Abstract
Myocarditis may present with a wide range of symptoms, ranging from mild dyspnea or chest pain that resolves without specific therapy to cardiogenic shock and death. Dilated cardiomyopathy with chronic heart failure is the major long-term sequela of myocarditis. Most often, myocarditis results from common viral infections; less commonly, specific forms of myocarditis may result from other pathogens, toxic or hypersensitivity drug reactions, giant-cell myocarditis, or sarcoidosis. The prognosis and treatment of myocarditis vary according to the cause, and clinical and hemodynamic data usually provide guidance to decide when to refer a patient to a specialist for endomyocardial biopsy. The aim of this review is to provide a practical and current approach to the evaluation and treatment of suspected myocarditis.
DEFINITION
The standard Dallas pathological criteria for the definition of myocarditis require that an inflammatory cellular infiltrate with or without associated myocyte necrosis be present on conventionally stained heart-tissue sections (Fig. 1A).1 These criteria are limited by variability in interpretation, lack of prognostic value, and low sensitivity, in part due to sampling error.2,3 These limitations have led to alternative pathological classifications with criteria that rely on cell-specific immunoperoxidase stains for surface antigens, such as anti-CD3, anti-CD4, anti-CD20, anti-CD68, and anti–human leukocyte antigen (Fig. 1B).4,5 Criteria that are based on immunoperoxidase staining have greater sensitivity and may have prognostic value.6
Figure 1. Lymphocytic and Histiocytic Infiltrate and T Lymphocytes in Heart-Tissue Sections from Patients with Acute Myocarditis.
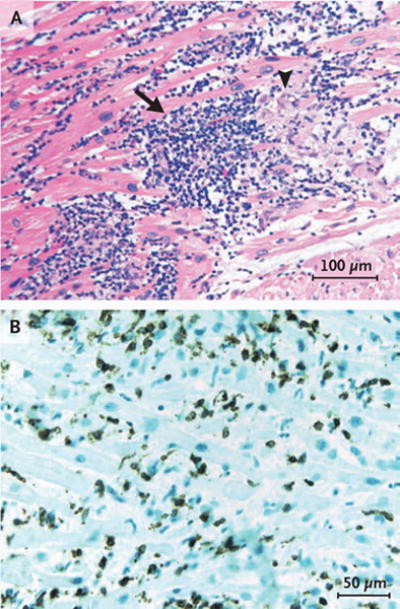
Panel A shows acute myocarditis with widespread lymphocytic and histiocytic infiltrate (arrow) and associated myocyte damage (arrowhead) (hematoxylin and eosin). Panel B shows CD3 immunostaining of T lymphocytes in a patient with acute myocarditis. Images provided courtesy of Dr. Dylan Miller.
Preliminary studies suggest that noninvasive cardiac magnetic resonance imaging (MRI) may provide an alternative method for diagnosis without the risks of biopsy. For example, regions of myocarditis are reported to correlate closely with regions of abnormal signal on cardiac MRI.7,8 The lack of consensus regarding the value of invasive studies such as endomyocardial biopsy and the overall good prognosis for patients with mild, acute dilated cardiomyopathy who have suspected myocarditis have led to recent recommendations that endomyocardial biopsy should be considered on the basis of the likelihood of finding specific treatable disorders.9
Clinicopathological criteria may distinguish fulminant lymphocytic myocarditis from acute lymphocytic myocarditis and introduce prognostically useful information that improves on purely pathological classifications.10 On the basis of clinicopathological criteria, fulminant lymphocytic myocarditis, which has a distinct onset with a viral prodrome within 2 weeks before the onset of symptoms and hemodynamic compromise but has a generally good prognosis, may be distinguished from acute lymphocytic myocarditis, which frequently does not have a distinct onset and hemodynamic compromise but more frequently results in death or the need for cardiac transplantation (Table 1).11,12 Two caveats are important when using such clinicopathological criteria. First, even though patients with fulminant lymphocytic myocarditis frequently recover, they are quite ill and need treatment with intravenous inotropic agents or mechanical circulatory support. Also, since both forms of myocarditis are rare, prognostic data on heart transplantation and survival are limited to relatively few patients.
Table 1.
Clinical Scenarios for the Diagnosis of Myocarditis.*
| Clinical Scenario | Duration of Illness | Pathological Correlates | Prognosis | Treatment |
|---|---|---|---|---|
| Acute myocardial infarction–like syndrome with normal coronary arteries | Several hours or days | Active lymphocytic myocarditis or, rarely, necrotizing eosinophilic myocarditis or giant-cell myocarditis | Good if lymphocytic myocarditis is present on biopsy | Supportive |
| Heart failure with normalsized or dilated left ventricle and hemodynamic compromise | Less than 2 wk | Active lymphocytic myocarditis or, less commonly, necrotizing eosinophilic myocarditis or giant-cell myocarditis | Good in fulminant lymphocytic myocarditis, but acute care often requires inotropic or mechanical circulatory support | Supportive; possible use of corticosteroids or IVIG in children |
| Heart failure with dilated left ventricle and new ventricular arrhythmias, high-degree heart block, or lack of response to usual care within 1 to 2 wk | A few weeks or months | Giant-cell myocarditis, eosinophilic myocarditis, or lymphocytic myocarditis | Poor; high likelihood of death or need for cardiac transplantation if giant-cell myocarditis is found on biopsy | Variable therapy according to histopathological results |
| Heart failure with dilated left ventricle without new ventricular arrhythmias or high-degree heart block | A few weeks or months | Nonspecific changes most likely, with the presence of viral genomes in 25 to 35% of patients and of lymphocytic myocarditis (Dallas criteria) in about 10% | Good in the first several years, but a risk of late disease progression with heart failure and cardiomyopathy | Supportive; definition of genomic predictors of risk under investigation |
| Heart failure with eosinophilia | Any duration | Eosinophilic or hypersensitivity myocarditis, eosinophilic endomyocarditis | Poor | Supportive, including identification and treatment of underlying cause; possible use of corticosteroids for hypersensitivity myocarditis |
| Heart failure with dilated left ventricle and new ventricular arrhythmias, high-degree heart block, or lack of response to usual care in 1 to 2 wk | More than several months | Cardiac sarcoidosis (idiopathic granulomatous myocarditis) or specific infection (e.g., Trypanosoma cruzi and Borrelia burgdorferi); nonspecific changes most likely | Increased risk of need for pacemaker or implantable cardioverter–defibrillator if sarcoidosis is confirmed on biopsy | Supportive; corticosteroids for biopsy-proven cardiac sarcoidosis |
| Heart failure with dilated left ventricle without new ventricular arrhythmias or high-degree heart block | More than several months | Nonspecific changes most likely; increased number of inflammatory cells shown by sensitive immunostaining in up to 40% of patients and the presence of viral genomes in 25 to 35% | Depends on functional class ejection fraction and the presence or absence of inflammation and viral genomes on biopsy | Supportive; antiviral treatment and immunosuppression under investigation |
IVIG denotes intravenous immune globulin.
CLINICAL MANIFESTATIONS AND INCIDENCE
Acute myocarditis is frequently first diagnosed as nonischemic dilated cardiomyopathy in a patient with symptoms that have been present for a few weeks to several months. However, manifestations range from subclinical disease to sudden death, with new-onset atrial or ventricular arrhythmias, complete heart block, or an acute myocardial infarction–like syndrome. Cardiac symptoms are variable and may include fatigue, decreased exercise tolerance, palpitations, precordial chest pain, and syncope. Chest pain in acute myocarditis can result from an associated pericarditis or, occasionally, from coronary-artery spasm.13
Although a viral prodrome with fever, myalgia, and respiratory or gastrointestinal symptoms is classically associated with myocarditis, reported symptoms are highly variable.14 Of the 3055 patients with suspected acute or chronic myocarditis who were screened in the European Study of the Epidemiology and Treatment of Inflammatory Heart Disease,15 72% had dyspnea, 32% had chest pain, and 18% had arrhythmias. Most studies of acute myocarditis report a slight preponderance in male patients,16–18 which may be due to a protective effect of natural hormone variations on immune responses in women.19 The clinical presentation of myocarditis in children differs from that in adults; children often have a more fulminant presentation.20 Because of the wide spectrum of clinical presentations, clinicians need to consider myocarditis in the differential diagnosis in many cardiac syndromes.
