Abstract
Background
Cerebral vasospasm (CV) is a major cause of delayed morbidity and mortality in patients with subarachnoid hemorrhage (SAH). Various cerebral protectants have been tested in patients with aneurysmal SAH. We aimed to research the success rate of treatment of CV via intra-arterial milrinone injection and aggressive pharmacological therapy for refractory CV.
Methods
A total of 25 consecutive patients who received intra-arterial milrinone and nimodipine treatment for CV following SAH between 2014 and 2017 were included in the study. Patients who underwent surgical clipping were excluded. Refractory vasospasm was defined as patients with CV refractory to therapies requiring ≥3 endovascular interventions. Overall, six patients had refractory CV. Long-term neurological outcome was assessed 6–18 months after SAH using a modified Rankin score and Barthel index.
Results
The median modified Rankin scores were 1 (min: 0, max: 3) and Barthel index scores were 85 (min: 70, max: 100) From each vasospastic territory maximal 10–16 mg milrinone was given to patients; a maximum of 24 mg milrinone was given to each patient in a session and a maximum of 42 mg milrinone was given to a patient in a day. Both milrinone and nimodipine were given to three patients. There was a large vessel diameter increase after milrinone and nimodipine injections. No patient died due to CV; only one patient had motor dysfunction on the right lower extremity.
Conclusion
Higher doses of milrinone can be used effectively to control refractory CV. For exceptional patients with refractory CV, high dose intra-arterial nimodipine and milrinone infusion can be used as a rescue therapy.
Keywords: Endovascular therapy, refractory cerebral vasospasm, intra-arterial milrinone infusion, subarachnoid hemorrhage
Introduction
Cerebral vasospasm (CV) is a major cause of delayed morbidity and mortality in patients with subarachnoid hemorrhage (SAH). Approximately two thirds of patients with aneurysmal SAH demonstrate angiographic vasospasm, and up to half of these patients demonstrate neurological deterioration secondary to delayed ischemia. Due to improvements in surgical and endovascular management of ruptured intracranial aneurysms, delayed ischemia is now a leading cause of death and disability in patients with aneurysmal SAH.1 Prevention or effective treatment of these secondary events after initial bleed could considerably reduce mortality and morbidity related to SAH. Various cerebral protectants have been tested in patients with aneurysmal SAH, but only calcium channel blockers have demonstrated a beneficial effect on outcome, especially oral nimodipine.2
The management of CV is based on endovascular techniques, such as pharmacological angioplasty, using intra-arterial papaverine or mechanical angioplasty using a balloon to dilate the stenotic vessels. However, the results of these therapies are neither always satisfactory nor free from adverse effects. Recently, new therapeutic options have appeared, like statins, milrinone, nicardipine, verapamil and fasudil, magnesium sulfate, antiplatelet or anticoagulant agents, and cisternal irrigation with thrombolytics, all of them with encouraging results but still under investigation.3
In this study, we chose to assess the efficacy and safety of intra-arterial milrinone treatment for CV; the efficacy and safety of higher dose intra-arterial milrinone and combined milrinone-nimodipine infusion for refractory CV.
Materials and methods
We retrospectively analyzed the medical records of all consecutive patients who received intra-arterial milrinone, and nimotop treatment for CV following SAH between 2014 and 2017. We also assessed the effects of these agents on CV and refractory CV. Informed consent was signed by a direct family member. Inclusion criteria were as follows: SAH confirmed by computed tomography, aneurysm confirmed by digital subtraction angiography (DSA) (Artis Zee, Siemens Germany), and symptomatic CV. Patients with traumatic SAH were excluded. Aneurysms were excluded during the first 72 hours after admission. Patients with cerebral aneurysms who underwent surgical clipping were excluded. All patients underwent endovascular treatment for their cerebral aneurysm under general anesthesia within 24 hours after admission. The following standard protocol of care was applied to all patients on admission: oral nimodipine (360 mg/d) was given orally or in the gastric tube if patients remained ventilated; anti-edema treatment was applied; analgesic treatment consisted of intravenous (IV) paracetamol (4 g/d) and IV or subcutaneous opioids; anti-epileptic treatment if required, and IV magnesium sulfate was given to prevent hypomagnesemia if there was a hypomagnesemia. Anti-edema treatment, mannitol, was administered at a dose of 0.25 g/kg/d divided into four equal doses. Dexamethasone was administered at a dose of 16 mg/day again divided into four equal doses. Anti-edema therapy was ceased by reducing one of the doses in every 3 days if there was no progression on control tomography. Patients received fluids that normal saline 30–40 ml/kg/d. Normovolemia and systolic arterial blood pressure values of 140–180 mmHg were maintained. When mean arterial pressure was <90 mmHg, norepinephrine infusion was started using incremental doses until the 90 mmHg threshold was reached. Blood glucose levels were managed by a protocol in order to maintain glucose levels between 80–150 mg/dl. After aneurysms were secured, hematomas were surgically removed. All patients received dalteparin 5000 IU subcutaneously starting on day 2 after securing the aneurysms. If required, cerebrospinal fluid drainage was made via lumbar drainage catheter or lumbar puncture. All patients with SAH were followed up in intensive care for 14 days.
