Key Points
Question
Is there an association between exposure to pesticide residues in fruits and vegetables and pregnancy outcomes?
Findings
In a cohort of 325 women undergoing infertility treatment with assisted reproductive technology, intake of high–pesticide residue fruits and vegetables was associated with a lower probability of live birth, while low–pesticide residue fruit and vegetable intake was not associated with this outcome.
Meaning
Dietary pesticide exposure within the range of typical human exposure may be associated with adverse reproductive consequences.
This cohort study examines the association of preconception intake of pesticide residues in fruits and vegetables with outcomes of infertility treatment with assisted reproductive technologies.
Abstract
Importance
Animal experiments suggest that ingestion of pesticide mixtures at environmentally relevant concentrations decreases the number of live-born offspring. Whether the same is true in humans is unknown.
Objective
To examine the association of preconception intake of pesticide residues in fruits and vegetables (FVs) with outcomes of infertility treatment with assisted reproductive technologies (ART).
Design, Setting, and Participants
This analysis included 325 women who completed a diet assessment and subsequently underwent 541 ART cycles in the Environment and Reproductive Health (EARTH) prospective cohort study (2007-2016) at a fertility center at a teaching hospital. We categorized FVs as having high or low pesticide residues using a validated method based on surveillance data from the US Department of Agriculture. Cluster-weighted generalized estimating equations were used to analyze associations of high– and low–pesticide residue FV intake with ART outcomes.
Main Outcomes and Measures
Adjusted probabilities of clinical pregnancy and live birth per treatment cycle.
Results
In the 325 participants (mean [SD] age, 35.1 [4.0] y; body mass index, 24.1 [4.3]), mean (SD) intakes of high– and low–pesticide residue FVs were 1.7 (1.0) and 2.8 (1.6) servings/d, respectively. Greater intake of high–pesticide residue FVs was associated with a lower probability of clinical pregnancy and live birth. Compared with women in the lowest quartile of high-pesticide FV intake (<1.0 servings/d), women in the highest quartile (≥2.3 servings/d) had 18% (95% CI, 5%-30%) lower probability of clinical pregnancy and 26% (95% CI, 13%-37%) lower probability of live birth. Intake of low–pesticide residue FVs was not significantly related to ART outcomes.
Conclusions and Relevance
Higher consumption of high–pesticide residue FVs was associated with lower probabilities of pregnancy and live birth following infertility treatment with ART. These data suggest that dietary pesticide exposure within the range of typical human exposure may be associated with adverse reproductive consequences.
Introduction
More than 90% of the US population has detectable concentrations of pesticides or their metabolites in their urine or blood samples. While pesticide exposure occurs through a variety of routes, the primary route in the general population is through diet–especially intake of conventionally grown fruits and vegetables (FVs). In the United States, pesticides are regulated and evaluated by the US Environmental Protection Agency to ensure the safety of the food supply for human consumption. Nonetheless, there has been a growing concern that permitted levels of pesticide residues in food defined by traditional toxicological testing may be too high, especially for susceptible populations such as pregnant women or infants.
In rodent models, ingestion of pesticide mixtures in early pregnancy at a concentration assumed to be without adverse health effects increased the percentage of apoptosis in embryos and decreased the number of live pups born. Evidence from human studies is scarce. Women occupationally exposed to pesticides and women living in or near agricultural areas may have increased risk of infertility and adverse pregnancy outcomes. However, whether exposure within the range of typical human exposure, such as through diet, has any effect on reproductive outcomes in humans is unknown.
We previously developed and validated a low-cost, questionnaire-based method—the Pesticide Residue Burden Score (PRBS)—to estimate exposure to pesticide residues from FVs in epidemiologic studies. In the present study, we aimed to investigate the associations between preconception intake of high– and low–pesticide residue FVs and outcomes of assisted reproductive technologies (ART) in a prospective cohort of women undergoing infertility treatment.
Methods
Study Population
Women in this study were participants in the Environment and Reproductive Health (EARTH) Study, an ongoing prospective cohort established in 2006 to identify determinants of fertility among couples presenting to the Massachusetts General Hospital Fertility Center (Boston, Massachusetts). Women were eligible to participate if they were between 18 and 45 years and planned to use their own gametes for infertility treatment. Women whose treating physician later determined that using donor eggs was clinically necessary remained in the study. Among women referred by physicians, approximately 60% of those approached by the research nurses enrolled in the study. Diet assessment was introduced to the study in 2007. The current analysis includes 325 women (contributing 541 ART cycles) whose diet was assessed and who contributed at least 1 subsequent ART cycle between April 2007 and August 2016. Women who did not complete a diet assessment (n = 113) or whose ART cycles started prior to assessment completion (n = 7) were excluded from the present analysis. The study was approved by the Human Studies Institutional Review Boards of the Massachusetts General Hospital, Harvard T.H. Chan School of Public Health, and the Centers for Disease Control and Prevention. All participants signed an informed consent after the study procedures were explained by trained study staff.
On entry, height and weight were measured by trained study staff to calculate body mass index (BMI, calculated as weight in kilograms divided by height in meters squared). Study staff also administered a brief questionnaire to collect data on demographic characteristics, medical history, and lifestyle factors. Participants completed a detailed take-home questionnaire with additional questions on reproductive history and lifestyle factors. On this take-home questionnaire, participants were asked how often they consumed organic FVs during the past 3 months. We considered women to be organic FV consumers if they consumed organic FVs at least 3 times per week (the median in this population); women with lower intake of organic FVs (<3 times/wk) were considered to be conventional FV consumers.
Outcome Assessment
Clinical information was abstracted by trained study staff from the patients’ electronic medical records. We have previously described details of patient clinical management elsewhere. Briefly, clinical staff monitored patients during gonadotropin stimulation for serum estradiol, follicle size and counts, and endometrial thickness for 2 days before oocyte retrieval, and administered human chorionic gonadotropin (β-hCG) to induce ovulation approximately 36 hours before oocyte retrieval. Embryologists classified oocytes as germinal vesicle, metaphase I, metaphase II (MII), or degenerated, and determined fertilization rate as the number of oocytes with 2 pronuclei divided by the number of MII oocytes at 17 to 20 hours after insemination. Cell cleavage rates of embryos were considered to be normal with a division of 2 to 4 cells on day 2 and 4 to 8 cells on day 3 of culture. A division below 2 cells on day 2 and 6 cells on day 3 was considered as slow while a division of 4 or more cells on day 2 and 8 or more cells on day 3 was designated accelerated. For this study, early ART end points referred to any end points prior to embryo transfer, including markers of ovarian responses to stimulation (peak estradiol levels, endometrial thickness, MII and total oocytes), fertilization rate, and embryo quality. We excluded egg donor and cryogenic cycles for the analysis of early ART end points.