Most people with myocarditis who present with acute dilated cardiomyopathy have relatively mild disease that resolves with few short-term sequelae, but certain clinical clues signify those at high risk for more difficulty (Table 1). Rash, fever, peripheral eosinophilia, or a temporal relation with recently initiated medications or the use of multiple medications suggest a possible hypersensitivity myocarditis. Giant-cell myocarditis should be considered in patients with acute dilated cardiomyopathy associated with thymoma, autoimmune disorders, ventricular tachycardia, or high-grade heart block. An unusual cause of myocarditis, such as cardiac sarcoidosis, should be suspected in patients who present with chronic heart failure, dilated cardiomyopathy and new ventricular arrhythmias, or second-degree or third-degree heart block or who do not have a response to standard care.21
The true incidence of myocarditis in the community is unknown. Endomyocardial biopsy is used infrequently because of perceived risks and the lack of a widely accepted and sensitive histologic standard. Seroepidemiologic data are difficult to interpret because of the heterotopic effect of enteroviruses, which may cause an amnestic antibody response to other coxsackievirus B strains.22 However, the observation that viral genomes are more common in cardiac tissue from patients with chronic dilated cardiomyopathy than in tissue from patients with valvular or ischemic cardiomyopathy supports the concept that viral myocarditis leads to a substantial disease burden in the community. Furthermore, myocarditis is an important cause of sudden death,23 as well as childhood cardiomyopathy.24 A recent long-term study of pediatric myocarditis demonstrated that the greatest burden of myocarditis may not be apparent for 6 to 12 years after diagnosis when children die or need to undergo cardiac transplantation for chronic dilated cardiomyopathy.25
CAUSATIVE AGENTS
Viral and postviral myocarditis remain major causes of acute and chronic dilated cardiomyopathy. Seroepidemiologic and molecular studies linked coxsackievirus B to outbreaks of myocarditis from the 1950s through the 1990s. The spectrum of viruses that were detected in endomyocardial biopsy samples shifted from coxsackievirus B to adenovirus in the late 1990s and, in the past 5 years, to parvovirus B19 and other viruses, according to reports from the United States and Germany.6,26 In Japan and in a serologic study of myocarditis in the United States, hepatitis C virus was also linked to myocarditis and dilated cardiomyopathy.27,28 Many other viruses have also been associated less frequently with myocarditis; these viruses include Epstein–Barr virus, cytomegalovirus, and human herpesvirus 6. The large number of observations that link viruses with myocarditis have led to ongoing treatment trials of antiviral therapy in patients with virus-associated cardiomyopathy.
In addition to viruses, certain other infectious causes of myocarditis should be considered in patients with acute or chronic cardiomyopathy. Myocarditis can result from infection with Borrelia burgdorferi (Lyme disease), and patients with myocarditis due to Lyme disease are occasionally coinfected with ehrlichia or babesia.29 Lyme myocarditis should be suspected in patients with a history of travel to regions where the disease is endemic or of a tick bite, particularly if they also have atrioventricular conduction abnormalities.30 In areas of rural Central and South America, Trypanosoma cruzi infection can present as acute myocarditis or chronic cardiomyopathy, sometimes with right bundle-branch block or left anterior fascicular block.31 In this disorder, echocardiography or contrast ventriculography may reveal a left ventricular apical aneurysm, regional wall-motion abnormalities, or diffuse cardiomyopathy. Regional wall-motion abnormalities or perfusion defects that are not in the distribution of a coronary artery may also be seen in noninfectious disorders, such as cardiac sarcoidosis and arrhythmogenic right ventricular cardiomyopathy or dysplasia.
Myocarditis is the most common cardiac pathological finding at autopsy of patients infected with the human immunodeficiency virus (HIV), with a prevalence of 50% or more. Cardiomyopathy in patients with HIV infection may be caused by an inhibition of cardiac contractility by HIV type 1 glycoprotein 120, coinfections, or antiviral medications.32
Drug-induced hypersensitivity reactions and systemic hypereosinophilic syndromes can cause a specific myocarditis that often responds to withdrawal of the offending agent or to treatment of the underlying disorder, though adjuvant corticosteroid therapy is often required.33 Numerous medications, including some anticonvulsants, antibiotics, and antipsychotics, have been implicated in hypersensitivity myocarditis. Eosinophilic myocarditis is characterized by a predominantly eosinophilic infiltrate in the myocardium and may occur in association with systemic diseases, such as the hypereosinophilic syndrome, the Churg–Strauss syndrome, Löffler’s endomyocardial fibrosis, cancer, and parasitic, helminthic, or protozoal infections.34–36 Eosinophilic myocarditis has been reported after vaccination for several diseases, including smallpox.37,38 Clinical manifestations of eosinophilic myocarditis include congestive heart failure, endocardial and valvular fibrosis, and endocardial thrombi. A rare disorder, acute necrotizing eosinophilic myocarditis is an aggressive form of eosinophilic myocarditis with an acute onset and a high death rate.39
Two idiopathic and histologically similar disorders, giant-cell myocarditis and cardiac sarcoidosis, are rare but important causes of cardiomyopathy. Giant-cell myocarditis, an acute disorder with a high risk of death or need for cardiac transplantation, is considered to be primarily autoimmune in nature because of its association with a variety of autoimmune disorders,40 thymoma,41 and drug hypersensitivity.42 Giant-cell myocarditis is sometimes distinguished from the much more common postviral myocarditis by the presence of ventricular tachycardia, heart block, and a downhill clinical course, despite optimal clinical care. Patients who present with apparently chronic dilated cardiomyopathy yet with new ventricular arrhythmias or second-degree or third-degree heart block or who do not have a response to optimal care are more likely to have cardiac sarcoidosis, a granulomatous myocarditis.21
Myocarditis may occur concomitantly with other cardiomyopathies and may have an adverse effect on the clinical course of the other condition. For example, the prognosis in cardiac amyloidosis is much worse if histologic evidence of myocarditis is present.43 Myocarditis has been associated with clinical deterioration in hypertrophic cardiomyopathy, and in such cases, evidence of a persistent viral genome may be identified in the myocardium.44 A high percentage of patients with arrhythmogenic right ventricular cardiomyopathy or dysplasia have associated myocarditis; some of these cases are associated with a viral infection,45–47 the prognostic value of which is not known. Recently, there was a report that active coxsackievirus B infection was present in up to 40% of patients who died of acute myocardial infarction; involved cardiomyocytes showed evidence of cytoskeletal disruption in these patients.48
PATHOGENETIC FEATURES
Most information about the molecular pathogenesis of viral and autoimmune myocarditis comes from rodent models and isolated cell systems, rather than from studies of human tissue.49 In these models, viruses appear to enter cardiac myocytes or macrophages through specific receptors and coreceptors. For example, the receptor for coxsackievirus B and adenoviruses 2 and 5 is the human Coxsackie adenovirus receptor.50,51 A coreceptor that plays a role in viral entrance for serotypes B1, B2, and B5 is the coxsackievirus B coreceptor decay-accelerating factor; it appears that differential binding to this coreceptor influences viral virulence.52 The virulence of coxsackievirus B is also modified by variations in its viral genome53 as well as in host factors, such as selenium deficiency54 and mercury exposure.55,56 A better understanding of the genetic and environmental determinants of virulence is needed to understand why the great majority of infections with “cardiotropic” viruses, including enterovirus, adenovirus, and parvovirus B19, do not cause cardiomyopathy.22,57,58
The innate immune response is essential for host defense early during an infection (Fig. 2). Viruses, streptococcal M protein, and certain host proteins can trigger an innate immune response through several mechanisms, which involve toll-like receptors and pattern-recognition receptors in patients with tissue injury.59 The development of myocarditis requires MyD88, a key protein in dendritic-cell toll-like receptor signaling.60 Coxsackievirus B infection up-regulates toll-like receptor 4 on macrophages, stimulates the maturation of antigen-presenting cells, leads to proinflammatory cytokine release,61 and decreases regulatory T-cell function.62 The production of increased levels of type 1 helper T (Th1) and type 2 helper T (Th2) cytokines, which occurs 6 to 12 hours into an innate immune response, is associated with the development of cardiomyopathy.63 Thus, the nature of the innate immune response can determine the subsequently acquired T-cell and B-cell responses. It is not known whether an autoreactive immune response will lead to viral clearance and normal heart function or ultimately progress to a chronic immune-mediated cardiomyopathy in individual patients.
Figure 2. Pathogenesis of Myocarditis.
