Abstract
Diffusion tensor imaging (DTI) studies in 22q11.2 Deletion Syndrome (22q11DS), a neurogenetic condition associated with psychosis, report brain white matter (WM) microstructure aberrations. Several studies report that WM disruptions in 22q11DS are similar to deficits in idiopathic schizophrenia. Yet, DTI results in 22q11DS are inconsistent. We used DTI to compare WM structure in 22q11DS individuals to healthy controls (HC) and explored WM differences in 22q11DS with (+) and without (−) psychosis spectrum symptoms. We examined 39 22q11DS individuals and 39 age, sex and race equivalent HC. DTI was performed at 3T using a 64-direction protocol. Fractional anisotropy (FA) was lower, while radial diffusivity was higher in 22q11DS within the cingulum bundle. Mean diffusivity was lower in the inferior longitudinal fasciculus, while axial diffusivity (AD) was lower in the cingulum bundle, forceps major, and several posterior to anterior fasciculi. 22q11DS+ had lower FA in the cingulum bundle and lower AD in the uncinate fasciculus compared to 22q11DS−. Overall, we found aberrant WM microstructure in individuals with 22q11DS compared to age and sex matched HC and exploratory analysis indicated subtle WM deficits associated with psychosis. The findings highlight the dysfunction of WM microstructure in 22q11DS and its potential importance in elucidating WM abnormalities in psychosis.
Keywords: 22q11.2 deletion syndrome, diffusion tensor imaging, psychosis, fractional anisotropy, cingulum bundle
1.0 Introduction
The 22q11.2 deletion syndrome (22q11DS) is the most common known genetic microdeletion in humans (McDonald-McGinn et al., 2015). 22q11DS is associated with craniofacial, cardiovascular, cognitive, endocrine, immune, and gastrointestinal disorders (McDonald-McGinn et al., 2015). Notably, 22q11DS is also associated with an increased risk for psychiatric disorders (Gothelf et al., 2007; Murphy et al., 1999; Pulver et al., 1994; Schneider et al., 2014; Tang et al., 2014), including psychosis (Bassett et al., 1998; Shprintzen et al., 1992). Individuals with 22q11DS who develop psychosis show similar symptoms to individuals with idiopathic schizophrenia (Murphy et al., 1999), and approximately 1–2% of cases of idiopathic schizophrenia have 22q11.2 deletions (Bassett et al., 2010). Thus, understanding brain dysfunction in 22q11DS may elucidate critical neural mechanisms in psychosis.
Structural brain abnormalities, including lower gray matter volume (Schneider et al., 2014; Tan et al., 2009), cortical thickness (Bearden et al., 2007; Schaer et al., 2008), surface area (Jalbrzikowski et al., 2013) and gyral complexity (Schaer et al., 2008; Schaer et al., 2006; Schmitt et al., 2015), are common in 22q11DS. Neural dysfunction in 22q11DS extends into structural connectivity of the brain, which can be measured using diffusion tensor imaging (DTI). DTI facilitates the in vivo study of brain white matter (WM) microstructure (Mori et al., 2008), and typical DTI measures include fractional anisotropy (FA), mean (MD), axial (AD) and radial diffusivity (RD). Alterations in these metrics are reported in 22q11DS (Barnea-Goraly et al., 2003; da Silva Alves et al., 2011; Deng et al., 2015; Kates et al., 2015; Kikinis et al., 2012; Martin et al., 2014; Ottet et al., 2013; Padula et al., 2015; Radoeva et al., 2012; Scariati et al., 2016; Sundram et al., 2010; Villalon-Reina et al., 2013), and some of the patterns observed are similar to deficits in SZ (Kyriakopoulos et al., 2008; Roalf et al., 2015; Thomason and Thompson, 2011), adolescents with psychosis (Davenport et al., 2010; White et al., 2007) and youth at risk for developing psychosis (Epstein et al., 2013; Lee et al., 2013). In contrast to structural gray matter findings, DTI results in 22q11DS are more inconsistent. The most common findings are lower FA and AD, but several studies report the opposite pattern and indicate conflicting deficits (See Supplemental Table 1). Accordingly, additional large scale studies that compare WM microstructure in 22q11DS to healthy individuals are warranted.
The overlap in brain structural abnormalities between individuals with 22q11DS and schizophrenia has led to intensive examination of the links between brain development, genetic defects and the development of psychotic symptoms (Kates et al., 2015). Patients with 22q11DS who develop schizophrenia have similar symptoms compared to patients with idiopathic schizophrenia (Murphy et al., 1999). Indeed, both 22q11DS and idiopathic schizophrenia share brain morphological changes (Eliez et al., 2001; Eliez et al., 2000; Glahn et al., 2008; Jalbrzikowski et al., 2013; Schaer et al., 2006), including alterations in WM structure and organization (Asami et al., 2014; Epstein et al., 2013; Kyriakopoulos et al., 2008; Lee et al., 2013; Roalf et al., 2013b). Thus, direct comparison of WM differences between 22q11DS patients with and without psychotic symptoms may elucidate deficits specific to psychosis.
The goals of this study were to 1) evaluate WM microstructural abnormalities in a large age, gender and race equivalent sample of participants with 22q11DS and healthy controls (HC) and 2) compare diffusion metrics in 22q11DS with and without psychotic symptoms. We hypothesized that: A) participants with 22q11DS will have lower FA and lower AD compared to HC throughout brain white matter; and B) 22q11DS participants with psychotic or prodromal symptoms will show lower FA and AD in white matter as compared to 22q11DS participants without psychotic-like symptoms.
2.0 Methods and Materials
2.1 Subjects
Individuals with 22q11DS (n=54; Table 1) were recruited for a prospective study Brain-Behavior and Genetics Studies of the 22q11DS at the University of Pennsylvania and Children’s Hospital of Philadelphia (CHOP). This sample overlaps with a previous report of abnormal brain structure in 22q11DS (Schmitt et al., 2014). Briefly, inclusion criteria included age ≥ 8 years, proficient in English, estimated IQ >70 by available records and the Wide Range Achievement Test IV (Wilkinson, 1993), and medically stable. 22q11DS participants were between 13–30 years of age. Exclusion criteria included pervasive developmental disorder or IQ < 70, medical disorders that may affect brain function (e.g., uncontrolled seizures, head trauma, CNS tumor and infection) or visual deficits (e.g., blindness). Deletion status was confirmed using multiplex ligation dependent probe amplification (Jalali et al., 2008). The University of Pennsylvania and the CHOP Institutional Review Boards approved all study procedures. Informed consent/assent was obtained from minors and accompanying parent at the time of initial evaluation.
Table 1.
Sample demographics, clinical and cognitive measures for Healthy Comparison (HC) and 22q11DS. This information is also shown for 22q11DS with (+) and without (−) psychosis symptoms
| Full Sample | 22q11DS subsamples | |||
|---|---|---|---|---|
|
| ||||
| HC (n=39) | 22q11DS (n=39) | 22q11DS+ (n=27) | 22q11DS− (n=12) | |
| Sex, n | ||||
| Male | 20 | 20 | 15 | 5 |
| Female | 19 | 19 | 12 | 7 |
| Race, n | ||||
| Non-Caucasian | 6 | 6 | 6 | 0 |
| Caucasian | 33 | 33 | 21 | 12 |
| Age (years) | 19.99 (1.73) | 19.83 (4.25) | 19.25 (4.50) | 21.17 (3.42) |
| Education (years) | 13.05* (1.59) | 11.05 (2.84) | 10.50 (2.92) | 12.36 (2.20) |
| Maternal Education (years) | 14.30 (3.83) | 14.65* (2.13) | 14.42 (2.12) | 14.09 (2.18) |
| Paternal Education (years) | 14.43 (2.57) | 14.02 (2.30) | 14.00 (2.50) | 14.09 (1.87) |
| Global Functioning (GAF) | 85.79* (7.24) | 60.44 (17.13) | 53.92 (15.08) | 77.40 (8.22) |
| Medication APS/ADS/Stim/Other | - | 3/13/6/6 | 2/13/2/2 | 1/0/4/4 |
| Clinical Aymptoms | ||||
| SIPS Positive | 1.12* (2.00) | 7.87 (6.19) | 10.25 (5.82) | 2.50 (2.57) |
| SIPS Negative | 1.07* (1.89) | 10.53 (8.25) | 13.92 (7.62) | 2.91 (2.53) |
| SIPS Disorganized | 0.31* (0.61) | 5.43 (5.78) | 7.04 (6.21) | 1.83 (1.95) |
| Cognitive Performance | ||||
| Accuracy (z-score) | 0.52* (0.44) | −1.28 (0.66) | −1.28 (0.68) | −1.27 (0.65) |
| Speed (z-score) | 0.35* (0.37) | −0.22 (0.74) | −0.27 (0.78) | −0.09 (0.63) |
| Accuracy Variability | 0.67* (0.18) | 1.08 (0.32) | 1.17 (0.32) | 0.90 (0.26) |
| Speed Variability | 0.71* (0.47) | 1.09 (0.63) | 1.14 (0.69) | 0.91 (0.40) |
APS= Antipsychotic medication; ADS= Antidepressant medication; Stim=Stimulant; Other = Other CNS medication. Cognitive scores are global indicies of performance across all tasks.
denotes p<0.05 between groups.
