Abstract
A novel hypothesis proposes that “cardio-cephalic neural crest (NC) syndrome,” i.e. cephalic NC including cardiac NC, contributes to the concurrent occurrence of vascular diseases in the cardio- and cerebrovascular regions. NC is a transient structure present in early embryogenesis. Cephalic NC provides mesenchymal cells to the vascular media in these regions. Concurrent cardio- and cerebrovascular lesions have been reported in PHACE syndrome, ACTA2 mutation syndrome, and less frequently in the spontaneous occlusion of the circle of Willis (so-called moyamoya disease). Cardiovascular lesions in these syndromes include coarctation of the aorta, persistent truncus arteriosus, patent ductus arteriosus, and coronary artery disease, and cerebrovascular lesions include agenesis and stenosis/occlusion of the internal carotid arteries, and moyamoya phenomenon. These concurrent vascular lesions both in the cardio- and cerebrovascular regions might be related to cephalic NC. This hypothesis, although not proven, may facilitate a better understanding of the above-mentioned NC-related vascular pathologies and lead to appropriate diagnostic and therapeutic approaches for clinicians and chart future direction for researchers.
Keywords: Neural crest, neurocristopathy, PHACE syndrome, ACTA2, moyamoya disease
Introduction
Concurrent occurrences of cardio- and cerebrovascular diseases have been reported in PHACE syndrome,1,2 and ACTA2 mutation syndrome,3,4 and less frequently in the spontaneous occlusion of the circle of Willis, also called moyamoya disease.5–7 Their concurrence could be by chance, but some cases were understandably explained by neural crest (NC) embryology, although their links remain speculative.8 NC exists transiently early in embryogenesis, and it arises from the neural folds, which develop from the most lateral part of the neural plate. NC cells are pluripotent and characteristic to vertebrates phylogenetically.9 NC provides a wide variety of cells, including pigment cells, adrenal medullary cells, bone and cartilage, connective tissues, glias and neurons of the peripheral nervous system, and smooth muscle cells (SMCs) of the cerebral, branchial, and coronary arteries, and the aorta.10 The relationships between cardio- and cerebrovascular diseases have not been considered in light of NC, and their diagnostic and therapeutic approaches are usually determined independently. The author would like to propose a novel hypothesis to explain the concurrence of cardio- and cerebrovascular diseases through cephalic NC embryology.
The hypothesis
Cephalic NC including cardiac NC contributes to the concurrent occurrence of vascular lesions in the cardio- and cerebrovascular regions in PHACE syndrome and ACTA2 mutation syndrome, and presumably in moyamoya disease.
Evaluation of the hypothesis
NC can be divided into cephalic and trunk NCs along the anterior-posterior axis of the embryo. Cephalic NC extends from the diencephalon to the fifth somites. Cardiac NC extends from the otic placodes to the third somites. Thus, cardiac NC is located within cephalic NC. Cephalic NC provides mesenchymal cells, which are called ectomesenchyme or mesenctoderm, including the SMCs and pericytes of the cerebral, branchial, coronary arteries, and the aorta. In contrast to cephalic NC, trunk NC does not provide mesenchymal cells. In fact, cephalic NC contributes to arteries of the prosencephalon (internal carotid arteries and their branches) and arteries of the face,11 aortic arches, outflow regions of the heart,12 and coronary arteries.13
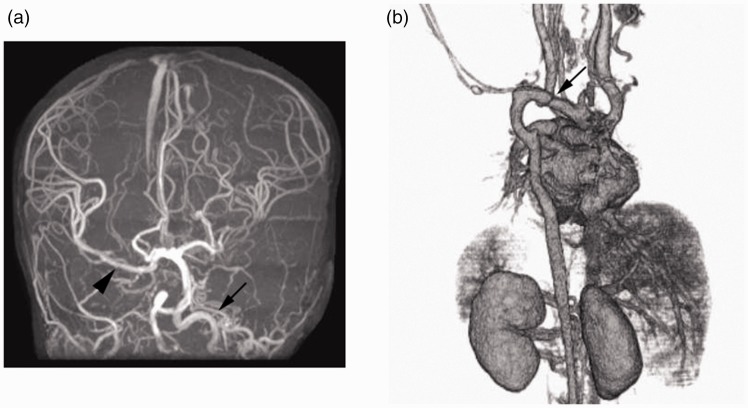
In the brain, the prosencephalon (telencephalon and diencephalon) is supplied by vessels of cephalic NC origin, while the remaining caudal brain (mesencephalon and rhombencephalon) is supplied by vessels of mesodermal origin.11 The endothelium of all the vessels in the body including the brain is of mesoderm origin. In PHACE syndrome,1,2 ACTA2 mutation syndrome,3,4 and moyamoya disease,5–7 the primitive internal carotid artery and its branches (anterior, middle, and posterior cerebral arteries) of cephalic NC origin are preferentially involved, whereas basilar artery and vertebral arteries of mesodermal origin are mostly not involved.14 This distinct distribution of the vascular pathologies is interestingly in accordance with that of cephalic NC (Figure 1).
