Abstract
Purpose
Carotid artery anatomy is thought to influence internal carotid artery access time (ICA-AT) in patients requiring mechanical thrombectomy for acute ischemic stroke. This study investigates the association between ICA-AT and carotid anatomy.
Material and methods
Computed tomography angiography (CTA) data of 76 consecutive patients presenting with acute ischemic stroke requiring mechanical thrombectomy for middle cerebral artery or carotid T occlusion were evaluated. The supraaortic extracranial vasculature was analyzed regarding take-off angles and curvature of the affected side. Digital subtraction angiography data were primarily analyzed regarding ICA-AT and secondarily regarding recanalization time and radiographic result.
Results
ICA-AT was significantly influenced by vessel tortuosity. Take-off angle of the left common carotid artery (p = 0.001) and the brachiocephalic trunk (p = 0.002) as well as the tortuosity of the common carotid artery (p = 0.002) had highest impact on ICA-AT. For recanalization time, however, we found only the take-off angle of the left common carotid artery to be of significance (p = 0.020). There was a tendency for ICA-AT to correlate with successful (mTICI ≥ 2 b) revascularization (average time of successful results was 24.3 minutes, of unsuccessful was 35.6 minutes; p = 0.065). Every evaluated segment with less carotid tortuosity showed a carotid AT below 25 minutes.
Conclusion
Supraaortic vessel tortuosity significantly influences ICA-AT in mechanical thrombectomy for an acute large vessel. There furthermore was a trend for lower successful recanalization rates with increasing ICA-AT.
Keywords: AIS, thrombectomy, carotid anatomy, tortuosity, ICA, access time
Introduction
Several recent studies have proven the benefit of intraarterial treatment for patients with acute ischemic stroke (AIS) in the anterior circulation.1–7 Rapid access is necessary for fast and successful recanalization of occluded cerebral vessels. Normally, thrombectomy is performed via a transfemoral approach; severe carotid artery tortuosity, however, may hamper fast and thus effective catheter access. So far there are no published data analyzing the influence of supraaortic vessel tortuosity on internal carotid access time (ICA-AT).
The purpose of the presented retrospective analysis was to determine the influence of carotid tortuosity via a transfemoral approach on ICA-AT and therefore indirectly recanalization time and radiographic recanalization result.
Material and methods
A total of 105 patients presenting with AIS between December 2013 and December 2014 in need of mechanical revascularization were included in the study. Routine stroke workup included native cranial computed tomography (CT), CT angiography (CTA) and CT-perfusion. Only patients with vessel occlusion of the anterior circulation were included for the retrospective analysis. Patients with moderate or severe carotid vessel stenosis were excluded. A femoral approach was obligatory for evaluation.
For thrombectomy the following selection criteria were met: (1) symptomatic patients with no contraindication, (2) mismatch between cerebral blood flow and cerebral blood volume perfusion CT, (3) occlusion of a proximal cerebral artery (carotid-T or M1) proven on CTA and (4) clinical symptoms matching the location of the occluded vessel. General endotracheal anesthesia was used for all procedures.
CTA data were acquired using a 40-slice CT scanner (Siemens Somatom AS, Siemens AG Healthcare, Erlangen, Germany) with 80 ml intravenous contrast bolus injection (flow rate of 5.0 ml/s) followed by a 20 ml saline flush. Acquisition parameters were as follows: scan length = aortic arch to apical hemispheres, tube-current-time product = 120 mAs, tube voltage = 100 kV, temporal resolution = 0.33 seconds, slice thickness = 0.6 mm.
