Abstract
Equol is a nonsteroidal estrogen that is produced by intestinal bacterial metabolism. Equol and equol-producing bacteria have been extensively investigated with soybean-based materials under anaerobic condition. In this study, an under-appreciated plant material, Pueraria lobata, was used to find new bacterial strains that produce equol under aerobic conditions. Three new intestinal bacteria, CS1, CS2, and CS3, were isolated, and internal transcribed spacer analysis revealed that belonging to genus Pediococcus and Lactobacillus. HPLC analysis showed that these strains produced equol or its related intermediates when fermenting P. lobata extract. In comparison to fermentation of P. lobata extract, soybean germ extract was also fermented. While the isolated strains did not produce equol in this extract, they produced other equol-related precursors. To test the modularity effect of these fermentation mixtures with the newly isolated bacteria, MCF-7 cell proliferation assay was performed, which showed that all extracts fermented with those strains has a modularity effect. Fermenting P. lobata extract with strain CS1 demonstrated the best modularity effect.
Introduction
Equol (4’,7-isoflavandiol) is an isoflavandiol commonly metabolized from daidzein and is a nonsteroidal estrogen of the isoflavone class [1]. It has been well established that equol is exclusively produced by intestinal bacterial metabolism, and its clinical importance for estrogenic activity and affinity for estrogen receptors have been studied [2]. Equol can be metabolized from biotransformation of the phytoestrogens, daidzein and genistein, which are isoflavones. These isoflavones are particularly prevalent in soybean foods [3], thus bioconversion of precursor isoflavones to equol or related intermediates by bacterial strains has been investigated in the context of soybean products. Such intestinal bacterial strains have been successfully isolated from human and animal feces [4–6] and a number of bacterial strains have been isolated and further characterized [7]. Fermentation to produce equol or related intermediates from soybean-based materials has been generally performed under anaerobic conditions, similar to the gastrointestinal environment where these bacterial strains dwell and produce equol.
Pueraria lobata, which has been more commonly called arrowroot or kudzu, is a perennial legume that is widely distributed in East Asia and tropical and subtropical countries [8]. Traditionally, it has been used medicinally in Korea, Japan, and China, and the roots have been shown to suppress alcohol intake, to reduce blood pressure, and to prevent osteoporosis [9]. In vitro culture assay revealed that certain P. lobata plant parts contain a significant amount of daidzein glycosides, such as puerarin and daidzin. The concentrations were higher in the root callus than in the callus from the leaves and stem segments [9]. Thus, fermenting P. lobata root extract could be reasonably expected to produce equol or its related intermediates.
In this study an under-appreciated plant material, P. lobata root extract, was used to identify new bacterial strains that can produce equol from isoflavones. In addition, aerobic culture has benefits over anaerobic culture in terms of cost and resource use. Thus, aerobic culture was used to identify new equol-producing bacterial strains.
Materials and methods
P. lobata and Soybean germ extract preparation
Pueraria lobata (Willd.) Ohwi roots were provided by Professor Tae-Ho Park from the greenhouse at Daegu University (Daegu, South Korea) and soybean germ were purchased from Dr Chung’s Food Co., Ltd. (Seoul, Korea).
Since the total amount of daidzein glycosides, such as puerarin and daidzin, is highest in the P. lobata root callus [9], root segments were used in the extract for equol bioconversion. Using the root extract of P. lobata for the bacterial cultures necessitated lyophilization of the extract contents. Pueraria lobata (Willd.) Ohwi roots were chopped into small pieces and extracted three times in 20% ethanol for 24 hr each at room temperature. Various ethanol concentrations were tested, and 20% gave the best yield. The extract was subsequently filtered to remove any particulates and was concentrated under vacuum at 50°C. Then, the concentrated crude extract was lyophilized to obtain a powder and stored at -20°C for further experiments (20.5% yield).
One kilogram of soybean was mixed with 10 L of 30% ethanol and then were extracted at 80 °C for 3 hr. The mixture was subsequently filtered to remove any particulate materials and vacuum concentrated at 50 °C. The concentrated crude extract was lyophilized to obtain a powder that was stored at -70 °C for further experiments.