Figure 1.
Cardio-cephalic neural crest syndrome (illustrative case).
In this 2-year-old girl with PHACE syndrome, magnetic resonance angiography of the brain ((a) frontal view) shows agenesis of the right internal carotid artery and hypogenesis of the left internal carotid artery (arrowhead), both of which are embryologically of cephalic neural crest origin. The right middle cerebral artery (arrowhead) is supplied by the vertebrobasilar system through the right posterior communicating artery. Computed tomography angiography of the cardiovascular system ((b) posterioranterior view) shows coarctation of the aorta (arrow), which is embryologically of cardiac neural crest origin.
It is historically well known that experimental manipulation (abrasion) of cardiac NC results in various anomalies of the branchial arteries, aorta and outflow region of the heart.15 Representative anomalies are persistent truncus arteriosus and double outlet right ventricle. Recently, it was proved that cephalic NC also contributes to the formation of coronary arteries.13 Although cardiovascular anomalies are classically related to cardiac NC, NC contributing to coronary arteries is located in the more rostral preotic region.13
Neurocristopathy
Canadian pediatric pathologist Bolande proposed that diseases of NC should be called “neurocristopathy” in 1974.16 It originally meant failure of correct migration or differentiation of NC cells. Neurocristopathy was further classified into dysgenetic and neoplastic forms. Dysgenetic neurocristopathy, for example, includes congenital melanotic nevi, cafe au lait spots, albinism, Pierre Robin syndrome, Treacher Collins syndrome, facial clefting syndrome, and Hirschsprung’s disease, and the neoplastic form is typified by neuroblastoma, pheochromocytoma, carotid body tumor, paraganglioma, peripheral neuroectodermal tumor, and meningioma.17 The author believes additional forms of neurocristopathy, i.e. vascular forms, can be added. Vascular diseases concurrently involving vessels of the prosencephalon and cardiovascular regions including the aorta, coronary arteries and outflow region of the heart could be regarded as vascular neurocristopathy. Since neither “dysgenetic” nor “neoplastic” is an appropriate word to express the roles of NC in PHACE syndrome, ACTA2 mutation syndrome and moyamoya disease, especially their vascular anomalies, vascular neurocristopathy seemed to be more appropriate for them. The role of NC cells in the pathogenesis of vascular anomalies in these syndromes has not been studied in detail. Thus, by putting forward vascular forms of neurocristopathy, it is clearer that NC cells are involved in these syndromes and contribute to their pathogeneses.
Neurocristopathy and PHACE syndrome
PHACE syndrome may present with cerebral arterial anomalies (agenesis of the internal carotid artery or carotid rete formation, for example) and coarctation of the aorta in addition to facial infantile hemangioma, posterior cranial fossa anomaly (Dandy-Walker cyst and/or cerebellar hypoplasia), and eye abnormality.1,2 Cerebrovascular anomalies in PHACE syndrome were found in 56% (39/70 patients): dysgenesis such as looping, ectasia, kinking (56%), narrowing (39%), absence of a normal artery (20%), persistent embryonic carotid-vertebral basilar connections such as trigeminal and stapedial arteries (20%), and abnormalities in arterial course or origin (47%).18
Cardiovascular anomalies in PHACE syndrome were found in 41% (62/150 patients): aberrant origin of a subclavian artery (21%), coarctation of aorta (19%), ventricular septal defect (13%), venous anomalies (8%), and right aortic arch (7%).19 Not well described in the literature, the basilar artery and vertebral artery are involved in PHACE syndrome less frequently. Cerebellar anomalies (Dandy-Walker cyst and hypoplasia of the cerebellar hemisphere) and anomalies of the basilar artery and vertebral artery (stenosis/occlusion and agenesis) are not directly related to the prosencephalon or cephalic NC, but they are related to rhombencephalon or adjacent mesoderm. Since the NC and neural plate share a common lineage, and NC cells develop under the inductive influence of the adjacent epithelium and possibly the mesoderm,20 extensive involvement of cephalic NC cells and their proximity to the rhombencephalon might be related to the anomalies of the cerebellum and vertebro-basilar arterial system in PHACE syndrome.