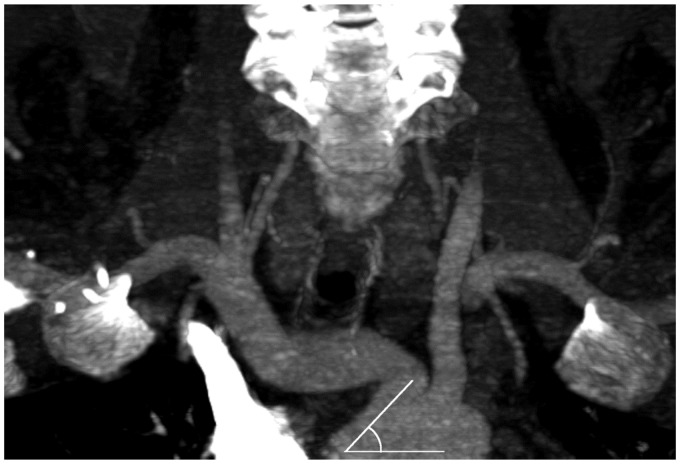
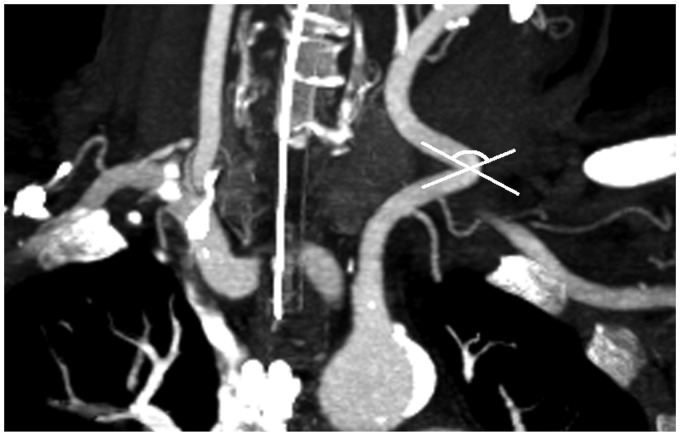
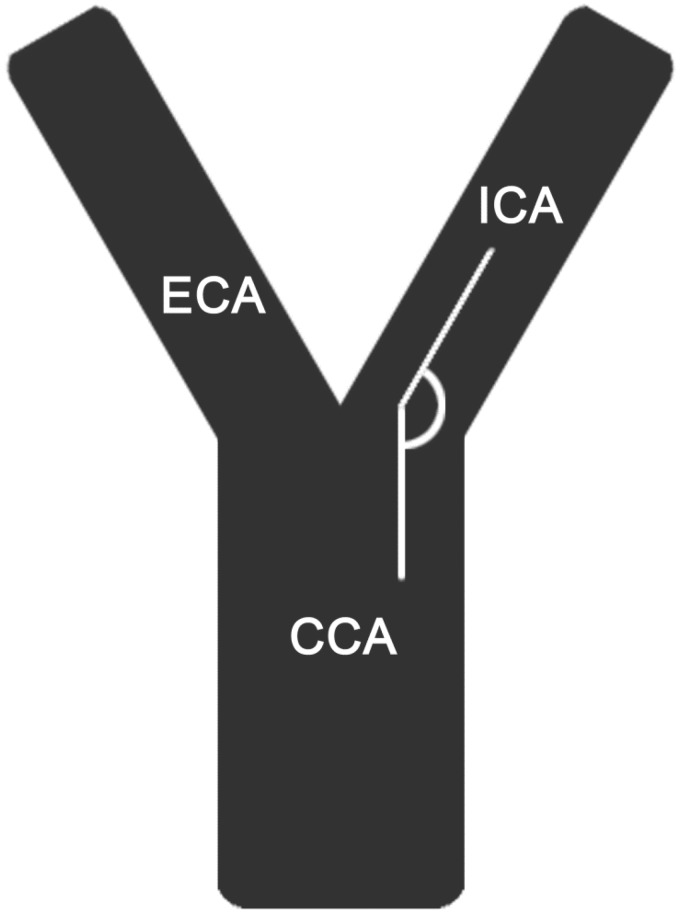
Vessel tortuosity was analyzed in CTA by a neuroradiologist with 12 years’ experience in diagnostic neuroradiology. Tortuosity was measured from the centerline of blood flow in inclined sagittal and coronal planes expressing the most relevant angle of each evaluated segment using commercially available software (iSite Pacs, Philips Healthcare, Amsterdam, the Netherlands). The following vessel parameters were evaluated: (1) take-off angle of the brachiocephalic trunk (Figure 1), (2) tortuosity of the brachiocephalic trunk including the transition segment of the common carotid artery (CCA), (3) take-off angle of the left CCA (Figure 1), (4) tortuosity of the CCA (Figure 2), (5) tortuosity of the ICA, (6) ICA-CCA angulation (Figure 3) and (7) if existing, take-off angle of the left CCA from the brachiocephalic trunk, hereafter referred to as bovine origin to the left CCA. Only the affected side of vessel occlusion was considered. A straight transition of the vessel was defined as 0 degrees and a looping of the vessel as 360 degrees.