Clinical outcomes were assessed per initiated cycle, including implantation (defined as a serum β-hCG level >6 mIU/mL [to convert to IU/L, multiply by 1.0] typically measured approximately 17 days after oocyte retrieval), clinical pregnancy (defined as presence of intrauterine gestational sac[s] on ultrasonography at 6 weeks), and live birth (as the birth of a neonate on or after 24 weeks of gestation).
We categorized total pregnancy loss into (1) early pregnancy loss, defined as a positive urine β-hCG test followed by the absence of signs of clinical pregnancy, including chemical pregnancy loss and ectopic pregnancy; and (2) clinical pregnancy loss, defined as an intrauterine pregnancy demise after a clinical pregnancy, including spontaneous abortion, stillbirth, and therapeutic abortion. No molar pregnancies occurred in this cohort.
Exposure Assessment
Diet was assessed before initiation of ART using a self-administered, previously validated food frequency questionnaire. Women reported how often they typically consumed specified amounts of each food, beverage, and supplement over the past year. Two data-derived dietary pattern scores, the prudent and Western pattern, were used to summarize overall food choices.
We used the annual reports from the US Department of Agriculture Pesticide Data Program (PDP) to classify FVs according to their mean pesticide residue status in the US food supply. Details of the PRBS methods have been described elsewhere. We considered 3 measures of contamination from the PDP to classify FVs: (1) the percentage of samples tested with any detectable pesticides, (2) the percentage of samples tested with pesticides exceeding the tolerance level, and (3) the percentage of samples with 3 or more individual detectable pesticides. The pesticide residue data in FVs were averaged by annual PDP reports from 2006 through 2015, corresponding to the periods when the diet history of the participants was captured by the food frequency questionnaire.
Next, we categorized foods according to tertiles for each of the 3 measurements of contamination and assigned a score of 0 to FVs in the bottom tertile, 1 to FVs in the middle tertile, and 2 for FVs in the top tertile. The PRBS for each food was the sum of scores across the 3 PDP contamination measures. We considered FVs with a PRBS of 4 or greater on a scale of 0 to 6 to be high–pesticide residue foods while FVs with a PRBS of less than 4 to be low–pesticide residue foods. Based on these criteria, 14 FVs were categorized as high pesticide residue and 22 as low pesticide residue (Table 1).
Table 1. Fruit and Vegetable Items in the Food Frequency Questionnaire (FFQ) and Pesticide Data Program (PDP), and Corresponding Scores for First, Second, and Third Measure, and Pesticide Residue Burden Score (PRBS).
| Definition of Measure Contamination | Score | PRBS | |||
|---|---|---|---|---|---|
| Items in FFQ | Items in PDP | First | Second | Third | |
| Peas or lima beans, FFC | Sweet pea, frozen | 0 | 0 | 0 | 0 |
| Dried plums or prunes | Dried plum | 0 | 0 | 0 | 0 |
| Onions | Onions | 0 | 0 | 0 | 0 |
| Beans or lentils | Beans | 0 | 0 | 0 | 0 |
| Avocado | Avocado | 0 | 0 | 0 | 0 |
| Corn, FFC | Corn, frozen | 0 | 0 | 0 | 0 |
| Cabbage or cole slaw | Cabbage | 0 | 0 | 0 | 0 |
| Orange juice, regular or calcium fortified | Orange juice | 0 | 0 | 0 | 0 |
| Tomato sauce | Tomato paste | 0 | 0 | 0 | 0 |
| Apple juice or cider | Apple juice | 0 | 0 | 1 | 1 |
| Cauliflower | Cauliflower | 1 | 0 | 0 | 1 |
| Grapefruit | Grapefruit | 1 | 0 | 0 | 1 |
| Cantaloupe | Cantaloupe | 0 | 1 | 1 | 2 |
| Tofu | Soybeans | 2 | 0 | 0 | 2 |
| Bananas | Bananas | 1 | 1 | 1 | 3 |
| Eggplant, summer squash, zucchini | Eggplant, summer squash, 0.5:0.5a | 0 | 2 | 1 | 3 |
| Yam or sweet potatoes | Sweet potatoes | 1 | 2 | 0 | 3 |
| Oranges | Oranges | 2 | 0 | 1 | 3 |
| Broccoli | Broccoli | 1 | 1 | 1 | 3 |
| Carrots | Carrots | 1 | 0 | 2 | 3 |
| Head lettuce, leaf lettuce | Lettuce | 1 | 0 | 2 | 3 |
| Celery | Celery | 1 | 0 | 2 | 3 |
| Tomatoes | Tomatoes | 1 | 2 | 1 | 4 |
| Apple sauce | Apple sauce | 2 | 0 | 2 | 4 |
| Blueberry, FFC | Blueberry, fresh, frozen, 0.5:0.5a | 2 | 0 | 2 | 4 |
| Kale, mustard, chard greens | Kale | 1 | 2 | 1 | 4 |
| Winter squash | Winter squash | 1 | 2 | 1 | 4 |
| Fresh apple or pear | Apple, pear, 0.7:0.3a | 2 | 1 | 2 | 5 |
| String beans | Green beans | 1 | 2 | 2 | 5 |
| Grape or raisin | Grape, raisin, 0.6:0.4a | 2 | 1 | 2 | 5 |
| Potatoes | Potatoes | 2 | 2 | 1 | 5 |
| Spinach, cooked | Spinach, frozen | 1 | 2 | 2 | 5 |
| Peach or plum | Peach, plum, 0.7:0.3a | 2 | 2 | 2 | 6 |
| Strawberries, FFC | Strawberries, fresh | 2 | 2 | 2 | 6 |
| Spinach, raw | Spinach, fresh | 2 | 2 | 2 | 6 |
| Green/yellow/red peppers | Sweet peppers | 2 | 2 | 2 | 6 |
Abbreviation: FFC, fresh, frozen, or canned.
