Abstract
Childhood maltreatment is associated with posttraumatic stress disorder (PTSD) and elevated rates of adolescent and adult psychopathology including major depression, bipolar disorder, substance use disorders, and other medical comorbidities. Gray matter volume changes have been found in maltreated youth with (versus without) PTSD. However, little is known about the alterations of brain structural covariance network topology derived from cortical thickness in maltreated youth with PTSD. High-resolution T1-weighted magnetic resonance imaging scans were from demographically matched maltreated youth with PTSD (N = 24), without PTSD (N =64), and non-maltreated healthy controls (n = 67). Cortical thickness data from 148 cortical regions was entered into interregional partial correlation analyses across participants. The supra-threshold correlations constituted connections in a structural brain network derived from four types of centrality measures (degree, betweenness, closeness, and eigenvector) estimated network topology and the importance of nodes. Between-group differences were determined by permutation testing. Maltreated youth with PTSD exhibited larger centrality in left anterior cingulate cortex than the other two groups, suggesting cortical network topology specific to maltreated youth with PTSD. Moreover, maltreated youth with versus without PTSD showed smaller centrality in right orbitofrontal cortex, suggesting that this may represent a vulnerability factor to PTSD following maltreatment. Longitudinal follow-up of the present results will help characterize the role that altered centrality plays in vulnerability and resilience to PTSD following childhood maltreatment.
Keywords: Childhood maltreatment, Posttraumatic stress disorder, Structural covariance network, Cortical thickness, Centrality, Anterior cingulate cortex
1. Introduction
Childhood maltreatment is associated with increased risk for multiple forms of psychopathology (De Bellis and Zisk, 2014; McLaughlin et al., 2013). Children who have been maltreated exhibit difficulties in multiple forms of emotion regulation (De Bellis and Zisk, 2014; McCrory et al, 2011, 2013; McLaughlin et al., 2015) and social cognition (Kay and Green, 2016). About 20–30% of maltreated youth meet criteria for chronic posttraumatic stress disorder (PTSD) (McLeer et al., 1998; McLeer and Ruggiero, 1999), which is accompanied by heightened threat response and difficulty inhibiting fear response to traumatic reminders. We recently reported that maltreated youth with chronic PTSD, when compared to maltreated youth without PTSD, have smaller volumes in brain areas associated with fear extinction, emotion and memory processing, including left amygdala, right hippocampus and right ventromedial prefrontal cortex (Morey et al., 2016).
It has been widely accepted that the brain is organized into complex networks that evolve throughout postnatal and adolescent development (Di Martino et al., 2014; Zhang and Sejnowski, 2000). This brain development involves a highly choreographed process of neuronal growth and migration throughout the cortical mantle that may be derailed by exposure to various environmental insults (Evsyukova et al., 2013; Houston et al., 2014). Severe maltreatment may initially influence gray matter integrity by activating glutamate circuits and triggering pro-inflammatory processes that initiate a cascade of neural events, which manifest as reduced synaptic density and strength, as well as dendritic retraction and reduced arborization (Popoli et al., 2012). Indeed, prior work suggests that environmental threats such as childhood maltreatment may modify the development of brain networks (Hart and Rubia, 2012; Morey et al., 2015; Spielberg et al., 2015). However, knowledge about brain networks in maltreated youth with or without PTSD is scarce.
Graph theoretical measures have been recently employed to investigate structural and functional brain networks in patients with various neuropsychiatric disorders (Bassett et al., 2008; Bernhardt et al., 2008; He et al., 2008, 2009; Yao et al., 2010). Cortical morphometric network analyses (Bassett et al., 2008; He et al., 2008; He and Evans, 2010; Lerch et al., 2006; Sporns, 2011) are based on structural inferences made between specific pairings of cortical regions that vary in tandem with respect to cortical thickness or gray matter volume (Gong et al., 2012; Lerch et al., 2006). Although the neurobiological interpretation of the covariance in cortical thickness across regions is yet unclear, it has been proposed that the correlation strength increases between regions that are concurrently affected by common factors, and decreases between regions that are differentially affected by such factors (Mueller et al., 2015). The structural covariance network method based on cortical thickness/gray matter volume is relatively insensitive to the noisy components that accompany task-based and resting-state functional magnetic resonance imaging (fMRI). Thus it is posited that the structural covariance network may reflect the current state of the highly choreographed developmental processes of neuronal growth and migration throughout the cortical mantle (Gong et al., 2012; Lerch et al., 2006; Marin et al., 2010).
The supra-threshold structural covariance between anatomically delineated brain regions may be treated as a connection in the brain network and further quantified with graph theoretical measures, although there may be no direct connections between regions. Previous studies have also indicated that cortical thickness covariance partly reflects underlying fiber connections where 35–40% of thickness correlations showed convergent diffusion connections, but should not be taken as a proxy measure of fiber connections (Gong et al., 2012). Centrality is an indicator of the importance of a region within a network of interconnected nodes. Graph theory suggests that nodes with high centrality play a crucial role in controlling network information transfer and neural communication (He et al., 2008).
Structural covariance network analyses based on cortical thickness have shown that adults previously exposed to childhood maltreatment exhibited lower brain network centrality than adults without childhood maltreated in left anterior cingulate gyrus, and increased centrality in right precuneus and right anterior insula (Teicher et al., 2014). Moreover, military veterans with PTSD demonstrated decreased centrality in left orbitofrontal cortex and anterior cingulate cortices (ACC), and increased centrality in left insula and right orbitofrontal cortex (Mueller et al., 2015). However, these studies are focused on PTSD in adults, often decades after exposure to childhood maltreatment, and were not able to uncover the brain network alterations associated with acute effects of child maltreatment and subsequent PTSD. Here, we examined the brain structure covariance network of maltreated youth with and without PTSD.
