Abstract
The 2014–15 Ebola virus (EBOV) outbreak in West Africa highlighted the urgent need for specific therapeutic interventions for infected patients. The human-mouse chimeric monoclonal antibody (mAb) cocktail ZMapp™, previously shown to be efficacious in EBOV (variant Kikwit) lethally infected nonhuman primates (NHPs) when administration was initiated up to 5 days, was used in some patients during the outbreak. Here we show that a two-antibody cocktail, MIL77E, is fully protective in NHPs when administered at 50 mg/kg 3 days after challenge with a lethal dose of EBOV, variant Makona, the virus responsible for the ongoing 2014–15 outbreak, while a similar formulation of ZMapp™ protected 2 of 3 NHPs. The chimeric MIL77E mAb cocktail is produced in engineered CHO cells and is based on mAbs c13C6 and c2G4 from ZMapp™. The use of only 2 antibodies in MIL77E opens the door to a pan-ebolavirus cocktail.
Introduction
Ebola virus (EBOV; species Zaire ebolavirus, family Filoviridae) is responsible for outbreaks of Ebola virus disease (EVD) in Africa. The 2014–15 West African outbreak is notable for many reasons(1, 2): it is the first outbreak of EBOV to occur outside of Central Africa; it is the first filovirus outbreak to last over one year; and the first to spread to more than several hundred individuals (over 27 000 cases including over 11 000 deaths, as of June 2015). The outbreak was caused by a novel EBOV variant, named Makona(3, 4). Since past EBOV outbreaks were localized geographically and often self-limiting, the scale of the 2014–15 outbreak took the international community by surprise. A number of foreign aid workers, mostly from Europe and North America, were also infected while assisting in Ebola Treatment Units(5). Many of these workers were repatriated to their home countries and given a number of experimental treatments. One of those treatments was ZMapp™, a cocktail of three monoclonal antibodies (mAbs) directed against the EBOV glycoprotein (GP). ZMapp™ combines the best-performing antibodies from two different cocktails (6): MB-003, containing the mAbs 13C6, 6D8, and 13F6(7); and ZMAb, containing the mAbs 1H3, 2G4, and 4G7 (8). The final formulation of ZMapp™ contains mAbs c13C6, c2G4, and c4G7, and was shown to rescue 100% of lethally-infected non-human primates (NHPs) even when the treatment began as late as 5 days after exposure thus capable of reversing advanced EBOV disease(6).
The first humans to receive ZMapp™ were two American aid workers who had contracted EVD while working in Liberia during the summer of 2014. At the time of treatment under compassionate use guidelines, both patients had signs of advanced EVD including hypovolemia, hypocalcemia, hypokalemia, hypoalbuminemia, and one patient also had substantial liver injury as a result of the infection(9). The administration of ZMapp™ coincided with a subsequent decrease in viremia and both patients survived the infection. ZMapp™ was subsequently used compassionately with 7 other patients, and four survived EVD(10). ZMapp™ is currently being evaluated in a randomized controlled trial in West Africa(11). Attempts to manufacture ZMapp™ on a larger scale have been met with challenges due in part to low 4G7 yields in both plant and mammalian expression systems. To attempt to provide more manufacturing capacity for these mAbs, PHAC and Mapp Biopharmaceutical collaborated with Beijing Mabworks to produce ZMapp™ –like mAbs in modified CHO cells; this version of the cocktail is called MIL77 and is composed of: MIL77-1 (containing the variable regions of c2G4), MIL77-2 (containing the variable regions of c4G7), and MIL77-3 (containing the variable regions of c13C6). The antibodies in MIL77 also had their framework regions modified to be more similar to human framework regions. The CHO cells used for expression of MIL77 are engineered to prevent fucosylation, similar to the N-glycosylation present in plant-produced ZMapp™; the absence of fucose increases the affinity of the mAbs for the FcγRIIIa (CD16)(12). Mabworks confirmed that MIL77-2 (based on mAb c4G7) had much lower expression than MIL77-1 and -3 in their system. Although initial studies suggested c2G4 and c4G7 bind separate epitopes(13), more detailed structural analysis of ZMapp™ revealed that the two mAbs bind overlapping epitopes on the EBOV GP(14, 15), suggesting that these two treatment components are redundant. Our objective was to confirm whether the cocktail produced in the modified CHO cells had similar properties and comparable efficacy to the plant-produced ZMapp™, and to evaluate the impact of removing mAb c4G7 (MIL77-2).
Results
MIL77-1 contains 5 different amino acids compared with the ZMapp™ c2G4; there were 3 changes for MIL77-2 (compared with ZMapp™ c4G7); and there were 20 changes for MIL77-3 (compared with ZMapp™ c13C6). None of the observed changes were in the complementarity-determining regions. The sequences of the variable regions in the antibodies were determined by mass spectrometry and LC-MS/MS tryptic peptide mapping.
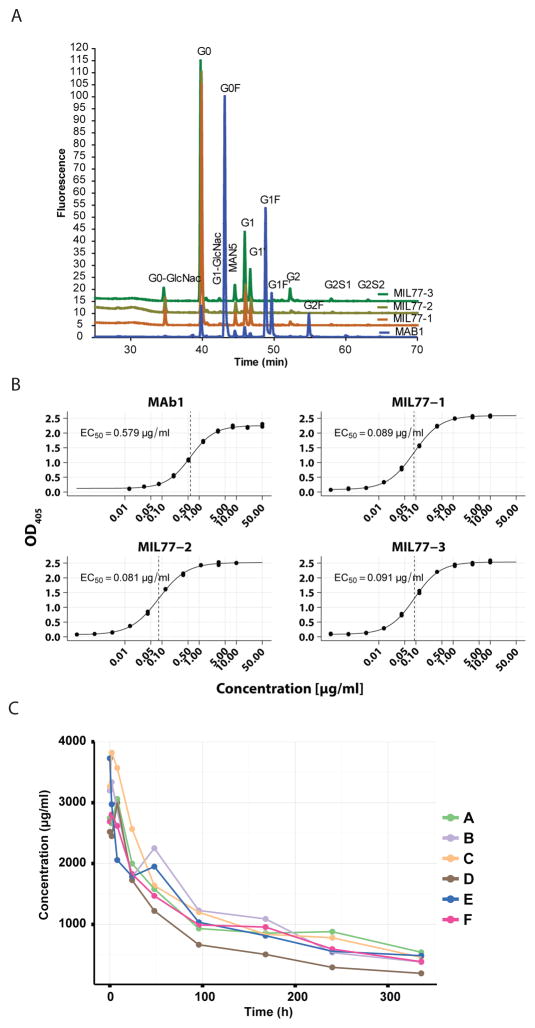
To assess the glycosylation of MIL77, the N-linked oligosaccharides were labeled with 2-aminobenzamide (2-AB) and analyzed using hydrophilic interaction liquid chromatography (HILIC). As shown in Figure 1A, less than 1% of the glycans of MIL77 contained fucose, all in the form of G0F. The structures of the oligosaccharides were confirmed to be G0, G1, and G1′ by LC-MS/MS characterization of the enzymatic released N-oligosaccharides with 2-AB tag HILIC separated (Supplementary Table S1).