Ratio weighted for pesticide residue for each produce according to the ratio of consumption of each produce from the US Department of Agriculture report.
Statistical Analysis
Women were classified according to quartiles of total FV intake, high–pesticide residue FV intake, and low–pesticide residue FV intake. We conducted Kruskal-Wallis tests (for continuous variables) and Fisher exact tests (for categorical variables) to compare baseline characteristics across quartiles of FV intake. To evaluate the relationship of FV intake with ART outcomes, we used cluster-weighted generalized estimating equations to account for within-person correlations in the presence of nonignorable cluster size. Each observation was weighted inversely to the number of cycles they contributed to the analysis. We evaluated ART outcomes per initiated cycle to estimate effects relevant in practice and mirror intention-to-treat analyses for studies of ART. However, in a post-hoc analysis, we evaluated the association of FV intake with risk of pregnancy loss only among cycles in which implantation was achieved. Population marginal means were used to present population averages adjusted for the covariates at their average levels for continuous variables and weighted average levels of categorical variables in the model. Tests for linear trend were performed using the median intake of FVs in each quartile as a continuous variable.
Confounding was evaluated using directed acyclic graphs based on prior knowledge.
Specifically, variables previously reported to be associated with live birth/pregnancy loss as well as associated with FV intake were considered as potential confounders. In addition, we included dietary pattern scores to distinguish relations between FV intake from those of overall food choices. The final multivariable models were adjusted for age (years), BMI, smoking status (current/former vs never), race (white vs nonwhite), supplemental folate intake (micrograms per day), organic FV consumption frequency (<3 vs ≥3 times/wk), residential pesticide exposure history (yes vs no), prudent and Western dietary patterns, total energy intake (kilocalories per day), and infertility diagnosis (male factor vs female factor vs unexplained). The model for high–pesticide residue FV intake was additionally adjusted for low-pesticide FV intake and vice versa because they may confound each other. To minimize residual confounding, we performed separate sensitivity analyses restricting to women younger than 40 years, women without a history of miscarriage, autologous cycles, and cycles initiated within 1 year of food frequency questionnaire completion. We also estimated the effect of substituting 1 serving/d of low–pesticide residue FVs for high–pesticide residue FVs on clinical outcomes. All statistical analyses were performed in SAS, version 9.4 (SAS Institute). P values were 2 sided. Findings were considered statistically significant when P < .05.
Results
A total of 325 women underwent 541 ART cycles (range, 1-6), of which 228 (42%) resulted in a live birth (eFigure 1 in the Supplement). Women had a mean (SD) intake of 1.7 (1.0) servings/d of high–pesticide residue FVs and 2.8 (1.6) servings/d of low–pesticide residue FVs. Intakes of high– and low–pesticide residue FVs were positively correlated with each other (Spearman r = 0.57). Women who consumed more high–pesticide residue FVs were more likely to report regular organic FV consumption, had higher total calorie and micronutrient intake, higher adherence to the prudent dietary pattern, and a slightly higher prevalence of diminished ovarian reserve. Similar trends were observed with greater intake of low–pesticide residue FVs except that no difference in prevalence of diminished ovarian reserve was observed. Other characteristics were similar across quartiles of high- or low-pesticide FV intake (Table 2).
Table 2. Demographic, Dietary, and Reproductive Characteristics of the Study Population According to Quartiles of High– and Low–Pesticide Residue Fruit and Vegetable (FV) Intake Among 325 Women in the Environment and Reproductive Health (EARTH) Study.
| Characteristic | Overall (N = 325) |
High–Pesticide Residue FV Intake | Low–Pesticide Residue FV Intake | ||||
|---|---|---|---|---|---|---|---|
| Q1 (n = 81) |
Q4 (n = 81) |
P Valuea | Q1 (n = 82) |
Q4 (n = 81) |
P Valuea | ||
| Intake, median (range), servings/d | 0.5 (0.3-1.0) | 2.2 (2.3-6.8) | 1.4 (0.5-1.7) | 4.6 (3.6-11.5) | |||
| Demographic | |||||||
| Age, mean (SD), y | 35.1 (4.0) | 35.3 (4.2) | 35.3 (3.8) | .08 | 35.1 (3.8) | 34.7 (4.2) | .28 |
| BMI, mean (SD) | 24.1 (4.3) | 23.1 (2.7) | 24.3 (5.2) | .51 | 23.8 (3.3) | 24.2 (4.8) | .86 |
| White race, No. (%) | 272 (84) | 71 (88) | 61 (75) | .16 | 71 (88) | 63 (78) | .25 |
| Education,b No. (%) | |||||||
| Less than college graduate | 29 (9) | 5 (6) | 8 (10) | .12 | 8 (10) | 11 (14) | .52 |
| College graduate | 98 (31) | 22 (28) | 21 (26) | 27 (35) | 21 (27) | ||
| Graduate degree | 188 (60) | 52 (66) | 51 (64) | 42 (55) | 47 (60) | ||
