Abstract
Introduction
Naturally, development of adaptive immunity following HRV infection affects the immune response. However, it is currently unclear whether or not HRV re-exposure within a short time frame leads to an altered innate immune response. The “experimental cold model” is used to investigate the pathogenesis of HRV infection and allows us to investigate the effects of repeated exposure on both local and systemic innate immunity.
Methods
40 healthy male and female (1:1) subjects were nasally inoculated with HRV-16 or placebo. One week later, all subjects received HRV-16. Baseline seronegative subjects (n = 18) were included for further analysis.
Results
Infection rate was 82%. Primary HRV infection induced a marked increase in viral load and IP-10 levels in nasal wash, while a similar trend was observed for IL-6 and IL-10. Apart from an increase in IP-10 plasma levels, HRV infection did not induce systemic immune effects nor lower respiratory tract inflammation. With similar viral load present during the second HRV challenge, IP-10 and IL-6 in nasal wash showed no increase, but gradually declined, with a similar trend for IL-10.
Conclusion
Upon a second HRV challenge one week after the first, a less pronounced response for several innate immune parameters is observed. This could be the result of immunological tolerance and possibly increases vulnerability towards secondary infections.
Introduction
In the recent decade, it has become clear that bacterial sepsis may induce an immunosuppressed state called “sepsis-induced immunoparalysis”[1, 2], a form of immunological tolerance which renders the host unable to clear primary infections leading to increased vulnerability towards secondary infections[3]. This immunologically tolerant state is characterized by both innate as well as adaptive immunodysfunction, such as functional defects in leukocytes, downregulation of cytokines and immunostimulatory membrane-bound receptors, accompanied by the upregulation of negative costimulatory molecules[3, 4]. A similar phenomenon is observed with virulent respiratory virus infections, such as influenza, which predispose to secondary bacterial or fungal infections[5, 6].
Human rhinoviruses (HRVs) are the most frequent cause of the common cold[7, 8] with prevalence estimates as high as 80% of the adult population[9]. HRV infection results in the production of inflammatory cytokines and chemokines[10–14], and subsequent recruitment of immune cells into the nasopharyngeal[15] and bronchial mucosa, and secretions[11–14]. In addition, HRV-specific neutralizing antibodies are produced, resulting in seroconversion approximately 28 days later[11, 16]. HRVs consist of three species, HRV-A, HRV-B and the more recently discovered HRV-C[7]. Due to its high virulence, HRV-C can cause systemic and severe respiratory infections in previously healthy subjects[7]. This has mainly been reported for children, although several adult cases have also been described[7, 17]. Although HRV-A and HRV-B may cause severe infections in young children, immunocompromised patients, and/or patients with pre-existing respiratory diseases[18–21], it is unknown to what extent these species induce systemic immunological effects or lower respiratory tract disease in healthy adult subjects. Furthermore, although in vitro studies indicate that HRV can also induce immunosuppressive or tolerance mechanisms[22, 23], this has never been investigated in humans in vivo.
In the present study, we investigated the local respiratory tract and systemic immune response following challenge with HRV-16 (a HRV-A species) using the so-called “experimental cold model”. This model is widely used to investigate the pathogenesis of HRV infection, including the effects on pulmonary function[24], allergies[10], chronic obstructive pulmonary disease (COPD)[25], and asthma exacerbations[24, 26]. Subjects were re-challenged with the same virus one week after the first challenge, to investigate whether the primary HRV infection modulates the innate immune response against the second HRV exposure. A one-week interval was chosen, as at this time point the innate immune response induced by the first infection is expected to be largely resolved and specific antibodies have not yet been produced to a large extent[16, 27, 28]. We recently demonstrated that seropositivity for HRV is associated with a virtually nullified immune response upon HRV challenge [29]. Therefore, we only analyzed data from seronegative subjects in the current study.
Our data indicate that, despite similar viral loads upon both challenges, a second HRV challenge one week after the first results in less pronounced response of several innate immune parameters. This could be the result of immunological tolerance and may be associated with increased vulnerability towards secondary infections.
Materials and methods
Subjects
This randomized, placebo-controlled study was part of a larger trial also investigating effects of serostatus and gender on the HRV-induced immune response[29]. This study is registered at ClinicalTrials.gov (NCT01823640, participant recruitment and follow-up: March-May 2013). The authors confirm that all ongoing and related trials for this intervention are registered. As this was a pilot study, no power calculation was performed. After approval by the local medical ethics committee CMO Arnhem-Nijmegen (NL42503.091.12; CMO 2012/476), 40 healthy, non-smoking, male and female subjects (ratio 1:1), aged 18–35 years gave written informed consent to participate in the study. All study procedures were in accordance with the declaration of Helsinki. Subjects were screened and excluded if they had a (febrile) illness within four weeks before the HRV-challenge, a pre-existent lung disease, or a history of allergic rhinitis. Subjects were not allowed to take (prescription) drugs throughout the study.
