Abstract
High mobility group box 1 (HMGB1) is a damage-associated molecular pattern (DAMP) protein that mediates inflammatory responses after infection or injury. Previously, we reported a peptide inhibitor of HMGB1 (P5779) that acts by directly interrupting HMGB1/MD-2 binding. Here, fingerprint similarity search and docking studies suggest folic acid derived-drugs function as P5779 mimetopes. Molecular dynamic (MD) simulation studies demonstrate that folic acid mimics the binding of P5779 at the TLR4 and MD-2 intersection. In surface plasmon resonance (SPR) studies, these drugs showed direct binding to TLR4/MD-2 but not HMGB1. Furthermore, these P5779 mimetopes inhibit HMGB1 and MD-2 binding and suppress HMGB1-induced TNF release in human macrophages in the nanomolar range. We assert from our findings that their demonstrated anti-inflammatory effects may be working through TLR4-dependent signaling.
Introduction
High mobility group box 1 (HMGB1) protein has dual roles, DNA binding and cytokine-like inflammatory activity [1]. It is an essential and ubiquitous DNA architectural factor that regulates cellular processes, including transcription, chromatin remodeling, recombination and DNA repair by modulating chromosomal DNA structure. In response to infections and injuries, HMGB1 is secreted from activated immune cells or passively released from injured cells. Excessive extracellular HMGB1 contributes to the pathogenesis of infection- and injury-elicited inflammatory diseases. The disulfide HMGB1 activates immune cells to produce cytokines/chemokines via Toll-like receptor 4 (TLR4), receptor for advanced glycation endproducts (RAGE), or other receptors [2]. The extracellular HMGB1 has been established as a pathogenic mediator of both infection- and injury-elicited inflammatory diseases.
Previously we reported that HMGB1 induces inflammatory responses via the TLR4 signaling pathway and that interaction with the TLR4/MD-2 complex requires the disulfide HMGB1. By screening the HMGB1 peptide libraries, a tetramer designated P5779 (FSSE) was identified as a specific MD-2 antagonist that inhibits HMGB1/MD-2 interaction and thereby TLR4 signaling [3]. Furthermore, P5779 protected mice against hepatic ischemia/reperfusion injury, chemical toxicity and sepsis. Our current study takes an in silico insight into the interaction of P5779, as well as the inactive scramble peptides, and the TLR4/MD-2 complex, to explore the mechanism of action. To overcome the poor pharmacokinetic profiles of P5779, we applied a virtual fingerprint similarity search on Drugbank database (version 4.3) to identify P5779 mimicking small molecule drugs. A set of pteroglutamic acid analogue drugs, such as folic acid, methotrexate (MTX), etc., shared similar binding poses with P5779 on the TLR4/MD-2 complex. Direct binding studies using SPR, further illustrated these molecules bind to MD-2 and inhibit the HMGB1/MD-2 interaction. Finally, in vitro studies demonstrated the ability of these molecules to inhibit HMGB1-induced TNF release in human-derived macrophages with higher potency compared with P5779. Based on our results, we propose that folic acid (an FDA approved supplement) and related analogue drugs with good pharmacokinetic profiles, can be used as P5779 mimetics to attenuate DAMP-mediated inflammation.
Materials and methods
Reagents
P5779 peptide was custom-made from Genemed Synthesis, Inc. with more than 95% purity as determined by HPLC. Recombinant HMGB1 was expressed in E. coli and purified to homogeneity as reported previously[4]. Folic acid and analogues were purchased from Sigma-Aldrich.
Docking study
The protein preparation wizard in Maestro (Schrödinger, LLC, New York, NY, 2016) [5]was used to prepare the TLR4/MD-2 complex (PDB: 3FXI, chain A and C) structure imported from the Protein Data Bank. LPS, ions and water molecules were removed from the structure. A receptor grid, which can accommodate ligands with length up to 20Å, was generated and located in the center to cover the entire MD-2 structure by GLIDE (Schrödinger, LLC, New York, NY, 2016) [6–8]. Compounds were constructed with 2D Sketcher or imported from the DrugBank database (version 4.3). All ligands underwent energy minimization and ligand preparation using LigPrep. Extra precision (XP) mode of Glide dock [7, 8] was executed using a flexible ligand and a rigid receptor routine. At most, 10 docking poses were obtained from each molecule.
Fingerprint-based similarity search
We conducted a P5779 fingerprint-based similarity search against the DrugBank database (version 4.3) [9]. The constructed P5779 structure was used as the template. A total of 1,982 FDA approved or investigational drugs from the database was prepared by energy minimization using MMFF94x forcefield. Three fingerprint types, MACC structure key (BIT-MACC), shape (ESsape3D) and pharmacophore atom triangle (piDAPH3), were calculated against each molecule in the database. The Tanimoto coefficient [10] was applied to score compound similarity with BIT-MACCS. For ESshape3D and phDAPH3 fingerprints, the similarity scores are calculated as the inverse of the distances between two corresponding fingerprints [11]. All the hits were then subjected to docking and MM-GBSA refinement in comparison with P5779. Molecules with docking scores better than P5779 were considered as P5779 mimetic hits.