Fig. 1.
Characterization of MIL77. A) The hydrophilic interaction liquid chromatography (HILIC) analysis of the PNGase F enzymatic released N-glycans with 2-AB fluorescence tag. MAb1, a recombinant humanized IgG1 monoclonal antibody produced with normal CHOK1 cells that has typical N-glycan profile, is used as a control. B) Affinity of MIL77 components for human CD16 (FcγRIIIa), determined by ELISA. C) Time-concentration curves for 6 animals given 150 mg/kg of MIL77 (1 + 2 + 3).
As displayed in Figure 1B, we evaluated the affinity of the components of MIL77 for FcγRIIIa (CD16) using ELISA. The median binding concentrations (BC50) were found to be similar for the three antibodies and in the range of 0.08–0.09 μg/ml. The results suggest that the mAbs do bind to CD16 at physiological concentrations and that CD16-mediated mechanisms would be available to supplement the documented neutralizing activity of the antibodies(14, 16).
In order to ensure that the antibodies were not altered by the changes in the framework regions, we performed anti-EBOV glycoprotein ELISA and anti-EBOV-eGFP neutralization assays, respectively. The ELISA titration curves were very similar, with BC50 values with overlapping 95% confidence intervals for c2G4/MIL77-1 and c13C6/MIL77-3 while c4G7/MIL77-2 had very close BC50 values (Supplementary Figure S1). The neutralization curves were also very similar for all 3 pairs of antibodies (Supplementary Figure S2). We also confirmed that the cross-inhibition profile of the antibodies had been maintained (Supplementary Figure S3). The results are consistent with those described previously(14, 15). MIL77-1 and MIL77-2 showed strong inhibition of each other’s signals, consistent with the previous observations for c2G4 and c4G7, respectively. MIL77-3, based on c13C6, showed no inhibition of MIL77-1 and -2.
We then measured the half-life of the MIL77 cocktail in cynomolgus macaques. Six macaques (3 males, 3 females) were given a dose of 150 mg/kg of MIL77 (50 mg/kg of each component) intravenously in a volume of 12 ml/kg, at a rate of 1 ml/kg/min. Plasma concentrations of MIL77 (as a cocktail) were evaluated by ELISA (Figure 1C). The pharmacokinetic parameters were estimated based on a non-compartmental model and were: the average elimination half-life of 161.0 ± 39.5 hr (6.7 ± 1.6 days), maximum concentration of 3203.8 ± 323.4 μg/ml, AUC up to the last measurable concentration (AUClast) of 332.7 ± 51.8 hr*mg/ml, and systemic clearance at 0.4 ± 0.1 ml/hr*kg.
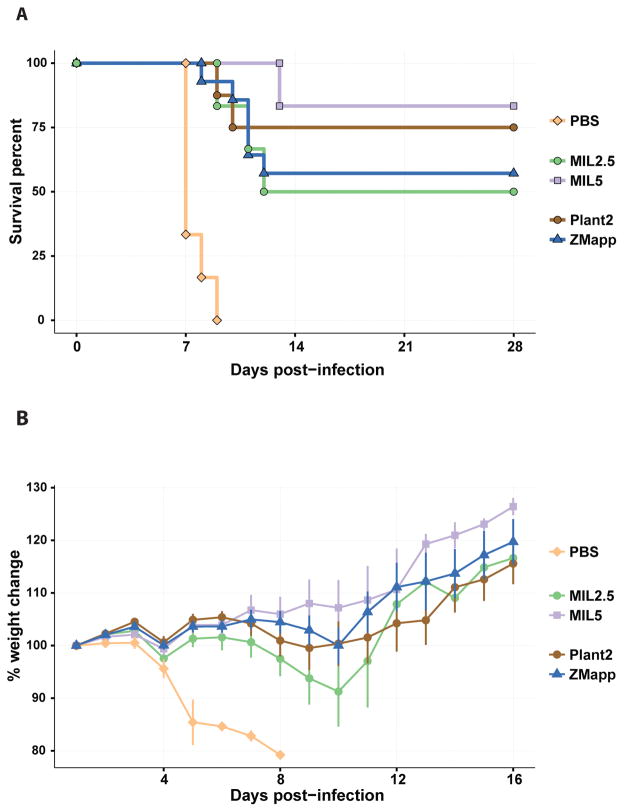
In order to confirm that MIL77 is at least as protective as ZMapp™, we infected 5 groups of guinea pigs with a guinea pig-adapted variant of EBOV (EBOV/GA). The animals were treated with PBS (6 animals), or MIL77 (2.5 or 5 mg/animal; 6 animals per dose), or ZMapp™ (c13C6 + c2G4 + c4G7, 1:1:1 ratio; 5 mg/animal, 1.66 mg/animal/mAb; 14 animals), or plant-produced c13C6 + c2G4 at a 1:2 ratio (Plant2; 5 mg/animal; 8 animals). All groups showed partial survival, except the PBS-treated animals which died around day 8 post-infection (Figure 2A). The log-rank test revealed no significant differences between the variously treated groups (χ2 = 2, df = 3, p = 0.565; comparing all groups except the controls using the log-rank test). Weight changes during the first 16 days of the challenge also supports equal or superior efficacy for MIL77 compared with the two plant-derived cocktails (Figure 2B). Both the Plant2 and MIL77 (5 mg) treatments showed better protection (although not statistically significant) than ZMapp™. For this reason, we decided to study the efficacy of only the 2-mAb cocktail version of MIL77 which corresponds to Plant2. The 2-mAb cocktail was designed with a skewed ratio in order to maintain the neutralization efficiency of the combination. The mAbs c2G4 (MIL77-1) and c4G7 (MIL77-2) are highly neutralizing, whereas mAb c13C6 (MIL77-3) is weakly neutralizing in the presence of complement. The original ZMapp™ cocktail has a 1:2 ratio of weak:strong neutralizing mAbs (1:(1 + 1) of c13C6:(c2G4 + c4G7)).
Fig. 2.
Protection of guinea pigs by MIL77. A) Survival curves showing the survival of various groups of guinea pigs infected with EBOV/GA on Day 0 and treated on Day 3 with the specified treatment (5 mg/animal unless specified). B) Weight change of guinea pigs from A over the first 16 days of the experiment. Groups: ZMapp: c13C6 + c2G4 + c4G7, ratio = 1:1:1, total dose = 5 mg/animal; Plant2: c13C6 + c2G4, ratio = 1:2, total dose = 5 mg/animal; MIL2.5: 2.5 mg of MIL77 (1 + 2 + 3); MIL5: 5 mg of MIL77 (1 + 2 + 3); PBS: 1ml of PBS.