Figure 1.
Take-off angles of the brachiocephalic trunk and common carotid artery.
Figure 2.
Tortuosity of the left common carotid artery measured from the centerline of blood flow.
Figure 3.
Take-off angle of the transition of common carotid artery and internal carotid artery.
ECA: external carotid artery.
Digital subtraction angiography (DSA) was performed using a biplane angiography system (Artis zee, Siemens Healthcare, Forchheim, Germany) for mechanical thrombectomy immediately after CT. DSA data were evaluated regarding carotid AT, i.e. time range between successful groin puncture and sufficient placement of the guiding catheter in the extracranial ICA. Time of sufficient ICA catheterization was either exactly documented by single-shot angiography or approximated by the time of the first intracranial series. Recanalization time was documented, i.e. time range between successful groin puncture and first pass after successful vessel recanalization. Success of vessel recanalization was classified by the Modified Treatment in Cerebral Ischemia (mTICI) scale. Angiographic result of mechanical thrombectomy was considered successful if the mTICI value post-treatment was rated as 2 b or higher.8
Statistical analysis
Statistical analyses were performed using SPSS (IBM, New York, USA), version 22. Spearman’s rank correlation coefficient was used to determine relations between carotid AT, recanalization time, radiographic recanalization results and vessel tortuosity. For further subanalyses calculation of a cut-off value for carotid AT based on success of recanalization (mTICI< or ≥2 b) was determined using a t-test. T-test for unpaired samples was utilized for analysis of vessel tortuosity between the two outcome groups (shorter or longer than the cut-off time value for ICA-AT). Spearman’s rank correlation coefficient was also used to determine relations between both outcome groups regarding vessel tortuosity. Statistical significance was considered at the level of p < 0.05.
Results
Seventy-six out of 105 consecutive AIS patients (70.6 ± 15.2 years) were eligible for retrospective evaluation. Mechanical thrombectomy was carried out by four neuroradiologists with each of them having at least five years of experience in endovascular stroke therapy using stent retrievers.
In most cases ICA access was primed by a sidewinder-configured catheter (70 of 76 cases = 92.1%). Only six cases were performed using a vertebralis-configured catheter. Carotid access was achieved by using either a long guiding sheath (n = 62) or a balloon-mounted guiding catheter (n = 14).
Average ICA-AT measured 26.7 (±19.6 minutes, range 4.0 to 129.0 minutes). The first successful pass was achieved at an average time of 63.3 (±38.3 minutes, range 23.0 to 184.0 minutes). Recanalization was successful (mTICI ≥ 2b) in 56 cases (73.7%).
There was a significant correlation between carotid AT and vessel tortuosity of each evaluated vessel segment. ICA-AT was most significantly influenced by the take-off angle of the left CCA (p = 0.000), followed equally by the take-off angle of the brachiocephalic trunk (p = 0.002) and the tortuosity of the CCA (p = 0.002) (Table 1). ICA-AT also significantly correlated with recanalization time (p = 0.001, n = 58) (Table 1).
Table 1.
Correlation between anatomical differences and procedure times. Correlation coefficients (Spearman) and respective p values are given below.
| ICA-AT | Recanalization time | mTICI | Age | NIHSS score | Sex correlation | |
|---|---|---|---|---|---|---|
| Take-off angle brachiocephalic trunk | −0.517 (0.002) | −0.305 (0.101) | 9.204 (9.247) | 0.062 (0.727) | 0.057 (0.756) | 0.089 (0.615) |
| Transition brachiocephalic trunk to CCA | 0.471 (0.005) | 0.151 (0.425) | 9.144 (9.418) | 0.062 (0.727) | 0.039 (0.831) | 0.301 (0.084) |
| Take-off angle left CCA | −0.601 (0.000) | −0.400 (0.026) | 9.232 (9.139) | −0.344 (0.026) | 0.225 (0.153) | −0.165 (0.297) |
| Tortuosity CCA | 0.358 (0.002) | 0.103 (0.433) | −0.129 (0.270) | 0.322 (0.005) | 0.011 (0.925) | 0.120 (0.304) |
| Tortuosity ICA | 0.292 (0.011) | 0.092 (0.484) | −0.124 (0.289) | 0.291 (0.011) | 0.095 (0.423) | 0.147 (0.207) |
| Transition CCA to ICA | −0.286 (0.013) | −0.216 (0.098) | 0.054 (0.643) | −0.096 (0.415) | 0.142 (0.230) | 0.055 (0.639) |
| Recanalization time | 0.476 (0.001) | − | − | − | − | − |
ICA-AT: internal carotid artery access time; mTICI: Modified Treatment in Cerebral Ischemia; NIHSS: National Institutes of Health Stroke Scale; CCA: common carotid artery; ICA: internal carotid artery.