MD simulation
The ligand-protein complex from the docking study was subjected to MD simulation performed using Desmond program (Schrödinger, LLC, New York, NY, 2016) [12, 13]. The complex was solvated with the TIP3P water model [14] in a 10Å ×10Å×10Å orthorhombic box. The simulation was carried out with Optimized Potential for Liquid Simulations (OPLS) force field. The default Desmond protocol was applied to equilibrate the prepared system and was followed by a 50ns NPT simulation to equilibrate the system. The equilibrated system was saved every 5 ps of time intervals. Potential energy of the entire system was calculated. Stability of the docked complex was evaluated from their root mean square deviation (RMSD) plots. A root mean square fluctuation (RMSF) plot of the backbone atoms of each residue was also created. A snapshot of the protein-peptide complexes were generated using PyMOL (The PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC.).
Surface plasmon resonance (SPR) analysis
Biacore T200 (GE Healthcare, USA) was used for real-time binding interaction studies. Recombinant human TLR4/MD2 (R&D 3146-TM-050) complex protein and MD2 (R&D 1787-MD) protein were purchased from R&D Systems (Minneapolis, USA). For binding analyses, CM5 series chips (GE Healthcare) were activated and the reference flow-cell was blocked by 1 M ethanolamine (pH = 8.5). The ligand protein MD-2 dilution (10 ug/mL in 10 mM Acetate buffer pH 5) or HMGB1 (20 ug/mL in 10 mM acetate buffer pH = 4.5) or TLR4/MD2 complex (10 ug/mL in 10 mM acetate buffer pH 4.5) were subjected to the sample flow-cell at the flow rate of 10 uL/min until the surface Plasmon resonance reached 1000–2000 RU. Analytes (Folic acid analogues) diluted into 5 different concentrations were sequentially injected at a flow rate of 30uL/min for 60s or 120s (TLR4/MD-2). The dissociation time was set for 1 minute or 2 minutes (TLR4/MD-2). The equilibrium dissociation constant (KD) was obtained to evaluate the binding affinity by using the BIAEvaluation 2.0 software (GE Healthcare) supposing a 1:1 binding ratio. At least 3 independent experiments were performed.
For the inhibition assay, wild type HMGB1 (20 ug/mL in 10 mM Acetate buffer pH = 4.5) was immobilized onto a CM5 at a flow rate of 10 uL/min until the surface Plasmon resonance reached 400 RU. MD-2 (0.5 uM) was incubated with or without folic acid analogues (the concentrations were varied (2X) from 10uM to 312.5 nM) for 15 minutes prior to injection of the mixture on the chip at 30 uL/min for 60s. The dissociation time was set for 1 minute. Binding experiments were conducted in 1XPBS + 0.05% Tween 20 as the running buffer, and at least 3 independent experiments were performed.
Preparation of HMGB1 protein
Recombinant HMGB1 was expressed and purified as described previously [4, 15]. The cytokine-stimulating disulfide HMGB1 was characterized by LC-MS/MS [16]. HMGB1 was extracted with Triton X-114 to remove any contaminating LPS. The LPS content in HMGB1 was measured by the Chromogenic Limulus Amebocyte Lysate Assay (Lonza). The LPS content in HMGB1 protein was < 0.1 EU/mg protein as measured by the limulus assay.
Cell isolation and culture
Peripheral-blood mononuclear cells were isolated from the blood of normal anonymous volunteers (Long Island Blood Services) over a Ficoll-Hypaque (Pharmacia Biotech) density gradient. Human primary monocytes were isolated by adherence and allowed to differentiate into macrophages for 7 days in complete DMEM medium containing 10% heat-inactivated human AB serum, 100 U/mL penicillin, 100 ug/mL streptomycin, and 1 ng/mL MCSF in 96-well culture plates at 105 cells/well. All experiments were carried out in serum-free Opti-MEM I medium.
Cytokine measurement
Human primary macrophages in 96-well plates were stimulated with HMGB1 at 1 ug/mL, plus increasing amounts of P5779 (or folic acid analogues) in serum-free Opti-MEM I medium as indicated for 16h. TNF release was measured using an ELISA kit (R&D Systems, Inc) according to the manufacturer instruction.
Data analysis
For the SPR inhibition assay, analysis of covariance (ANCOVA) was used to model inhibition as a function of log (base 10) concentration and treatment group. Prior to applying ANCOVA, both the normality and homogeneity of slopes assumptions were verified. Upon finding a significant treatment group effect, Dunnett’s test was used to compare each group to P5779. For the cytokine measurement, one-way analysis of variance (ANOVA) was used to compare mean TNF levels among the 10 treatment groups (including HMGB alone serving as the control group). The normality and homogeneity of variance assumptions were verified. Upon finding a significant treatment group effect, Dunnett’s test was used to compare each group to HMGB-1 alone. A result was considered statistically significant if p < 0.05.