Psychopathology was assessed using the Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS)(Kaufman et al., 1997), Structured Interview for Prodromal Syndromes (SIPS)(Miller et al., 2003), and the psychotic and mood diagnoses modules of the Structured Clinical Interview for DSM-IV (Modules C and D)(First and Gibbon, 2004). As in previous reports, positive, negative and disorganized SIPS symptoms were rated on a 7-point scale (0 - “absent,” 1 - “questionably present,” 2 - “mild,” 3 - “ moderate,” 4 - “moderately severe,” 5 - “severe but not psychotic,” and 6 - “severe and psychotic”)(Schmitt et al., 2014). Individuals with at least one positive symptom rated ≥ 3 or at least two negative and/or disorganized symptoms rated ≥ 3 were considered at heighten psychosis risk (e.g. prodromal). Seventeen individuals were considered psychosis-spectrum based upon only the positive symptoms. Ten individuals were considered psychosis-spectrum individuals based upon negative/disorganized symptoms.
Age and gender matched healthy individuals from the follow-up study to the Philadelphia Neurodevelopmental Cohort (PNC; (Gur et al., 2012; Roalf et al., 2016; Satterthwaite et al., 2015) served as a comparison group. Matching was conducted in a similar manner as a previous study (Tang et al., 2016) using an optimizing algorithm (Konsake and Bergstralh, 2004) in SAS programming language. Variable selected to match included age, gender and then race. Note that all analyses were conducted using a larger sample (n=141) of individuals who returned as part of the PNC; these analyses are presented in the Supplement (See “Diffusion Tensor Imaging Results using 141 healthy comparison participants from the Philadelphia Neurodevelopment Cohort”). HC had no DSM-IV Axis I psychotic disorder, no subthreshold prodromal symptomatology, no history of psychosis in a first-degree biological relative, and no personal Axis II Cluster A diagnosis. Participants were excluded for any history of neurological disorder, head trauma with loss of consciousness, lifetime history of substance dependence, substance abuse within the preceding 6 months, any medical condition that might affect brain function or any contraindication for MRI. In addition, the Global Assessment of Functioning (GAF) (Hall, 1995) scale was administered to assess overall daily functioning and the Penn Computerized Neurocognitive Battery(CNB(Gur et al., 2001) examined neurocognitive functioning. CNB performance in 22q11DS has previously been reported(Gur et al., 2014) and here we present overall performance accuracy, speed and variability (Roalf et al., 2013a; Roalf et al., 2014).
2.2 Neuroimaging
Neuroimaging was performed on all participants without contraindications for MRI. A standard protocol was followed to familiarize individuals with MRI scanning procedures. All participants underwent mock scanning prior to MRI scanning. This procedure was completed using a decommissioned GE MRI scanner with similar audio/video projection equipment. The environment mimicked the actual MR environment including the use of earplugs, headphones, and foam padding cushions. MRI scanner noise was simulated, head-motion was monitored and feedback was provided. All MRI scans were acquired on the same 3T Siemens Tim Trio whole-body scanner, used the same 32-channel head coil and acquisition protocol at the Hospital of the University of Pennsylvania.
2.2.1 Diffusion Weighted Imaging Acquisition
DTI scans were obtained using a twice-refocused spin-echo (TRSE) single-shot EPI sequence (TR=8100 ms, TE =82 ms, FOV = 240mm2/240mm2; Matrix= RL: 128/AP:128/Slices:70, in-plane resolution (x & y) 1.875mm2; slice thickness=2 mm, gap=0; FlipAngle=90°/180°/180°, volumes=71, GRAPPA factor =3, bandwidth = 2170 Hz/pixel, PE direction = AP; 11 minutes) (Roalf et al., 2016; Satterthwaite et al., 2014a). The complete sequence consisted of 64 diffusion-weighted directions with b = 1000 s/mm2 and 7 interspersed scans where b = 0 s/mm2 (Satterthwaite et al., 2014a). In addition, a B0 field map was acquired and used in the pre-processing (Roalf et al., 2016).
2.2.2 DTI quality control and image processing
DTI quality control (QC), pre- and post-processing are published in detail elsewhere (Roalf et al., 2016) and in the Supplemental Methods. Briefly, images were first checked for data quality using manual and automated methods (Roalf et al., 2016). QC metrics include temporal signal-to-noise (TSNR), mean relative motion, mean and maximum outlier counts.
Following QC, diffusion data were skull stripped by generating a brain mask for each subject by registering a binary mask of a standard image (FMRIB58_FA) to each subject’s brain using FLIRT (Smith, 2002). When necessary, manual adjustments were made to this mask. Next, eddy currents and movement were estimated and corrected using FSL’s eddy tool (Andersson and Sotiropoulos, 2016; Graham et al., 2016; Roalf et al., 2016). Eddy is an improvement upon the typical eddy/motion correction used as part of FSL’s Diffusion Tool Box (Behrens et al., 2003). This tool simultaneously models the effects of diffusion eddy current and head movement on DTI images in order to reduce the amount of resampling and is a vast improvement of the standard FSL eddy correct tool (Andersson and Sotiropoulos, 2016; Graham et al., 2016). Next, the diffusion gradient vectors were rotated to adjust for motion using the 6-parameter motion output generated from eddy. Then, the B0 field map was estimated and distortion correction was applied to the DTI data using FSL’s FUGUE (Smith, 2002). Finally, the diffusion tensor was modeled and metrics (FA and MD) were estimated at each voxel using FSL’s DTIFIT.
Registration from native space to a template space was completed using DTI-TK (Zhang et al., 2014; Zhang et al., 2006). First, the FA and MD output of DTIFIT was converted to DTI-TK format. Next, template was generated from the tensor volumes using 14 representative diffusion data sets that were considered “Excellent” from the PNC sample. One individual from each of the 14 ages (age range 8–21) was randomly selected. These 14 DTI volumes were averaged together to create an initial template. Next, data from the 14 subjects were registered to this template in an iterative manner. Unlike standard intensity-based registration algorithms, this process utilizes the full tensor information in an attempt to best align the underlying white matter tracts using iterations of rigid, affine and diffeomorphic registration leading to the generation of a successively refined template. Ultimately, one high-resolution refined template was created and used for registration of the remaining diffusion datasets. All DTI maps were then registered (rigid, affine, diffeomorphic) to the high-resolution study-specific template using DTI-TK. Whole brain analysis was performed using a customized implementation of tract-based spatial statistics (TBSS) (Bach et al., 2014). FA, MD, AD, and RD were compared along a study specific white matter skeleton. Then, standard regions of interest (ROI; ICBM-JHU White Matter Tracts; Harvard-Oxford Atlas) were registered from MNI152 space to the study-specific template using ANTs registration (Avants et al., 2011). Mean diffusion metrics were extracted from these ROIs using FSL the ‘fslmeants’ command.
2.3 Statistical Analysis
Demographic and clinical differences between groups were examined with chi-square and t-tests, which were corrected for unequal variance using the Welch approximation. Prior work has demonstrated that brain development is not a linear process (Giedd et al., 1999), including white matter development (Peters et al., 2014). Thus, group-level analyses of DTI data were flexibly modeled using penalized splines within a general additive model (GAM; (Wood, 2004, 2011). The GAM is a commonly used statistical approach (Satterthwaite et al., 2014b; Satterthwaite et al., 2016) that assesses a penalty for increasing nonlinearity to avoid over-fitting. The GAM was employed for both whole brain analysis in randomise (5000 permutations) using threshold-free cluster estimation (TFCE). Only F-statistics could be generated for the voxelwise GAM. Thus follow-up ROI analyses were completed. All models included factors for sex, race and TSNR (Roalf et al., 2016). A significance threshold of p<5.0×10−3 was used to control for Type-I error probability across both the whole brain TBSS analysis and the analyses of 17 ROIs. All ROI statistics were performed using R (3.1.2) statistical software (R-Core-Team, 2012). The relationships between DTI metrics and clinical measures were examined within each group using Pearson correlations.
