Abstract
Due to multiple impacts, Cystoseira forests are experiencing a significant decline, which is affecting the ecosystem services they provide. Despite conservation efforts, there is an urgent need to develop best practices and large-scale restoration strategies. To implement restoration actions, we developed an ex situ protocol for the cultivation of Cystoseira. amentacea var. stricta, aimed at reducing the time needed for laboratory culture, thus avoiding prolonged maintenance and minimizing costs. Specifically, we tested the effects of temperature, light and substratum on settlement and growth of early life stages using a factorial experiment. Temperature (20 and 24°C) and photoperiod (15L:9D) were selected to reflect the conditions experienced in the field during the reproductive period. Two light intensities (125 and 250 μmol photons m−2s−1) were selected to mimic the condition experienced in the absence of canopy (i.e. barren—higher light intensity) or in the understory (lower light intensity) during gamete release. The tested substrata were flat polished pebbles and rough clay tiles. The release of gametes and the successive survival and development of embryo and germlings were followed for two weeks. Regardless of the culture conditions, rougher tiles showed higher zygote settlement, but the substrata did not affect the successive development. Zygote mortality after one week averaged 50% and at the end of the second week, embryonic survival was higher under lower light and temperature conditions, which also determined the growth of larger embryos.
Introduction
The genus Cystoseira C. Agardh, brown algae belonging to the order Fucales, is distributed along the Mediterranean and Atlantic coasts from the intertidal to the lower sublittoral. This genus is ecologically relevant as an “ecosystem engineer” [1], and plays a key functional role in controlling spatial habitat heterogeneity, productivity, and nutrient cycling in temperate rocky reefs. In particular, Cystoseira forests provide refuge and food for many invertebrates and fishes and modulate the structure of the associated benthic community [2]. Currently, some Cystoseira populations (depending on species and location) are declining/lost throughout the Mediterranean [3,4,5,6,7, 8, 9], largely due to multiple human impacts such as urbanization, overfishing and climate change. Consequently, many systems have shifted from complex and productive assemblages to simpler, less-productive habitats such as barrens, turf-forming algae and other ephemeral opportunistic seaweeds, thus impacting the provision of ecosystem services [3,10–16]. Cystoseira species are listed as “of community interest” according to the Habitat Directive (92/43/EEC) [17], and are indicators of environmental quality in Mediterranean coastal waters according to the Water Framework Directive (2000/60/EC) [18] (i.e., EEI [19] and CARLIT [20, 21]. Several species are protected by the Bern Convention, recognized as a priority by the Barcelona Convention and considered vulnerable by several international organizations (i.e. IUCN, RAC/SPA, MedPan).
Despite the implementation of significant conservation efforts, most degraded systems have not recovered, emphasizing the urgency to develop an active intervention to restore endangered habitats [16]. The threat of losing Cystoseira species is magnified by their low dispersal capacity due to rapid egg fertilization and zygote sinking [22–25], which hampers natural recovery in the absence of adults, even if in some Cystoseira species the potential dispersal distance can be enhanced by the transport in floating rafts [26,27]. As a result, interest in habitat restoration is increasing according to the Biodiversity Strategy to 2020 (Target 2; European Commission, 2011), which recommends the reintroduction of relevant species into areas where they were present historically and where the factors that led to their loss have been removed.
Small-scale Cystoseira transplants have been attempted utilizing several techniques [28–31]. The most frequently tested method is the transplantation of juveniles or adult thalli: the only major challenge to this approach is the appropriate fixing of individuals or installation in the target area.
Outplanting, which consists of producing recruits from fertile material in hatcheries for placement in the sea, has been explored for the genus Cystoseira to a lesser degree [29,32]. In contrast, many studies have been performed using other large fucoid seaweeds [33–41] with a particular focus on the long-term maintenance of seedlings in culture [41–47].
Usually, the need for large numbers of germlings for outplanting represents a bottleneck in the design of large-scale restoration actions, so it is especially challenging to plan an efficient, effortless and cost-effective seedling production system that fits the breeding needs of a specific species. Considering the high potential of Cystoseira to generate gametes and zygotes under optimal conditions, the cultivation of germlings starting from fertile receptacles represents a sustainable option for restoring endangered species without depleting natural populations. From this perspective, the development of effective cultivation protocols tailored to the eco-physiological needs of different species is a compulsory milestone.
The aim of this study was to develop an ex situ protocol for the restoration of Cystoseira amentacea var. stricta Montagne, a sensitive caespitose intertidal Mediterranean species whose reduction/loss has primarily been recorded in several locations in the NW Mediterranean [6,48]. The protocol aimed to maximize zygote settlement, minimize embryo development time and generate a dense coverage of healthy germlings for outplanting. Based on these objectives, we tested the effects of easily adjustable variables (temperature, light and substratum) on the settlement and growth of early life stages to develop best practices for the restoration of this sensitive species.
Materials and methods
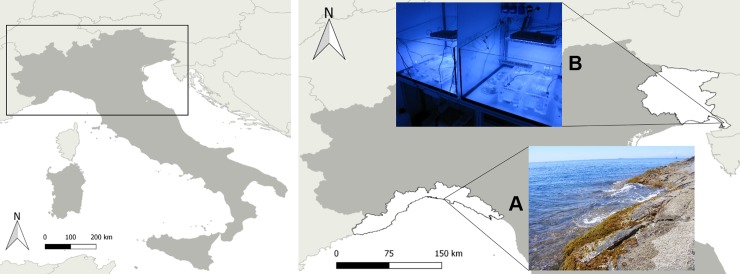
In June 2016, during the reproductive period of C. amentacea var. stricta, healthy apical fronds of ca. 3 cm in length holding mature receptacles were collected in the intertidal zone at Bogliasco, Genoa (NW Italy, 44°22'40.37"N—9°4'35.14"E) (Fig 1). No specific permits were required for collecting specimens in the selected location because it is not part of a protected or private area. Additionally, non-destructive sampling was performed, as only apical branches were collected. In particular, the site is characterized by a tide in the range of 30 cm (the barometric tide may dominate the water level) and an average spring temperature of 20°C. After sampling, apices wrapped with seawater-wetted towels were delivered within 24 h under dark, cold and humid conditions to the laboratory in Trieste (NE Italy) (Fig 1) for culture in environmentally controlled rooms.
Fig 1.
Map showing the geographical location of the collection site (A) and the laboratory site (B) in northern Italy.
