Abstract
Orbital arteriovenous malformations (AVMs) are rare vascular lesions that may be managed with endovascular embolization followed by surgical resection. Embolization is often accomplished with n-butyl-2-cyanoacrylate (nBCA), which is considered to be a safe and effective liquid occlusive agent. Localized vascular inflammation has been associated with endovascular nBCA use in histopathologic studies, but reports of systemic hypersensitivity reactions following endovascular embolization with nBCA are rare. We present a case of a 26-year-old male who developed an intermittent systemic urticarial reaction without cardiopulmonary compromise beginning four weeks after nBCA embolization of an orbital AVM. Subsequent skin allergy testing performed by an allergist confirmed hypersensitivity to nBCA glue and the patient has since been successfully managed with daily oral antihistamines. Awareness of this rare potential complication of endovascular embolization with nBCA will aid in the counseling and management of patients with AVMs.
Keywords: AVM, cyanoacrylate, endovascular
Introduction
Arteriovenous malformations (AVMs) of the orbit are rare congenital anomalies consisting of abnormal vascular connections between arteries and veins.1 Presenting symptoms include periorbital mass, proptosis and pain.1 Exam findings include chemosis, bruit or decreased visual acuity.1 Angiography is required for definitive diagnosis and therapy.2 Though expectant management is appropriate in some cases, orbital AVMs may enlarge over time leading to exacerbation of symptoms.2,3 Treatment involves surgical resection, often accompanied by endovascular embolization given the difficulty of identifying component vessels and margins intraoperatively.3
N-buytl-2-cyanoacrylate (nBCA) is a synthetic cyanoacrylate widely used for adjuvant embolization of AVMs. This agent produces permanent vessel occlusion by polymerizing on contact with blood.1 Though nBCA is known to induce a localized chronic inflammatory response,4 few cases of systemic hypersensitivity reactions following its use have been reported. Here we present a case in which the patient developed an urticarial systemic reaction after nBCA embolization of a left-sided orbital AVM.
Written informed consent to publish information on this case and associated photographic data was provided by the patient.
Case presentation
A 26-year-old male with no known allergies was referred for evaluation of a left-sided supraorbital mass, first noted by the patient nine months prior to presentation. Examination was notable only for a bluish, non-tender mass of the left eyebrow with a palpable pulse.
Magnetic resonance imaging (MRI) demonstrated a 4 cm mass in the superior lateral margin of the left orbit. Cerebral angiogram confirmed the lesion as an AVM with feeding vessels from the left superficial temporal, internal maxillary and ophthalmic arteries.
Management of the lesion involved embolization of the left supratrochlear, superficial temporal and deep temporal artery feeding vessels with nBCA glue (Figure 1). On the following day, the bulk of the lesion was excised surgically. Both procedures were well tolerated and the patient was discharged home on the day of surgery.
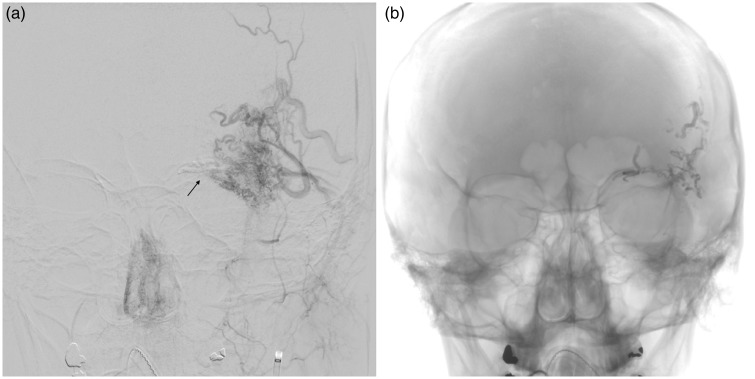
Figure 1.
