Abstract
Previous studies have demonstrated the association between EGFR mutations and distant metastasis. However, the association for subsequent brain metastasis (BM) in stages I-III non-small cell lung cancer (NSCLC) patients remains inconclusive. We conducted a retrospective analysis to clarify the impact of EGFR mutations on the incidence of BM and associated survival in patients with stage I-III NSCLC. A total of 491 patients screened for EGFR mutations were retrospectively enrolled. Brain MRI or CT was used to detect the BM. Cumulative incidence of subsequent BM and overall survival (OS) after diagnosis of BM were estimated by the Kaplan-Meier method and compared using log-rank test. We performed Cox proportional hazard regression for predictors of subsequent BM and determinants of OS after BM. The cumulative incidence of BM seemed higher in patients harboring EGFR mutations than those without EGFR mutations although it did not reach statistical significance (hazard ratio [HR] = 1.75, 95% confidence interval [CI] = 0.73~1.81). After adjusting possible confounders, including age, smoking, stage, and tumor size, EGFR mutation became one of the predictors for subsequent BM (HR = 1.89, 95% CI = 1.12~3.17, p = 0.017). Though there was no statistical difference in survival after BM between patients with EGFR mutations and wild-type EGFR (median survival: 17.8 vs. 12.2 months, HR = 0.79, 95% CI = 0.45–1.40), patients with EGFR 19 deletion (Del) tended to have a longer survival after BM than the non-EGFR 19 Del group (median survival: 29.4 vs. 14.3 months, HR 0.58, 95% CI = 0.32–1.09, p = 0.089). In conclusion, our data suggested EGFR mutation to be one of the predictors for subsequent BM in stage I-III patients. Given the small sample size, more studies are warranted to corroborate our results.
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide; in 2016, there were 158,080 lung cancer deaths in the USA alone [1]. In recent years, advances in our understanding of molecular abnormalities in lung cancer has helped define disease subgroups and develop specific molecular targets in the presence of driver mutations, thus providing valuable information for cancer treatment. The administration of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR- TKIs), such as gefitinib, erlotinib and afatinab, is a major breakthrough in the management of advanced non-small cell lung cancer (NSCLC) [2]. EGFR mutation has been demonstrated to be the strongest predictor for the benefits of these EGFR-TKIs [3], which have shown to be superior to chemotherapy in terms of overall response rate (ORR), progression-free survival (PFS), and quality of life in untreated patients with EGFR mutation-positive NSCLC [2, 4–11]. Despite advances in systemic therapy and improvements in survival for advanced NSCLC, brain metastasis (BM) remains an important cause of morbidity and mortality. Nearly 50% of patients with metastatic NSCLC will develop BM during their disease courses [12]. In addition, the prognosis for patients with BM remains poor. The median overall survival (OS) was around 2–3 months among patients treated with systemic corticosteroids alone, and 3–6 months for those with whole brain radiation therapy (WBRT) [13, 14]. Though some studies suggested that patients with EGFR mutations had a higher incidence of BM compared with those with wild-type EGFR [15–17], others showed no significant association [18–21]. The definite association for BM in early-stage NSCLC patients is not fully understood due to the small sample size and lower proportion of patients available for EGFR mutation analyses in these studies. On the other hand, multiple case reports have described favorable outcomes with new or recurrent BM to EGFR TKI therapy, particularly in patients with sensitizing EGFR mutations [22–26]. Although the development of brain metastases in general predicts a poor outcome in lung cancer, it is not known whether EGFR mutation-positive patients with brain metastases have a better prognosis as compared to EGFR mutation-negative patients, especially those in stages I to III lung cancer.
The purpose of this study was to examine the significance of EGFR mutations on the incidence of brain metastases in a population of patients with a stage I to III lung cancer. We also evaluate the survival after the diagnosis of BM in relation to EGFR mutation status.
Materials and methods
Patients
The study was reviewed and approved by the Review Board and Ethics Committee of National Cheng Kung University Hospital (A-ER-105-327, S1 Fig) and all data were fully anonymized and the requirement for written informed consent was waived, given this study’s retrospective nature. This research was carried out in accordance with approved guidelines and the Declaration of Helsinki. We retrospectively reviewed patients between January 2010 and June 2016. The inclusion criteria for the study population consisted of patients with pathologically confirmed non-small cell lung cancer and receiving treatment at National Cheng Kung University Hospital. All patients received staging work-up including chest computed tomography (CT) scan and bone scan or brain images (CT or MRI) according to the clinical guidelines proposed by the National Comprehensive Cancer Network. The clinical stage was classified according to the tumor, node, metastasis (TMN) system proposed by the American Joint Committee on Cancer (7th edition). Patients who were diagnosed as having stage IV disease during initial staging work-up were excluded.
Data collection and follow-up
The inpatient and outpatient medical records of all patients were reviewed, and we collected data regarding the demographic and clinical characteristics, which include patient gender, age, smoking history, clinical/pathological stage, size of primary lung lesion, pathological subtype, treatment modalities, use of targeted therapy, date of initial diagnosis, date of subsequent BM, BM treatment, EGFR mutations, and time to recurrence, death date, and cause of death. Each patient was followed up until March 1, 2017. The presence of BM was defined as the presence of one or more enhanced lesions on CT or brain magnetic resonance imaging (MRI) and diagnosed when patients became symptomatic. Patients with lepto-meningeal metastases were also identified as BM. The time to subsequent BM was defined as the time between the date of initial diagnosis and the date of BM diagnosis; whereas the survival after diagnosis of BM was followed from the date of BM diagnosis to the date of death or being censored.
EGFR mutations analysis
Tumor tissue from primary lung tumors were obtained for EGFR mutation analysis. Tissue samples that consisted of >80% tumor content, as determined via microscopy with hematoxylin and eosin staining, were selected for the study. DNA was extracted using the QIAcube automated extractor (Qiagen) with the QIAamp DNA FFPE tissue kit (Qiagen) and eluted in ATE (QIAmp Tissue Elution) buffer (Qiagen), according to the manufacturer’s instructions. Macrodissection was performed to enrich the final proportion of tumor DNA for analysis. The presence of EGFR mutations was determined using the EGFR PCR Kit (EGFR RUO Kit) and therascreen EGFR RGQ PCR Kit (EGFR IVD Kit). These kits combine Scorpions and ARMS technologies to detect the mutations using real-time quantitative PCR. Approximately 25 ng of DNA was loaded to each well and the assay was done according to the manufacturer’s instructions [27]. This assay system was designed to detect the common and uncommon EGFR mutations, including 19 deletions in exon 19, 3 insertions in exon 20, and the point mutations G719X (in exon 18), S768I (in exon 20), and L858R and L861Q (in exon 21). We then switched to the EGFR IVD Kit, which adds T790M (exon 20), an important TKI-resistant mutation. Analysis was done using the Rotor-Gene Q series built-in software version 2.0.3 (Build 2) for the EGFR RUO Kit and EGFR IVD Kit (Qiagen, Manchester, UK). Real-time curves were generated using FAM-labeled probes for both the control tube (exon 2, as a control) and each mutation in separate tubes. To calculate a ΔCT value for each mutation reaction, the following equation was used: [Mutation CT]–[Control CT] = ΔCT. Manufacturer-supplied ΔCT thresholds were used as LODs to call a mutation (≤ΔCT threshold is positive for mutation) [28].