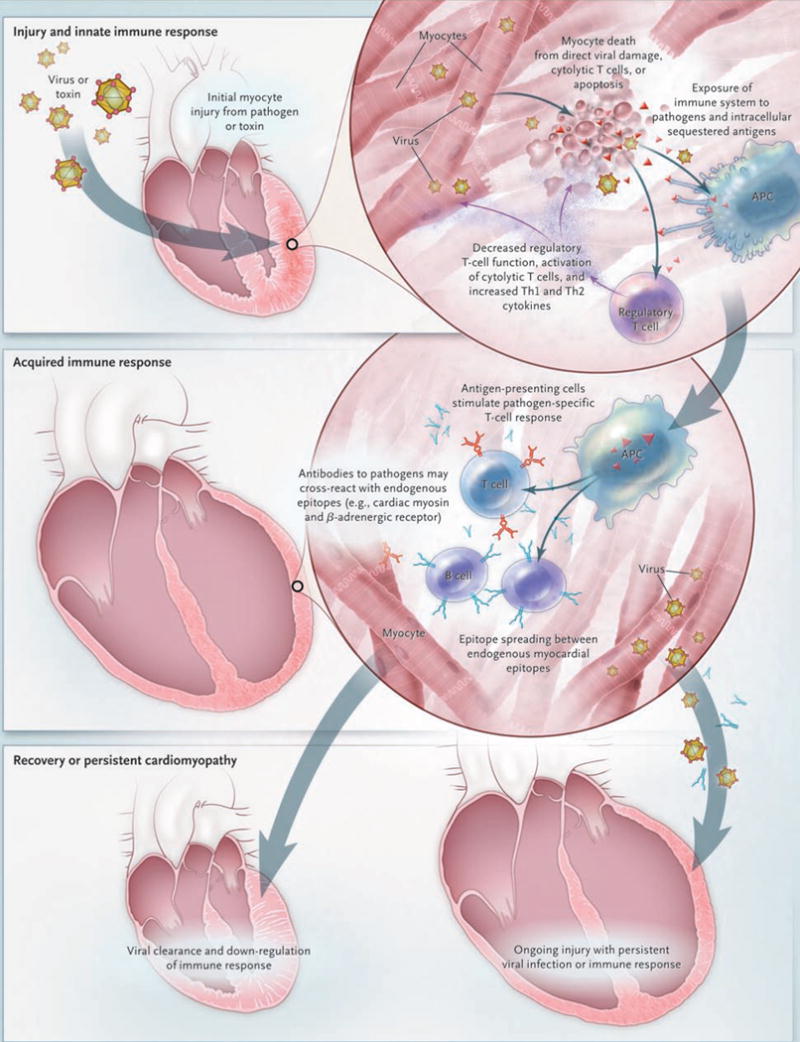
The current understanding of the cellular and molecular pathogenesis of postviral and autoimmune myocarditis is based solely on animal models. In these models, the progression from acute injury to chronic dilated cardiomyopathy may be simplified into a three-stage process. Acute injury leads to cardiac damage, exposure of intracellular antigens such as cardiac myosin, and activation of the innate immune system. Over weeks, specific immunity that is mediated by T lymphocytes and antibodies directed against pathogens and similar endogenous heart epitopes cause robust inflammation. In most patients, the pathogen is cleared and the immune reaction is down-regulated with few sequelae. However, in other patients, the virus is not cleared and causes persistent myocyte damage, and heart-specific inflammation may persist because of mistaken recognition of endogenous heart antigens as pathogenic entities. APC denotes antigen-presenting cell.
CD4+ T lymphocytes are key mediators of cardiac damage in experimental autoimmune myocarditis.64,65 For example, circulating T cells that have a low avidity for self antigens are normally harmless but can cause immune-mediated heart disease if stimulated with large amounts of self antigens.66 T-cell responses that are associated with the production of both Th1 and Th2 cytokines have been implicated in the pathogenesis of myocarditis after viral infection.67 Recently, a third T helper subgroup, Th17 cells, which produce interleukin-17,68 have been implicated in myocarditis as well.69 Both CD4+ and CD8+ T cells are important in a murine model of coxsackievirus B myocarditis.70 The prominent role of T lymphocytes in multiple models of experimental myocarditis supports the rationale for the use of anti–T-cell therapy in severe human cardiomyopathy with prominent autoimmune features.71
Circulating CD4+ T cells are generally under the control of at least one subgroup of regulatory T cells (T reg).72 Ono and colleagues73 demonstrated that one subgroup of regulatory T cells that express CD4, transcription factor forkhead box p3 (FOXP3), and that have a high level of the corticosteroid-induced tumor necrosis factor receptor influence the course of autoimmune myocarditis. CD4+CD25+FOXp3+ T cells are also important negative regulators of inflammation in coxsackievirus B myocarditis.74 Regulatory T-cell subgroups have not yet been studied in human myocarditis.
Autoantibodies to a variety of cardiac antigens are common in suspected or histologically confirmed lymphocytic myocarditis and dilated cardiomyopathy.75,76 Streptococcal M protein and coxsackievirus B share epitopes with cardiac myosin, an intracellular antigen, and cross-reactive antibodies may result in the production of auto-antibodies because of this antigenic mimicry.77 After viral clearance, cardiac myosin may provide an endogenous source of antigen in chronic myocarditis and stimulate chronic inflammation through autoimmune mechanisms. A series of studies during the past decade or so have described cross-reactivity between cardiac myosin and the endogenous human cell-surface protein laminin, suggesting that laminin could serve as an ongoing stimulus in chronic myocarditis.78,79 Recently, antibodies to cardiac myosin that cross-react with the β1-adrenergic receptor have been described,77 and these antibodies may contribute to cardiomyocyte apoptosis.80 However, distinguishing antibody autoreactivity, which occurs commonly in the course of normal immune reactions, from autoimmune disease, in which anti-cardiac antibodies contribute to ongoing cardiomyopathy, is a challenge for investigators.
Myocardial damage during enterovirus infection may also occur independently of immune reactions. For example, protein products of the enteroviral genome, including viral protease 2A, can cleave host proteins, including dystrophin, which may lead to cardiomyopathy.81 This induction of dystrophin deficiency augments the cardiomyopathy that accompanies the enterovirus.82 Data from experimental models indicate that coxsackievirus B may persist in the myocardium with a partially deleted genome, leading to a low-grade, noncytolytic, chronic infection in the heart.83 Such observations, if replicated in human patients with dilated cardiomyopathy, might help to explain how enterovirus infection can cause chronic dilated cardiomyopathy in the absence of myocarditis.84
DIAGNOSIS
Biomarkers of cardiac injury are elevated in a minority of patients with acute myocarditis but may help confirm the diagnosis. Troponin I has high specificity (89%) but limited sensitivity (34%) in the diagnosis of myocarditis.85 Clinical and experimental data suggest that increased levels of cardiac troponin I are more common than increased levels of creatine kinase MB in acute myocarditis.86 A few serologic and imaging biomarkers have been associated with poor clinical outcome. For example, relatively high serum levels of Fas ligand and interleukin-10 may predict an increased risk of death,87,88 although these assays are not widely available.
In acute myocarditis, the electrocardiogram may show sinus tachycardia with nonspecific ST-segment and T-wave abnormalities. Occasionally, the changes on electrocardiography are suggestive of an acute myocardial infarction and may include ST-segment elevation, ST-segment depression, and pathologic Q waves. Pericarditis not infrequently accompanies myocarditis clinically and is often manifested in pericarditis-like changes seen on electrocardiography. The sensitivity of the electrocardiogram for myocarditis is low (47%).89 The presence of Q waves or left bundle-branch block is associated with higher rates of death or cardiac transplantation.18,90
Echocardiography is useful primarily to rule out other causes of heart failure, since there are no specific features of acute myocarditis. Echocardiographic patterns of dilated, hypertrophic, restrictive, and ischemic cardiomyopathies have been described in histologically proven myocarditis. Segmental or global wall-motion abnormalities in myocarditis can simulate myocardial infarction.91 In the Myocarditis Treatment Trial, increased sphericity and left ventricular volume occurred in acute, active myocarditis.92 Fulminant myocarditis may be distinguished from acute myocarditis by a smaller left ventricular cavity size and increased wall thickness.93 The loss of right ventricular function was the most powerful predictor of death or the need for cardiac transplantation in a series of 23 patients with biopsy-confirmed myocarditis.94
Cardiac MRI is being used with increasing frequency as a diagnostic test in suspected acute myocarditis95,96 and may be used to localize sites for endomyocardial biopsy (Fig. 3). In a study by Mahrholdt et al., histopathological evaluation of biopsy specimens directed by contrast cardiac MRI with delayed enhancement demonstrated active myocarditis in 19 of 21 patients, although such evaluation without delayed enhancement showed active myocarditis in only 1 of 7 patients.7 A combination of T1-weighted and T2-weighted images had the best combination of sensitivity and specificity.97
Figure 3. Contrast-Enhanced Magnetic Resonance Imaging (MRI) of the Heart of a 24-Year-Old Man with Acute Myocarditis.