A total of 250 patients with aneurysmal SAH were admitted to our hospital between 2014 and 2017; 50 patients were treated surgically and 200 patients were treated endovascularly. Aneurysms were treated during the first 24 h after admission. The neurological status at admission was assessed by means of the World Federation of Neuro Surgeons (WFNS) and Glasgow Coma Score (GCS). The extent of the SAH was graded according to the Fisher scale. Long-term neurological outcome was assessed 6–18 months after SAH using modified Rankin score (Table1) and Barthel index (0–100 points)
The diagnosis of symptomatic CV was based on the clinical course, such as headache, decrease in the level of consciousness, three levels or more reduction in GCS values, and focal neurological deficit not explained by other causes. GCS values of all patients were evaluated by anesthesiologists every 2 hours. Overall, 25 patients with symptomatic CV eventually underwent DSA to confirm and assess the severity of vasospasm. CV was defined as a reduction in vessel diameter greater than 40% with respect to admission diameter. The magnitude of reduction was used to grade the severity of CV as moderate (40–60%) or severe (≥60%). The severity of CV was assessed by comparing the diameter of arteries at the point of maximum narrowing in the recent angiographic study with their diameter at the same corresponding point in the baseline angiographic study, performed at the time of admission. Measurements were performed by two radiologists. They reviewed the images together and they reached a consensus for measurements. And the other parameters such as modified Rankin score, GCS, WFNS scores, Barthel index were evaluated and recorded by anesthesiologists.
Table 1.
Modified Rankin score.
| Score | Description |
|---|---|
| 0 | No symptoms at all |
| 1 | No significant disability despite symptoms; able to carry out all usual duties and activities |
| 2 | Slight disability; unable to carry out all previous activities, but able to look after own affairs without assistance |
| 3 | Moderate disability; requiring some help, but able to walk without assistance |
| 4 | Moderately severe disability; unable to walk without assistance and unable to attend to own bodily needs without assistance |
| 5 | Severe disability; bedridden, incontinent and requiring constant nursing care and attention |
| 6 | Dead |
CV was divided into proximal and distal. The supraclinoid segment of the internal carotid artery (ICA), M1 segment of middle cerebral artery (MCA), A1 segment of anterior cerebral artery, basilar artery, and P1 segment of the posterior cerebral artery were involved in proximal CV. The distal CV included involvement distal to these arteries.
Refractory vasospasm was defined patients with CV refractory to therapies requiring ≥3 endovascular interventions during the course of treatment following aneurismal SAH. Patients with refractory CV were named patient one, patient two, and patient three, etc.
Patients who developed CV themselves or next of kin when neurological status was altered, were informed of the procedure. Patients with CV were treated openly with intra-arterial vasodilators, such as milrinone only or combined milrinone and nimodipine. Additionally, one patient was treated with IV milrinone infusion. Blood pressure, potassium levels, heart rate were under control while the vasodilators agents were infused. Vasodilator agents were administered through a 5-French diagnostic catheter (Terumo®-Europe, Leuven, Belgium) positioned in the cervical segment of the ICA or dominant vertebral artery. Milrinone was mixed with normal saline to a concentration of 0.1 mg/ml and was administered within 4 minutes. As the standard treatment of CV in our center, milrinone was administered at a total of 6 mg for each territory, totaling 18 mg. After giving 4 mg milrinone, generally we performed a control angiography. Drug doses were set for all patients with CV according to the control angiograms. If there was a refractory CV, higher dose milrinone was usually given (total 24 mg). If refractory CV was only one vascular territory, then 8–16 mg milrinone was given from ipsilateral ICA. Generally, after our endovascular procedure, CV was reduced but some patients had CV more than once during the day; we also repeated the procedure each time. If there was not enough increase in vessel diameter despite using high dose milrinone, nimodipine was injected after injection of the milrinone. Nimodipine was given at 5 mg in 20 ml saline over 5–10 minutes.