Statistical analysis
The frequencies and descriptive statistics of demographic and clinical variables were collected. Categorical variables were compared using a Chi-square test or Fisher exact test; whereas continuous variables were compared using Student’s t-test or Wilcoxon rank-sum test. The cumulative incidence of BM [29] and overall survival (OS) of patients after diagnosis of BM were estimated by the Kaplan-Meier method and compared using a log-rank test. We performed Cox proportional hazard regression models for predictors of subsequent BM and determinants of OS after BM diagnosis. The determination of predictors and prognostic factors is based on prior studies investigating the risk factors of brain metastasis or the prognostic factors of survival in early-stage lung cancer [30, 31]. Age at diagnosis, sex, smoking status, tumor stage, tumor size, and EGFR mutations, were chosen as the predictors and prognostic factors. Statistical Analysis System® software version 9.4 (SAS Institute, Cary, North Carolina, USA) was used to perform the analysis. All the reported p-values are two-sided.
Results
Patient characteristics
A total of 491 patients were enrolled in this study. The demographic and clinical characteristics are summarized in Table 1. Among these patients, 280 (57%) had EGFR mutations and 211 (43%) had wild-type EGFR. Among patients with EGFR mutations, 97 (34.6%) had exon 19 deletions, 152 (54.3%) had L858R substitution, and 31 (11.1%) had mutations in other sites or double mutations. EGFR mutations were predominantly found in adenocarcinoma (270 patients, 96.4%). There were higher proportions of patients with EGFR mutations who were female (59.3% vs. 35.5%, p = 0.019), non-smokers (77.1% vs. 48.8%, p < 0.001), and older than 60 years, (61.1% vs. 38.9%, p = 0.031). In addition, a higher proportion of patients with EGFR mutations had a tumor size of less than 30mm (60.4% vs. 43.6%, p < 0.001) and earlier stages (p < 0.001).
Table 1. Basic characteristics.
| Variables | Total (%) N = 491 |
EGFR Mutation Status, n (%) | p | |
|---|---|---|---|---|
| WT n = 211 |
Mutant n = 280 |
|||
| Gender | 0.019 | |||
| Female | 241 (49.1) | 75 (35.5) | 166 (59.3) | |
| Male | 250 (50.9) | 136 (64.5) | 114 (40.7) | |
| Age | 0.031 | |||
| ≥60 | 293 (59.7) | 122 (57.8) | 171 (61.1) | |
| <60 | 198 (40.3) | 89 (42.2) | 109 (38.9) | |
| Mean | 62.8 | 61.9 | 63.4 | |
| Smoking History | < 0.001 | |||
| No | 319 (65.0) | 103 (48.8) | 216 (77.1) | |
| Yes | 172 (35.0) | 108 (51.2) | 64 (22.9) | |
| Stage | < 0.001 | |||
| IA | 144 (29.3) | 56 (26.5) | 88 (31.4) | |
| IB | 86 (17.5) | 26 (12.3) | 60 (21.4) | |
| IIA | 22 (4.5) | 7 (3.3) | 15 (5.4) | |
| IIB | 22 (4.5) | 8 (3.8) | 14 (5.0) | |
| IIIA | 113 (23.0) | 45 (21.3) | 68 (24.3) | |
| IIIB | 104 (21.2) | 69 (32.7) | 35 (12.5) | |
| Pathology | ||||
| Adeno | 444 (90.4) | 174 (82.5) | 270 (96.4) | |
| SqCC | 19 (3.9) | 18 (8.5) | 1 (0.4) | |
| Others | 28 (5.7) | 19 (9.0) | 9 (3.2) | |
| Tumor size | < 0.001 | |||
| ≤30mm | 261 (53.2) | 92 (43.6) | 169 (60.4) | |
| >30mm | 225 (45.8) | 115 (54.5) | 109 (38.9) | |
| Mean | 33.8 | 38.5 | 30.2 | |
| ECOG | 0.482 | |||
| 0 | 373 (76.0) | 153 (72.5) | 220 (78.6) | |
| 1 | 98 (20.0) | 49 (23.2) | 49 (17.5) | |
| 2 | 15 (3.1) | 7 (3.3) | 8 (2.9) | |
| >2 | 5 (1.0) | 2 (0.9) | 3 (1.1) | |
Risk factors for BM
The cumulative incidence of BM seemed higher in patients harboring EGFR mutations than those without EGFR mutations (Fig 1); however, it did not reach statistical significance. Cox proportional hazards models were conducted to adjust possible confounders of subsequent BM (Table 2). After adjusting possible confounders, including age, smoking, stage, and tumor size, EGFR mutation was one of the predictors for subsequent BM (HR = 1.89, 95% CI = 1.12~3.17, p = 0.017).
Fig 1. Cumulative incidence of brain metastasis (BM) in EGFR mutant versus wild-type patients.
Table 2. Cox proportional hazard regression models for predictors of subsequent BM.