We were specifically interested in orbitofrontal cortex (OFC) and ACC (Kelly et al., 2013; Kringelbach, 2005), which are consistently related to emotion processing, safety signal learning, and decision-making processes that may be disrupted by childhood maltreatment (McLaughlin et al, 2014b, 2015) and PTSD (James et al., 2014; Morey et al., 2012). Previous research has also shown structural and functional disruptions to OFC and ACC in survivors of childhood maltreatment (Kelly et al., 2013; Lim et al., 2014; Teicher et al., 2014) and those with PTSD (Morey et al., 2016; Mueller et al., 2015; Spielberg et al., 2015). We hypothesized that maltreatment would be broadly associated with altered centrality in OFC and in the ACC, particularly children with PTSD as disruptions in these neural networks may increase their vulnerability to the disorder. Specifically, we predicted that these areas would show lower centrality in maltreated youth with PTSD than maltreated youth without PTSD and non-maltreated controls. Based on findings from previous studies of brain structural covariance network architecture in adults exposed to maltreatment (Teicher et al., 2014) or with PTSD (Mueller et al., 2015), we also anticipated between-group differences in insula/inferior frontal cortex (Ins/IFC) and temporal pole (TP).
2. Methods
2.1. Participants
Study participants were drawn from three neuroimaging cohorts of childhood maltreatment: one cohort of maltreated adolescents (aged 13-20) completed in Boston (Boston Children's Hospital) and two cohorts of maltreated children (aged 8-19) completed in Seattle (University of Washington). The investigation was carried out in accordance with the latest version of the Declaration of Helsinki, and was approved by the local institutional review board. Written informed consent was obtained from each participant after the nature of the procedures had been fully explained. The combined sample consisted of all participants who completed an MRI and included maltreated youth with chronic PTSD (N =24), without PTSD (N = 64), and non-maltreated controls (n = 67). As shown in Table 1, there were no between-group differences in age and sex. However, maltreatment (both with and without PTSD) was associated with lower parental education. Exclusion criteria included psychiatric medication use (with the exception of stimulant medications for attention-deficit/hyperactivity disorder [ADHD], which were discontinued 24 h before the scan), dental braces, claustrophobia, active substance dependence, pervasive developmental disorder, inability to speak English, and presence of active safety concerns.
Table 1.
Demographic information.
| TEST | Mean (SD) | Statistic (p-value)* | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| CONT (n = 67) | MALT (n= 64) | PTSD (n=24) | PTSD vs MALT | MALT vs CONT | PTSD vs CONT | |
| Age [years] | 14.8 (2.7) | 15.4 (2.8) | 14.7 (2.7) | –1.011 (0.315) | 1.138 (0.257) | –0.195 (0.846) |
| Sex [m(f)] | 31 (36) | 28 (36) | 11 (13) | 0.031 (0.861) | 0.084 (0.772) | 0.001 (0.971) |
| Parental Education | 3.4 (0.8) | 2.3 (1.2) | 2.1 (1.2) | –0.824 (0.412) | –5.729 (< 0.001) | –5.806 (<0.001) |
| CTQ Physical | 5.1 (0.4) | 7.9 (4.1) | 11.3 (5.0) | 3.294 (0.001) | 5.582 (<0.001) | 10.142 (<0.001) |
| CTQ Sexual | 5.0 (0.4) | 7.4 (4.6) | 8.5 (6.1) | 0.927 (0.356) | 4.241 (<0.001) | 4.733 (<0.001) |
| CTQ Emotional | 6.1 (1.7) | 9.3 (4.1) | 12.1 (4.9) | 2.722 (0.008) | 5.847 (<0.001) | 8.637 (<0.001) |
| Depression | –0.5 (0.8) | 0.3 (1.0) | 0.7 (1.0) | 1.378 (0.172) | 5.647 (<0.001) | 5.839 (<0.001) |
| DISC | 7.0 (5.4) | 13.2 (7.9) | 29.5 (9.2) | 2.847 (0.007) | 3.156 (0.003) | 5.390 (<0.001) |
| SCARED | 12.4 (9.8) | 22.5 (9.3) | 31.0 (17.4) | 2.221 (0.031) | 4.354 (<0.001) | 5.628 (<0.001) |
Note: the statistical values for Sex are from Chi-Square tests, while others from t tests. CONT = Control, Malt = Maltreated youth without PTSD, PTSD = Maltreated youth with PTSD, m(f) = number of males (females), CTQ = Childhood Trauma Questionnaire, DISC = anxiety symptoms index from the Diagnostic Interview Schedule for Children Version IV, SCARED = Screen for Child Anxiety Related Disorders.
2.2. Measures
Maltreatment was assessed using the Childhood Trauma Questionnaire (CTQ), a self-report measure, and the Childhood Experiences of Care and Abuse (CECA) interview, which was administered by trained research assistants. The CTQ assesses frequency of physical, sexual, and emotional abuse during childhood and has excellent psychometric properties including internal consistency, test–retest reliability, and convergent and discriminant validity with interviews and clinician reports of maltreatment (Bernstein et al., 1997). The CECA assesses multiple aspects of caregiving experiences, including physical and sexual abuse. Interrater reliability for maltreatment reports is excellent, and validation studies find high agreement between siblings on reports of maltreatment (Bifulco et al., 1997). Participants who reported physical or sexual abuse during the CECA interview or who had a score on the physical or sexual abuse subscales of the CTQ above a validated threshold (Walker et al., 1999) were classified as maltreated. Participants (or parents) who endorsed exposure to maltreatment on the UCLA PTSD Reaction Index (PTSD-RI) (Steinberg et al., 2013), or who reported high levels of exposure to interpersonal violence on the Screen for Adolescent Violence Exposure (SAVE) (Hastings and Kelley, 1997) were also classified as maltreated.
PTSD was assessed in the Boston sample using the Diagnostic Interview Schedule for Children Version IV (DISC-IV (Shaffer et al., 2000);), in the Seattle samples using child- and parent-report versions of the PTSD-RI and the child- and parent-report versions of the Clinician Administered PTSD Scale for Children and Adolescents (CAPS-CA) (Nader et al., 1996) respectively. All three instruments have demonstrated excellent psychometric properties (Harrington, 2008; Shaffer et al., 2000; Steinberg et al., 2013). Depression was assessed using Children's Depression Inventory (CDI and CDI2) (Kovacs, 2010). Anxiety was assessed using the Screen for Child Anxiety Related Disorders (Birmaher et al., 1999) in the Seattle sample and the anxiety symptoms index from the DISC-IV in the Boston sample. Within-study standardized scores for depression and anxiety were calculated for each participant.