Bacterial culture and media preparation
Fecal suspensions were plated onto several different media plates: Gifu Anaerobic Medium (GAM), Trypticase Soy Agar (TSA), Eosin Methylene Blue (EMA), Reinforced Clostridial Media (RCM), Brain heart infusion (BHI), and de Man, Rogosa and Sharpe (MRS). To isolate equol-producing strains under aerobic conditions, colonies from the plates were further cultured in liquid GAM medium (Becton, Dickinson and Company, New Jersey, USA) containing 2% P. lobata extract (w/v) or 2% soybean germ extract (w/v) at 28°C or 37°C for 72 hr with agitation. GAM medium was chosen because the candidate strains grew fastest in that medium.
We did not obtain ethics approval for the study, because ethics approval was not necessary. Particularly in the clinical diagnosis, the human-derived substances refer to blood, organ tissue, urine, etc. Feces used in this study were not stored as human-derived materials nor used in the clinical diagnosis, rather just used to isolate microorganisms. Thus, IRB was not required.
Thin layer chromatography for equol detection
TLC experiments to screen for isoflavones were performed with the following method from a previous publication [10] with slight modifications. In brief, bacterial culture supernatant was transferred to RP-18 silica gel TLC plates (20 × 20 cm, Merck, 60 F, 254 nm, Darmstadt, Germany) with a solvent system of acetonitrile: water: acetic acid (60:40:1). Isoflavones on TLC were visualized with a UV transilluminator (321 nm) by spraying with anisaldehyde reagent. Pure equol (Cayman Chemical, Michigan, USA) was used as a positive control in the TLC experiments.
High-performance liquid chromatography (HPLC) analysis
The HPLC system was a DGU-20A3R HPLC apparatus (Shimadzu, Japan) with a photodiode array detector (SPD-M20A, Shimadzu, Japan). A Skypack C18 column (SK chemical, Seoul, Korea, 4.6 × 250 mm, 5 μm) was used to analyze all samples. The mobile phases were water: acetic acid (100:1, solvent system A) and water: acetonitrile: acetic acid (50:50:1, solvent system B) in gradient mode.
Strain identification by sequencing the ribosome 16S internal transcribed spacer (ITS) regions
Bacterial strains CS1, CS2, and CS3, were cultured at 28°C in an aerobic incubator for 16 hr and were collected by centrifugation at 10,000 × g for 3 min. Genomic DNA was extracted with a genomic DNA prep kit (Solgent, Daejeon, Korea) by following the manufacturer’s protocol. The 16S rRNA gene was amplified by polymerase chain reaction (PCR) with primers described previously [11]. The PCR mixture (20 μL) contained 2× Taq PCR premix (Qiagen), 10 pM each primer (S2 Table), double distilled water, and 1 μL of genomic DNA. The PCR conditions were as follows: 94°C for 2 min; 30 cycles of 94°C for 10 s, 45°C for 20 s, and 72°C for 60 s; and 72°C for 5 min. The PCR product was mixed with 6× gel loading dye and subjected to electrophoresis on a 0.7% agarose gel to confirm the PCR product. The amplified product was excised from the gel and purified with a QIAquick Gel Extraction Kit (Qiagen).
Phylogenetic tree analysis
A phylogenetic tree was constructed by using internal transcribed spacer (ITS) sequences with the ETE toolkit [12]. Of 29 published equol-producing strains [7], only one, Eggerthella sp. YY7918, has a complete genome sequence [13]. In addition, 14 bacterial species that the other equol-producing strains belong to and that have genome sequences at NCBI were used to prepare ITS sequences between the 16S and 23S rRNA genes. Each number is expressed as a percentage of 100 replications. The scale bar represents genetic distance (the number of mutations/evolutionary events between species since their divergence).