| Variables | Total N = 491 |
Univariate | Multivariate | ||
|---|---|---|---|---|---|
| p | p | HR | 95%CI | ||
| Sex | 0.493 | 0.644 | 0.872 | 0.488–1.559 | |
| Female | 241 | ||||
| Male | 250 | ||||
| Dx age (each one year older) | 0.011 | 0.006 | 0.968 | 0.947–0.991 | |
| Smoking | 0.014 | 0.016 | 2.062 | 1.147–3.707 | |
| No | 319 | ||||
| Yes | 172 | ||||
| Stage | |||||
| IA | 144 | ||||
| IB | 86 | 0.370 | 0.710 | 1.207 | 0.449–3.246 |
| IIA | 22 | 0.078 | 0.147 | 2.383 | 0.737–7.710 |
| IIB | 22 | 0.025 | 0.065 | 3.194 | 0.932–10.946 |
| IIIA | 113 | <0.001 | <0.001 | 4.078 | 1.925–8.636 |
| IIIB | 104 | <0.001 | <0.001 | 4.854 | 2.113–11.154 |
| Tumor size | 0.002 | 0.171 | 1.451 | 0.851–2.475 | |
| ≤30mm | |||||
| >30mm | |||||
| EGFR mutation | 0.511 | 0.017 | 1.885 | 1.120–3.171 | |
| Wild-type | 211 | ||||
| Mutant | 280 | ||||
Overall survival after BM and associated factors
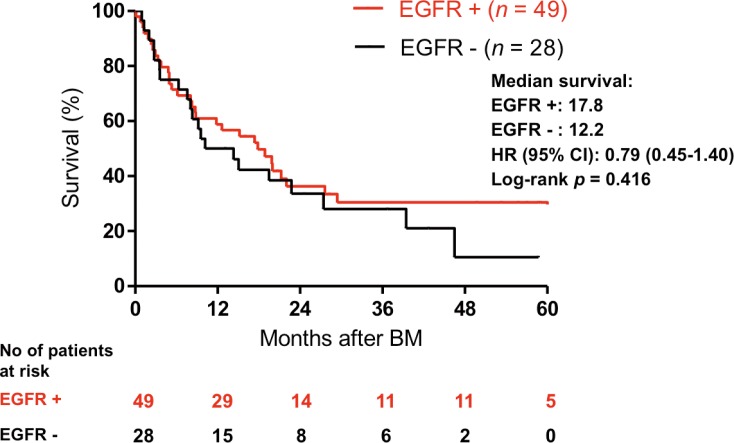
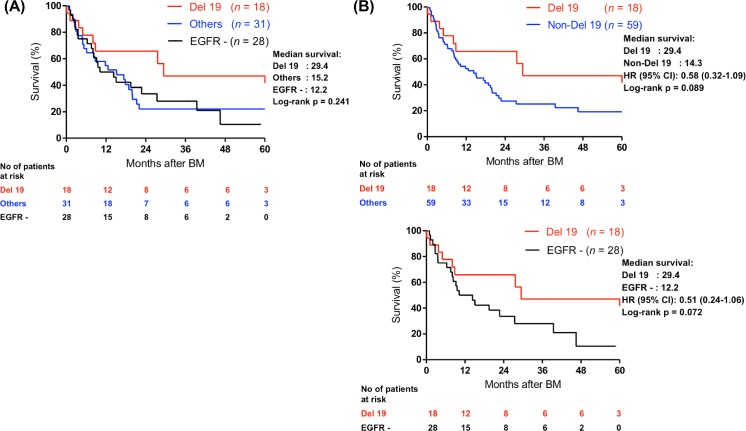
Though patients with EGFR mutations tended to have a longer OS after BM than patients with wild-type EGFR (Fig 2), it did not reach statistical significance (median survival: 17.8 vs. 12.2 months, HR = 0.79, 95% CI = 0.45–1.40). The age when patients were diagnosed with BM was the only significant prognostic factor of survival in the univariate analysis (Table 3). Sex, smoking history, stage, tumor size, EGFR mutations and whole brain radiotherapy had no statistical influence on survival. Previous studies revealed that patients with exon 19 deletions were associated with a longer progression-free survival compared to those with other mutations [32]. We therefore investigated if patients with EGFR 19 deletions had a longer OS after BM diagnosis in comparison with other mutations or wild type EGFR. Patients with exon 19 deletions had a longer median survival than that for patients harboring other EGFR mutations (29.4 months versus 14.3 months, HR = 0.58 (95% CI: 0.32–1.09)) and wild-type EGFR (29.4 months versus 12.2 months, HR = 0.51 (95% CI: 0.24–1.06)), but the differences were not statistical significant (Fig 3).
Fig 2. Kaplan-Meier curve for overall survival in patients with mutant EGFR mutation versus those with wild type EGFR after the diagnosis of brain metastasis.
Table 3. Cox proportional hazard regression models for prognostic factors of survival after diagnosis of BM.
| Variables | Total N = 78 |
Univariate | Multivariate | ||
|---|---|---|---|---|---|
| p | p | HR | 95%CI | ||
| Sex | 0.247 | 0.567 | 0.796 | 0.364–1.740 | |
| Female | 37 | ||||
| Male | 41 | ||||
| Dx age (each one year older) | 0.032 | 0.010 | 1.036 | 1.009–1.064 | |
| Smoking | 0.088 | 0.190 | 1.573 | 0.800–3.091 | |
| No | 42 | ||||
| Yes | 36 | ||||
| Stage | |||||
| IA | 11 | ||||
| IB | 8 | 0.926 | 0.792 | 0.852 | 0.260–2.794 |
| IIA | 4 | 0.288 | 0.209 | 2.605 | 0.584–11.616 |
| IIB | 4 | 0.633 | 0.471 | 1.910 | 0.329–11.095 |
| IIIA | 28 | 0.568 | 0.554 | 0.757 | 0.301–1.905 |
| IIIB | 23 | 0.114 | 0.128 | 2.050 | 0.813–5.170 |
| Tumor size | 0.367 | 0.609 | 0.827 | 0.398–1.716 | |
| ≤30mm | 35 | ||||
| >30mm | 43 | ||||
| EGFR mutation status 1 | 0.417 | 0.304 | 0.687 | 0.335–1.407 | |
| Wild-type | 29 | ||||
| Mutant | 49 | ||||
| EGFR mutation status 2 | 0.095 | 0.192 | 0.598 | 0.276–1.294 | |
| Wild-type | 29 | ||||
| Exon 19 deletion | 18 | 0.118 | 0.139 | 0.486 | 0.187–1.265 |
| Other mutations | 31 | 0.938 | 0.471 | 0.765 | 0.368–1.587 |
| Radiation therapy | 0.157 | 0.422 | 0.513 | 0.100–2.619 | |
| No | 22 | ||||
| Yes | 56 | ||||
Fig 3. Kaplan-Meier estimations for overall survival in patients with different EGFR mutation status after the diagnosis of brain metastasis.
(A) Exon 19 deletions versus other mutations and wild-type. (B) Exon 19 versus other mutations and exon 19 deletions versus wild type EGFR.
Discussion
In this study, we retrospectively reviewed and evaluated the different characteristics of BM according to the EGFR mutation status in patients with NSCLC. Although it did not reach statistical significance, we found patients with EGFR mutations seemed to have a higher cumulative incidence of BM than patients with wild-type EGFR (Fig 1). And EGFR mutation, a younger age, history of smoking, and locally advanced diseases predicted subsequent BM using Cox proportional hazard regression (Table 2). The median survival after diagnosis of BM tended to be longer in patients with EGFR mutations than those with wild-type EGFR and patients with exon 19 deletions had a median survival twice longer than that of patients who harbored other EGFR mutations or wild-type EGFR. However, the differences were also not statistical significant.