Study design
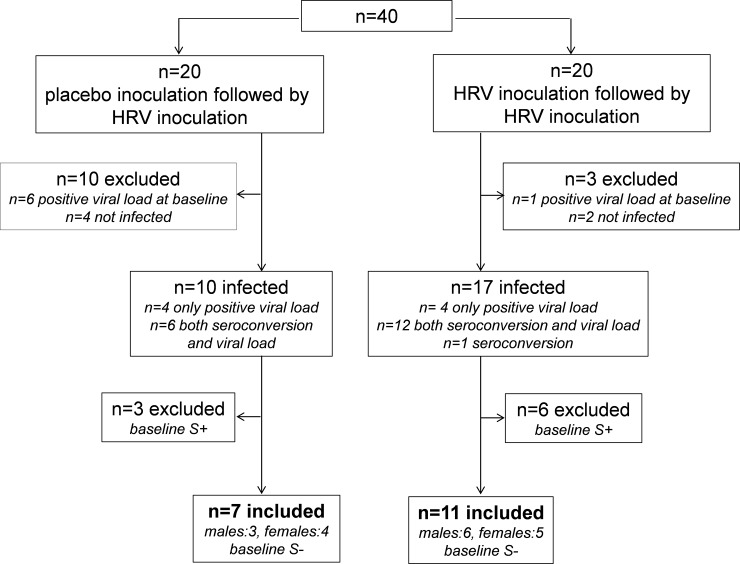
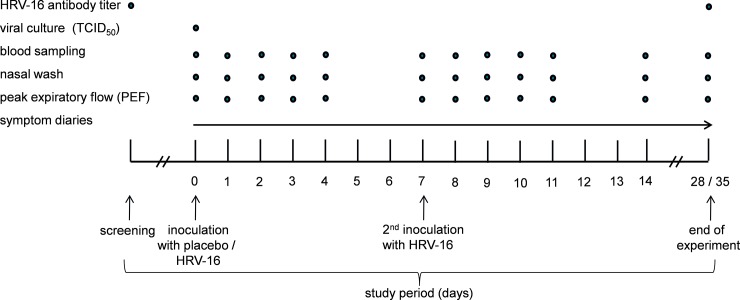
A flowchart of the study, based on infection rate (further detailed in ‘antibody titer analysis’ section) is depicted in Fig 1 and the study design is depicted in Fig 2. Stratification was based on sex and serostatus as follows: Subjects with the same sex were grouped in two groups of 10 subjects (2x n = 10 males; 2x n = 10 females; in total n = 40) subjects. Each group of 10 subjects consisted of roughly 50% seronegatives and 50% seropositives, based on the baseline antibody titers to HRV-16, measured at the screening visit (assay and cutoff value detailed in “antibody titer analysis” section below). Subsequently, one group of the same sex (n = 10) was randomized to the placebo-HRV group, while the other (n = 10) was randomized to the HRV-HRV group. Randomization was carried out by an independent nurse using the sealed envelope method. Subjects were inoculated by an independent nurse with either HRV-16 by instillation of 102 TCID50 (Tissue Culture Infectious Dose in 50% of subjects, based on previous studies[30]) units of HRV-16, diluted in 0.5 mL 0.9% saline, (n = 20; 10 males; 10 females), or placebo (0.9% saline; n = 20; 10 males; 10 females) into each nostril. Subjects remained in the recumbent position for two minutes after instillation and refrained from touching their nose for 30 minutes. Seven days later, all 40 subjects underwent challenge with HRV-16. For the analyses described in this manuscript, only seronegative subjects were included, as serostatus was shown to exert profound effects on the HRV-induced immune response, which was virtually absent in seropositive subjects[29]. Furthermore, we did not stratify our analyses for gender, as we found no differences in any parameters between males and females[29].
Fig 1. Flowchart of the study.
S+ indicates seropositive at baseline; S- indicates seronegative at baseline.
Fig 2. Experimental design of the study.
HRV-16 virus
Safety-tested Good Manufacturing Practice (GMP+) grade inoculum pools of HRV-16 were supplied by Respivert Ltd. (MM#400472 Lot#R2011038, London, UK). Each cryovial contained 0.2 mL of HRV-16 at a dose of 2*102 TCID50 units/mL and was diluted in Hartmann’s solution to a concentration of 102 TCID50 units/mL and stored at -80°C. Per infection day, one aliquot of HRV-16 was cultured in fourfold on a MRC-5 cell monolayer in 10-fold dilutions of samples, positive and negative controls in order to assess infectivity. Cell plates were placed at 33°C with 5% CO2 for seven days and observed for the development of cytopathic effects (CPE). The Reed-Muench formula was used to calculate the viral titer[31]. Viral titers were all in the expected infectivity range (101−102 TCID50 units/mL) and positive and negative control wells positive and negative for CPE, respectively.
Antibody titer analysis
A standard end-point neutralization assay for HRV-16 was used to quantify levels of HRV-neutralizing antibodies in the serum of every subject at baseline (virus neutralization titer [VNT] <1:4) and seropositive subjects (VNT ≥1:4) to exclude subjects who were seropositive to HRV-16 for the analyzes described in this manuscript, to assess seroconversion (≥fourfold increase in antibody titer at day 28 post-challenge; day 28 in the HRV-HRV group and day 35 in the placebo-HRV group; Fig 1). The assay was performed on HEL cells at 37°C and 5% CO2. Two-step serum dilutions starting at 1:4 were incubated with 102 TCID50 HRV-16 for 1 h at 37°C before inoculation on HEL cells. Serum controls were included on each plate to test for toxicity, and a positive control of anti-HRV-16 antiserum was added to each test plate. The formation of CPE was examined daily and after 7 days the cultures were scored for CPE. The Reed-Muench formula was used to calculate the antibody titer[31]. Cells with >50% CPE/well were scored antibody negative and ≤50% CPE as antibody positive. HRV-infection was defined as positive viral load and/or seroconversion (≥fourfold increase in antibody titer at day 28 post-inoculation) compared with baseline[24].