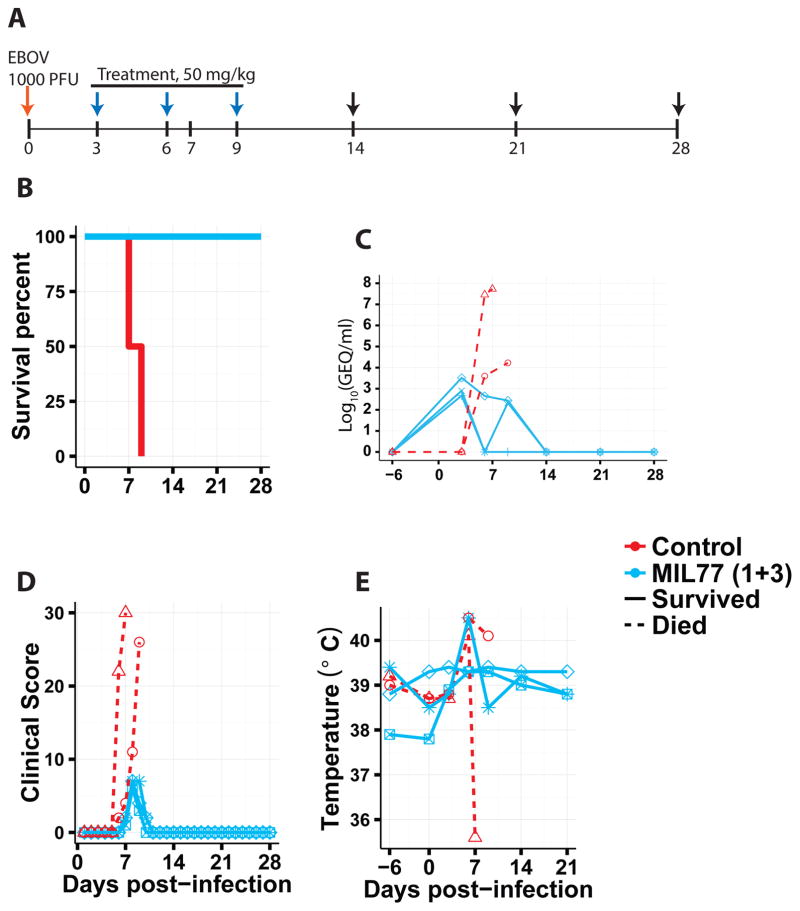
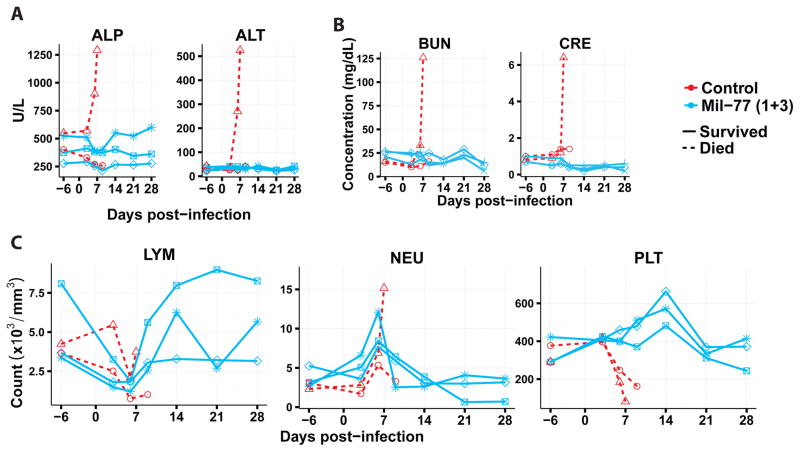
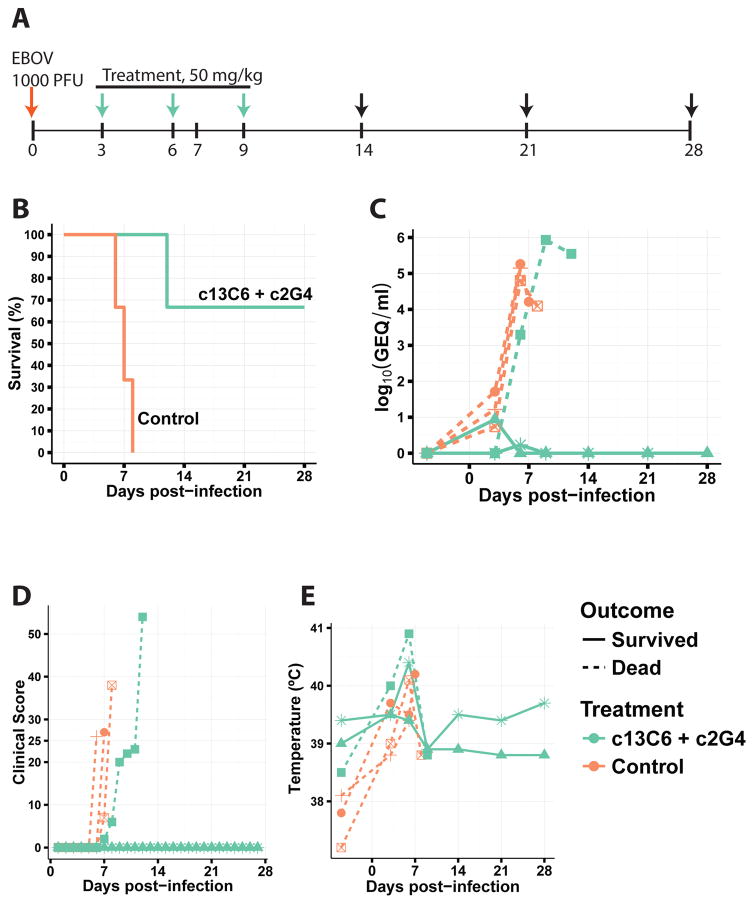
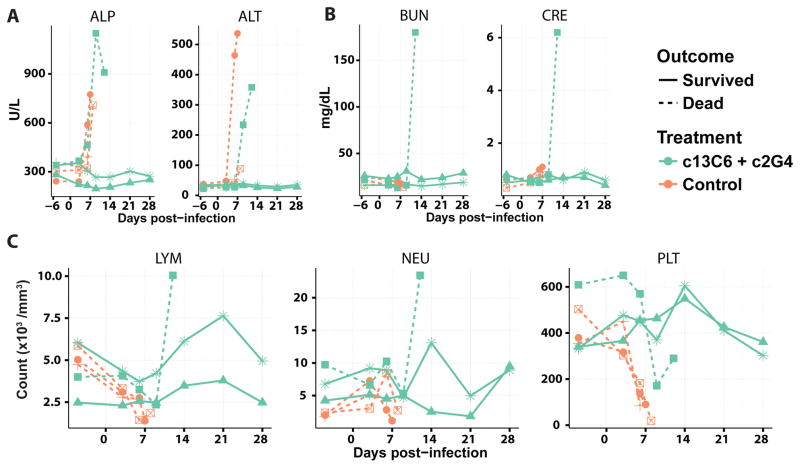
Next, we characterized the efficacy of the two-mAb cocktail containing only MIL77-1 and -3 (2:1 ratio; MIL77E for Ebola virus) in NHPs. Five rhesus macaques were challenged with the Makona variant of EBOV (EBOV/Mak-C05) on day 0. Three animals received the cocktail and two animals were given a control mAb. The animals were treated on days 3, 6, and 9 post-exposure with 50 mg/kg of the cocktail (Figure 3A). MIL77E protected all 3 animals. The two control NHPs were euthanized on days 7 and 8 (Figure 3B). All animals showed viremia of at least 2.5 log10 GEQ/ml at the time of the first treatment (Figure 3C) and changes in the clinical score (Figure 3D). Most animals had at least a slight increase in temperature compared to baseline (Figure 3E). The control animals showed signs of liver damage as evidenced by increased alkaline phosphatase and alanine aminotransferase levels (Figure 4A), and kidney damage as evidenced by elevated total bilirubin and blood urea nitrogen levels (Figure 4B). Lymphopenia appeared between days 3–9 along with an increase in circulating neutrophils, only the control animals showed signs of thrombocytopenia (Figure 4C).