Results
Identification of binding site on TLR4/MD-2
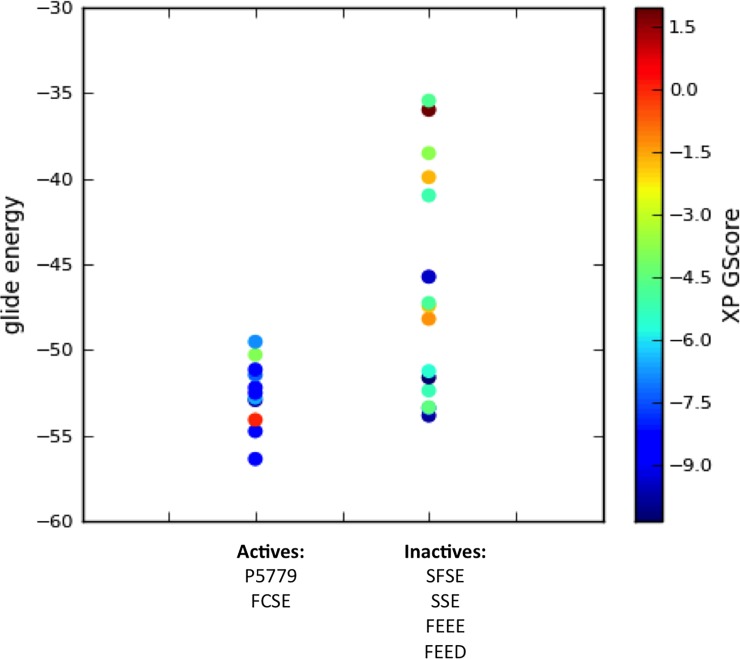
In previous studies, we reported a tetramer peptide P5779 (FSSE, the serine homologue of FC106SE on HMGB1) directly bound to the TLR4 extracellular adapter MD-2 and inhibited HMGB1-induced TNF release in human macrophages. A series of control trimer or tetramer peptides spanning the Cys106 region, including SFSE, FEED, FEEE, and SSE, showed no binding to MD-2 and were not active in vitro [3]. Here, we carried out an extra precision glide docking study with all the active and inactive peptides on the TLR4/MD-2 complex (PDB: 3FXI, chain A and C) to identify an accurate binding site. The lower score/energy indicates a better fit for the protein target (S1 Table). We observed that the active peptides (FCSE and P5779) have lower binding energy and better glide scores compared to the inactive ones (Fig 1). This plot showed a trend of correlation between the calculated binding free energy and bioactivity of these molecules, indicating the defined docking area is likely the binding site for P5779 and active molecules.
Fig 1. Docking score and glide energy plots of active and inactive tetramers on TLR4/MD-2 complex.
A trend correlation of biological activity and docking energy (active compound docks better with lower glide energy) suggests a promising bind site.
Fingerprint similarity search and structure-based screening for P5779 mimetics
To overcome the weak pharmacokinetic properties of the peptide tetramer P5779 and to enrich the HMGB1 antagonist compound catalog, we carried out a three-step similarity search followed by a structure-based docking method to screen P5779-like small molecules within the Drugbank database (version 4.3). The advantage of combining methods is to capture chemical information in different aspects and reduce the redundancy of artifacts that would be introduced by a single approach.
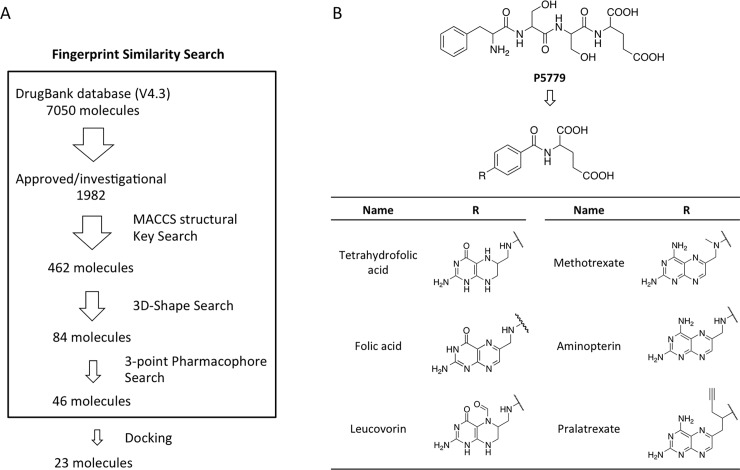
The Drugbank database covers 7,050 drug entries, including FDA approved drugs, nutraceuticals, and experimental compounds. A total of 1,982 FDA approved/investigational drugs were submitted to a three-step similarity search using three fingerprint types to examine different aspects of the consensus structure (Fig 2A)[17]. The screening gave 46 potential hits out from the initial 1,982 drugs. These molecules were subsequently docked to the binding site identified on TLR4/MD-2 complex in parallel with P5779 as a reference. Molecules with docking scores ranking below P5779 dock poses were eliminated, which yielded 23 hits. Among these hits, we found the most populated group of 6, tetrahydrofolic acid, folic acid, methotrexate (MTX), pralatrexate, aminopterin and leucovorin, shared a common 2-benzamidosuccinic acid structure and a pteridine ring system (Fig 2B). The structure similarity of the folic acid analogues and P5779 suggested they could potentially display comparable biological effects.