As EGFR mutant lung cancer patients survive longer because of the use of EGFR-TKIs, it would be unclear whether EGFR mutant lung cancer patients have BM due to their longer observation period or because EGFR mutant cancer cells tend to invade the brain. However, only stages I to III non-small cell lung cancer (NSCLC) patients were enrolled in our study, and most of these patients did not receive EGFR-TKIs. The effect of treatment with EGFR-TKIs on the incidence of BM would thus be minimal. The literature on the relationship between EGFR mutation status and subsequent brain metastases of stages I to III remains limited and inconclusive (Table 4) [21, 33–35]. Akamatsu et al. investigated the impact of outcomes according to EGFR mutation status in patients with stage III Adenocarcinoma, and found that those with EGFR mutations tended to develop BM as compared to those with wild-type EGFR after concurrent chemoradiotherapy (6/13 versus 4/31, p = 0.04). However, whether the EGFR mutation status was the independent factor could not be clarified due to the small sample size (n = 10) [33]. Stanic et al. investigated the correlation between EGFR mutation status and subsequent BM, and showed that EGFR status had no influence upon the cumulative incidence of this. Tanaka et al. investigated the impact of EGFR mutations on the efficacy of concurrent chemoradiation therapy (CRT), and found that concurrent CRT resulted in a shorter progression-free survival in EGFR-mutant stage III adenocarcinoma patients than in wild-type patients, mainly because of the distant metastasis. However, the correlation between EGFR mutation status and subsequent BM metastasis was not clarified. Yagishita et al. found that EGFR mutation is associated with a longer local control after definitive chemoradiotherapy in patients with stage III nonsquamous non-small-cell lung cancer. Though more patients with EGFR mutations developed brain relapses than those with wild-type EGFR (16 versus 12), the correlation was not further investigated [35]. A summary of the studies directly examining EGFR mutation status and brain metastases is presented in Table 4. We have the largest number of patients in comparison with other works, since EGFR mutation status was checked during the study period. The mean age in our study does not differ from that of other studies, although there was a higher proportion of female patients in our group as compared to other works. However, this ratio is acceptable, since most cancer types were adenocarcinomas, and this is compatible with the findings of another study investigating the association between adenocarcinoma and EGFR mutation in Taiwan [36]. We also noted those patients with EGFR mutation tended to be older and their brain tumor size tended to be smaller. These are important points, since such factors will affect BM and survival. According to the analysis of Taiwan’s nationwide lung cancer registry focusing on epidermal growth factor receptor mutation and smoking status, the EGFR mutation rate of younger lung cancer patients was significantly lower than that in the older group [37]. Moreover, in a study with a total of 401 Chinese NSCLC patients (280 males and 121 females) investigating the correlation between EGFR mutations and incidence of distant metastases and tumor size in patients with non-small-cell lung cancer, the tumor size in EGFR mutation group was significantly smaller than that in the wild-type group (p< 0.001), as shown in our study [38]. The EGFR mutation rate (57%) found in the current work is higher than in the other four studies. However, according to a recent systematic review and global map of EGFR mutation incidence in NSCLC [39], the frequency of EGFR mutations among adenocarcinoma patients in the Asia-Pacific area ranges from 20% to 76%, and the mean frequency is 57% in Taiwan. Our study further identified that EGFR mutation was independently associated with subsequent BM (odds ratio 2.246) in a multiple logistic regression model. Other risk factors, such as younger age and locally advanced diseases, have been demonstrated to be associated with BM in other studies [40, 41]. As for the correlation between smoking history and brain metastasis, we are the first work demonstrating their correlation. Recent studies have shown that smoking tobacco is associated with cancer metastasis [42, 43], but the associated mechanism underlying the correlation between metastasis remains unclear.
Table 4. Summary of studies examining the association between EGFR mutations and brain metastasis in patients with stages I to III NSCLC.
| Author | Patient (n) |
Country | Mean age | Sex (F, %) |
EGFR mutation (%) |
Stage (%) | Association between BM and EGFR mutations |
|---|---|---|---|---|---|---|---|
| Hiroaki Akamatsu [33] | 44 | Japan | 65.2 | 27.3 | 29.5 | III | Significant |
| Karmen Stanic [21] | 245 | Slovenia | N/A | N/A | 30.6 | I to III | Non-significant |
| Kosuke Tanaka [34] | 104 | Japan | 62.0 | 38.0 | 28.0 | III | Not mention |
| Shigehiro Yagishita [35] | 198 | Japan | 60.0 | 30.2 | 17.0 | III | Not mention |
| Current study | 491 | Taiwan | 62.8 | 49.0 | 57 | I to III | Significant and independent |
N/A: Not available
The molecular mechanism for the linkage between EGFR mutations and BM remains unclarified. It is proposed that EGFR downstream signaling and other pathways which activate EGFR signaling contribute the metastasis to the brain in patients harboring EGFR mutations. Mutant EGFR could induce IL-6 activation and then up-regulate the downstream gp130/JAK/STAT3 pathway [44], and STAT3 cooperates with microRNA-21 (miR-21) contributing to lung-to-brain metastases [45]. Moreover VEGF, which creates a favorable environment that promotes metastasizing to the brain, was found to be upregulated by STAT3 and EGFR [46]. Other pathways, such as Met [47], C/EBPβ-LIP/CUG-binding protein 1 (CUGBP1) [48] and phosphoinositide 3-kinase/protein kinase B / phospholipase C γ [49], have been shown to promote BM via activation by EGFR. However, more studies are needed to elucidate the exact role of EGFR mutation in BM at the molecular level.
The median survival from the diagnosis of BM to death was 15.2 months for all patients with BM. The EGFR mutation status seemed to influence the median survival time after BM (17.8 vs. 12.2 months) but with no statistical significance (HR 0.79, 95% CI = 0.45–1.40). Stanic [21] and Baek et al. [50] also investigated the impact of EGFR mutation median survival on patients with BM, and found that during the later course of the disease there was no significant difference between EGFR mutant and wild-type patients (p = 0.7 and p = 0.23, Table 5). Han et al. demonstrated that EGFR mutation is an independent predictive and prognostic risk factor for BM, and a positive predictive factor for OS in patients with BM [51]. However, whether EGFR mutation served as an independent prognosis factor was not revealed. In another study with more patients enrolled, the EGFR mutation status strongly influenced the median survival time if BM had been already discovered at diagnosis [52, 53]. However, whether the relationship can be observed in larger cohorts of patients with stages I to III remains unclear. We also found EGFR mutations were more common among elderly patients, and that such patients tended to have worse survival after diagnosis of BM compared to younger patients, with borderline significance (HR = 1.036). However, the benefits of TKIs in NSCLC with regard to elderly and younger patients, both in terms of PFS and OS, remain controversial [54, 55].
Table 5. The four studies selected for examining the association between EGFR mutations and overall survival of NSCLC patients with subsequent brain metastasis.
| Author | Patient EGFRM |
Patient EGFRW |
Medium OS EGFRM | Medium OS EGFRW | Hazard ratio | Exon 19 vs. Other mutation and wild type (HR) |
|---|---|---|---|---|---|---|
| Karmen Stanic [21]* | 26 | 64 | N/A | N/A | N/A (p = 0.7) | N/A |
| Guang Han [51]* | 48 | 28 | 23.8 | 14.2 | N/A (p = 0.028) | N/A |
| Min Young Baek [50] | 7 | 13 | 14.5 | 2.5 | N/A (p = 0.23) | N/A |
| Current study | 49 | 28 | 17.8 | 12.2 | 0.687 (p = 0.30) |
0.58 (p = 0.089) |
*EGFR mutation was an independent prognosis factor under univariate and multivariate analysis.