2.3. MRI acquisition and analyses
T1-weighted scans were collected at the Harvard Center for Brain Sciences using a Siemens Tim Trio 3T scanner in the Boston sample and at the University of Washington Integrated Brain Imaging Center using a Phillips Achieva 3T scanner in the Seattle samples. A 32-channel head coil was used at both locations. Structural images in Boston and scans in Seattle sample-1 were T1-weighted multi-echo MPRAGE volumes (TR =2530ms, TE = 1640-7040μs, flip angle=7°, 176 slices, in-plane voxel size=1mm3). Scans in Seattle sample-2 were T1-weighted MPRAGE volumes with TE= 3.5ms and all other parameters equivalent. FOV was 220×220mm for the Siemens scanner and 256×256 mm for the Phillips scanner.
T1-weighted scans were processed using FreeSurfer version 5.3 (Fischl and Dale, 2000). Automatic image segmentation and parcellation was used to estimate cortical thickness. The results were inspected and manually edited to optimize accurate placement of gray/white and gray/CSF borders. Cortical thickness was estimated in each cortical segment from the 2009 Destrieux atlas in FreeSurfer (Destrieux et al., 2010).
2.4. Network analyses
The influence of age, sex, and study site were regressed out from the association with cortical thickness values. After that, group-specific matrices containing partial correlation coefficients between all pairs of cortical regions were calculated. The matrices were further thresholded to create binary graphs representing edges (suprathreshold partial correlations) between nodes (brain areas) (Bassett et al., 2008; Teicher et al., 2014). Only positive correlations were kept given that the negative correlations are not mediated by direct fiber pathways (Gong et al., 2012). Moreover, the thresholds were group-specific so that the graphs of all groups had the same wiring cost (number of edges divided by maximum possible number of edges). This step is important because imposing the same threshold on all groups may reflect between-group differences are not only network topological characteristics (effects of interest) but also represent connection strength (effects of no interest). To get the group-specific threshold, the minimum wiring cost was calculated to produce a fully connected network for each group (0.1974 for the Control group; 0.2920 for Maltreated youth without PTSD; 0.3608 for Maltreated youth with PTSD). The largest minimum wiring cost was chosen to calculate a group-specific threshold for between-group comparisons to ensure that all nodes (cortical areas) were included in the network while minimizing the number of redundant paths (supra-threshold connections) across groups. The minimum wiring cost method has been successfully used in previous studies on maltreatment (Teicher et al., 2014).
2.5. Centrality measures
There are numerous brain network topology measures. We selected the measures that would enable direct comparisons of our results with previous findings (Mueller et al., 2015; Teicher et al., 2014). Four types of centrality measures were analyzed using Brain Connectivity Toolbox (BCT (Rubinov and Sporns, 2010);): (a) degree centrality is the number of directly interconnected nodes, and reflects how much a node serves as a focal point of communication; (b) betweenness centrality is the frequency with which a node falls between pairs of other nodes on their shortest interconnecting path, and reflects the potential of a node to control communication; (c) closeness centrality, adapted from the distance function in BCT, reflects the normalized number of steps required to access every other node from a given node in a network, and reflects a node's speed to spread information throughout the network; (d) eigenvector centrality is a measure of a node's overall influence based on the idea that the importance of a node is recursively related to the importance of the nodes associated with it.
2.6. Statistical analyses
The variance in the groups' centrality measures should be equal despite the different sample sizes across groups (Winkler et al., 2015). Areas showing unequal variance (supplementary information Table 1S) on a two-sample F-test for equal variances (at the 1% significance level) were excluded from further analysis. To test the reliability of the centrality measures, the 99% confidence interval [CI] was calculated through the Jackknife resampling method (Efron and Tibshirani, 1993), which is well suited for structural covariance network analyses (Teicher et al., 2014).
The centrality measures were highly correlated to each other (all Pearson correlation coefficients > 0.59, p's < 0.001), suggesting that the four centralities reflect very similar graph characteristics. We then applied principle component analysis (PCA) in Matlab using default settings including mean-centered values and singular value decomposition algorithm. Before running PCA, we scaled each centrality measure from 0 to 1, to avoid scale difference across centrality measures. Applying PCA across the entire sample loaded four centrality measures through linear combinations onto four components (variance explained: 96.81%, 1.67%, 1.34% and 0.18% for components 1-4, respectively). We included the first two PCA components for analyses because they captured variance from all four centrality measures as evident from the coefficient matrix in Table 2S. Specifically, degree, closeness, and eigenvector centrality measures loaded primarily on the first component, while betweenness centrality loaded primarily on the second component.
Teicher et al. (2014) reported between-group differences when at least two of four centralities were significantly different between groups (p < .05). However, their method elevates Type II error (false positives) given that the group differences are often shared by more than two centralities, given the collinearity of centrality measures, even if these differences were tiny and did not survive multiple comparison correction. Moreover, their approach might also induce Type I error (false negatives) given that group differences detected in only one centrality measure that differ from other centrality measures, would not be reported even when highly significant. In our study, the application of PCA reduced the probability of Type 1 and Type II errors by transforming the centralities into linearly uncorrelated components, which increased power by reducing the number of comparisons. Between-group differences in centrality were investigated with permutation testing to calculate the probability that differences in PCA components could have occurred by chance based on 10,000 random permutations of subjects to two groups (He et al., 2008; Zalesky et al., 2010). Bonferroni correction was applied to pairwise comparisons between three groups with two PCA components of centrality, i.e. p < .05/(3×2) = 0.008.
3. Results
3.1. Demographic and clinical information
Participants' age, sex, parental education, depression, anxiety, and CTQ scores are summarized in Table 1.
3.2. Centrality measures
The centrality measures of all nodes were within their corresponding 99% CI, supporting the reliability of the findings. Significant between-group differences (p < .05; corrected) based on PCA components 1 and 2 are summarized in Table 2 and Fig. 1. The corresponding centrality values are also reported in Table 2 to facilitate an understanding of their topological meaning. As summarized in Table 2S, degree, closeness and eigenvector centralities loaded on PCA component 1, and betweenness centrality loaded on component 2. Moreover, the corresponding coefficients were all positive values, indicating that a larger score on the principal component was associated with greater centrality for one or more centrality measures.
Table 2.