Fig 2. Fingerprint similarity search and structure-based screening for P5779 mimetics.
(A) Flowchart of virtual screening. (B) Structures of P5779 and the hits.
Molecular dynamic simulation of P5779 and folic acid with TLR4/MD-2
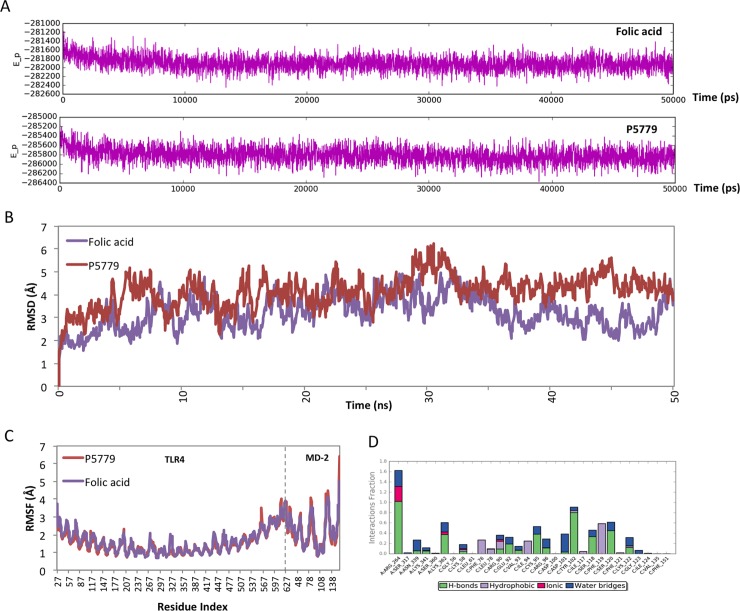
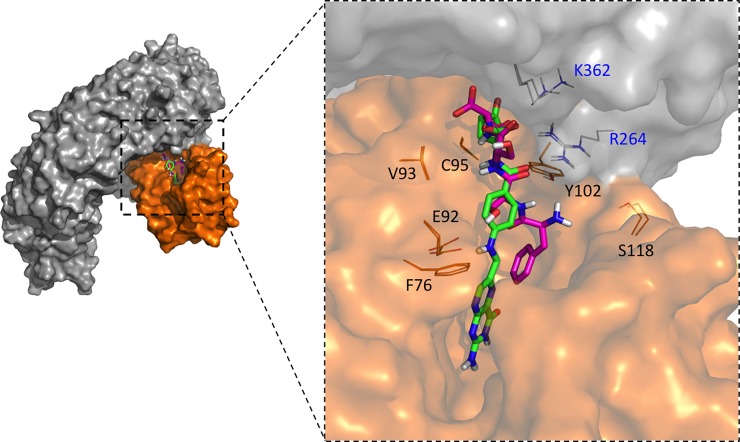
To exam the dynamic interactions of TLR4/MD-2 complex with P5779 or folic acid, we carried out 50 ns molecular dynamic simulation studies. The potential energy plot of folic acid or P5779 with TLR4/MD-2 (Fig 3A) suggested a well-equilibrated complex system through the simulation. The root mean square deviation (RMSD) during the simulation was analyzed with respect to initial structures in order to provide the structural deviation with respect to time (Fig 3B). At the equalization stage, the small deviation (< 2Å) indicated a stable binding of both molecules to TLR4/MD-2 protein complex. Furthermore, root-mean-square fluctuation (RMSF) of the protein backbone atoms from their time-average position was examined to address the high conformational arrangement area upon ligand binding. The RMSF plot showed higher fluctuations around of MD-2 and suggested that folic acid binding to TLR4/MD-2 has comparable influence on the receptor protein as P5779 binding (Fig 3C). Overlapping the binding poses showed that folic acid binds to TLR4/MD-2 complex in a similar manner as P5779. The glutamic acid group on folic acid binds to R264 and K362 on TLR4. The E92 and Y102 on MD-2 form hydrogen bonding with the amine and carbonyl group on the para-amino benzoic acid region of the folic acid (Fig 4). The protein-ligand contacts plot summarized the interactions throughout the folic acid/TLR4/MD-2 simulation. We found very stable interactions between folic acid and R264, K362 and Y102 (MD-2) during the simulation (Fig 3D).