The temperature and photoperiod were selected to reflect typical seasonal conditions at the sampling site during the reproductive phase of C. amentacea var. stricta (from late spring to summer). The photoperiod was set to a 15:9 h light:dark cycle, and light intensities were chosen to mimic two possible scenarios occurring in nature during the gamete release, fertilization and the early life growth stages of Cystoseira: in the absence of a canopy, as on barren ground (higher light intensity) or in the understory (lower light intensity). Light was provided by LED lamps (AM366 Sicce USA Inc., Knoxville, USA), and irradiance was measured with a LI-COR LI-190/R Photometer (LICOR-Biosciences, Lincoln, NE, USA). Light irradiance (L) was set at 125 μmol photons m−2s−1 (L-) or at 250 μmol photons m−2s−1 (L+), while temperature (T) was set at 20°C (T-) or at 24°C (T+) The medium used in the experiments was Stosch's enriched filtered and autoclaved seawater (VSE) [49,50]. Aquaria were filled with 4 L of culture medium, renewed every 4 days to minimize any possible effects of nutrient limitation and continuously aerated by air pumps. Two substrata with differing natures and roughness were tested: flat polished pebbles and rough clay tiles.
A factorial laboratory experiment was performed that combined irradiance, temperature and substratum. Four combinations of culturing conditions consisting of two crossed levels of each environmental condition (L+T+, L+T-, L-T+, L-T-) and two substrata (Pebbles and Tiles) were tested in a two-way crossed design.
Fertile apices were gently cleaned with a brush and rinsed with sterile seawater to remove adherent biofouling and surface detritus. Fronds were then placed in individual aquaria: three apices with mature receptacles on each substratum in separate aquaria per condition (in triplicate). Three additional replicates were placed on glass slides under each condition to observe zygote development with an inverted microscope (Leica, DM IL LED), and photographs were obtained with a Canon Powershot G9, avoiding stress on the treatment replicates.
Data analysis
Egg release and settlement
After 2 h, gametes were released on all substrata under all conditions. Next, the receptacles were removed, and their fresh weight (FW) was measured. Due to the high density of eggs released on each substratum and to reduce manipulation stress as much as possible, counts were performed by processing photographic data. For each substratum, eggs were counted in 5 randomly selected 1x1 cm quadrants in photos obtained from a Leica MZ6 stereo microscope with a Nikon Coolpix 4500 camera. The number of eggs per unit of receptacle FW was analyzed as a response variable to compare settlement on different substrata under different conditions. Two-way crossed ANOVA was performed using both factors and their interaction as fixed factors. The data were square-root transformed to satisfy the assumptions of normality and homoscedasticity.
Embryo development
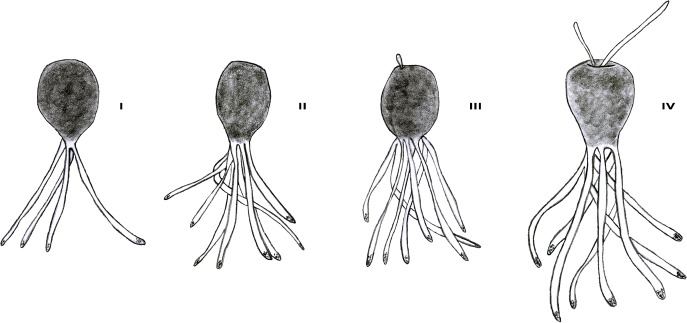
At the end of the first week, replicate embryos in all four growth stages were visible on glass slides and were counted: I-embryos with 4 primary rhizoids, II-embryos with 8 rhizoids, III-embryos with short apical hair/s, and IV-embryos with long apical hair/s (Fig 2). To analyze the differences among conditions, a PERMANOVA was applied using the percentage of individuals at each stage as a response variable and conditions as factors. Pairwise comparisons of significant terms were performed.
Fig 2. Early stages of C. amentacea var. strictaat week 1: I-embryos with 4 primary rhizoids, II-embryos with 8 rhizoids, III-embryos with short apical hair/s, and IV-embryos with long apical hair/s.
Embryo survival
After 24 h (T0), the number of zygotes was counted on each substratum (5 1x1 cm quadrants) by processing photographic data. Counts were then repeated at week 1 and week 2 to calculate the germling survival rate. We applied an ANCOVA for both week 1 and week 2 with unequal slopes for survival rate as a response variable and density (i.e., number of fertilized eggs) as a covariate, and substrata, conditions and their interactions were used as fixed factors. Assumptions were validated after applying the arcsine square root transformation (suitable for proportional data). Post hoc SNK tests were performed on significant interaction terms.
Subsequent germling growth
At week 2, three subsequent developmental stages were identifiable: I-round-shaped, II-elongated, and III-elongated with branch (Fig 3). The area of ten randomly chosen individuals per shape was measured in each replicate substratum and used as a response variable. The area was quantified by processing photographic data using ImageJ software [51]. Conditions and substrata were used as crossed fixed factors in a PERMANOVA, and pairwise comparisons were performed on significant terms.
Fig 3. C. amentacea var. stricta germling stages at week 2: I-round-shaped, II-elongated, and III- elongated with branching.
Results
Morphogenesis
C. amentacea var. stricta is a monoic species with female and male gametes produced in the same conceptacle (Fig 4A). In our trials, gamete release began soon after the receptacles were placed in the aquaria, and the mean diameter of the eggs was 122±3 μm (n = 20). Fertilization occurred externally, and the development of a fecundation membrane around the zygote facilitated its adhesion to a substratum (Fig 4B). The zygote cytoplasm, which was initially homogeneous, became metabolically differentiated (polarization) with the establishment of a vertical growth axis (connecting the rhizoid and thallus pole). Twelve hours after fertilization (AF), the first division perpendicular to the growth axis was observed, leading to the formation of two equally sized cells (Fig 4C). The second division, which was parallel to the first, occurred in the lower cell 20–22 h AF (Fig 4D), while the third division, perpendicular to the first, appeared in the upper cell (Fig 4D). Within 32–34 h AF, many divisions occurred without an increase in embryo volume.
Fig 4. Early development of Cystoseira amentacea var. stricta.
A. Detail of a conceptacle with an oogonium and antheridia (arrowhead). B. Zygote with a central large nucleus. C. First zygote division (arrow). D. Second zygote division (II) parallel to the first (I) and third (III) and fourth divisions (IV) perpendicular to the first. E. Embryo with rhizoidal buds (arrow). F. Embryo with secondary rhizoids. Note the detachment of the fecundation membrane (arrow) during embryo elongation. G. Hyaline hairs growing from the invagination in the apical region of the embryo. H. Embryo with long apical hairs and numerous rhizoids (arrow). I. Germling with cryptostomata (arrows). Bar = 200 μm.
Within the first week, the rhizoids developed as follows: via perpendicular divisions, the rhizoid mother cell gave rise to four cells that differentiated into four primary rhizoids (Figs 4E and 2) that grew further, forming long filaments (ca. 150–200 μm long). After detachment of the fecundation membrane, the length of the embryo increased through subsequent cell divisions, and secondary rhizoids were formed (Fig 4F). Thus, the embryo assumed an erect position, and an invagination with hyaline hairs appeared in the apical region (Fig 4H and 4G). At week 1 AF, the more developed embryos were 353±26 μm long and 259±37 μm wide (n = 20). At week 2 AF, germlings with numerous rhizoids grew further [466±26 μm long and 275±28 μm wide (n = 20)] and small lateral branches with some cryptostomata began to appear. At week 3 AF, numerous cryptostomata were observed (Fig 4I), and iridescence, which is typical of adult plants, was visible on the thallus surface. At this time point, the germlings were 1.38±0.13 mm long and 0.46±0.06 mm wide (n = 20). At the end of the third week, few tiles were transported back in the field at Bogliasco. Juveniles were 4.73±0.05 mm long and 0.81±0.09 mm wide after 1 month in the field, and they grew up to 9 cm in 9 months (April 2017).