Parameters collected included the following clinical data and radiological findings: sex, age, WFNS score, Fisher score, aneurysm location, angiographic characteristics of CV, arterial diameter before and after intra-arterial vasodilators, number of CV recurrences, modified Rankin score, and Barthel index.
Statistics
All analyses were done using the IBM SPSS statistics 21 (SPSS for Windows 9.0, Chicago, IL, USA) software package. Continuous variables were presented as mean (standard deviation (SD)), whereas categorical variables were presented as frequencies. In order to test whether the data were normally distributed, the Kolmogorov–Smirnov test was applied. A p-value of <0.05 was considered significant.
Results
During 4 years, 200 cerebral aneurysms were treated in our interventional radiology unit. A total of 25 patients received intra-arterial milrinone and nimotop treatment for CV following SAH and endovascular interventions were performed 50 times in these patients. Overall, 7 patients were male and 18 patients were female. The mean age of patients with CV was 59.32 ± 9.82 years (mean ± SD). Demographic properties and clinical scores of patients are shown in Table 2. There was a large vessel diameter increase after milrinone and combined milrinone-nimodipine injections (Figure 1(a) and (b)). Table 3 shows the diameters of vessels before and after infusion. Long-term neurological outcome was assessed 6–18 months after SAH using modified Rankin score and Barthel index. The median modified Rankin scores were 1 (min: 0, max: 3) and Barthel index scores were 85 (min: 70, max: 100). One patient died because of infection in intensive care.
Table 2.
Demographic properties and clinical scores of patients.
| Patient number | 25 |
| Sex | |
| Patients with CV | 18 female, 7 male |
| Patients with refractory CV | 4 female, 2 male |
| Age * | |
| Patients with CV | 59.32 ± 9.82 |
| Patients with refractory CV | 57 ± 8.57 |
| Aneurysm localization | |
| middle cerebral artery bifurcation | 8 (32%) |
| internal carotid artery | 6 (24%) |
| anterior communicating artery | 10 (40%) |
| posterior inferior cerebellar artery | 1 (4%) |
| Mean days from SAH to CV * | 7.92 ± 3.46 |
| Barthel index scores ** | |
| Patients with CV | 85 (min: 70, max: 100) |
| Patients with refractory CV | 90 (min: 80, max: 100) |
| modified Rankin scores ** | |
| Patients with CV | 1 (min: 0, max: 3) |
| Patients with refractory CV | 1 (min: 0, max: 2) |
| Average WFNS scores ** | |
| Patients with CV | 3 (min: 1, max: 4) |
| Patients with refractory CV | 3 (min: 2, max: 4) |
| Average Glasgow scores ** | |
| Patients with CV | 13 (min: 10, max: 15) |
| Patients with refractory CV | 13 (min: 12, max: 14) |
| Fisher Scores ** | |
| Patients with CV | 3 (min: 2, max: 4) |
| Patients with refractory CV | 3 (min: 3, max: 4) |
Values are means ± standard deviation
Values are median levels and minimum–maximum levels
CV: cerebral vasospasm; SAH: subarachnoid hemorrhage; WFNS: World Federation of Neuro Surgeons.
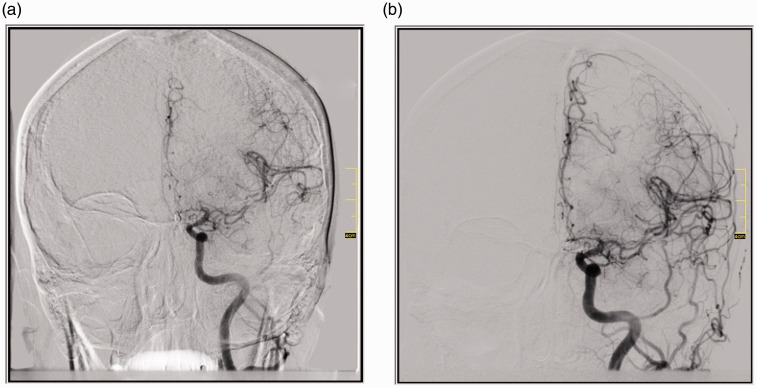
Figure 1.
Patient with ruptured anterior communicating artery aneurysm. (a) Cerebral vasospasm on A1, A2, A3 segments of the anterior cerebral artery and M1, M2 and distal segments of the middle cerebral artery; (b) After the infusion there was a large vessel diameter increase on territories with vasospasm.
