowan R, Curley DP, Kutok JL, Akashi K, Williams IR, Speck NA, Gilliland DG. 2005. Loss of Runx1 perturbs adult hematopoiesis and is associated with a myeloproliferative phenotype. Blood 106:494–504. doi: 10.1182/blood-2004-08-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guo H, Ma O, Speck NA, Friedman AD. 2012. Runx1 deletion or dominant inhibition reduces Cebpa transcription via conserved promoter and distal enhancer sites to favor monopoiesis over granulopoiesis. Blood 119:4408–4418. doi: 10.1182/blood-2011-12-397091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hock H, Hamblen MJ, Rooke HM, Traver D, Bronson RT, Cameron S, Orkin SH. 2003. Intrinsic requirement for zinc finger transcription factor Gfi-1 in neutrophil differentiation. Immunity 18:109–120. doi: 10.1016/S1074-7613(02)00501-0. [DOI] [PubMed] [Google Scholar]
- 97.Ostuni R, Natoli G, Cassatella MA, Tamassia N. 2016. Epigenetic regulation of neutrophil development and function. Semin Immunol 28:83–93. doi: 10.1016/j.smim.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 98.Borregaard N. 2010. Neutrophils, from marrow to microbes. Immunity 33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 99.Klimenkova O, Ellerbeck W, Klimiankou M, Ünalan M, Kandabarau S, Gigina A, Hussein K, Zeidler C, Welte K, Skokowa J. 2014. A lack of secretory leukocyte protease inhibitor (SLPI) causes defects in granulocytic differentiation. Blood 123:1239–1249. doi: 10.1182/blood-2013-06-508887. [DOI] [PubMed] [Google Scholar]
- 100.Nolte MA. 2014. SLPI is essential for granulopoiesis. Blood 123:1121–1123. doi: 10.1182/blood-2014-01-546986. [DOI] [PubMed] [Google Scholar]
- 101.Heath V, Suh HC, Holman M, Renn K, Gooya JM, Parkin S, Klarmann KD, Ortiz M, Johnson P, Keller J. 2004. C/EBPalpha deficiency results in hyperproliferation of hematopoietic progenitor cells and disrupts macrophage development in vitro and in vivo. Blood 104:1639–1647. doi: 10.1182/blood-2003-11-3963. [DOI] [PubMed] [Google Scholar]
- 102.Lekstrom-Himes JA. 2001. The role of C/EBP(epsilon) in the terminal stages of granulocyte differentiation. Stem Cells 19:125–133. doi: 10.1634/stemcells.19-2-125. [DOI] [PubMed] [Google Scholar]
- 103.Rørvig S, Østergaard O, Heegaard NH, Borregaard N. 2013. Proteome profiling of human neutrophil granule subsets, secretory vesicles, and cell membrane: correlation with transcriptome profiling of neutrophil precursors. J Leukoc Biol 94:711–721. doi: 10.1189/jlb.1212619. [DOI] [PubMed] [Google Scholar]
- 104.Parmley RT, Rice WG, Kinkade JM Jr, Gilbert C, Barton JC. 1987. Peroxidase-containing microgranules in human neutrophils: physical, morphological, cytochemical, and secretory properties. Blood 70:1630–1638. [PubMed] [Google Scholar]
- 105.Egesten A, Breton-Gorius J, Guichard J, Gullberg U, Olsson I. 1994. The heterogeneity of azurophil granules in neutrophil promyelocytes: immunogold localization of myeloperoxidase, cathepsin G, elastase, proteinase 3, and bactericidal/permeability increasing protein. Blood 83:2985–2994. [PubMed] [Google Scholar]
- 106.Pryzwansky KB, Breton-Gorius J. 1985. Identification of a subpopulation of primary granules in human neutrophils based upon maturation and distribution. Study by transmission electron microscopy cytochemistry and high voltage electron microscopy of whole cell preparations. Lab Invest 53:664–671. [PubMed] [Google Scholar]
- 107.Schultz J, Kaminker K. 1962. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch Biochem Biophys 96:465–467. doi: 10.1016/0003-9861(62)90321-1. [DOI] [PubMed] [Google Scholar]
- 108.Odobasic D, Kitching AR, Holdsworth SR. 2016. Neutrophil-mediated regulation of innate and adaptive immunity: the role of myeloperoxidase. J Immunol Res 2016:2349817. doi: 10.1155/2016/2349817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oelgeschläger M, Nuchprayoon I, Lüscher B, Friedman AD. 1996. C/EBP, c-Myb, and PU.1 cooperate to regulate the neutrophil elastase promoter. Mol Cell Biol 16:4717–4725. doi: 10.1128/MCB.16.9.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ford AM, Bennett CA, Healy LE, Towatari M, Greaves MF, Enver T. 1996. Regulation of the myeloperoxidase enhancer binding proteins Pu1, C-EBP alpha, -beta, and -delta during granulocyte-lineage specification. Proc Natl Acad Sci U S A 93:10838–10843. doi: 10.1073/pnas.93.20.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang X, Scott E, Sawyers CL, Friedman AD. 1999. C/EBPalpha bypasses granulocyte colony-stimulating factor signals to rapidly induce PU.1 gene expression, stimulate granulocytic differentiation, and limit proliferation in 32D cl3 myeloblasts. Blood 94:560–571. [PubMed] [Google Scholar]