Nasal wash
Nasal washings for viral load and cytokine quantification were collected daily from subjects according to the method described by Naclerio[32]. Nasal wash from two nostrils was pooled, centrifuged (6000 rpm, 4°C, 20 minutes), and stored at -80°C until analysis.
Viral load
Non-specific HRV viral load was determined from nasal wash as described previously[33] and performed at the laboratory of medical microbiology at the Radboudumc according to QCMD regulations. Briefly, nucleic acids were extracted from each sample using the MagNA Pure LC (with Total Nucleic Acid Isolation Kit) and PCRs were performed on the LightCycler 480 with Probes Master Mix (Roche Diagnostics, Almere, The Netherlands) using commercial validated primer and probe-mixes (Diagenode, Liège, Belgium; Table B in S1 File). Cycling conditions were 95°C for five minutes, followed by 40 cycles of 95°C (15 sec), 55°C (15 sec) and 72°C (20 sec). Virus amount was recorded semi-quantitatively based on the cycle threshold value (Ct value). All samples in which virus was detected (Ct<40) were considered as having a positive viral load. For samples in which no virus was detected, the Ct value was set as 40 to allow fold change calculations. Fold change from baseline (Ct = 40) was calculated using the formula 2∆Ct.
Symptoms
We previously showed that the HRV-16 batch used in this study does not result in significant induction of cold symptoms[29]. Therefore, we focused on lower respiratory tract and systemic symptoms in the current study. To assess these, all subjects filled out an online symptom diary (LimeSurvey Project Hamburg, Germany), using questions from the validated Wisconsin Upper Respiratory Symptom Survey-24 (WURSS-24)[34], indicative for a lower respiratory tract/systemic respiratory infection (headache, sinusitis, cough, chest tightness, dyspnoea, fever, shivering, body ache and malaise). Symptoms were assessed prior to viral/placebo challenge and then twice daily, to take into account circadian variations, until day 28. The severity of each symptom was rated on a six-point scale.
Peak Expiratory Flow
To evaluate whether HRV influenced pulmonary function (lower respiratory tract effects), Peak Expiratory Flow (PEF) was measured during each visit using a peak flow meter (PFM20, Omron Healthcare Europe B.V., Hoofddorp, The Netherlands). PEF was determined twice during each visit and the highest value was used. An affected lower respiratory tract was defined as a >20% decrease in PEF of the predicted values of their corresponding age, gender, and stature[35].
Cytokine analysis
Nasal wash and ethylenediaminetetraacetic acid (EDTA) anticoagulated blood samples were collected at various time points (Fig 1) centrifuged (6000 rpm, 4°C, 20 minutes) and stored at -80°C until analysis. Concentrations of interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-8, IL-10 were measured using a simultaneous Luminex assay (Milliplex; Millipore, Billerica, MA, USA). Interferon gamma-induced protein (IP-10) was measured using ELISA (R&D Systems, Minneapolis, MN, USA). Lower detection limits were 3.2 pg/mL for IFN-γ, TNF-α, IL-1β, IL-6, IL-8, IL-10, and 156 pg/mL for IP-10.
Statistical analysis
According to the Kolmogov-Smirnov test, all data were non-normally distributed. Therefore, demographic data are presented as medians [interquartile range] and between-group comparisons were made using Mann-Whitney U tests. All other data are presented as geometric mean and 95% CI. Because there was an interindividual variation in the peak of acute infection ranging from day 1–4 after HRV inoculation, differences were analyzed on log-transformed peak levels in the first four days post-challenge using paired and unpaired Student’s t-tests. Statistical analyses were performed using Graphpad Prism version 5.0 (Graphpad Software, San Diego, CA, USA). Two-sided p-values <0.05 were considered statistically significant.
Results
Baseline subject characteristics
Baseline characteristics of the entire study population (n = 40, before exclusion of subject as detailed below) are listed in Table A in S1 File). There were no significant differences in baseline characteristics between the groups. No adverse events occurred during the trial.
Infection rate
A flowchart based on infection rate is depicted in Fig 1. Seven subjects displayed a positive viral load in nasal wash before challenge with HRV, hence they were excluded from all subsequent analyses. In 27 of the remaining 33 subjects (82%), HRV challenge resulted in infection (positive viral load and/or seroconversion; antibody titers of all subject provided in Table C in S1 File). The six subjects who showed neither positive viral load at any of the time-points post-challenge, nor seroconversion were categorized ‘not infected’ and were also excluded from all subsequent analyses. Furthermore, as explained in the introduction and materials and methods section, 9 subjects who were HRV-seropositive at baseline were excluded from the subsequent analyses. Characteristics of the 18 remaining baseline HRV-seronegative infected subjects who were included for the final analysis showed no significant differences between groups (Table 1).
Table 1. Demographic characteristics of the 18 seronegative subjects who displayed positive infection that were included in the final analysis.
Parameters were assessed during the screening visit. M: male, F: female. BMI: body mass index. Data are presented as medians [interquartile range].
| placebo-HRV n = 7 M (n = 3) F (n = 4) |
HRV- HRV n = 11 M (n = 6) F (n = 5) |
total group n = 18 |
p value between groups | |
|---|---|---|---|---|
| Age (yrs) | 22[21–26] | 22[22–25] | 22[21–25] | 0.51 |
| Height (cm) | 175[166–182] | 175[168–184] | 175[168–183] | 0.82 |
| Weight (kg) | 73[66–88] | 71[64–78] | 72[66–79] | 0.56 |
| BMI (kg/m2) | 23[22–25] | 23[21–24] | 23[22–25] | 0.41 |
Viral load
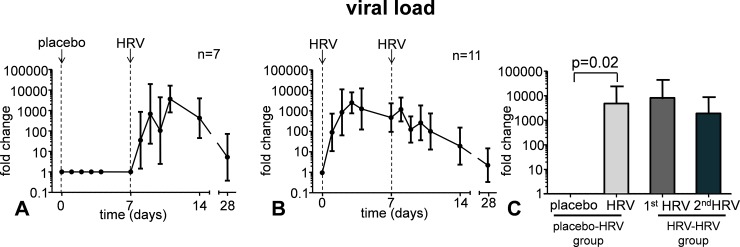
Viral load in nasal wash increased following HRV infection (Fig 3). At day 7, viral load was still increased, and remained at a similar level upon a second challenge with the same virus (Fig 3).