Fig 3. MD simulation trajectory (50 ns) analysis of P5779 and folic acid with TLR4/MD-2 complex.
(A) The potential energy plots showing the relatively stable complex system. (B) RMSD plot representing small deviation along the stable region preceded by small rearrangement from the initial conformation. (C) RMSF graph representing the extent of conformational arrangement upon P5779 and folic acid binding to TLR4/MD-2 complex. (D) Protein-ligand contacts during the folic acid/TLR4/MD-2 simulation.
Fig 4. Overlap of folic acid (green) and P5779 (purple) binding poses on TLR4/MD-2.
Folic acid analogues directly bind to MD-2
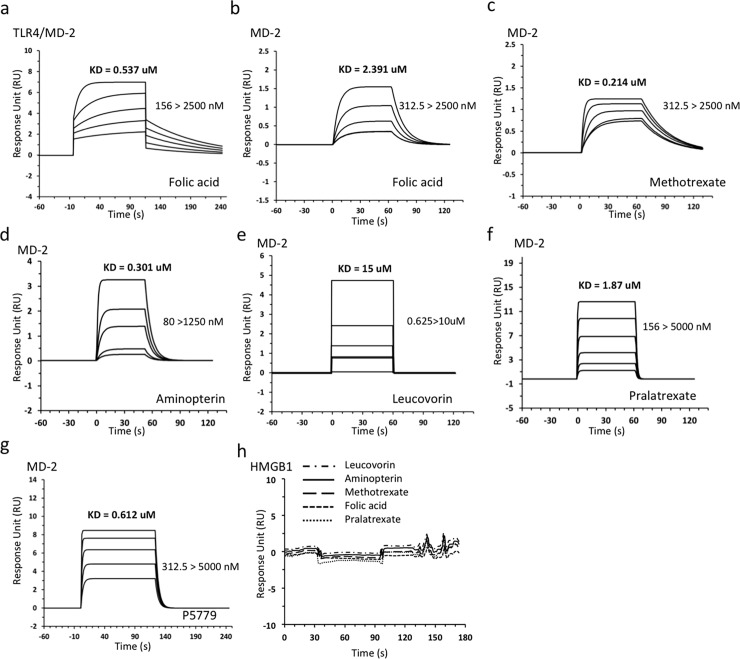
The tetramer peptide P5779 has been identified to bind to MD-2 with a KD value of approximately 0.65 μM and inhibited HMGB1/MD-2 interaction with an IC50 of 29 nM [3]. We asked whether the P5779-like folic acid analogues bind to MD-2. Real time surface plasmon resonance (SPR) analysis (Biacore T200) was employed to test the binding activity between folic acid analogues and MD-2. SPR analysis was carried out by immobilizing MD-2 on the CM5 chip as a ligand; analyzing the binding of compounds in gradient concentration as analytes. The KD value was obtained was as a measure of the binding affinity. Tetrahydrofolic acid was excluded in this experiment due to its decomposition over time. P5779 was used as a positive control. Not surprisingly, SPR analysis showed all five folic acid analogues bound directly to MD-2 protein (Fig 5B–5F). Compared to P5779 (Fig 5G), MTX and aminopterin exhibited higher binding affinity to MD-2 with the KD values of 214 nM and 301 nM, respectively (Fig 5C and 5D). Folic acid showed moderate binding to MD-2 (Fig 5B) but higher affinity to the TLR4/MD-2 complex (Fig 5A) suggesting that TLR4 contributes to the binding as well. This supports our observation with the modeling studies that P5779 binds at the TLR4/MD-2 interface. Finally, none of these analogues demonstrated binding to HMGB1 (Fig 5H).
Fig 5. SPR studies of folic acid analogues show direct binding to MD-2 of the TLR/MD-2 complex.
Folic acid analogues inhibit HMGB1/MD-2 binding
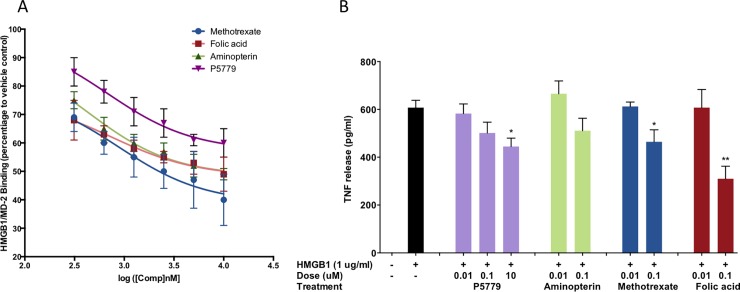
Extracellular HMGB1 activates TLR4 dependent signal by directly binding to TLR4/MD-2. We therefore interrogated whether folic acid and its analogues could interrupt the HMGB1/MD-2 interaction. An SPR inhibitory assay was designed accordingly. We immobilized HMGB1 on the CM5 chip. MTX and aminopterin with good MD-2 binding affinity, as well as the natural vitamin folic acid, were prepared in different concentrations (0–10 uM). Molecules were incubated with 0.5 μM MD-2 for 15 minutes and the mixture was subsequently subjected to SPR analysis as analytes. Inhibitory effect was plotted as the percentage inhibition compared to the control. The folic acid analogues exhibited inhibitory effects against HMGB1/MD-2 binding up to 60% in a concentration-dependent manner (Fig 6A). ANCOVA analysis showed a significant difference between groups with all three groups significantly lower than P5779 (Dunnett’s test: each p<0.0001). Altogether, we demonstrate that the P5779 structurally similar folic acid analogues can act as MD-2 antagonists and inhibit HMGB1/MD-2 binding.