A previous study has showed that exon 19 deletions are associated with prolonged survival among EGFR-mutant metastatic lung adenocarcinoma patients treated with EGFR-TKI [32]. Theoretically, there may be more BM observed throughout the disease course of those patients with exon 19 deletions. However, our analysis did not find any difference in subsequent BM in stages I to III NSCLC between exon 19 deletions and other mutations (19% vs. 20%). On the other hand, the prognostic value of different EGFR mutations in resected NSCLC remains controversial. In our study, patients with exon 19 deletions tended to have a longer survival after BM (29.4 months) than patients with other mutations (15.2 months) or wild-type EGFR (12.2 months). The difference was not significant after adjusting for other factors. Larger retrospective studies are needed to verify if stages I to III patients with exon 19 deletions and subsequent BM has better survival with investigation of associated mechanism.
The efficiency of systemic chemotherapy combined whole brain radiotherapy (WBRT) for the treatment of patient with BM is limited, with reported response rates ranging from 40–60% (overall survival [OS] 6–12 months) [56, 57]. Conversely, response rates of brain metastases to EGFR tyrosine kinase inhibitor (TKI) treatment in patients with NSCLC harboring EGFR mutations reach 60–80%, with median OS around 15–20 months, demonstrating an improved clinical outcome [58]. The different response rates to BM come from the good efficiency of EGFR-TKI in passing through the blood brain barrier and targeting the BM of NSCLC patients harboring sensitive EGFR mutations [59, 60]. Besides, some patients receiving WBRT developed cognitive problems, particularly in terms of short-term memory, which were not observed in patients receiving EGFR-TKI [61]. On the other hand, though recent research demonstrated that advanced NSCLC patients with exon 19 deletion might have longer PFS compared to those with L858 mutation after first-line EGFR-TKIs [32, 62], the reason for the observed difference remained inconclusive. Some mechanisms suggested by preclinical studies were proposed to explain the difference in efficacy of EGFR TKIs according to EGFR mutation subtype. Carey et. al. performed an in vitro kinetic analysis of peptide phosphorylation reactions with purified intracellular domains from EGFR wild-type, L858R, and EGFR del746-750. The results of a kinetic assay indicated a higher affinity of gefitinib and erlotinib for recombinant EGFR with the exon 19 deletion than that with the L858R mutation [63]. Another study showed cell lines with different EGFR mutations expressed different EGFR phosphorylation status and downstream signaling before and after EGFR-TKI treatment. The human embryonic kidney cell (293) cell line was transfected with a vector with inserts containing the entire length of EGFR with L858R or EGFR del746–750, and the baseline levels of EGFR autophosphorylation were not different in both conditions. However. gefitinib induced a more marked decrease in EGFR autophosphorylation at tyrosine residues 1173, 845, and 1045 and a lesser decrease at Y992 in del746–750 cells, compared with the autophosphorylation levels in L858R cells. The phosphorylation levels of major downstream signals of EGFR, including Akt and Erk1/2, decreased more sharply in del746–750 cells than in L858R cells [64]. Therefore, the different phosphotyrosine patterns between these two mutations may be associated with differential response durations of the EGFR TKIs. A recent study further showed that the exon 19 deletion group had a longer median PFS than the L858R mutation group (6.7 vs. 3.9 months, p<0.001) in patient with BM [65]. Some research showed that NSCLC patients with exon 19 deletion had more and smaller metastases with a reduced extent of peritumoral brain edema compared with patients with wild-type EGFR alleles. The characteristics of BM in patients with L858R mutation were also similar to those of the metastases in wild-type patients [66]. Recent clinical study showed the survival of patients with Exon 19 Del is better than those with L858R because the former group developed higher proportion of EGFR T790M which was correlated with a better prognosis than other acquired mutations such as met positive or KRAS/PIK3CA/ALK-altered population [67]. However, more efforts are needed to investigate if these molecular mechanisms and characteristics of BM are the key issues of the more favorable efficacy in terms of exon 19 deletion compared with L858R mutation in patients with BM.
There are several limitations to this study that should be noted. First, it is a retrospective study from a single-institution and not all patients received testing of EGFR mutation during the enrolled period (see S2 Fig and S1 Table). Though EGFR mutation may be studied on tumor resection or on tumor recurrence, most of the tumors were checked for EGFR mutation at initial diagnosis. Second, there are many significant differences with regard to clinical characteristics between the EGFR mutations and EGFR wild-type groups, including case number, age, stage distribution and tumor size. However, EGFR mutations remained one of the independent risk factors after multiple regression to adjust for confounders. Third, we did not investigate if the choice of first line TKIs affect the prognosis of patient with subsequent BM with Exon 19 Del. A previous study found overall survival was significantly longer for patients with Exon 19 Del-positive tumours treated with irreversible first-line TKI than in the chemotherapy group. And the survival difference was not observed in other reversible first-line TKIs [68]. Fourth, the incidence of BM diagnosis may be underestimated because serial brain image examination was not part of standard follow-up. It is thus possible that asymptomatic disease was not detected. Fifth, we did not evaluate the influence of other genetic changes, such as KRAS mutation, Met amplification, or EML4-ALK translocation. However, this potential bias may be small because the frequencies of other driver mutations are relatively low, and more than one driver mutation is rarely found concurrently in the same tumor. Finally, there is a relatively low number of BM patients in this cohort, especially those with subsequent BM after 36 months of diagnosis (Fig 1), when compared to other studies aimed at examining the correlation between EGFR mutation and BM in late-stage patients. Therefore, the statistical significance may be over-estimated and a larger cohort is thus required to verify the difference in risk of subsequent BM and associated survival between EGFR mutation-positive and wild-type EGFR patients.
Previous studies have showed that a brain MRI is not indicated due to the low incidence of asymptomatic BM in patients with operable NSCLC [69]. Our results implied the importance of brain imaging, especially for patients with EGFR mutations, even those with stages I to III. Moreover, recent studies have highlighted the role of EGFR-TKIs in the adjuvant treatment of NSCLC [70, 71]; consequently, our study may provide a clue in selecting the EGFR-TKIs with a high concentration in brain in order to prevent a higher incidence of BM in these patients.
Conclusion
Our data suggested that EGFR mutation is one of the predictive factors for the development of BM. Though it did not reach statistical significance, NSCLC brain-metastatic patients with exon 19 deletions tended to have a longer survival than those with other EGFR mutations and wild-type EGFR. These observations help delineate subsets of patients who tend to develop BM and who might reach a longer BM survival. Further studies designed to investigate the molecular and genetic factors that impact survival should help further improve our understanding of the heterogeneous outcomes in these patients.