E-screen assay
To evaluate estrogenic-like effects, a sensitive E-screen assay by cell proliferation was performed with human ER-positive breast adenocarcinoma cells (MCF-7). This assay measures the proliferation of MCF-7 cells as an indirect proxy of the estrogenicity of compounds. The technique was adopted from the previous study [14]. In brief, trypsinized MCF-7 cells were seeded in 24-well plates at an initial concentration of 20,000 cells per well in 10% FBS in RPMI. To permit their adhesion, cells were incubated for 24 h (37 °C, 5% CO2), and were washed with phosphate-buffered saline (PBS). The Serum Replacement 2 (0.5×) supplemented phenol red-free RPMI was substituted for the seeding medium. The synthetic estrogen or the fermentation mixture were added to the experimental medium at concentrations from 0.1 nM to 1 μM. After 144 hr, the medium was removed from wells to stop the assay. Cells were fixed and were stained with sulforhodamine-B (SRB). Bound dye was solubilized with 10 mM Tris base (pH 10.5) in a shaker. At last, aliquots were read in a Biotek EL800 Multiscan apparatus (Winoosky, USA) at 510 nm. The measured estrogenic activity was shown as mean ± standard error of mean of the proliferative effect (PE).
Quantitative reverse transcription polymerase chain reaction
Human breast ER+ cancer, MCF-7 Cells were treated with samples for 48 hours. Total RNA from cells was extracted with TRIzol reagent (Invitrogen) following the manufacturer’s instruction, and complementary DNA was synthesized using Moloney murine leukemia virus reverse transcriptase with random primers. The expression levels of genes were determined by quantitative PCR. Complementary DNA was generated with PrimeScriptTM 1st strand cDNA Synthesis Kit (Takara) according to the manufacturer’s protocol. qRT-PCR was performed using SYBR Premix Ex Taq™ (TaKaRa).
Results
Isolation of human intestinal bacteria capable of producing equol
To isolate possible bacterial strains that might be able to convert daidzein glycosides to equol, 14 healthy volunteers (6 females and 8 males, 25–41 years old) consumed 100 mL of P. lobata extract (1:10 extract in water) for 14 days. Fresh fecal specimens were suspended in 1 ml sterile phosphate buffer saline (PBS) solution. Then 0.1 ml of fecal suspension was plated onto various media, such as GAM, TSA, EMA, RCM, BHI, and MRS. A number of colonies were selected from the plates and were further cultured under aerobic conditions in liquid GAM medium containing 2% (w/v) P. lobata extract at 28°C or 37°C. GAM medium was used because the isolated strains grew fastest in GAM medium under aerobic conditions. After a 24-hour incubation, the presence of equol in samples was assessed by thin layer chromatography (TLC) experiments.
TLC was used to screen isoflavones by following a previously published method [10] with a slight modification. Of the approximately 200 colonies from plates of various media, 3 had a TLC band indicating the presence of equol molecules in the culture. These three bacterial strains were used for further analyses and are referenced as CS1, CS2, and CS3.
Characterization of production capability of equol and related intermediates
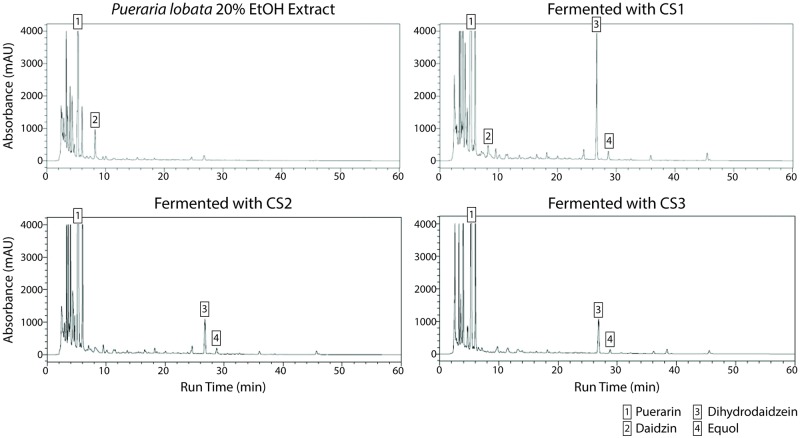
To measure the consumption of precursor molecules and the production of equol or related products, the three newly isolated bacterial strains, CS1, CS2, and CS3 in Fig 1, and Table 1, were further examined by HPLC. The HPLC experiments showed that a 20% ethanol extract of P. lobata had a high amount of puerarin and a decent amount of daidzin (Fig 1). After fermentation with the three bacterial strains, the concentrations of puerarin and daidzin, which are equol precursors, decreased, while the dihydrodiadzein (DHD, an equol precursor) and equol concentrations increased (Fig 1). This result indicates that all three bacterial strains contributed in bioconversion of the precursor molecules into DHD or equol.