Egg release and settlement
The number of settled eggs was higher on Tiles (avg = 5226, SE = 566) than on Pebbles (avg = 2429; SE = 199), highlighting a significant effect of substratum roughness (p<0.0001; S1 Table). Conversely, no significant differences were detected between conditions or within the interaction term.
Early embryo development
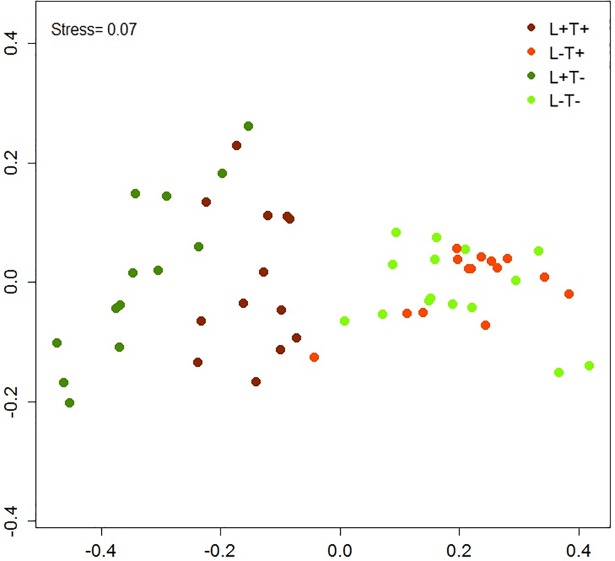
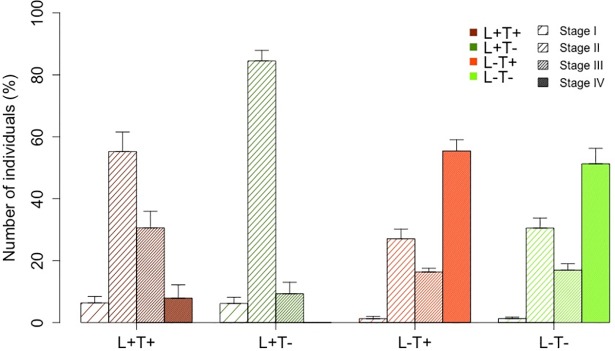
The percentage of individuals was calculated for each of the four stages observed in the glass slide replicates (Fig 2). An MDS ordination plot (Fig 5) showed three different groups: L+T-, L+T+ and one group comprising L- conditions (L-T- and L-T+). PERMANOVA confirmed significant differences among these groups (p<0.001; S2 Table). Furthermore, a bar plot (Fig 6) revealed a higher percentage of embryos in stage IV (embryos with long apical hair/s) under L-conditions.
Fig 5. MDS ordination plot of the percent composition of embryonic developmental stages at week 1 under the different conditions.
L+ T+ = high light–high temperature, L+ T- = high light–low temperature, L- T+ = low light–high temperature, L- T- = low light–low temperature.
Fig 6. Bar plot of the percent composition of embryonic developmental stages at week 1 under the different conditions.
L+ T+ = high light–high temperature, L+ T- = high light–low temperature, L- T+ = low light–high temperature, L- T- = low light–low temperature. I = embryos with 4 primary rhizoids, II = embryos with 8 rhizoids, III = embryos with short apical hair/s, IV =: mbryos with long apical hair/s.
Embryo survival
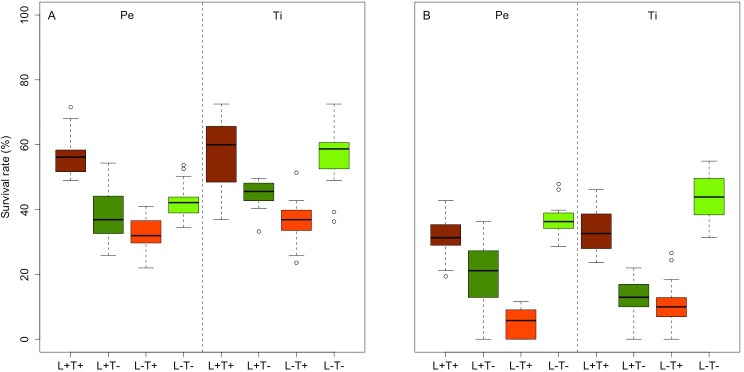
The ANCOVA results indicated strong significant differences among conditions and small differences between the two substrata at week 1 (p<0.0001 and p<0.03, respectively; S3 Table). At week 2 the interaction term (conditionXsubstratum) was significant (p<0.001; S4 Table): on both substrata, the extreme conditions did not differ from each other (L+T+ = L-T-), but they differed significantly from the other two conditions (L+T-; L-T+). On Tiles, L+T- and L-T+ did not differ significantly, although they differed on Pebbles. Additionally, ANCOVA indicated that L+T- was significantly different between substrata, while survival was slightly higher on Pebbles. As shown in boxplots (Fig 7), the survival rate at week 1 was higher under extreme conditions (L+T+; L-T-) compared to the other two conditions (L+T-; L-T+). The survival rate from week 1 to week 2 conspicuously decreased (between 50 and 95%) for the L+T+, L+T- and L-T+ conditions, while the survival rate under L-T- remained more stable with a mortality below 30%.
Fig 7.
Boxplot of the survival rates at week 1 (A) and week 2 (B) among conditions and substrata. L+ T+ = high light–high temperature, L+ T- = high light–low temperature, L- T+ = low light–high temperature, L- T- = low light–low temperature. Pe = Pebbles, Ti = Tiles.
Subsequent germling growth
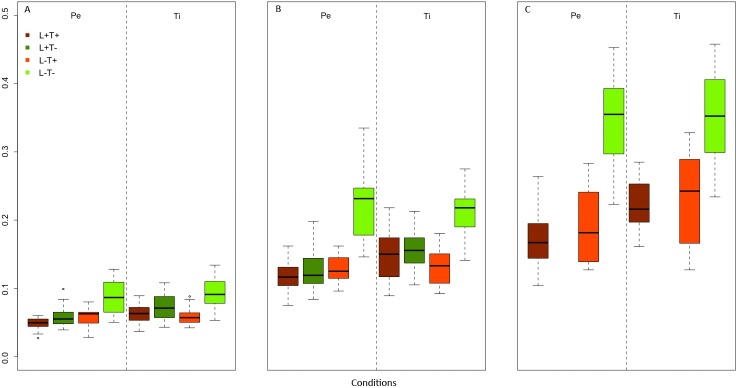
PERMANOVA performed on the germling area at different stages (Fig 3) at week 2 showed significant differences among conditions (p = 0.001) with L-T-condition different from the others conditions (S5 Table; Fig 8).