Supporting information
(TIF)
(TIF)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by MOST 104-2314-B-006-046-MY3 and MOST105-2314-B-076-MY2 from the Ministry of Science and Technology, Taiwan, and NCKUH-10605004 from the National Cheng Kung University Hospital.
References
- 1.Venur VA, Ahluwalia MS. Targeted Therapy in Brain Metastases: Ready for Primetime? Am Soc Clin Oncol Educ Book. 2016;35:e123–30. doi: 10.14694/EDBK_100006 [DOI] [PubMed] [Google Scholar]
- 2.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–57. doi: 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 3.Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866–74. doi: 10.1200/JCO.2010.33.4235 [DOI] [PubMed] [Google Scholar]
- 4.Han JY, Park K, Kim SW, Lee DH, Kim HY, Kim HT, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30(10):1122–8. doi: 10.1200/JCO.2011.36.8456 [DOI] [PubMed] [Google Scholar]
- 5.Socinski MA, Evans T, Gettinger S, Hensing TA, VanDam Sequist L, Ireland B, et al. Treatment of stage IV non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e341S–e68S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–8. doi: 10.1016/S1470-2045(09)70364-X [DOI] [PubMed] [Google Scholar]
- 7.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–46. doi: 10.1016/S1470-2045(11)70393-X [DOI] [PubMed] [Google Scholar]
- 8.Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–22. doi: 10.1016/S1470-2045(13)70604-1 [DOI] [PubMed] [Google Scholar]
- 9.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–42. doi: 10.1016/S1470-2045(11)70184-X [DOI] [PubMed] [Google Scholar]
- 10.Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94. doi: 10.1056/NEJMoa1214886 [DOI] [PubMed] [Google Scholar]
- 11.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–77. doi: 10.1056/NEJMoa1408440 [DOI] [PubMed] [Google Scholar]
- 12.Sorensen JB, Hansen HH, Hansen M, Dombernowsky P. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol. 1988;6(9):1474–80. doi: 10.1200/JCO.1988.6.9.1474 [DOI] [PubMed] [Google Scholar]
- 13.Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol. 2005;23(25):6207–19. doi: 10.1200/JCO.2005.03.145 [DOI] [PubMed] [Google Scholar]
- 14.Monaco EA 3rd, Faraji AH, Berkowitz O, Parry PV, Hadelsberg U, Kano H, et al. Leukoencephalopathy after whole-brain radiation therapy plus radiosurgery versus radiosurgery alone for metastatic lung cancer. Cancer. 2013;119(1):226–32. doi: 10.1002/cncr.27504 [DOI] [PubMed] [Google Scholar]
- 15.Welsh JW, Komaki R, Amini A, Munsell MF, Unger W, Allen PK, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013;31(7):895–902. doi: 10.1200/JCO.2011.40.1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eichler AF, Kahle KT, Wang DL, Joshi VA, Willers H, Engelman JA, et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol. 2010;12(11):1193–9. doi: 10.1093/neuonc/noq076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto S, Takahashi K, Iwakawa R, Matsuno Y, Nakanishi Y, Kohno T, et al. Frequent EGFR mutations in brain metastases of lung adenocarcinoma. Int J Cancer. 2006;119(6):1491–4. doi: 10.1002/ijc.21940 [DOI] [PubMed] [Google Scholar]
- 18.Enomoto Y, Takada K, Hagiwara E, Kojima E. Distinct features of distant metastasis and lymph node stage in lung adenocarcinoma patients with epidermal growth factor receptor gene mutations. Respir Investig. 2013;51(3):153–7. doi: 10.1016/j.resinv.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 19.Jeon JH, Kang CH, Kim HS, Seong YW, Park IK, Kim YT. Prognostic and predictive role of epidermal growth factor receptor mutation in recurrent pulmonary adenocarcinoma after curative resection. Eur J Cardiothorac Surg. 2015;47(3):556–62. doi: 10.1093/ejcts/ezu177 [DOI] [PubMed] [Google Scholar]
- 20.Li B, Sun SZ, Yang M, Shi JL, Xu W, Wang XF, et al. The correlation between EGFR mutation status and the risk of brain metastasis in patients with lung adenocarcinoma. J Neurooncol. 2015;124(1):79–85. doi: 10.1007/s11060-015-1776-3 [DOI] [PubMed] [Google Scholar]
- 21.Stanic K, Zwitter M, Hitij NT, Kern I, Sadikov A, Cufer T. Brain metastases in lung adenocarcinoma: impact of EGFR mutation status on incidence and survival. Radiol Oncol. 2014;48(2):173–83. doi: 10.2478/raon-2014-0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gounant V, Wislez M, Poulot V, Khalil A, Lavole A, Cadranel J, et al. Subsequent brain metastasis responses to epidermal growth factor receptor tyrosine kinase inhibitors in a patient with non-small-cell lung cancer. Lung Cancer. 2007;58(3):425–8. doi: 10.1016/j.lungcan.2007.07.010 [DOI] [PubMed] [Google Scholar]
- 23.Kim JE, Lee DH, Choi Y, Yoon DH, Kim SW, Suh C, et al. Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer. 2009;65(3):351–4. doi: 10.1016/j.lungcan.2008.12.011 [DOI] [PubMed] [Google Scholar]
- 24.Gridelli C, Maione P, Galetta D, Colantuoni G, Del Gaizo F, Ferrara C, et al. Three cases of long-lasting tumor control with erlotinib after progression with gefitinib in advanced non-small cell lung cancer. J Thorac Oncol. 2007;2(8):758–61. doi: 10.1097/JTO.0b013e3180cc25b0 [DOI] [PubMed] [Google Scholar]
- 25.Lai CS, Boshoff C, Falzon M, Lee SM. Complete response to erlotinib treatment in brain metastases from recurrent NSCLC. Thorax. 2006;61(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popat S, Hughes S, Papadopoulos P, Wilkins A, Moore S, Priest K, et al. Recurrent responses to non-small cell lung cancer brain metastases with erlotinib. Lung Cancer. 2007;56(1):135–7. doi: 10.1016/j.lungcan.2006.11.009 [DOI] [PubMed] [Google Scholar]