Cerebral angiogram conducted on the day of embolization. (a) Anteroposterior (AP) view of the left external carotid artery showing supply to the supraorbital arteriovenous malformation from deep and superficial temporal arteries. Arrow shows glue from ophthalmic artery embolization. (b) AP view post-embolization showing glue cast in the arterial pedicles.
Two weeks postoperatively, swelling was noted in the region of the ruminant AVM, which resolved with a two-week tapering course of prednisone by mouth. Upon completion of the steroids, the patient began to note intermittent diffuse urticaria (Figure 2). He presented to the emergency department two weeks thereafter with severe diffuse urticaria that improved with diphenhydramine. On exam, the patient was normotensive and breathing comfortably with diffuse raised pruritic lesions on the bilateral upper arms, torso and back. After observation, he was discharged home.
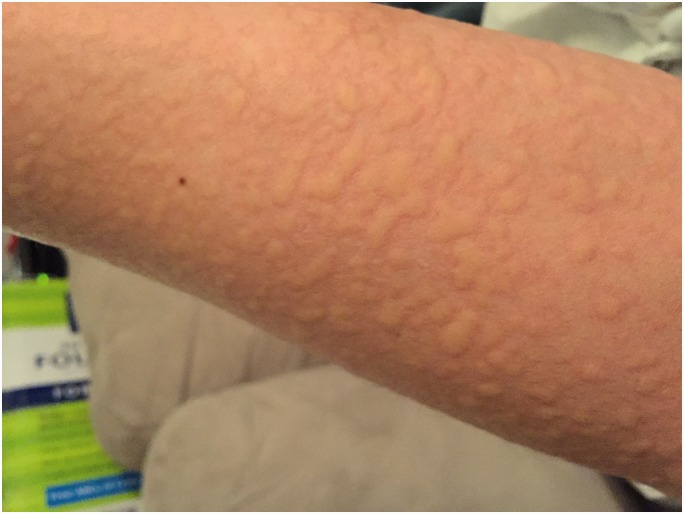
Figure 2.
Patient-provided image of urticarial reaction on the left arm four weeks after n-butyl-2-cyanoacrylate embolization.
He was referred to an allergist and skin testing revealed hypersensitivity to nBCA glue, but not to the oil or tantalum. Postoperative computed tomography scan of the orbits demonstrated residual glue in the feeders. The patient began a regimen of oral loratidine, cetirizine and ranitidine. On follow-up one week later, the patient denied any symptoms of allergic reaction since and no signs of allergy were evident on exam. He has since been stable on a regimen of daily oral loratidine and diphenhydramine as needed with exercise.
Discussion
Preferred therapy for the treatment of orbital AVMs can involve preoperative embolization followed by surgical removal of the lesion.3 Several occlusive agents are available for endovascular therapy, including particulate agents (e.g. coils and polyvinyl alcohol (PVA)), liquid sclerosants (e.g. ethanol) and liquid occlusive agents (e.g. cyanoacrylates or ethylene vinyl alcohol copolymer (Onyx)). Particulate embolic agents are rarely used for AVMs as they offer limited benefit in comparison to internal ligation;4 microscopic particulate agents are rarely able to reach the nidus of AVMs in practice; and liquid sclerosing agents are tissue-toxic.5 Thus, liquid occlusive agents such as cyanoacrylates are typically preferred for embolization of AVMs.4
nBCA is currently the most widely used cyanoacrylate for endovascular treatment of AVMs. Preparations of nBCA for endovascular use (e.g. Trufill®) contain tantalum powder (for radiopacity), and iodized oil (for radiopacity and viscosity).6
nBCA has been shown to induce a local inflammatory response within one month of use, possibly due to generation of heat and formaldehyde during polymerization.4 Quinn et al.7 presented histologic evidence of eosinophilic vasculitis in three patients treated with nBCA for cerebral AVMs, which the authors speculate could predispose to vessel rupture. However, the clinical significance of these findings remains unclear, and nBCA has been shown to result in fewer adverse clinical events compared to PVA, the previous preferred agent for endovascular embolization of AVMs.8