Average ICA-AT was 40.8 (±21.2 minutes) in patients (n = 34) with a bovine origin to the left CCA, whereas patients with the left CCA originating directly from the aorta (n = 42) presented with an average ICA-AT of 22.5 (±14.0 minutes). Thus carotid AT was strongly significantly decreased in patients with the left CCA originating directly from the aorta (p = 0.015) (Table 2).
Table 2.
Comparison of patients with bovine origin and direct aortic origin of the left CCA.
| Bovine origin to the left CCA (n = 34) | CCA originating from the aorta (n = 42) | p value | |
|---|---|---|---|
| Age in years | 70.6 ± 16.0 | 68.7 ± 17.2 | 0.568 |
| NIHSS | 14.1 ± 8.6 | 14.5 ± 7.2 | 0.795 |
| Male sex, no. (%) | 15 (68.2) | 16 (74.0) | 0.505 |
| ICA-AT in minutes | 40.8 ± 21.2 | 22.5 ± 14.0 | 0.015 |
| Recanalization time in minutes | 56.8 ± 13.6 | 51.4 ± 29.7 | 0.728 |
CCA: common carotid artery; NIHSS: National Institutes of Health Stroke Scale; ICA-AT: internal carotid artery access time.
Likewise recanalization time significantly depended on the take-off angle of the left CCA (p = 0.020). Brachiocephalic trunk take-off angles below 100 degrees were associated with longer ICA-AT, whereas larger angles were associated with a shorter ICA-AT. Table 3 shows for each evaluated vessel segment a difference in vessel tortuosity between two groups with a carotid AT of >25 or <25 minutes. From this it can be deduced which angles lead to which times.
Table 3.
Comparison of patients requiring short and long ICA-ATs (cut-off value = 25 minutes).
| ≥25 min ICA-AT | <25 min ICA-AT | p value | |
|---|---|---|---|
| Take-off angle of brachiocephalic trunk for patients with right-sided stroke | 71.9 ± 26.0 (n = 15) | 100.4 ± 25.2 (n = 17) | 0.620 |
| Transition angle of brachiocephalic trunk to CCA for patients with right-sided stroke | 59.0 ± 50.2 (n = 15) | 18.2 ± 27.2 (n = 17) | 0.001 |
| Take-off angle of left CCA for patients with left-sided stroke | 60.1 ± 20.6 (n = 15) | 90.9 ± 19.9 (n = 26) | 0.812 |
| Tortuosity of CCA for all patients | 84.1 ± 40.4 (n = 30) | 60.6 ± 57.1 (n = 43) | 0.381 |
| Tortuosity of ICA for all patients | 104.2 ± 62.5 (n = 30) | 93.7 ± 90.5 (n = 43) | 0.088 |
| Transition from CCA and ICA for all patients | 145.4 ± 31.1 (n = 30) | 162.2 ± 25.4 (n = 43) | 0.164 |
ICA-ATs: internal carotid artery access times; CCA: common carotid artery; min: minutes.
Recanalization time was increased by 5 minutes if the left CCA originated from the brachiocephalic trunk compared to a direct take-off of the left CCA from the aorta (56.8 ± 13.6 minutes vs. 51.4 ± 29.7 minutes; p = 0.728) (Table 2).
Radiographic recanalization results in terms of successful (mTICI = 2b or 3) and unsuccessful (mTICI = 1 or 2a) showed a tendency to depend on ICA-AT (average ICA-AT of successful recanalization was 24.3 minutes, of unsuccessful recanalization was 35.6 minutes; p = 0.065) (Table 4). Thus a cut-off value regarding successful or unsuccessful recanalization could be calculated at approximately 25 minutes ICA-AT in the presented group. A total of 71% of the successful procedures were performed within 25 minutes ICA-AT.
Table 4.