Fig. 3.
Protection of NHPs by MIL77. A) Timeline of the experiment. Arrows: red = challenge day, blue = treatment + exam, black = exam. B) Survival. C) Viremia measured by RT-qPCR. D) Clinical score. E) Rectal temperature.
Fig. 4.
Clinical parameters of NHPs challenged with EBOV/Mak and treated with PBS or MIL77. A) Liver function, illustrated by alkaline phosphatase (ALP) and alanine aminotransferase (ALT). B) Kidney function, illustrated by total bilirubin (TBIL) and blood urea nitrogen (BUN). C) Blood count, illustrated by lymphocyte count (LYM), neutrophil count (NEU), and platelet count (PLT).
We also characterized the efficacy of plant-produced c13C6 and c2G4 (50 mg/kg total, 1:2 ratio, as with MIL77E) in protecting three NHPs against EBOV/Mak-C05. Six NHPs (3 treated and 3 control animals) were infected with EBOV/Mak-C05 and were treated every 72 hours starting on day 3 post-infection (Figure 5A). The three control animals were euthanized on days 6, 7, and 8, whereas only one treated animal was euthanized on day 12 (Figure 5B). Five of the 6 animals, including 2 of the 3 treated animals, showed at least mild viremia at the time of the first treatment (Figure 5C). Two of the 3 treated animals showed no signs of disease, maintaining a clinical score of or close to 0 for the entire 28 days (Figure 5D). Only the four NHPs which were euthanized showed fever (Figure 5E). The animals which succumbed to EBOV disease showed signs of liver damage as evidenced by increased alkaline phosphatase and alanine aminotransferase levels (Figure 6A), but only the non-surviving treated animal showed kidney damage as evidenced by elevated creatinine and blood urea nitrogen levels (Figure 6B). Lymphopenia appeared between days 3–9 along with an increase in circulating neutrophils, only the non-surviving animals showed signs of thrombocytopenia (Figure 6C).
Fig. 5.
Protection of NHPs by ZMapp™ (c13C6 + c2G4). A) Timeline of the experiment. Arrows: red = challenge day, green = treatment + exam, black = exam. B) Survival. C) Viremia measured by RT-qPCR. D) Clinical score. E) Rectal temperature.
Fig. 6.
Clinical parameters of NHPs challenged with EBOV/Mak and treated with PBS or ZMapp™ (c13C6 + c2G4). A) Liver function, illustrated by alkaline phosphatase (ALP) and alanine aminotransferase (ALT). B) Kidney function, illustrated by = blood urea nitrogen (BUN) and creatinine (CRE). C) Blood count, illustrated by lymphocyte count (LYM), neutrophil count (NEU), and platelet count (PLT).
Discussion
Our goal was to evaluate whether a new form of CHO-produced ZMapp™ could provide a level of protection similar to the previously published efficacy, even against EBOV/Mak-C05. The initial in vitro characterization of the new cocktail, composed of the antibodies MIL77-1, MIL77-2, and MIL77-3, suggested it is afucosylated, as expected based on the modified CHO cells used for production. An ELISA was used to assess the affinity of the afucosylated antibodies to human CD16a, this assay showed that the median binding concentration for all three antibodies was around 80–90 ng/ml. While the MIL77 antibodies are based on the ZMapp antibodies, the framework regions have been humanized in order to reduce the chances of patients developing anti-treatment responses. In order to ensure that the humanization did not compromise the paratope, we performed side-by-side ELISA and EBOV-eGFP neutralization assays. No meaningful differences were found between the original chimeric antibodies and the new humanized antibodies.
An initial in vivo experiment was performed to assess the half-life of the new humanized antibodies. This experiment suggests that the half-life in cynomolgus macaques is approximately 6.7 days (standard deviation of 1.7 days) in the absence of virus. We next investigated the efficacy of the new antibodies against a guinea pig-adapted EBOV. The results suggested that MIL77 was at least as protective as ZMapp™ and that a two-mAb combination of antibodies (c13C6 + c2G4) could perform similarly the complete ZMapp™. We then assessed the efficacy of both the MIL77E (MIL77-1 + MIL77-3) and 2-mAb ZMapp™ (c13C6 + c2G4) cocktails in protecting cynomolgus macaques against disease caused by infection with EBOV/Mak-C05. While ZMapp™ protected only 2 of 3 animals, MIL77E protected 3 of 3 treated animals, with the treatment beginning 72 hours after infection.
The only other 2-mAb cocktail we could find was evaluated by Marzi et al(17) and protected 1 of 3 challenged animals, although the dosing schedule was very different, with administration of antibodies at days −1, 1, and 3 post-infection.
While we believe that the results presented here represent an important advance in the treatment of EBOV disease, it is important to keep in mind some of the limitations of the study. First, in order to ensure humane treatment of the animals, the experimenters were not blinded to the experimental treatments. This is because the control animals are euthanized at lower thresholds than experimental animals since they are historically known to die of the infection. Second, the NHP studies had relatively low power to detect small to medium differences in survival rates. However, we have, in the past, found that guinea pig studies were highly predictive, if not of the survival rate, at least of the ranking of various antibody-based treatments in NHPs. The combination of results from the two studies is strongly suggestive that the efficacy of MIL77E is at least similar to that of ZMapp™.