Areas showing significant between-group differences in any of the first two PCA components that cumulatively account for more than 98% variance.
| Area | No | ROI | PCA | Group | Deg | Bet | Clo | Clo |
|---|---|---|---|---|---|---|---|---|
| MALT > CONT | ||||||||
| L Supramarginal gyrus | 26 | 1 | CONT | 54 | 54.5 | 0.676 | 0.076 | |
| MALT | 103 | 273.9 | 0.848 | 0.137 | ||||
| L Sulcus intermedius primus (of Jensen) | 55 | 1 | CONT | 2 | 0.0 | 0.430 | 0.003 | |
| MALT | 79 | 151.4 | 0.765 | 0.110 | ||||
| MALT < CONT | ||||||||
| R Planum polare of the superior temporal gyrus | 35 | TP | 1 | CONT | 71 | 226.8 | 0.731 | 0.091 |
| MALT | 15 | 11.1 | 0.530 | 0.013 | ||||
| PTSD > MALT | ||||||||
| L Central sulcus (Rolando's fissure) | 45 | 1 | MALT | 18 | 203.1 | 0.552 | 0.017 | |
| PTSD | 92 | 211.6 | 0.807 | 0.127 | ||||
| R Central sulcus (Rolando's fissure) | 45 | 1 | MALT | 19 | 24.5 | 0.554 | 0.019 | |
| PTSD | 87 | 138.8 | 0.789 | 0.123 | ||||
| R Middle frontal sulcus | 53 | 1 | MALT | 59 | 68.2 | 0.698 | 0.085 | |
| PTSD | 96 | 157.9 | 0.819 | 0.133 | ||||
| L Anterior part of the cingulate gyrus and sulcus (ACC) | 6 | ACC | 2 | MALT | 67 | 122.9 | 0.725 | 0.090 |
| PTSD | 93 | 586.3 | 0.813 | 0.117 | ||||
| PTSD < MALT | ||||||||
| L Sulcus intermedius primus (of Jensen) | 55 | 1 | MALT | 79 | 151.4 | 0.765 | 0.110 | |
| PTSD | 0 | 0.0 | 0.000 | 0.000 | ||||
| R Orbital gyri | 24 | OFC | 1 | MALT | 94 | 286.5 | 0.818 | 0.123 |
| PTSD | 22 | 48.5 | 0.558 | 0.020 | ||||
| L Postcentral gyrus | 28 | 2 | MALT | 52 | 161.0 | 0.675 | 0.067 | |
| PTSD | 45 | 15.3 | 0.643 | 0.066 | ||||
| L Subparietal sulcus | 71 | 2 | MALT | 98 | 228.5 | 0.830 | 0.131 | |
| PTSD | 67 | 33.8 | 0.719 | 0.101 | ||||
| PTSD > CONT | ||||||||
| L Superior occipital sulcus and transverse occipital sulcus | 58 | 1 | CONT | 36 | 7.0 | 0.607 | 0.055 | |
| PTSD | 87 | 120.7 | 0.789 | 0.123 | ||||
| L Anterior part of the cingulate gyrus and sulcus | 6 | ACC | 2 | CONT | 46 | 98.5 | 0.647 | 0.061 |
| PTSD | 93 | 586.3 | 0.813 | 0.117 | ||||
| L Middle-anterior part of the cingulate gyrus and sulcus | 7 | ACC | 2 | CONT | 60 | 46.1 | 0.695 | 0.085 |
| PTSD | 78 | 333.6 | 0.762 | 0.099 | ||||
| R Inferior frontal sulcus | 52 | Ins/IF | C2 | CONT | 65 | 49.8 | 0.710 | 0.095 |
| PTSD | 75 | 291.5 | 0.748 | 0.100 | ||||
| PTSD < CONT | ||||||||
| R Planum polare of the superior temporal gyrus | 35 | TP | 1 | CONT | 71 | 226.8 | 0.731 | 0.091 |
| PTSD | 14 | 151.2 | 0.517 | 0.014 | ||||
Note: The significant results survived Bonferroni correction for the multiple comparisons (i.e. p < .05/(3×2)=0.008). PCA, the PCA component where the significant difference was detected. Number (No) and Area Name (Area) are according to Destrieux et al. (2010). ROI, region of interest based on research hypotheses and interest. ACC, anterior cingulate cortex; Ins/IFC, insula and inferior frontal cortex; TP, temporal pole; OFC, orbitofrontal cortex. Deg = degree centralty; Bet = betweenness centrality; Clo = closeness centrality; Eig = eigenvector centrality; CONT=Non-maltreated controls; MALT = Maltreated youth without PTSD; PTSD =Maltreated youth with PTSD.
Fig. 1.
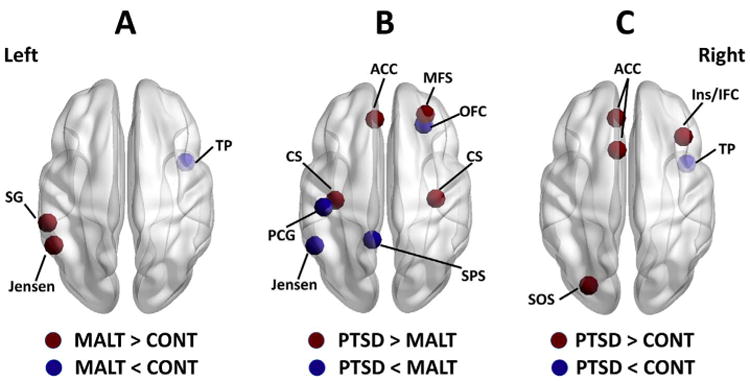
Nodes showing significant between-group differences in graph metrics. (A) Maltreated youth without PTSD (MALT group) versus non-maltreated controls (CONT group). (B) Maltreated youth with PTSD (PTSD group) versus maltreated youth without PTSD. (C) Maltreated youth with PTSD versus non-maltreated controls. Abbreviations: ACC, anterior cingulate cortex (including areas 6 and 7 in the aparc. a2009s template (Destrieux et al., 2010)); CS, central sulcus (area 45); Ins/IFC, insula and inferior frontal cortex (area 52); Jensen, sulcus inter-medius primus (of Jensen) (area 55); MFS, middle frontal sulcus (area 53); PCG, postcentral gyrus (area 28); SG, su-pramarginal gyrus (area 26); SOS, superior occipital sulcus and transverse occipital sulcus (area 58); SPS, subparietal sulcus (area 71); TP, temporal pole (area 35); OFC, orbi-tofrontal cortex (area 24).