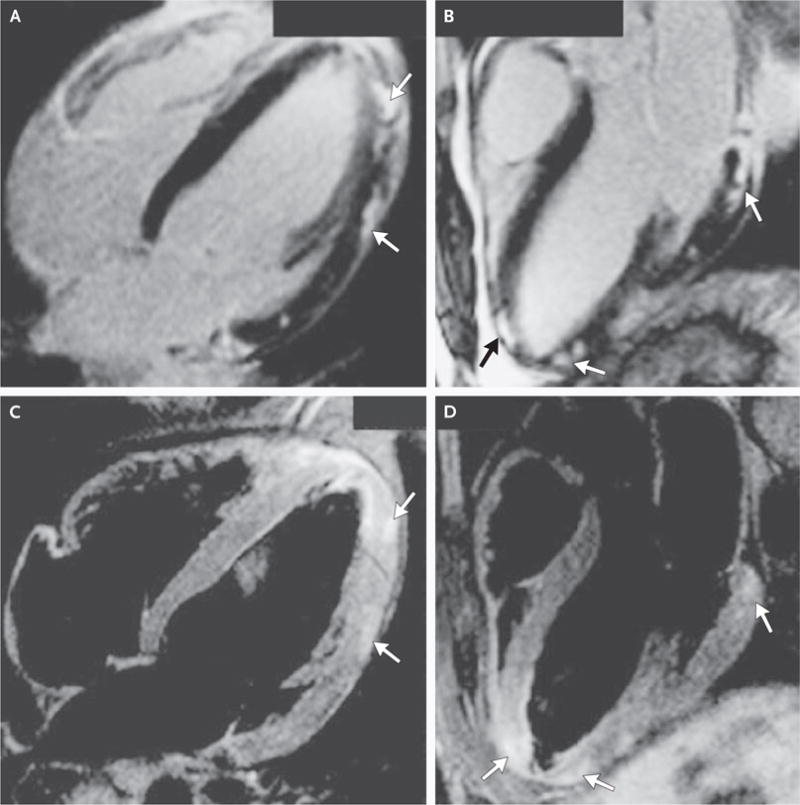
Cardiac MRI is being increasingly used to evaluate suspected acute myocarditis and to localize sites for endomyocardial biopsy, with additional detail shown with delayed gadolinium enhancement (Panel A, arrows), in a four-chamber view (Panel B, arrows), and in T2-weighted three-chamber views (Panels C and D, arrows). Scans provided courtesy of Dr. Jeannette Schultz-Menger.
The role of endomyocardial biopsy in the evaluation of cardiovascular disease was recently addressed in a scientific statement by the American Heart Association in concert with the American College of Cardiology and the European Society of Cardiology.9 Two scenarios, which described the most common presentations of fulminant myocarditis and giant-cell myocarditis, received a class I recommendation. Endomyocardial biopsy should be performed in patients with unexplained, new-onset heart failure of less than 2 weeks’ duration in association with a normalsize or dilated left ventricle and hemodynamic compromise, for suspected fulminant myocarditis. Endomyocardial biopsy should also be performed in patients with unexplained, new-onset heart failure of 2 weeks’ to 3 months’ duration in association with a dilated left ventricle and new ventricular arrhythmias or Mobitz type II or second-degree or third-degree heart block and in patients who do not have a response to usual care within 1 to 2 weeks, for suspected giant-cell myocarditis. The role of endomyocardial biopsy in patients who do not present with these clinical scenarios has not been as well established. Patients with an indication for endomyocardial biopsy who present to a medical center where the relevant expertise is unavailable should generally be sent to a medical center with biopsy capability.
TREATMENT
Patients who present with myocarditis with acute dilated cardiomyopathy should be treated according to the current guidelines of the American Heart Association, the American College of Cardiology, the European Society of Cardiology, and the Heart Failure Society of America.98–100 The mainstay of therapy for acute myocarditis is supportive therapy for left ventricular dysfunction. Most patients will improve with a standard heart-failure regimen that includes the administration of angiotensin-converting–enzyme inhibitors or angiotensin-receptor blockers, beta-blockers such as metoprolol and carvedilol, and diuretics, if needed. In patients whose condition deteriorates despite optimal medical management, case series suggest a role for mechanical circulatory support, such as ventricular assist devices or extracorporeal membrane oxygenation, as a bridge to transplantation or recovery.101–103 The overall rate of survival after cardiac transplantation for myocarditis is similar to that for other causes of cardiac failure.104
Since no clinical trials of therapy for heart failure have been conducted specifically in patients with myocarditis, the only relevant studies describe animal models. Patients recovering from acute myocarditis should refrain from aerobic activity for a period of months after the clinical onset of the disease, based on studies in rodents with myocarditis in which increased death rates were associated with sustained exercise.105 The reintroduction of aerobic activities somewhat depends on the severity of left ventricular dysfunction and the extent of recovery.106 The use of candesartan improved survival in a murine model of viral myocarditis (60%, vs. 18% with no candesartan treatment).107 The use of carteolol, a nonselective beta-blocker, improved histopathological results and reduced wall thickness in coxsackievirus B myocarditis.108 The use of nonsteroidal antiinflammatory drugs was associated with increased mortality.109–111 Taken together, these data support the application of the current heart-failure guidelines to patients with heart failure from myocarditis.
In patients with acute myocarditis, therapy for arrhythmias is also supportive, since such arrhythmias usually resolve after the acute phase of the disease, which can last several weeks. The 2006 guidelines of the American Heart Association, the American College of Cardiology, and the European Society of Cardiology recommend that arrhythmias be managed conventionally in patients with myocarditis.112 However, in acute myocarditis, temporary pacemakers may be required for patients with symptomatic bradycardia or complete heart block. Patients with symptomatic or sustained ventricular arrhythmias may need amiodarone and possibly an implantable cardioverter–defibrillator, even if active inflammation is still present. The prognostic importance and treatment of nonsustained ventricular arrhythmias in acute myocarditis have not been systematically evaluated.
The finding of viral genomes on endomyocardial biopsy has been used to guide treatment in acute and chronic cardiomyopathy. In some but not all studies, the presence of viral genomes was associated with subsequent worsening of heart function, the need for cardiac transplantation, and death.6,113 Data regarding the use of antiviral agents are currently limited to animal models and small case series. In murine viral myocarditis, antiviral therapy with ribavirin and interferon alfa reduced the severity of myocardial lesions and mortality.114,115 Antiviral therapy has been used in only one case series of fulminant myocarditis.116 Because most patients with acute myocarditis are diagnosed weeks after viral infection, it is unlikely that antiviral therapy would be provided early enough to be of benefit in acute viral myocarditis. In contrast, interferon beta has been used successfully in patients with viral persistence in chronic, stable dilated cardiomyopathy.117 Viral clearance was achieved in all patients after antiviral treatment, with a significant increase in left ventricular function in the treatment group. Successful antiviral therapy or vaccines would need to be tailored to current viruses, since viruses that have been detected in the heart have changed from enterovirus in the 1980s to adenovirus in the 1990s and now to parvovirus B19 and human herpesvirus 6 — and because coinfections are common.113,118
Antiviral and immunomodulatory effects that have been shown in experimental models and uncontrolled case series suggest that intravenous immune globulin (IVIG) might have a therapeutic use in myocarditis. However, in the Intervention in Myocarditis and Acute Cardiomyopathy trial, patients with acute dilated cardiomyopathy who were treated with IVIG did no better than those given placebo.119 Therefore, the routine use of IVIG for acute myocarditis in adults is not recommended. IVIG has not been evaluated rigorously for the treatment of chronic dilated cardiomyopathy with inflammation or viral persistence. IVIG may have a role in the treatment of acute pediatric myocarditis.20,120
Results from several randomized, controlled trials of immunosuppression for acute myocarditis were negative or only marginally positive.16,121 These studies suggest that immunosuppression is not beneficial in the routine treatment of acute lymphocytic myocarditis. Future trials involving patients with acute myocarditis are probably not feasible since the disease affects so few patients, has a highly variable clinical prognosis, and is associated with substantial improvement in left ventricular function with usual care.122 Unlike lymphocytic myocarditis, transplant-free survival in patients with giant-cell myocarditis may be prolonged with a combination of cyclosporine and corticosteroids.40 There may be a broader role for immunosuppression in patients with chronic, moderate-to-severe cardiomyopathy, whose condition is unlikely to improve further after optimal care has been given for 6 to 12 months. In one trial involving 84 patients with chronic dilated cardiomyopathy and human leukocyte antigen expression on cardiomyocytes, the use of azathioprine and prednisone was associated with improvement in cardiac function and in New York Heart Association functional class.123 Other approaches to modify immune activation that are under investigation in this population include immunoadsorption and immunomodulation.124,125
SUMMARY AND FUTURE DIRECTIONS
This review discusses an approach to suspected myocarditis according to the likelihood of finding a treatable disorder. A major issue for the future is whether the diagnosis of myocarditis will continue to require histologic confirmation. Cardiac MRI is a promising tool but requires additional validation for noninvasive diagnosis and prognosis in acute and chronic myocarditis. On the horizon, analysis of messenger RNA and protein markers from peripheral-blood components may be able to detect a clinically meaningful inflammatory signal in high-risk populations without the risk of endomyocardial biopsy.126 Treatment of subpopulations of chronic viral-associated and nonviral myocarditis with biopsy-guided therapy is an area of active investigation. Our understanding of the immunologic regulation of viral cardiac infection is derived primarily from research in animal models. The insights from these models may be explored in human studies in the next decade to develop new diagnostic tests and possibly pathway-specific treatments.
