Abstract
The pro-inflammatory potency and causal relationship with asthma of inhaled endotoxins have underscored the importance of accurately assessing the endotoxin content of organic dusts. The Limulus Amebocyte Lysate (LAL) assay has emerged as the preferred assay but its ability to measure endotoxin in intact bacteria and organic dusts with similar sensitivity as purified endotoxin is unknown. We used metabolically radiolabeled Neisseria meningitidis and both rough and smooth Escherichia coli to compare dose-dependent activation in the LAL with purified endotoxin from these bacteria and shed outer membrane (OM) blebs. Bacteria labeled with [14C]-3-OH-fatty acids were used to quantify the endotoxin content of the samples. Purified meningococcal and E. coli endotoxins and OM blebs displayed similar specific activity in the LAL assay to the purified LPS standard. In contrast, intact bacteria exhibited 5-fold lower specific activity in the LAL assay but showed similar MD-2-dependent potency as purified endotoxin in inducing acute airway inflammation in mice. Pretreatment of intact bacteria and organic dusts with 0.1M Tris-HCl/10mM EDTA increased by 5-fold the release of endotoxin. These findings demonstrate that house dust and other organic dusts should be extracted with Tris/EDTA to more accurately assess the endotoxin content and pro-inflammatory potential of these environmental samples.
Keywords: asthma, endotoxin, house dust, lipopolysaccharide, organic dust
1. Introduction
Organic dusts are airborne particles of vegetable, animal, and/or microbial origin. These dusts are present at many industrial and agricultural workplaces as well as in homes 1, 2. A constituent of virtually all organic dusts are Gram-negative bacteria (GNB) endotoxins 1, 3, 4. Endotoxins are highly abundant integral membrane glycolipids present uniquely in the outer membrane (OM) of GNB, in shed OM-rich nanoparticles (OM blebs) from these bacteria, and as supramolecular aggregates after extraction and purification of endotoxin from the GNB OM 5–7. Endotoxins are recognized causal agents for asthma, recurrent wheeze and organic dust toxics syndrome 8–10. Many GNB endotoxins are remarkably potent stimuli of human and murine inflammatory and immune responses 5, 11. The ability of inhaled or aspirated purified endotoxin to simulate many of the effects of inhaled organic dusts on airway immunity and pathophysiology 3, 4, 12, 13 has stimulated extensive efforts to measure endotoxin contamination of diverse organic dusts and test experimental mitigation strategies directed at blocking or modulating endotoxin-triggered innate immune responses.
Two large U.S. nationwide representative studies have demonstrated significant association of increased household endotoxin exposure with asthma and wheeze outcomes 2, 14 and have demonstrated that higher household endotoxin concentrations are associated with low household income; children in the home; and carpeting, cockroaches, pets, smoking, and mold in households 14, 15. A growing number of studies have also demonstrated a protective effect of growing up on a farm with associated exposures to endotoxin and other microbial agents against development of allergies and atopic asthma 16–18. Stein et al. demonstrated that Amish farm children have 4- and 6-fold lower asthma and allergic sensitization rates and seven-fold higher endotoxin exposures than Hutterite farm children, 16 attributable to early stimulation of innate immune responses.
Endotoxins (lipopolysaccharides, LPS; lipooligosaccharides, LOS) are comprised of a structurally unique and generally conserved lipid A region, normally embedded in the GNB-OM, linked via a unique 8-carbon acidic sugar (keto-deoxy-manno-octanoic acid, KDO) to a more variable core ± O-antigen oligo- or poly-saccharide chain that extends outward into the extracellular surroundings 5. The most efficient mechanisms of mammalian recognition and response to endotoxin depend on the polyanionic and hydrophobic properties of the KDO-lipid A region and host (e.g., mouse or human) innate immune proteins that bind OM-endotoxin with high affinity 19, 20. These interactions trigger extraction and transfer of individual molecules of endotoxin to cellular pro-inflammatory MD-2/TLR4 receptors via interactions with MD-2 11, 21.
In environmental samples, endotoxin may exist in diverse physical states, including as aggregates containing thousands of endotoxin molecules/aggregate, or still embedded within the OM of intact or fragmented bacteria, or of naturally shed nanosized OM vesicles (blebs). For three decades, assessment of organic dust contamination with endotoxin and environmental exposure to endotoxin has relied on the chromogenic Limulus amebocyte lysate (LAL) assay 1, 22 with little regard to the physical form of the endotoxin. This assay is based on the presence in this ancient horseshoe crab, Limulus polyphemus, of an endotoxin-activated pro-coagulant protease 23. Strengths of this assay include its simplicity, quantitative sensitivity, and its generally similar structure-activity-relationship for many extracted and purified natural endotoxin species in activation of the LAL and MD-2/TLR4-dependent inflammatory responses 24–27.
However, the efficiency of detection in the LAL assay when endotoxin is present as an integral membrane component of intact bacteria or of even more complex particulate samples (e.g., house dust, agricultural organic dust, ambient air samples) has not been studied. Potent pro-inflammatory effects in vivo of bacteria-derived endotoxin depend on specific extracellular and cell surface host proteins that can extract and transfer individual molecules of endotoxin from tightly-packed endotoxin-rich monolayers (e.g., outer leaflet of GNB OM) to MD-2/TLR4 20, 21. The absence of functionally analogous proteins in the LAL assay could lead to selective under-recognition of endotoxin in intact bacteria and other complex particulate preparations in comparison to purified endotoxin standards that are pre-extracted and purified by specific mixtures of organic and aqueous solvents. Such a variable recognition of endotoxin by the LAL assay depending on the physical context of its presence would preclude accurate exposure assessment and establishment of meaningful health-based exposure standards.
To address these issues, we used metabolically labeled Neisseria meningitidis and rough and smooth Escherichia coli and endotoxins extracted and purified from these metabolically labeled bacteria, and compared the ability of purified endotoxin and endotoxin in intact bacteria to activate the LAL and induce acute airway inflammation in mice. Our findings demonstrate that bioactive endotoxin in intact bacteria, capable of inducing airway inflammation, has reduced activity in the LAL assay, resulting in significant under-estimation of the bioactive endotoxin content of intact bacteria and organic dusts that may contain intact bacteria. This limitation of the LAL assay can be largely overcome by pre-treatment of these particulate samples with Tris/EDTA, a procedure that induces release of OM LPS 28.
2. Materials and Methods
2.1 Growth and metabolic radiolabeling of bacteria
AceE mutants of Neisseria meningitidis serogroup B strain (N. menigitidis, NMB) and of Escherichia coli (E. coli) CL99 29 were metabolically labeled in, respectively, supplemented Morse’s defined broth medium or nutrient broth/0.9% saline plus [1,2-14C]acetic acid sodium salt (110 mCi/mmol) and unlabeled sodium acetate to final concentrations of 2 μCi/ml and 2.0 mM sodium acetate. Parallel cultures of E. coli CL99 were grown ± 2 mM D-galactose to yield “rough” bacteria producing exclusively Rc chemotype LPS (- gal) and “smooth” bacteria containing longer chain LPS (+ gal) 30, 31. Each of the cultures were grown to late log phase/early stationary phase of bacterial growth (ca. 6–8 hr at 37°C).