Maltreatment and Centrality
As shown in Fig. 1A, we found larger values of PCA component 1 in maltreated youth without PTSD than non-maltreated controls in the left Supramarginal gyrus, and the left sulcus intermedius primus (of Jensen). On the other hand, maltreated youth without PTSD exhibited smaller values of PCA component 1 than non-maltreated controls in the right temporal pole (including the right planum polare of the superior temporal gyrus). No significant difference between maltreated youth without PTSD and controls was detected for PCA component 2.
PTSD and Centrality
Maltreated youth with PTSD compared to maltreated youth without PTSD showed larger values of PCA component 1 in bilateral central sulcus and the right middle frontal sulcus, and smaller values in the left sulcus intermedius primus (of Jensen) and the right OFC (including the right orbital gyri) (see Fig. 1B). Moreover, maltreated youth with PTSD compared to maltreated youth without PTSD had a larger value of PCA component 2 in the left ACC (including the left anterior cingulate gyrus and sulcus), and smaller values in the left post central gyrus and the left subparietal sulcus.
Maltreated youth with PTSD, as compared to non-maltreated controls, showed larger value of PCA component 1 in the left superior occipital sulcus and transverse occipital sulcus, and smaller value in the right temporal pole (including the right planum polare of the superior temporal gyrus) (see Fig. 1C). Moreover, maltreated youth with PTSD compared to non-maltreated controls showed larger value of PCA component 2 in the left ACC (including the left anterior cingulate gyrus and sulcus, and the left middle-anterior part of the cingulate gyrus and sulcus) and the right insula/IFC (including the right inferior frontal sulcus).
Site by Group Effect
Our data were from two different sites (i.e. Boston and Seattle). To investigate whether our findings were biased by the data from a specific site, we compared centralities between Seattle and Boston in MALT and CONT group (but not the PTSD group due to the limited sample size in Boston, i.e. N = 2), respectively. A few regions showed differences between sites (Table 4S). Importantly, none of these regions overlapped with the main findings except area 58, i.e. left superior occipital sulcus and transverse occipital sulcus, which is not an ROI. Therefore, our main findings were not deemed to be biased by the data from a specific site. However, caution is warranted in interpreting these patterns given the significantly reduced sample size when the sample is split by site.
4. Discussion
Our work demonstrates for the first time that maltreatment and PTSD in children and adolescents (from 8 to 20 years old) are associated with atypical brain network architectures as measured by structural covariance networks based on cortical thickness. Previous studies have investigated such abnormal network architectures only in adults who previously experienced childhood maltreatment or PTSD (Mueller et al., 2015; Teicher et al., 2014). Child maltreatment and PTSD were each associated with differences in network centrality in ACC in these prior works. Here, we compared PCA components derived from linear combinations of centralities. Higher scores on the PCA components reflect greater centrality in all cases.
Maltreated youth with PTSD exhibited larger centrality than both maltreated youth without PTSD and controls in the left ACC and smaller centrality in the right OFC than maltreated youth without PTSD. These findings suggest that structural brain networks show distinct changes in maltreated youth with and without PTSD. Furthermore, maltreated youth with PTSD showed larger centrality than controls in the right insula/inferior frontal cortex. This replicates a prior finding in adults (Mueller et al., 2015) and suggests that PTSD-related network abnormalities in the insula and inferior frontal areas are consistent between youth and adults. Additionally, maltreated youth with and without PTSD compared to non-maltreated controls showed smaller centrality in the right temporal pole, an area that is associated with social cognition (Hillis, 2014; Molenberghs et al., 2016). These findings advance our understanding of brain network abnormalities in youth exposed to maltreatment and in maltreated youth with PTSD.
The larger ACC centrality we find in maltreated youth with PTSD compared to maltreated youth without PTSD and to non-maltreated youth may reflect stronger network information transfer (He et al., 2008) between other brain areas and ACC, which is known to be involved in fear expression (Milad et al., 2007) and resolving cognitive interference in PTSD (Shin et al., 2011). This result is broadly in line with previous findings of attentional biases toward threat (Fani et al., 2012) and difficulties in regulating negative emotions (Shepherd and Wild, 2014) in PTSD. Pediatric maltreatment-related PTSD may be accompanied by higher utilization of ACC and ACC-involved brain networks to resolve emotional and cognitive conflicts. Interestingly, adults with PTSD exhibit lower ACC centrality than healthy controls (Mueller et al., 2015). This inconsistency may suggest a divergent pattern of ACC centrality influenced by developmental stage at PTSD onset. Alternatively, it might reflect developmental differences in the influence of environmental exposure and psychopathology on network structure covariance. Finally, our between-group differences could be caused by greater severity or chronicity of maltreatment in youth who develop PTSD compared to the maltreated youth without PTSD and non-maltreated controls. These speculative explanations will require empirical testing with longitudinal designs aimed at delineating the trajectory of ACC alterations resulting from maltreatment and PTSD across development.
Maltreated youth with PTSD had smaller right OFC centrality than maltreated youth without PTSD. Cortical thickness in OFC is positively related to extinction memory processes (Milad et al., 2005) that have been posited to play a central role in the etiology and maintenance of PTSD (Milad and Quirk, 2012; Jovanovic et al., 2010). Impaired recall of extinction learning has been observed in those with PTSD, along with reduced activation in OFC and greater activation in dorsal ACC during extinction recall (Milad et al., 2008, 2009). Our previous work demonstrated that maltreated youth with PTSD have reduced volume in OFC than those without PTSD (Morey et al., 2016). Consistent with these previous findings, we find that maltreatment-related PTSD may also involve disruptions in the structural covariance of networks involving OFC. Future research is needed on this topic to determine the specific nature of those differences. For instance, military veterans with PTSD had smaller degree centrality in the left medial OFC and larger betweenness centrality in the right medial OFC than controls (Mueller et al., 2015). The exact role of OFC in the brain network in PTSD and how it is influenced by other factors will need to be addressed in future studies.