Fig 3.
Viral load levels in nasal wash following placebo inoculation and HRV challenge (panel A) and following two HRV challenges separated by one week time (panel B). Panel C shows the peak levels in the first four days post-challenge in the group that received placebo, followed by a HRV challenge (bars 1 and 2), and in the group that were challenged with HRV twice (bars 3 and 4). Data are represented as geometric mean and 95% CI.
Cytokines in nasal wash
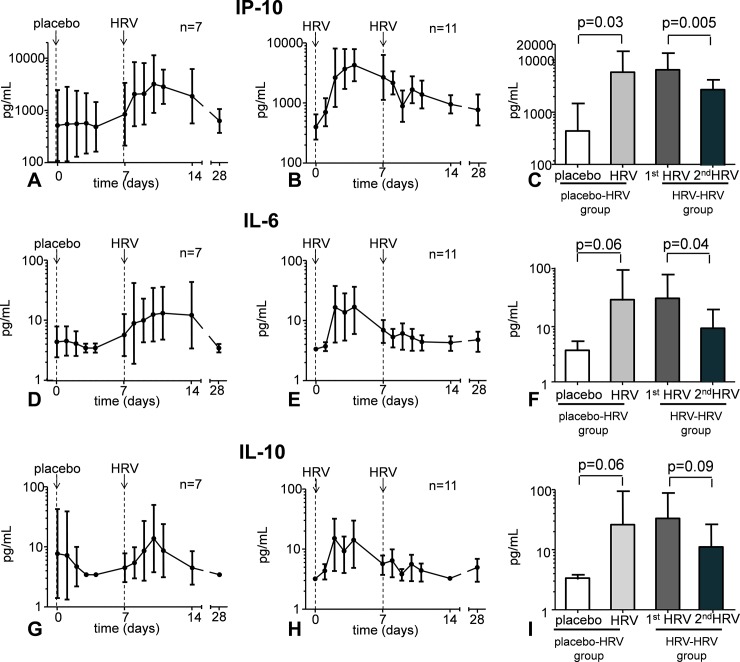
HRV infection did not increase the production of IFN-γ, TNF-α, IL-1β and IL-8. However, a transient increase in levels of IP-10 in nasal wash, and a similar trend for IL-6 and IL-10 was observed (Fig 4). In subjects who were challenged with HRV twice, the first HRV inoculation resulted in an identical immune response compared with subjects who received HRV once (Fig 4).
Fig 4.
Cytokine levels in nasal wash following placebo inoculation and HRV challenge (panels A, D, and G) and following two HRV challenges separated by one week time (panels B, E, and H). Panels C, F, and I show the peak levels in the first four days post-challenge in the group that received placebo, followed by a HRV challenge (bar 1 and 2), and in the group that were challenged with HRV twice (bar 3 and 4). Data are represented as geometric mean and 95% CI. Lower detection limits were 3.2 pg/mL for IL-6 and IL-10, and 156 pg/mL for IP-10.
However, following the second HRV challenge, levels of IP-10 and IL-6 in nasal wash showed no further increase, but decreased significantly. A similar trend was observed for IL-10 (Fig 4). IFN-γ, IL-1β, and TNF-α levels in nasal wash were below the detection limit in virtually all subjects, and no clear profiles were observed following HRV infection.
Systemic and lower respiratory tract responses
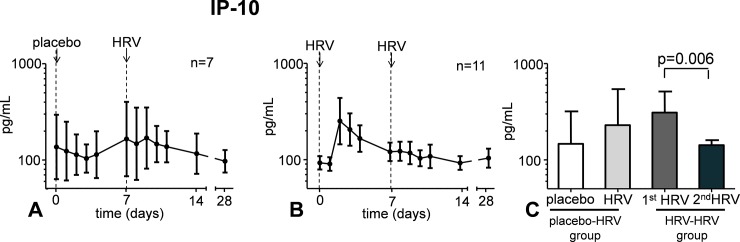
Plasma levels of IFN-γ, TNF-α, IL-1β, IL-6, IL-8, and IL-10 were below the detection limit in virtually all subjects at all time points. In the few subjects who displayed detectable levels of these cytokines, values were very low (approximately 10 pg/mL), in most cases already present as baseline, and did not increase following HRV infection. IP-10 levels were detectable in plasma already at baseline, but HRV infection after placebo inoculation did not result in significantly increased plasma levels (Fig 5). In subjects who were challenged with HRV twice, a more pronounced increase was observed after the first inoculation, although this response was not significantly different compared with that observed in subjects who received HRV once. Upon the second HRV challenge however, a significant decrease was observed compared with the first (Fig 5).
Fig 5.
