Abstract
Patients with atopic dermatitis are frequently colonized by Staphylococcus aureus. If S. aureus is present, then the subject tends to have more severe disease. However, it is unclear if S. aureus is a cause of atopic dermatitis or a consequence of the abnormal epithelial environment. In this issue of the Journal of Investigative Dermatology, Meylan et al. present evidence from a prospective clinical trial that shows that S. aureus colonization precedes onset of atopic dermatitis in children. These observations suggest that S. aureus may cause atopic dermatitis in some individuals.
Atopic dermatitis (AD) is a complex multifactorial disease that has been associated with both genetic risk factors and environmental stimuli. It has long been known that subjects with AD are more likely to be colonized by Staphylococcus aureus (Leyden et al., 1974). Advances in next-generation sequencing have improved descriptions of the composition of the skin bacterial community known as the microbiome and furthered understanding of the degree of dysbiosis that is present on AD skin. Both 16S and metagenomic analyses of bacterial DNA have shown that the skin microbiome on AD subjects has decreased bacterial diversity and increased S. aureus colonization (Kong et al., 2012). Indeed, recent work has shown that not only is S. aureus increased on AD skin but that specific strains of S. aureus are associated with more severe AD (Byrd et al., 2017). These observations, considered together with work that has shown how normal commensal skin bacteria can promote health, support the hypothesis that dysbiosis in AD is critical to driving disease severity and/or tendency for relapse (Williams and Gallo, 2015).
In this study, we observed a distinct increase of S. aureus prevalence at age 3 months in infants who later developed AD.
S. aureus colonization in longitudinal studies
Although multiple clinical studies had previously shown increased S. aureus colonization in children and adult patients with existing AD, it remains unclear if S. aureus precedes the onset of clinically apparent disease. To address this, large prospective longitudinal studies analyzing the microbiome from birth are needed. In 2017, Kennedy et al. described a study of 50 infants from birth to 2 years of age who were swabbed at four different skin sites at three time points in the first 6 months of life. Using 16S ribosomal RNA gene DNA sequencing, the researchers did not detect significant increases in S. aureus colonization on the 10 infants who developed AD, and they therefore concluded that S. aureus colonizes skin after onset of AD. However, a potential causal association of AD with the microbiome was seen with other nonidentified members of the Staphylococcus genus, and their presence correlated with a better outcome of AD (Kennedy et al., 2017). Meylan et al. (2017) now present a larger longitudinal study of 149 infants who were sampled in the axillae and the antecubital fossae seven times during the first 2 years of life. AD developed in 36 of these subjects. This study was performed with higher-resolution, but lower-sensitivity, culture-based techniques for identification of bacterial species. This study detected S. aureus colonization before clinical onset of AD. Furthermore, another member of the Staphylococcus genus, Staphylococcus hominis, was observed to be statistically less abundant in AD and potentially protective (Meylan et al., 2017).
Recent experimental evidence that S. aureus can induce AD-like phenotypes
Experiments done in vitro and in mice suggest that S. aureus could promote disease through secretion of multiple virulence factors. Some of the most well-studied virulence factors include toxic shock syndrome toxin-1, enterotoxins, proteases (e.g., aureolysin, V8, and exfoliative toxins), and lysins (e.g., α-toxin and phenol-soluble modulins). It has been clearly shown that these factors can elicit both toxicity and/or inflammatory responses in skin cells that are directly exposed to microbes. Although in vitro studies of the effect of S. aureus on skin cells is important mechanistically, in vivo murine models of live S. aureus colonization have provided even stronger evidence that S. aureus colonization can induce AD skin phenotypes. We have shown that colonization of murine skin by S. aureus directly induces serine protease activity that disrupts the epidermal barrier (Williams et al., 2017) and that expression of T helper type 2 cytokines in the skin (a hallmark of the AD phenotype) is dependent on proteases secreted by S. aureus (Nakatsuji et al., 2016). Additionally, Nakamura et al. (2013) have observed that S. aureus delta toxin increases allergic responses and promotes both inflammation and desquamation of the skin surface. Collectively, these and several other reports provide strong supporting mechanistic evidence that can explain how S. aureus colonization could promote AD in genetically susceptible individuals.
Current therapeutic strategies to combat S. aureus
The etiologic role of S. aureus in AD suggested by the work discussed suggests that targeted elimination of S. aureus should be beneficial. Previously, methods including topical antimicrobials, systemic antibiotics, and bleach baths have been used alone or in different combinations to remove bacteria. Although these treatments are occasionally effective in treating AD, it has not been clear if the treatments eliminate S. aureus colonization. Furthermore, antibiotic treatments can also perturb the normal bacterial flora, and this can have a negative impact on potential benefits of the microbiome. In 2017, we described a potential method to specifically target S. aureus colonization on AD skin using a skin microbiome transplant (Nakatsuji et al., 2017). In this approach, we isolated coagulase-negative Staphylococcus species that secrete lantibiotics with strong antimicrobial activity against S. aureus. The presence of these coagulase-negative Staphylococcus strains was strongly associated with protection against S. aureus in the normal population, and when an expanded culture of these beneficial bacteria was applied to the skin of AD subjects, this significantly reduced S. aureus colonization. Ongoing trials will determine if this approach can be successful for longer periods of time and if this can improve the phenotype of AD.