- 112.Saito N, Pulford KA, Breton-Gorius J, Massé JM, Mason DY, Cramer EM. 1991. Ultrastructural localization of the CD68 macrophage-associated antigen in human blood neutrophils and monocytes. Am J Pathol 139:1053–1059. [PMC free article] [PubMed] [Google Scholar]
- 113.Mirinics ZK, Calafat J, Udby L, Lovelock J, Kjeldsen L, Rothermund K, Sisodia SS, Borregaard N, Corey SJ. 2002. Identification of the presenilins in hematopoietic cells with localization of presenilin 1 to neutrophil and platelet granules. Blood Cells Mol Dis 28:28–38. doi: 10.1006/bcmd.2002.0486. [DOI] [PubMed] [Google Scholar]
- 114.Feuk-Lagerstedt E, Samuelsson M, Mosgoeller W, Movitz C, Rosqvist A, Bergström J, Larsson T, Steiner M, Prohaska R, Karlsson A. 2002. The presence of stomatin in detergent-insoluble domains of neutrophil granule membranes. J Leukoc Biol 72:970–977. [PubMed] [Google Scholar]
- 115.Nanda A, Brumell JH, Nordström T, Kjeldsen L, Sengelov H, Borregaard N, Rotstein OD, Grinstein S. 1996. Activation of proton pumping in human neutrophils occurs by exocytosis of vesicles bearing vacuolar-type H+-ATPases. J Biol Chem 271:15963–15970. doi: 10.1074/jbc.271.27.15963. [DOI] [PubMed] [Google Scholar]
- 116.Allen RC. 2015. Neutrophil leukocyte: combustive microbicidal action and chemiluminescence. J Immunol Res 2015:794072. doi: 10.1155/2015/794072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Klebanoff SJ. 1999. Myeloperoxidase. Proc Assoc Am Physicians 111:383–389. [DOI] [PubMed] [Google Scholar]
- 118.Okochi Y, Aratani Y, Adissu HA, Miyawaki N, Sasaki M, Suzuki K, Okamura Y. 2016. The voltage-gated proton channel Hv1/VSOP inhibits neutrophil granule release. J Leukoc Biol 99:7–19. doi: 10.1189/jlb.3HI0814-393R. [DOI] [PubMed] [Google Scholar]
- 119.Ramsey IS, Ruchti E, Kaczmarek JS, Clapham DE. 2009. Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst. Proc Natl Acad Sci U S A 106:7642–7647. doi: 10.1073/pnas.0902761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Allen RC. 1975. Halide dependence of the myeloperoxidase-mediated antimicrobial system of the polymorphonuclear leukocyte in the phenomenon of electronic excitation. Biochem Biophys Res Commun 63:675–683. doi: 10.1016/S0006-291X(75)80437-2. [DOI] [PubMed] [Google Scholar]
- 121.Clark RA. 1999. Activation of the neutrophil respiratory burst oxidase. J Infect Dis 179(Suppl 2):S309–S317. doi: 10.1086/513849. [DOI] [PubMed] [Google Scholar]
- 122.Segal AW. 1988. The molecular and cellular pathology of chronic granulomatous disease. Eur J Clin Invest 18:433–443. doi: 10.1111/j.1365-2362.1988.tb01037.x. [DOI] [PubMed] [Google Scholar]
- 123.Klinke A, Nussbaum C, Kubala L, Friedrichs K, Rudolph TK, Rudolph V, Paust HJ, Schröder C, Benten D, Lau D, Szocs K, Furtmüller PG, Heeringa P, Sydow K, Duchstein HJ, Ehmke H, Schumacher U, Meinertz T, Sperandio M, Baldus S. 2011. Myeloperoxidase attracts neutrophils by physical forces. Blood 117:1350–1358. doi: 10.1182/blood-2010-05-284513. [DOI] [PubMed] [Google Scholar]
- 124.Gorudko IV, Sokolov AV, Shamova EV, Grudinina NA, Drozd ES, Shishlo LM, Grigorieva DV, Bushuk SB, Bushuk BA, Chizhik SA, Cherenkevich SN, Vasilyev VB, Panasenko OM. 2013. Myeloperoxidase modulates human platelet aggregation via actin cytoskeleton reorganization and store-operated calcium entry. Biol Open 2:916–923. doi: 10.1242/bio.20135314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Grigorieva DV, Gorudko IV, Sokolov AV, Kostevich VA, Vasilyev VB, Cherenkevich SN, Panasenko OM. 2016. Myeloperoxidase stimulates neutrophil degranulation. Bull Exp Biol Med 161:495–500. doi: 10.1007/s10517-016-3446-7. [DOI] [PubMed] [Google Scholar]
- 126.Holle JU, Windmöller M, Lange C, Gross WL, Herlyn K, Csernok E. 2013. Toll-like receptor TLR2 and TLR9 ligation triggers neutrophil activation in granulomatosis with polyangiitis. Rheumatology (Oxford) 52:1183–1189. doi: 10.1093/rheumatology/kes415. [DOI] [PubMed] [Google Scholar]
- 127.Dang Y, Lowe GM, Edwards SW, Galvani DW. 1993. The effects of GM-CSF on myeloperoxidase release in normal and myelodysplastic neutrophils. Leuk Res 17:1037–1044. doi: 10.1016/0145-2126(93)90160-M. [DOI] [PubMed] [Google Scholar]
- 128.Chatham WW, Turkiewicz A, Blackburn WD Jr. 1994. Determinants of neutrophil HOCl generation: ligand-dependent responses and the role of surface adhesion. J Leukoc Biol 56:654–660. [DOI] [PubMed] [Google Scholar]