Average ICA-AT and recanalization times sorted by mTICI results.
| mTICI | Average ICA-AT in minutes | Average recanalization time in minutes |
|---|---|---|
| 1 | 31.0 ± 19.1 (n = 8) | 87.5 ± 59.9 (n = 4) |
| 2a | 36.6 ± 19.6 (n = 10) | 69.6 ± 14.7 (n = 8) |
| 2b | 24.8 ± 23.6 (n = 30) | 60.2 ± 38.5 (n = 23) |
| 3 | 23.8 ± 13.1 (n = 25) | 59.9 ± 33.9 (n = 23) |
ICA-AT: internal carotid artery access time; mTICI: Modified Treatment in Cerebral Ischemia.
Table 5 shows for both the right and left hemisphere a higher mean ICA-AT for unsuccessful procedures and a lower ICA-AT for successful maneuvers. The mean ICA-AT is higher for both high and low National Institutes of Health Stroke Scale (NIHSS) scores in case of unsuccessful maneuvers and lower for successful maneuvers.
Table 5.
Average ICA-AT after adjusting for site of stroke and NIHSS score.
| mTICI < 2b | mTICI ≥ 2b | p value | |
|---|---|---|---|
| Average ICA-AT for right hemisphere (n = 33) in minutes | 29.1 ± 14.9 | 26.4 ± 25.4 | 0.814 |
| Average ICA-AT for left hemisphere (n = 43) in minutes | 36.7 ± 21.8 | 22.3 ± 12.5 | 0.009 |
| Average ICA-AT for NIHSS 15–42 (n = 29) in minutes | 28.6 ± 12.1 | 23.8 ± 27.3 | 0.475 |
| Average ICA-AT for NIHSS 0–14 (n = 36) in minutes | 37.8 ± 24.6 | 24.0 ± 13.5 | 0.004 |
ICA-AT: internal carotid artery access time; NIHSS: National Institutes of Health Stroke Scale; mTICI: Modified Treatment in Cerebral Ischemia.
Discussion
Patient data and angiographic results of the presented study are comparable to the populations and results of other thrombectomy studies.2,7 Most patients treated for AIS are older patients presenting with elongated vessels and a high variability in supraaortic vessel tortuosity.9
Age and vessel anatomy in pre-interventional CTA as well as personal preferences influenced choice of a specific catheter. Since most patients were older than 60 years and/or presented with a difficult anatomy (70 out of 76 cases), a SIM-2 configured diagnostic catheter 5F, 125 cm was used to advance the guide catheter into the ICA. Only a few cases (n = 7) presented with an easy anatomy with straight vessels and therefore a straight vertebralis-configured catheter was used.
Basically, in patients with slightly elongated vessels using a sidewinder catheter in combination with a stiff wire should be considered. Only in very young patients or patients with obviously straight vessels and easy anatomy (or when the left vertebral artery is the target vessel) should it be started with a vertebralis-configured catheter. In cases with a bovine origin to the left CCA, we thus recommend the use of a sidewinder configuration (or similar).
Faster recanalization correlates with better outcomes in AIS.10 Rapid catheterization of the ICA is crucial and may be hampered by difficult vessel anatomy.11,12
The first hurdle approaching the ICA is given by the take-off angle of the brachiocephalic trunk or the left CCA from the aorta.
In the presented evaluation the take-off angles of the brachiocephalic trunk and the left CCA from the aorta have the highest impact on carotid AT. The more pointed the departure angles are the more difficult the approach and therefore the longer the ATs are. A departure angle of the brachiocephalic trunk of <100 degrees and <60 degrees of the left CCA facilitates a fast carotid access. Carotid AT is almost doubled if the left CCA departs from the brachiocephalic trunk, i.e. bovine origin to the left CCA.
The retrospective study design and small patient number limit the accuracy of the results. A certain measurement inaccuracy needs to be taken in account for the manual measurements of the angles based on centerline blood flow by only one reader. In cases without clear documentation of the time point at final placement of the catheter tip in the ICA, the end-point of the carotid AT was documented by the time point of the first intracranial series. A certain inaccuracy of time measurements must therefore be considered.
There is a tendency to have additional increased tortuosity along the path from the femoral-iliac arteries up to the ICA in the elderly and hypertensive population. During or before the procedures no images revealing distal aortic or femoral-iliac anatomy were stored. Imaging of the aorta and femoral-iliac arteries was performed independently of thrombectomy in only a few patients. Because of the retrospective character of this study, we were not able to analyze diffuse tortuosity, which might have additionally affected outcome and procedure times.