We also detected larger centrality in the insula and inferior frontal cortex in maltreated youth with PTSD compared to the controls. This is consistent with findings of larger betweenness centrality in the right insula (within the prefrontal-limbic network) in military veterans with PTSD as compared to healthy controls (Mueller et al., 2015). This pattern suggests that larger insula and IFC centrality is present across both youth and adults with PTSD. The insula is a key node in the salience network (Seeley et al., 2007), which is involved in coordinating emotional and behavioral responses to salient environmental stimuli. Enhanced activation in the salience network in response to negative emotional cues, and even at rest, has been reported by multiple investigators in children and adults with PTSD (Shin et al, 2005, 2006; Yan et al., 2013) and prospectively predicts the onset of PTSD symptoms (McLaughlin et al., 2014a). Functional connectivity differences in this network have also been reported in those with PTSD (Wolf and Herringa, 2016). For example, Sripada et al. (2012) found that veterans with PTSD had stronger functional connectivity between the insula and other regions of the salience network, including both the amygdala and hippocampus, compared to veterans without PTSD. These studies, combined with our present findings, suggest that aberrant functions and abnormal roles of insula and IFC are potential markers of PTSD in both adults and youth. We failed to replicate the findings by Teicher et al. (2014) who reported larger centrality in right insula in adults who previously experienced childhood maltreatment compared to non-maltreated controls. This may be due to differences in the age of participants or differences in the statistical analyses between the two studies. The inferior frontal cortex (especially the right IFC) plays a critical role in response inhibition (Hampshire et al., 2010) and cognitive control over emotional responses (Etkin et al., 2015; Wager et al., 2008). Impaired abilities in fear inhibition (Jovanovic et al., 2010), emotion regulation for negative events (Shepherd and Wild, 2014), and response inhibition for non-emotional stimuli (Swick et al., 2012) have been observed in people with PTSD. Functional neuroimaging studies have also reported reduced activation in right inferior frontal gyrus during an inhibitory control task in veterans with PTSD as compared to combat-exposed controls (van Rooij et al., 2014). Future studies are required to uncover the specific role of insula and inferior frontal cortex in brain networks associated with pediatric maltreatment, with and without PTSD.
We found smaller centrality in the right temporal pole (TP) for both maltreated youth with and without PTSD compared to controls. The TP is involved in several socially relevant cognitive processes, particularly theory of mind (Molenberghs et al., 2016), which reflects the ability of an individual to attribute mental states to others. Childhood maltreatment may be associated with reductions in social cognitive ability as well as related disruptions to brain networks involving the TP. Consistent with this, impaired theory of mind (Nazarov et al., 2014) and decreased altruism (Nazarov et al., 2016) have been reported in women with PTSD stemming from childhood trauma. In addition, cortical thickness is lower in the left TP in adolescents exposed to physical and/or sexual abuse (Gold et al., 2016). Interestingly, we detected the between-group difference in the right TP, while Teicher et al. (2014) reported that adults exposed maltreatment have smaller centrality in the left TP compared to control subjects with no history of trauma exposure. The left TP is frequently recruited during language processing (Tsapkini et al., 2011), while right TP recruitment has been associated with emotional empathy (Hillis, 2014). The differences between our results and prior findings may reflect development-related changes in TP, and should be further investigated in longitudinal studies.
4.1. Limitations
There are a number of limitations of this study. Firstly, subcortical regions known to be affected by maltreatment, such as amygdala and hippocampus (Lim et al., 2014; Morey et al., 2016), were not considered in the present study, given that our analyses were based exclusively on the large-scale covariance in cortical thickness. Both cortical thickness and subcortical volumes were involved in structural network analyses in some recent studies (Mueller et al., 2015). However, the validity of this method requires further testing. Secondly, the relationship between structural network characteristics and behavioral performance/demographic information is hard to calculate. This limitation is inherent to the cortical network analyses methods because only one network is formed for each group. Network analyses based on the other types of neuroimaging data, such as resting-state functional magnetic resonance imaging, are derived from inter-regional relationships across measures within each subject and may complement our knowledge of the correlations between individual differences and network topology. Thirdly, our sample size is relatively small compared with some previous studies (He et al., 2008; Yao et al., 2010), particularly for maltreated youth with PTSD. However, it is difficult to recruit a large sample of maltreated youth who are medically healthy and non-medicated due to co-occurrence of confounding factors such as prenatal substance exposure, alcohol and substance dependence, use of psychotropic medications, and medical illness (Smith et al., 2007). Future collaborations among several research teams studying childhood maltreatment and PTSD with standardized methods across sites will greatly increase sample sizes, which will generate more robust and replicable findings. Fourth, our data were collected at multiple sites using different acquisition parameters and experimental procedures. Similar measures were used to assess maltreatment across sites, and participants from all three groups were recruited from both sites. Nevertheless, PTSD prevalence was not equivalent between sites, and site-specific differences in PTSD measures, scanner characteristics, and other sample differences could contribute to the between-group differences. Additionally, heterogeneity in assessments of psycho-pathology precluded a more complete examination of these differences between groups, which could influence structural covariance. Therefore, to control for these effects, study site was included as a regressor of no interest.
5. Conclusions
Here, we investigated the brain topological centrality associated with childhood maltreatment and PTSD in youth by applying the structural covariance network method based on correlations in cortical thickness. Larger centrality in the left ACC reflects PTSD-specific topology following maltreatment. Maltreated youth with PTSD also showed smaller centrality in the right OFC compared to maltreated youth without PTSD, and larger centrality in the right insula and inferior frontal cortex compared to trauma-exposed controls. Moreover, both maltreated youth with and without PTSD exhibited smaller centrality in the right temporal pole than controls. Longitudinal research will help determine how brain network changes influence the risk and resilience to PTSD following childhood maltreatment.
Supplementary Material
Acknowledgments
This research was supported by the U.S. Department of Veterans Affairs (VA) Mid-Atlantic Mental Illness Research, Education, and Clinical Center (MIRECC) core funds. Dr. Morey also received financial support from the US Department of Veterans Affairs (VA) Office of Research and Development (5I01CX000748-01, 5I01CX000120-02). Additional financial support was provided by the National Institute of Neurological Disorders and Stroke (R01NS086885-01A1).
Footnotes
Financial disclosure: The authors declare no conflict of interest or financial disclosures.
Appendix A. Supplementary data: Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jpsychires.2017.12.015.