Fig 1. Analysis of equol-related components in P. lobata extract medium before and after fermentation.
HPLC analysis of puerarin, dihydrodiadzein, daidzin, and equol in P. lobata extract medium before and after fermentation.
Table 1. Concentration of puerarin, daidzin, DHD, and equol in P. lobata extract medium before and after fermentation.
(ND: not detected).
| Extract (mg/g) | CS1 (mg/g) | CS2 (mg/g) | CS3 (mg/g) | |
|---|---|---|---|---|
| Puerarin | 195 | 194 | 192 | 201 |
| Daidzin | 78.1 | 10.3 | N/D | N/D |
| Dihydrodaidzein | N/D | 196 | 82 | 84 |
| Equol | N/D | 12.3 | 7.4 | 7.9 |
The DHD and equol concentrations were measured by lyophilizing culture samples before and after fermentation with the newly isolated bacterial strains (Table 1). Strain CS1 produced the most abundant amount of equol and its precursor, DHD. The other two strains, CS2 and CS3, also produced 60.2% and 64.2% as much equol as CS1 and 41.8% and 42.9% as much DHD as CS1.
In summary, all of the three strains proved the ability to consume daidzein glycosides and to produce equol and its precursor, DHD. However, strain CS1 was the most efficient strain in converting daidzein glycosides to equol and DHD.
Identification of equol-producing strains
Isolation of the equol-producing bacterial strains entailed further identification of those strains. Bacterial strains can be identified by biochemical, morphological, cultural, molecular, and physiological properties. Sequence-based analysis of 16S rRNA, particularly the internal transcribed spacer (ITS), has been widely used to identify various organisms including bacteria [15], eukaryotic microorganisms [16], fungi [17], plants [18], and animals [19]. Thus, the ribosomal ITS regions from the three bacterial strains were amplified by PCR and were sequenced (S1 Table).
ITS sequences were submitted for BLAST analysis to identify bacterial strains (Table 2). Strain CS1 had an identical ITS sequence to Pediococcus pentosaceus, CS2 had an identical ITS sequence to Lactobacillus casei or Lactobacillus paracasei, and CS3 had an identical ITS sequence to Lactobacillus sakei or Lactobacillus graminis. Those three strains belong to a genus of gram-positive lactic acid bacteria in the Lactobacillaceae family. Multiple intestinal bacteria in a wide range of families have been identified to biotransform isoflavones to equol or related intermediates [7], and a few of these strains belong to genus Lactobacillus [20, 21].
Table 2. Identification of equol-producing strains by ITS sequence analysis.
| Strain | Species | Query Coverage | Sequence Identity |
|---|---|---|---|
| CS1 | Pediococcus pentosaceus | 100% | 99% |
| CS2 | Lactobacillus casei | 100% | 99% |
| Lactobacillus paracasei | 100% | 99% | |
| CS3 | Lactobacillus sakei | 100% | 99% |
| Lactobacillus graminis | 100% | 99% |
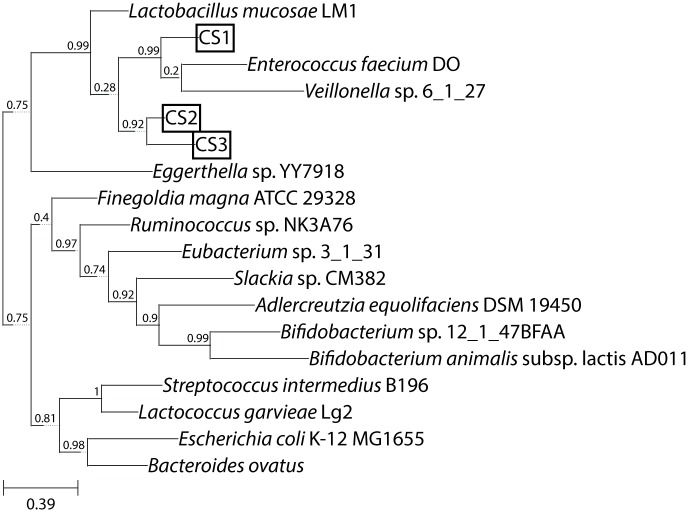
The bacterial strain, Eggerthella sp. YY7918, has a proven ability to produce equol [22] and has a complete genome sequence [13]. Thus, the ITS sequence was prepared from the genome sequence of Eggerthella sp. YY7918. Among 29 equol-producing strains [7], 14 bacterial strains belong to bacterial species or genus that have the published genome sequences at NCBI, thus ITS sequences were prepared from those genome sequences as well. Phylogenetic tree analysis showed that strains CS2 and CS3 are quite close to each other, and strain CS1 is also close to the other two strains. All three strains are relatively close to Lactobacillus mucosae and Eggerthella sp. YY7918. (Fig 2)
Fig 2. Phylogenetic tree of the newly isolated strains and other bacterial strains to which the previously reported equol-producing strains belong.