Fig 8.
Boxplots of the area of the different stages (A: stage I; B: stage II; C: stage III) at week 2 among conditions for both substrata. L+ T+ = high light–high temperature, L+ T- = high light–low temperature, L- T+ = low light–high temperature, L- T- = low light–low temperature. Pe = Pebbles, Ti = Tiles.
Discussion
Given the worldwide concern over the loss of key habitat-forming organisms, such as the large brown macroalgae of the order Fucales, and the downstream cascade effects on the services provided by such organisms, there is an urgent need to develop best practices and restoration strategies. Studies that provide sound information on how to best undertake habitat restoration are crucial for managing coastal ecosystems.
Outplanting appears to be an ecologically sustainable approach that consists of two main steps: culturing germlings in the laboratory and transferring them into the field [37,41,45,52]. For Cystoseira, outplanting appears to be a feasible management option that can provide many healthy specimens for re-introduction to the environment without impacting natural populations [29,32,53].
In this study, we focused on the first step of the outplanting process: developing an effective protocol for cultivating the early stages of C. amentacea var. stricta. This approach is challenging because most eco-physiological studies of Cystoseira, both in the field and in the laboratory, have focused on the adult stages. Nevertheless, the single/few-celled stages are characterized by simplicity and sensitivity, so compared to adults, any environmental variable will exert greater effects on germling mortality and growth rate [54,55]. Thus, the needs of these ontogenetic stages must be understood because findings related to the macrothallus stages cannot be extrapolated to the microscopic stages [56]. Thus, species-specific best practices for the cultivation of germlings must be developed to implement a successful large-scale restoration strategy.
First, we tested whether it was possible to collect samples far from the breeding facility (ca. 600 km) without damaging the reproductive materials to exclude the possible negative effects related to the distance of the target site from the hatchery. Transporting under dark and cold conditions allowed immediate gamete release, thus avoiding thermal and light shock in the laboratory; indeed, receptacles that were placed in aquaria soon after their arrival in the laboratory immediately released gametes. Starting from eggs fecundation, we described the morphogenesis and successive germlings development of C. amentacea var. stricta. Based on zygotes segmentation and number of primary rhizoids, three groups of Cystoseira species have been identified by [22]. C. amentacea var. stricta belongs to the first group, that is characterized by spherical eggs, zygotes that adhere to substrata by the fecundation membrane, and four primary rhizoids [57].
Nutrient limitation affects many processes, such as photosynthetic capacity [58], protein content [59,60], photoprotection mechanisms [61–63], egg behavior and settlement, embryonic development and growth rate [64–69], so the culture medium was enriched to allow the germlings to invest their photosynthetic energy in growth processes [70,71]. Better growth of C. amentacea var. stricta germlings with nutrient enrichment has also been observed by [57]. The positive effect of nitrate supply on C. stricta growth rate has been demonstrated in adults cuttings cultivation, although with small differences between apical and subapical segments [72]. Together with culture conditions, the choice of substrata must also favor the adhesion of gametes and zygotes and their successive development. Regardless of the culture conditions, rougher tiles showed higher zygote settlement than smoother pebbles, although the substrata did not affect successive germling growth or survival under any of the tested conditions.
Embryonic mortality after one week was elevated under all conditions (50% on average), which was expected given the very high stochastic gamete and zygote mortality of Cystoseira in the natural environment. At the end of the first week, embryonic survival was positively affected by two of the tested conditions: L+T+ and L-T-. At week 2, survival was still higher under the L-T- condition but significantly decreased under L+T+ treatment. The other two combined conditions showed the lowest embryonic survival throughout the entire experiment. Low-light and low-temperature conditions also favor higher embryonic survival rate in Sargassum vachellianum Greville cultivations [73]. Lower light intensity also reduced the time required for embryo development, allowing a greater number of individuals to reach developmental stage IV (larger embryos) within the first week regardless of temperature. After two weeks at low irradiance, lower temperature also strongly determined the growth of larger embryos.
Our findings corroborate that environmental conditions (specifically light and temperature) may interact and exert synergistic or antagonistic effects on physiological responses in unpredictable ways that differ according to developmental stage. The distribution of C. amentacea var. stricta is restricted to the intertidal, so this species is naturally exposed to high levels of irradiance that potentially exceed its light energy requirements, as has been reported for other species that live close to the water surface [70,74–76]. Generally, sun-adapted species [sensu 77] develop efficient photoprotection mechanisms to tolerate light stress in addition to dynamic photoinhibition [75,76,78–86].Our study highlighted the light-shade adaptation of C. amentacea var. stricta germlings, which showed enhanced growth at lower irradiance (125 μmol photons m−2 s−1). Other Cystoseira species have been cultivated under different conditions that have primarily depended on laboratory facilities such as Cystoseira susanensis Nizamuddin (16±1°C | 40 μmol photons m−2 s−1 [87] and C. barbata (Stackhouse) C. Agardh (16–17°C | 120 μmol photons m−2 s−1 [29]. The morphological development of C. amentacea var. stricta embryos cultivated at 18±1°C and an average light intensity of 70 μmol photons m−2s−1 has been described by [57]. After ca. 2 months in these conditions, embryos cultivated in seawater were 332.60 ± 22.3μm long, while in VSE medium they were 5.14 ± 0.08 mm [57]. In our experiment germlings cultivated in VSE were 1.38±0.13 mm long after three weeks and 4.73±0.05 mm long after one month in the field. We observed cryptostomata and lateral branches after two weeks of cultivation, while in [57] study cryptostomata were observed after 52 days and ramifications appeared after 106 days.
Studies examining adult thalli of Cystoseira have demonstrated the absence of photosynthetic inhibition, even with very high irradiance [88]. In C. barbata (Stackhouse) C. Agardh f. aurantia (Kuetzing) Giaccone, photoinhibition only occurs at irradiances higher than 1500 μmol photons m−2s−1 [89], while photosynthesis in C. mediterranea Sauvageau is not saturated at an irradiance of 1600 μmol photons m−2s−1[75]. Notably, the light requirements of adults should not be extrapolated to the microscopic stages because the presence of non-photosynthetic tissues in complex thalli increases the need for light energy [76], and the irradiance reaching the embryos is restricted by adult fronds in nature [35,42,90]. Cystoseira zygotes and germlings settle under adult plants, where they find a protective screen against high irradiance and other stressors. In nature, such community self-protection could be particularly important during spring-summer, when C. amentacea var. stricta produces new recruits in the study area. Conversely, the lower irradiance requirements of germlings permit high-density cultures because self-shading is not a restricting factor.
At higher temperatures (24°C), the proliferation of biofouling was enhanced, particularly at lower light intensity, progressively affecting the development of C. amentacea var. stricta embryos. Based on these results, we determined that the lower values tested for irradiance (125 μmol photons m−2 s−1) and temperature (20°C) were the best hatchery conditions to accelerate the development of high numbers of healthy, large embryos.