Fig 6.
Folic acid, MTX, and Aminopterin inhibit HMGB1/MD-2 directly binding (A) and suppress HMGB1-induced TNF release in human macrophages (B). Experimental data are expressed as means ± S.E. for triplicate estimates of individual experiments (n = 3). A p < 0.05 was considered statistically significant. * Indicates p < 0.05; ** indicates p < 0.01.
Folic acid analogues inhibit HMGB1-induced TNF release in human macrophages
To evaluate the therapeutic potential of the folic acid analogues, we studied whether they inhibit HMGB1-induced cytokine production in vitro. Human primary macrophages were stimulated with HMGB1 at 1 μg/mL, plus increasing amounts of P5779 (or MTX, or aminopterin, or folic acid) in serum-free Opti-MEM I medium as indicated for 16h. TNF release was measured by ELISA as reported in Fig 6B. Both P5779 and the three folic acid analogues inhibited HMGB1-induced TNF release in primary human macrophages in a dose-responsive manner. Compared to tetrapeptide P5779, the folic acid and MTX exhibit a similar inhibitory effect at a lower concentration.
Discussion
The peptide tetramer P5779 was identified as an MD-2 antagonist by directly binding to the TLR4 adapter protein MD-2 and was able to consequently disrupHMGB1-TLR4/MD-2 binding and downstream effects (TNF release). P5779 protected mice against hepatic ischemia/reperfusion injury, chemical toxicity and sepsis without interfering with LPS-induced cytokine/chemokine production [3]. Thus P5779 could have a valuable therapeutic impact in attenuating DAMP-mediated inflammation while preserving antimicrobial immune responsiveness. Like most peptide therapeutics, P5779 suffers from poor pharmacokinetic properties. In the current study, an integrated in silico virtual screening found a series of P5779-mimetic FDA approved molecules, which retained the critical interactions of P5779 and TLR4/MD-2. These pteroglutamic acid analogues, including MTX, folic acid, pralatrexate, aminopterin and leucovorin, showed direct binding to TLR4/MD-2 and inhibited HMGB1/MD-2 binding in SPR studies. Finally, we establish that the top two analogues (folic acid and MTX), reduce HMGB1-induced TNF release in human macrophages.
High-doses of antifolates, such as MTX, aminopterin, or pralatrexate, act by competitively inhibiting the binding of dihydrofolate reductase (DHFR) to folate, and disrupting de novo synthesis of DNA and RNA, thereby retarding the proliferation of cancer cells [18]. Their cytotoxicity’s and side effects, such as inflammation in the digestive tract, hepatotoxicity, pulmonary damage, myelosuppression and nephrotoxicity, are well known. Interestingly, their analogue molecule folic acid has been shown to have anti-inflammatory and hepato-protective effects in an acetaminophen-induced acute liver failure model and to counteract the effect of acetaminophen [19], and is widely given in combination with MTX to decrease the toxicity.
Apparently, the therapeutic effect versus the toxicity of MTX are dose dependent. In addition to its use in cancer, MTX is also widely used for treatment of inflammatory diseases, such as rheumatoid arthritis, SLE, psoriasis and Crohn’s disease for decades, due to its beneficial immunosuppressive and anti-inflammatory effects when administered in low dosage [20]. Although not yet fully understood, it is believed MTX’s low dosage immunosuppressive effect is not through DHFR. Multiple mechanisms appear to be involved in the MTX anti-inflammatory effect, including: the inhibition of adenosine A2A receptor (A2AR) [21]; inhibition of T cell activation; selective down-regulation of B cells; increasing CD95 sensitivity; inhibition of methyltransferase activity [22]; and inhibition of the binding of interleukin 1β to its cell surface receptor (IL-1R) [23].