| Never smokers, No. (%) | 235 (72) | 55 (68) | 61 (75) | .35 | 54 (67) | 52 (64) | .05 |
| Residential pesticide use, No. (%) | 244 (75) | 61 (75) | 56 (69) | .32 | 64 (79) | 57 (70) | .62 |
| Dietary | |||||||
| Organic FV consumers, No. (%) | 118 (36) | 11 (14) | 45 (56) | <.001 | 17 (21) | 47 (58) | <.001 |
| Intake, mean (SD) | |||||||
| Alcohol, g/d | 8.9 (10.7) | 8.6 (12.8) | 9.9 (11.2) | .44 | 8.2 (11.7) | 11.1 (12.1) | .14 |
| Caffeine, g/d | 126.6 (107.7) | 131.6 (122.6) | 130 (103.7) | .48 | 132.8 (112.2) | 138.7 (106.0) | .11 |
| Supplemental folate, μg/d | 630 (401) | 602 (354) | 641 (394) | .91 | 627 (415) | 644 (371) | .63 |
| Vitamin A, IU/d | 10847 (6626) | 6046 (2610) | 17036 (8088) | <.001 | 5725 (2194) | 18236 (7733) | <.001 |
| Vitamin C, mg/d | 102.2 (58.5) | 61.9 (27.9) | 146.6 (69.1) | <.001 | 58.4 (25.4) | 163.0 (67.4) | <.001 |
| Beta carotene, μg/d | 5292 (3555) | 2730 (1393) | 8703 (4365) | <.001 | 2621 (1236) | 9198 (4178) | <.001 |
| Beta cryptoxanthin, μg/d | 104.6 (93.1) | 60.3 (41.2) | 141.7 (124.0) | <.001 | 49.8 (32.1) | 189.3 (130.1) | <.001 |
| Lycopene, μg/d | 4803 (3223) | 3566 (2176) | 5852 (3869) | <.001 | 3321 (1743) | 6225 (3900) | <.001 |
| Lutein and zeaxanthin, μg/d | 4233 (3319) | 2058 (927) | 7653 (4374) | <.001 | 5725 (2194) | 6947 (4206) | <.001 |
| Use of multivitamin, No. (%) | 283 (88) | 72 (90) | 70 (88) | .81 | 71 (88) | 73 (91) | .31 |
| Total energy intake, mean (SD), kcal/d | 1800 (584) | 1472 (438) | 2077 (625) | <.001 | 1792 (899) | 1939 (732) | <.001 |
| Prudent pattern score, mean (SD) | 0.0 (1) | −0.9 (0.4) | 1.0 (1.1) | <.001 | −0.8 (0.5) | 1.1 (1.1) | <.001 |
| Western pattern score, mean (SD) | 0.0 (0.9) | −0.1 (0.7) | −0.2 (1.1) | .12 | −0.1 (0.8) | 0.0 (1.2) | .68 |
| Baseline Reproductive | |||||||
| Prior miscarriage history, No. (%) | 69 (21) | 15 (19) | 19 (23) | .84 | 12 (15) | 21 (26) | .29 |
| Infertility diagnosis, No. (%) | |||||||
| Male factor | 106 (33) | 18 (22) | 21 (26) | .03 | 21 (26) | 23 (28) | .24 |
| Female factor | 95 (29) | 25 (31) | 30 (37) | 27 (33) | 27 (33) | ||
| Diminished ovarian reserve | 26 (8) | 7 (9) | 11 (14) | 6 (7) | 7 (9) | ||
| Ovulatory | 26 (8) | 5 (6) | 7 (9) | 9 (11) | 7 (9) | ||
| Tubal | 23 (7) | 7 (9) | 9 (11) | 9 (11) | 8 (10) | ||
| Uterine | 5 (2) | 3 (4) | 0 | 2 (2) | 0 | ||
| Endometriosis | 15 (5) | 3 (4) | 3 (4) | 1 (1) | 5 (6) | ||
| Unexplained | 124 (38) | 38 (47) | 30 (37) | 33 (40) | 31 (38) | ||
| Initial treatment protocol, No. (%) | |||||||
| Antagonist | 37 (11) | 9 (11) | 12 (15) | .34 | 9 (11) | 11 (14) | .84 |
| Flarec | 35 (11) | 5 (6) | 7 (9) | 11 (14) | 6 (7) | ||
| Luteal phase agonistd | 253 (78) | 67 (83) | 62 (77) | 61 (75) | 64 (79) | ||
| Embryo transfer day, No. (%) | |||||||
| No embryos transferred | 31 (10) | 11 (14) | 6 (7) | .42 | 10 (12) | 5 (6) | .44 |
| Day 2 | 16 (5) | 3 (4) | 5 (6) | 5 (6) | 5 (6) | ||
| Day 3 | 132 (41) | 28 (35) | 35 (44) | 33 (41) | 34 (42) | ||
| Day 5 | 121 (37) | 31 (38) | 28 (35) | 29 (36) | 33 (41) | ||
| Egg donor or cryogenic cycle | 24 (7) | 8 (10) | 6 (7) | 4 (5) | 4 (5) | ||
| Embryos transferred, No. (%) | |||||||
| 0 | 31 (10) | 11 (14) | 6 (7) | .24 | 10 (12) | 5 (6) | .29 |
| 1 | 64 (20) | 9 (11) | 20 (25) | 11 (14) | 18 (22) | ||
| 2 | 157 (48) | 44 (54) | 36 (45) | 42 (52) | 43 (53) | ||
| ≥3 | 48 (15) | 9 (11) | 12 (15) | 14 (17) | 11 (14) | ||
| Egg donor or cryogenic cycle | 24 (7) | 8 (10) | 6 (7) | 4 (5) | 4 (5) | ||
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; GnRH, gonadotropin-releasing hormone; Q, quartile.
For continuous variables, a Kruskal-Wallis test was used to compare characteristics across quartiles of fruit and vegetable intake. For categorical variables, a Fisher exact test was used to compare characteristics across quartiles of fruit and vegetable intake.
Ten women had missing data on education.
Follicular phase GnRH agonist/flare protocol.
Luteal phase GnRH agonist protocol.
Total FV intake was unrelated to probability of implantation, clinical pregnancy, and live birth (Table 3). However, when FVs were classified as having high or low pesticide residues, divergent patterns of associations with clinical pregnancy and live birth emerged (Table 3). Specifically, high–pesticide residue FV intake was inversely associated with probability of clinical pregnancy and live birth per initiated cycle. Compared with women in the lowest quartile of high–pesticide residue FV intake (<1 serving/d), women in the highest quartile (≥2.3 servings/d) had 18% (95% CI, 5%-30%) lower probability of clinical pregnancy and 26% (95% CI, 13%-37%) lower probability of live birth. These associations persisted in sensitivity analyses (eTable 1 in the Supplement). Low–pesticide residue FV intake was associated with a higher, albeit nonsignificant, probability of clinical pregnancy and live birth (Table 3). We found no associations between intake of high– or low–pesticide residue FVs with markers of response to ovarian stimulation, fertilization rate, or embryo quality (eTable 2 and eTable 3 in the Supplement).