Summarily the ITS analysis showed that the strain CS1 belongs to Pediococcus pentosaceus, and the strains CS2 and CS3 belong to Lactobacillus. These species were known to be closely related to other published equol-producing bacteria.
Fermentation of soybean germ extract
Equol production has been most extensively investigated in the context of soy-based diets, and the precursor molecules to equol and DHD, daidzein glycosides have been considered to be soy isoflavones [7]. Thus, it became of interests to see if the newly isolated bacteria, which could produce equol and related intermediates from P. lobata root extract, can also produce equol from soy-based extract.
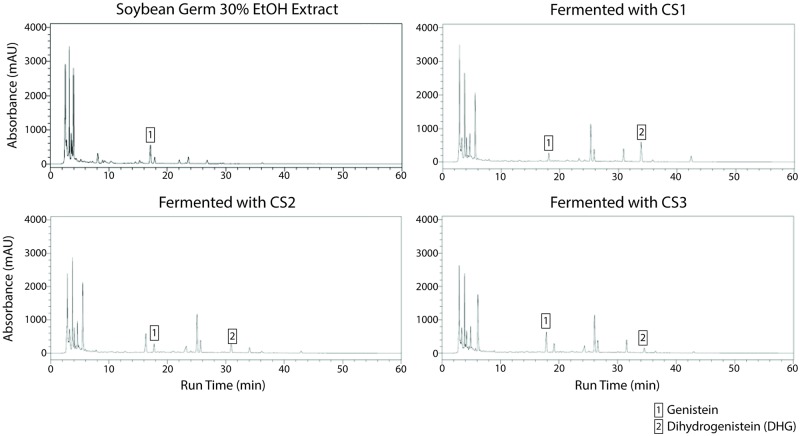
Soybean germ was chosen for the soy-based extract because soy germ is a significant source of bioactive phytochemicals [23]. HPLC analysis showed that the soybean germ extract had genistein, another equol precursor, but no dihydrogenistein (DHG), which is the next intermediate for equol production. After fermenting the extract with CS1, CS2, and CS3 at 28°C for 24 hr, the amount of genistein decreased, and the amount of DHG increased (Fig 3, Table 3).
Fig 3. Analysis of equol-related components in soybean germ extract medium before and after fermentation.
HPLC analysis of genistein and dihydrogenistein in soybean germ extract medium before and after fermentation.
Table 3. Concentration of genistein and DHG in soybean germ extract medium before and after fermentation.
| Soybean extract (mg/g) | CS1 (mg/g) | CS2 (mg/g) | CS3 (mg/g) | |
|---|---|---|---|---|
| Genistein | 135.7 | 2.1 | 129.3 | 131.6 |
| Dihydrogenistein | N/D | 122.8 | 34.3 | 31.8 |
However, no equol was found in the fermentation samples from any of the bacterial strains. This result indicates that those strains can produce equol and its related intermediate, DHD, from P. lobata root extract but not from soybean germ extract. Among the three strains, CS1 had the best ability to produce DHG from genistein.