- 129.Lau D, Mollnau H, Eiserich JP, Freeman BA, Daiber A, Gehling UM, Brümmer J, Rudolph V, Münzel T, Heitzer T, Meinertz T, Baldus S. 2005. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc Natl Acad Sci U S A 102:431–436. doi: 10.1073/pnas.0405193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Perera NC, Wiesmüller KH, Larsen MT, Schacher B, Eickholz P, Borregaard N, Jenne DE. 2013. NSP4 is stored in azurophil granules and released by activated neutrophils as active endoprotease with restricted specificity. J Immunol 191:2700–2707. doi: 10.4049/jimmunol.1301293. [DOI] [PubMed] [Google Scholar]
- 131.Owen CA, Campbell EJ. 1999. The cell biology of leukocyte-mediated proteolysis. J Leukoc Biol 65:137–150. [DOI] [PubMed] [Google Scholar]
- 132.Belaaouaj A, McCarthy R, Baumann M, Gao Z, Ley TJ, Abraham SN, Shapiro SD. 1998. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat Med 4:615–618. doi: 10.1038/nm0598-615. [DOI] [PubMed] [Google Scholar]
- 133.Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 134.Tkalcevic J, Novelli M, Phylactides M, Iredale JP, Segal AW, Roes J. 2000. Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity 12:201–210. doi: 10.1016/S1074-7613(00)80173-9. [DOI] [PubMed] [Google Scholar]
- 135.Garwicz D, Lindmark A, Persson AM, Gullberg U. 1998. On the role of the proform-conformation for processing and intracellular sorting of human cathepsin G. Blood 92:1415–1422. [PubMed] [Google Scholar]
- 136.Horwitz M, Benson KF, Duan Z, Li FQ, Person RE. 2004. Hereditary neutropenia: dogs explain human neutrophil elastase mutations. Trends Mol Med 10:163–170. doi: 10.1016/j.molmed.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 137.Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, Bainton DF, Lehrer RI. 1985. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest 76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Daher KA, Selsted ME, Lehrer RI. 1986. Direct inactivation of viruses by human granulocyte defensins. J Virol 60:1068–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lehrer RI, Ganz T, Szklarek D, Selsted ME. 1988. Modulation of the in vitro candidacidal activity of human neutrophil defensins by target cell metabolism and divalent cations. J Clin Invest 81:1829–1835. doi: 10.1172/JCI113527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wimley WC, Selsted ME, White SH. 1994. Interactions between human defensins and lipid bilayers: evidence for formation of multimeric pores. Protein Sci 3:1362–1373. doi: 10.1002/pro.5560030902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Territo MC, Ganz T, Selsted ME, Lehrer R. 1989. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest 84:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang D, Chen Q, Chertov O, Oppenheim JJ. 2000. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J Leukoc Biol 68:9–14. [PubMed] [Google Scholar]
- 143.Gautam N, Olofsson AM, Herwald H, Iversen LF, Lundgren-Akerlund E, Hedqvist P, Arfors KE, Flodgaard H, Lindbom L. 2001. Heparin-binding protein (HBP/CAP37): a missing link in neutrophil-evoked alteration of vascular permeability. Nat Med 7:1123–1127. doi: 10.1038/nm1001-1123. [DOI] [PubMed] [Google Scholar]
- 144.Chertov O, Michiel DF, Xu L, Wang JM, Tani K, Murphy WJ, Longo DL, Taub DD, Oppenheim JJ. 1996. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J Biol Chem 271:2935–2940. doi: 10.1074/jbc.271.6.2935. [DOI] [PubMed] [Google Scholar]
- 145.Elsbach P. 1998. The bactericidal/permeability-increasing protein (BPI) in antibacterial host defense. J Leukoc Biol 64:14–18. [DOI] [PubMed] [Google Scholar]
- 146.Weiss J, Olsson I. 1987. Cellular and subcellular localization of the bactericidal/permeability-increasing protein of neutrophils. Blood 69:652–659. [PubMed] [Google Scholar]
- 147.Levy O, Martin S, Eichenwald E, Ganz T, Valore E, Carroll SF, Lee K, Goldmann D, Thorne GM. 1999. Impaired innate immunity in the newborn: newborn neutrophils are deficient in bactericidal/permeability-increasing protein. Pediatrics 104:1327–1333. doi: 10.1542/peds.104.6.1327. [DOI] [PubMed] [Google Scholar]