- 27.Wei F, Lin CC, Joon A, Feng Z, Troche G, Lira ME, et al. Noninvasive saliva-based EGFR gene mutation detection in patients with lung cancer. Am J Respir Crit Care Med. 2014;190(10):1117–26. doi: 10.1164/rccm.201406-1003OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen YL, Lu CC, Yang SC, Su WP, Lin YL, Chen WL, et al. Verification of wild-type EGFR status in non-small cell lung carcinomas using a mutant-enriched PCR on selected cases. J Mol Diagn. 2014;16(5):486–94. doi: 10.1016/j.jmoldx.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 29.Stish BJ, Pisansky TM, Harmsen WS, Davis BJ, Tzou KS, Choo R, et al. Improved Metastasis-Free and Survival Outcomes With Early Salvage Radiotherapy in Men With Detectable Prostate-Specific Antigen After Prostatectomy for Prostate Cancer. J Clin Oncol. 2016;34(32):3864–71. doi: 10.1200/JCO.2016.68.3425 [DOI] [PubMed] [Google Scholar]
- 30.Bajard A, Westeel V, Dubiez A, Jacoulet P, Pernet D, Dalphin JC, et al. Multivariate analysis of factors predictive of brain metastases in localised non-small cell lung carcinoma. Lung Cancer. 2004;45(3):317–23. doi: 10.1016/j.lungcan.2004.01.025 [DOI] [PubMed] [Google Scholar]
- 31.Batevik R, Grong K, Segadal L, Stangeland L. The female gender has a positive effect on survival independent of background life expectancy following surgical resection of primary non-small cell lung cancer: a study of absolute and relative survival over 15 years. Lung Cancer. 2005;47(2):173–81. doi: 10.1016/j.lungcan.2004.08.014 [DOI] [PubMed] [Google Scholar]
- 32.Lin JJ, Cardarella S, Lydon CA, Dahlberg SE, Jackman DM, Janne PA, et al. Five-Year Survival in EGFR-Mutant Metastatic Lung Adenocarcinoma Treated with EGFR-TKIs. J Thorac Oncol. 2016;11(4):556–65. doi: 10.1016/j.jtho.2015.12.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akamatsu H, Kaira K, Murakami H, Serizawa M, Koh Y, Ono A, et al. The impact of clinical outcomes according to EGFR mutation status in patients with locally advanced lung adenocarcinoma who recieved concurrent chemoradiotherapy. Am J Clin Oncol. 2014;37(2):144–7. doi: 10.1097/COC.0b013e31826e04f9 [DOI] [PubMed] [Google Scholar]
- 34.Tanaka K, Hida T, Oya Y, Oguri T, Yoshida T, Shimizu J, et al. EGFR Mutation Impact on Definitive Concurrent Chemoradiation Therapy for Inoperable Stage III Adenocarcinoma. J Thorac Oncol. 2015;10(12):1720–5. doi: 10.1097/JTO.0000000000000675 [DOI] [PubMed] [Google Scholar]
- 35.Yagishita S, Horinouchi H, Katsui Taniyama T, Nakamichi S, Kitazono S, Mizugaki H, et al. Epidermal growth factor receptor mutation is associated with longer local control after definitive chemoradiotherapy in patients with stage III nonsquamous non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2015;91(1):140–8. doi: 10.1016/j.ijrobp.2014.08.344 [DOI] [PubMed] [Google Scholar]
- 36.Wu SG, Chang YL, Yu CJ, Yang PC, Shih JY. Lung adenocarcinoma patients of young age have lower EGFR mutation rate and poorer efficacy of EGFR tyrosine kinase inhibitors. ERJ Open Res. 2017;3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsu CH, Tseng CH, Chiang CJ, Hsu KH, Tseng JS, Chen KC, et al. Characteristics of young lung cancer: Analysis of Taiwan's nationwide lung cancer registry focusing on epidermal growth factor receptor mutation and smoking status. Oncotarget. 2016;7(29):46628–35. doi: 10.18632/oncotarget.9338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan J, Chen M, Xiao N, Li L, Zhang Y, Li Q, et al. EGFR mutations are associated with higher incidence of distant metastases and smaller tumor size in patients with non-small-cell lung cancer based on PET/CT scan. Med Oncol. 2016;33(1):1 doi: 10.1007/s12032-015-0714-8 [DOI] [PubMed] [Google Scholar]
- 39.Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015;5(9):2892–911. [PMC free article] [PubMed] [Google Scholar]
- 40.Hubbs JL, Boyd JA, Hollis D, Chino JP, Saynak M, Kelsey CR. Factors associated with the development of brain metastases: analysis of 975 patients with early stage nonsmall cell lung cancer. Cancer. 2010;116(21):5038–46. doi: 10.1002/cncr.25254 [DOI] [PubMed] [Google Scholar]
- 41.Ji Z, Bi N, Wang J, Hui Z, Xiao Z, Feng Q, et al. Risk factors for brain metastases in locally advanced non-small cell lung cancer with definitive chest radiation. Int J Radiat Oncol Biol Phys. 2014;89(2):330–7. doi: 10.1016/j.ijrobp.2014.02.025 [DOI] [PubMed] [Google Scholar]
- 42.Abrams JA, Lee PC, Port JL, Altorki NK, Neugut AI. Cigarette smoking and risk of lung metastasis from esophageal cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2707–13. doi: 10.1158/1055-9965.EPI-08-0232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murin S, Inciardi J. Cigarette smoking and the risk of pulmonary metastasis from breast cancer. Chest. 2001;119(6):1635–40. [DOI] [PubMed] [Google Scholar]
- 44.Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117(12):3846–56. doi: 10.1172/JCI31871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh M, Garg N, Venugopal C, Hallett R, Tokar T, McFarlane N, et al. STAT3 pathway regulates lung-derived brain metastasis initiating cell capacity through miR-21 activation. Oncotarget. 2015;6(29):27461–77. doi: 10.18632/oncotarget.4742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Mol Diagn. 2013;15(4):415–53. doi: 10.1016/j.jmoldx.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 47.Breindel JL, Haskins JW, Cowell EP, Zhao M, Nguyen DX, Stern DF. EGF receptor activates MET through MAPK to enhance non-small cell lung carcinoma invasion and brain metastasis. Cancer Res. 2013;73(16):5053–65. doi: 10.1158/0008-5472.CAN-12-3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baldwin BR, Timchenko NA, Zahnow CA. Epidermal growth factor receptor stimulation activates the RNA binding protein CUG-BP1 and increases expression of C/EBPbeta-LIP in mammary epithelial cells. Mol Cell Biol. 2004;24(9):3682–91. doi: 10.1128/MCB.24.9.3682-3691.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nie F, Yang J, Wen S, An YL, Ding J, Ju SH, et al. Involvement of epidermal growth factor receptor overexpression in the promotion of breast cancer brain metastasis. Cancer. 2012;118(21):5198–209. doi: 10.1002/cncr.27553 [DOI] [PubMed] [Google Scholar]