Acknowledgments
Dr. Cooper reports receiving consulting fees from Acambis, Asahi Kasei Kuraray Medical, and Crucell and serving as a board member and president of the Myocarditis Foundation.
Footnotes
No other potential conflict of interest relevant to this article was reported.
References
- 1.Aretz HT, Billingham ME, Edwards WD, et al. Myocarditis: a histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1:3–14. [PubMed] [Google Scholar]
- 2.Chow LH, Radio SJ, Sears TD, McManus BM. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J Am Coll Cardiol. 1989;14:915–20. doi: 10.1016/0735-1097(89)90465-8. [DOI] [PubMed] [Google Scholar]
- 3.Baughman KL. Diagnosis of myocarditis: death of Dallas criteria. Circulation. 2006;113:593–5. doi: 10.1161/CIRCULATIONAHA.105.589663. [DOI] [PubMed] [Google Scholar]
- 4.Herskowitz A, Ahmed-Ansari A, Neumann DA, et al. Induction of major histocompatibility complex antigens within the myocardium of patients with active myocarditis: a nonhistologic marker of myocarditis. J Am Coll Cardiol. 1990;15:624–32. doi: 10.1016/0735-1097(90)90637-5. [DOI] [PubMed] [Google Scholar]
- 5.Maisch B, Portig I, Ristic A, Hufnagel G, Pankuweit S. Definition of inflammatory cardiomyopathy (myocarditis): on the way to consensus: a status report. Herz. 2000;25:200–9. doi: 10.1007/s000590050007. [DOI] [PubMed] [Google Scholar]
- 6.Kindermann I, Kindermann M, Kandolf R, et al. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118:639–48. doi: 10.1161/CIRCULATIONAHA.108.769489. [Erratum, Circulation 2008;118(12):e493.] [DOI] [PubMed] [Google Scholar]
- 7.Mahrholdt H, Goedecke C, Wagner A, et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109:1250–8. doi: 10.1161/01.CIR.0000118493.13323.81. [DOI] [PubMed] [Google Scholar]
- 8.Gutberlet M, Spors B, Thoma T, et al. Suspected chronic myocarditis at cardiac MR: diagnostic accuracy and association with immunohistologically detected inflammation and viral persistence. Radiology. 2008;246:401–9. doi: 10.1148/radiol.2461062179. [DOI] [PubMed] [Google Scholar]
- 9.Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116:2216–33. doi: 10.1161/CIRCULATIONAHA.107.186093. [DOI] [PubMed] [Google Scholar]
- 10.Lieberman EB, Hutchins GM, Herskowitz A, Rose NR, Baughman KL. Clinicopathologic description of myocarditis. J Am Coll Cardiol. 1991;18:1617–26. doi: 10.1016/0735-1097(91)90493-s. [DOI] [PubMed] [Google Scholar]
- 11.Hare JM, Baughman KL. Fulminant and acute lymphocytic myocarditis: the prognostic value of clinicopathological classification. Eur Heart J. 2001;22:269–70. doi: 10.1053/euhj.2000.2272. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy RE, III, Boehmer JP, Hruban RH, et al. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med. 2000;342:690–5. doi: 10.1056/NEJM200003093421003. [DOI] [PubMed] [Google Scholar]
- 13.McCully RB, Cooper LT, Schreiter S. Coronary artery spasm in lymphocytic myocarditis: a rare cause of acute myocardial infarction. Heart. 2005;91:202. doi: 10.1136/hrt.2004.035675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magnani JW, Dec GW. Myocarditis: current trends in diagnosis and treatment. Circulation. 2006;113:876–90. doi: 10.1161/CIRCULATIONAHA.105.584532. [DOI] [PubMed] [Google Scholar]
- 15.Hufnagel G, Pankuweit S, Richter A, Schönian U, Maisch B. The European Study of Epidemiology and Treatment of Cardiac Inflammatory Diseases (ESETCID): first epidemiological results. Herz. 2000;25:279–85. doi: 10.1007/s000590050021. [DOI] [PubMed] [Google Scholar]
- 16.Mason JW, O’Connell JB, Herskowitz A, et al. A clinical trial of immunosuppressive therapy for myocarditis. N Engl J Med. 1995;333:269–75. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 17.Caforio A, Calabrese F, Angelini A, et al. A prospective study of biopsy-proven myocarditis: prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur Heart J. 2007;28:1326–33. doi: 10.1093/eurheartj/ehm076. [DOI] [PubMed] [Google Scholar]
- 18.Magnani JW, Danik HJ, Dec GW, Jr, DiSalvo TG. Survival in biopsy-proven myocarditis: a long-term retrospective analysis of the histopathologic, clinical, and hemodynamic predictors. Am Heart J. 2006;151:463–70. doi: 10.1016/j.ahj.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz J, Sartini D, Huber S. Myocarditis susceptibility in female mice depends upon ovarian cycle phase at infection. Virology. 2004;330:16–23. doi: 10.1016/j.virol.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 20.Amabile NF, Fraisse A, Bouvenot A, Chetaille P, Ovaert C. Outcome of acute fulminant myocarditis in children. Heart. 2006;92:1269–73. doi: 10.1136/hrt.2005.078402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yazaki Y, Isobe M, Hiramitsu S, et al. Comparison of clinical features and prognosis of cardiac sarcoidosis and idiopathic dilated cardiomyopathy. Am J Cardiol. 1998;82:537–40. doi: 10.1016/s0002-9149(98)00377-4. [DOI] [PubMed] [Google Scholar]
- 22.Pallansch MA. Coxsackievirus B epidemiology and public health concerns. Curr Top Microbiol Immunol. 1997;223:13–30. doi: 10.1007/978-3-642-60687-8_2. [DOI] [PubMed] [Google Scholar]
- 23.Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980-2006. Circulation. 2009;119:1085–92. doi: 10.1161/CIRCULATIONAHA.108.804617. [DOI] [PubMed] [Google Scholar]
- 24.Nugent AW, Daubeney PEF, Chondros P, et al. The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med. 2003;348:1639–46. doi: 10.1056/NEJMoa021737. [DOI] [PubMed] [Google Scholar]
- 25.Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–76. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 26.Kühl U, Pauschinger M, Noutsias M, et al. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation. 2005;111:887–93. doi: 10.1161/01.CIR.0000155616.07901.35. [DOI] [PubMed] [Google Scholar]
- 27.Matsumori A, Shimada T, Chapman NM, Tracy SM, Mason JW. Myocarditis and heart failure associated with hepatitis C virus infection. J Card Fail. 2006;12:293–8. doi: 10.1016/j.cardfail.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Matsumori A. Hepatitis C virus infection and cardiomyopathies. Circ Res. 2005;96:144–7. doi: 10.1161/01.RES.0000156077.54903.67. [DOI] [PubMed] [Google Scholar]
- 29.Jahangir A, Kolbert C, Edwards W, Mitchell P, Dumler JS, Persing DH. Fatal pancarditis associated with human granulocytic Ehrlichiosis in a 44-year-old man. Clin Infect Dis. 1998;27:1424–7. doi: 10.1086/515014. [DOI] [PubMed] [Google Scholar]
- 30.McAlister HF, Klementowicz PT, Andrews C, Fisher JD, Feld M, Furman S. Lyme carditis: an important cause of reversible heart block. Ann Intern Med. 1989;110:339–45. doi: 10.7326/0003-4819-110-5-339. [DOI] [PubMed] [Google Scholar]
- 31.Rassi A, Jr, Rassi A, Little WC, et al. Development and validation of a risk score for predicting death in Chagas’ heart disease. N Engl J Med. 2006;355:799–808. doi: 10.1056/NEJMoa053241. [DOI] [PubMed] [Google Scholar]
- 32.Chen F, Shannon K, Ding S, et al. HIV type 1 glycoprotein 120 inhibits cardiac myocyte contraction. AIDS Res Hum Retroviruses. 2002;18:777–84. doi: 10.1089/08892220260139512. [DOI] [PubMed] [Google Scholar]