3.0 Results
3.1 Participant Characteristics, Clinical and Cognitive Scores
22q11DS and HC were similar in age, gender, race, maternal and paternal education (Table 1). HC had higher education attainment [t(55.91)=3.75, p<4.10×10−4]. As expected, 22q11DS participants had higher positive, negative, and disorganized symptoms, and had lower overall functioning assessed by the GAF (Table 1).
3.2 Diffusion Tensor Imaging
3.2.1 Quality Control Metrics
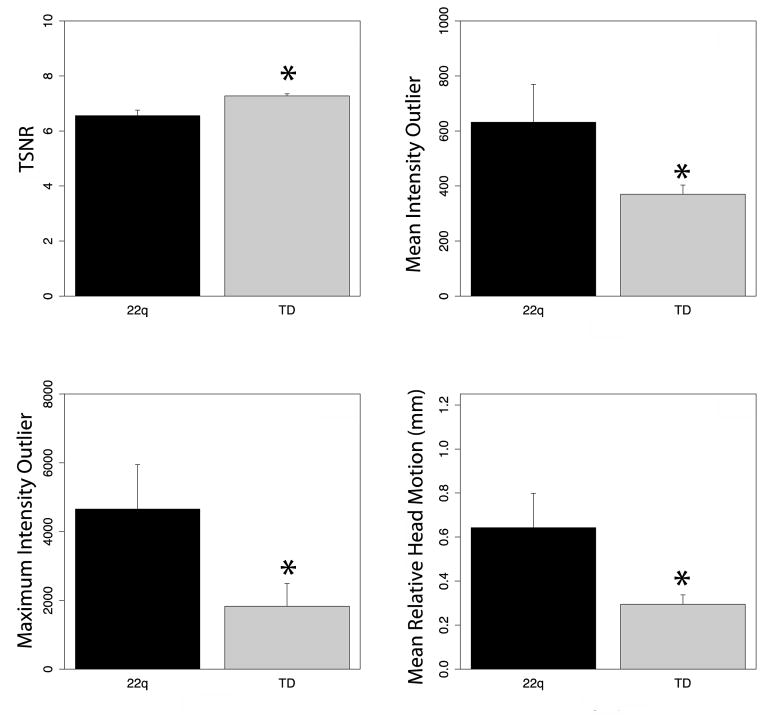
Overall 22q11DS individuals had poorer QC metrics as compared to HC (Figure 1). 22q11DS had lower TSNR [t(49.23)=6.49, p<3.96×10−8], higher motion [t(43.71)=4.23, p<1.16×10−4], average intensity outliers [t(42.46)=3.61, p<7.86×10−4] and maximal intensity outliers [t(56.26)=3.82, p<3.35×10−4]. Subsequently, TSNR was included as a factor in subsequent analyses.
Figure 1. Diffusion Tensor Imaging data quality.
Data quality was lower in 22q11DS than healthy comparison participants. Hence, data quality (TSNR) was included in statistical analysis of group differences in diffusion metrics. *p<0.05.
3.2.2 Whole brain analysis
Group differences in AD and MD were found in the whole brain TBSS analysis (Figure 2). The groups differed in AD within one cluster centered and the juncture between the CST and SLF. Differences in MD were found in a large cluster including the SLF, Forceps Major, Forceps Minor and ILF. Post-hoc follow-up indicated diffusivity values were lower in 22q11DS as compared to HC. No group differences in FA or RD reached significance at the voxel level. As the TBSS approach was limited to comparison along only the white matter skeleton, complementary ROI analyses were completed to further probe potential white matter differences since these ROI more thoroughly measure the extent of white matter microstructure.
Figure 2.
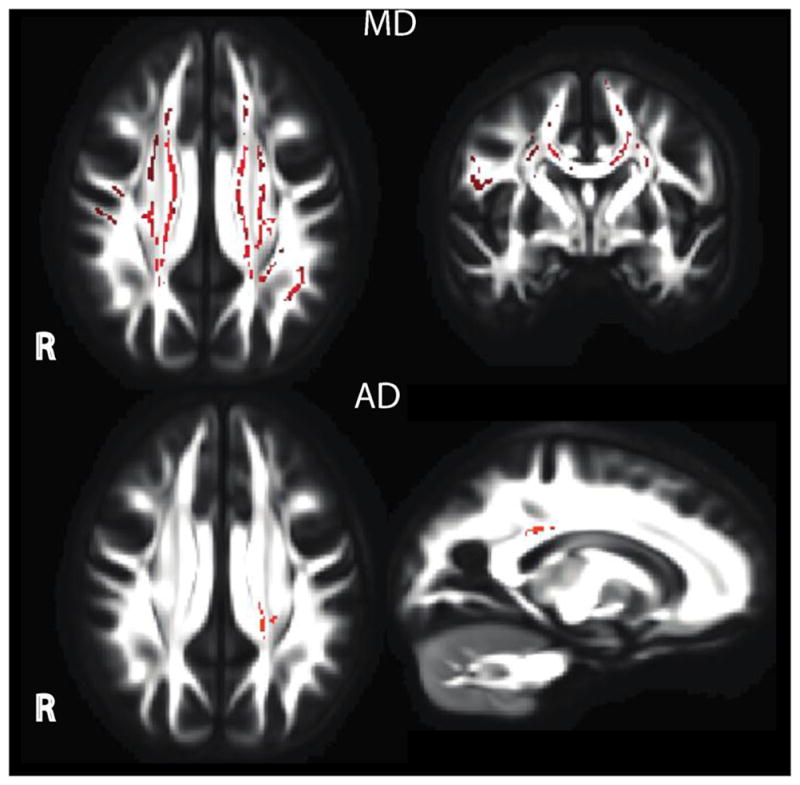
Whole brain comparison using tract-based spatial statistics. Healthy comparison differed from 22q11DS in both whole brain MD and AD. Lower AD was found in one cluster centered near the SLF and CST. Lower MD in 22q11DS was found in a large cluster including the SLF, Forceps Major, Forceps Minor and ILF.
3.2.3 Region-of-Interest Analysis
Diffusion metrics are displayed in full for each region of interest in Supplemental Table 2 and plots are show for all regions in Supplemental Figures 1–4.
3.2.3.1 Fractional Anisotropy (FA)
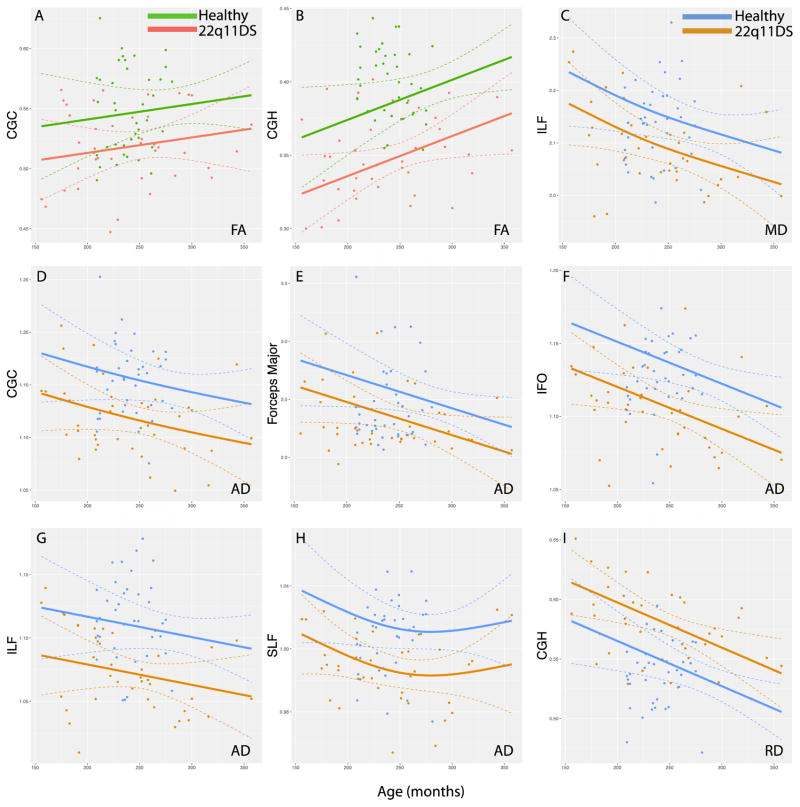
22q11DS participants had lower FA in the CGC (p<2.86×10−3) and CGH (p<2.98×10−6; Figure 3) as compared to HC. Lower TSNR was associated with lower FA in the ATR (p<4.41×10−4) and ILF (p<1.44×10−3). There were no effects of age, sex or race.