- 148.Iovine NM, Elsbach P, Weiss J. 1997. An opsonic function of the neutrophil bactericidal/permeability-increasing protein depends on both its N- and C-terminal domains. Proc Natl Acad Sci U S A 94:10973–10978. doi: 10.1073/pnas.94.20.10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Valenti P, Antonini G. 2005. Lactoferrin: an important host defence against microbial and viral attack. Cell Mol Life Sci 62:2576–2587. doi: 10.1007/s00018-005-5372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Siqueiros-Cendón T, Arévalo-Gallegos S, Iglesias-Figueroa BF, García-Montoya IA, Salazar-Martínez J, Rascón-Cruz Q. 2014. Immunomodulatory effects of lactoferrin. Acta Pharmacol Sin 35:557–566. doi: 10.1038/aps.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Baveye S, Elass E, Mazurier J, Spik G, Legrand D. 1999. Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin Chem Lab Med 37:281–286. doi: 10.1515/CCLM.1999.049. [DOI] [PubMed] [Google Scholar]
- 152.Legrand D, Elass E, Carpentier M, Mazurier J. 2005. Lactoferrin: a modulator of immune and inflammatory responses. Cell Mol Life Sci 62:2549–2559. doi: 10.1007/s00018-005-5370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lu YC, Yeh WC, Ohashi PS. 2008. LPS/TLR4 signal transduction pathway. Cytokine 42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 154.Kruzel ML, Bacsi A, Choudhury B, Sur S, Boldogh I. 2006. Lactoferrin decreases pollen antigen-induced allergic airway inflammation in a murine model of asthma. Immunology 119:159–166. doi: 10.1111/j.1365-2567.2006.02417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Actor JK, Hwang SA, Kruzel ML. 2009. Lactoferrin as a natural immune modulator. Curr Pharm Des 15:1956–1973. doi: 10.2174/138161209788453202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Eipper S, Steiner R, Lesner A, Sienczyk M, Palesch D, Halatsch ME, Zaczynska E, Heim C, Hartmann MD, Zimecki M, Wirtz CR, Burster T. 2016. Lactoferrin is an allosteric enhancer of the proteolytic activity of cathepsin G. PLoS One 11:e0151509. doi: 10.1371/journal.pone.0151509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hendrixson DR, Qiu J, Shewry SC, Fink DL, Petty S, Baker EN, Plaut AG, St Geme JW III. 2003. Human milk lactoferrin is a serine protease that cleaves Haemophilus surface proteins at arginine-rich sites. Mol Microbiol 47:607–617. doi: 10.1046/j.1365-2958.2003.03327.x. [DOI] [PubMed] [Google Scholar]
- 158.Klausen P, Niemann CU, Cowland JB, Krabbe K, Borregaard N. 2005. On mouse and man: neutrophil gelatinase associated lipocalin is not involved in apoptosis or acute response. Eur J Haematol 75:332–340. doi: 10.1111/j.1600-0609.2005.00511.x. [DOI] [PubMed] [Google Scholar]
- 159.Kjeldsen L, Bainton DF, Sengeløv H, Borregaard N. 1994. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood 83:799–807. [PubMed] [Google Scholar]
- 160.Lawrence SM, Eckert J, Makoni M, Pereira HA. 2015. Is the use of complete blood counts with manual differentials an antiquated method of determining neutrophil composition in newborns? Ann Clin Lab Sci 45:403–413. [PubMed] [Google Scholar]
- 161.Cohen G, Ilic D, Raupachova J, Hörl WH. 2008. Resistin inhibits essential functions of polymorphonuclear leukocytes. J Immunol 181:3761–3768. doi: 10.4049/jimmunol.181.6.3761. [DOI] [PubMed] [Google Scholar]
- 162.Boström EA, Tarkowski A, Bokarewa M. 2009. Resistin is stored in neutrophil granules being released upon challenge with inflammatory stimuli. Biochim Biophys Acta 1793:1894–1900. doi: 10.1016/j.bbamcr.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 163.Liu W, Yan M, Liu Y, McLeish KR, Coleman WG Jr, Rodgers GP. 2012. Olfactomedin 4 inhibits cathepsin C-mediated protease activities, thereby modulating neutrophil killing of Staphylococcus aureus and Escherichia coli in mice. J Immunol 189:2460–2467. doi: 10.4049/jimmunol.1103179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Clemmensen SN, Bohr CT, Rørvig S, Glenthøj A, Mora-Jensen H, Cramer EP, Jacobsen LC, Larsen MT, Cowland JB, Tanassi JT, Heegaard NH, Wren JD, Silahtaroglu AN, Borregaard N. 2012. Olfactomedin 4 defines a subset of human neutrophils. J Leukoc Biol 91:495–500. doi: 10.1189/jlb.0811417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stenberg Å, Karlsson A, Feuk-Lagerstedt E, Christenson K, Bylund J, Oldenborg A, Vesterlund L, Matozaki T, Sehlin J, Oldenborg PA. 2014. Signal regulatory protein alpha is present in several neutrophil granule populations and is rapidly mobilized to the cell surface to negatively fine-tune neutrophil accumulation in inflammation. J Innate Immun 6:553–560. doi: 10.1159/000357820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Khanna-Gupta A, Zibello T, Sun H, Lekstrom-Himes J, Berliner N. 2001. C/EBP epsilon mediates myeloid differentiation and is regulated by the CCAAT displacement protein (CDP/cut). Proc Natl Acad Sci U S A 98:8000–8005. doi: 10.1073/pnas.141229598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shigemura T, Yamazaki T, Shiohara M, Kobayashi N, Naganuma K, Koike K, Agematsu K. 2014. Clinical course in a patient with neutrophil-specific granule deficiency and rapid detection of neutrophil granules as a screening test. J Clin Immunol 34:780–783. doi: 10.1007/s10875-014-0082-8. [DOI] [PubMed] [Google Scholar]