Plasma IP-10 levels following placebo inoculation and HRV challenge (panel A) and following two HRV challenges separated by one week time (panel B). Panel C shows the peak levels in the first four days post-challenge in the group that received placebo, followed by a HRV challenge (bar 1 and 2) and in the group that were challenged with HRV twice (bar 3 and 4). Data are represented as geometric mean and 95% CI. Lower detection limit was 156 pg/mL.
HRV infection resulted neither in systemic symptoms, nor in symptoms indicating a descending respiratory tract infection (Figure A in S1 File). Furthermore, HRV infection did not affect PEF, as all subjects produced values >80% of their individual predicted values at all time-points, and PEF did not change after HRV challenge (Figure B in S1 File).
Discussion
In the present study, we show that the response of several innate immune parameters is less pronounced upon a second HRV challenge seven days after the first challenge.
We chose to investigate the influence of two consecutive HRV challenges within a short time frame. As a consequence, the observed attenuated response of several innate immune parameters to the second HRV exposure may be the result of tolerance. These results indicate that viruses causing relatively mild illness also induce attenuation of innate immune responses and may lead to increased vulnerability towards secondary fungal / bacterial infections, or reactivation of viruses that reside latent in the human host[4]. This could have consequences for vulnerable patient groups, such as the elderly, and might influence vaccination strategies. This finding is supported by in vitro studies that have shown that HRV can induce immunosuppressive mechanisms[22, 23]. The effects observed in the present study are reminiscent of “endotoxin tolerance”, observed after challenging healthy volunteers with bacterial lipopolysaccharide[36, 37], and might resemble some aspects of the severely immunosuppressed state observed in patients with sepsis[3] and influenza infections[5]. As such, it is tempting to speculate that previous infection with relatively mild viruses such as HRV renders patients increased vulnerable towards secondary bacterial and/or viral infections. However, additional studies are warranted to assess the clinical relevance of our observations. The tolerance effect observed could be due to desensitization effects on local immune cells or a type-I interferon induced CD8 T-cell mediated anti-viral state where no replication occurs[38]. For instance, it was shown that HRV can survive in alveolar macrophages and impair the cytokine responses to a second challenge with bacterial ligands[23]. Along these lines, for influenza and respiratory syncytial virus (RSV), previous work has demonstrated a post-viral desensitization of alveolar macrophages to Toll-like receptor (TLR) ligands, associated with reduced NF-kappaB activation and chemokine production[5].
In our study, we only included seronegative subjects, because previous work has demonstrated that serostatus alters both HRV-induced symptom scores[16, 39] as well as the HRV-induced immune response, which we showed to be virtually nullified in seropositive subjects[29]. As such, next to the pathophysiological consequences, our results indicate that a crossover design is neither feasible using a short interval, due to a suppressed innate immune response, nor using a longer interval, due to antibody formation that starts approximately one week after HRV infection[16] and severely impacts the immune response[29].
The mucosal immune response observed in the present study, reflected by the increase in cytokines in nasal wash, is in line with various previous in vitro and in vivo studies[11–13, 40]. In response to HRV infection, IP-10 was in contrast to other cytokines, produced in nasal wash of all subjects following infection. As such, our results are in accordance with previous studies that have demonstrated that IP-10 is a sensitive marker for HRV infection[12, 41, 42]. IP-10 plasma levels did not significantly increase upon a single HRV challenge although a trend was apparent, especially following the first HRV inoculation in subjects who were challenged with HRV twice. In addition, more subjects that were challenged twice demonstrated positive infection (11 in the HRV-HRV group vs. 7 in the placebo-HRV group; Fig 2). Apparently in this short time frame, the host is not yet capable to induce an effective innate immune response to eliminate HRV, supportive of the immunological tolerance theory. The mild increase of IP-10 plasma levels is in line with studies in asthmatic and COPD patients[41, 42]. In the absence of alterations of all other systemic markers measured, this relatively minor increase might suggest spillover from the nasopharynx to the circulation, although production by cells outside the nasopharynx cannot be excluded.
Apart from the increase in IP-10, neither systemic symptoms or immune effects were found after HRV infection, nor effects on the lower respiratory tract were observed. These findings suggest that, unlike what has been described for vulnerable groups such as young children, immunocompromised patients, and/or patients with pre-existing airway diseases[18–21], HRV-16 does not exert these effects in healthy subjects.
This study has several limitations. First, we included a relatively low number of subjects, which probably explains that several differences did not reach statistical significance.
Second, we only studied healthy subjects, therefore the observed effects could be different in a diseased population, such as those suffering from sepsis, which might display dysregulated cytokine responses[37].
Third, we did not store the nasal wash cellular fraction and therefore could not assess cellular markers such as induction of antiviral genes such as type I or III interferons, which play important roles in respiratory viral infections[43].
Finally, we chose to perform the second HRV challenge one week after the first, as at this time point the innate immune response induced by the first infection is expected to be largely resolved, but apparently, viral load and levels of the cytokines in nasal wash had not returned to baseline yet. However, one would expect a further increase in these parameters after a second HRV challenge in case of a normal immune response, while a similar viral load and further decrease in cytokines was apparent. In addition, although tolerance is a plausible explanation for the attenuated immune response, one could speculate about the possibility that, albeit in relatively little quantities, anti-HRV specific antibodies are already present at this time, which could also lead to attenuation of the immune response upon the second HRV-16 challenge. However, studies that investigated kinetics of HRV-induced neutralizing antibodies make this theory less likely[44, 45]. These studies demonstrate that HRV-neutralizing antibodies in both nasal wash and serum are low seven days after HRV inoculation, and their titers begin to rise at approximately two weeks after inoculation[44], although it should be mentioned that the conventional detection tests used in these studies might be less sensitive in detecting HRV-specific antibodies than the tests that are used nowadays. Nonetheless, our data also show that viral load remained elevated until day 28, when the majority of subjects displayed seroconversion. Therefore, it appears not to be possible to find a suitable time-window to investigate the effects of consecutive HRV challenges without taking into account the possibly confounding effects of the development of an adaptive immune response, leading to the production of anti-HRV specific antibodies. Using a different HRV strain for the second HRV challenge would eliminate the possible influence of HRV-16-specific antibodies on the immune response.