Further studies are required to improve the second step in the outplanting process to increase the number of juveniles that can reach the adult stage once they are reintroduced into the field. Grazing pressure, timing and density dependent effects need to be considered to achieve the best restoration results.
Supporting information
Significant effects are in bold.
(PDF)
Significant effects are in bold.
aPairwise comparisons among conditions: L+T-≠L+T+≠ L-T- = L-T+.
(PDF)
Significant effects are in bold.
aSNK test among conditions: L+T+≠L-T-≠L+T-≠L-T.
(PDF)
Significant effects are in bold.
aSNK test among substrata within condition: Cond. L+T-, T≠S; all other Cond., T = S.
bSNK test among conditions within substratum: Sub. S, (L+T+ = L-T-)≠L+T-≠L-T+; Sub. T, (L+T+ = L-T-)≠(L+T- = L-T+).
(PDF)
Condition and substratum are crossed fixed factors. Significant effects are in bold.
aPairwise comparisons among conditions: L-T-≠L+T+ = L-T+ = L+T-.
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was conducted with the contribution of the LIFE financial instrument of the European Community, project ROC-POP-LIFE (LIFE16 NAT/IT/000816). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jones CG, Lawton JH, Shachak M. Organisms as ecosystem engineers. Oikos. 1994;69(3):373–386. doi: 10.2307/3545850 [Google Scholar]
- 2.Mineur F, Arenas F, Assis J, Davies AJ, Engelen AH, Fernandes F, et al. European seaweeds under pressure: Consequences for communities and ecosystem functioning. J Sea Res. 2015;98:91–108. [Google Scholar]
- 3.Boudouresque CF. Marine biodiversity in the Mediterranean: status of species, populations and communities. Sci Rep Port-Cros Natl Park, Fr. 2004;20:97–146. [Google Scholar]
- 4.Thibaut T, Pinedo S, Torras X, Ballesteros E. Long-term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Alberes coast (France, North-western Mediterranean). Mar Pollut Bull. 2005;50(12):1472–1489. doi: 10.1016/j.marpolbul.2005.06.014 [DOI] [PubMed] [Google Scholar]
- 5.Micheli F, Levin N, Giakoumi S, Katsanevakis S, Abdulla A, Coll M, et al. Setting priorities for regional conservation planning in the Mediterranean Sea. PLoS ONE. 2013;8(4):e59038.8 doi: 10.1371/journal.pone.0059038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thibaut T, Blanfuné A, Markovic L, Verlaque M, Boudouresque CF, Perret-Boudouresque M, Maćic V, Botti L. Unexpected abundance and long-term relative stability of the brown alga C. amentacea var. stricta, hitherto regarded as a threatened species, in the north-western Mediterranean Sea. Mar Pollut Bull. 2014;89(1):305–323. doi: 10.1016/j.marpolbul.2014.09.043 [DOI] [PubMed] [Google Scholar]
- 7.Thibaut T, Blanfuné A, Boudouresqu CF, Verlaque M. Decline and local extinction of Fucales in the French Riviera: the harbinger of future extinctions? Mediterr Mar Sci. 2015;16:206–224. doi: 10.12681/mms.1032 [Google Scholar]
- 8.Blanfuné A, Boudouresque CF, Verlaque M, Thibaut T. The fate of Cystoseira crinita, a forest-forming Fucales (Phaeophyceae, Stramenopiles), in France (North Western Mediterranean Sea). Estuar Coast Shelf Sci. 2016;181:196–208. doi: 10.1016/j.ecss.2016.08.049 [Google Scholar]
- 9.Thibaut T, Blanfuné A, Boudouresque CF, Cottalorda JM, Hereu B, Susini ML, et al. Unexpected temporal stability of Cystoseira and Sargassum forests in Port-Cros, one of the oldest Mediterranean marine National Parks. Cryptogam., Algol. 2016;37:1–30. doi: 10.7872/crya/v37.iss1.2016.61 [Google Scholar]
- 10.Munda IM. Changes and degradation of seaweed stands in the Northern Adriatic. Hydrobiologia. 1993; 260(1):239–253. doi: 10.1007/BF00049025 [Google Scholar]
- 11.Sala E, Boudouresque CF, Harmelin-Vivien M. Fishing, trophic cascades, and the structure of algal assemblages: evaluation of an old but untested paradigm. Oikos. 1998;82(3):425–439. doi: 10.2307/3546364 [Google Scholar]
- 12.Falace A, Alongi G, Cormaci M, Furnari G, Curiel D, Cecere E, et al. Changes in the benthic algae along the Adriatic Sea in the last three decades. Chemistry and ecology 2010; 26(S1):77–90. doi: 10.1080/02757541003689837 [Google Scholar]
- 13.Fraschetti S, Guarnieri G, Bevilacqua S, Terlizzi A, Claudet J, Russo GF, et al. Conservation of Mediterranean habitats and biodiversity countdowns: what information do we really need? Aquat Conserv. 2011;21(3):299–306. doi: 10.1002/aqc.1185 [Google Scholar]
- 14.Giakoumi S, Cebrian E, Kokkoris GD, Ballesteros E, Sala E. Relationships between fish, sea urchins and macroalgae: The structure of shallow rocky sublittoral communities in the Cyclades, Eastern Mediterranean. Estuar Coast Shelf Sci. 2012;109:1–10. doi: 10.1016/j.ecss.2011.06.004 [Google Scholar]
- 15.Templado J. Future trends of Mediterranean biodiversity In: Goffredo S, Dubinsky Z, editors. The Mediterranean Sea. Springer, Dordrecht Netherlands; 2014. pp. 479–498. doi: 10.1007/978-94-007-6704-1_28 [Google Scholar]
- 16.Marzinelli EM, Campbell AH, Vergés A, Coleman MA, Kelaher BP, Steinberg PD. Restoring seaweeds: does the declining fucoid Phyllospora comosa support different biodiversity than other habitats? J Appl Phycol. 2014;26(2):1089–1096. doi: 10.1007/s10811-013-0158-5 [Google Scholar]
- 17.EEC, 1992. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Official Journal L 206, 22/07/1992 p.0007-0050.
- 18.EC, 2000. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Official Journal L 327, 22/12/2000 p.0001-0073.