Overall, MIL77E offered a level of protection comparable to treatments using a similar formulation of the ZMapp™ antibodies. To the best of our knowledge, this is the first study to show that a two-mAb cocktail can confer 100% protection in NHPs infected with EBOV. The efficacy of MIL77E at later starting times remains to be assessed. Additionally, MIL77 has been administered compassionately to 4 people (2 infected patients therapeutically and 2 high-risk exposure patients prophylactically) and showed very encouraging results without any reported side effects(18, 19). Since MIL77E includes one mAb specific for EBOV, and one mAb based on c13C6, which also cross-reacts with other ebolaviruses in vitro(20), the addition of a third potent Sudan virus (SUDV)-specific mAb could lead to the generation of a single product targeting both EBOV and SUDV, the causative agents of the majority of EVD outbreaks in Africa. Finally, a two-mAb cocktail will also simplify the production and approval processes as well as increase the safety profile of the treatment.
Materials and Methods
Study Design
The half-life experiment was designed to provide biological triplicates for both male and female cynomolgus macaques. The challenge NHP experiments were designed to declare a true difference of 89% to be significant 80% of the time at the 5% significance level, based on the power calculation of the comparison of two proportions without continuity correction. The guinea pig studies were initially designed as three separate studies. The first was designed to compare ZMapp with its 2-mAb version and was initially designed to declare a true difference of 80% to be significant 80% of the time at the 5% significance level (n = 4/group) using the comparison of two proportions without continuity correction; this experiment was repeated a second time to confirm the initial results. The third experiment was designed to compare ZMapp to MIL77 at two doses of MIL77: 2.5 and 5 mg/animal. The experiment was powered to declare a true difference of 70% to be significant 80% of the time at the 5% significance level based on the comparison of two proportions without continuity correction. All power calculations were performed using the online power calculators located at http://www.sample-size.net/. None of the experiments were conducted under blinding to ensure humane treatment of control animals. All animals were randomized into control or treatment groups.
Viruses
The challenge virus used for guinea pigs was Ebola virus VECTOR/C.porcellus-lab/COD/1976/Mayinga-GPA (EBOV/GA) (order Mononegavirales, family Filoviridae, species Zaire ebolavirus; GenBank accession number AF272001.1). The virus used to challenge the non-human primates was Ebola virus/H.sapiens-tc/GIN/2014/Makona-C05 (EBOV/Mak-C05) (order Mononegavirales, family Filoviridae, species Zaire ebolavirus;GenBank accession number KJ660348).
Animals
Outbred 6–8-week-old female Hartley strain guinea pigs (from Charles River) were used in these experiments. The animals were infected with 1000 LD50 of EBOV/GA. The treatments consisted of one dose of either: ZMapp™ (5 mg; n = 14); c1H3 + c2G4 (1:3; 5 mg; n = 8) referred to as Plant2; MIL77 (1 + 2 + 3; 5 mg; n = 6); MIL77 (1 + 2 + 3; 2.5 mg; n = 6); or PBS (n = 6). The animals were monitored daily for 28 days for survival and clinical symptoms, and 16 days for weight. The study was not blinded and no animals were excluded from the analysis.
For the NHP study, five male and female rhesus macaques (Macaca mulatta), ranging from 3.7–10.9 kg (2–5 years old) were purchased from Primgen (USA). The study was not blinded and no animals were excluded from the analysis. The animals were assigned to groups based on sex and weight. The NHPs were fed standard monkey chow along with fruits, vegetables, and treats. Husbandry enrichment consisted of visual stimulation and toys. All animals were challenged with 1000 TCID50 of EBOV/Mak-C05 on Day 0. The treatments were administered on Days 3, 6, and 9 and consisted of either PBS or 50 mg/kg (total) of MIL77-1 + MIL77-3 (1:2 ratio; 16.7 and 33.3 mg/kg, respectively). The animals were monitored closely for signs of disease and changes in food and water consumption. The rectal temperature and weight were measured on treatment days and Days 14, 21, and 28. Blood samples and swabs were collected on exam days to evaluate viremia, blood counts, and serum biochemistry.
Six cynomolgus macaques (3 males and 3 females) were given a single intravenous infusion of the combined injection at 150 mg/kg including 50 mg/kg each of MIL77-1, MIL77-2 and MIL77-3. The drug was intravenously infused at dose volume of 12 mL/kg and dose rate of 1 mL/kg/min. The whole blood samples (approximate 1.0 mL) were collected at predose, and 1min, 2 h, 8 h, 24 h, 48 h, 96 h, 168 h, 240 h and 336 h post dose. Plasma concentrations were quantitatively analyzed by an enzyme-linked immunosorbent assay (ELISA). ELISA plates were coated with the EBOV glycoprotein GP. Reaction was initiated by addition of the serum to be measured. Then horseradish peroxidase labeled goat anti-Human IgG (adsorption of monkey serum) was used as secondary antibody. It was developed using TMB. The concentrations of samples were calculated based on the calibration curve fitted by the software of OriginPro 7.5.
Monoclonal antibody production
Production of ZMappTM and plant-produced recombinant c2G4 and c1H3 was carried out as described previously(7, 21, 22).
The primary sequences of the three MIL77 antibodies (MIL77-1, -2, and -3) were constructed and optimized based on the variable region sequences of corresponding ZMappTM antibodies (2G4, 4G7 and 13C6). The constant regions of MIL77 mAbs were all constructed based on human consensus sequence of IgG1 subgroup-III VH for heavy chain and κ subgroup-I VL for light chain. The genes encoding the heavy chain and light chain of MIL77-1, MIL77-2, and MIL77-3 were cloned into Mabworks’ proprietary GS expression vector and introduced into the glyco-engineered CHOK1-AF cells for expression. The CHOK1-AF cell line was developed by engineering the CHO-K1 (CCL-61, ATCC) using the zinc-finger nuclease technology to knock-out the SLC35C1 gene, which encodes the GDP-fucose transporter, a critical factor in regulating the fucosylation of glycans(23).
The MIL77 drug product materials used for the guinea pig efficacy and all of the in vitro tests were produced from the 30 L pilot-scale production campaigns in fed-batch process using chemically-defined cell culture media at Mabworks’ (Beijing, China) and HiSun Pharma’s (Taizhou, China) GMP facilities. The MIL77 materials used for the NHP efficacy and pharmcokinetic studies were produced from the 300 L production campaigns using the same fed-batch process at the HiSun GMP facilities. All of the analytical characterization and QC testing of the drug product batches were performed according to Chinese Food and Drug Control regulations. The certificate of analysis was issued to each batch that was tested and released by the Quality Department, Mabworks (Beijing, China).