References
- Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 2008;28:9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, Worsley KJ, Besson P, Concha L, Lerch JP, Evans AC, et al. Mapping limbic network organization in temporal lobe epilepsy using morphometric correlations: insights on the relation between mesiotemporal connectivity and cortical atrophy. Neuroimage. 2008;42:515–524. doi: 10.1016/j.neuroimage.2008.04.261. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the childhood trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Psychiatr. 1997;36:340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Bifulco A, Brown GW, Lillie A, Jarvis J. Memories of childhood neglect and abuse: corroboration in a series of sisters. J Child Psychol Psyc. 1997;38:365–374. doi: 10.1111/j.1469-7610.1997.tb01520.x. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatr. 1999;38(10):1230–1236. 1. doi: 10.1097/00004583-199910000-00011. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Zisk A. The biological effects of childhood trauma. Child and adolescent psychiatric clinics of North America. 2014;23:185–222. doi: 10.1016/j.chc.2014.01.002. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53:1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Fair DA, Kelly C, Satterthwaite TD, Castellanos FX, Thomason ME, et al. Unraveling the miswired connectome: a developmental perspective. Neuron. 2014;83:1335–1353. doi: 10.1016/j.neuron.2014.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. An Introduction to the Bootstrap. xvi. Chapman & Hall; New York: 1993. p. 436. [Google Scholar]
- Etkin A, Buchel C, Gross JJ. The neural bases of emotion regulation. Nat Rev Neurosci. 2015;16:693-+. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- Evsyukova I, Plestant C, Anton ES. Integrative mechanisms of oriented neuronal migration in the developing brain. Annu Rev Cell Dev Biol. 2013;29:299–353. doi: 10.1146/annurev-cellbio-101512-122400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Tone EB, Phifer J, Norrholm SD, Bradley B, Ressler KJ, et al. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol Med. 2012;42:533–543. doi: 10.1017/S0033291711001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AL, Sheridan MA, Peverill M, Busso DS, Lambert HK, Alves S, et al. Childhood abuse and reduced cortical thickness in brain regions involved in emotional processing. Journal of Child Psychology and Psychiatry. 2016;57:1154–1164. doi: 10.1111/jcpp.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong GL, He Y, Chen ZJ, Evans AC. Convergence and divergence of thickness correlations with diffusion connections across the human cerebral cortex. Neuroimage. 2012;59:1239–1248. doi: 10.1016/j.neuroimage.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington T. The Clinician-administered PTSD Scale for Children and Adolescents. A Validation Study The University of Tulsa; 2008. [Google Scholar]
- Hart H, Rubia K. Neuroimaging of child abuse: a critical review. Front Hum Neurosci. 2012;5(52):6. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings TL, Kelley ML. Development and validation of the screen for adolescent violence exposure (SAVE) J Abnorm Child Psychol. 1997;25:511–520. doi: 10.1023/a:1022641916705. [DOI] [PubMed] [Google Scholar]
- He Y, Chen Z, Evans A. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer's Disease. J Neurosci. 2008;28:4756–4766. doi: 10.1523/JNEUROSCI.0141-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Dagher A, Chen Z, Charil A, Zijdenbos A, Worsley K, et al. Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain. 2009;132:3366–3379. doi: 10.1093/brain/awp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Evans A. Graph theoretical modeling of brain connectivity. Curr Opin Neurol. 2010;23:341–350. doi: 10.1097/WCO.0b013e32833aa567. [DOI] [PubMed] [Google Scholar]
- Hillis AE. Inability to empathize: brain lesions that disrupt sharing and understanding another's emotions. Brain. 2014;137:981–997. doi: 10.1093/brain/awt317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston SM, Herting MM, Sowell ER. The neurobiology of childhood structural brain development: conception through adulthood. Curr Top Behav Neurosci. 2014;16:3–17. doi: 10.1007/7854_2013_265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LM, Strom TQ, Leskela J. Risk-taking behaviors and impulsivity among veterans with and without PTSD and mild TBI. Mil Med. 2014;179:357–363. doi: 10.7205/MILMED-D-13-00241. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, et al. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety. 2010;27:244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay CL, Green JM. Social cognitive deficits and biases in maltreated adolescents in U.K. out-of-home care: relation to disinhibited attachment disorder and psychopathology. Dev Psychopathol. 2016;28:73–83. doi: 10.1017/S0954579415000292. [DOI] [PubMed] [Google Scholar]
- Kelly PA, Viding E, Wallace GL, Schaer M, De Brito SA, Robustelli B, et al. Cortical thickness, surface area, and gyrification abnormalities in children exposed to maltreatment: neural markers of vulnerability? Biol Psychiatr. 2013;74:845–852. doi: 10.1016/j.biopsych.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Children's Depression Inventory 2™ (CDI 2) 2010 [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, et al. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage. 2006;31:993–1003. doi: 10.1016/j.neuroimage.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Lim L, Radua J, Rubia K. Gray matter abnormalities in childhood maltreatment: a voxel-wise meta-analysis. Am J Psychiat. 2014;171:854–863. doi: 10.1176/appi.ajp.2014.13101427. [DOI] [PubMed] [Google Scholar]
- Marin O, Valiente M, Ge XC, Tsai LH. Guiding neuronal cell migrations. Csh Perspect Biol. 2010;2 doi: 10.1101/cshperspect.a001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Kelly PA, Bird G, Sebastian CL, Mechelli A, et al. Amygdala activation in maltreated children during pre-attentive emotional processing. Br J Psychiatr. 2013;202:269–276. doi: 10.1192/bjp.bp.112.116624. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, et al. Heightened neural reactivity to threat in child victims of family violence. Curr Biol. 2011;21:R947–R948. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, et al. Amygdala response to negative stimuli predicts ptsd symptom onset following a terrorist attack. Depress Anxiety. 2014a;31:834–842. doi: 10.1002/da.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Koenen KC, Hill ED, Petukhova M, Sampson NA, Zaslavsky AM, et al. Trauma exposure and posttraumatic stress disorder in a national sample of adolescents. J Am Acad Child Psychiatr. 2013;52:e814. doi: 10.1016/j.jaac.2013.05.011. 815–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Peverill M, Gold AL, Alves S, Sheridan MA. Child maltreatment and neural systems underlying emotion regulation. J Am Acad Child Psychiatr. 2015;54:753–762. doi: 10.1016/j.jaac.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Lambert HK. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev. 2014b;47:578–591. doi: 10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeer SV, Dixon JF, Henry D, Ruggiero K, Escovitz K, Niedda T, et al. Psychopathology in non-clinically referred sexually abused children. J Am Acad Child Psychiatr. 1998;37:1326–1333. doi: 10.1097/00004583-199812000-00017. [DOI] [PubMed] [Google Scholar]