Figure 3.
Significant differences in mean FA, MD, AD an RD between 22q11DS and healthy comparison participants are displayed across the age spectrum (in months). Linear and non-linear associations with age were analyzed using a general additive model. Significant group mean FA differences across age are displayed in A–B; significant group mean MD results in C; significant group mean AD results in D–H; and significant group mean RD results in I. All analyses are corrected for temporal signal-to-noise ratio. Significance threshold was p<5.0×10−3. Complete display of all ROI plots are available in the Supplement. (Color)
3.2.3.2 Mean Diffusivity (MD)
22q11DS participants had lower MD in the ILF (p<2.86×10−3) as compared to HC (Figure 3). Lower TSNR values were associated with higher MD in the ATR (p<4.50×10−3) and IFO (p<3.31×10−3). Higher MD values in the CGH were associated with younger age (p<3.90×10−3). There were no effects sex or race.
3.2.3.3 Axial Diffusivity (AD)
Axial diffusivity was lower in 22q11DS in the CGC (p<2.34×10−4), Forceps major (p<4.36×10−3), IFO (p<4.08×10−5), ILF (p<1.73×10−4), and SLF (p<7.70×10−5) as compared to HC (Figure 3). There were no effects of age, sex, race or TSNR.
3.2.3.4 Radial Diffusivity (RD)
Radial diffusivity was higher in 22q11DS in the CGH (p<8.20×10−5) as compared to HC. (Figure 3). Lower TSNR was associated with higher RD in the ATR (p<1.88×10−3) and IFO (p<4.26×10−3). There were no effects of age, sex or race.
3.2.3.5 Exploratory analysis of DTI in 22q11DS with psychotic symptoms
We considered, in an exploratory manner, that 22q11DS psychosis spectrum participants (n=27; 22q11DS+), who included individuals with a psychotic disorder (n=4) and prodromal individuals (n=23), may exhibit subtle differences from 22q11DS without psychotic symptoms (n=12; 22q11DS−). Thus, we repeated the above analysis on only 22q11DS individuals and, given the exploratory nature, used an uncorrected statistical threshold due to small sample size (p<0.05). The subsamples did not differ in age, education, maternal or paternal education, sex or race distribution. 22q11DS+ had higher positive, F(1,35)=19.153, p<1.0×10−4, negative F(1,35)=24.19, p<2.0×10−5, and disorganized F(1,35)=8.16, p<7.0×10−3 symptoms, and lower GAF F(1,32)=20.53, p<7.72×10−5 than 221DS−.
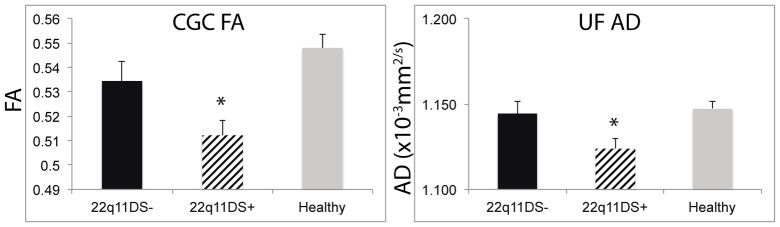
Diffusion metrics are displayed in full for each region of interest in Supplemental Table 3. 22q11DS subgroups did not differ on DTI QC metrics (all p > 0.65). Whole brain TBSS analysis did not reveal any significant differences. But, exploratory regional analysis indicated that 22q11DS+ had lower mean FA in the CGC (p=0.02) and lower mean AD in the UF (p=0.02) compared to 22q11DS− (Figure 4). Indeed, 22q11DS− showed similar mean FA within the CGC (0.534±0.028) to HC (0.548±0.035), while 22q11DS+ had lower mean FA than both (0.512±0.032). 22q11DS+ and 22q11DS− did not differ in mean MD or mean RD in any region. There were no associations with clinical or cognitive symptoms. It should be noted that many investigations of 22q11DS limit criteria of psychosis-spectrum symptoms to individuals with SIPS Positive Symptom Scores > 3. This is based on the notion that negative symptoms in 22q11DS are predominant features of the overall behavioral phenotype associated with the syndrome. The CGC effects remain in the same direction and trends accordingly when only positive symptoms were used (22q11DS− > than 22q11DS+ (p=0.13); See “Exploratory analysis of psychosis spectrum symptoms” in the Supplement for more detail).
Figure 4.
Exploratory analyses between 22q11DS+ and 22q11DS−. 22q11DS+ had lower FA in the CGC and lower AD in the UF as compared to 22q11DS−. Healthy comparison participants are show for comparison. Mean +/− SEM are reported. *p<0.05.
4.0 Discussion
Overall, we find aberrant WM microstructure in individuals with 22q11DS compared to age and sex matched healthy individuals. Along the whole brain white matter skeleton we find lower in AD in the ILF and in MD in the SLF, ILF, and the Forceps Major and Minor. Regionally, we report 1) lower FA in the CGC and CGH; 2) lower AD in the CGC, Forceps major, IFO, ILF and SLF; 3) lower MD in the ILF; and 4) higher RD in the CGH in 22q11DS compared to healthy individuals. Intriguingly, our exploratory analysis of 22q11DS with (+) and without (−) psychosis symptoms indicated lower FA in the CGC and lower AD in the UF in 22q11DS+, albeit this result would not have survived statistical correction for multiple comparisons.
Our results confirm and extend previous DTI studies in 22q11DS. Overall, all previous studies report alterations in white matter FA, but the direction of FA effects is inconsistent (See Supplemental Table 1), which may be the result of relatively small sample sizes, heterogeneity of deficits in 22q11DS, or a failure to account for potential data confounds. Here, we include a large sample of individuals with 22q11DS and employ a strategy to mitigate the influence of data quality. Ultimately, we only report significantly lower FA within the CGC and CGH, lower MD in the ILF, and lower AD within the CGC, Forceps major, IFO, ILF, and SLF, while also finding higher RD in the CGH in 22q11DS. Similar results—specifically alterations in portions of the cingulum bundle (Jalbrzikowski et al., 2014; Kates et al., 2015; Kikinis et al., 2012), IFO (Kikinis et al., 2012), ILF (Kikinis et al., 2012) and SLF (Kikinis et al., 2012) have been reported in 22q11DS. Lower FA within the cingulum bundle (Kelly et al., 2017; Roalf et al., 2013b) and SLF aligns with patterns of disruption seen in schizophrenia, including in the recent results from the ENIGMA-DTI consortium (Kelly et al., 2017). Below, we expand upon several of the specific findings and their relevance for 22q11DS.
White matter alterations in the cingulum bundle are commonly reported in 22q11DS (Jalbrzikowski et al., 2013; Kates et al., 2015; Simon et al., 2005). In addition, the cingulum bundle has been intensely studied in schizophrenia due to its role in emotion processing and executive functioning, and has received recent attention in 22q11DS (Kates et al., 2015). Here, we found lower FA and AD in the cingulate gyrus of the cortex (CGC), and lower FA and complementary higher RD in the cingulum bundle proximal to the hippocampus (CGH). Lower FA and AD in the CGC aligns with a recent study of 47 22q11DS participants (Kates et al., 2015). The cingulum bundle is a major fiber tract that connects limbic and cortical brain regions, has connections with several regions including the thalamus, amygdala, hippocampus, and dorsolateral and dorsomedial prefrontal cortex (Croxson et al., 2005; Di Rosa et al., 2008; Goldman-Rakic et al., 1984), and is critical for memory and executive functioning—two abilities disrupted in 22q11DS (Gur et al., 2014). Disruption within the cingulum white matter, a midline structure, correlates with anatomic imaging and connectomic data (Vaáša et al., 2016) that localize many 22q11DS deficits to the midline. Moreover, our exploratory comparisons of 22q11DS with (+) and without (−) psychosis symptoms indicated lower FA in the CGC. While this difference was small and would not have survived stringent multiple comparison correction, it suggests that this disruption may be associated with features associated with psychosis. Lower FA of the cingulum bundle in psychosis is linked to lower attention, poorer executive function (Nestor et al., 2007) and greater inconsistency in neurocognitive performance (Roalf et al., 2013b). In addition, the cingulum bundle is associated with conflict monitoring (Bush et al., 2002), providing feedback to guide prospective decisions (Carter et al., 2001), and is important for other neurocognitive tasks (Carter et al., 2001; Peters et al., 2012). These results further suggest disorganization of critical axons that connect the anterior cingulate with the limbic and motor cortices (Nestor et al., 2008; Nestor et al., 2007; Roalf et al., 2015; Roalf et al., 2013b; Takei et al., 2009) in 22q11DS and, moreover, that this deficit may be related to psychosis.