Neurocristopathy and ACTA2 mutation syndrome
ACTA2 mutation syndrome may present typically with dilatation and stenosis of the supraclinoidal portions of internal carotid arteries, moyamoya phenomenon resulting in premature stroke (defined as an age at onset less than 55 years in men and less than 60 years in women), thoracic aorta aneurysm and dissection, premature coronary artery disease (same definition as in premature stroke), and patent ductus arteriosus in addition to fixed dilated pupils and malfunction of bowel movement because the ACTA2 gene encodes the alpha-actin in the contractile SMCs.3,4,19 According to the locations of missense mutations, frequency of phenotypes, such as stroke, coronary artery disease, and thoracic aortic aneurysm and dissection symptoms, is variable.19 Among 127 carriers with ACTA2 mutation, 76 patients (60%) had thoracic arterial aneurysm and dissection, 26 patients (20.4%) had coronary artery disease, and 15 patients (11.8%) had ischemic strokes, including moyamoya disease.
Neurocristopathy and moyamoya disease
Moyamoya disease presents with stenosis/occlusion of bilateral intracranial internal carotid arteries and moyamoya phenomenon in all cases by disease definition. In this disease, the susceptibility gene of RNF213 p.R4810K variant was recently discovered.21,22 Although less frequently, there have been case reports on the concurrent occurrence of moyamoya disease and cardiovascular diseases, which included coronary artery disease,7–9 coarctation of the aorta,5,6 tetralogy of Fallot,6 ventricular septal defect,6 and aortic and mitral valve stenosis.6 Recently, Nam et al. reported that 4.6% (21 among 456 Korean patients with moyamoya disease) were found to have coronary artery disease. Their median age of 44 years old was apparently younger than that of patients with common coronary artery disease alone. They conclude that coronary artery disease might be systemic manifestation of moyamoya disease.23 Lutterman et al.6 suspected NC involvement in congenital cardiac anomalies, but they did not suspect concomitant NC involvement in moyamoya disease. It is interesting that the pathological changes of the arterial walls are SMC proliferation both in ACTA2 mutation syndrome and moyamoya disease.19,24
Incidences of cerebro- and cardiovascular involvements
As presented above, incidences of cerebrovascular involvement in PHACE syndrome, ACTA2 mutation syndrome, and moyamoya disease are 41%,18 11.8%,19 and 100%, respectively. Incidences of cardiovascular involvement in these syndromes are 56%,25 80.4% (if simply assembling the incidences of 60% in thoracic arterial aneurysm and dissection, and 20.4% in coronary artery disease),19 and 4.6%,23 respectively. Although cardiovascular presentation in moyamoya disease is low, it might be reasonably correlated to this disease.
Role of RNF213 and ACTA2 genes
Some neurocristopathy may develop after normal migration and differentiation of NC cells. For example, SMCs of the arteries that originally arose from cephalic NC may present pathological changes (steno-occlusive changes) later in life like in ACTA2 mutation syndrome and moyamoya disease.8 Although the relationship between cardio- and cerebrovascular diseases in these diseases are not well elucidated and could be coincidental, the author believes there are unproved roles of ACTA2 mutation and RNF213 variant that play a critical role for the manifestation of steno-occlusive changes of the internal carotid arteries and their branches. In fact, RNF213 p.R4810K variant is significantly related to not only steno-occlusive changes at terminal portions of the internal carotid arteries, but to coronary artery disease in the Japanese population with an odds ratio of 2.11.26
The RNF213 protein is a large protein and the RNF213 p.R4810K variant is found in 95% of patients with familial moyamoya disease and 73% of patients with solitary moyamoya disease; it is found in 1.4% of normal Asian controls.21,22 The mechanism by which the RNF213 p.R4810K variant works as a susceptibility gene for moyamoya disease in Eastern Asian population is still unknown.