Table 3.
Diameter of vessels before and after infusion.
| Anterior cerebral artery | Middle cerebral artery | Vertebral artery | |
|---|---|---|---|
| Patients with CV; Diameter of vessels before the infusion | 1.41 ± 0.43 | 2.16 ± 0.49 | 0.96 ± 0.35 |
| Patients with CV; Diameter of vessels after the infusion | 2.19 ± 0.51 | 2.95 ± 0.45 | 1.96 ± 0.61 |
| Patients with refractory CV; Diameter of vessels before the infusion | 1.56 ± 0.49 | 2.19 ± 0.50 | 0.96 ± 0.35 |
| Patients with refractory CV; Diameter of vessels after the infusion | 2.42 ± 0.51 | 2.98 ± 0.52 | 1.96 ± 0.61 |
| p-value | <0.001 | <0.001 | 0.006 |
CV: cerebral vasospasm.
A total of six patients had refractory CV; four were female and two were male. The mean age of patients with refractory CV was 57 ± 8.57 years. A total of 29 endovascular interventions were performed. A total of 44 vasospastic territories were treated. Overall, 3 patients underwent the endovascular procedure 3 times; 1 patient 4 times, 1 patient 5 times and 1 patient 11 times. There was a large vessel diameter increase after milrinone and combined milrinone-nimodipine injections (Figure 2(a) and 2(b); Table 3). Long-term neurological outcome was assessed 6–18 months after SAH using modified Rankin score and Barthel index (Table 4).
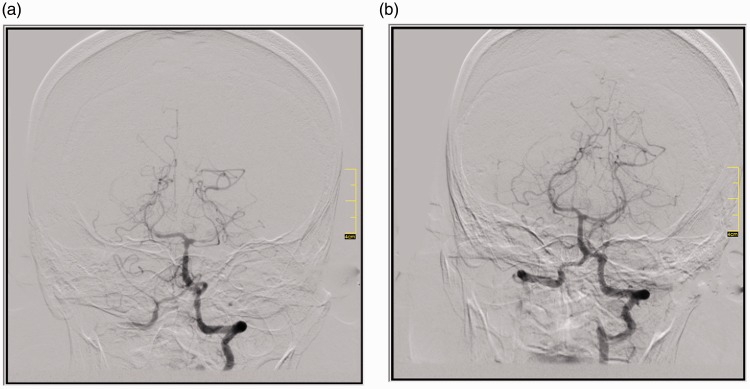
Figure 2.
Patient with ruptured posterior inferior cerebellar artery aneurysm. (a) Cerebral vasospasm on P1 and distal segments of the left posterior cerebral artery and the distal segments of right posterior cerebral artery; (b) There was a large vessel diameter increase on vasospastic territories after infusion.
Table 4.
Patients with refractory cerebral vasospasm.
| Patient | Days from SAH to CV | İntervention number | Affected territory numbers | Vasodilator agent | Maximum milrinone dose for all affected territories | Glasgow Scale | WFNS | Fisher score | Modified Rankin score | Barthel Index |
|---|---|---|---|---|---|---|---|---|---|---|
| One | 5, 6, 8 | 3 | one | Milrinone | 14 mg | 14 | 3 | 3 | 1 | 90 |
| Two | 9, 10, 11 | 3 | three | Milrinone | 24 mg | 12 | 4 | 4 | 2 | 85 |
| Three | 8, 9, 10 | 4 | one | Milrinone | 22 mg | 13 | 2 | 3 | 0 | 100 |
| Nimodipine | ||||||||||
| Four | 5, 7, 12, 13, 17 | 5 | one | Milrinone | 14 mg | 12 | 4 | 3 | 2 | 80 |
| Five | 10, 11, 12 | 3 | three | Milrinone | 18 mg | 13 | 3 | 3 | 0 | 100 |
| Nimodipine | ||||||||||
| Six | 3, 4, 5, 6, 8, 9, 11, 12, 13 | 11 | three | Milrinone | 24 mg | 13 | 3 | 3 | 2 | 85 |
| Nimodipine |
CV: cerebral vasospasm; SAH: subarachnoid hemorrhage; WFNS: World Federation of Neuro Surgeons.
Patient one had a CV on days 5, 6, and 8 of SAH, although standard protocol treatment to prevent CV was performed. She had CV on one territory. A total of 8 mg milrinone was given on day 5 from the right ICA, 10 mg milrinone was given on day 6 and 14 mg milrinone was given on day 8.