- 168.Kjeldsen L, Sengeløv H, Lollike K, Nielsen MH, Borregaard N. 1994. Isolation and characterization of gelatinase granules from human neutrophils. Blood 83:1640–1649. [PubMed] [Google Scholar]
- 169.Jacobsen LC, Theilgaard-Mönch K, Christensen EI, Borregaard N. 2007. Arginase 1 is expressed in myelocytes/metamyelocytes and localized in gelatinase granules of human neutrophils. Blood 109:3084–3087. doi: 10.1182/blood-2006-06-032599. [DOI] [PubMed] [Google Scholar]
- 170.Jog NR, Rane MJ, Lominadze G, Luerman GC, Ward RA, McLeish KR. 2007. The actin cytoskeleton regulates exocytosis of all neutrophil granule subsets. Am J Physiol Cell Physiol 292:C1690–C1700. doi: 10.1152/ajpcell.00384.2006. [DOI] [PubMed] [Google Scholar]
- 171.Borregaard N, Sørensen OE, Theilgaard-Mönch K. 2007. Neutrophil granules: a library of innate immunity proteins. Trends Immunol 28:340–345. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 172.Gullberg U, Bengtsson N, Bülow E, Garwicz D, Lindmark A, Olsson I. 1999. Processing and targeting of granule proteins in human neutrophils. J Immunol Methods 232:201–210. doi: 10.1016/S0022-1759(99)00177-5. [DOI] [PubMed] [Google Scholar]
- 173.Gullberg U, Andersson E, Garwicz D, Lindmark A, Olsson I. 1997. Biosynthesis, processing and sorting of neutrophil proteins: insight into neutrophil granule development. Eur J Haematol 58:137–153. doi: 10.1111/j.1600-0609.1997.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 174.Wada T, Akagi T, Muraoka M, Toma T, Kaji K, Agematsu K, Koeffler HP, Yokota T, Yachie A. 2015. A novel in-frame deletion in the leucine zipper domain of C/EBPε leads to neutrophil-specific granule deficiency. J Immunol 195:80–86. doi: 10.4049/jimmunol.1402222. [DOI] [PubMed] [Google Scholar]
- 175.Yamanaka R, Barlow C, Lekstrom-Himes J, Castilla LH, Liu PP, Eckhaus M, Decker T, Wynshaw-Boris A, Xanthopoulos KG. 1997. Impaired granulopoiesis, myelodysplasia, and early lethality in CCAAT/enhancer binding protein epsilon-deficient mice. Proc Natl Acad Sci U S A 94:13187–13192. doi: 10.1073/pnas.94.24.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lekstrom-Himes J, Xanthopoulos KG. 1999. CCAAT/enhancer binding protein epsilon is critical for effective neutrophil-mediated response to inflammatory challenge. Blood 93:3096–3105. [PubMed] [Google Scholar]
- 177.Larsen MT, Häger M, Glenthøj A, Asmar F, Clemmensen SN, Mora-Jensen H, Borregaard N, Cowland JB. 2014. miRNA-130a regulates C/EBP-ε expression during granulopoiesis. Blood 123:1079–1089. doi: 10.1182/blood-2013-08-523233. [DOI] [PubMed] [Google Scholar]
- 178.Nakajima H, Watanabe N, Shibata F, Kitamura T, Ikeda Y, Handa M. 2006. N-terminal region of CCAAT/enhancer-binding protein epsilon is critical for cell cycle arrest, apoptosis, and functional maturation during myeloid differentiation. J Biol Chem 281:14494–14502. doi: 10.1074/jbc.M600575200. [DOI] [PubMed] [Google Scholar]
- 179.Lemansky P, Gerecitano-Schmidek M, Das RC, Schmidt B, Hasilik A. 2003. Targeting myeloperoxidase to azurophilic granules in HL-60 cells. J Leukoc Biol 74:542–550. doi: 10.1189/jlb.1202616. [DOI] [PubMed] [Google Scholar]
- 180.Niemann CU, Abrink M, Pejler G, Fischer RL, Christensen EI, Knight SD, Borregaard N. 2007. Neutrophil elastase depends on serglycin proteoglycan for localization in granules. Blood 109:4478–4486. doi: 10.1182/blood-2006-02-001719. [DOI] [PubMed] [Google Scholar]
- 181.Glenthøj A, Cowland JB, Heegaard NH, Larsen MT, Borregaard N. 2011. Serglycin participates in retention of α-defensin in granules during myelopoiesis. Blood 118:4440–4448. doi: 10.1182/blood-2011-06-362947. [DOI] [PubMed] [Google Scholar]
- 182.Lemansky P, Smolenova E, Wrocklage C, Hasilik A. 2007. Neutrophil elastase is associated with serglycin on its way to lysosomes in U937 cells. Cell Immunol 246:1–7. doi: 10.1016/j.cellimm.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 183.Faurschou M, Sørensen OE, Johnsen AH, Askaa J, Borregaard N. 2002. Defensin-rich granules of human neutrophils: characterization of secretory properties. Biochim Biophys Acta 1591:29–35. doi: 10.1016/S0167-4889(02)00243-4. [DOI] [PubMed] [Google Scholar]
- 184.Sengelov H, Kjeldsen L, Borregaard N. 1993. Control of exocytosis in early neutrophil activation. J Immunol 150:1535–1543. [PubMed] [Google Scholar]