To my knowledge, there has been no report on the genetic factors contributing to the formation of PHACE syndrome, but heterozygous RNF213 p.W4677L (inherited from the mother) and RNF213 p.Q469H (inherited from the father) variants were observed only in one Caucasian girl with quasi-moyamoya disease, who also had PHACE syndrome.27 These two inherited heterozygous variants in the RNF213 gene might play a role in the development of moyamoya disease, but were not directly related to PHACE syndrome. The vascular SMC-specific isoform of alfa-actin (encoded by ACTA2) is a major component of the contractile apparatus in SMCs in the arterial system throughout the body. It is suspected that the ACTA2 mutation results in increased proliferation of SMCs contributing to occlusive arterial diseases.19
Relationships between NC cells and ACTA2 and RNF213 genes mutations are strongly suspected as discussed above, but no firm data are available up to now. Proliferation of SMCs in the arterial wall is a common pathological change in ACTA2 mutation syndrome and moyamoya disease. Although somatic mutation of the RNF213 gene in moyamoya disease is not well known, heterozygous mutation was found in the surgically removed specimen in ACTA2 mutation syndrome.19 The presence of ACTA2 missense mutations might lead to increase proliferation of SMCs, and then steno-occlusive changes of the involved arteries.
Weakness of hypothesis
The weakness of this hypothesis is that there is no direct proof of NC contribution to the above-mentioned vascular diseases. Although the distribution of these vascular lesions is in accordance with that of the destinations of cephalic/cardiac NC migration, it is probably unlikely that only NC lineages are selectively involved in the formation of the NC-related syndromes without interaction with the endothelium of mesodermal origin. In fact, preferential involvement of GNAQ somatic mutation is found in the endothelium in Sturge-Weber syndrome, which is another example of cephalic NC disease.28 It is possible that non-autonomous interactions between NC and mesodermal cell lineages play important roles in the formation of the cardio-cephalic NC syndrome.
Implications of hypothesis
There have been no NC-directed studies on cerebrovascular diseases. Thus, this novel hypothesis of cardio-cephalic NC syndrome may invoke a new research field in neurocristopathy and provide better understanding and proper diagnostic and therapeutic approaches for clinicians as well as chart future direction for basic scientists.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Frieden U, Reese V, Cohen D. PHACE syndrome. The association of posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities. Arch Dermatol 1996; 132: 307–311. [DOI] [PubMed] [Google Scholar]
- 2.Metry D, Heyer G, Hess C, et al. Consensus statement on diagnostic criteria for PHACE syndrome. Pediatrics 2009; 124: 1447–1456. [DOI] [PubMed] [Google Scholar]
- 3.Khan N, Schinzel A, Shuknecht B, et al. Moyamoya angiopathy with dolichoectatic internal carotid arteries, patent ductus arteriosus and pupillary dysfunction: A new genetic syndrome? Eur Neurol 2004; 51: 72–77. [DOI] [PubMed] [Google Scholar]
- 4.Milewicz DM, Østergaad JR, Ala-Kokko LM, et al. De novo ACTA2 mutation causes a novel syndrome of multisystemic smooth muscle dysfunction. Am J Med Genet Part A 2010; 152A: 2437–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baltaxe HA, Bloch S, Mooring PK. Coarctation of the thoracic aorta associated with cerebral arterial occlusive disease. AJN Am J Roentgenol 1982; 3: 577–580. [DOI] [PubMed] [Google Scholar]
- 6.Lutterman J, Scott M, Nass R, et al. Moyamoya syndrome associated with congenital heart disease. Pediatrics 1998; 101: 57–60. [DOI] [PubMed] [Google Scholar]
- 7.Komiyama M, Nishikawa M, Yasui T, et al. Moyamoya disease and coronary artery disease. Case report. Neurol Med Chir (Tokyo) 2001; 41: 37–41. [DOI] [PubMed] [Google Scholar]