Patient two had a CV on days 9–11 of SAH. She had CV on two territories on days 9 and 10 and three territories on day 11. Then 6 mg milrinone was given from each ICA (total 12 mg) on day 9. A dose of 10 mg milrinone was given from each ICA (total 20 mg) on day 10. Then 10 mg milrinone was given from right ICA, 8 mg from left ICA and 6 mg from vertebral artery (total 24 mg) on day 11.
Patient three had CV on one territory for four times; two were on day 8 and the others days 9 and 10 of SAH. At the first intervention on day 8, 10 mg milrinone and 5 mg nimodipine were given from right ICA. Then 12 mg milrinone (total 22 mg in 1 day) and 5 mg nimodipine were given at a second intervention. A dose of 12 mg milrinone was given on day 9 from right ICA. A total of 14 mg milrinone and 5 mg nimodipine were given from right ICA on day 10.
Patient four had CV on one territory for five times on days 5, 7, 12, 13, and 17 of SAH. A total of 8 mg milrinone was given on days 5 and 7. Then 10 mg milrinone was given on day 12, 14 mg milrinone was given on day 13 and 12 mg milrinone was given on day17. Then she had no CV but she had hydrocephalus and a third ventriculostomy operation was performed on the patient.
Patient five had CV on days 10–12 of SAH. She had CV on three territories on day 10, two territories on day 11 and one territory on day 12. Then 6 mg milrinone was given from each ICA and left vertebral artery (total 18 mg) on day 10. On day 11, 10 mg milrinone was given from left ICA and 6 mg from left vertebral artery (total 16 mg). A total of 12 mg milrinone and 5 mg nimodipine was given on day 12 from left ICA.
Patient six had CV on different territories for 11 times on days 3–6, 8, 9, 11–13 of SAH. He had CV two times in a day on days 4 and 13. A total of 6 mg milrinone was injected from each ICA (total 12 mg) on day 3. On day 4, 8 mg milrinone was injected from each ICA (total 16 mg) and 7 hours later he had a second CV in a day, then 10 mg milrinone was injected from each ICA (total 20 mg for that intervention and total 36 mg in a day). Then 10 mg milrinone was injected from each ICA on days 5, 6, 8, 9, 11, and 12 (total 20 mg). A dose of 5 mg nimodipine was given on some sessions. At the same time, milrinone was injected via (IV) on days 10–12 continuously. On day 13, 10 mg milrinone was injected from left ICA and 8 mg from vertebral artery (total 18 mg) and 6 hours later he had a second CV in a day, then 12 mg milrinone was injected from each ICA (total 24 mg for that intervention and total 42 mg in a day). Then, he had no CV. He was discharged with only motor dysfunction on right low extremity on day 23.
No patients died because of refractory CV. Only, patient six had a permanent alteration on one extremity.
Discussion
CV remains the major cause of secondary brain damage after SAH and is a major contributor to mortality and morbidity besides the initial impact of bleeding.4 Angiographic vasospasm may occur in up to 70% of patients, which is typically observed between 5 and 14 days after the onset of SAH;5 however, symptomatic vasospasm may only occur in about 30% of patients. Nimodipine has been shown to improve neurologic outcome and decrease the incidence of CV.6
Recently, balloon and chemical angioplasty with super selective intra-arterial injection of vasodilators has emerged as the primary intervention for treating medically refractory ischemia from cerebral vasospasm and in many centers is being used as a first-line treatment or even prophylactically.6 Balloon angioplasty is very effective to reverse CV, but this procedure can generate physical damage to cerebral arteries. The use of balloon angioplasty is limited by the localization of CV, because smaller distal arteries are not accessible to angioplasty. This technique may be complementary with chemical angioplasty. In our study, balloon angioplasty was performed only one patient (CV on supra-clinoid ICA). All of patients had also CV on distal arteries; some of them had CV on proximal and distal arteries.