- 19.Orfanidis S, Panayotidis P, Stamatis N. An insight to the ecological evaluation index (EEI). Ecol Indic. 2003;3(1):27–33. doi: 10.1016/S1470-160X(03)00008-6 [Google Scholar]
- 20.Ballesteros E, Torras X, Pinedo S, García M, Mangialajo L, and De Torres M. A new methodology based on littoral community cartography dominated by macroalgae for the implementation of the European Water Framework Directive. Mar Pollut Bull. 2007;55(1):172–180. doi: 10.1016/j.marpolbul.2006.08.038 [DOI] [PubMed] [Google Scholar]
- 21.Blanfuné A, Thibaut T, Boudouresque CF, Mačić V, Markovic L, Palomba L, et al. The CARLIT method for the assessment of the ecological quality of European Mediterranean waters: Relevance, robustness and possible improvements. Ecol Indic. 2017;72:249–259. doi: 10.1016/j.ecolind.2016.07.049 [Google Scholar]
- 22.Guern M. Embryologie de quelques espèces du genre Cystoseira Agardh 1821 (Fucales). Vie Milieu. 1962;13:649–679. [Google Scholar]
- 23.Clayton MN. The adaptive significance of life history characters in selected orders of marine brown macroalgae. Austral Ecol. 1990;15(4):439–452. doi: 10.1111/j.1442-9993.1990.tb01469.x [Google Scholar]
- 24.Johnson LE, Brawley SH. Dispersal and recruitment of a canopy-forming intertidal alga: the relative roles of propagule availability and post-settlement processes. Oecologia. 1998;117(4):517–526. doi: 10.1007/s004420050688 [DOI] [PubMed] [Google Scholar]
- 25.Gaylord B, Reed DC, Raimondi PT, Washburn L, McLean SR. A physically based model of macroalgal spore dispersal in the wave and current dominated near shore. Ecology. 2002;83(5):1239–1251. doi: 10.1890/0012-9658(2002)083[1239:APBMOM]2.0.CO;2 [Google Scholar]
- 26.Thibaut T, Bottin L, Aurelle D, Boudouresque CF, Blanfuné A, Verlaque M, et al. Connectivity of populations of the seaweed Cystoseira amentacea within the Bay of Marseille (Mediterranean Sea): genetic structure and hydrodynamic connections. Cryptogam., Algol. 2016;37(4):233–255. doi: 10.7872/crya/v37.iss4.2016.233 [Google Scholar]
- 27.Buonomo R, Assis J, Fernandes F, Engelen AH, Airoldi L, Serrão EA. Habitat continuity and stepping-stone oceanographic distances explain population genetic connectivity of the brown alga Cystoseira amentacea. Mol Ecol. 2017;26:766–780. doi: 10.1111/mec.13960 [DOI] [PubMed] [Google Scholar]
- 28.Falace A, Bressan G. Seasonal variations of Cystoseira barbata (Stackhouse) C. Agardh frond architecture. Hydrobiologia. 2006;555:93–206. doi: 10.1007/s10750-005-1116-2 [Google Scholar]
- 29.Falace A, Zanelli E, Bressan G. Algal transplantation as a potential tool for artificial reef management and environmental mitigation. Bull Mar Sci. 2006;8:161–166. [Google Scholar]
- 30.Susini ML, Mangialajo L, Thibaut T, Meinesz A. Development of a transplantation technique of Cystoseira amentacea var. stricta and Cystoseira compressa. Hydrobiologia. 2007;580(1):241–244. doi: 10.1007/s10750-006-0449-9 [Google Scholar]
- 31.Sales M, Cebrian E, Tomas F, Ballesteros E. Pollution impacts and recovery potential in three species of the genus Cystoseira (Fucales, Heterokontophyta). Estuar Coast Shelf Sci. 2011;92(3):347–357. doi: 10.1016/j.ecss.2011.01.008 [Google Scholar]
- 32.Verdura J, Cefali ME, Orfila E, Verges A, Sanchez M, Cebrian E. Optimal environmental conditions in Cystoseira sp early life stages. Eur J Phycol. 2015;50(1):214–214. [Google Scholar]
- 33.Vasquez JA, Tala F. Repopulation of intertidal areas with Lessonia nigrescens in northern Chile. J Appl Phycol. 1995;7:347–349. doi: 10.1007/BF00003791 [Google Scholar]
- 34.Stekoll MS, Deysher L. Recolonization and restoration of upper intertidal Fucus gardneri (Fucales, Phaeophyta) following the Exxon Valdez oil spill. Hydrobiologia. 1996. 326(1):311–316. [Google Scholar]
- 35.Dudgeon S, Petraitis PS. First year demography of the foundation species, Ascophyllum nodosum, and its implications. Oikos. 2005;109(2):405–415. doi: 10.1111/j.0030-1299.2005.13782.x [Google Scholar]
- 36.Hwang E, Park C, Baek J. Artificial seed production and cultivation of the edible brown alga, Sargassum fulvellum (Turner) C. Agardh: Developing a new species for seaweed cultivation in Korea. J Appl Phycol. 2006;18:251–257. doi: 10.1007/978-1-4020-5670-3_4 [Google Scholar]
- 37.Hays CG. Adaptive phenotypic differentiation across the intertidal gradient in the alga Silvetia compressa. Ecology. 2007;88(1):149–157. doi: 10.1890/0012-9658(2007)88[149:APDATI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 38.Lamote M, Johnson LE. Temporal and spatial variation in the early recruitment of fucoid algae: the role of microhabitats and temporal scales. Mar Ecol Prog Ser. 2008;368:93–102. doi: 10.3354/meps07592 [Google Scholar]
- 39.Pang S, Liu F, Shann T, Gao S, Zhang Z. Cultivation of the brown alga Sargassum horneri: Sexual reproduction and seedling production in tank culture under reduced solar irradiance in ambient temperature. J App Phycol. 2009;21:413–422. doi: 10.1007/s10811-008-9386-5 [Google Scholar]
- 40.Yatsuya K. Techniques for the restoration of Sargassum beds on barren grounds. Bull Fish Res Agen. 2010;32:69–73. [Google Scholar]
- 41.Yu YQ, Zhang QS, Tang YZ, Zhang SB, Lu ZC, Chu SH, et al. Establishment of intertidal seaweed beds of Sargassum thunbergii through habitat creation and germling seeding. Ecol Eng. 2012;44:10–17. doi: 10.1016/j.ecoleng.2012.03.016 [Google Scholar]
- 42.Zhao Z, Zhao F, Yao J, Lu J, Ang PO, Duan D. Early development of germlings of Sargassum thunbergii (Fucales, Phaeophyta) under laboratory conditions In: Borowitzka MA, Critchley AT, Kraan S, Peters A, Sjøtun K, Notoya M., editors. Nineteenth International Seaweed Symposium. Developments in Applied Phycology, vol 2 Springer, Dordrecht; 2008. pp 475–481. doi: 10.1007/978-1-4020-9619-8_57 [Google Scholar]
- 43.Sun JZ, Zhuang DG, Sun QH, Pang SJ. Artificial cultivation trials of Sargassum horneri at Nanji islands of China. South China. Fish Sci. 2009;5(6):41–46. [Google Scholar]