We also found significantly lower AD in several long-range white matter tracts in 22q11DS. Lower AD in the IFO, ILF, and SLF corroborate several recent reports (Bakker et al., 2016; Kates et al., 2015; Kikinis et al., 2012; Villalon-Reina et al., 2013) and suggest that specific aspects (e.g. myelination or axonal diameter) of the white matter microstructure may be disrupted. Importantly, these large association white matter fibers connect rostral and caudal brain regions. For example, the SLF connects aspects of the parietal lobe with frontal cortex, including the DLPFC. As such, disruption of the SLF is associated with poorer working memory, an ability often affected in 22q11DS (Bava et al., 2011; Montojo et al., 2014). Moreover, one previous study found lower AD in 22q11DS (Kikinis et al., 2012), but no associations with cognition were reported. The IFO, another region with significantly lower AD in 22q11DS, forms the main connection between the fusiform and lingual gyri and the prefrontal cortex (Martino et al., 2010). Disruption of the right IFO is associated with deficits in semantic processing (Duffau et al., 2005) and recognition of facial expressions, including emotional content (Philippi et al., 2009; Thomas et al., 2008). Finally, it appears that lower AD is a more common finding in 22q11DS than in schizophrenia (Kikinis et al., 2012) and it was the most common finding in the current study. Importantly, our diffusivity results in 22q11DS align with the results of a recent direct comparison of 22q11DS and ultra high risk psychosis (UHR) patients (Bakker et al., 2016). In this study, the most pronounced alterations were found in AD, but in different directions for 22q11DS (lower) and UHR (higher). While it is tempting to speculate that lower AD is specifically associated with changes in axonal diameter in 22q11DS, it is incomplete information and mapping the outcome of complex diffusion analyses onto specific microstructural features is extremely difficult, if not impossible (Wheeler-Kingshott and Cercignani, 2009). Nonetheless, the current findings illuminate significant neuroanatomical deficits in white matter structural connectivity in 22q11DS and in individuals with 22q11DS and psychosis features.
Our exploratory findings in 22q11DS+ individuals suggest that the use of 22q11DS as a model for understanding psychosis is likely useful, but significantly larger samples of 22q11DS+ participants are needed to confirm these findings. Moreover, lower FA and AD and higher radial diffusivity in other white matter regions align with some whole brain findings in 22q11DS (Bakker et al., 2016; Barnea-Goraly et al., 2003; da Silva Alves et al., 2011) and psychosis (Asami et al., 2014; Epstein et al., 2013; Kyriakopoulos et al., 2008; Lee et al., 2013; Roalf et al., 2013b). While it is tempting to draw conclusions as to how white matter alterations in 22q11DS can inform us about structural changes in psychosis, more research is necessary to elucidate the specific features that overlap and those that are distinct to each disorder.
4.1 Limitations
While our study benefitted from a large sample, rigorous attention to data quality, and nonlinear analytics, several limitations should be noted. First, only high functioning 22q11DS participants without a neurological diagnosis and with IQ > 70 were included in the sample and some individuals did not qualify for MRI due to contraindications. This may have precluded the sicker individuals from participating, and as such our results may underestimate white matter deficits in 22q11DS. Notably, high functioning individuals with 22q11DS are anxious and have learning disabilities (Tang et al., 2014). These factors may have increased the rate of questionable data within this sample, which was considerably higher than the exclusion rate in a much larger sample of typically developing youth (~10%) (Roalf et al., 2016) collected on the same scanner, by the same investigators, using the same DTI acquisition protocol. However, procedures were in place to minimize anxiety as much as possible, including the use of a prescribed protocol to familiarize individuals with MRI scanning procedures. Secondly, we employ an uncommon, but powerful statistical approach (GAM) (Wood, 2004, 2011) that did not assume a linear trajectory with age. This approach differs from most analysis of DTI data in 22q11DS, yet we believe that this is a significant strength of the current approach. We do not report the effects of medication in the 22q11DS group. However, previous reports have not consistently shown a relationship between DTI metrics and medications (Kanaan et al., 2009; Kyriakopoulos et al., 2011), but at least one report in 22q11DS suggests a reparative effect (Kates et al., 2015). Finally, discrepancies between our whole brain and ROI approach is not surprising given that when a significant cluster is quite large and spans multiple anatomical regions, it is difficult make inferences about a specific anatomical region with confidence, as one can only infer that there is significant signal somewhere within the expansive cluster. While TFCE is more robust to this issue than other cluster based approaches, it still suffers from low spatial specificity when clusters are large (Woo et al., 2014). Hence, ROI based analyses aid in the regional interpretation of potential differences based upon group.
Finally, we offer a note of caution regarding data quality. Our results provide a specific example of the confounding effects of poor data quality on DTI analysis. While not the focus of the current study, it should be noted that data quality is an important factor to consider when comparing individuals with and without 22q11DS. We reported lower overall data quality in 22q11DS, as measured by TSNR, head motion, and image intensity outliers. As a result, we included TSNR as a covariate in all regional analyses. Nonetheless, lower TSNR was associated with significant differences in FA and diffusivity metrics. These issues are certainly not specific to 22q11DS, but indicate a systemic problem in the use of DTI in clinical populations. Importantly, not all group differences could be explained by data quality issues, and as such real differences in white matter are likely a true reflection of aberrant neuronal processes in 22q11DS.
In conclusion, we find individuals with 22q11DS to have consistent alterations in white matter microstructure, some of which are specifically relevant for psychosis.
Supplementary Material
Table 2. ICBM-JHU White Matter Tracts.
Abbreviations for regions used in DTI analysis.
| Abbreviation | Region-of-Interest |
|---|---|
| ATR | Anterior Thalamic Radiation |
| CST | Corticospinal Tract |
| CGC | Cingulate Gyrus-Cingulum Bundle |
| CGH | Cingulate Gyrus proximal to the Hippocampus |
| Forceps Major | Forceps Major |
| Forceps Minor | Forceps Minor |
| IFO | Inferior Fronto-Occipital Fasciculus |
| ILF | Inferior Longitudinal Fasciculus |
| SLF | Superior Longitudinal Fasciculus |
| UF | Uncinate Fasciculus |
WM microstructure in individuals with 22q11DS is aberrant compared to age and sex matched healthy individuals.
The general pattern of DTI dysfunction was lower FA, MD, and AD, while also finding higher RD in 22q11DS
White matter microstructure within the cingulum bundle was lower in 22q11DS with psychosis symptoms
Acknowledgments
Thanks to the acquisition and recruitment team: Jeff Valdez, Raphael Gerraty, Marisa Riley, Jack Keefe, Elliott Yodh, Jason Blake, Prayosha Villa, R. Sean Gallagher and Rosetta Chiavacci. Thanks to Sushila Kabadi and Margo Gawronska for their help with the literature review in 22q11DS.
Funding Sources: This work was supported by the National Institute of Mental Health grants MH089983, MH089924, MH087626, MH087636. Additional support was provided by K01MH102609 to DRR; K23MH098130 and R01 MH107703 to TDS; T32MH065218-11 to SNV; K01ES026840 to JES; K23MH108736 to JJY and the Dowshen Program for Neuroscience at the University of Pennsylvania. The funding sources were not directly involved in study design, collection, data analysis or interpretation, nor manuscript writing.