Hydrophilic Interaction Liquid Chromatography (HILIC) of MIL77 N-Linked glycans
The N-glycans were released by incubating the desalted MIL77 with PNGase F overnight (~15 h) at 37 °C. After the enzymatic incubation, the antibody was precipitated by the addition of ethanol and centrifugation. The supernatants were collected and brought to dryness by using of Speedvac at ≤35°C. The labeling solution was prepared by adding 60 mg/mL of sodium cyanoborohydride (Na[BH3(CN)]) and 50 mg/mL of 2-AB dissolved in70:30 (%, v/v) DMSO–acetic acid. The labeling solution (20 μL) was added to the dried glycans and heated for 3h at 65°C.
The labeled N-glycans were analyzed on a BEH Glycan column (Waters, 2.1 mm ×150 mm, 1.7 μm particle) with mobile phases A, 100mM ammonium formate, pH 4.5; and mobile phase B, acetonitrile. The gradient was maintained at 80% B for 20 min, to 75% B in 5 min, to 60% B in 50 min then to 35% in 5min and maintained for 5min at a flow rate of 0.25 mL/min with the column temperature maintained at 65°C. The Fluorescent wavelengths were set at λex = 330 nm and λem = 420 nm. The structures of the 2-AB labeled N-glycans were verified by monitoring the outlets of the HILIC gradient on a 4600 TripleTOF mass spectrometer (AB Sciex, Foster City, CA) with Analyst TF 1.7 software. Data 500-3000 m/z were acquired in the Positive TOF MS mode and the acquisition time was 0.25s. MS settings were: GS1 55, GS2 55, CUR 25, ISVF 5500, TEM 550, CE 15 and DP 100. Mass calibration was conducted using sodium trifluoroacetate for every injection. The errors between the observed and theoretical mass charge ratios (m/z) were all within the inherent determination errors of the mass spectrometer.
The analysis results are displayed in Supplementary Table 1.
Binding Affinities of MIL77 Antibodies to human FcγRIIIa(158V)
The 96-well plates (corning) were coated with anti-His antibody (Genescript, Nanjing, China) (1 μg/ml) in carbonate buffer, pH9.6, at 4 °C overnight. Plates were washed with phosphate buffered saline (PBS) containing 0.05% polysorbate, pH 7.4 and blocked with PBS containing 5% non-fat milk, pH 7.4. After a 1.5 h incubation at 37°C, plates were washed and FcγRIIIa (158V) (1 μg/ml) in PBS containing 0.05% polysorbate 20, pH 7.4 (assay buffer) was added. After a 1 h incubation, plates were washed. IgG was preincubated with goat F(ab′)2 anti-human κ antibody (Sigma-Aldrich) at a 1:2 (w/w) ratio in assay buffer for 1 h to form complex to increase binding avidity. The complexed IgG (for MAB1, 0.013–50 μg/ml in 2.5-fold serial dilution in duplicate, for MIL77-1, MIL77-2 and MIL77-3, 0.0005–10 μg/ml in 3-fold serial dilution in duplicate) was added to the plates. After a 2 h incubation, plates were washed and bound IgG was detected by adding horseradish peroxidase (HRP) labeled goat F(ab′)2 anti-human IgG F(ab′)2 (Sigma-Aldrich). After a final 1 h incubation, plates were washed and the substrate 3,3′,5,5′-tetramethyl benzidine (TMB) (InnoReagents) was added. The reaction was stopped by adding 1 M sulfuric acid. Absorbance was read at 450 nm on a Microplate Reader (Molecular Devices). For data analysis, the OD 450 and concentrations of antibodies were fit using a four-parameter logistic curve and EC50 was obtained.
Binding Affinity of MIL77 antibodies to recombinant EBOV GPΔTM
For the affinity ELISA, Costar half-area 96-well plates were coated with 30 μl of 1.25 μg/ml of recombinant EBOV GPΔTM (IBT Bioservices) overnight at 4°C. The plates were then blocked with 100 μl of PBS 5% skim milk (BD) for 1 hour at 37°C. Two-fold serial dilutions of the test antibodies (30 μl) in PBS 2% skim milk was added and incubated at 37°C for 2 hours. The plates were washed with 4 x 150 μl of PBS 0.1% Tween 20 with a plate washer (BioTek). The secondary antibody, goat anti-human IgG (H + L) -HRP (KPL), was added (30 μl; in PBS 2% skim milk) at a concentration of 0.5 μg/ml for 1 hour at 37°C. The plates were washed again and 50 μl of TMB Single Solution (ThermoFisher) was added. The plates were incubated in the dark at room temperature for 30 minutes and the absorbance was read at 650 nm on a BioTek Synergy HT plate reader (BioTek). Wells where the absorbance was reported as “OVRFLW” were discarded from the analysis. At every incubation step, the plates were sealed with a new plate sealing film (Excel Scientific). The binding affinity results are present in the supplementary Figure S1.
Neutralization assay
The fluorescent neutralization assay was performed in 96-well tissue culture plates (Corning). The virus, EBOV/May-eGFP (passage 4), was incubated with the indicated concentration of mAbs c13C6, c2G4, c4G7, MIL77-1,2,3, ranging from 0.05 to 100 μg/ml, for 1hr at 37˚C in plain DMEM with (c13C6, MIL77-3) or without complement (c2G4, MIL77-1, c4G7, MIL77-2) (Sigma). Vero E6 cells at 90–100% confluence were infected in triplicate with the virus-antibody mixture at an MOI of 0.1 TCID50 per cell. Infection was carried out for 1 hr at 37˚C, 5% CO2. The inoculum was removed and replaced with DMEM/2% FBS. Plates were incubated for 72 hr. Fluorescent intensities of GFP were measured using a Synergy HT microplate reader (Biotek). The neutralization percentage was calculated as: 100 − ((Fluo − Background)/(max(Fluo) − Background)) * 100. A four-parameter curve was fitted to the percent neutralization curves using GraphPad Prism 5. The results are present in the supplementary Figure S2.
Binding Specificity Analysis of MIL77 Antibodies
The 96-well plates were coated with Ebola GP (1ug/ml Ebola GP was provided by the Institute of Microbiology, Chinese Academy of Sciences, Beijing, China) in carbonate buffer, pH9.6, at 4°C overnight. The plates were washed with PBS containing 0.05% polysorbate 20, pH7.4, and were then blocked with PBS containing 1.5% casein for 1 h at 37°C. Biotinylated-MIL77-1, -2, or -3 (0.44 μg/ml each) was used to diluted MIL77-1, -2 and -3 in 3-fold serial dilution(ranging from 0.0017 to 300 ug/ml). The Biotin-MIL77 and MIL77 complex were then added to the plates. After 1 h incubation at 37°C, the plates were washed and the bound Biotin-MIL77 was detected by adding horseradish peroxidase (HRP) labeled Streptavidin (purchased from Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD, USA). After a further 45 min incubation at room temperature, the plates were washed and the substrate 3,3′,5,5′-Tetramethylbenzidine (TMB, purchased from eBioscience, San Diego, CA, USA) was added. The reaction was stopped by adding 1 M phosphoric acid. Absorbance was measured at 450 nm using a plate reader. The results are present in the supplementary Figure S3.