- 44.Yan XH, Zhang J. Embryology of zygote and development of germling in Sargassum vachellianum Greville (Fucales, Phaeophyta). J Appl Phycol. 2014;26:577–585. doi: 10.1007/s10811-013-0102-8 [Google Scholar]
- 45.Yoon JT, Sun SM, Chung G. Sargassum bed restoration by transplantation of germlings grown under protective mesh cage. J Appl Phycol. 2014;26(1):505–509. doi: 10.1007/s10811-013-0058-8 [Google Scholar]
- 46.Kerrison P, Le HN. Environmental factors on egg liberation and germling production of Sargassum muticum. J Appl Phycol. 2016;28(1):481–489. doi: 10.1007/s10811-015-0580-y [Google Scholar]
- 47.Liu W, Wu H, Liu M, Duan D. Improvement of the zygote utilization and reduction of the seedling loss in the early stage of seedling production of Sargassum thunbergii (Fucales, Phaeophyta). Ocean. Limnol. 2016;34(3):492–497. doi: 10.1007/s00343-016-5041-1 [Google Scholar]
- 48.Mangialajo L, Ruggieri N, Asnaghi V, Chiantore M, Povero P, Cattaneo-Vietti R. Ecological status in the Ligurian Sea: the effect of coastline urbanisation and the importance of proper reference sites. Mar Pollut Bull. 2007; 55(1):30–41. doi: 10.1016/j.marpolbul.2006.08.022 [DOI] [PubMed] [Google Scholar]
- 49.Von Stosch HA. Wirkungen von Jod und Arsenit auf Meeresalgen in Kultur. Proc. Int. Seaweed Symp. 1963;4:142–50. [Google Scholar]
- 50.Guiry MD, Cunningham EM. Photoperiodic and temperature responses in the reproduction of north-eastern Atlantic Gigartina acicularis (Rhodophyta: Gigartinales). Phycologia. 1984;23(3):357–367. doi: 10.2216/i0031-8884-23-3-357.1 [Google Scholar]
- 51.Schneider CA, Rasband WS., Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012; 9:671–675. doi: 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dudgeon S, Petraitis PS. First year demography of the foundation species, Ascophyllum nodosum, and its implications. Oikos. 2005;109:405–415. doi: 10.1111/j.0030-1299.2005.13782.x [Google Scholar]
- 53.Sales M, Ballesteros E, Vidal E, Tomas F, Moranta J, Cebrian E. New method for restoring degraded Cystoseira forests. Eur J Phycol. 2015;50(1):108–109. [Google Scholar]
- 54.Yakovleva IM, Dring M, Titlyanov EA. Tolerance of North Sea algae to UV and visible radiation. Rus. J. Plant. Physiol. 1998;45(1):45–54. [Google Scholar]
- 55.Wiencke C, Gómez I, Pakker H, Flores-Moya A, Altamirano M, Hanelt D, et al. Impact of UV-radiation on viability, photosynthetic characteristics and DNA of brown algal zoospores:implications for depth zonation. Mar Ecol Prog Ser. 2000;197:217–229. [Google Scholar]
- 56.Altamirano M, Flores-Moya A. Figueroa FL. Effects of UV radiation and temperature on growth of germlings of three species of Fucus (Phaeophyceae). Aquat Bot. 2009;75:9–20. [Google Scholar]
- 57.Susini ML. Statut et biologie de Cystoseira amentaceavar. stricta. PhD Thesis. Université Nice-Sophia Antipolis, France. 2006. Available from: ftp://nephi.unice.fr/users/ecomers/2006/2006%20Susini%20Th%C3%A8se.pdf
- 58.Pérez-Lloréns JL, Vergara JJ, Pino RR, Hernandez I, Peralta G, Niell FX The effect of photoacclimation on the photosynthetic physiology of Ulva curvata and Ulva rotundata (Ulvales, Chlorophyta). Eur J Phycol. 1996;31:349–359. doi: 10.1080/09670269600651581 [Google Scholar]
- 59.Vergara JJ, Bird KT, Niell FX. Nitrogen assimilation following NH4+ pulses in the red alga Gracilariopsis lemaneiformis: effect on C metabolism. Mar Ecol Prog Ser. 1995;122(1–3):253–263. doi: 10.3354/meps122253 [Google Scholar]
- 60.Martínez B, Rico JM. Seasonal variation of P content and major N pools in Palmaria palmata (Rhodophyta). J Phycol. 2002;38:1082–1089. doi: 10.1046/j.1529-8817.2002.01217.x [Google Scholar]
- 61.Korbee-Peinado N, Abdala-Díaz RT, Figueroa FL, Helbling EW. Ammonium and UV radiation simulate the accumulation of mycosporine-like amino acids in Porphyra columbina (Rhodophyta) from Patagonia, Argentina. J Phycol. 2004;40:248–259. doi: 10.1046/j.1529-8817.2004.03013.x [Google Scholar]
- 62.Korbee N, Figueroa FL, Aguilera J. Effect of light quality on the accumulation of photosynthetic pigments, proteins and mycosporine-like amino acids in the red alga Porphyra leucosticta (Bangiales, Rhodophyta). J Photochem Photobiol. 2005;80:71–78. doi: 10.1016/j.jphotobiol.2005.03.002 [DOI] [PubMed] [Google Scholar]
- 63.Huovinen P, Matos J, Pinto IS, Figueroa FL. The role of ammonium in photoprotection against high irradiance in the red alga Grateloupia lanceola. Aquat Bot. 2006;84:308–316. doi: 10.1016/j.aquabot.2005.12.002 [Google Scholar]
- 64.Chapman ARO, Markham JW, Lüning K. Effects of nitrate concentration on growth and physiology of Laminaria saccharina (Phaeophyta) in culture. J Phycol. 1978;14(2):195–198. doi: 10.1111/j.1529-8817.1978.tb02448.x [Google Scholar]
- 65.Amsler CD, Neushul M. Nutrient stimulation of spore settlement in the kelps Pterygophora californica and Macrocystis pyrifera. Mar Biol. 1990;107:297–304. [Google Scholar]
- 66.Reed DC, Brzezinski MA, Coury DA, Graham WM, Petty RL. Neutral lipids in macroalgal spores and their role in swimming. Mar Biol. 1999;133:737–744. doi: 10.1007/s002270050515 [Google Scholar]
- 67.Kinlan BP, Graham MH, Sala E, Dayton PK. Arrested development of giant kelp (Macrocystis pyrifera, Phaeophyceae) embryonic sporophytes: a mechanism for delayed recruitment in perennial kelps. J Phycol. 2003;39:47–57. doi: 10.1046/j.1529-8817.2003.02087.x [Google Scholar]
- 68.Morelisen B, Dudley BD, Geange SW, Phillips NE. Gametophyte reproduction and development of Undaria pinnatifida under varied nutrient and irradiance conditions. J Exp Mar Biol Ecol. 2013;448:197–206. doi: 10.1016/j.jembe.2013.07.009 [Google Scholar]
- 69.Flavin K, Flavin N, Flahive B. Kelp farming manual. A guide to the processes, techniques and equipment for farming kelp in New England Waters. Ocean Approved LLC. Portland; 2013.