- 8.Komiyama M. Moyamoya disease is a vascular form of neurocristopathy: Disease of the embryologic cephalic neural crest. Childs Nerv Syst 2017; 33: 567–568. [DOI] [PubMed] [Google Scholar]
- 9.Gans C, Northcutt RG. Neural crest and the origin of vertebrates: A new head. Science 1983; 220: 268–274. [DOI] [PubMed] [Google Scholar]
- 10.Le Douarin NM, Kalcheim C. The neural crest, 2nd ed Cambridge, UK: Cambridge University Press, 1999. [Google Scholar]
- 11.Etchevers HC, Vincent C, Le Douarin NM, et al. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 2001; 128: 1059–1068. [DOI] [PubMed] [Google Scholar]
- 12.Kirby ML. Cardiac morphogenesis—recent research advances. Pediatr Res 1987; 21: 219–224. [DOI] [PubMed] [Google Scholar]
- 13.Arima Y, Miyagawa-Tomita S, Maeda K, et al. Preotic neural crest cells contribute to coronary artery smooth muscle involving endothelin signaling. Nat Commu 2012; 3: 1267. [DOI] [PubMed] [Google Scholar]
- 14.Komiyama M. Moyamoya disease is a progressive occlusive arteriopathy of the primitive internal carotid artery. Interv Neuroradiol 2003; 9: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to normal aorticopulmonary septation. Science 1983; 220: 1059–1061. [DOI] [PubMed] [Google Scholar]
- 16.Bolande R. The neurocristopathies: A unifying concept of disease arising in neural crest maldevelopment. Hum Pathol 1974; 5: 409–429. [DOI] [PubMed] [Google Scholar]
- 17.Bolande RP. Neurocristopathy: Its growth and development in 20 years. Pediatr Pathol Lab Med 1997; 17: 1–25. [PubMed] [Google Scholar]
- 18.Hess CP, Fullerton HJ, Metry DW, et al. Cervical and intracranial arterial anomalies in 70 patients with PHACE syndrome. AJNR Am J Neuroradiol 2010; 31: 1980–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo DC, Papke CL, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and Moyamoya disease, along with thoracic aortic disease. Am J Hum Genet 2009; 84: 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krings T, Geibprasert S, Luo CB, et al. Segmental neurovascular syndromes in children. Neuroimaging Clin N Am 2007; 17: 245–258. [DOI] [PubMed] [Google Scholar]
- 21.Kamada F, Aoki Y, Narisawa A, et al. A genome-wide association study identifies RNF213 as the first moyamoya disease gene. J Hum Genet 2011; 56: 34–40. [DOI] [PubMed] [Google Scholar]
- 22.Liu W, Morito D, Takashima S, et al. Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS One 2011; 6: e22542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nam TM, Jo KI, Yeon JY, et al. Coronary heart disease in moyamoya disease: Are they concomitant or coincidence? J Korean Med Sci 2015; 30: 470–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosoda Y. A pathological study of so-called spontaneous occlusion of the circle of Willis (cerebrovascular moyamoya disease). Folia Angiologica 1976; 24: 85–86. [Google Scholar]
- 25.Bayer ML, Frommelt PC, Blei F, et al. Congenital cardiac, aortic arch, and vascular bed anomalies in PHACE syndrome (from the International PHACE Syndrome registry). Am J Cardiol 2013; 112: 1948–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morimoto T, Mineharu Y, Ono K, et al. Significant association of rnf213 p.R4810K, a moyamoya susceptibility variant, with coronary artery disease. PLoS One 2017; 12: e0175649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schilter KF, Steiner JE, Demos W, et al. RNF213 variants in a child with PHACE syndrome and moyamoya vasculopathy. Am J Med Genet A. Epub ahead of print 7 July 2017. DOI: 10.1002/ajmg.a.38258. [DOI] [PMC free article] [PubMed]
- 28.Couto JA, Huang L, Vivero MP, et al. Endothelial cells from capillary malformations are enriched for somatic GNAQ mutations. Plast Reconstr Surg 2016; 137: 77e–82e. [DOI] [PMC free article] [PubMed] [Google Scholar]