Table 3. Clinical Outcomes per Initiated Cycle According to Fruit and Vegetable Intake, Considering Pesticide Residue Status, Among 325 Women (Contributing 541 Cycles) From the Environment and Reproductive Health (EARTH) Study.
| Fruit and Vegetable Intake, Q (Range), Servings/d | Probability (95% CI) | |||||
|---|---|---|---|---|---|---|
| Implantation | Clinical Pregnancy | Live Birth | ||||
| Unadjusted | Adjusteda,b | Unadjusted | Adjusteda,b | Unadjusted | Adjusteda,b | |
| Total | ||||||
| Q1 (0.7-2.7) | 0.70 (0.61-0.78) | 0.71 (0.59-0.8) | 0.64 (0.55-0.72) | 0.63 (0.51-0.74) | 0.54 (0.45-0.63) | 0.51 (0.39-0.63) |
| Q2 (2.7-3.8) | 0.64 (0.55-0.73) | 0.64 (0.54-0.73) | 0.60 (0.51-0.69) | 0.60 (0.49-0.69) | 0.53 (0.43-0.62) | 0.51 (0.41-0.61) |
| Q3 (3.9-5.3) | 0.68 (0.59-0.76) | 0.69 (0.6-0.77) | 0.64 (0.55-0.72) | 0.65 (0.55-0.73) | 0.57 (0.48-0.66) | 0.60 (0.50-0.69) |
| Q4 (5.3-14.9) | 0.6 (0.51-0.69) | 0.59 (0.44-0.72) | 0.56 (0.46-0.65) | 0.56 (0.41-0.69) | 0.47 (0.37-0.57) | 0.46 (0.32-0.61) |
| P value for trendc | .20 | .38 | .24 | .62 | .37 | .80 |
| High pesticide residued | ||||||
| Q1 (0.3-1.0) | 0.69 (0.59-0.78) | 0.70 (0.57-0.80) | 0.67 (0.56-0.76) | 0.67 (0.55-0.77) | 0.65 (0.54-0.75) | 0.65 (0.52-0.76) |
| Q2 (1.0-1.6) | 0.74 (0.65-0.81) | 0.74 (0.65-0.82) | 0.71 (0.62-0.78) | 0.70 (0.60-0.78) | 0.58 (0.49-0.67) | 0.55 (0.45-0.64) |
| Q3 (1.6-2.2) | 0.64 (0.55-0.73) | 0.64 (0.55-0.73) | 0.58 (0.49-0.67) | 0.58 (0.48-0.67) | 0.49 (0.40-0.59) | 0.49 (0.39-0.59) |
| Q4 (2.3-6.8) | 0.55 (0.45-0.65) | 0.56 (0.43-0.68) | 0.48 (0.38-0.58) | 0.49 (0.37-0.62) | 0.39 (0.29-0.50) | 0.39 (0.28-0.52) |
| P value for trendc | .02 | .08 | .004 | .04 | .002 | .02 |
| Low pesticide residuee | ||||||
| Q1 (0.5-1.7) | 0.58 (0.47-0.68) | 0.59 (0.48-0.7) | 0.50 (0.40-0.60) | 0.50 (0.39-0.61) | 0.39 (0.29-0.49) | 0.38 (0.28-0.50) |
| Q2 (1.7-2.5) | 0.71 (0.62-0.79) | 0.71 (0.6-0.79) | 0.66 (0.56-0.75) | 0.65 (0.54-0.74) | 0.56 (0.46-0.66) | 0.56 (0.45-0.66) |
| Q3 (2.5-3.5) | 0.64 (0.55-0.73) | 0.65 (0.56-0.73) | 0.62 (0.53-0.71) | 0.63 (0.53-0.71) | 0.57 (0.48-0.67) | 0.58 (0.48-0.67) |
| Q4 (3.6-11.5) | 0.70 (0.60-0.78) | 0.70 (0.56-0.81) | 0.66 (0.56-0.75) | 0.67 (0.53-0.78) | 0.59 (0.48-0.69) | 0.57 (0.43-0.69) |
| P value for trendc | .24 | .43 | .09 | .16 | .05 | .14 |
Abbreviation: Q, quartile.
Model was adjusted for age, body mass index, smoking status, race, folate supplementation, organic fruit and vegetable consumption frequency, residential pesticide exposure history, total energy intake, Western and prudent pattern scores, and infertility diagnosis. Adjusted means are calculated at the mean level of continuous covariates and weighted average over levels of categorical covariates.
Adjusted proportions were calculated at mean levels for continuous covariates and weighted average over categorical covariates.
Tests for trend were performed using the median intake in each quartile as a continuous variable in the model.
Model additionally adjusted for low-pesticide fruit and vegetable intake.
Model additionally adjusted for high-pesticide fruit and vegetable intake.
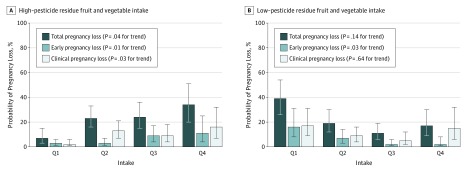
Next, we examined the associations of FV intake with risks of pregnancy loss (Figure 1). High–pesticide residue FV intake was positively associated with probability of total pregnancy loss. The adjusted probabilities of total pregnancy loss were 7% (95% CI, 3%-15%), 23% (95% CI, 16%-33%), 24% (95% CI, 15%-36%), and 34% (95% CI, 20%-51%) for women in increasing quartiles of high–pesticide residue FV intake (P = .04 for trend). When total pregnancy loss was divided into early and clinical pregnancy loss, the trends were similar. On the other hand, low–pesticide residue FV intake was inversely associated with early pregnancy loss but unrelated to clinical pregnancy loss.
Figure 1. Probabilities of Total, Early, and Clinical Pregnancy Loss According to High– or Low–Pesticide Residue Fruit and Vegetable Intake Among 256 Women With Successful Implantation (316 Cycles) From the EARTH Study.
Data are presented as predicted probabilities in each quartile (Q) adjusting for age, body mass index, smoking status, race, folate supplementation, organic fruit and vegetable consumption frequency, residential pesticide exposure history, total energy intake, Western and prudent pattern scores, and infertility diagnosis. The model for high–pesticide residue fruit and vegetable intake was additionally adjusted for low–pesticide residue fruit and vegetable intake and vice versa. Error bars indicate 95% confidence interval.