Footnotes
Financial Disclosures/Conflicts of Interest:
David R. Roalf PhD reports no potential conflicts of interest or financial disclosures related to this work.
J. Eric Schmitt MD PhD reports no potential conflicts of interest or financial disclosures related to this work.
Simon N. Vandekar BS reports no potential conflicts of interest or financial disclosures related to this work.
Theodore D. Satterthwaite, MD reports no potential conflicts of interest or financial disclosures related to this work.
Russell T. Shinohara, PhD reports no potential conflicts of interest or financial disclosures related to this work.
Kosha Ruparel MSE reports no potential conflicts of interest or financial disclosures related to this work.
Mark A. Elliott PhD reports no potential conflicts of interest or financial disclosures related to this work.
Karthik Prabhakaran reports no potential conflicts of interest or financial disclosures related to this work.
Donna M. McDonald-McGinn MS CGC reports no potential conflicts of interest or financial disclosures related to this work.
Elaine H. Zackai MD reports no potential conflicts of interest or financial disclosures related to this work.
Ruben C. Gur PhD reports no potential conflicts of interest or financial disclosures related to this work.
Beverly S. Emanuel PhD reports no potential conflicts of interest or financial disclosures related to this work.
Raquel E. Gur MD PhD reports no potential conflicts of interest or financial disclosures related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson JL, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asami T, Hyuk Lee S, Bouix S, Rathi Y, Whitford TJ, Niznikiewicz M, Nestor P, McCarley RW, Shenton ME, Kubicki M. Cerebral white matter abnormalities and their associations with negative but not positive symptoms of schizophrenia. Psychiatry Research: Neuroimaging. 2014;222:52–59. doi: 10.1016/j.pscychresns.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M, Laun FB, Leemans A, Tax CM, Biessels GJ, Stieltjes B, Maier-Hein KH. Methodological considerations on tract-based spatial statistics (TBSS) Neuroimage. 2014;100:358–369. doi: 10.1016/j.neuroimage.2014.06.021. [DOI] [PubMed] [Google Scholar]
- Bakker G, Caan M, Schluter R, Bloemen ON, da Silva-Alves F, de Koning M, Boot E, Vingerhoets W, Nieman D, de Haan L. Distinct white-matter aberrations in 22q11. 2 deletion syndrome and patients at ultra-high risk for psychosis. Psychol Med. 2016;46:2299–2311. doi: 10.1017/S0033291716000970. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Krasnow B, Ko A, Reiss A, Eliez S. Investigation of white matter structure in velocardiofacial syndrome: a diffusion tensor imaging study. Am J Psychiatry. 2003;160:1863–1869. doi: 10.1176/appi.ajp.160.10.1863. [DOI] [PubMed] [Google Scholar]
- Bassett AS, Costain G, Fung WLA, Russell KJ, Pierce L, Kapadia R, Carter RF, Chow EW, Forsythe PJ. Clinically detectable copy number variations in a Canadian catchment population of schizophrenia. J Psychiatr Res. 2010;44:1005–1009. doi: 10.1016/j.jpsychires.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Hodgkinson K, Chow EW, Correia S, Scutt LE, Weksberg R. 22q11 deletion syndrome in adults with schizophrenia. Am J Med Genet. 1998;81:328. [PMC free article] [PubMed] [Google Scholar]
- Bava S, Boucquey V, Goldenberg D, Thayer RE, Ward M, Jacobus J, Tapert SF. Sex differences in adolescent white matter architecture. Brain Res. 2011;1375:41–48. doi: 10.1016/j.brainres.2010.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Van Erp TG, Dutton RA, Tran H, Zimmermann L, Sun D, Geaga JA, Simon TJ, Glahn DC, Cannon TD. Mapping cortical thickness in children with 22q11. 2 deletions. Cereb Cortex. 2007;17:1889–1898. doi: 10.1093/cercor/bhl097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, MacDonald AW, 3rd, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry. 2001;158:1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Johansen-Berg H, Behrens TE, Robson MD, Pinsk MA, Gross CG, Richter W, Richter MC, Kastner S, Rushworth MF. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. The Journal of Neuroscience. 2005;25:8854–8866. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Alves F, Schmitz N, Bloemen O, van der Meer J, Meijer J, Boot E, Nederveen A, de Haan L, Linszen D, van Amelsvoort T. White matter abnormalities in adults with 22q11 deletion syndrome with and without schizophrenia. Schizophr Res. 2011;132:75–83. doi: 10.1016/j.schres.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Davenport ND, Karatekin C, White T, Lim KO. Differential fractional anisotropy abnormalities in adolescents with ADHD or schizophrenia. Psychiatry Research: Neuroimaging. 2010;181:193–198. doi: 10.1016/j.pscychresns.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Goodrich-Hunsaker NJ, Cabaral M, Amaral DG, Buonocore MH, Harvey D, Kalish K, Carmichael OT, Schumann CM, Lee A. Disrupted fornix integrity in children with chromosome 22q11. 2 deletion syndrome. Psychiatry Research: Neuroimaging. 2015;232:106–114. doi: 10.1016/j.pscychresns.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rosa E, Crow TJ, Chance SA. Axon bundle spacing in the anterior cingulate cortex of the human brain. J Clin Neurosci. 2008;15:1389–1392. doi: 10.1016/j.jocn.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio-Mazoyer N, Capelle L. New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain. 2005;128:797–810. doi: 10.1093/brain/awh423. [DOI] [PubMed] [Google Scholar]
- Eliez S, Blasey CM, Schmitt EJ, White CD, Hu D, Reiss AL. Velocardiofacial syndrome: are structural changes in the temporal and mesial temporal regions related to schizophrenia? Am J Psychiatry. 2001;158:447–453. doi: 10.1176/appi.ajp.158.3.447. [DOI] [PubMed] [Google Scholar]
- Eliez S, Schmitt JE, White CD, Reiss AL. Children and adolescents with velocardiofacial syndrome: a volumetric MRI study. Am J Psychiatry. 2000 doi: 10.1176/appi.ajp.157.3.409. [DOI] [PubMed] [Google Scholar]
- Epstein KA, Cullen K, Mueller B, Lee S, Kumra S. White Matter Abnormalities and Cognitive Impairment in Early-Onset Schizophrenia-Spectrum Disorders. J Am Acad Child Adolesc Psychiatry. 2013;53:362–372. doi: 10.1016/j.jaac.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II) John Wiley & Sons Inc; 2004. [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, Bullmore E, Fox PT. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64:774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Feinstein C, Thompson T, Gu E, Penniman L, Van Stone E, Kwon H, Eliez S, Reiss AL. Risk factors for the emergence of psychotic disorders in adolescents with 22q11. 2 deletion syndrome. Am J Psychiatry. 2007;164:663–669. doi: 10.1176/ajp.2007.164.4.663. [DOI] [PubMed] [Google Scholar]
- Graham MS, Drobnjak I, Zhang H. Realistic simulation of artefacts in diffusion MRI for validating post-processing correction techniques. Neuroimage. 2016;125:1079–1094. doi: 10.1016/j.neuroimage.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Turner TH, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001;25:766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, Loughead J, Connolly JJ, Qiu H, Mentch FD, Abou-Sleiman PM, Hakonarson H, Gur RE. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012;26:251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Yi J, McDonald-McGinn D, Tang S, Calkins M, Whinna D, Souders M, Savitt A, Zackai E, Moberg P. Neurocognitive development in 22q11. 2 deletion syndrome: comparison with youth having developmental delay and medical comorbidities. Mol Psychiatry. 2014 doi: 10.1038/mp.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RC. Global assessment of functioning: a modified scale. Psychosomatics. 1995;36:267–275. doi: 10.1016/S0033-3182(95)71666-8. [DOI] [PubMed] [Google Scholar]