- 33.Taliercio CP, Olney BA, Lie JT. Myocarditis related to drug hypersensitivity. Mayo Clin Proc. 1985;60:463–8. doi: 10.1016/s0025-6196(12)60870-2. [DOI] [PubMed] [Google Scholar]
- 34.Corssmit EP, Trip MD, Durrer JD. Löffler’s endomyocarditis in the idiopathic hypereosinophilic syndrome. Cardiology. 1999;91:272–6. doi: 10.1159/000006923. [DOI] [PubMed] [Google Scholar]
- 35.Spodick DH. Eosinophilic myocarditis. Mayo Clin Proc. 1997;72:996. doi: 10.1016/S0025-6196(11)63373-9. [DOI] [PubMed] [Google Scholar]
- 36.Corradi D, Vaglio A, Maestri R, et al. Eosinophilic myocarditis in a patient with idiopathic hypereosinophilic syndrome: insights into mechanisms of myocardial cell death. Hum Pathol. 2004;35:1160–3. doi: 10.1016/j.humpath.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Barton M, Finkelstein Y, Opavsky M, et al. Eosinophilic myocarditis temporally associated with conjugate meningococcal C and hepatitis B vaccines in children. Pediatr Infect Dis J. 2008;27:831–5. doi: 10.1097/INF.0b013e31816ff7b2. [DOI] [PubMed] [Google Scholar]
- 38.Arness MK, Eckart RE, Love SS, et al. Myopericarditis following smallpox vaccination. Am J Epidemiol. 2004;160:642–51. doi: 10.1093/aje/kwh269. [DOI] [PubMed] [Google Scholar]
- 39.Cooper LT, Zehr KJ. Biventricular assist device placement and immunosuppression as therapy for necrotizing eosinophilic myocarditis. Nat Clin Pract Cardiovasc Med. 2005;2:544–8. doi: 10.1038/ncpcardio0322. [DOI] [PubMed] [Google Scholar]
- 40.Cooper LT, Jr, Berry GJ, Shabetai R. Idiopathic giant-cell myocarditis — natural history and treatment. N Engl J Med. 1997;336:1860–6. doi: 10.1056/NEJM199706263362603. [DOI] [PubMed] [Google Scholar]
- 41.Kilgallen CM, Jackson E, Bankoff M, Salomon RN, Surks HK. A case of giant cell myocarditis and malignant thymoma: a postmortem diagnosis by needle biopsy. Clin Cardiol. 1998;21:48–51. doi: 10.1002/clc.4960210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daniels P, Tazelaar H, Edwards W, Cooper L. Giant cell myocarditis as a manifestation of drug hypersensitivity. Cardiovasc Pathol. 2000;9:287–91. doi: 10.1016/s1054-8807(00)00049-1. [DOI] [PubMed] [Google Scholar]
- 43.Rahman JE, Helou EF, Gelzer-Bell R, et al. Noninvasive diagnosis of biopsyproven cardiac amyloidosis. J Am Coll Cardiol. 2004;43:410–5. doi: 10.1016/j.jacc.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 44.Frustaci A, Verardo R, Caldarulo M, Acconcia MC, Russo MA, Chimenti C. Myocarditis in hypertrophic cardiomyopathy patients presenting acute clinical deterioration. Eur Heart J. 2007;28:733–40. doi: 10.1093/eurheartj/ehl525. [DOI] [PubMed] [Google Scholar]
- 45.Chimenti C, Pieroni M, Maseri A, Frustaci A. Histologic findings in patients with clinical and instrumental diagnosis of sporadic arrhythmogenic right ventricular dysplasia. J Am Coll Cardiol. 2004;43:2305–13. doi: 10.1016/j.jacc.2003.12.056. [DOI] [PubMed] [Google Scholar]
- 46.Basso C, Ronco F, Marcus F, et al. Quantitative assessment of endomyocardial biopsy in arrhythmogenic right ventricular cardiomyopathy/dysplasia: an in vitro validation of diagnostic criteria. Eur Heart J. 2008;29:2760–71. doi: 10.1093/eurheartj/ehn415. [DOI] [PubMed] [Google Scholar]
- 47.Bowles NE, Ni J, Marcus F, Towbin JA. The detection of cardiotropic viruses in the myocardium of patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol. 2002;39:892–5. doi: 10.1016/s0735-1097(02)01688-1. [DOI] [PubMed] [Google Scholar]
- 48.Andréoletti L, Ventéo L, Douche-Aourik F, et al. Active Coxsackieviral B infection is associated with disruption of dystrophin in endomyocardial tissue of patients who died suddenly of acute myocardial infarction. J Am Coll Cardiol. 2007;50:2207–14. doi: 10.1016/j.jacc.2007.07.080. [DOI] [PubMed] [Google Scholar]
- 49.Tam PE. Coxsackievirus myocarditis: interplay between virus and host in the pathogenesis of heart disease. Viral Immunol. 2006;19:133–46. doi: 10.1089/vim.2006.19.133. [DOI] [PubMed] [Google Scholar]
- 50.Bergelson JM, Cunningham JA, Drouguett G, et al. Isolation of a common receptor for Coxsackie B virus and adenoviruses types 2 and 5. Science. 1997;275:1320–3. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 51.Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. 2006;124:119–31. doi: 10.1016/j.cell.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 52.Martino TA, Petric M, Brown M, et al. Cardiovirulent coxsackieviruses and the decay-accelerating factor (CD55) receptor. Virology. 1998;244:302–14. doi: 10.1006/viro.1998.9122. [DOI] [PubMed] [Google Scholar]
- 53.Tracy S, Höfling K, Pirruccello S, Lane PH, Reyna SM, Gauntt CJ. Group B coxsackievirus myocarditis and pancreatitis: connection between viral virulence phenotypes in mice. J Med Virol. 2000;62:70–81. doi: 10.1002/1096-9071(200009)62:1<70::aid-jmv11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 54.Beck MA, Shi Q, Morris VC, Levander OA. Rapid genomic evolution of a nonvirulent coxsackievirus B3 in selenium-deficient mice results in selection of identical virulent isolates. Nat Med. 1995;1:433–6. doi: 10.1038/nm0595-433. [DOI] [PubMed] [Google Scholar]
- 55.Ilbäck NG, Wesslén L, Fohlman J, Friman G. Effects of methyl mercury on cytokines, inflammation and virus clearance in a common infection (coxsackie B3 myocarditis) Toxicol Lett. 1996;89:19–28. doi: 10.1016/s0378-4274(96)03777-0. [DOI] [PubMed] [Google Scholar]
- 56.Cooper LT, Rader V, Ralston NV. The roles of selenium and mercury in the pathogenesis of viral cardiomyopathy. Congest Heart Fail. 2007;13:193–9. doi: 10.1111/j.1527-5299.2007.06410.x. [DOI] [PubMed] [Google Scholar]
- 57.Gifford R, Dalldorf G. Morbid anatomy of experimental Coxsackie virus infection. Am J Pathol. 1951;27:1047–63. [PMC free article] [PubMed] [Google Scholar]
- 58.Gauntt CJ, Pallansch MA. Coxsackievirus B3 clinical isolates and murine myocarditis. Virus Res. 1996;41:89–99. doi: 10.1016/0168-1702(95)01250-8. [Erratum, Virus Res 1996;45:69.] [DOI] [PubMed] [Google Scholar]
- 59.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 60.Fuse K, Chan G, Liu Y, et al. Myeloid differentiation factor-88 plays a crucial role in the pathogenesis of Coxsackievirus B3-induced myocarditis and influences type I interferon production. Circulation. 2005;112:2276–85. doi: 10.1161/CIRCULATIONAHA.105.536433. [DOI] [PubMed] [Google Scholar]
- 61.Fairweather D, Frisancho-Kiss S, Rose NR. Viruses as adjuvants for autoimmunity: evidence from Coxsackievirus-induced myocarditis. Rev Med Virol. 2005;15:17–27. doi: 10.1002/rmv.445. [DOI] [PubMed] [Google Scholar]
- 62.Frisancho-Kiss S, Davis SE, Nyland JF, et al. Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol. 2007;178:6710–4. doi: 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]
- 63.Fairweather D, Frisancho-Kiss S, Gatewood S, et al. Mast cells and innate cytokines are associated with susceptibility to autoimmune heart disease following coxsackievirus B3 infection. Autoimmunity. 2004;37:131–45. doi: 10.1080/0891693042000196200. [DOI] [PubMed] [Google Scholar]