- McLeer SV, Ruggiero K. Diagnostic stability following termination of child sexual abuse. Scientific Proceedings of the Annual Meeting of the American Academy of Child & Adolescent Psychiatry. 1999;XV:105. [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang YC, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatr. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63(63):129–151. doi: 10.1146/annurev.psych.121208.131631. http://dx.doi.org/10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior Cingulate cortex in fear expression. Biol Psychiatr. 2007;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Johnson H, Henry JD, Mattingley JB. Understanding the minds of others: a neuroimaging meta-analysis. Neurosci Biobehav Rev. 2016;65:276–291. doi: 10.1016/j.neubiorev.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Morey RA, Gold AL, LaBar KS, Beall SK, Brown VM, Haswell CC, et al. Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Arch Gen Psychiatr. 2012;69:1169–1178. doi: 10.1001/archgenpsychiatry.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Haswell CC, Hooper SR, De Bellis MD. Amygdala, Hippocampus, and ventral medial prefrontal cortex volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Neuropsychopharmacol. 2016;41:791–801. doi: 10.1038/npp.2015.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Lancaster SC, Haswell CC. Trauma Re-experiencing symptoms modulate topology of intrinsic functional networks. Biol Psychiatr. 2015;78:156–158. doi: 10.1016/j.biopsych.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Ng P, Neylan T, Mackin S, Wolkowitz O, Mellon S, et al. Evidence for disrupted gray matter structural connectivity in posttraumatic stress disorder. Psychiatr Res Neuroimaging. 2015;234:194–201. doi: 10.1016/j.pscychresns.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Nader K, Kriegler JA, Blake DD, Pynoos RS, Newman E, Weathers FW. Clinician-administered PTSD Scale, Child and Adolescent Version White River Junction. VT: National Center for PTSD; 1996. [Google Scholar]
- Nazarov A, Frewen P, Parlar M, Oremus C, MacQueen G, McKinnon M, et al. Theory of mind performance in women with posttraumatic stress disorder related to childhood abuse. Acta Psychiatr Scand. 2014;129:193–201. doi: 10.1111/acps.12142. [DOI] [PubMed] [Google Scholar]
- Nazarov A, Walaszczyk V, Frewen P, Oremus C, Lanius R, McKinnon MC. Moral reasoning in women with posttraumatic stress disorder related to childhood abuse. Eur J Psychotraumato. 2016;7 doi: 10.3402/ejpt.v7.31028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Psychiatr. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shepherd L, Wild J. Emotion regulation, physiological arousal and PTSD symptoms in trauma-exposed individuals. J Behav Ther Exp Psychiatr. 2014;45:360–367. doi: 10.1016/j.jbtep.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Bush G, Milad MR, Lasko NB, Brohawn KH, Hughes KC, et al. Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: a monozygotic twin study of posttraumatic stress disorder. Am J Psychiat. 2011;168:979–985. doi: 10.1176/appi.ajp.2011.09121812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Psychobiology of Posttraumatic Stress Disorder: A Decade of Progress. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial pre-frontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatr. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Smith DK, Johnson AB, Pears KC, Fisher PA, DeGarmo DS. Child maltreatment and foster care: unpacking the effects of prenatal and postnatal parental substance use. Child Maltreat. 2007;12:150–160. doi: 10.1177/1077559507300129. [DOI] [PubMed] [Google Scholar]
- Spielberg JM, McGlinchey RE, Milberg WP, Salat DH. Brain network disturbance related to posttraumatic stress and traumatic brain injury in veterans. Biol Psychiatr. 2015;78:210–216. doi: 10.1016/j.biopsych.2015.02.013. [DOI] [PubMed] [Google Scholar]
- Sporns O. Networks of the Brain. xi. MIT Press; Cambridge Mass: 2011. p. 412. 418 pp. of platespp. [Google Scholar]
- Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, et al. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med. 2012;74:904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg AM, Brymer MJ, Kim S, Briggs EC, Ippen CG, Ostrowski SA, et al. Psychometric properties of the UCLA PTSD reaction index: Part I. J Trauma Stress. 2013;26:1–9. doi: 10.1002/jts.21780. [DOI] [PubMed] [Google Scholar]
- Swick D, Honzel N, Larsen J, Ashley V, Justus T. Impaired response inhibition in veterans with post-traumatic stress disorder and mild traumatic brain injury. J Int Neuropsychol Soc. 2012;18:917–926. doi: 10.1017/S1355617712000458. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Ohashi K, Polcari A. Childhood maltreatment: altered network centrality of cingulate, precuneus, temporal pole and insula. Biol Psychiatr. 2014;76:297–305. doi: 10.1016/j.biopsych.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K, Frangakis CE, Hillis AE. The function of the left anterior temporal pole: evidence from acute stroke and infarct volume. Brain. 2011;134:3094–3105. doi: 10.1093/brain/awr050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij SJH, Rademaker AR, Kennis M, Vink M, Kahn RS, Geuze E. Impaired right inferior frontal gyrus response to contextual cues in male veterans with PTSD during response inhibition. J Psychiatr Neurosci. 2014;39:330–338. doi: 10.1503/jpn.130223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA, Unutzer J, Rutter C, Gelfand A, Saunders K, VonKorff M, et al. Costs of health care use by women HMO members with a history of childhood abuse and neglect. Arch Gen Psychiatr. 1999;56:609–613. doi: 10.1001/archpsyc.56.7.609. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Webster MA, Vidaurre D, Nichols TE, Smith SM. Multi-level block permutation. Neuroimage. 2015;123:253–268. doi: 10.1016/j.neuroimage.2015.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf RC, Herringa RJ. Prefrontal-amygdala dysregulation to threat in pediatric posttraumatic stress disorder. Neuropsychopharmacol. 2016;41:822–831. doi: 10.1038/npp.2015.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XD, Brown AD, Lazar M, Cressman VL, Henn-Haase C, Neylan TC, et al. Spontaneous brain activity in combat related PTSD. Neurosci Lett. 2013;547:1–5. doi: 10.1016/j.neulet.2013.04.032. [DOI] [PubMed] [Google Scholar]
- Yao ZJ, Zhang YC, Lin L, Zhou YA, Xu CL, Jiang TZ, et al. Abnormal cortical networks in mild cognitive impairment and Alzheimer's disease. PLoS Comput Biol. 2010;6 doi: 10.1371/journal.pcbi.1001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Zhang K, Sejnowski TJ. A universal scaling law between gray matter and white matter of cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5621–5626. doi: 10.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