In conclusion, we report that a second HRV challenge one week after the first results in a less pronounced response of several innate immune parameters. This could indicate that relatively mild viruses can also induce immunosuppression, possibly leading to increased vulnerability towards secondary infections.
Supporting information
(DOC)
(DOC)
(DOC)
Acknowledgments
The authors thank the research nurses (Marieke van der A, Chantal Luijten-Arts, Hellen van Wezel) of the ICU department and Hicham el Moussaoui of the Laboratory of Paediatric Infectious Diseases for their help during the HRV experiments and Cor Jacobs for the flow cytometry analysis. Moreover, we thank Hetty van Eijk and Katja Wolthers from the University of Amsterdam for performing the HRV-16 neutralizing antibody assay. We also thank Chantelle Norton and Lindsey Cass of Respivert Ltd. for providing HRV-16 and Dr. Foekje Stelma of the Department Virology for processing HRV-16 and Jenneke Leentjens for advice.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by an EFRO grant (2011-013287). StB is an employee of NIZO food research BV, a commercial party that participated in this EFRO grant. EFRO provided support in the form of salaries for authors [RMK, MK, StB, MIdJ, PP] and research materials, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Apart from the design and analysis of the online symptom diary and review of the manuscript, NIZO food research BV, in the person of StB, did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of all authors are articulated in the 'author contributions' section.
References
- 1.Leentjens J, Kox M, van der Hoeven JG, Netea MG, Pickkers P. Immunotherapy for the adjunctive treatment of sepsis: from immunosuppression to immunostimulation. Time for a paradigm change? Am J Respir Crit Care Med. 2013;187(12):1287–93. Epub 2013/04/18. doi: 10.1164/rccm.201301-0036CP . [DOI] [PubMed] [Google Scholar]
- 2.Hamers L, Kox M, Pickkers P. Sepsis-induced immunoparalysis: mechanisms, markers, and treatment options. Minerva anestesiologica. 2014. Epub 2014/06/01. . [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. The Lancet infectious diseases. 2013;13(3):260–8. doi: 10.1016/S1473-3099(13)70001-X ; PubMed Central PMCID: PMC3798159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koch RM, Kox M, de Jonge MI, van der Hoeven JG, Ferwerda G, Pickkers P. Patterns in Bacterial- and Viral-Induced Immunosuppression and Secondary Infections in the ICU. Shock. 2016. doi: 10.1097/SHK.0000000000000731 . [DOI] [PubMed] [Google Scholar]
- 5.Didierlaurent A, Goulding J, Patel S, Snelgrove R, Low L, Bebien M, et al. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. The Journal of experimental medicine. 2008;205(2):323–9. Epub 2008/01/30. doi: 10.1084/jem.20070891 ; PubMed Central PMCID: PMC2271005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van de Veerdonk FL, Kolwijck E, Lestrade PP, Hodiamont CJ, Rijnders BJ, van Paassen J, et al. Influenza-Associated Aspergillosis in Critically Ill Patients. Am J Respir Crit Care Med. 2017. doi: 10.1164/rccm.201612-2540LE . [DOI] [PubMed] [Google Scholar]
- 7.Lau SK, Yip CC, Woo PC, Yuen KY. Human rhinovirus C: a newly discovered human rhinovirus species. Emerging health threats journal. 2010;3:e2 Epub 2010/01/01. doi: 10.3134/ehtj.10.002 ; PubMed Central PMCID: PMC3167658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinke JW, Liu L, Turner RB, Braciale TJ, Borish L. Immune Surveillance by Rhinovirus-Specific Circulating CD4+ and CD8+ T Lymphocytes. PLoS One. 2015;10(1):e0115271 Epub 2015/01/15. doi: 10.1371/journal.pone.0115271 ; PubMed Central PMCID: PMC4293146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atmar RL. Uncommon(ly considered) manifestations of infection with rhinovirus, agent of the common cold. Clin Infect Dis. 2005;41(2):266–7. doi: 10.1086/430927 . [DOI] [PubMed] [Google Scholar]
- 10.Avila PC, Abisheganaden JA, Wong H, Liu J, Yagi S, Schnurr D, et al. Effects of allergic inflammation of the nasal mucosa on the severity of rhinovirus 16 cold. J Allergy Clin Immunol. 2000;105(5):923–32. Epub 2000/05/16. S0091-6749(00)80015-9 [pii] doi: 10.1067/mai.2000.106214 . [DOI] [PubMed] [Google Scholar]
- 11.Doyle WJ, Gentile DA, Cohen S. Emotional style, nasal cytokines, and illness expression after experimental rhinovirus exposure. Brain Behav Immun. 2006;20(2):175–81. Epub 2005/07/19. S0889-1591(05)00101-7 [pii] doi: 10.1016/j.bbi.2005.05.005 . [DOI] [PubMed] [Google Scholar]