- 185.Fittschen C, Henson PM. 1994. Linkage of azurophil granule secretion in neutrophils to chloride ion transport and endosomal transcytosis. J Clin Invest 93:247–255. doi: 10.1172/JCI116952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mollinedo F, Calafat J, Janssen H, Martín-Martín B, Canchado J, Nabokina SM, Gajate C. 2006. Combinatorial SNARE complexes modulate the secretion of cytoplasmic granules in human neutrophils. J Immunol 177:2831–2841. doi: 10.4049/jimmunol.177.5.2831. [DOI] [PubMed] [Google Scholar]
- 187.Brumell JH, Volchuk A, Sengelov H, Borregaard N, Cieutat AM, Bainton DF, Grinstein S, Klip A. 1995. Subcellular distribution of docking/fusion proteins in neutrophils, secretory cells with multiple exocytic compartments. J Immunol 155:5750–5759. [PubMed] [Google Scholar]
- 188.Naegelen I, Plançon S, Nicot N, Kaoma T, Muller A, Vallar L, Tschirhart EJ, Bréchard S. 2015. An essential role of syntaxin 3 protein for granule exocytosis and secretion of IL-1α, IL-1β, IL-12b, and CCL4 from differentiated HL-60 cells. J Leukoc Biol 97:557–571. doi: 10.1189/jlb.3A0514-254RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Brochetta C, Vita F, Tiwari N, Scandiuzzi L, Soranzo MR, Guérin-Marchand C, Zabucchi G, Blank U. 2008. Involvement of Munc18 isoforms in the regulation of granule exocytosis in neutrophils. Biochim Biophys Acta 1783:1781–1791. doi: 10.1016/j.bbamcr.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 190.Monfregola J, Johnson JL, Meijler MM, Napolitano G, Catz SD. 2012. MUNC13-4 protein regulates the oxidative response and is essential for phagosomal maturation and bacterial killing in neutrophils. J Biol Chem 287:44603–44618. doi: 10.1074/jbc.M112.414029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pivot-Pajot C, Varoqueaux F, de Saint Basile G, Bourgoin SG. 2008. Munc13-4 regulates granule secretion in human neutrophils. J Immunol 180:6786–6797. doi: 10.4049/jimmunol.180.10.6786. [DOI] [PubMed] [Google Scholar]
- 192.Ménasché G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F, de Saint Basile G. 2000. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet 25:173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- 193.Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, Lambert N, Ouachée-Chardin M, Chedeville G, Tamary H, Minard-Colin V, Vilmer E, Blanche S, Le Deist F, Fischer A, de Saint Basile G. 2003. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell 115:461–473. doi: 10.1016/S0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 194.Froon AH, Dentener MA, Greve JW, Ramsay G, Buurman WA. 1995. Lipopolysaccharide toxicity-regulating proteins in bacteremia. J Infect Dis 171:1250–1257. doi: 10.1093/infdis/171.5.1250. [DOI] [PubMed] [Google Scholar]
- 195.Wong HR, Doughty LA, Wedel N, White M, Nelson BJ, Havrilla N, Carcillo JA. 1995. Plasma bactericidal/permeability-increasing protein concentrations in critically ill children with the sepsis syndrome. Pediatr Infect Dis J 14:1087–1091. doi: 10.1097/00006454-199512000-00011. [DOI] [PubMed] [Google Scholar]
- 196.Nupponen I, Turunen R, Nevalainen T, Peuravuori H, Pohjavuori M, Repo H, Andersson S. 2002. Extracellular release of bactericidal/permeability-increasing protein in newborn infants. Pediatr Res 51:670–674. doi: 10.1203/00006450-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 197.Froon AH, Bonten MJ, Gaillard CA, Greve JW, Dentener MA, de Leeuw PW, Drent M, Stobberingh EE, Buurman WA. 1998. Prediction of clinical severity and outcome of ventilator-associated pneumonia. Comparison of simplified acute physiology score with systemic inflammatory mediators. Am J Respir Crit Care Med 158:1026–1031. doi: 10.1164/ajrccm.158.4.9801013. [DOI] [PubMed] [Google Scholar]
- 198.Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. 2014. Early-onset neonatal sepsis. Clin Microbiol Rev 27:21–47. doi: 10.1128/CMR.00031-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ambruso DR, Johnston RB Jr. 1981. Lactoferrin enhances hydroxyl radical production by human neutrophils, neutrophil particulate fractions, and an enzymatic generating system. J Clin Invest 67:352–360. doi: 10.1172/JCI110042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Welin A, Amirbeagi F, Christenson K, Björkman L, Björnsdottir H, Forsman H, Dahlgren C, Karlsson A, Bylund J. 2013. The human neutrophil subsets defined by the presence or absence of OLFM4 both transmigrate into tissue in vivo and give rise to distinct NETs in vitro. PLoS One 8:e69575. doi: 10.1371/journal.pone.0069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liu W, Yan M, Sugui JA, Li H, Xu C, Joo J, Kwon-Chung KJ, Coleman WG, Rodgers GP. 2013. Olfm4 deletion enhances defense against Staphylococcus aureus in chronic granulomatous disease. J Clin Invest 123:3751–3755. doi: 10.1172/JCI68453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lawrence SM, Corriden R, Nizet V. 2017. Age-appropriate function and dysfunction of the neonatal neutrophil. Front Pediatr 5:23. doi: 10.3389/fped.2017.