2.2 Purification of endotoxin (aggregates) and outer membrane (OM) blebs from metabolically labeled bacteria
After harvesting the metabolically labeled bacteria, [14C]LOS (NMB) and [14C]LPS ( E. coli) were purified from the bacterial pellets and [14C] OM blebs were isolated from the conditioned bacterial medium as previously described 6, 29. The yield of OM blebs from the conditioned media of E. coli cultures was insufficient for subsequent functional studies and thus only recovered purified OM blebs from NMB were used in this study. Test materials were analyzed for the presence of β-glucans using the endpoint Glucatell assay (Associates of Cape Cod) and were all below the limit of detection of 25 pg/mL.
2.3 Assay of [14C] endotoxin content of metabolically labeled samples
We took advantage of the unique presence of 3-OH-fatty acids in LOS of meningococci (3-OH-12:0 and 3-OH-14:0) 29 and LPS of E. coli (virtually exclusively 3-OH-14:0) 5 to normalize and quantify the endotoxin content of metabolically labeled intact bacteria and OM blebs vs. purified endotoxin (LOS or LPS) aggregates. In brief, samples were treated sequentially with 4N HCl and 4N NaOH at 90°C to release ester- and amide-linked fatty acids from the parent [14C]-labeled lipids 32. Samples were then subjected to Bligh-Dyer extraction and the released [14C] free fatty acids were recovered in the chloroform phase. Individual [14C] fatty acids were resolved by reverse-phase TLC (0.2 mm HPTLC, RP-18; Merck) using acetonitrile/acetic acid (1:1, v/v) as the solvent system 29. Resolved radiolabeled species were detected and quantified by image analysis using a tritium screen that permitted quantitation of as little as 200 cpm and identified by co-migration with authentic [14C] fatty acid standards. Quantitation was done using ImageQuant software (Amersham Biosciences). Comparison of the ratio of the [14C] 3-OH-14:0-derived radioactivity to that of the total cpm of purified endotoxin vs. purified OM blebs and intact bacteria indicated that 20% of the total radioactivity of the metabolically labeled intact bacteria was derived from LOS (or LPS) and 40% of the radiolabeled material in OM blebs was [14C] LOS. This permitted calculation of the relative LOS (or LPS) content of the purified endotoxin, OM blebs, and intact bacteria stocks used in this study based on the concentration of total [14C] radioactivity in each stock, as determined by liquid scintillation spectroscopy.
The specific radioactivity of [14C]LOS (or LPS) was determined by liquid chromatography/mass spectrometry (LC-MS) after isolation of [14C]3-OH-14:0 by reverse-phase-HPLC 33. In brief, chemically hydrolyzed samples containing free fatty acids in the chloroform phase after Bligh-Dyer extraction were dried under nitrogen, dissolved in 90% methanol/0.05% acetic acid and applied to a 5 μm Grace Prevail organic acid 10 mm x 250 mm column. Applied samples were eluted with the same solvent at a flow rate of 1 ml/min on a Beckman System Gold HPLC (Beckman Coulter, Inc.) and collected in 0.5 ml fractions. Elution of [14C]-labeled free fatty acids was monitored by liquid scintillation spectroscopy. Fractions for LC-MS analysis were selected on the basis of co-elution with purified [14C]3-OH-14:0 standard, confirmed by subsequent LC-MS analysis using a Waters Q-TOF Premier with an Acquity UPLC system. Comparison of the test samples to a dose curve of known quantities of commercially-obtained purified 3-OH-14:0 permitted calculation of the mass of 3-OH-14:0 in the test samples and, hence, the specific radioactivity of NMB and E. coli 3-OH-14:0 (range of 25–50 cpm/ng 3-OH-14:0, corresponding to 6–10 cpm/ng LOS (or LPS)).
2.4 Collection and extraction of house and barn dusts
Reservoir house dust was obtained from archived samples collected from a previous study on indoor dust exposures 34. Samples were collected using a High Volume Small Surface Sampler 35 and were collected from four locations in each of ten homes as previously described 34. Barn reservoir dust samples were brushed off horizontal surfaces (e.g., stanchions) into 50 mL endotoxin-free polypropylene tubes in a farrowing swine barn. Collected house and barn dust were sieved through a 0.355 mm mesh, barcoded and stored at −20ºC until extraction.
To compare the efficiency of different pre-treatments on endotoxin activity from house and barn dusts, 50 mg aliquots of individual dust samples were weighed on a microbalance (MT-5, Mettler-Toledo) and incubated in 2 ml cryovials with either pyrogen-free water (PFW) ± 0.05% Tween 20, 10 mM EDTA, or 0.1M Tris-HCl/10 mM EDTA (Tris/EDTA) at a concentration of 50.0 mg dust/ml. The Tris/EDTA had a pH of 7.8. After 1 hr incubation with shaking at 22°C, the samples were centrifuged at 600 x g for 20 min at 4°C and the recovered supernatant (extract) was tested in vitro (LAL assay; see below) and in vivo (induction of acute airway inflammation in mice; see below).
2.5 Measurement of endotoxin activity by kinetic, chromogenic LAL assay
The kinetic chromogenic LAL assay (Kinetic-QCL; Lonza) for endotoxin measurement was performed as previously described 9. Briefly, 3- or 4-fold dilutions in PFW of the sample preparations were made in depyrogenated borosilicate tubes; dilutions in the 102–105 range are presented. Two-fold dilutions of Control Standard Endotoxin (CSE, E. coli O55:B5) were assayed to create a 12-point standard curve ranging from 0.0244 to 50.0 Endotoxin Units (EU)/ml. The preparations and controls were assayed in 96-well microplates (Corning) and the rate of change in absorbance was measured at 405 nm every 30 s. for 90 min. using a microplate reader (SpectraMax 384 Plus, Molecular Devices, with Softmax PRO 4.0). Samples pre-treated (1 hr at 22°C) with Tris/EDTA were diluted >100-fold with PFW to avoid inhibitory effects of Tris/EDTA on the LAL assay observed at higher concentrations of Tris/EDTA. All samples assayed by the LAL assay were diluted to yield at least 4 separate dose points that fell within the CSE curve. Quality assurance measures for the LAL assay were as previously described 14.
2.6 Assay of acute airway inflammation following intranasal (i.n.) instillation of endotoxin-containing samples
Animal care, exposure, and necropsy were performed as previously described 7. Experimental protocols were approved by the Institutional Animal Care and Use Committee and adhered to NIH Guidelines. Briefly, 6 week old C3HeB/FeJ wild type (WT) mice (Jackson Laboratory) and MD-2−/− mice on a C57BL/6 background (Courtesy of Douglas T. Golenbock, University of Massachusetts) were intranasally instilled with 50 μl of three different endotoxin doses (3, 30, 300 ng/mouse) in the form of whole bacteria, OM blebs, or aggregates originated from NMB and whole bacteria or aggregates from E. coli (smooth and rough strains) or with equivalent 100-fold dilutions (in PFW) of PFW vs. Tris/EDTA barn dust extracts. I.n. instillation was performed under brief isoflurane anesthesia. Controls were sham exposed with the extraction diluent, either Tris/EDTA diluted to 1:100 in PFW (or PFW alone) or with PBS + 0.1% human serum albumin. Untreated sentinel mice from the same shipment remained in the vivarium and were necropsied at the beginning and end of the experiments to confirm the health condition of the animals. Groups of 4–6 mice (see legends to Figs. 2, 3, and 5) were used for each experimental condition.