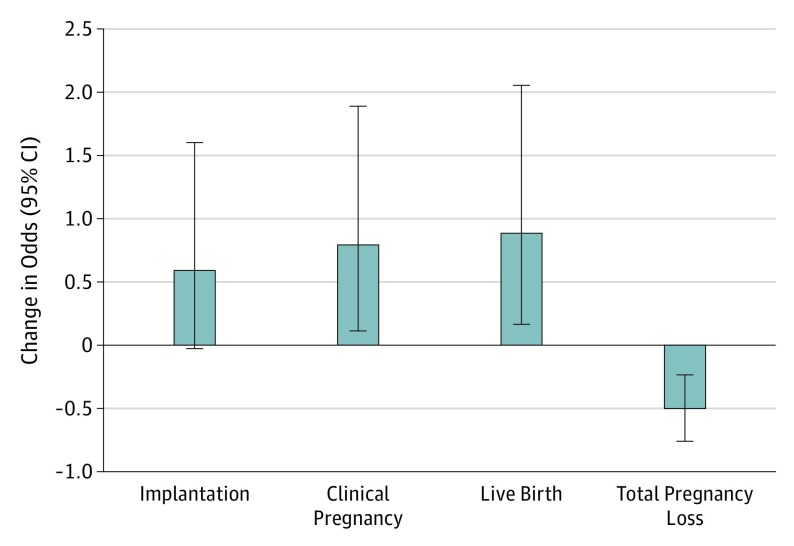
Last, we estimated the effect of replacing high–pesticide residue FVs with low–pesticide residue FVs on the odds of clinical outcomes (Figure 2). Consuming 1 serving/d of low–pesticide residue FVs in lieu of 1 serving/d of high–pesticide residue FVs was associated with 79% (95% CI, 11%-188%) higher odds of clinical pregnancy and 88% (95% CI, 16%-205%) higher odds of live birth.
Figure 2. Estimated Changes in Odds Ratios of Clinical Outcomes by Replacing 1 Serving/d of High–Pesticide Residue Fruits and Vegetables With 1 Serving/d of Low–Pesticide Residue Fruits and Vegetables.
Data were adjusted for age, body mass index, smoking status, race, folate supplementation, organic fruit and vegetable consumption frequency, residential pesticide exposure history, total energy intake, Western and prudent pattern scores, and infertility diagnosis. Error bars indicate 95% confidence interval.
Discussion
We evaluated the association between preconception intake of FVs, considering their pesticide residue status, and ART outcomes among women undergoing infertility treatment. We observed that greater intake of high–pesticide residue FVs was associated with lower probabilities of clinical pregnancy and live birth per initiated cycle. The observed association with live births was driven by a higher risk of early and clinical pregnancy loss. On the other hand, low–pesticide residue FV intake was associated with a lower risk of early pregnancy loss. Replacing high–pesticide residue FVs with low–pesticide residue FVs was estimated to provide the greatest benefit for achieving clinical pregnancy and live birth.
While FVs are an important part of a healthy diet, they also serve as the primary vehicle for pesticide residue exposure in the general population. Earlier studies have shown that many pesticides used in agriculture have deleterious effects on reproductive health outcomes, such as decreased fertility, spontaneous abortion, stillbirth, or developmental abnormalities, while a few others reported no associations. Of note, in one of these studies, among 684 participants (73 cases, 611 controls) from agricultural counties of California, Bell et al found that the adjusted odds ratio of fetal death for those exposed to 3 or more pesticide classes was 2.6 (95% CI, 1.3-5.3), while those exposed to 1 or 2 pesticide classes had an odds ratio of 1.1 (95% CI, 0.6-2.1). In another study of women living on Ontario farms, Arbuckle et al showed that exposure to both fungicides and herbicides before conception doubled the risk of spontaneous abortion relative to women exposed only to fungicides, suggesting that pesticide mixtures may confer a greater risk of fetal loss. Nonetheless, most of these studies have focused on occupational workers or women living in or near agricultural areas. The influence of exposure to pesticide residues primarily through foods on pregnancy outcomes in the general population remains unknown.
To the best of our knowledge, this is the first prospective study evaluating the relationship of dietary pesticide exposure to reproductive success in humans. The most closely related study to ours is a prospective study of 28 192 Norwegian women, which found that women choosing organically grown vegetables during pregnancy had reduced risk of preeclampsia regardless of adjustment for various healthy food scores. One possible explanation was that organic vegetable consumption may reduce exposure to pesticides. Some forms of miscarriage and preeclampsia may be related, representing a continuum whose origin is oxidative stress–induced placental dysfunction. Pesticide-induced placental dysfunction may explain the relationship of lower rates of clinical pregnancy loss associated with lower intake of high–pesticides FVs in the present study, as well as lower prevalence of preeclampsia associated with organic vegetable consumption in the earlier study. However, given the paucity of the data, future studies are warranted to replicate these findings.
Our results are also in agreement with experimental animal data. Cavieres et al showed that pregnant mice exposed to a pesticide mixture at a level lower than drinking water standards during a period spanning preimplantation and organogenesis produced a significant decrease in implantation sites and number of live pups born. Further, Greenlee and colleagues showed that a mixture of agricultural chemicals at 1 reference dose (ie, an estimate of daily oral exposure that is likely to be without an appreciable risk of deleterious effects during lifetime) increased blastomere apoptosis and suppressed cell proliferation of morulae, which may result in embryonic demise or pregnancy loss. It is possible that pesticides may impair pregnancy maintenance by affecting peri-implantation embryo development, which is known as a period of heightened susceptibility to malformations.(pp421-423)
Limitations
Our study has some limitations. First, exposure to pesticides was not directly assessed but was rather estimated from self-reported FV intake paired with pesticide residue surveillance data. Although we have adjusted for organic FV intake, data on whether individual FVs were consumed as organic or conventional were not collected, possibly leading to exposure misclassification. However, our previous work has shown that higher intake of high–pesticide residue FVs was significantly associated with higher levels of urinary pesticide metabolites, supporting the use of the PRBS as an adequate measure to characterize exposure to pesticides through diet. Second, our methodology does not allow linking specific pesticides to adverse reproductive effects. Further confirmation studies, preferably accounting for common chemical mixtures used in agriculture by biomarkers, are needed. Third, as in all observational studies, we cannot rule out the possibility that residual (eg, significant differences in organic FV consumption across quartiles of high–pesticide residue FV intake) or unmeasured confounding may still be explaining some of our observed associations. However, women with greater high-pesticide FV intake and those of greater low-pesticide FV intake had similar patterns of baseline characteristics, suggesting that the observed associations are due to intake of pesticide residues rather than to residual confounding. Furthermore, results were consistent after accounting for many factors that could potentially affect the risk of pregnancy loss. An additional limitation is that findings may not be generalizable to the general population because participants were recruited through a fertility clinic and intake of FVs in our cohort was double the median intake in the US population (2 servings/d). However, the infertility cohort allowed us to examine the association of dietary pesticide exposure with many pregnancy outcomes that are not observable among couples becoming pregnant on their own such as very early pregnancy losses. In addition, demographic characteristics of the study participants were comparable to those of women seeking fertility treatment in the United States, suggesting that results may be generalizable to women seeking infertility treatment. Additional strengths of the study include its prospective study design and well-documented outcome measures.