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ambruso DR, Bentwood B, Henson PM, Johnston RB Jr. 1984. Oxidative metabolism of cord blood neutrophils: relationship to content and degranulation of cytoplasmic granules. Pediatr Res 18:1148–1153. doi: 10.1203/00006450-198411000-00019. [DOI] [PubMed] [Google Scholar]
- 204.Ambruso DR, Stork LC, Gibson BE, Thurman GW. 1987. Increased activity of the respiratory burst in cord blood neutrophils: kinetics of the NADPH oxidase enzyme system in subcellular fractions. Pediatr Res 21:205–210. doi: 10.1203/00006450-198702000-00019. [DOI] [PubMed] [Google Scholar]
- 205.Urlichs F, Speer CP. 2004. Neutrophil function in preterm and term infants. Neoreviews 5:e417–e429. doi: 10.1542/neo.5-10-e417. [DOI] [Google Scholar]
- 206.Drossou V, Kanakoudi F, Tzimouli V, Sarafidis K, Taparkou A, Bougiouklis D, Petropoulou T, Kremenopoulos G. 1997. Impact of prematurity, stress and sepsis on the neutrophil respiratory burst activity of neonates. Biol Neonate 72:201–209. doi: 10.1159/000244485. [DOI] [PubMed] [Google Scholar]
- 207.Gahr M, Blanke R, Speer CP. 1985. Polymorphonuclear leukocyte function in term and preterm newborn infants. Biol Neonate 48:15–20. doi: 10.1159/000242147. [DOI] [PubMed] [Google Scholar]
- 208.Komatsu H, Tsukimori K, Hata K, Satoh S, Nakano H. 2001. The characterization of superoxide production of human neonatal neutrophil. Early Hum Dev 65:11–19. doi: 10.1016/S0378-3782(01)00188-8. [DOI] [PubMed] [Google Scholar]
- 209.Strunk T, Temming P, Gembruch U, Reiss I, Bucsky P, Schultz C. 2004. Differential maturation of the innate immune response in human fetuses. Pediatr Res 56:219–226. doi: 10.1203/01.PDR.0000132664.66975.79. [DOI] [PubMed] [Google Scholar]
- 210.Micanovic R, Chitteti BR, Dagher PC, Srour EF, Khan S, Hato T, Lyle A, Tong Y, Wu XR, El-Achkar TM. 2015. Tamm-Horsfall protein regulates granulopoiesis and systemic neutrophil homeostasis. J Am Soc Nephrol 26:2172–2182. doi: 10.1681/ASN.2014070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tak T, Tesselaar K, Pillay J, Borghans JA, Koenderman L. 2013. What's your age again? Determination of human neutrophil half-lives revisited. J Leukoc Biol 94:595–601. doi: 10.1189/jlb.1112571. [DOI] [PubMed] [Google Scholar]
- 212.Bektas S, Goetze B, Speer CP. 1990. Decreased adherence, chemotaxis and phagocytic activities of neutrophils from preterm neonates. Acta Paediatr Scand 79:1031–1038. doi: 10.1111/j.1651-2227.1990.tb11379.x. [DOI] [PubMed] [Google Scholar]
- 213.Boll IT, Fuchs G. 1970. A kinetic model of granulocytopoiesis. Exp Cell Res 61:147–152. doi: 10.1016/0014-4827(70)90268-5. [DOI] [PubMed] [Google Scholar]
- 214.Donohue DM, Reiff RH, Hanson ML, Betson Y, Finch CA. 1958. Quantitative measurement of the erythrocytic and granulocytic cells of the marrow and blood. J Clin Invest 37:1571–1576. doi: 10.1172/JCI103750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Carr R. 2000. Neutrophil production and function in newborn infants. Br J Haematol 110:18–28. doi: 10.1046/j.1365-2141.2000.01992.x. [DOI] [PubMed] [Google Scholar]
- 216.Edwards SW. 1994. Biochemistry and physiology of the neutrophil, 1st ed Cambridge University Press Publishing, New York, NY. [Google Scholar]
- 217.Christensen RD, Rothstein G. 1984. Pre- and postnatal development of granulocytic stem cells in the rat. Pediatr Res 18:599–602. doi: 10.1203/00006450-198407000-00006. [DOI] [PubMed] [Google Scholar]
- 218.Christensen RD, Harper TE, Rothstein G. 1986. Granulocyte-macrophage progenitor cells in term and preterm neonates. J Pediatr 109:1047–1051. doi: 10.1016/S0022-3476(86)80297-9. [DOI] [PubMed] [Google Scholar]
- 219.Deshmukh HS, Liu Y, Menkiti OR, Mei J, Dai N, O'Leary CE, Oliver PM, Kolls JK, Weiser JN, Worthen GS. 2014. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med 20:524–530. doi: 10.1038/nm.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bizzarro MJ, Dembry LM, Baltimore RS, Gallagher PG. 2008. Changing patterns in neonatal Escherichia coli sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis. Pediatrics 121:689–696. doi: 10.1542/peds.2007-2171. [DOI] [PubMed] [Google Scholar]