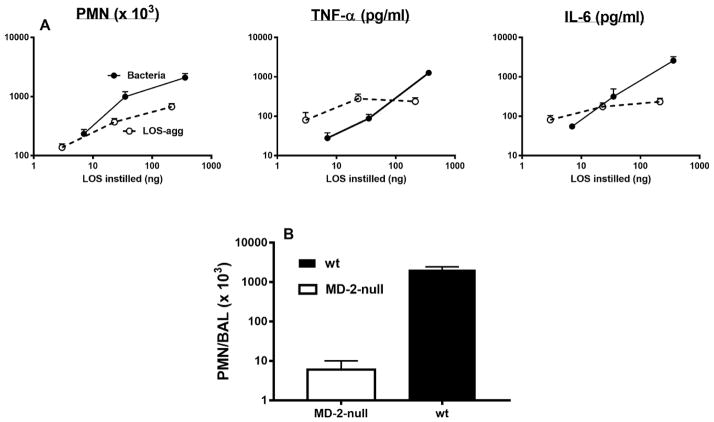
Fig. 2.
(A) Comparison of dose-dependent induction of acute airway inflammation in mice by purified LOS-agg or intact bacteria (NMB) measured 4 h after i.n. administration. The doses of LOS-agg and of intact bacteria are normalized based on their LOS content (see legend to Fig. 1). (B) Induction of accumulation of PMN recovered in BAL 4 h after instillation of 2 x 107 meningococci (containing 300 ng LOS) is MD-2-dependent. Data shown represent the mean ± SEM of results obtained from groups of 5 (A) or 4 (B) mice and represent the results of one of two similar experiments. Mice exposed to vehicle (PBS) alone had < 1x103 PMN in recovered BAL.
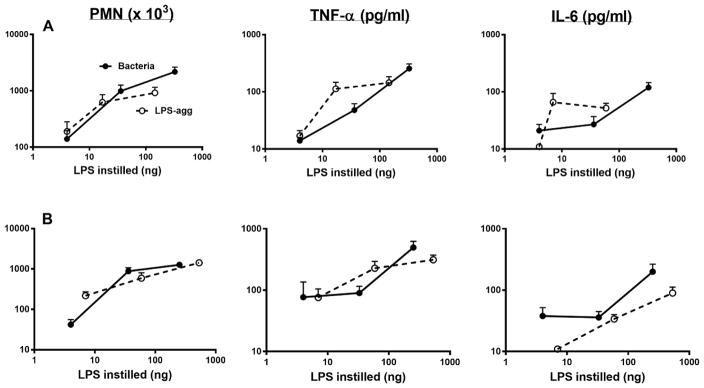
Fig. 3.
Comparison of dose-dependent induction of acute airway inflammation in mice by purified LPS-agg or intact bacteria derived from E. coli CL99 grown in the absence (A) or presence (B) of 2 mM D-galactose (“rough” and “smooth” bacteria, respectively) measured 4 h after intra-nasal administration. See legend to Fig. 2 for further details.
Fig. 5.
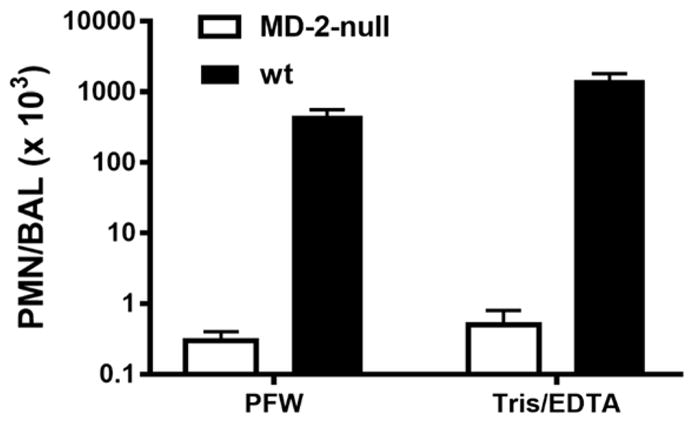
The increased airway inflammation in mice induced 4 h after i.n. instillation of barn dust was MD-2-dependent for both Tris/EDTA and PFW dust extracts. See methods and the legend to Fig. 2B for further experimental details. Results shown represent the mean ± SEM of groups of 4 mice/treatment. The difference in recovery of PMN following bronchoalveolar lavage of WT mice exposed by i.n. installation to Tris/EDTA vs. PFW barn dust extract is statistically significantly (p<0.02).
The mice were euthanized 4 h. after i.n. instillation to monitor early, acute airway inflammatory responses. After bronchoalveolar lavage (BAL) using 4 x 1.0 ml of saline, lungs were excised and fixed in zinc formalin for archival. The BAL fluid was assayed for IL-6 and TNF-α by ELISA (Invitrogen). Total cell counts of the BAL fluid were performed using a hemocytometer and cell differential counting was performed by microscopy after fixing and staining the slides with Diff-Quik. Differential cells were enumerated independently by two qualified technicians.
2.7 Statistical analyses
Statistical analyses were conducted separately for each bacterial species to test if the form of presentation affected the endotoxin assessment in the LAL assay as compared to the assessment from scintillation counting. A generalized linear model (GLM) was built using the form of presentation (aggregates, intact bacteria and OM blebs), experiment replicate, and scintillation counting of the endotoxin concentration using SAS Version 9.2 (SAS Inc.). A model was created for each of the three bacterial species (NMB, E. coli smooth and E. coli rough) using the in vivo response or the log ratio of endotoxin concentration from the LAL assay to [14C]-endotoxin concentration as the dependent variables. The dependent variables were log-normally distributed and were therefore log-transformed. Because each experiment was run in multiple replicates, we tested for interaction between replicate and form of presentation. In all models the interaction was not significant (p-values were > 0.15). Inclusion of the effect of scintillation counting for endotoxin concentration was necessary because each preparation and experiment had a somewhat different concentration of endotoxin (i.e. the doses were not exactly 3, 30, 300 ng/mouse). In all models, the differences between the three forms of presentation were assessed using the ratios of the corresponding modeled least squares (LS) means with the associated p-value testing whether the ratio was different from 1.0.
Statistical analysis also tested if the extraction methods contributed a significant change in the ability of the LAL to detect endotoxin in the intact bacteria form. A GLM was built using the log ratio of endotoxin concentration from the LAL assay to 14C-endotoxin concentration as the response variable and the extraction method, experiment replicate, and the scintillation count as the covariates. The LS means were calculated using PFW or PFW + shaking as the reference extraction method to determine if the new pre-treatment method significantly increased detection in the LAL assay.