- Jalali G, Vorstman J, Errami A, Vijzelaar R, Biegel J, Shaikh T, Emanuel B. Detailed analysis of 22q11. 2 with a high density MLPA probe set. Hum Mutat. 2008;29:433–440. doi: 10.1002/humu.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M, Jonas R, Senturk D, Patel A, Chow C, Green MF, Bearden CE. Structural abnormalities in cortical volume, thickness, and surface area in 22q11. 2 microdeletion syndrome: relationship with psychotic symptoms. NeuroImage: Clinical. 2013;3:405–415. doi: 10.1016/j.nicl.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M, Villalon-Reina JE, Karlsgodt KH, Senturk D, Chow C, Thompson PM, Bearden CE. Altered white matter microstructure is associated with social cognition and psychotic symptoms in 22q11. 2 microdeletion syndrome. Front Behav Neurosci. 2014:8. doi: 10.3389/fnbeh.2014.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan R, Barker G, Brammer M, Giampietro V, Shergill S, Woolley J, Picchioni M, Toulopoulou T, McGuire P. White matter microstructure in schizophrenia: effects of disorder, duration and medication. The British Journal of Psychiatry. 2009;194:236–242. doi: 10.1192/bjp.bp.108.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Olszewski AK, Gnirke MH, Kikinis Z, Nelson J, Antshel KM, Fremont W, Radoeva PD, Middleton FA, Shenton ME. White matter microstructural abnormalities of the cingulum bundle in youths with 22q11. 2 deletion syndrome: associations with medication, neuropsychological function, and prodromal symptoms of psychosis. Schizophr Res. 2015;161:76–84. doi: 10.1016/j.schres.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kelly S, Agartz N, Andreassen OA, Fatourous-Bergman H, Brouwer R, Cahn W, Calhoun V, Cannon D, Gabriel Castrillon J, Chiapponi C, Corvin A, Trung Doan N, Ehrlich S, Crespo-Cacorro B, Flycket L, Fukunaga M, Glahn DC, Gollub R, Gur RE, Tordesillas-Gutierrez D, Hashimoto R, Hatton S, Hibar DP, Hickie I, Horacek H, Lopez Jaramillo C, Jonsson E, Kahn RS, Kubicki M, Knochel C, Oertel-Knochel V, Kikinis Z, Lange C, Lagopoulos J, Lyall A, Magnotta VA, Mandl RC, MacDonald C, Melicher T, Newell D, Pasternak O, Piras F, Pearlson GD, Pol HEH, Roalf DR, Roiz-Santianez R, DeRosse P, Rotenberg D, Satterthwaite TD, Spalletta G, Spaniel F, Stablein M, Tonnessen S, Vanegas A, Vargas C, Voineskos A, Westyle L, White T, Zhao J, Thompson P, Donohoe G. White matter differences in schizophrenia: Meta-analytic findings from ENIGMA-Schizophrenia DTI. Mol Psychiatry. 2017 in press. [Google Scholar]
- Kikinis Z, Asami T, Bouix S, Finn C, Ballinger T, Tworog-Dube E, Kucherlapati R, Kikinis R, Shenton M, Kubicki M. Reduced fractional anisotropy and axial diffusivity in white matter in 22q11. 2 deletion syndrome: a pilot study. Schizophr Res. 2012;141:35–39. doi: 10.1016/j.schres.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsake J, Bergstralh E. vmatch 2004 [Google Scholar]
- Kyriakopoulos M, Bargiotas T, Barker GJ, Frangou S. Diffusion tensor imaging in schizophrenia. Eur Psychiatry. 2008;23:255–273. doi: 10.1016/j.eurpsy.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Kyriakopoulos M, Samartzis L, Dima D, Hayes D, Corrigall R, Barker G, Correll C, Frangou S. P03-111-Does antipsychotic medication affect white matter in schizophrenia and bipolar disorder? a review of diffusion tensor imaging literature. Eur Psychiatry. 2011;26:1280. [Google Scholar]
- Lee S-H, Kubicki M, Asami T, Seidman LJ, Goldstein JM, Mesholam-Gately RI, McCarley RW, Shenton ME. Extensive white matter abnormalities in patients with first-episode schizophrenia: a diffusion tensor imaging (DTI) study. Schizophr Res. 2013;143:231–238. doi: 10.1016/j.schres.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AK, Robinson G, Reutens D, Mowry B. Cognitive and structural neuroimaging characteristics of schizophrenia patients with large, rare copy number deletions. Psychiatry Research: Neuroimaging. 2014;224:311–318. doi: 10.1016/j.pscychresns.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Martino J, Vergani F, Robles SG, Duffau H. New insights into the anatomic dissection of the temporal stem with special emphasis on the inferior fronto-occipital fasciculus: implications in surgical approach to left mesiotemporal and temporoinsular structures. Neurosurgery. 2010;66:4–12. doi: 10.1227/01.NEU.0000348564.28415.FA. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Sullivan KE, Marino B, Philip N, Swillen A, Vorstman JA, Zackai EH, Emanuel BS, Vermeesch JR, Morrow BE. 22q11. 2 deletion syndrome. Nature reviews Disease primers. 2015;1:15071. doi: 10.1038/nrdp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Montojo CA, Ibrahim A, Karlsgodt KH, Chow C, Hilton A, Jonas RK, Vesagas TK, Bearden C. Disrupted working memory circuitry and psychotic symptoms in 22q11. 2 deletion syndrome. NeuroImage: Clinical. 2014;4:392–402. doi: 10.1016/j.nicl.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56:940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Niznikiewicz M, Gurrera RJ, McCarley RW, Shenton ME. Neuropsychological disturbance in schizophrenia: a diffusion tensor imaging study. Neuropsychology. 2008;22:246–254. doi: 10.1037/0894-4105.22.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Spencer KM, Niznikiewicz M, McCarley RW, Shenton ME. Attentional networks and cingulum bundle in chronic schizophrenia. Schizophr Res. 2007;90:308–315. doi: 10.1016/j.schres.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottet MC, Schaer M, Cammoun L, Schneider M, Debbane M, Thiran JP, Eliez S. Reduced fronto-temporal and limbic connectivity in the 22q11. 2 deletion syndrome: vulnerability markers for developing schizophrenia. PLoS One. 2013;8:e58429. doi: 10.1371/journal.pone.0058429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula MC, Schaer M, Scariati E, Schneider M, Van De Ville D, Debbané M, Eliez S. Structural and functional connectivity in the default mode network in 22q11. 2 deletion syndrome. J Neurodev Disord. 2015;7:1. doi: 10.1186/s11689-015-9120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BD, Ikuta T, DeRosse P, John M, Burdick KE, Gruner P, Prendergast DM, Szeszko PR, Malhotra AK. Age-related differences in white matter tract microstructure are associated with cognitive performance from childhood to adulthood. Biol Psychiatry. 2014;75:248–256. doi: 10.1016/j.biopsych.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BD, Szeszko PR, Radua J, Ikuta T, Gruner P, DeRosse P, Zhang J-P, Giorgio A, Qiu D, Tapert SF. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophr Bull. 2012;38:1308–1317. doi: 10.1093/schbul/sbs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi CL, Mehta S, Grabowski T, Adolphs R, Rudrauf D. Damage to association fiber tracts impairs recognition of the facial expression of emotion. The Journal of Neuroscience. 2009;29:15089–15099. doi: 10.1523/JNEUROSCI.0796-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver AE, Nestadt G, Goldberg R, Shprintzen RJ, Lamacz M, Wolyniec PS, Morrow B, Karayiorgou M, Antonarakis SE, Housman D. Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. The Journal of nervous and mental disease. 1994;182:476–477. doi: 10.1097/00005053-199408000-00010. [DOI] [PubMed] [Google Scholar]
- R-Core-Team. R: A Language and Enviroment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. [Google Scholar]