Modularity effect of fermentation mixture to estrogen receptor
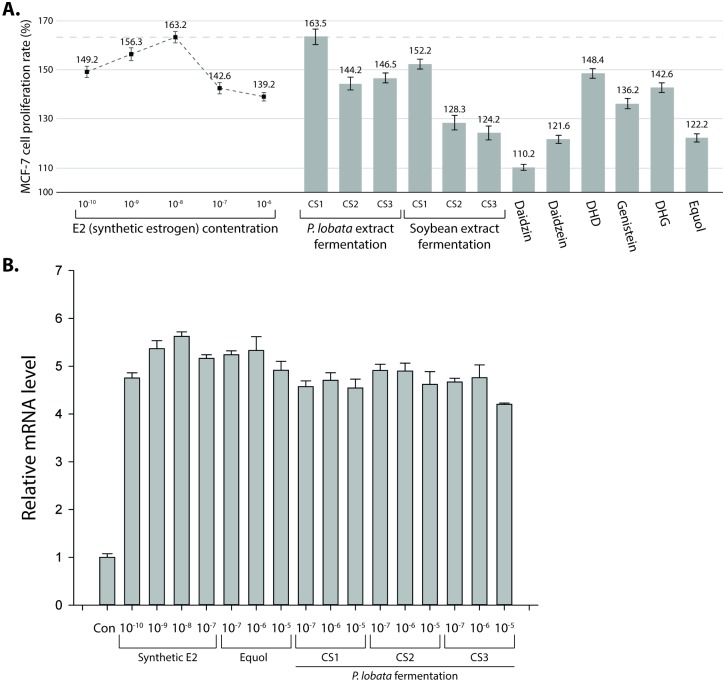
To measure the modularity effect in the P. lobata extract fermentation, MCF-7 cell proliferation assay was performed. The in vitro MCF-7 cell proliferation assay has been widely used to measure estrogenic activity of molecules [24]. As a control, synthetic estrogen (E2, 17 β-estradiol) at different concentrations was used (Fig 4). Synthetic estrogen at 10−8 μg/ml induced the most proliferation. Above this concentration, the proliferation rate decreased as the synthetic estrogen concentration increased, indicating that higher E2 concentrations were toxic to the cells. The proliferation rate was also measured with the P. lobata fermentation mixture, the soybean fermentation mixture, daidzin, daidzein, DHD, genistein, DHG, and equol. Equol-related intermediates, such as DHD, genistein, and DHG, had a better modularity effect than equol itself.
Fig 4. The modularity effect of the fermentation mixture.
(A) MCF-7 cell proliferation assay shows the modularity effect of the fermentation mixture. (B) RT-qPCR experiment shows the expression change of the estrogen receptor. The relative mRNA level was normalized to the level of the control.
In order to provide an independent line of evidence showing the modularity effect of fermentation mixture with three newly isolated strains, RT-qPCR experiment was performed to measure the transcriptional expression of the estrogen receptor (Fig 4B). The higher level of the estrogen receptor could serve as a proxy of a better modularity effect. As observed from MCF-7 cell proliferation assay, treatment of the synthetic estrogen, and equol increased the expression of the estrogen receptor. Like the synthetic estrogen and equol, the fermentation mixture also increased the expression of the estrogen receptor, suggesting the modularity effect.
Fermentation by CS1, CS2, and CS3 had a modularity effect on the estrogen receptor. Among the three strains, CS1 produced a fermentation mixture with the highest effect, which was higher than that of equol or related intermediates. The P. lobata fermentation mixture had a higher modularity effect than did the soybean fermentation mixture. These results are particularly interesting because equol and its related intermediates have been more extensively investigated in soy materials. The modularity effect of P. lobata extract fermented with CS1 was similar to 10−8 μg/ml E2 in the MCF-7 cell proliferation assay.
Discussion
In this study, kudzu arrowroot (Pueraria lobata) instead of soy-based materials which have been more widely investigated for isoflavones, was used to find bacterial strains that can produce equol or related intermediates from their precursors under aerobic conditions. TLC screening experiments with human feces were used to isolate three new bacterial strains, CS1, CS2, and CS3, that can convert isoflavones in P. lobata root extract into equol. The amount of equol and DHD produced was measured by HPLC analysis, and CS1 was the most active. In fermentation experiments with soybean germ extract, the strains did not produce equol, but created DHG, which is an equol precursor. Fermenting P. lobata or soybean germ extract with the three strains had a higher modularity effect than did pure equol in a majority of cases.