Blood count and biochemistry
Complete blood counts were obtained using a VetScan HM5 (Abaxis Veterinary Diagnostics), and serum biochemistry was analyzed using a VetScan VS2 (Abaxis Veterinary Diagnostics) using comprehensive profile discs. For the blood counts, the following parameters are reported (all measured parameters are in Supplementary Materials): lymphocyte count, neutrophil count, and platelet count. For serum biochemistry, the following parameters are graphed (all measured parameters are in Supplementary Materials): alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin (TBIL), blood urea nitrogen (BUN), and potassium (K+).
EBOV titration by RT-qPCR
Total RNA was extracted from 140 μl of whole blood using the QIAmp Viral RNA Mini Kit (Qiagen), the elution volume is 60 μl. EBOV/Mak-C05 was detected using the LightCycler 480 RNA Master Hydrolysis Probes kit (Roche), using the L gene (RNA-dependent RNA polymerase) as the target gene (nucleotides 16472–16538, AF086833). The reaction conditions consisted of: reverse transcription at 63°C for 3 min, initial denaturation at 95°C for 30s, and 45 cycles of 95°C for 30 s with 60°C for 30 s (data read during elongation) on an ABI StepOnePlus (Life Technologies). The lower detection limit for this assay is 107 GEQ/ml, using 4 μl of template/reaction. The sequences of the primers used were: EBOVLF2 (CAGCCAGCAATTTCTTCCAT), EBOVLR2 (TTTCGGTTGCTGTTTCTGTG), and EBOVLP2FAM (FAM-ATCATTGGCGTACTGGAGGAGCAG-BHQ1).
Statistical analysis
Comparisons of survival were carried out using Revolution R Open (version 8.0.2) with a checkpoint date of April 20th 2015 and the “survival” package(24). The threshold for statistical significance was set at 0.05. The regressions for the binding of the mAbs to FcγRIII and Ebola virus GPΔTM were also carried out in the same version of R.
Calculation of the pharmacokinetic parameters was carried out in WinNonLin (PharSight) version 5.0.2 using non-compartmental analysis on each individual animal. The final values are expressed as mean ± standard deviation.
Supplementary Material
Acknowledgments
The authors thank Kevin Tierney, Geoff Soule, and Kaylie Tran from PHAC-NML for their assistance with the animal care and technical assistance.
Funding: This work was supported by the Public Health Agency of Canada (PHAC), a Canadian Safety and Security Program (CSSP) grant to G.P.K. and X.Q, and an NIH grant to G.P.K., LZ and E.O.S. (1U19AI109762).
Gary Wong is supported by the Banting Post-doctoral Fellowship from the Canadian Institutes of Health Research (CIHR), and the President’s International Fellowship Initiative from the Chinese Academy of Sciences (CAS).
This project is partially supported by The China National Key Subject of Drug Innovation (Funding No: 2015ZX09102024-005, to Beijing Mabworks) and the Major Program of the National Natural Science Foundation of China Grant No: 81590766 to J. Feng.
Footnotes
Author contributions:
X.Q., G.P.K. designed the challenge studies. X.Q., J.A., G.W., M.J., G.I., E.O.S., S.B., G.F.G., L.Z., B.Z., G.P.K. wrote and revised the manuscript. X.Q., J.A., S.H., G.W., H.W., L.F., A.K., H.F.B., A.B. performed the challenge experiments and analyzed the corresponding data. M.L., L.L., F.L., P.Y., B.S., J.F., B.Z. performed all experiments and analyses regarding Figure 1 and Supplementary Figure S3. L.Z. provided ZMapp™. B.Z. provided MIL77.
Competing interests:
Her Majesty the Queen in right of Canada holds a patent on the mAbs 1H3, 2G4, and 4G7, PCT/CA2009/000070, “Monoclonal antibodies for Ebola and Marburg viruses.”
L.Z. is CEO of Mapp Biopharmaceuticals Inc. Mapp Biopharmaceuticals Inc owns the license to the antibody cocktails used in the study.
Material and data availability:
Scripts and data are availablable through the Open Science Framework; link: https://osf.io/7makz/. ZMapp™ and MIL77 are available upon request from Mapp Biopharmaceuticals Inc, pending a material transfer agreement.