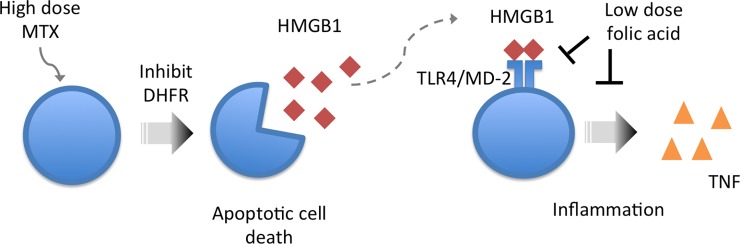
Our studies implicate an additional mechanism for the anti-inflammatory effect of the pteroglutamic acid analogues. High dose antifolate MTX and analogs are widely used as chemotherapeutic drugs, which inhibit DNA synthesis and induce apoptosis. During apoptotic cell death, the extracellular HMGB1 can be released [24] and therefore triggers HMGB1-induced inflammation, which can lead to a cascade of inflammatory responses [25]. Our observations support that the pteroglutamic acid analogues, when given in low dosage, seem to regulate HMGB1-induced inflammation through directly binding to TLR4/MD-2 and therefore inhibit HMGB1-induced inflammation (Fig 7). Other research also suggests the folic acid mediates effects through the TLR4 receptor [26]. Interestingly, a recent study hypothesized the anti-inflammatory effect of MTX is due to direct binding to HMGB1 and inhibition of HMGB1-induced TLR4 signaling. Biotinylated MTX (bio-MTX) was shown to bind to HMGB1 in SPR assays [27]. However, the modification on the molecule could interfere with its binding to the protein, although the authors argued that mmobilizing the mixture of both α and β—biotinylated MTX on the chip in SPR study compensated for the issue [27]. In contrast, our study using non-biotinylated MTX and analogues showed no binding to HMGB1, but tight binding to TLR4/MD-2. The contradictory results reveal that the binding of bio-MTX to HMGB1 might be due to a false binding of the modified molecule. Overall, we conclude that the pteroglutamic acid analogue drugs with improved pharmacokinetic properties are structurally similar to the indirect HMGB1 antagonist peptide P5779. Most noteworthy, folic acid, a B vitamin supplement with minimal toxicity and a natural substrate of DHFR, showed good inhibitory effect on HMGB1-induced TNF release in human macrophages. Furthermore, our studies suggest a new mechanism for the well-known MTX anti-inflammatory effects and are worth additional investigation.
Fig 7. Proposed mechanism of the anti-inflammatory effect of pteroglutamic acid analogues.
Supporting information
(PDF)
Acknowledgments
We gratefully acknowledge Dr. Sonya VanPatten's technical help and writing assistance. We thank Dr. Martin Lesser for conducting the statistical analysis. This work was supported by funding from the Feinstein Institute for Medical Research and National Institutes of Health (NIH) Grant 1S10OD012042-01 (former number: 1S10RR033072-01) to Y.A-A.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by funding from the Feinstein Institute for Medical Research and National Institutes of Health (NIH) Grant 1S10OD012042-01 (Former number 1S10 RR033072-01) to Y.A-A. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Czura CJ, Wang H, Tracey KJ. Dual roles for HMGB1: DNA binding and cytokine. Journal of endotoxin research. 2001;7(4):315–21. . [DOI] [PubMed] [Google Scholar]
- 2.Yang H, Antoine DJ, Andersson U, Tracey KJ. The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. Journal of leukocyte biology. 2013;93(6):865–73. doi: 10.1189/jlb.1212662 ; PubMed Central PMCID: PMC4051189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang H, Wang H, Ju Z, Ragab AA, Lundback P, Long W, et al. MD-2 is required for disulfide HMGB1-dependent TLR4 signaling. The Journal of experimental medicine. 2015;212(1):5–14. doi: 10.1084/jem.20141318 ; PubMed Central PMCID: PMC4291531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Wang H, Mason JM, Levine J, Yu M, Ulloa L, et al. Recombinant HMGB1 with cytokine-stimulating activity. Journal of immunological methods. 2004;289(1–2):211–23. doi: 10.1016/j.jim.2004.04.019 . [DOI] [PubMed] [Google Scholar]
- 5.Sastry GM, Adzhigirey M, Day T, Annabhimoju R, Sherman W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J Comput Aided Mol Des. 2013;27(3):221–34. doi: 10.1007/s10822-013-9644-8 . [DOI] [PubMed] [Google Scholar]
- 6.Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, et al. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. Journal of medicinal chemistry. 2004;47(7):1739–49. doi: 10.1021/jm0306430 . [DOI] [PubMed] [Google Scholar]
- 7.Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, et al. Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. Journal of medicinal chemistry. 2004;47(7):1750–9. doi: 10.1021/jm030644s . [DOI] [PubMed] [Google Scholar]
- 8.Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, et al. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. Journal of medicinal chemistry. 2006;49(21):6177–96. doi: 10.1021/jm051256o . [DOI] [PubMed] [Google Scholar]