The primary goal of this study was to isolate and identify new bacterial strains that could produce equol from P. lobata extract under aerobic conditions. HPLC analysis showed that these strains produced a reasonable amount of equol or DHD. Previous studies demonstrated that bioconversion to make isoflavones principally depends on the fermentation conditions including the fermentation temperature, time, medium, and starter culture [25]. In this study, fermentation was only performed under one condition, thus the fermentation process could be further optimized by altering oxygen concentration, pH, nitrogen or carbon source supplementation, different base medium, fermentation temperature, or fermentation time. Particularly, strain CS1 produced the most abundant amount of equol and related intermediates; thus, this strain would be the best candidate for process optimization.
During fermentation with soybean germ extract, HPLC analysis revealed that the amount of genistein decreased. This result implies that genistein could be converted into other forms of isoflavones other than DHG. The fermented extract showed a significant peak at around 25 min that could be further be isolated and be analyzed to identify a new isoflavone or their intermediate.
Particularly, using an under-appreciated fermentation material, P. lobata extract, led to the isolation and identification of new equol-producing bacterial strains. Thus, fermentation of other legumes with the bacterial strains identified in this study could identify new functional molecules.
MCF-7 cell proliferation assay showed that the fermentation mixture, which contains equol and intermediates including DHD, had a much higher modularity effect than did equol itself. Thus, fermentation with new materials and identification of new fermentation products could result in the discovery of functional molecules or mixtures.
Supporting information
(DOC)
(DOC)
Acknowledgments
This work was carried out with the support of "Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01128101), Rural Development Administration, Republic of Korea. This work was also supported by a grant from Kyung Hee University in 2016."(KHU-20160596, and KHU-20160597)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was carried out with the support of "Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01128101) to SCK, Rural Development Administration, Republic of Korea. This work was also supported by a grant from Kyung Hee University in 2016."(KHU-20160596, and KHU-20160597). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rufer CE, Glatt H, Kulling SE. Structural elucidation of hydroxylated metabolites of the isoflavan equol by gas chromatography-mass spectrometry and high-performance liquid chromatography-mass spectrometry. Drug Metab Dispos. 2006;34(1):51–60. doi: 10.1124/dmd.105.004929 . [DOI] [PubMed] [Google Scholar]
- 2.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132(12):3577–84. . [DOI] [PubMed] [Google Scholar]
- 3.Xu X, Wang HJ, Murphy PA, Cook L, Hendrich S. Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women. J Nutr. 1994;124(6):825–32. . [DOI] [PubMed] [Google Scholar]
- 4.Maruo T, Sakamoto M, Ito C, Toda T, Benno Y. Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. Int J Syst Evol Microbiol. 2008;58(Pt 5):1221–7. doi: 10.1099/ijs.0.65404-0 . [DOI] [PubMed] [Google Scholar]
- 5.Minamida K, Tanaka M, Abe A, Sone T, Tomita F, Hara H, et al. Production of equol from daidzein by gram-positive rod-shaped bacterium isolated from rat intestine. J Biosci Bioeng. 2006;102(3):247–50. doi: 10.1263/jbb.102.247 . [DOI] [PubMed] [Google Scholar]
- 6.Minamida K, Ota K, Nishimukai M, Tanaka M, Abe A, Sone T, et al. Asaccharobacter celatus gen. nov., sp. nov., isolated from rat caecum. Int J Syst Evol Microbiol. 2008;58(Pt 5):1238–40. doi: 10.1099/ijs.0.64894-0 . [DOI] [PubMed] [Google Scholar]
- 7.Setchell KD, Clerici C. Equol: history, chemistry, and formation. J Nutr. 2010;140(7):1355S–62S. doi: 10.3945/jn.109.119776 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee E, Park T-H. Isoflavones and biotransformed dihydrodaidzein in hairy roots of Korean wild arrowroot. Journal of Plant Biotechnology. 2016;43(1):125–31. doi: 10.5010/jpb.2016.43.1.125 [Google Scholar]