3. Results
3.1. Comparison of endotoxin-specific activity in LAL assay of purified endotoxin aggregates and endotoxin in OM blebs and in intact bacteria
Key to our studies was the ability to sensitively and accurately quantify the endotoxin content of samples independent of the form of presentation of endotoxin in these samples (e.g., whether as an integral constituent of the GNB OM or as aggregates of purified endotoxin after extraction and isolation). For this purpose, we initially made use of endotoxin (LOS) present in or derived from NMB that had been metabolically labeled with [14C]-acetate during several generations of growth. This yields uniform and selective radiolabeling of the acyl chains of LOS 36. Since the fatty acid composition of each of the LOS molecules is essentially the same (2 mol each of 3-OH-12:0, 3-OH-14:0, and 12:0), metabolic radiolabeling of the LOS with [14C]-acetate yields essentially equivalent radiolabeling of each LOS molecule. Among the various metabolically labeled fatty acids of GNB, the 3-OH-fatty acids are unique to endotoxin 37. Thus, sequential treatment of the various samples with 4N HCl followed by 4N NaOH at 100° C to release amide- and ester-linked fatty acids (e.g., from LOS) followed by separation of the 3-OH-fatty acids from non-hydroxylated fatty acids by either TLC and [14C] image analysis or HPLC and liquid scintillation counting of the collected samples 33 permitted precise quantification of the endotoxin content of each sample (i.e. a gold standard). In each set of comparative analyses, purified endotoxin, shed OM blebs, and intact bacteria were derived from the same population of metabolically labeled bacteria. In parallel, analysis of recovered HPLC-purified 3-OH-14:0 by LC-MS permitted calculation of the LOS concentration added in each sample as represented in the x-axes of Fig. 1 and 2.
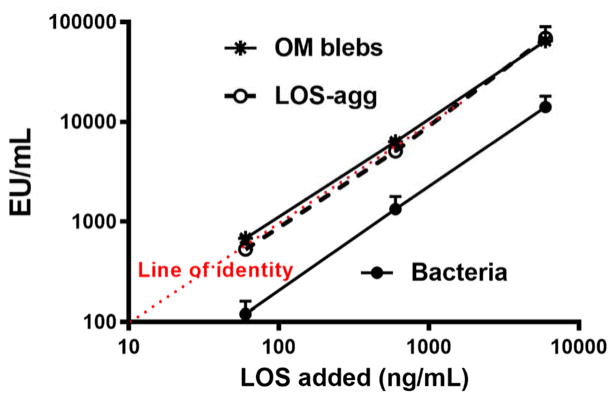
Fig. 1.
Comparison of dose-dependent activation of Limulus amebocyte lysates by meningococcal endotoxin (LOS) presented either in purified form (LOS-agg), or as an integral membrane component of purified OM blebs or intact bacteria, as indicated with dispersion using PFW. The doses of each are normalized based on their LOS content, measured by 3-OH-14:0 analyses as described in Methods. Data shown at each dose represent the mean ± SEM of calculated EU/ml derived from testing of three or more dilutions of the indicated sample using the LAL assay. These results are representative of three or more separate experiments. The “line of identity” represents the activity of the CSE (10 EU/ng).
Fig. 1 shows a comparison of the LAL-activating potency of meningococcal endotoxin presented either as aggregates of purified endotoxin (LOS-agg), purified OM blebs, or intact bacteria, plotted against the concentration of LOS added based on the [14C]LOS content of these samples. Over an overlapping and nearly 100-fold concentration range of the various sample stocks tested, the endotoxin activity of LOS-agg and OM blebs were closely similar whereas the activity of the same endotoxin when an integral component of intact bacteria was about 5-fold lower in the LAL assay. Comparison of the calculated EU/ng of endotoxin (LOS) tested to that of the purified E. coli in the LAL assay, showed that meningococcal LOS either in the form of LOS-agg or as a component of OM blebs had closely similar LAL-activating potency as the purified E. coli LPS standard (10 EU/ng) whereas the potency of LOS in whole bacteria was 4-fold lower (Table 1; p<0.001).
Table 1.
Comparison of the activity of endotoxin detected in the LAL assay (with PFW extraction), normalized to the concentration measured from the [14C] labelling. Data clearly show reduced activity of endotoxins in the LAL assay when presented as part of intact bacteria.
| Endotoxin source | EU/ng endotoxin* |
|---|---|
| Neisseria meningitidis | |
| LOS-agg | 8.8 ± 1.0 |
| OM blebs | 11.3 ± 0.6 |
| Intact bacteria | 2.5 ± 0.5 |
| Escherichia coli (“rough”) | |
| LPS-agg | 11.8 ± 2.1 |
| Intact bacteria | 2.1 ± 1.1 |
| Escherichia coli (“smooth”) | |
| LPS-agg | 11.9 ± 2.6 |
| Intact bacteria | 2.0 ± 0.8 |
EU/ng endotoxin was calculated as described in Methods and the legend to Fig. 1.
Results shown represent the mean ± SEM of at least six separate determinations for each sample.
Similar analyses were extended to LPS of “rough” and “smooth” E. coli, using metabolically labeled 3-OH-14:0 to assess and normalize the amount of LPS tested, either in the form of aggregates of purified LPS (LPS-agg) or as part of whole bacteria. As shown above for LOS of Neisseria meningitidis, purified LPS from rough and smooth E. coli had similar LAL-activating potency as the purified E. coli LPS standard whereas in the whole bacteria, endotoxin activity was about 5-fold reduced (Table 1; p<0.01). Thus, in each of the three populations of GNB tested, presentation of LOS/LPS as part of whole bacteria resulted in an approximately 5-fold lower specific endotoxin activity (EU/ng endotoxin) in comparison to the same LOS/LPS that we purified from these bacteria or the purified LPS standard used as reference in the LAL assays.
3.2. Similar potency of purified NMB and E. coli endotoxin and of intact bacteria in induction of acute airway inflammation in mice
In contrast to the reduced LAL-activating potency of intact bacteria (vs. corresponding amounts of purified LOS or LPS), each of the three species of intact bacteria – NMB (Fig. 2A), rough E. coli (Fig. 3A), and smooth E. coli (Fig. 3B) had similar potency to purified LOS or LPS in inducing acute airway inflammation following i.n. instillation in mice. This was apparent both by measurement of the mobilization of polymorphonuclear leukocytes and accumulation of TNF-α and IL-6 recovered by BAL 4 h after i.n. administration. Since the bacteria produce and contain other bioactive products (e.g., pathogen-associated molecular patterns) in addition to endotoxin that could also acutely activate innate immune responses in the airway, it seemed possible that the greater ability of intact GNB to induce airway inflammation vs. activate LAL could be due to the added contribution of these endotoxin-independent inflammogens. To test this possibility, we compared the ability of NMB to induce airway accumulation of polymorphonuclear leukocytes (PMN) in wild-type mice expressing both MD-2 and TLR4, as needed for sensitive responses to intranasally administered endotoxin 7 vs. MD-2 null mice. As shown in Fig. 2B, mobilization of PMN in the airway by intact NMB was reduced 100-fold in mice lacking MD-2 indicating that the measured response was MD-2 dependent and attributed to the endotoxin MD-2-dependent inflammatory pathway. Taken together, these findings indicate that in contrast to the reduced ability of endotoxin in intact NMB to activate LAL, its potency in inducing acute airway inflammation in mice is similar to that of purified endotoxin.