- 9.Wishart DS, Knox C, Guo AC, Shrivastava S, Hassanali M, Stothard P, et al. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic acids research. 2006;34(Database issue):D668–72. doi: 10.1093/nar/gkj067 ; PubMed Central PMCID: PMC1347430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willett P, Barnard J, Downs G. Chemical Similarity Searching. J Chem Inf Comput Sci. 1998;38(6):983–96. doi: 10.1021/ci9800211 [Google Scholar]
- 11.Ballester PJ, Richards WG. Ultrafast shape recognition to search compound databases for similar molecular shapes. J Comput Chem. 2007;28(10):1711–23. doi: 10.1002/jcc.20681 . [DOI] [PubMed] [Google Scholar]
- 12.Guo Z, Mohanty U, Noehre J, Sawyer TK, Sherman W, Krilov G. Probing the alpha-helical structural stability of stapled p53 peptides: molecular dynamics simulations and analysis. Chemical biology & drug design. 2010;75(4):348–59. doi: 10.1111/j.1747-0285.2010.00951.x . [DOI] [PubMed] [Google Scholar]
- 13.Shivakumar D, Williams J, Wu Y, Damm W, Shelley J, Sherman W. Prediction of Absolute Solvation Free Energies using Molecular Dynamics Free Energy Perturbation and the OPLS Force Field. Journal of chemical theory and computation. 2010;6(5):1509–19. doi: 10.1021/ct900587b . [DOI] [PubMed] [Google Scholar]
- 14.William L. Jorgensen JC, and Jeffry D. Madura. Comparison of simple potential functions for simulating liquid water. The Journal of chemical physics. 1983;79(926). dx.doi.org/10.1063/1.445869. [Google Scholar]
- 15.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–51. . [DOI] [PubMed] [Google Scholar]
- 16.Yang H, Lundback P, Ottosson L, Erlandsson-Harris H, Venereau E, Bianchi ME, et al. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1). Molecular medicine. 2012;18:250–9. doi: 10.2119/molmed.2011.00389 ; PubMed Central PMCID: PMC3324950. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Cereto-Massague A, Ojeda MJ, Valls C, Mulero M, Garcia-Vallve S, Pujadas G. Molecular fingerprint similarity search in virtual screening. Methods. 2015;71:58–63. doi: 10.1016/j.ymeth.2014.08.005 . [DOI] [PubMed] [Google Scholar]
- 18.Gargaro AR, Soteriou A, Frenkiel TA, Bauer CJ, Birdsall B, Polshakov VI, et al. The solution structure of the complex of Lactobacillus casei dihydrofolate reductase with methotrexate. Journal of molecular biology. 1998;277(1):119–34. doi: 10.1006/jmbi.1997.1560 . [DOI] [PubMed] [Google Scholar]
- 19.Al-Sowyan NS. Efficacy and Safety of Folic Acid During Toxic Hepatits Induced by Acute Overdose of Paracetamol. International Journal of Pharmacology. 2009;5(3):208–14. [Google Scholar]
- 20.Liu J, Zhao J, Hu L, Cao Y, Huang B. Low dosages: new chemotherapeutic weapons on the battlefield of immune-related disease. Cell Mol Immunol. 2011;8(4):289–95. doi: 10.1038/cmi.2011.6 ; PubMed Central PMCID: PMCPMC4002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cronstein BN, Eberle MA, Gruber HE, Levin RI. Methotrexate inhibits neutrophil function by stimulating adenosine release from connective tissue cells. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(6):2441–5. ; PubMed Central PMCID: PMCPMC51248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wessels JA, Huizinga TW, Guchelaar HJ. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology (Oxford). 2008;47(3):249–55. doi: 10.1093/rheumatology/kem279 . [DOI] [PubMed] [Google Scholar]
- 23.Brody M, Bohm I, Bauer R. Mechanism of action of methotrexate: experimental evidence that methotrexate blocks the binding of interleukin 1 beta to the interleukin 1 receptor on target cells. Eur J Clin Chem Clin Biochem. 1993;31(10):667–74. . [DOI] [PubMed] [Google Scholar]
- 24.Bell CW, Jiang W, Reich CF, 3rd, Pisetsky DS. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol. 2006;291(6):C1318–25. doi: 10.1152/ajpcell.00616.2005 . [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Wang H, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. Journal of leukocyte biology. 2005;78(1):1–8. doi: 10.1189/jlb.1104648 . [DOI] [PubMed] [Google Scholar]
- 26.Souza AC, Tsuji T, Baranova IN, Bocharov AV, Wilkins KJ, Street JM, et al. TLR4 mutant mice are protected from renal fibrosis and chronic kidney disease progression. Physiol Rep. 2015;3(9). doi: 10.14814/phy2.12558 ; PubMed Central PMCID: PMCPMC4600397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuroiwa Y, Takakusagi Y, Kusayanagi T, Kuramochi K, Imai T, Hirayama T, et al. Identification and characterization of the direct interaction between methotrexate (MTX) and high-mobility group box 1 (HMGB1) protein. PloS one. 2013;8(5):e63073 doi: 10.1371/journal.pone.0063073 ; PubMed Central PMCID: PMCPMC3643934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.