Papaverine and nimodipine were the most used agents for chemical angioplasty via intra-arterial infusion. Milrinone, nicardipine, verapamil and fasudil were the other agents for chemical angioplasty.7 The most widely studied intra-arterial vasodilator, papaverine, is an opium alkaloid. But no outcome improvement could be demonstrated in comparison with standard medical treatment. Moreover, papaverine is associated with serious neurotoxic side effects including: brain stem depression, increased intracranial pressure, mydriasis, neurological deficits, seizures, and coma possibly related to precipitation of papaverine hydrochloride crystals.8 For these side effects we did not use papaverine for the treatment of CV. Nimodipine is a dihydropyridine agent thought to inhibit voltage-gated calcium channels in the arterial wall smooth muscle cells and results in vasodilatation with much longer half-life than papaverine. It is the only United States Food and Drug Administration-approved oral agent for vasospasm. The terminal elimination half-life is approximately 8–9 hours but earlier elimination rates are much more rapid, equivalent to a half-life of 1–2 hours; a consequence is the need for frequent dosing.9 Intra-arterial nimodipine infusion is well studied than other calcium channel blockers (CCBs) for CV. Biondi et al. used intra-arterial nimodipine for treatment of CV and found angiographic improvement in 43% of patients and clinical improvement in 76% of patients.1 Other studies have demonstrated that, although nimodipine may not positively affect trans-cranial Doppler velocities, its use may still effect improvements in neurological outcome. It is possible that nimodipine exerts neuroprotective effects not completely accounted for by cerebral vasodilation. These ancillary effects may be due to direct neuroprotective properties induced by blockage of free radical attack on the intraneuronal mitochondria, an improvement of carbon dioxide reactivity and cerebral oxygen metabolism, or a reduction of tissue damage caused by calcium overload at reperfusion.1 Notwithstanding these observations, the intra-arterial use of nimodipine for CV remains uncommon.10 We did not choose the intra-arterial nimodipine routinely to treat CV. For exceptional patients with refractory CV we used high dose combined milrinone and nimodipine infusion.
Intra-arterial nicardipine is most commonly used in the treatment of CV after SAH, which also brings various complications, including pulmonary edema, prolonged hypotension and renal failure. Interestingly, given those complications caused by nicardipine and the results of many studies that nicardipine does not improve poor outcomes of CV, its use in clinical practice is controversial. It should be cautioned to use intra-arterial nicardipine as the treatment of vasospasm, and physicians should be ready to manage the potential severe adverse effects.11 We did not use nicardipine for CV treatment. Verapamil has been used to treat coronary vasospasm in a long time according to the literature. Its use in the treatment of refractory coronary spasm is safe and effective, which is also advantageous in availability and low price.12 Like nimodipine, the CCB verapamil also blocks voltage-gated calcium inflow into the smooth muscle cells of the artery. Although verapamil is a CCB, it is not selective to cerebral vasculature.13 Fasudil hydrochloride is a Rho kinase inhibitor, which has an inhibitory effect on protein phosphorylation. Fasudil is attributed to having a unique and effective anti-CV effect without significantly lowering blood pressure. Preoperatively prophylactic use of anti-spasm drugs significantly reduces intraoperative and postoperative complications.14 Liu Guang Jian et al.15 conducted a systematic assessment and meta-analysis on fasudil, which demonstrated that occurrence of CV and cerebral infarction, was greatly reduced by fasudil in SAH patients, and clinical outcomes of the patients (as assessed by the Glasgow Outcome Scale) were significantly improved. Due to the limited number of samples and trials, the conclusion still requires further verification by large randomized controlled clinical trials.
Milrinone is a phosphodiesterase III inhibitor that affects cyclic adenosine monophosphate (cAMP) pathways with both inotropic and vasodilatory effects. The potential role of increased cardiac output in improving cerebral tissue perfusion independently of mean arterial pressure has been suggested by several authors.16 The most common side effects were hypokalemia and tachycardia. Side effects of milrinone are less when compared with other drugs which were used to treat CV. In our study, two patients had tachycardia during operation and heart rate normalized by anesthesiologists. There were no significant changes in blood pressure or potassium levels and never required milrinone discontinuation. Milrinone has a short half-life (2.3 h) and it is used for short periods in congestive heart failure.17 Milrinone doses adjustment generally was set suitable of Fraticelli et al.’s study. In their study, 22 patients were assessed. Intra-arterial milrinone was infused in the cerebral territories involved and followed by continuous IV infusion until day 14 after initial bleeding. Overall, three patients underwent mechanical angioplasty.2 In our study, IV infusion of milrinone was given in only one patient for 3 days. Patients with refractory CV were not in their study. In our study, there were 25 patients and 6 of them had refractory CV. We used higher doses of milrinone for patients with refractory CV. They gave 8 mg milrinone for each territory in their studies and we gave 10–16 mg. Shankar et al.18 assessed 14 patients with CV. Overall, nine patients underwent coil occlusion and five patients underwent surgical clipping for ruptured intracranial aneurysms. Intra-arterial milrinone was given for CV to all patients and three patients underwent mechanical angioplasty. The maximum dose of milrinone during single intra-arterial treatment in any patient was 15 mg in their series and 24 mg in our series. All patients in our study underwent endovascular intervention.