3.2. Treatment of E. coli with Tris-EDTA increases detection of bioactive endotoxin by the LAL assay
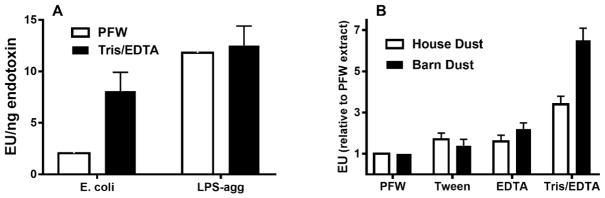
A possible explanation for the reduced activation of the LAL assay by LOS or LPS presented as part of intact bacteria is that the dense packing of endotoxin in the OM of intact bacteria impedes interactions of the Limulus lysate with the endotoxin molecules necessary for activation and subsequent quantification using the LAL. To test this hypothesis, we pre-treated E. coli and, for comparison, LPS purified from the same bacteria with 0.1M Tris-HCl/10 mM EDTA vs. PFW before diluting at least 100-fold with PFW and assaying LAL activation. Under these conditions, pre-treatment with Tris/EDTA caused release of nearly 50% of the bacterial OM LPS resulting in significantly (p<0.01) increased LAL activation from the intact bacteria sample (Fig. 4A). The increased potency of LAL activation by E. coli following Tris/EDTA pre-treatment was 3- to 5-fold, resulting in measured EU/ng endotoxin concentration that was, on average, two-thirds that of purified LPS-agg. Tris/EDTA pre-treatment of LPS-agg, by contrast, had little or no effect on the potency of LAL activation, indicating no alteration by Tris-EDTA of the activity of the Limulus lysate. These findings are consistent with the hypothesis that detection of bioactive endotoxin of intact GNB is impeded by the dense packing of LPS within the outer membrane of intact bacteria which can be overcome by pre-treatment of the bacteria with Tris/EDTA.
Fig. 4.
Pre-treatment of intact bacteria (A) and organic dusts (B) with Tris/EDTA significantly (p<0.01) increased detection of bioactive endotoxin by the LAL assay. In contrast, similar treatment of LPS-agg has no significant effect. See Methods for additional experimental details. Results shown represent the mean ± SEM of four separate determinations.
3.3. Detection of bioactive endotoxin in house and barn dust is also enhanced by Tris/EDTA pre-treatment
The above findings prompted us to consider the possibility that the bioactive endotoxin content of other complex particulate samples may also be underestimated by the LAL assay given current protocols for sample handling. To test this hypothesis, we compared endotoxin activity of two different aliquots each of house dust and of barn dust following pre-treatment and extraction either with: PFW; PFW supplemented with 0.05% Tween 20 (Tween); 10 mM EDTA (EDTA); or 0.1M Tris-HCl/10 mM EDTA (Tris/EDTA). As shown in Fig. 4B, endotoxin activity of both organic dusts was significantly greater when pre-treated with Tris/EDTA in comparison to the other tested conditions. Extending the comparison between PFW vs. Tris/EDTA pre-treatments to several additional aliquots of the same batch mixtures of house and barn dust confirmed a reproducible and significant (p<0.001) increase in endotoxin activity extracted from both organic dusts by Tris/EDTA (Table 2). The effect of Tris/EDTA pre-treatment was greater on barn dust (5.6-fold) than on house dust (3.1-fold). Both PFW and Tris/EDTA dust extracts induced acute MD-2-dependent airway inflammation in mice, that was also greater when the same dust was extracted with Tris/EDTA (Fig. 5).
Table 2.
Increased detection of endotoxin activity in barn and house dust following pre-treatment with Tris/EDTA.
| Organic Dust | Pretreatment | EU/mg dust* | Fold Increase (P-value) |
|---|---|---|---|
| Barn Dust | Pyrogen-free water | 305 ± 10 | |
| Tris/EDTA | 1701 ± 83 | 5.6 (<0.001) | |
| House Dust | Pyrogen-free water | 73 ± 5 | |
| Tris/EDTA | 228 ± 11 | 3.1 (<0.001) |
Several aliquots (50 mg each) of a single collection of barn dust and of house dust were dispersed and extracted in 1 ml of PFW or Tris/EDTA, as indicated. Each of the recovered supernatants was tested by the LAL assay for endotoxin and expressed as EU/mg of dust.
Results represent the mean ± SEM of at least ten separate determinations.
3.4. Tris-EDTA pretreatment increases detection by LAL assay of endotoxin activity from house dust collected at each of several different sites
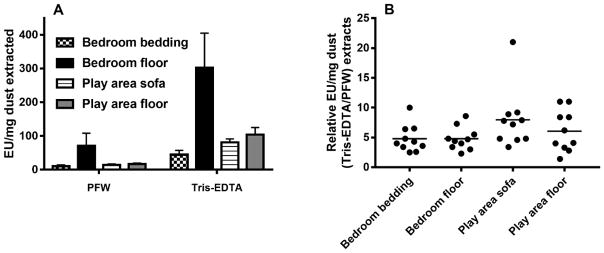
The studies reported thus far on house and barn dust were of several independent aliquots of dust each derived from the same mixture of homogenized reservoir dusts from a dozen or more homes or of barn dust (see Methods). Thus, to extend our testing to a broader array of separate individual dust samples, we compared the effect of Tris/EDTA vs. PFW extraction on 40 samples of reservoir house dust representing samples from 10 different homes, with each home having dust collected at four different locations. Both after PFW and Tris/EDTA extraction, the highest levels of bioactive endotoxin were measured in children’s bedroom floor samples (Fig. 6A), with the play area sofa and floor-derived dust containing 3- to 4-fold lower endotoxin activity and the children’s bedroom bedding nearly 10-fold less endotoxin activity. Despite these differences in endotoxin content, each of the 40 house dust samples displayed significantly greater endotoxin activity when extracted by Tris/EDTA (vs. PFW) before LAL assay (Fig. 6B). From the 10 different homes in which dust was collected, there was an approximately 5-fold increase in measured endotoxin activity (EU/mg dust) of the Tris/EDTA vs. PFW extracts (p<0.01) at each site in the house in which dust was collected. However, the extent to which Tris-EDTA treatment amplified detection of bioactive endotoxin in individual dust samples varied by almost 10-fold (Fig. 6B) suggesting varying degrees of endotoxin contained within intact bacteria.
Fig. 6.