- 12.Spurrell JC, Wiehler S, Zaheer RS, Sanders SP, Proud D. Human airway epithelial cells produce IP-10 (CXCL10) in vitro and in vivo upon rhinovirus infection. Am J Physiol Lung Cell Mol Physiol. 2005;289(1):L85–95. Epub 2005/03/15. doi: 10.1152/ajplung.00397.2004 . [DOI] [PubMed] [Google Scholar]
- 13.Koetzler R, Zaheer RS, Wiehler S, Holden NS, Giembycz MA, Proud D. Nitric oxide inhibits human rhinovirus-induced transcriptional activation of CXCL10 in airway epithelial cells. J Allergy Clin Immunol. 2009;123(1):201–8 e9. Epub 2008/11/07. S0091-6749(08)01738-7 [pii] doi: 10.1016/j.jaci.2008.09.041 . [DOI] [PubMed] [Google Scholar]
- 14.Jarjour NN, Gern JE, Kelly EA, Swenson CA, Dick CR, Busse WW. The effect of an experimental rhinovirus 16 infection on bronchial lavage neutrophils. J Allergy Clin Immunol. 2000;105(6 Pt 1):1169–77. Epub 2000/06/16. S0091674900171234 [pii]. . [DOI] [PubMed] [Google Scholar]
- 15.Sanders SP, Proud D, Permutt S, Siekierski ES, Yachechko R, Liu MC. Role of nasal nitric oxide in the resolution of experimental rhinovirus infection. J Allergy Clin Immunol. 2004;113(4):697–702. Epub 2004/04/22. doi: 10.1016/j.jaci.2004.01.755 . [DOI] [PubMed] [Google Scholar]
- 16.Barclay WS, al-Nakib W, Higgins PG, Tyrrell DA. The time course of the humoral immune response to rhinovirus infection. Epidemiology and infection. 1989;103(3):659–69. Epub 1989/12/01. ; PubMed Central PMCID: PMC2249538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau SK, Yip CC, Lin AW, Lee RA, So LY, Lau YL, et al. Clinical and molecular epidemiology of human rhinovirus C in children and adults in Hong Kong reveals a possible distinct human rhinovirus C subgroup. J Infect Dis. 2009;200(7):1096–103. Epub 2009/08/28. doi: 10.1086/605697 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jennings LC, Anderson TP, Beynon KA, Chua A, Laing RT, Werno AM, et al. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008;63(1):42–8. Epub 2007/06/19. doi: 10.1136/thx.2006.075077 . [DOI] [PubMed] [Google Scholar]
- 19.Brownlee JW, Turner RB. New developments in the epidemiology and clinical spectrum of rhinovirus infections. Current opinion in pediatrics. 2008;20(1):67–71. Epub 2008/01/17. doi: 10.1097/MOP.0b013e3282f41cb6 . [DOI] [PubMed] [Google Scholar]
- 20.Miller EK, Lu X, Erdman DD, Poehling KA, Zhu Y, Griffin MR, et al. Rhinovirus-associated hospitalizations in young children. J Infect Dis. 2007;195(6):773–81. Epub 2007/02/15. doi: 10.1086/511821 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee WM, Lemanske RF Jr., Evans MD, Vang F, Pappas T, Gangnon R, et al. Human Rhinovirus Species and Season of Infection Determine Illness Severity. Am J Respir Crit Care Med. 2012. Epub 2012/08/28. doi: 10.1164/rccm.201202-0330OC . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stockl J, Vetr H, Majdic O, Zlabinger G, Kuechler E, Knapp W. Human major group rhinoviruses downmodulate the accessory function of monocytes by inducing IL-10. J Clin Invest. 1999;104(7):957–65. Epub 1999/10/08. doi: 10.1172/JCI7255 ; PubMed Central PMCID: PMC408557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliver BG, Lim S, Wark P, Laza-Stanca V, King N, Black JL, et al. Rhinovirus exposure impairs immune responses to bacterial products in human alveolar macrophages. Thorax. 2008;63(6):519–25. Epub 2008/02/05. thx.2007.081752 [pii] doi: 10.1136/thx.2007.081752 . [DOI] [PubMed] [Google Scholar]
- 24.Bardin PG, Fraenkel DJ, Sanderson G, van Schalkwyk EM, Holgate ST, Johnston SL. Peak expiratory flow changes during experimental rhinovirus infection. Eur Respir J. 2000;16(5):980–5. Epub 2001/01/12. . [DOI] [PubMed] [Google Scholar]
- 25.Mallia P, Message SD, Gielen V, Contoli M, Gray K, Kebadze T, et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med. 2011;183(6):734–42. Epub 2010/10/05. 201006-0833OC [pii] doi: 10.1164/rccm.201006-0833OC ; PubMed Central PMCID: PMC3081284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bardin PG, Sanderson G, Robinson BS, Holgate ST, Tyrrell DA. Experimental rhinovirus infection in volunteers. Eur Respir J. 1996;9(11):2250–5. Epub 1996/11/01. . [DOI] [PubMed] [Google Scholar]
- 27.Message SD, Johnston SL. The immunology of virus infection in asthma. Eur Respir J. 2001;18(6):1013–25. Epub 2002/02/07. . [DOI] [PubMed] [Google Scholar]
- 28.Fleming HE, Little FF, Schnurr D, Avila PC, Wong H, Liu J, et al. Rhinovirus-16 colds in healthy and in asthmatic subjects: similar changes in upper and lower airways. Am J Respir Crit Care Med. 1999;160(1):100–8. Epub 1999/07/03. doi: 10.1164/ajrccm.160.1.9808074 . [DOI] [PubMed] [Google Scholar]