- 70.Celis-Plá PSM, Korbee N, Gómez-Garreta A, Figueroa FL. Seasonal photoacclimation patterns in the intertidal macroalga Cystoseira tamariscifolia (Ochrophyta). Sci. Mar. 2014;78(3):377–388. doi: 10.3989/scimar.04053.05A [Google Scholar]
- 71.Celis-Plá PSM., Martínez B, Quintano E, García-Sánchez M, Pedersen A, Navarro NP, et al. Short-term ecophysiological and biochemical responses of Cystoseira tamariscifolia and Ellisolandia elongata to environmental changes. Aquat Biol. 2014;22:227–243. doi: 10.3354/ab00573 [Google Scholar]
- 72.Epiard-Lahaye M. Effects of ammonium, nitrate and phosphate on the growth of Cystoseira stricta (Phaeophyta, Fucales) cuttings in culture. Cryptogam., Algol. 1988;9(3):211–229. [Google Scholar]
- 73.Yan XH, Zhang J. Embryology of zygote and development of germling in Sargassum vachellianum Greville (Fucales, Phaeophyta). J Appl Phycol. 2014;26:577–585. doi: 10.1007/s10811-013-0102-8 [Google Scholar]
- 74.Delgado O, Rodriguez-Prieto C, Frigola L, Ballesteros E. Drought tolerance and light requirements on high and low sublittoral species of Mediterranean macroalgae of the genus Cystoseira C. Agardh (Fucales, Phaeophyceae). Bot Mar. 1995;38:127–132. doi: 10.1515/botm.1995.38.1–6.127 [Google Scholar]
- 75.Hader DP, Figueroa FL. Photo-ecophysiology of marine macroalgae. Photochem Photobiol. 1997;66(1):1–14. doi: 10.1111/j.1751-1097.1997.tb03132.x [Google Scholar]
- 76.Hanelt D, Figueroa FL Physiological and photomorphogenic effects of light on marine macrophytes In: Wiencke C., Bischof K., editors. Seaweed biology: Novel insights into ecophysiology, ecology and utilization. Ecological Studies. Springer-Verlag; 2012. 219 pp. 3–23. [Google Scholar]
- 77.Ramus J. The capture and transduction of light energy In: Lobban CS, Wynne MJ, editors. The Biology of the Seaweeds. Oxford: Blackwell Scientific; 1981. pp. 458–492. [Google Scholar]
- 78.Mattoo AK, Hoffman-Falk H, Marder JB, Edelman M. Regulation of protein metabolism: coupling of photosynthetic electron transport to in vivo degradation of rapidly metabolized 32-kilodalton protein of the chloroplast membranes. Proc Nat. Aca. Sci. 1984;81:1380–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ohad I, Kyle DJ, Artntzen CJ. Membrane protein damage and repair: Removal and replacement of inactivated 32-kilodalton polypeptides in chloroplast. J Cell Biol. 1984;99:481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guenther JE, Melis A. The physiological significance of Photosystem II heterogeneity in chloroplasts. Photosynth Res. 1990;23(1):105–109. doi: 10.1007/BF00030070 [DOI] [PubMed] [Google Scholar]
- 81.Critchley C, Russel AW. Photoinhibition of photosynthesis in vivo: The role of protein turnover in photosystem II. Physio. Plant. 1994;92(1):188–196. doi: 10.1111/j.1399-3054.1994.tb06670.x [Google Scholar]
- 82.Figueroa FL, Gómez I. Photosynthetic acclimation to solar UV radiation of marine red algae from the warm temperate coast of southern Spain. A review. J Appl Phycol. 2001;13(3):233–245. doi: 10.1023/A:1011126007656 [Google Scholar]
- 83.Franklin LA, Osmond CB, Larkum AWD. Photoinhibition, UV-B and algal photosynthesis In: Larkum AWD, Douglas SE, Raven JA., editors. Photosynthesis in Algae. Advances in Photosynthesis and Respiration Vol. 14 Kluwer, Dordrecht; 2003. pp 351–384. doi: 10.1007/978-94-007-1038-2_16 [Google Scholar]
- 84.Abdala-Dıaz RT, Cabello-Pasini A, Pérez-Rodrìguez E, Conde Alvarez RM, Figueroa FL. Daily and seasonal variations of optimum quantum yield and phenolic compounds in Cystoseira tamariscifolia (Phaeophyta). Mar Biol. 2006;148:459–465. doi: 10.1007/s00227-005-0102-6 [Google Scholar]
- 85.Wilhelm C, Selmar D. Energy dissipation is an essential mechanism to sustain the viability of plants: the physiological limits of improved photosynthesis. J. Plant Physiol. 2011;168:79–87. doi: 10.1016/j.jplph.2010.07.012 [DOI] [PubMed] [Google Scholar]
- 86.Celis-Plá PSM, Hall-Spencer JM, Horta PA, Milazzo M, Korbee N, Cornwall CE, et al. Macroalgal responses to ocean acidification depend on nutrient and light levels. Front Mar Sci. 2015;2:26 doi: 10.3389/fmars.2015.00026 [Google Scholar]
- 87.Alongi G, Catra M, Cormaci M. Observations sur Cystoseira susanensis (Cystoseiraceae, Phaeophyta): une espèce méditerranéenne rare et peu connue. Cryptogam, Algol. 1999;20(1):25–33. doi: 10.1016/S0181-1568(99)80004-X [Google Scholar]
- 88.Coudret A, Jupin H. Action de énergie lumineuse sur le métabolisme de Cystoseira elegans Sauvageau. C.R. Acad. Sci. Paris.Sér. III 1985; 301:827–832. [Google Scholar]
- 89.Baghdadli D, Tremblin G, Pellegrini M, Coudret A. Effects of environmental parameters on net photosynthesis of a free-living brown seaweed Cystoseira barbata forma repens: determination of optimal photosynthetic culture conditions. J Appl Phycol. 1990;2:281–287. doi: 10.1007/BF02179786 [Google Scholar]
- 90.Van Tamelen PG, Stekoll MS, Deysher L. Recovery. Processes of the brown alga Fucus gardneri following the ‘Exxon Valdez’ oil spill: settlement and recruitment. Mar Ecol Prog Ser, 1997;160: 265–277. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Significant effects are in bold.
(PDF)
Significant effects are in bold.
aPairwise comparisons among conditions: L+T-≠L+T+≠ L-T- = L-T+.
(PDF)
Significant effects are in bold.
aSNK test among conditions: L+T+≠L-T-≠L+T-≠L-T.
(PDF)
Significant effects are in bold.
aSNK test among substrata within condition: Cond. L+T-, T≠S; all other Cond., T = S.
bSNK test among conditions within substratum: Sub. S, (L+T+ = L-T-)≠L+T-≠L-T+; Sub. T, (L+T+ = L-T-)≠(L+T- = L-T+).
(PDF)
Condition and substratum are crossed fixed factors. Significant effects are in bold.
aPairwise comparisons among conditions: L-T-≠L+T+ = L-T+ = L+T-.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.