There are some studies in the literature about refractory CV. Helbok et al.19 used intra-arterial nimodipine and they reported good results. But only one patient was in their study. Kim et al.20 compared the patients with CV who underwent single or multiple intra-arterial nimodipine infusions. They reported that no improvement in GCS after the intra-arterial nimodipine infusion and no decrease of MCA velocity on ultrasonography over 50 cm/s or Lindegaard ratio >4. 3 after the multiple intra-arterial nimodipine infusion. Ott et al.10 assessed intra-arterial nimodipine infusion for refractory CV. They had good outcomes. In their studies, if post-infusion angiography showed complete recurrence of vasospasm the infusion catheter was taken out. When vasospasm persisted after short-time local intra-arterial nimodipine infusion, the catheter was left in place and continuous intra-arterial nimodipine infusion was started in the intensive care unit. After short-time milrinone infusion, CV did not persist in all interventions and the vessel diameter increased in all of the operations in our study.
Romero et al.3 used intra-arterial milrinone infusion in their studies for refractory CV. The criteria for refractory vasospasm were based on the persistence of angiographic vasospasm after an intra-arterial papaverine (300 mg) infusion and patients with refractory vasospasm were treated with selective intra-arterial milrinone infusion at the affected vessel, at a 0.25 mg/min infusion, until a satisfactory angiographic response was observed or when the 15-mg maximal dose was infused in their studies. In Romero’s study patients with refractory CV were followed up for 3 months. We did not use papaverine infusion and refractory vasospasm was defined patients with CV refractory to therapies requiring ≥3 endovascular interventions during the course of treatment following aneurismal SAH in our study. We used higher milrinone doses and long-term neurological outcome of patients were followed up for 6–18 months. Anand et al.21 described one case where they used intra-arterial nimodipine (4 mg) and milrinone (10 mg) with IV milrinone infusion. The intra-arterial bolus injection of nimodipine was the first-line agent used in their setup. In our study, if there were no good outcomes after intra-arterial milrinone injection, intra-arterial nimodipine infusion was started in patients with refractory CV.
In the above studies on CV, aneurysms were generally treated with surgical clipping and endovascular coiling. All patients in our study were treated with endovascular coiling. Thus, the risk of increased vasospasm due to surgery was also eliminated.
There were some limitations in our study. It was not randomized, it had no control group and the sample size was small. We did not obtain systematic cardiac output measurements in these patients and therefore were unable to quantify the effects of milrinone on hemodynamic targets such as cardiac output and systemic vascular resistance. There were not enough studies about milrinone infusion for refractory CV in the literature. Nonetheless to our knowledge this study is the largest series about combined intra-arterial milrinone and nimodipine infusion for refractory CV and has the longest time outcomes in the literature. It systematically analyzed safety, efficacy and functional outcome.
Our results should be interpreted with caution, considering them as preliminary to a bigger and better methodological study designed to confirm our results and allow stronger conclusions.