Integrating a model and future directions
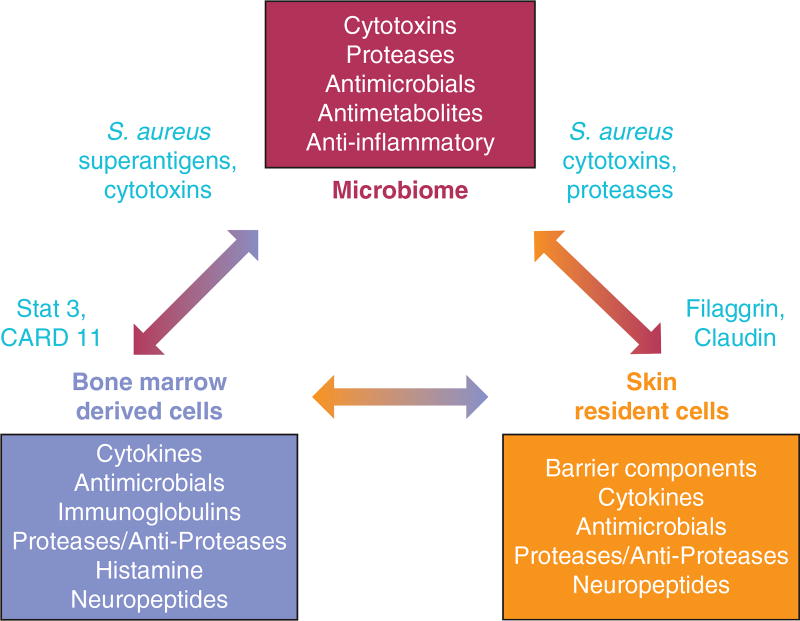
Overall, the essential role that the microbiome plays in shaping the human immune system suggests that AD is a clinical phenotype that reflects imbalance between the functions of the epidermis, the resident microbiome, and circulating cells of the immune system. As seen in Figure 1, each element of this triad is required for normal immune homeostasis. AD probably is a clinical phenotype that can be driven by a defect in any of these elements, and one of the key drivers of disease can be colonization by an abnormal skin microbiome that includes S. aureus. Additional basic research is needed to help guide clinical trials to confirm the role of S. aureus in AD.
Figure 1. Cells that participate in skin health and disruption during atopic dermatitis.
Interactions among the skin microbiome (bacteria, fungi, and viruses), resident cells in the skin (keratinocytes, fibroblasts, adipocytes, neural elements, and vasculature), and bone marrow-derived cells of the immune system (dendritic cells, lymphocytes, are granulocytes) are essential for homeostasis in healthy skin. Each box denotes some of the responsible molecules and functions for each of these three cellular systems. In atopic dermatitis, molecular or cellular defects in these systems are associated with disease (indicated in red). Disease may manifest from individual or a combination of defective functions. S. aureus, Staphylococcus aureus.
Footnotes
CONFLICT OF INTEREST
RLG is a consultant for Matrisys, and Sente, Inc. Matrisys has licensed IP from UCSD for microbiome therapy. MRW states no conflict of interest.
References
- Byrd AL, Deming C, Cassidy SKB, Harrison OJ, Ng WI, Conlan S, et al. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci Transl Med. 2017;9(397):eaa14651. doi: 10.1126/scitranslmed.aal4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy EA, Connolly J, Hourihane JO, Fallon PG, McLean WH, Murray D, et al. Skin microbiome before development of atopic dermatitis: early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J Allergy Clin Immunol. 2017;139:166–72. doi: 10.1016/j.jaci.2016.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850–9. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525–30. doi: 10.1111/j.1365-2133.1974.tb06447.x. [DOI] [PubMed] [Google Scholar]
- Meylan P, Lang C, Mermoud S, Johannsen A, Norrenberg S, Hohl D, et al. Skin colonization by Staphylococcus aureus precedes the clinical diagnosis of atopic dermatitis in infancy. J Invest Dermatol. 2017;137:2497–504. doi: 10.1016/j.jid.2017.07.834. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Munoz-Planillo R, Hasegawa M, et al. Staphylococcus delta-toxin induces allergic skin disease by activating mast cells. Nature. 2013;503(7476):397–401. doi: 10.1038/nature12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. 2017;9(378):eaah4680. doi: 10.1126/scitranslmed.aah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Chen TH, Two AM, Chun KA, Narala S, Geha RS, et al. Staphylococcus aureus exploits epidermal barrier defects in atopic dermatitis to trigger cytokine expression. J Invest Dermatol. 2016;136:2192–200. doi: 10.1016/j.jid.2016.05.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MR, Gallo RL. The role of the skin microbiome in atopic dermatitis. Curr Allergy Asthma Rep. 2015;15:65. doi: 10.1007/s11882-015-0567-4. [DOI] [PubMed] [Google Scholar]
- Williams MR, Nakatsuji T, Sanford JA, Vrbanac AF, Gallo RL. Staphylococcus aureus induces increased serine protease activity in keratinocytes. J Invest Dermatol. 2017;137:377–84. doi: 10.1016/j.jid.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]