- 64.Eriksson U, Ricci R, Hunziker L, et al. Dendritic cell-induced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nat Med. 2003;9:1484–90. doi: 10.1038/nm960. [Erratum, Nat Med 2004;10: 105.] [DOI] [PubMed] [Google Scholar]
- 65.Kodama M, Hanawa H, Saeki M, et al. Rat dilated cardiomyopathy after autoimmune giant cell myocarditis. Circ Res. 1994;75:278–84. doi: 10.1161/01.res.75.2.278. [DOI] [PubMed] [Google Scholar]
- 66.Zehn D, Bevan MJ. T cells with a low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity. 2006;25:261–70. doi: 10.1016/j.immuni.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huber SA, Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J Virol. 1994;68:5126–32. doi: 10.1128/jvi.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–7. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 69.Rangachari M, Mauermann N, Marty RR, et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203:2009–19. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Opavsky MA, Penninger J, Aitken K, et al. Susceptibility to myocarditis is dependent on the response of alphabeta T lymphocytes to coxsackieviral infection. Circ Res. 1999;85:551–8. doi: 10.1161/01.res.85.6.551. [DOI] [PubMed] [Google Scholar]
- 71.Perens G, Levi DS, Alejos JC, Wetzel GT. Muronomab-CD3 for pediatric acute myocarditis. Pediatr Cardiol. 2007;28:21–6. doi: 10.1007/s00246-006-1322-3. [DOI] [PubMed] [Google Scholar]
- 72.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 73.Ono M, Shimizu J, Miyachi Y, Sakaguchi S. Control of autoimmune myocarditis and multiorgan inflammation by glucocorticoid-induced TNF receptor family-related protein(high), Foxp3-expressing CD25+ and CD25− regulatory T cells. J Immunol. 2006;176:4748–56. doi: 10.4049/jimmunol.176.8.4748. [DOI] [PubMed] [Google Scholar]
- 74.Huber SA, Feldman AM, Sartini D. Coxsackievirus B3 induces T regulatory cells, which inhibit cardiomyopathy in tumor necrosis factor-alpha transgenic mice. Circ Res. 2006;99:1109–16. doi: 10.1161/01.RES.0000249405.13536.49. [DOI] [PubMed] [Google Scholar]
- 75.Caforio AL, Mahon NJ, Tona F, Mc-Kenna WJ. Circulating cardiac autoantibodies in dilated cardiomyopathy and myocarditis: pathogenetic and clinical significance. Eur J Heart Fail. 2002;4:411–7. doi: 10.1016/s1388-9842(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 76.Schulze K, Becker BF, Schauer R, Schultheiss HP. Antibodies to ADP-ATP carrier — an autoantigen in myocarditis and dilated cardiomyopathy — impair cardiac function. Circulation. 1990;81:959–69. doi: 10.1161/01.cir.81.3.959. [DOI] [PubMed] [Google Scholar]
- 77.Li Y, Heuser JS, Cunningham LC, Kosanke SD, Cunningham MW. Mimicry and antibody-mediated cell signaling in autoimmune myocarditis. J Immunol. 2006;177:8234–40. doi: 10.4049/jimmunol.177.11.8234. [DOI] [PubMed] [Google Scholar]
- 78.Galvin JE, Hemric ME, Ward K, Cunningham MW. Cytotoxic mAb from rheumatic carditis recognizes heart valves and laminin. J Clin Invest. 2000;106:217–24. doi: 10.1172/JCI7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Antone SM, Adderson EE, Mertens NM, Cunningham MW. Molecular analysis of V gene sequences encoding cytotoxic anti-streptococcal/anti-myosin monoclonal antibody 36.2.2 that recognizes the heart cell surface protein laminin. J Immunol. 1997;159:5422–30. [PubMed] [Google Scholar]
- 80.Huber SA, Budd RC, Rossner K, Newell MK. Apoptosis in coxsackievirus B3-induced myocarditis and dilated cardiomyopathy. Ann N Y Acad Sci. 1999;887:181–90. doi: 10.1111/j.1749-6632.1999.tb07932.x. [DOI] [PubMed] [Google Scholar]
- 81.Badorff C, Lee GH, Lamphear BJ, et al. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat Med. 1999;5:320–6. doi: 10.1038/6543. [DOI] [PubMed] [Google Scholar]
- 82.Badorff C, Knowlton KU. Dystrophin disruption in enterovirus-induced myocarditis and dilated cardiomyopathy: from bench to bedside. Med Microbiol Immunol. 2004;193:121–6. doi: 10.1007/s00430-003-0189-7. [DOI] [PubMed] [Google Scholar]
- 83.Kim KS, Tracy S, Tapprich W, et al. 5′-Terminal deletions occur in coxsackievirus B3 during replication in murine hearts and cardiac myocyte cultures and correlate with encapsidation of negativestrand viral RNA. J Virol. 2005;79:7024–41. doi: 10.1128/JVI.79.11.7024-7041.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Y, Bourlet T, Andreoletti L, et al. Enteroviral capsid protein VP1 is present in myocardial tissues from some patients with myocarditis or dilated cardiomyopathy. Circulation. 2000;101:231–4. doi: 10.1161/01.cir.101.3.231. [DOI] [PubMed] [Google Scholar]
- 85.Smith SC, Ladenson JH, Mason JW, Jaffe AS. Elevations of cardiac troponin I associated with myocarditis: experimental and clinical correlates. Circulation. 1997;95:163–8. [PubMed] [Google Scholar]
- 86.Lauer B, Niederau C, Kühl U, et al. Cardiac troponin T in patients with clinically suspected myocarditis. J Am Coll Cardiol. 1997;30:1354–9. doi: 10.1016/s0735-1097(97)00317-3. [DOI] [PubMed] [Google Scholar]
- 87.Sheppard R, Bedi M, Kubota T, et al. Myocardial expression of fas and recovery of left ventricular function in patients with recent-onset cardiomyopathy. J Am Coll Cardiol. 2005;46:1036–42. doi: 10.1016/j.jacc.2005.05.067. [DOI] [PubMed] [Google Scholar]
- 88.Nishii M, Inomata T, Takehana H, et al. Serum levels of interleukin-10 on admission as a prognostic predictor of human fulminant myocarditis. J Am Coll Cardiol. 2004;44:1292–7. doi: 10.1016/j.jacc.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 89.Morgera T, Di Lenarda A, Dreas L, et al. Electrocardiography of myocarditis revisited: clinical and prognostic significance of electrocardiographic changes. Am Heart J. 1992;124:455–67. doi: 10.1016/0002-8703(92)90613-z. [DOI] [PubMed] [Google Scholar]
- 90.Nakashima H, Katayama T, Ishizaki M, Takeno M, Honda Y, Yano K. Q wave and non-Q wave myocarditis with special reference to clinical significance. Jpn Heart J. 1998;39:763–74. doi: 10.1536/ihj.39.763. [DOI] [PubMed] [Google Scholar]
- 91.Angelini A, Calzolari V, Calabrese F, et al. Myocarditis mimicking acute myocardial infarction: role of endomyocardial biopsy in the differential diagnosis. Heart. 2000;84:245–50. doi: 10.1136/heart.84.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mendes LA, Picard MH, Dec GW, Hartz VL, Palacios IF, Davidoff R. Ventricular remodeling in active myocarditis: Myocarditis Treatment Trial. Am Heart J. 1999;138:303–8. doi: 10.1016/s0002-8703(99)70116-x. [DOI] [PubMed] [Google Scholar]
- 93.Felker GM, Boehmer JP, Hruban RH, et al. Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol. 2000;36:227–32. doi: 10.1016/s0735-1097(00)00690-2. [DOI] [PubMed] [Google Scholar]
- 94.Mendes LA, Dec GW, Picard MH, Palacios IF, Newell J, Davidoff R. Right ventricular dysfunction: an independent predictor of adverse outcome in patients with myocarditis. Am Heart J. 1994;128:301–7. doi: 10.1016/0002-8703(94)90483-9. [DOI] [PubMed] [Google Scholar]
- 95.Laissy JP, Messin B, Varenne O, et al. MRI of acute myocarditis: a comprehensive approach based on various imaging sequences. Chest. 2002;122:1638–48. doi: 10.1378/chest.122.5.1638. [DOI] [PubMed] [Google Scholar]