Tris/EDTA pre-treatment increased detection by LAL assay of endotoxin-like activity from house dust collected at each of several different locations. Each location indicated was sampled (see Methods) in each of 10 different homes. An equal portion of each of the filters containing collected dust was treated with either 100 mM Tris-HCl/10 mM EDTA or PFW and tested by LAL assay. (A) Mean ± SEM of endotoxin activity of PFW vs. Tris/EDTA extracts from each location, expressed as EU/mg dust extracted. The differences are statistically significant (p<0.01) for each of the four sites of collection. (B) Relative activity of Tris/EDTA pre-treatment as compared to PFW of each collected house dust, expressed as EU/mg dust extracted.
4. Discussion
We have demonstrated that the recognition of bioactive endotoxin by the LAL assay is significantly reduced when endotoxin is presented as an integral constituent of the outer membrane of intact GNB. This was observed both in encapsulated (NMB) and non-encapsulated (E. coli CL99) bacteria producing endotoxin containing lipid A linked to core polysaccharide alone as well as in smooth E. coli producing LPS species containing longer polysaccharide chains (Table 1). The similarity of our observations in NMB and E. coli in which there are also compositional differences within the lipid A and core oligosaccharide regions 5, 38 strongly suggests that the differences in specific endotoxin activity between intact bacteria and aggregates of purified endotoxin are not dependent on species-specific structural properties of bioactive endotoxins. Thus, the differences we observed are likely to be manifested across the spectrum of environmental GNB and their endotoxins. Extensions of our observations can make use of the metabolic labeling and analytical approaches described in this study. What accounts for the reduced activation of the LAL assay by endotoxin present in intact GNB is not yet fully clear. The similar specific activity of endotoxin in purified, spontaneously shed meningococcal OM blebs and in aggregates of purified meningococcal LOS (Fig. 1, Table 1) indicate that the reduced activity of the same endotoxin in intact bacteria (Fig. 1, Table 1) is not simply a consequence of presentation of endotoxin as an integral OM constituent and/or of its interactions with other OM components.
One possibly relevant distinguishing characteristic between intact bacteria and OM blebs may be the density of intermolecular packing of LOS within the outer leaflet of the OM where endotoxin resides, influenced at least in part by differences in membrane curvature 39. In comparison to intact bacteria, the higher curvature of the small vesicles (50–150 nm) and increased presence of phospholipids in the outer leaflet as observed in NMB OM blebs 6, is likely to result in lower surface density and reduced intermolecular packing of endotoxin 39, 40. The dense packing of endotoxin in intact bacteria requires tightly bound divalent cations (Ca2+ and Mg2+) to reduce the electrostatic repulsion that would otherwise be manifest between neighboring endotoxin molecules due to the presence in each molecule of multiple anionic moieties within and near the lipid A region 28, 39. This is illustrated by the effects of Tris/EDTA treatment which by displacing and then chelating the divalent cations, induces the release of up to 50% of the OM endotoxin 28. The increased ability of the endotoxin in intact bacteria to activate LAL following Tris/EDTA treatment and subsequent dilution with PFW (above 100-fold) (Fig. 4A) is thus consistent with a role for endotoxin surface density and packing in limiting endotoxin-induced LAL activation. That the same treatment does not increase the specific activity of aggregates of purified LOS toward the LAL (Fig. 4A) may reflect looser intermolecular packing of LOS within the aggregates vs. intact bacteria that is more favorable for LAL activation.
Endotoxin activity, as measured in this study, reflects binding and activation of Limulus Factor C, an endotoxin-binding protein stored within granules of hemocytes with endotoxin-induced proteolytic activity 23. Our findings imply that the activation of Factor C by endotoxin is regulated by the form of presentation of endotoxin (i.e. diminished when endotoxin is presented as part of intact bacteria vs. its presentation as aggregates of purified endotoxin or shed OM blebs). Effects of variables in endotoxin presentation on interactions with other endotoxin-binding proteins, most notably the bactericidal/permeability-increasing protein (BPI), have been previously demonstrated 30, 41. Diminished affinity of BPI for endotoxin of intact bacteria vs. aggregates of purified endotoxin was most pronounced in bacteria that expressed LPS with longer polysaccharide chains, apparently reflecting greater steric hindrance of BPI access to the poly-anionic inner core-lipid A region to which it binds when the polysaccharide chains of LPS are longer and more densely packed 39. Although we did not observe significantly lower specific activity in the LAL assay of LPS in intact smooth vs. rough E. coli, this may be due to the relatively low fraction and moderate length of LPS that contains O-antigen chains in the smooth E. coli CL99 used in this study 42.
From the perspective of the assessment of bioactive endotoxin content of environmental samples, our most striking observation was the demonstration that Tris/EDTA treatment also markedly increased detection of endotoxin activity of both barn and house dust samples by the LAL assay. The adequacy of sample handling in environmental assessment studies – especially for a class of compounds as amphipathic as endotoxins – has long been a source of concern. In 2000, the European Committee for Standardization (CEN) published a protocol for assessment of airborne endotoxin in occupational settings 43. While this represented movement toward method harmonization it was found lacking 44. It failed to specify the extraction solution or storage conditions to use and provided no data to support the recommended methodology. Spaan et al. undertook extensive studies to address these limitations by systematically investigating the effects of sample handling and storage, storage of extracts, filter type and assay medium using air samples collected in work sites 44. The conclusion of this work was “the extraction method appeared to be the most important determinant.” The authors recommended using PFW with 0.05% Tween-20 (PFW-Tween) rather than PFW alone. In follow-up work this group further evaluated extraction solutions and found that PFW-Tween yielded better results than PFW alone, PFW-triethylamine phosphate or PFW-Tris 45. These studies did not evaluate the efficacy of Tris/EDTA nor did they employ an external means of evaluating total endotoxin such as with radiolabeling as done here.
The aforementioned studies recommended use of 0.05% Tween 20 in PFW to improve dispersion of endotoxin in these complex particulate samples 44, 45. In limited testing in this study, we reproduced the approximately 2-fold increase reported previously in endotoxin units measured by the LAL assay of both house and barn dust treated with PFW supplemented with 0.05% Tween 20 (Fig. 4B). By comparison, Tris/EDTA treatment had a greater effect, increasing on average LAL activation by house and barn dust approximately 3- and 6-fold, respectively (Fig. 4B) and increasing in mice the induction of MD-2-dependent airway inflammation. The ability of Tris/EDTA treatment to increase endotoxin activity measured by the LAL assay was highly reproducible, as judged both by repeated sampling and assay of single mixtures of house and barn dust extracts (Table 2) and testing of 40 independent house dust collections (Fig. 6). Conceivably, the increased activation of LAL by pre-treatment of Tris/EDTA vs. PFW of these dusts reflects increased extraction of bioactive endotoxin from intact GNB present within these particulate samples. The greater effect of Tris/EDTA treatment of barn vs. house dust (Fig. 4B; Table 2) is consistent with such a hypothesis. However, we cannot exclude other possible constraints on endotoxin presentation within organic dusts and, therefore, other possible means by which Tris/EDTA treatment increases detection of dust-derived endotoxin by the LAL assay. Fungal-derived glucans that are also commonly present in dust samples require much harsher conditions for solubilization (e.g., autoclaving or 0.3 N sodium hydroxide) and thus are not present in the centrifuged extracts recovered after pre-treatment with PFW or Tris/EDTA.