References and Notes
- 1.Center for Disease Control and Prevention. Outbreaks Chronology: Ebola Virus Disease. 2015 (available at http://www.cdc.gov/vhf/ebola/outbreaks/history/chronology.html)
- 2.Centers for Disease Control and Prevention. Chronology of Marburg Hemorrhagic Fever Outbreaks. Marbg hemorrhagic fever (marbg HF) 2014 (available at http://www.cdc.gov/vhf/marburg/resources/outbreak-table.html)
- 3.Baize S, Pannetier D, Oestereich L, Rieger T, Koivogui L, Magassouba N, Soropogui B, Sow MS, Keïta S, De Clerck H, Tiffany A, Dominguez G, Loua M, Traoré A, Kolié M, Malano ER, Heleze E, Bocquin A, Mély S, Raoul H, Caro V, Cadar D, Gabriel M, Pahlmann M, Tappe D, Schmidt-Chanasit J, Impouma B, Diallo AK, Formenty P, Van Herp M, Günther S. Emergence of Zaire Ebola Virus Disease in Guinea - Preliminary Report. N Engl J Med. 2014;1–8 doi: 10.1056/NEJMoa1404505. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn J, Andersen K, Baize S, Bào Y, Bavari S, Berthet N, Blinkova O, Brister J, Clawson A, Fair J, Gabriel M, Garry R, Gire S, Goba A, Gonzalez J-P, Günther S, Happi C, Jahrling P, Kapetshi J, Kobinger G, Kugelman J, Leroy E, Maganga G, Mbala P, Moses L, Muyembe-Tamfum J-J, N’Faly M, Nichol S, Omilabu S, Palacios G, Park D, Paweska J, Radoshitzky S, Rossi C, Sabeti P, Schieffelin J, Schoepp R, Sealfon R, Swanepoel R, Towner J, Wada J, Wauquier N, Yozwiak N, Formenty P. Nomenclature- and Database-Compatible Names for the Two Ebola Virus Variants that Emerged in Guinea and the Democratic Republic of the Congo in 2014. Viruses. 2014;6:4760–4799. doi: 10.3390/v6114760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kilmarx PH, Clarke KR, Dietz PM, Hamel MJ, Husain F, McFadden JD, Park BJ, Sugerman DE, Bresee JS, Mermin J, McAuley J, Jambai A. Ebola Virus Disease in Health Care Workers — Sierra Leone, 2014. Morb Mortal Wkly Rep. 2014;63:1168–1171. [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu X, Wong G, Audet J, Bello A, Fernando L, Alimonti JB, Fausther-Bovendo H, Wei H, Aviles J, Hiatt E, Johnson A, Morton J, Swope K, Bohorov O, Bohorova N, Goodman C, Kim D, Pauly MH, Velasco J, Pettitt J, Olinger GG, Whaley K, Xu B, Strong JE, Zeitlin L, Kobinger GP. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014 doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olinger GG, Pettitt J, Kim D, Working C, Bohorov O, Bratcher B, Hiatt E, Hume SD, Johnson AK, Morton J, Pauly M, Whaley KJ, Lear CM, Biggins JE, Scully C, Hensley L, Zeitlin L. Delayed treatment of Ebola virus infection with plant-derived monoclonal antibodies provides protection in rhesus macaques. Proc Natl Acad Sci U S A. 2012;109:18030–5. doi: 10.1073/pnas.1213709109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiu X, Audet J, Wong G, Pillet S, Bello A, Cabral T, Strong JE, Plummer F, Corbett CR, Alimonti JB, Kobinger GP. Successful treatment of ebola virus-infected cynomolgus macaques with monoclonal antibodies. Sci Transl Med. 2012;4:138ra81. doi: 10.1126/scitranslmed.3003876. [DOI] [PubMed] [Google Scholar]
- 9.Lyon GM, Mehta aK, Varkey JB, Brantly K, Plyler L, McElroy aK, Kraft CS, Towner JS, Spiropoulou C, Stroher U, Uyeki TM, Ribner BS. Clinical care of two patients with Ebola virus disease in the United States. N Engl J Med. 2014;371:2402–2409. doi: 10.1056/NEJMoa1409838. [DOI] [PubMed] [Google Scholar]
- 10.Wong G, Kobinger GP. Backs against the Wall: Novel and Existing Strategies Used during the 2014–2015 Ebola Virus Outbreak. Clin Microbiol Rev. 2015;28:593–601. doi: 10.1128/CMR.00014-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NIAID. Putative Investigational Therapeutics in the Treatment of Patients with Known Ebola Infection. ClinicalTrialsgov. 2015 (available at https://www.clinicaltrials.gov/ct2/show/NCT02363322?term=Zmapp&rank=1)
- 12.Hiatt A, Bohorova N, Bohorov O, Goodman C, Kim D, Pauly MH, Velasco J, Whaley KJ, Piedra Pa, Gilbert BE, Zeitlin L. Glycan variants of a respiratory syncytial virus antibody with enhanced effector function and in vivo efficacy. Proc Natl Acad Sci U S A. 2014;111:5992–7. doi: 10.1073/pnas.1402458111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu X, Alimonti JB, Melito PL, Fernando L, Ströher U, Jones SM. Characterization of Zaire ebolavirus glycoprotein-specific monoclonal antibodies. Clin Immunol. 2011;141:218–27. doi: 10.1016/j.clim.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Murin CD, Fusco ML, Bornholdt Za, Qiu X, Olinger GG, Zeitlin L, Kobinger GP, Ward AB, Saphire EO. Structures of protective antibodies reveal sites of vulnerability on Ebola virus. Proc Natl Acad Sci U S A. 2014;111:17182–17187. doi: 10.1073/pnas.1414164111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Audet J, Wong G, Wang H, Lu G, Gao GF, Kobinger G, Qiu X. Molecular Characterization of the Monoclonal Antibodies Composing ZMAb: A Protective Cocktail Against Ebola Virus. Sci Rep. 2014;4:6881. doi: 10.1038/srep06881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bale S, Dias JM, Fusco ML, Hashiguchi T, Wong AC, Liu T, Keuhne AI, Li S, Woods VL, Chandran K, Dye JM, Saphire EO. Structural basis for differential neutralization of ebolaviruses. Viruses. 2012;4:447–70. doi: 10.3390/v4040447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marzi A, Yoshida R, Miyamoto H, Ishijima M, Suzuki Y, Higuchi M, Matsuyama Y, Igarashi M, Nakayama E, Kuroda M, Saijo M, Feldmann F, Brining D, Feldmann H, Takada A. Protective efficacy of neutralizing monoclonal antibodies in a nonhuman primate model of Ebola hemorrhagic fever. PLoS One. 2012;7:e36192. doi: 10.1371/journal.pone.0036192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fink S. A Chinese Ebola Drug Raises Hopes, and Rancor. New York Times. 2015;A9 [Google Scholar]
- 19.Mundasad S. British medic declared free of Ebola. BBC News. 2015 (available at http://www.bbc.com/news/health-32088310)
- 20.Dias JM, Kuehne AI, Abelson DM, Bale S, Wong AC, Halfmann P, Muhammad Ma, Fusco ML, Zak SE, Kang E, Kawaoka Y, Chandran K, Dye JM, Saphire EO. A shared structural solution for neutralizing ebolaviruses. Nat Struct Mol Biol. 2011;18:1424–7. doi: 10.1038/nsmb.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pettitt J, Zeitlin L, Kim DH, Working C, Johnson JC, Bohorov O, Bratcher B, Hiatt E, Hume SD, Johnson AK, Morton J, Pauly MH, Whaley KJ, Ingram MF, Zovanyi A, Heinrich M, Piper A, Zelko J, Olinger GG. Therapeutic intervention of Ebola virus infection in rhesus macaques with the MB-003 monoclonal antibody cocktail. Sci Transl Med. 2013;5:199ra113. doi: 10.1126/scitranslmed.3006608. [DOI] [PubMed] [Google Scholar]
- 22.Zeitlin L, Pettitt J, Scully C, Bohorova N, Kim D, Pauly M, Hiatt a, Ngo L, Steinkellner H, Whaley KJ, Olinger GG. Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc Natl Acad Sci. 2011;108:20690–20694. doi: 10.1073/pnas.1108360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang P, Haryadi R, Chan KF, Teo G, Goh J, Pereira NA, Feng H, Song Z. Identification of functional elements of the GDP-fucose transporter SLC35C1 using a novel Chinese hamster ovary mutant. Glycobiology. 2012;22:897–911. doi: 10.1093/glycob/cws064. [DOI] [PubMed] [Google Scholar]
- 24.Lin H, Zelterman D. Modeling Survival Data: Extending the Cox Model. Technometrics. 2002;44:85–86. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.