- 221.Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sánchez PJ, Ambalavanan N, Benjamin DK Jr, NICHD Neonatal Research Network. 2009. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 123:58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Greenwood C, Morrow AL, Lagomarcino AJ, Altaye M, Taft DH, Yu Z, Newburg DS, Ward DV, Schibler KR. 2014. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of Enterobacter. J Pediatr 165:23–29. doi: 10.1016/j.jpeds.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Balmer ML, Schürch CM, Saito Y, Geuking MB, Li H, Cuenca M, Kovtonyuk LV, McCoy KD, Hapfelmeier S, Ochsenbein AF, Manz MG, Slack E, Macpherson AJ. 2014. Microbiota-derived compounds drive steady-state granulopoiesis via MyD88/TICAM signaling. J Immunol 193:5273–5283. doi: 10.4049/jimmunol.1400762. [DOI] [PubMed] [Google Scholar]
- 224.Zhang D, Chen G, Manwani D, Mortha A, Xu C, Faith JJ, Burk RD, Kunisaki Y, Jang JE, Scheiermann C, Merad M, Frenette PS. 2015. Neutrophil ageing is regulated by the microbiome. Nature 525:528–532. doi: 10.1038/nature15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mauer AM, Athens JW, Ashenbrucker H, Cartwright GE, Wintrobe MM. 1960. Leukokinetics studies. II. A method for labeling granulocytes in vitro with radioactive diisopropylfluorophosphate (DFP). J Clin Invest 39:1481–1486. doi: 10.1172/JCI104167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Athens JW, Haab OP, Raab SO, Mauer AM, Ashenbrucker H, Cartwright GE, Wintrobe MM. 1961. Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J Clin Invest 40:989–995. doi: 10.1172/JCI104338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Boxer LA, Allen JM, Baehner RL. 1980. Diminished polymorphonuclear leukocyte adherence. Function dependent on release of cyclic AMP by endothelial cells after stimulation of beta-receptors by epinephrine. J Clin Invest 66:268–274. doi: 10.1172/JCI109853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kreisel D, Nava RG, Li W, Zinselmeyer BH, Wang B, Lai J, Pless R, Gelman AE, Krupnick AS, Miller MJ. 2010. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc Natl Acad Sci U S A 107:18073–18078. doi: 10.1073/pnas.1008737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Devi S, Wang Y, Chew WK, Lima R, González A-N, Mattar CN, Chong SZ, Schlitzer A, Bakocevic N, Chew S, Keeble JL, Goh CC, Li JL, Evrard M, Malleret B, Larbi A, Renia L, Haniffa M, Tan SM, Chan JK, Balabanian K, Nagasawa T, Bachelerie F, Hidalgo A, Ginhoux F, Kubes P, Ng LG. 2013. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J Exp Med 210:2321–2336. doi: 10.1084/jem.20130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Saverymuttu SH, Peters AM, Keshavarzian A, Reavy HJ, Lavender JP. 1985. The kinetics of 111indium distribution following injection of 111indium labeled autologous granulocytes in man. Br J Haematol 61:675–685. doi: 10.1111/j.1365-2141.1985.tb02882.x. [DOI] [PubMed] [Google Scholar]
- 231.Christensen RD, Yoder BA, Baer VL, Snow GL, Butler A. 2015. Early-onset neutropenia in small-for-gestational-age infants. Pediatrics 136:e1259–e1267. doi: 10.1542/peds.2015-1638. [DOI] [PubMed] [Google Scholar]
- 232.Koenig JM, Christensen RD. 1989. Incidence, neutrophil kinetics, and natural history of neonatal neutropenia associated with maternal hypertension. N Engl J Med 321:557–562. doi: 10.1056/NEJM198908313210901. [DOI] [PubMed] [Google Scholar]
- 233.Christensen RD, Henry E, Wiedmeier SE, Stoddard RA, Lambert DK. 2006. Low blood neutrophil concentrations among extremely low birth weight neonates: data from a multihospital health-care system. J Perinatol 26:682–687. doi: 10.1038/sj.jp.7211603. [DOI] [PubMed] [Google Scholar]
- 234.Schmutz N, Henry E, Jopling J, Christensen RD. 2008. Expected ranges for blood neutrophil concentrations of neonates: the Manroe and Mouzinho charts revisited. J Perinatol 28:275–281. doi: 10.1038/sj.jp.7211916. [DOI] [PubMed] [Google Scholar]
- 235.Boner A, Zeligs BJ, Bellanti JA. 1982. Chemotactic responses of various differentiational stages of neutrophils from human cord and adult blood. Infect Immun 35:921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Makoni M, Eckert J, Pereira HA, Nizet V, Lawrence SM. 2016. Alterations in neonatal neutrophil function attributable to increased immature neutrophil forms. Early Hum Dev 103:1–7. doi: 10.1016/j.earlhumdev.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]