Conclusions
In conclusion, intake of high–pesticide residue FVs was associated with lower probabilities of clinical pregnancy and live birth among women undergoing infertility treatment. Our findings are consistent with animal studies showing that low-dose pesticide ingestion may exert an adverse impact on sustaining pregnancy. Because, to our knowledge, this is the first report of this relationship in humans, confirmation of these findings is warranted.
eTable 1. Adjusted probability of clinical pregnancy and live birth according to high pesticide fruit and vegetable intake, restricting for different characteristics
eTable 2. Ovarian biomarker and ovarian stimulation outcomes according to quartile of fruit and vegetable intake, considering pesticide residue status, among 305 women (424 fresh cycles)
eTable 3. Fertilization and embryo quality according to fruit and vegetable intake, considering pesticide residue status, in 305 women (424 fresh cycles) from EARTH study
eFigure. Overview of 541 initiated cycles in the EARTH Study between April 2007 and August 2016
References
- 1.Centers for Disease Control and Prevention National Report on Human Exposure to Environmental Chemicals, Updated Tables, January 2017. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention. https://www.cdc.gov/exposurereport. Accessed September 10, 2015.
- 2.Lu C, Barr DB, Pearson MA, Waller LA. Dietary intake and its contribution to longitudinal organophosphorus pesticide exposure in urban/suburban children. Environ Health Perspect. 2008;116(4):537-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration Pesticide monitoring program fiscal year 2012 pesticide report. 2012. https://www.ams.usda.gov/datasets/pdp/pdpdata. Accessed September 10, 2017.
- 4.Fortes C, Mastroeni S, Pilla MA, Antonelli G, Lunghini L, Aprea C. The relation between dietary habits and urinary levels of 3-phenoxybenzoic acid, a pyrethroid metabolite. Food Chem Toxicol. 2013;52:91-96. [DOI] [PubMed] [Google Scholar]
- 5.Lu C, Toepel K, Irish R, Fenske RA, Barr DB, Bravo R. Organic diets significantly lower children’s dietary exposure to organophosphorus pesticides. Environ Health Perspect. 2006;114(2):260-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradman A, Quirós-Alcalá L, Castorina R, et al. . Effect of organic diet intervention on pesticide exposures in young children living in low-income urban and agricultural communities. Environ Health Perspect. 2015;123(10):1086-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oates L, Cohen M, Braun L, Schembri A, Taskova R. Reduction in urinary organophosphate pesticide metabolites in adults after a week-long organic diet. Environ Res. 2014;132:105-111. [DOI] [PubMed] [Google Scholar]
- 8.Vandenberg LN, Colborn T, Hayes TB, et al. . Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33(3):378-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes TB, Collins A, Lee M, et al. . Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc Natl Acad Sci U S A. 2002;99(8):5476-5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenlee AR, Ellis TM, Berg RL. Low-dose agrochemicals and lawn-care pesticides induce developmental toxicity in murine preimplantation embryos. Environ Health Perspect. 2004;112(6):703-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavieres MF, Jaeger J, Porter W. Developmental toxicity of a commercial herbicide mixture in mice: I. effects on embryo implantation and litter size. Environ Health Perspect. 2002;110(11):1081-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald AD, McDonald JC, Armstrong B, et al. . Fetal death and work in pregnancy. Br J Ind Med. 1988;45(3):148-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goulet L, Thériault G. Stillbirth and chemical exposure of pregnant workers. Scand J Work Environ Health. 1991;17(1):25-31. [DOI] [PubMed] [Google Scholar]
- 14.Pastore LM, Hertz-Picciotto I, Beaumont JJ. Risk of stillbirth from occupational and residential exposures. Occup Environ Med. 1997;54(7):511-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White FM, Cohen FG, Sherman G, McCurdy R. Chemicals, birth defects and stillbirths in New Brunswick: associations with agricultural activity. CMAJ. 1988;138(2):117-124. [PMC free article] [PubMed] [Google Scholar]
- 16.Savitz DA, Whelan EA, Kleckner RC. Self-reported exposure to pesticides and radiation related to pregnancy outcome—results from National Natality and Fetal Mortality Surveys. Public Health Rep. 1989;104(5):473-477. [PMC free article] [PubMed] [Google Scholar]
- 17.Taha TE, Gray RH. Agricultural pesticide exposure and perinatal mortality in central Sudan. Bull World Health Organ. 1993;71(3-4):317-321. [PMC free article] [PubMed] [Google Scholar]
- 18.Bell EM, Hertz-Picciotto I, Beaumont JJ. A case-control study of pesticides and fetal death due to congenital anomalies. Epidemiology. 2001;12(2):148-156. [DOI] [PubMed] [Google Scholar]
- 19.Levario-Carrillo M, Amato D, Ostrosky-Wegman P, González-Horta C, Corona Y, Sanin LH. Relation between pesticide exposure and intrauterine growth retardation. Chemosphere. 2004;55(10):1421-1427. [DOI] [PubMed] [Google Scholar]
- 20.Shirangi A, Fritschi L, Holman CD. Maternal occupational exposures and risk of spontaneous abortion in veterinary practice. Occup Environ Med. 2008;65(11):719-725. [DOI] [PubMed] [Google Scholar]
- 21.Naidoo S, London L, Burdorf A, Naidoo R, Kromhout H. Spontaneous miscarriages and infant deaths among female farmers in rural South Africa. Scand J Work Environ Health. 2011;37(3):227-236. [DOI] [PubMed] [Google Scholar]
- 22.Razi S, Rezaeian M, Dehkordi FG, Manshoori A, Goujani R, Vazirinejad R. Exposure to pistachio pesticides and stillbirth: a case-control study. Epidemiol Health. 2016;38:e2016016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Settimi L, Spinelli A, Lauria L, et al. . Spontaneous abortion and maternal work in greenhouses. Am J Ind Med. 2008;51(4):290-295. [DOI] [PubMed] [Google Scholar]