- 50.Baek MY, Ahn HK, Park KR, Park HS, Kang SM, Park I, et al. Epidermal growth factor receptor mutation and pattern of brain metastasis in patients with non-small cell lung cancer. Korean J Intern Med. 2018;33(1):168–175. doi: 10.3904/kjim.2015.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han G, Bi J, Tan W, Wei X, Wang X, Ying X, et al. A retrospective analysis in patients with EGFR-mutant lung adenocarcinoma: is EGFR mutation associated with a higher incidence of brain metastasis? Oncotarget. 2016;7(35):56998–7010. doi: 10.18632/oncotarget.10933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu F, De Caluwe A, Anderson D, Nichol A, Toriumi T, Ho C. EGFR mutation status on brain metastases from non-small cell lung cancer. Lung Cancer. 2016;96:101–7. doi: 10.1016/j.lungcan.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 53.Iuchi T, Shingyoji M, Sakaida T, Itakura M, Kageyama H, Yokoi S, et al. The incidence and clinical feature of brain metastasis from non-small cell lung cancer, and their associations with EGFR mutation. Eur J Cancer. 2013;49:S787–S. [Google Scholar]
- 54.Sacher AG, Dahlberg SE, Heng J, Mach S, Janne PA, Oxnard GR. Association Between Younger Age and Targetable Genomic Alterations and Prognosis in Non-Small-Cell Lung Cancer. JAMA Oncol. 2016;2(3):313–20. doi: 10.1001/jamaoncol.2015.4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Landre T, Nicolas P, Uzzan B, Chouahnia K, Taleb C, Boudabous H, et al. Is there a benefit of TKIs among the elderly compared with younger patients in non-small cell lung cancer? a meta-analysis. J Clin Oncol. 2015;33(15_suppl):e20515–e. [Google Scholar]
- 56.Dinglin XX, Huang Y, Liu H, Zeng YD, Hou X, Chen LK. Pemetrexed and cisplatin combination with concurrent whole brain radiotherapy in patients with brain metastases of lung adenocarcinoma: a single-arm phase II clinical trial. J Neurooncol. 2013;112(3):461–6. doi: 10.1007/s11060-013-1079-5 [DOI] [PubMed] [Google Scholar]
- 57.Barlesi F, Gervais R, Lena H, Hureaux J, Berard H, Paillotin D, et al. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07–01). Ann Oncol. 2011;22(11):2466–70. doi: 10.1093/annonc/mdr003 [DOI] [PubMed] [Google Scholar]
- 58.Dempke WC, Edvardsen K, Lu S, Reinmuth N, Reck M, Inoue A. Brain Metastases in NSCLC—are TKIs Changing the Treatment Strategy? Anticancer Res. 2015;35(11):5797–806. [PubMed] [Google Scholar]
- 59.Broniscer A, Panetta JC, O'Shaughnessy M, Fraga C, Bai F, Krasin MJ, et al. Plasma and cerebrospinal fluid pharmacokinetics of erlotinib and its active metabolite OSI-420. Clin Cancer Res. 2007;13(5):1511–5. doi: 10.1158/1078-0432.CCR-06-2372 [DOI] [PubMed] [Google Scholar]
- 60.Chen Y, Wang M, Zhong W, Zhao J Pharmacokinetic and pharmacodynamic study of Gefitinib in a mouse model of non-small-cell lung carcinoma with brain metastasis. Lung Cancer. 2013;82: 313–318. doi: 10.1016/j.lungcan.2013.08.013 [DOI] [PubMed] [Google Scholar]
- 61.Owen S, Souhami L. The management of brain metastases in non-small cell lung cancer. Frontiers in oncology. 2014;4:248 doi: 10.3389/fonc.2014.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Sheng J, Kang S, Fang W, Yan Y, Hu Z, et al. Patients with exon 19 deletion were associated with longer progression-free survival compared to those with L858R mutation after first-line EGFR-TKIs for advanced non-small cell lung cancer: a meta-analysis. PLoS One. 2014;9(9):e107161 doi: 10.1371/journal.pone.0107161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carey KD, Garton AJ, Romero MS, Kahler J, Thomson S, Ross S, et al. Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Cancer Res. 2006;66(16):8163–71. doi: 10.1158/0008-5472.CAN-06-0453 [DOI] [PubMed] [Google Scholar]
- 64.Zhu JQ, Zhong WZ, Zhang GC, Li R, Zhang XC, Guo AL, et al. Better survival with EGFR exon 19 than exon 21 mutations in gefitinib-treated non-small cell lung cancer patients is due to differential inhibition of downstream signals. Cancer Lett. 2008;265(2):307–17. doi: 10.1016/j.canlet.2008.02.064 [DOI] [PubMed] [Google Scholar]
- 65.Zheng Z, Jin X, Lin B, Su H, Chen H, Fei S, et al. Efficacy of Second-line Tyrosine Kinase Inhibitors in the Treatment of Metastatic Advanced Non-small-cell Lung Cancer Harboring Exon 19 and 21 EGFR Mutations. J Cancer. 2017;8(4):597–605. doi: 10.7150/jca.16959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sekine A, Kato T, Hagiwara E, Shinohara T, Komagata T, Iwasawa T, et al. Metastatic brain tumors from non-small cell lung cancer with EGFR mutations: distinguishing influence of exon 19 deletion on radiographic features. Lung Cancer. 2012;77(1):64–9. doi: 10.1016/j.lungcan.2011.12.017 [DOI] [PubMed] [Google Scholar]
- 67.Ke EE, Zhou Q, Zhang QY, Su J, Chen ZH, Zhang XC, et al. A Higher Proportion of the EGFR T790M Mutation May Contribute to the Better Survival of Patients with Exon 19 Deletions Compared with Those with L858R. J Thorac Oncol. 2017;12(9):1368–75. doi: 10.1016/j.jtho.2017.05.018 [DOI] [PubMed] [Google Scholar]
- 68.Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16(2):141–51. doi: 10.1016/S1470-2045(14)71173-8 [DOI] [PubMed] [Google Scholar]
- 69.Yohena T, Yoshino I, Kitajima M, Uehara T, Kanematsu T, Teruya T, et al. Necessity of preoperative screening for brain metastasis in non-small cell lung cancer patients without lymph node metastasis. Ann Thorac Cardiovasc Surg. 2004;10(6):347–9. [PubMed] [Google Scholar]
- 70.Huang Q, Li J, Sun Y, Wang R, Cheng X, Chen H. Efficacy of EGFR Tyrosine Kinase Inhibitors in the Adjuvant Treatment for Operable Non-small Cell Lung Cancer by a Meta-Analysis. Chest. 2016;149(6):1384–92. doi: 10.1016/j.chest.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 71.Wu Y-L, Zhong W, Wang Q, Xu S-T, Mao W-M, Wu L, et al. Gefitinib (G) versus vinorelbine+cisplatin (VP) as adjuvant treatment in stage II-IIIA (N1-N2) non-small-cell lung cancer (NSCLC) with EGFR-activating mutation (ADJUVANT): A randomized, Phase III trial (CTONG 1104). J Clin Oncol. 2017;35(15_suppl):8500–. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.