- 96.Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998;97:1802–9. doi: 10.1161/01.cir.97.18.1802. [DOI] [PubMed] [Google Scholar]
- 97.Abdel-Aty H, Boyé P, Zagrosek A, et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45:1815–22. doi: 10.1016/j.jacc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 98.Hunt S. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46(6):e1–e82. doi: 10.1016/j.jacc.2005.08.022. [Erratum, J Am Coll Cardiol 2006;47:1503-5.] [DOI] [PubMed] [Google Scholar]
- 99.Adams K, Lindenfeld J, Arnold J, Heart Failure Society of America Executive summary: HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2006;12:10–38. doi: 10.1016/j.cardfail.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 100.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the task force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10:933–89. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 101.Farrar DJ, Holman WR, McBride LR, et al. Long-term follow-up of Thoratec ventricular assist device bridge-to-recovery patients successfully removed from support after recovery of ventricular function. J Heart Lung Transplant. 2002;21:516–21. doi: 10.1016/s1053-2498(01)00408-9. [DOI] [PubMed] [Google Scholar]
- 102.Chen YS, Yu HY. Choice of mechanical support for fulminant myocarditis: ECMO vs. VAD? Eur J Cardiothorac Surg. 2005;27:931–2. doi: 10.1016/j.ejcts.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 103.Topkara VK, Dang NC, Barili F, et al. Ventricular assist device use for the treatment of acute viral myocarditis. J Thorac Cardiovasc Surg. 2006;131:1190–1. doi: 10.1016/j.jtcvs.2005.08.073. [DOI] [PubMed] [Google Scholar]
- 104.Moloney ED, Egan JJ, Kelly P, Wood AE, Cooper LT., Jr Transplantation for myocarditis: a controversy revisited. J Heart Lung Transplant. 2005;24:1103–10. doi: 10.1016/j.healun.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 105.Cabinian AE, Kiel RJ, Smith F, Ho KL, Khatib R, Reyes MP. Modification of exercise-aggravated coxsackievirus B3 murine myocarditis by T lymphocyte suppression in an inbred model. J Lab Clin Med. 1990;115:454–62. [PubMed] [Google Scholar]
- 106.Maron BJ, Ackerman MJ, Nishimura RA, Pyeritz RE, Towbin JA, Udelson JE. Task Force 4: HCM and other cardiomyopathies, mitral valve prolapse, myocarditis, and Marfan syndrome. J Am Coll Cardiol. 2005;45:1340–5. doi: 10.1016/j.jacc.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 107.Saegusa S, Fei Y, Takahashi T, et al. Oral administration of candesartan improves the survival of mice with viral myocarditis through modification of cardiac adiponectin expression. Cardiovasc Drugs Ther. 2007;21:155–60. doi: 10.1007/s10557-007-6024-4. [DOI] [PubMed] [Google Scholar]
- 108.Tominaga M, Matsumori A, Okada I, Yamada T, Kawai C. Beta-blocker treatment of dilated cardiomyopathy: beneficial effect of carteolol in mice. Circulation. 1991;83:2021–8. doi: 10.1161/01.cir.83.6.2021. [DOI] [PubMed] [Google Scholar]
- 109.Khatib R, Reyes MP, Smith F, Khatib G, Rezkalla S. Enhancement of coxsackievirus B4 virulence by indomethacin. J Lab Clin Med. 1990;116:116–20. [PubMed] [Google Scholar]
- 110.Costanzo-Nordin MR, Reap EA, O’Connell JB, Robinson JA, Scanlon PJ. A nonsteroid anti-inflammatory drug exacerbates Coxsackie B3 murine myocarditis. J Am Coll Cardiol. 1985;6:1078–82. doi: 10.1016/s0735-1097(85)80312-0. [DOI] [PubMed] [Google Scholar]
- 111.Rezkalla S, Khatib R, Khatib G, et al. Effect of indomethacin in the late phase of coxsackievirus myocarditis in a murine model. J Lab Clin Med. 1988;112:118–21. [PubMed] [Google Scholar]
- 112.Zipes D, Camm A, Borggrefe M, et al. ACC/AHA/ESC 2006 guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114(10):e385–e484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 113.Kuhl U, Pauschinger M, Seeberg B, et al. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation. 2005;112:1965–70. doi: 10.1161/CIRCULATIONAHA.105.548156. [DOI] [PubMed] [Google Scholar]
- 114.Matsumori A, Crumpacker CS, Abelmann WH. Prevention of viral myocarditis with recombinant human leukocyte interferon alpha A/D in a murine model. J Am Coll Cardiol. 1987;9:1320–5. doi: 10.1016/s0735-1097(87)80472-2. [DOI] [PubMed] [Google Scholar]
- 115.Okada I, Matsumori A, Matoba Y, Tominaga M, Yamada T, Kawai C. Combination treatment with ribavirin and interferon for coxsackievirus B3 replication. J Lab Clin Med. 1992;120:569–73. [PubMed] [Google Scholar]
- 116.Ray CG, Icenogle TB, Minnich LL, Copeland JG, Grogan TM. The use of intravenous ribavirin to treat influenza virus-associated acute myocarditis. J Infect Dis. 1989;159:829–36. doi: 10.1093/infdis/159.5.829. [Erratum, J Infect Dis 1989;160:564.] [DOI] [PubMed] [Google Scholar]
- 117.Kühl U, Pauschinger M, Schwimmbeck PL, et al. Interferon-beta treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation. 2003;107:2793–8. doi: 10.1161/01.CIR.0000072766.67150.51. [DOI] [PubMed] [Google Scholar]
- 118.Mahrholdt H, Wagner A, Deluigi CC, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581–90. doi: 10.1161/CIRCULATIONAHA.105.606509. [DOI] [PubMed] [Google Scholar]
- 119.McNamara DM, Holubkov R, Starling RC, et al. Controlled trial of intravenous immune globulin in recent-onset dilated cardiomyopathy. Circulation. 2001;103:2254–9. doi: 10.1161/01.cir.103.18.2254. [DOI] [PubMed] [Google Scholar]
- 120.Drucker NA, Colan SD, Lewis AB, et al. Gamma-globulin treatment of acute myocarditis in the pediatric population. Circulation. 1994;89:252–7. doi: 10.1161/01.cir.89.1.252. [DOI] [PubMed] [Google Scholar]
- 121.Parrillo JE, Cunnion RE, Epstein SE, et al. A prospective, randomized, controlled trial of prednisone for dilated cardiomyopathy. N Engl J Med. 1989;321:1061–8. doi: 10.1056/NEJM198910193211601. [DOI] [PubMed] [Google Scholar]
- 122.Stanton C, Mookadam F, Cha S, et al. Greater symptom duration predicts response to immunomodulatory therapy in dilated cardiomyopathy. Int J Cardiol. 2008;128:38–41. doi: 10.1016/j.ijcard.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 123.Wojnicz R, Nowalany-Kozielska E, Wojciechowska C, et al. Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy: two-year follow-up results. Circulation. 2001;104:39–45. doi: 10.1161/01.cir.104.1.39. [DOI] [PubMed] [Google Scholar]
- 124.Torre-Amione G, Anker SD, Bourge RC, et al. Results of a non-specific immunomodulation therapy in chronic heart failure (ACCLAIM trial): a placebo-controlled randomised trial. Lancet. 2008;371:228–36. doi: 10.1016/S0140-6736(08)60134-8. [DOI] [PubMed] [Google Scholar]
- 125.Staudt A, Hummel A, Ruppert J, et al. Immunoadsorption in dilated cardiomyopathy: 6-month results from a randomized study. Am Heart J. 2006;152(4):712e1–712.e6. doi: 10.1016/j.ahj.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 126.Oberg AL, Mahoney DW, Eckel-Passow JE, et al. Statistical analysis of relative labeled mass spectrometry data from complex samples using ANOVA. J Proteome Res. 2008;7:225–33. doi: 10.1021/pr700734f. [DOI] [PMC free article] [PubMed] [Google Scholar]