- 24.Arbuckle TE, Lin Z, Mery LS. An exploratory analysis of the effect of pesticide exposure on the risk of spontaneous abortion in an Ontario farm population. Environ Health Perspect. 2001;109(8):851-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiu YH, Afeiche MC, Gaskins AJ, et al. . Fruit and vegetable intake and their pesticide residues in relation to semen quality among men from a fertility clinic. Hum Reprod. 2015;30(6):1342-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Y, Chiu Y-H, Hauser R, Chavarro J, Sun Q. Overall and class-specific scores of pesticide residues from fruits and vegetables as a tool to rank intake of pesticide residues in United States: a validation study. Environ Int. 2016;92-93:294-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu Y-H, Williams PL, Mínguez-Alarcón L, et al. . Comparison of questionnaire-based estimation of pesticide residue intake from fruits and vegetables with urinary concentrations of pesticide biomarkers [published online September 20, 2017]. J Expo Sci Environ Epidemiol. doi: 10.1038/jes.2017.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chavarro JE, Ehrlich S, Colaci DS, et al. . Body mass index and short-term weight change in relation to treatment outcomes in women undergoing assisted reproduction. Fertil Steril. 2012;98(1):109-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114-1126. [DOI] [PubMed] [Google Scholar]
- 30.Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Hum Reprod. 2012;27(10):2899-2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.US Department of Agriculture (USDA) Pesticide data program, annual summary, 2006-2012. Washington, DC: USDA, Agricultural Marketing Service; 2006-2012. https://www.ams.usda.gov/datasets/pdp/pdpdata. Accessed September 10, 2017.
- 32.Chiu YH, Gaskins AJ, Williams PL, et al. . Intake of fruits and vegetables with low-to-moderate pesticide residues is positively associated with semen-quality parameters among young healthy men. J Nutr. 2016;146(5):1084-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williamson JM, Kim HY, Warner L. Weighting condom use data to account for nonignorable cluster size. Ann Epidemiol. 2007;17(8):603-607. [DOI] [PubMed] [Google Scholar]
- 34.Mumford SL, Schisterman EF, Cole SR, Westreich D, Platt RW. Time at risk and intention-to-treat analyses: parallels and implications for inference. Epidemiology. 2015;26(1):112-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Messerlian C, Gaskins AJ. Epidemiologic approaches for studying assisted reproductive technologies: design, methods, analysis, and interpretation. Curr Epidemiol Rep. 2017;4(2):124-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least squares means. Am Stat. 1980;34(4):216-221. [Google Scholar]
- 37.Practice Committee of the American Society for Reproductive Medicine Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98(5):1103-1111. [DOI] [PubMed] [Google Scholar]
- 38.Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ. 2000;320(7251):1708-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaskins AJ, Afeiche MC, Wright DL, et al. . Dietary folate and reproductive success among women undergoing assisted reproduction. Obstet Gynecol. 2014;124(4):801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf AM, Hunter DJ, Colditz GA, et al. . Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991-999. [DOI] [PubMed] [Google Scholar]
- 42.Millen BE, Abrams S, Adams-Campbell L, et al. . The 2015 Dietary Guidelines Advisory Committee scientific report: development and major conclusions. Adv Nutr. 2016;7(3):438-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bell EM, Hertz-Picciotto I, Beaumont JJ. Case-cohort analysis of agricultural pesticide applications near maternal residence and selected causes of fetal death. Am J Epidemiol. 2001;154(8):702-710. [DOI] [PubMed] [Google Scholar]
- 44.Willis WO, de Peyster A, Molgaard CA, Walker C, MacKendrick T. Pregnancy outcome among women exposed to pesticides through work or residence in an agricultural area. J Occup Med. 1993;35(9):943-949. [DOI] [PubMed] [Google Scholar]
- 45.Torjusen H, Brantsæter AL, Haugen M, et al. . Reduced risk of pre-eclampsia with organic vegetable consumption: results from the prospective Norwegian Mother and Child Cohort Study. BMJ Open. 2014;4(9):e006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11(6):342-352. [DOI] [PubMed] [Google Scholar]
- 47.Gupta S, Agarwal A, Banerjee J, Alvarez JG. The role of oxidative stress in spontaneous abortion and recurrent pregnancy loss: a systematic review. Obstet Gynecol Surv. 2007;62(5):335-347. [DOI] [PubMed] [Google Scholar]
- 48.Brantsæter AL, Torjusen H, Meltzer HM, et al. . Organic food consumption during pregnancy and hypospadias and cryptorchidism at birth: the Norwegian Mother and Child Cohort Study (MoBa). Environ Health Perspect. 2016;124(3):357-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bretveld RW, Thomas CM, Scheepers PT, Zielhuis GA, Roeleveld N. Pesticide exposure: the hormonal function of the female reproductive system disrupted? Reprod Biol Endocrinol. 2006;4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rozman KK, Klaassen CD. Casarett and Doull’s Toxicology: The Basic Science of Poisons. New York, NY: McGraw-Hill; 2007. [Google Scholar]
- 51.Moore LV, Dodd KW, Thompson FE, Grimm KA, Kim SA, Scanlon KS. Using behavioral risk factor surveillance system data to estimate the percentage of the population meeting US Department of Agriculture Food Patterns fruit and vegetable intake recommendations. Am J Epidemiol. 2015;181(12):979-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stephen EH, Chandra A. Use of infertility services in the United States: 1995. Fam Plann Perspect. 2000;32(3):132-137. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Adjusted probability of clinical pregnancy and live birth according to high pesticide fruit and vegetable intake, restricting for different characteristics
eTable 2. Ovarian biomarker and ovarian stimulation outcomes according to quartile of fruit and vegetable intake, considering pesticide residue status, among 305 women (424 fresh cycles)
eTable 3. Fertilization and embryo quality according to fruit and vegetable intake, considering pesticide residue status, in 305 women (424 fresh cycles) from EARTH study
eFigure. Overview of 541 initiated cycles in the EARTH Study between April 2007 and August 2016