- Radoeva PD, Coman IL, Antshel KM, Fremont W, McCarthy CS, Kotkar A, Wang D, Shprintzen RJ, Kates WR. Atlas-based white matter analysis in individuals with velo-cardio-facial syndrome (22q11. 2 deletion syndrome) and unaffected siblings. Behav Brain Funct. 2012;8:38. doi: 10.1186/1744-9081-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Gur RC, Almasy L, Richard J, Gallagher RS, Prasad K, Wood J, Pogue-Geile MF, Nimgaonkar VL, Gur RE. Neurocognitive performance stability in a muliplex multigenerational study of schizophrenia. Schizophr Bull. 2013a;39:529–537. doi: 10.1093/schbul/sbs078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Gur RE, Ruparel K, Calkins ME, Satterthwaite TD, Bilker W, Hakonarson H, Harris LJ, Gur RC. Within-Individual Variability in Neurocognitive Performance: Age and Sex-Related Differences in Children and Youths From Ages 8 to 21. Neuropsychology. 2014;28:506–518. doi: 10.1037/neu0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Gur RE, Verma R, Parker WA, Quarmley M, Ruparel K, Gur RC. White matter microstructure in schizophrenia: Associations to neurocognition and clinical symptomatology. Schizophr Res. 2015;161:42–49. doi: 10.1016/j.schres.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Quarmley M, Elliott MA, Satterthwaite TD, Vandekar SN, Ruparel K, Gennatas ED, Calkins ME, Moore TM, Hopson R. The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large-scale population-based cohort. Neuroimage. 2016;125:903–919. doi: 10.1016/j.neuroimage.2015.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Ruparel K, Verma R, Elliott MA, Gur RE, Gur RC. White matter organization and neurocognitive performance variability in schizophrenia. Schizophr Res. 2013b;143:172–178. doi: 10.1016/j.schres.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Connolly JJ, Ruparel K, Calkins ME, Jackson C, Elliott MA, Roalf DR, Hopsona KPR, Behr M, Qiu H. The Philadelphia Neurodevelopmental Cohort: A publicly available resource for the study of normal and abnormal brain development in youth. Neuroimage. 2015 doi: 10.1016/j.neuroimage.2015.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, Hopson R, Jackson C, Keefe J, Riley M, Mentch FD, Sleiman P, Verma R, Davatzikos C, Hakonarson H, Gur RC, Gur RE. Neuroimaging of the Philadelphia neurodevelopmental cohort. Neuroimage. 2014a;86:544–553. doi: 10.1016/j.neuroimage.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Shinohara RT, Wolf DH, Hopson RD, Elliott MA, Vandekar SN, Ruparel K, Calkins ME, Roalf DR, Gennatas ED. Impact of puberty on the evolution of cerebral perfusion during adolescence. Proceedings of the National Academy of Sciences. 2014b;111:8643–8648. doi: 10.1073/pnas.1400178111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Calkins ME, Vandekar SN, Erus G, Ruparel K, Roalf DR, Linn KA, Elliott MA, Moore TM. Structural brain abnormalities in youth with psychosis spectrum symptoms. JAMA psychiatry. 2016;73:515–524. doi: 10.1001/jamapsychiatry.2015.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scariati E, Padula M, Schaer M, Eliez S. Long-range dysconnectivity in frontal and midline structures is associated to psychosis in 22q11. 2 deletion syndrome. J Neural Transm. 2016:1–17. doi: 10.1007/s00702-016-1548-z. [DOI] [PubMed] [Google Scholar]
- Schaer M, Cuadra MB, Tamarit L, Lazeyras F, Eliez S, Thiran JP. A surface-based approach to quantify local cortical gyrification. Medical Imaging, IEEE Transactions on. 2008;27:161–170. doi: 10.1109/TMI.2007.903576. [DOI] [PubMed] [Google Scholar]
- Schaer M, Schmitt JE, Glaser B, Lazeyras F, Delavelle J, Eliez S. Abnormal patterns of cortical gyrification in velo-cardio-facial syndrome (deletion 22q11. 2): an MRI study. Psychiatry Research: Neuroimaging. 2006;146:1–11. doi: 10.1016/j.pscychresns.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Vandekar S, Yi J, Calkins ME, Ruparel K, Roalf DR, Whinna D, Souders MC, Satterthwaite TD, Prabhakaran K. Aberrant cortical morphometry in the 22q11. 2 deletion syndrome. Biol Psychiatry. 2015;78:135–143. doi: 10.1016/j.biopsych.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt JE, Yi JJ, Roalf DR, Loevner LA, Ruparel K, Whinna D, Souders MC, McDonald-McGinn DM, Yodh E, Vandekar S. Incidental Radiologic Findings in the 22q11. 2 Deletion Syndrome. American Journal of Neuroradiology. 2014;35:2186–2191. doi: 10.3174/ajnr.A4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Van der Linden M, Menghetti S, Glaser B, Debbané M, Eliez S. Predominant negative symptoms in 22q11. 2 deletion syndrome and their associations with cognitive functioning and functional outcome. J Psychiatr Res. 2014;48:86–93. doi: 10.1016/j.jpsychires.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ, Goldberg R, Golding-Kushner KJ, Marion RW. Late-onset psychosis in the velo-cardio-facial syndrome. Am J Med Genet. 1992;42:141–142. doi: 10.1002/ajmg.1320420131. [DOI] [PubMed] [Google Scholar]
- Simon TJ, Ding L, Bish JP, McDonald-McGinn DM, Zackai EH, Gee J. Volumetric, connective, and morphologic changes in the brains of children with chromosome 22q11. 2 deletion syndrome: an integrative study. Neuroimage. 2005;25:169–180. doi: 10.1016/j.neuroimage.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundram F, Campbell LE, Azuma R, Daly E, Bloemen OJ, Barker GJ, Chitnis X, Jones DK, van Amelsvoort T, Murphy KC. White matter microstructure in 22q11 deletion syndrome: a pilot diffusion tensor imaging and voxel-based morphometry study of children and adolescents. J Neurodev Disord. 2010;2:77–92. doi: 10.1007/s11689-010-9043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K, Yamasue H, Abe O, Yamada H, Inoue H, Suga M, Muroi M, Sasaki H, Aoki S, Kasai K. Structural disruption of the dorsal cingulum bundle is associated with impaired Stroop performance in patients with schizophrenia. Schizophr Res. 2009;114:119–127. doi: 10.1016/j.schres.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Tan GM, Arnone D, McIntosh AM, Ebmeier KP. Meta-analysis of magnetic resonance imaging studies in chromosome 22q11. 2 deletion syndrome (velocardiofacial syndrome) Schizophr Res. 2009;115:173–181. doi: 10.1016/j.schres.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Tang S, Yi J, Calkins M, Whinna D, Kohler C, Souders M, McDonald-McGinn D, Zackai E, Emanuel B, Gur R. Psychiatric disorders in 22q11. 2 deletion syndrome are prevalent but undertreated. Psychol Med. 2014;44:1267–1277. doi: 10.1017/S0033291713001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SX, Moore TM, Calkins ME, James JY, Savitt A, Kohler CG, Souders MC, Zackai EH, McDonald-McGinn DM, Emanuel BS. The Psychosis Spectrum in 22q11. 2 Deletion Syndrome Is Comparable to That of Nondeleted Youths. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2016.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Moya L, Avidan G, Humphreys K, Jung KJ, Peterson MA, Behrmann M. Reduction in white matter connectivity, revealed by diffusion tensor imaging, may account for age-related changes in face perception. J Cogn Neurosci. 2008;20:268–284. doi: 10.1162/jocn.2008.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Thompson PM. Diffusion imaging, white matter, and psychopathology. Clin Psychol (New York) 2011;7:63. doi: 10.1146/annurev-clinpsy-032210-104507. [DOI] [PubMed] [Google Scholar]
- Vaáša F, Griffa A, Scariati E, Schaer M, Urben S, Eliez S, Hagmann P. An affected core drives network integration deficits of the structural connectome in 22q11. 2 deletion syndrome. NeuroImage: Clinical. 2016;10:239–249. doi: 10.1016/j.nicl.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalon-Reina J, Jahanshad N, Beaton E, Toga AW, Thompson PM, Simon TJ. White matter microstructural abnormalities in girls with chromosome 22q11. 2 deletion syndrome, Fragile X or Turner syndrome as evidenced by diffusion tensor imaging. Neuroimage. 2013;81:441–454. doi: 10.1016/j.neuroimage.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler-Kingshott CA, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- White T, Kendi ATK, Lehéricy S, Kendi M, Karatekin C, Guimaraes A, Davenport N, Schulz SC, Lim KO. Disruption of hippocampal connectivity in children and adolescents with schizophrenia—a voxel-based diffusion tensor imaging study. Schizophr Res. 2007;90:302–307. doi: 10.1016/j.schres.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. Wide Range Achievement Test--Revision 3. Wilmington, DE: 1993. [Google Scholar]
- Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SN. Stable and efficient multiple smoothing parameter estimation for generalized additive models. Journal of the American Statistical Association. 2004:99. [Google Scholar]
- Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2011;73:3–36. [Google Scholar]
- Zhang B, Xu Y, Zhu B, Kantarci K. The role of diffusion tensor imaging in detecting microstructural changes in prodromal Alzheimer’s disease. CNS Neurosci Ther. 2014;20:3–9. doi: 10.1111/cns.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yushkevich PA, Alexander DC, Gee JC. Deformable registration of diffusion tensor MR images with explicit orientation optimization. Med Image Anal. 2006;10:764–785. doi: 10.1016/j.media.2006.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.