Conclusion
High doses of milrinone can be used effectively to control the refractory CV. For exceptional patients with refractory CV, high dose intra-arterial nimodipine and milrinone infusion can be use as a rescue therapy. Efficiency and reliability might be assessed in larger series as a distinct entity in vivo or in vitro.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Karimi RJ, Choudhry O, Prestigiacomo CJ. Intra-arterial pharmacotherapy of cerebral vasospasm. In: Prestigiacomo CJ, Bendok BR. (eds). Endovasular Surgical Neuroradiology: Theory and Clinical Practice, New York, USA: Thieme, 2015, pp. 210–217. [Google Scholar]
- 2.Fraticelli AT, Cholley BP, Losser MR, et al. Milrinone for the treatment of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke 2008; 39: 893–898. [DOI] [PubMed] [Google Scholar]
- 3.Romero CM, Morales D, Reccius A, et al. Milrinone as a rescue therapy for symptomatic refractory cerebral vasospasm in aneurysmal subarachnoid hemorrhage. Neurocrit Care 2009; 11: 165–171. [DOI] [PubMed] [Google Scholar]
- 4.Dabus G, Nogueira RG. Current options for the management of aneurysmal subarachnoid hemorrhage-induced cerebral vasospasm: a comprehensive review of the literature. Interv Neurol 2013; 2: 30–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velat GJ, Kimball MM, Mocco JD, et al. Vasospasm after aneurysmal subarachnoid hemorrhage: review of randomized controlled trials and meta-analyses in the literature. World Neurosurg 2011; 76: 446–454. [DOI] [PubMed] [Google Scholar]
- 6.Wu CT, Wong CS, Yeh CC, et al. Treatment of cerebral vasospasm after subarachnoid hemorrhage: a review. See comment in PubMed Commons belowActa Anaesthesiol Taiwan 2004; 42: 215–222. [PubMed] [Google Scholar]
- 7.Pierot L, Aggour M, Moret J. Vasospasm after aneurysmal subarachnoid hemorrhage: recent advances in endovascular management. Curr Opin Crit Care 2010; 16: 110–116. [DOI] [PubMed] [Google Scholar]
- 8.Mathis JM, Jensen ME, Dion JE. Technical considerations on intra-arterial papaverine hydrochloride for cerebral vasospasm. Neuroradiology 1997; 39: 90–98. [DOI] [PubMed] [Google Scholar]
- 9.Keyrouz SG, Diringer MN. Clinical review: prevention and therapy of vasospasm in subarachnoid hemorrhage. Crit Care 2007; 11: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ott S, Jedlicka S, Wolf S, et al. Continuous selective intra-arterial application of nimodipine in refractory cerebral vasospasm due to aneurysmal subarachnoid hemorrhage. Biomed Res Int 2014; 2014: 970741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang R, Jiang F, Feng Z, et al. Nicardipine in the treatment of aneurysmal subarachnoid haemorrhage: a meta-analysis of published data. Acta Neurol Belg 2013; 113: 3–6. [DOI] [PubMed] [Google Scholar]
- 12.Stuart RM, Helbok R, Kurtz P, et al. High-dose intra-arterial verapamil for the treatment of cerebral vasospasm after subarachnoid hemorrhage: prolonged effects on hemodynamic parameters and brain metabolism. Neurosurgery 2011; 68: 337–345. [DOI] [PubMed] [Google Scholar]
- 13.Keuskamp J, Murali R, Chao KH. High-dose intraarterial verapamil in the treatment of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg 2008; 108: 458–463. [DOI] [PubMed] [Google Scholar]
- 14.Muroi C, Seule M, Mishima K, et al. Novel treatments for vasospasm after subarachnoid hemorrhage. Curr Opin Crit Care 2012; 18: 119–126. [DOI] [PubMed] [Google Scholar]
- 15.Liu GJ, Wang ZJ, Wang YF, et al. Systematic assessment and meta-analysis of the efficacy and safety of fasudil in the treatment of cerebral vasospasm in patients with subarachnoid hemorrhage. Eur J Clin Pharmacol 2012; 68: 131–139. [DOI] [PubMed] [Google Scholar]
- 16.Joseph M, Ziadi S, Nates J, et al. Increases in cardiac output can reverse flow deficits from vasospasm independent of blood pressure: a study using xenon computed tomographic measurement of cerebral blood flow. Neurosurgery 2003; 53: 1044–1051. [DOI] [PubMed] [Google Scholar]
- 17.Bayram M, De Luca L, Massie MB, et al. Reassessment of dobutamine, dopamine and milrinone in the management of acute heart failure syndromes. Am J Cardiol 2005; 96: 47–48. [DOI] [PubMed] [Google Scholar]
- 18.Shankar JJ, Dos Santos MP, Deus-Silva L, et al. Angiographic evaluation of the effect of intra-arterial milrinone therapy in patients with vasospasm from aneurysmal subarachnoid hemorrhage. Neuroradiology 2011; 53: 123–128. [DOI] [PubMed] [Google Scholar]
- 19.Helbok R, Zangerle A, Chemelli A, et al. Continuous intra-arterial nimodipine infusion in refractory symptomatic vasospasm after subarachnoid hemorrhage. Springerplus 2016; 5: 1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SY, Kim KH, Cho JH, et al. Clinical variables correlated with numbers of intra-arterial nimodipine infusion in patients with medically refractory cerebral vasospasm. J Cerebrovasc Endovasc Neurosurg 2015; 17: 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anand S, Goel G, Gupta V. Continous intra-arterial dilatation with nimodipine and milrinone for refractory cerebral vasospasm. J Neurosurg Anesthesiol 2014; 26: 92–93. [DOI] [PubMed] [Google Scholar]