- 29.Koch RM, Kox M, Pickkers P, de Jonge MI, Radboudumc Immunoforce g. Effects of serostatus and gender on the HRV-16-induced local immune response. Vaccine. 2016;34(35):4087–91. doi: 10.1016/j.vaccine.2016.06.076 . [DOI] [PubMed] [Google Scholar]
- 30.Branigan PJ, Turner RB, LM J., Marciniak S, Delvecchio AM, Norton C, et al. Enhanced Local Interferon-Associated CXCL11 And CXCL12 Production Correlates With Elevated CXCL10 Response And Cold Severity Following Experimental Rhinovirus 16 Infection In Normal Healthy Volunteers Am J Respir Crit Care Med 2014;189;2014;A1710. [Google Scholar]
- 31.Reed LJaM. A simple method of estimating fifty per cent endpoints. The american Journal of Hygiene. 1938;27(No.3). [Google Scholar]
- 32.Naclerio RM, Meier HL, Kagey-Sobotka A, Adkinson NF Jr., Meyers DA, Norman PS, et al. Mediator release after nasal airway challenge with allergen. Am Rev Respir Dis. 1983;128(4):597–602. Epub 1983/10/01. doi: 10.1164/arrd.1983.128.4.597 . [DOI] [PubMed] [Google Scholar]
- 33.Scheltinga SA, Templeton KE, Beersma MF, Claas EC. Diagnosis of human metapneumovirus and rhinovirus in patients with respiratory tract infections by an internally controlled multiplex real-time RNA PCR. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2005;33(4):306–11. Epub 2005/07/05. doi: 10.1016/j.jcv.2004.08.021 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrett B, Hayney MS, Muller D, Rakel D, Ward A, Obasi CN, et al. Meditation or exercise for preventing acute respiratory infection: a randomized controlled trial. Annals of family medicine. 2012;10(4):337–46. Epub 2012/07/11. doi: 10.1370/afm.1376 ; PubMed Central PMCID: PMC3392293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quanjer PH, Lebowitz MD, Gregg I, Miller MR, Pedersen OF. Peak expiratory flow: conclusions and recommendations of a Working Party of the European Respiratory Society. The European respiratory journal Supplement. 1997;24:2S–8S. Epub 1997/02/01. . [PubMed] [Google Scholar]
- 36.Kox M, de Kleijn S, Pompe JC, Ramakers BP, Netea MG, van der Hoeven JG, et al. Differential ex vivo and in vivo endotoxin tolerance kinetics following human endotoxemia. Critical care medicine. 2011;39(8):1866–70. Epub 2011/04/16. doi: 10.1097/CCM.0b013e3182190d5d . [DOI] [PubMed] [Google Scholar]
- 37.Leentjens J, Kox M, Koch RM, Preijers F, Joosten LA, van der Hoeven JG, et al. Reversal of Immunoparalysis in Humans in vivo: A Double-blind Placebo-controlled Randomized Pilot-Study. Am J Respir Crit Care Med. 2012. Epub 2012/07/24. doi: 10.1164/rccm.201204-0645OC . [DOI] [PubMed] [Google Scholar]
- 38.Welsh RM, Bahl K, Marshall HD, Urban SL. Type 1 interferons and antiviral CD8 T-cell responses. PLoS pathogens. 2012;8(1):e1002352 Epub 2012/01/14. doi: 10.1371/journal.ppat.1002352 ; PubMed Central PMCID: PMC3252364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alper CM, Doyle WJ, Skoner DP, Buchman CA, Cohen S, Gwaltney JM. Prechallenge antibodies moderate disease expression in adults experimentally exposed to rhinovirus strain hanks. Clin Infect Dis. 1998;27(1):119–28. Epub 1998/07/24. . [DOI] [PubMed] [Google Scholar]
- 40.Johnston SL, Papi A, Bates PJ, Mastronarde JG, Monick MM, Hunninghake GW. Low grade rhinovirus infection induces a prolonged release of IL-8 in pulmonary epithelium. J Immunol. 1998;160(12):6172–81. Epub 1998/06/24. . [PubMed] [Google Scholar]
- 41.Wark PA, Bucchieri F, Johnston SL, Gibson PG, Hamilton L, Mimica J, et al. IFN-gamma-induced protein 10 is a novel biomarker of rhinovirus-induced asthma exacerbations. J Allergy Clin Immunol. 2007;120(3):586–93. Epub 2007/07/14. doi: 10.1016/j.jaci.2007.04.046 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quint JK, Donaldson GC, Goldring JJ, Baghai-Ravary R, Hurst JR, Wedzicha JA. Serum IP-10 as a biomarker of human rhinovirus infection at exacerbation of COPD. Chest. 2010;137(4):812–22. Epub 2009/10/20. doi: 10.1378/chest.09-1541 ; PubMed Central PMCID: PMC2851557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wack A, Terczynska-Dyla E, Hartmann R. Guarding the frontiers: the biology of type III interferons. Nature immunology. 2015;16(8):802–9. doi: 10.1038/ni.3212 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cate TR, Rossen RD, Douglas RG, Butler WT, Couch RB. Role of Nasal Secretion and Serum Antibody in Rhinovirus Common Cold. American Journal of Epidemiology. 1966;84(2):352-&. ISI:A19668383200017. [DOI] [PubMed] [Google Scholar]
- 45.Butler WT, Waldmann TA, Rossen RD, Douglas RG Jr., Couch RB. Changes in IgA and IgG concentrations in nasal secretions prior to the appearance of antibody during viral respiratory infection in man. J Immunol. 1970;105(3):584–91. . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.