It is important to recognize certain anatomical limitations in order to choose the right guiding materials in advance. Extremely difficult cases need to be admitted at an early stage to save time and eventually switch to alternative approaches including direct carotid puncture, which has been suggested as a bail-out strategy in some recent studies.13–16 Optimally, computer-aided simulations of cervical CTA data and fast angle measurements could be used in the future to predict the probability of successfully navigating a catheter into the ICA, and suggest the optimal coaxial catheter system, thereby helping to decide earlier if a direct ICA approach is useful. A transcervical access might be more risky; however, the presented data underline the importance of short ATs to achieve good recanalization results.
The presented data describe a cut-off value of approximately 25 minutes at which the success rate of mechanical thrombectomy clearly decreases. Furthermore, adjusting for cofounders like site of stroke and NIHSS suggests that correlation still exists. However, the correlation between ICA-AT and the success of thrombectomy need to be judged carefully since there is no information regarding onset time and total duration of the intervention in the given analysis.
In conclusion, there is a strong correlation between supraaortic vessel anatomy and ICA-AT in mechanical thrombectomy for AIS via a transfemoral approach. There was a trend toward lower successful recanalization as time to ICA access increased.
It is important to recognize and to accept extremely difficult cases at an early stage to save time and eventually switch to alternative approaches including direct carotid puncture.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Nikoubashman O, Jungbluth M, Schürmann K, et al. Neurothrombectomy in acute ischaemic stroke: A prospective single-centre study and comparison with randomized controlled trials. Eur J Neurol 2016; 23: 807–816. [DOI] [PubMed] [Google Scholar]
- 2.Berkhemmer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, Bonafe A, et al. Solitaire with the Intention for Thrombectomy as Primary Endovascular Treatment for Acute Ischemic Stroke (SWIFT PRIME) trial: Protocol for a randomized, controlled, multicenter study comparing the Solitaire revascularization device with IV tPA with IV tPA alone in acute ischemic stroke. Int J Stroke 2015; 10: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiner GM, Ducruet A. ESCAPE trial supports rapid endovascular thrombectomy in the management of large-vessel acute ischemic stroke. Neurosurgery 2015; 76: N15–N16. [DOI] [PubMed] [Google Scholar]
- 6.Molina CA, Chamorro A, Rovira À, et al. REVASCAT: A randomized trial of revascularization with SOLITAIRE FR device vs. best medical therapy in the treatment of acute stroke due to anterior circulation large vessel occlusion presenting within eight–hours of symptom onset. Int J Stroke 2015; 10: 619–626. [DOI] [PubMed] [Google Scholar]
- 7.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 8.Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: A consensus statement. Stroke 2013; 44: 2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res 2017; 120: 472–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz ML, Yeatts SD, Tomsick TA, et al. Recanalization and angiographic reperfusion are both associated with a favorable clinical outcome in the IMS III Trial. Interv Neurol 2016; 5: 118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin SC, Trocciola SM, Rhee J, et al. Analysis of anatomic factors and age in patients undergoing carotid angioplasty and stenting. Ann Vase Surg 2005; 19: 798–804. [DOI] [PubMed] [Google Scholar]
- 12.Naggara O, Touzé E, Beyssen B, et al. Anatomical and technical factors associated with stroke or death during carotid angioplasty and stenting. Stroke 2011; 42: 380–388. [DOI] [PubMed] [Google Scholar]
- 13.Wiesmann M, Kalder J, Reich A, et al. Feasibility of combined surgical and endovascular carotid access for interventional treatment of ischemic stroke. J Neurointerv Surg 2016; 8: 571–575. [DOI] [PubMed] [Google Scholar]
- 14.Mokin M, Snyder KV, Levy EI, et al. Direct carotid artery puncture access for endovascular treatment of acute ischemic stroke: Technical aspects, advantages, and limitations. J Neurointerv Surg 2015; 7: 108–113. [DOI] [PubMed] [Google Scholar]
- 15.Jadhav AP, Ribo M, Grandhi R, et al. Transcervical access in acute ischemic stroke. J Neurointerv Surg 2014; 6: 652–657. [DOI] [PubMed] [Google Scholar]
- 16.Castaño C, Remollo S, García MR, et al. Mechanical thrombectomy with ‘ADAPT’ technique by transcervical access in acute ischemic stroke. Neuroradiol J 2015; 28: 617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]