- 9.Matkowski A. In vitro isoflavonoid production in callus from different organs of Pueraria lobata (Wild.) Ohwi. J Plant Physiol. 2004;161(3):343–6. doi: 10.1078/0176-1617-01145 . [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama S, Kuzuguchi T. Rapid and convenient detection of urinary equol by thin-layer chromatography. J Nutr Sci Vitaminol (Tokyo). 2007;53(1):43–7. . [DOI] [PubMed] [Google Scholar]
- 11.Suzuki MT, Giovannoni SJ. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl Environ Microbiol. 1996;62(2):625–30. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huerta-Cepas J, Serra F, Bork P. ETE 3: Reconstruction, Analysis, and Visualization of Phylogenomic Data. Molecular biology and evolution. 2016;33(6):1635–8. doi: 10.1093/molbev/msw046 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokoyama S, Oshima K, Nomura I, Hattori M, Suzuki T. Complete genomic sequence of the equol-producing bacterium Eggerthella sp. strain YY7918, isolated from adult human intestine. Journal of bacteriology. 2011;193(19):5570–1. doi: 10.1128/JB.05626-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Resende FA, de Oliveira AP, de Camargo MS, Vilegas W, Varanda EA. Evaluation of estrogenic potential of flavonoids using a recombinant yeast strain and MCF7/BUS cell proliferation assay. PLoS One. 2013;8(10):e74881 doi: 10.1371/journal.pone.0074881 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruegger PM, Clark RT, Weger JR, Braun J, Borneman J. Improved resolution of bacteria by high throughput sequence analysis of the rRNA internal transcribed spacer. J Microbiol Methods. 2014;105:82–7. doi: 10.1016/j.mimet.2014.07.001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guillamon JM, Sabate J, Barrio E, Cano J, Querol A. Rapid identification of wine yeast species based on RFLP analysis of the ribosomal internal transcribed spacer (ITS) region. Arch Microbiol. 1998;169(5):387–92. . [DOI] [PubMed] [Google Scholar]
- 17.Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci U S A. 2012;109(16):6241–6. doi: 10.1073/pnas.1117018109 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.China Plant BOLG, Li DZ, Gao LM, Li HT, Wang H, Ge XJ, et al. Comparative analysis of a large dataset indicates that internal transcribed spacer (ITS) should be incorporated into the core barcode for seed plants. Proc Natl Acad Sci U S A. 2011;108(49):19641–6. doi: 10.1073/pnas.1104551108 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blouin MS. Molecular prospecting for cryptic species of nematodes: mitochondrial DNA versus internal transcribed spacer. Int J Parasitol. 2002;32(5):527–31. . [DOI] [PubMed] [Google Scholar]
- 20.Decroos K, Vanhemmens S, Cattoir S, Boon N, Verstraete W. Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch Microbiol. 2005;183(1):45–55. doi: 10.1007/s00203-004-0747-4 . [DOI] [PubMed] [Google Scholar]
- 21.Wang XL, Hur HG, Lee JH, Kim KT, Kim SI. Enantioselective synthesis of S-equol from dihydrodaidzein by a newly isolated anaerobic human intestinal bacterium. Appl Environ Microbiol. 2005;71(1):214–9. doi: 10.1128/AEM.71.1.214-219.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yokoyama S, Suzuki T. Isolation and characterization of a novel equol-producing bacterium from human feces. Bioscience, biotechnology, and biochemistry. 2008;72(10):2660–6. doi: 10.1271/bbb.80329 . [DOI] [PubMed] [Google Scholar]
- 23.Hubert J, Berger M, Nepveu F, Paul F, Dayde J. Effects of fermentation on the phytochemical composition and antioxidant properties of soy germ. Food Chem. 2008;109(4):709–21. doi: 10.1016/j.foodchem.2007.12.081 . [DOI] [PubMed] [Google Scholar]
- 24.Dickson RB, Huff KK, Spencer EM, Lippman ME. Induction of epidermal growth factor-related polypeptides by 17 beta-estradiol in MCF-7 human breast cancer cells. Endocrinology. 1986;118(1):138–42. doi: 10.1210/endo-118-1-138 . [DOI] [PubMed] [Google Scholar]
- 25.Yaakob H, Malek RA, Misson M, Jalil MFA, Sarmidi MR, Aziz R. Optimization of isoflavone production from fermented soybean using response surface methodology. Food Science and Biotechnology. 2011;20(6):1525–31. doi: 10.1007/s10068-011-0211-6 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.