Although topical cyanoacrylates such as n-butyl cyanoacrylate (MediBond®) have a well-known potential to cause allergic contact dermatitis,9 reports of allergic reaction to endovascular nBCA are exceedingly rare. In a trial by Vanlangenhove et al.10 comparing nBCA versus nBCA with the co-monomer methacryloxysulfolane (nBCA-MS) for embolization of varicoceles, one out of 58 patients in the nBCA-MS group developed urticaria and hypotension requiring admission to the intensive care unit immediately after embolization. Unfortunately, no information regarding allergy testing is provided, so it is unclear whether the patient reacted to the nBCA, MS or lipiodol.10
Of note, although positive skin allergy testing against nBCA (along with radiologic evidence of residual nBCA glue in the feeder arteries) supports the conclusion that this agent was responsible for the systemic allergic reaction observed in our patient, this does not imply that candidates for endovascular embolization with nBCA should undergo preoperative skin allergy testing. It is unclear whether such testing would be positive before embolization or if skin allergy testing against nBCA would be able to distinguish between patients who are at risk of developing a systemic hypersensitivity. Finally, such a systemic allergic response to endovascular nBCA is exceedingly rare, limiting the utility of preoperative screening for nBCA allergy. Nevertheless, clinicians should be aware of the rare possibility of this potentially severe adverse event.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Smoker W, Gentry L, Yee N, et al. Vascular lesions of the orbit: More than meets the eye. Radiographics 2008; 28: 185–204;. quiz 325. [DOI] [PubMed] [Google Scholar]
- 2.Rootman J, Heran MK, Graeb DA. Vascular malformations of the orbit: Classification and the role of imaging in diagnosis and treatment strategies*. Ophthal Plast Reconstr Surg 2014; 30: 91–104. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg RA, Garcia GH, Duckwiler GR. Combined embolization and surgical treatment of arteriovenous malformation of the orbit. Am J Ophthalmol 1993; 116: 17–25. [DOI] [PubMed] [Google Scholar]
- 4.Rosen RJ, Contractor S. The use of cyanoacrylate adhesives in the management of congenital vascular malformations. Semin Intervent Radiol 2004; 21: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Odeyinde SO, Kangesu L, Badran M. Sclerotherapy for vascular malformations: Complications and a review of techniques to avoid them. J Plast Reconstr Aesthetic Surg 2013; 66: 215–223. [DOI] [PubMed] [Google Scholar]
- 6.Baugh RF, Basura GJ, Ishii LE, et al. Clinical practice guideline: Bell’s palsy. Otolaryngol Head Neck Surg 2013; 149(3 Suppl): S1–S27. [DOI] [PubMed] [Google Scholar]
- 7.Quinn JC, Mittal N, Baisre A, et al. Vascular inflammation with eosinophils after the use of n-butyl cyanoacrylate liquid embolic system. J Neurointerv Surg 2011; 3: 21–24. [DOI] [PubMed] [Google Scholar]
- 8.n-BCA Trial Investigators. N-butyl cyanoacrylate embolization of cerebral arteriovenous malformations: Results of a prospective, randomized, multi-center trial. AJNR Am J Neuroradiol 2002; 23: 748–755. [PMC free article] [PubMed] [Google Scholar]
- 9.Advanced Medical Solutions. Cyanoacrylate materials safety data sheet, http://us.liquiband.com/media/43652/cyanoacrylate_msds.pdf (accessed 26 February 2017).
- 10.Vanlangenhove P, De Keukeleire K, Everaert K, et al. Efficacy and safety of two different n-butyl-2-cyanoacrylates for the embolization of varicoceles: A prospective, randomized, blinded study. Cardiovasc Intervent Radiol 2012; 35: 598–606. [DOI] [PubMed] [Google Scholar]