Importantly, our in vivo studies demonstrate that endotoxin presented in intact bacteria is equally potent for induction of airway inflammation as LOS or LPS aggregates (Figs. 2 and 3). Thus, use of the LAL assay without Tris/EDTA pre-treatment leads to an underestimation of exposure to bioactive endotoxin in environmental and occupational assessments. Hence, occupational exposure limits based on data from the conventional LAL assay will be set erroneously – our data suggest as much as five-fold too high (i.e. too permissive). Epidemiologic studies relating endotoxin exposures to asthma outcomes 2, 14, 16, 17, 46, 47 have not adequately accounted for household endotoxin contained within bacterial OM.
In summary, our findings strongly suggest that current protocols used for quantitative testing of organic dusts significantly underestimate the bioactive endotoxin content of these samples. The significance of this limitation and benefit of modified sample processing by Tris/EDTA is supported by evidence that the magnitude of acute airway inflammation induced by intact bacteria following i.n. instillation (Figs. 2 and 3) is better predicted by the endotoxin activity of Tris/EDTA than of PFW extracts of the bacteria (Fig. 4A). Moreover, the variable extent to which Tris/EDTA pre-treatment amplifies detection of bioactive endotoxin in individual house dust samples (Fig. 6B) implies an under-estimate of endotoxin content of dusts by current testing methods that varies among individual dust samples, under-cutting its utility for establishing risk-based exposure guidelines. Studies are underway to test whether the greater endotoxin content of organic dusts revealed after Tris/EDTA pre-treatment in the LAL assay better predicts the pro-inflammatory capacity of such dust when inhaled as airborne particles. Results of these studies should help determine if organic dusts should be pre-treated with Tris/EDTA to more accurately assess the endotoxin content and airway pro-inflammatory potential of these environmental samples.
Highlights.
The physical state of environmental endotoxin is unknown and likely diverse.
Limulus-based assays poorly detect endotoxin in intact bacteria.
In contrast, endotoxin in intact bacteria is an equally potent pulmonary inflammogens as purified endotoxin.
Extraction of dust with Tris/EDTA yields a 5-fold enhancement in LAL activity.
Exposure guidelines for endotoxin based on current sample handling are roughly 5-fold too permissive.
Acknowledgments
Research Funding Source: NIH P30 ES005605, NIH R01 AI059372
The authors gratefully acknowledge the assistance of Andrea Adamcakova-Dodd, Theresa Gioannini, Nervana Metwali, Sarah Perry, Athmane Teghanemt, Rachel Wolf, and De Sheng Zhang.
Footnotes
Declaration of Conflicting Interests
The authors declare that there is no conflict of interest.
References
- 1.Douwes J, Thorne P, Pearce N, Heederik D. Bioaerosol health effects and exposure assessment: progress and prospects. Ann Occup Hyg. 2003;47:187–200. doi: 10.1093/annhyg/meg032. [DOI] [PubMed] [Google Scholar]
- 2.Thorne PS, Kulhánková K, Yin M, Cohn R, Arbes SJ, Zeldin DC. Endotoxin exposure is a risk factor for asthma: the national survey of endotoxin in United States housing. Am J Respir Crit Care Med. 2005;172:1371–7. doi: 10.1164/rccm.200505-758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duquenne P, Marchand G, Duchaine C. Measurement of endotoxins in bioaerosols at workplace: a critical review of literature and a standardization issue. Ann Occup Hyg. 2013;57:137–72. doi: 10.1093/annhyg/mes051. [DOI] [PubMed] [Google Scholar]
- 4.Paba E, Tranfo G, Corsetti F, Marcelloni AM, Iavicoli S. Indoor exposure to airborne endotoxin: a review of the literature on sampling and analysis methods. Ind Health. 2013;51:237–55. doi: 10.2486/indhealth.ms1325. [DOI] [PubMed] [Google Scholar]
- 5.Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol. 2003;3:169–76. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- 6.Post DM, Zhang D, Eastvold JS, Teghanemt A, Gibson BW, Weiss JP. Biochemical and functional characterization of membrane blebs purified from Neisseria meningitidis serogroup B. J Biol Chem. 2005;280:38383–94. doi: 10.1074/jbc.M508063200. [DOI] [PubMed] [Google Scholar]
- 7.Hadina S, Weiss JP, McCray PB, Jr, Kulhankova K, Thorne PS. MD-2-dependent pulmonary immune responses to inhaled lipooligosaccharides: effect of acylation state. Am J Respir Cell Mol Biol. 2008;38:647–54. doi: 10.1165/rcmb.2007-0418OC. Epub 2008 Jan 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz DA, Thorne PS, Yagla SJ, et al. The role of endotoxin in grain dust-induced lung disease. Am J Respir Crit Care Med. 1995;152:603–8. doi: 10.1164/ajrccm.152.2.7633714. [DOI] [PubMed] [Google Scholar]
- 9.Thorne PS. Inhalation toxicology models of endotoxin- and bioaerosol-induced inflammation. Toxicology. 2000;152:13–23. doi: 10.1016/s0300-483x(00)00287-0. [DOI] [PubMed] [Google Scholar]
- 10.Liebers V, Brüning T, Raulf-Heimsoth M. Occupational endotoxin-exposure and possible health effects on humans. Am J Ind Med. 2006;49:474–91. doi: 10.1002/ajim.20310. [DOI] [PubMed] [Google Scholar]
- 11.Gioannini TL, Teghanemt A, Zhang D, et al. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci U S A. 2004;101:4186–91. doi: 10.1073/pnas.0306906101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jagielo PJ, Thorne PS, Watt JL, Frees KL, Quinn TJ, Schwartz DA. Grain dust and endotoxin inhalation challenges produce similar inflammatory responses in normal subjects. Chest. 1996;110:263–70. doi: 10.1378/chest.110.1.263. [DOI] [PubMed] [Google Scholar]
- 13.Singh J, Schwartz DA. Endotoxin and the lung: Insight into the host-environment interaction. J Allergy Clin Immunol. 2005;115:330–3. doi: 10.1016/j.jaci.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 14.Thorne PS, Mendy A, Metwali N, et al. Endotoxin Exposure: Predictors and Prevalence of Associated Asthma Outcomes in the United States. Am J Respir Crit Care Med. 2015;192:1287–97. doi: 10.1164/rccm.201502-0251OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorne PS, Cohn R, Mav D, Arbes SJ, Zeldin DC. Predictors of endotoxin levels in U.S. housing. Environ Health Persp. 2009;117:763–71. doi: 10.1289/ehp.11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein MM, Hrusch CL, Gozdz J, et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. N Engl J Med. 2016;375:411–21. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braun-Fahrlander C, Riedler J, Herz U, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–77. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 18.Ege MJ, Frei R, Bieli C, et al. Not all farming environments protect against the development of asthma and wheeze in children. J Allergy Clin Immunol. 2007;119:1140–7. doi: 10.1016/j.jaci.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 19.Raetz CR, Garrett TA, Reynolds CM, et al. Kdo2-Lipid A of Escherichia coli, a defined endotoxin that activates macrophages via TLR-4. J Lipid Res. 2006;47:1097–111. doi: 10.1194/jlr.M600027-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Gioannini TL, Weiss JP. Regulation of interactions of Gram-negative bacterial endotoxins with mammalian cells. Immunol Res. 2007;39:249–60. doi: 10.1007/s12026-007-0069-0. [DOI] [PubMed] [Google Scholar]
- 21.Gioannini TL, Teghanemt A, Zhang D, Levis EN, Weiss JP. Monomeric endotoxin:protein complexes are essential for TLR4-dependent cell activation. J Endotoxin Res. 2005;11:117–23. doi: 10.1179/096805105X35198. [DOI] [PubMed] [Google Scholar]
- 22.Novitsky TJ. Biomedical applications of the Limulus amebocyte lysate. In: Tanacredi JT, Botton ML, Smith DR, editors. Biology and Conservation of Horseshoe Crabs. New York: Springer; 2009. pp. 315–29. [Google Scholar]
- 23.Levin J, Bang FB. The role of endotoxin in the extracellular coagulation of limulus blood. Bulletin of the Johns Hopkins Hospital. 1964;115:265–74. [PubMed] [Google Scholar]
- 24.Erwin AL, Mandrell RE, Munford RS. Enzymatically deacylated Neisseria lipopolysaccharide (LPS) inhibits murine splenocyte mitogenesis induced by LPS. Infect Immun. 1991;59:1881–7. doi: 10.1128/iai.59.6.1881-1887.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thorne PS, Perry SS, Saito R, et al. Evaluation of the Limulus amebocyte lysate and recombinant factor C assays for assessment of airborne endotoxin. Appl Environ Microbiol. 2010;76:4988–95. doi: 10.1128/AEM.00527-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutsmann T, Howe J, Zähringer U, et al. Structural prerequisites for endotoxic activity in the Limulus test as compared to cytokine production in mononuclear cells. Innate Immun. 2010;16:39–47. doi: 10.1177/1753425909106447. [DOI] [PubMed] [Google Scholar]
- 27.Munford RS. Endotoxemia-menace, marker, or mistake? J Leukoc Biol. 2016 doi: 10.1189/jlb.3RU0316-151R. pii: jlb.3RU0316–151R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974;235:109–29. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- 29.Giardina PC, Gioannini T, Buscher BA, et al. Construction of acetate auxotrophs of Neisseria meningitidis to study host-meningococcal endotoxin interactions. J Biol Chem. 2001;276:5883–91. doi: 10.1074/jbc.M009273200. [DOI] [PubMed] [Google Scholar]
- 30.Weiss J, Beckerdite-Quagliata S, Elsbach P. Resistance of gram-negative bacteria to purified bactericidal leukocyte proteins: relation to binding and bacterial lipopolysaccharide structure. J Clin Invest. 1980;65:619–28. doi: 10.1172/JCI109707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madsen KM, Zhang L, Abu Shamat AR, Siegfried S, Cha JH. Ultrastructural localization of osteopontin in the kidney: induction by lipopolysaccharide. J Am Soc Nephrol. 1997;8:1043–53. doi: 10.1681/ASN.V871043. [DOI] [PubMed] [Google Scholar]
- 32.Weinrauch Y, Katz SS, Munford RS, Elsbach P, Weiss J. Deacylation of purified lipopolysaccharides by cellular and extracellular components of a sterile rabbit peritoneal inflammatory exudate. Infect Immun. 1999;67:3376–82. doi: 10.1128/iai.67.7.3376-3382.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barker JH, Kaufman JW, Zhang DS, Weiss JP. Metabolic labeling to characterize the overall composition of Francisella lipid A and LPS grown in broth and in human phagocytes. Innate Immun. 2014;20:88–103. doi: 10.1177/1753425913485308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chrischilles E, Ahrens R, Kuehl A, et al. Asthma prevalence and morbidity among rural Iowa schoolchildren. J Allergy Clin Immunol. 2004;113:66–71. doi: 10.1016/j.jaci.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 35.Svendsen E, Thorne P, O’Shaughnessy P, Zimmerman D, Reynolds S. House dust collection efficiency of the high volume small surface sampler on worn carpets. J Occup Environ Hyg. 2006;3:334–41. doi: 10.1080/15459620600700651. [DOI] [PubMed] [Google Scholar]
- 36.Post DM, Zhang D, Weiss JP, Gibson BW. Stable isotope metabolic labeling of Neisseria meningitidis lipooligosaccharide. J Endotoxin Res. 2006;12:93–8. doi: 10.1177/09680519060120020501. [DOI] [PubMed] [Google Scholar]
- 37.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Preston A, Mandrell RE, Gibson BW, Apicella MA. The lipooligosaccharides of pathogenic gram-negative bacteria. Crit Rev Microbiol. 1996;22:139–80. doi: 10.3109/10408419609106458. [DOI] [PubMed] [Google Scholar]
- 39.Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roier S, Zingl FG, Cakar F, et al. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat Commun. 2016;7:1–13. doi: 10.1038/ncomms10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capodici C, Chen S, Sidorczyk Z, Elsbach P, Weiss J. Effect of lipopolysaccharide (LPS) chain length on interactions of bactericidal/permeability-increasing protein and its bioactive 23-kilodalton NH2-terminal fragment with isolated LPS and intact Proteus mirabilis and Escherichia coli. Infect Immun. 1994;62:259–65. doi: 10.1128/iai.62.1.259-265.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiss J, Hutzler M, Kao L. Environmental modulation of lipopolysaccharide chain length alters the sensitivity of Escherichia coli to the neutrophil bactericidal/permeability-increasing protein. Infect Immun. 1986;51:594–9. doi: 10.1128/iai.51.2.594-599.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.CEN NEN-EN 14031. Workplace atmospheres - Determination of airborne endotoxins. 2000 Available at: https://www.nen.nl/NEN-Shop/Norm/NENEN-140312000-Ontw.-en.htm.
- 44.Spaan S, Heederik DJ, Thorne PS, Wouters IM. Optimization of airborne endotoxin exposure assessment: effects of filter type, transport conditions, extraction solutions, and storage of samples and extracts. Appl Environ Microbiol. 2007;73:6134–43. doi: 10.1128/AEM.00851-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spaan S, Doekes G, Heederik D, Thorne PS, Wouters IM. Effect of extraction and assay media on analysis of airborne endotoxin. Appl Environ Microbiol. 2008;74:3804–11. doi: 10.1128/AEM.02537-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen CM, Thiering E, Doekes G, et al. Geographical variation and the determinants of domestic endotoxin levels in mattress dust in Europe. Indoor Air. 2012;22:24–32. doi: 10.1111/j.1600-0668.2011.00740.x. [DOI] [PubMed] [Google Scholar]
- 47.Perzanowski MS, Miller RL, Thorne PS, et al. Endotoxin in inner-city homes: associations with wheeze and eczema in early childhood. J Allergy Clin Immunol. 2006;117:1082–9. doi: 10.1016/j.jaci.2005.12.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]