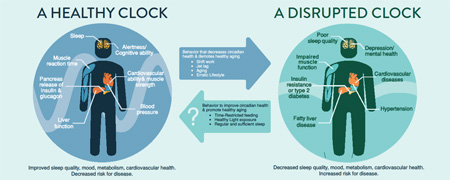
Abstract
Circadian rhythms optimize physiology and health by temporally coordinating cellular function, tissue function, and behavior. These endogenous rhythms dampen with age and thus compromise temporal coordination. Feeding-fasting patterns are an external cue that profoundly influence the robustness of daily biological rhythms. Erratic eating patterns can disrupt the temporal coordination of metabolism and physiology leading to chronic diseases that are also characteristic of aging. However, sustaining a robust feeding-fasting cycle, even without altering nutrition quality or quantity, can prevent or reverse these chronic diseases in experimental models. In humans, epidemiological studies have shown erratic eating patterns increase the risk of disease, whereas sustained feeding-fasting cycles, or prolonged overnight fasting, is correlated with protection from breast cancer. Therefore, optimizing the timing of external cues with defined eating patterns can sustain a robust circadian clock, which may prevent disease and improve prognosis.
Keywords: circadian rhythms, metabolism, time-restricted feeding, aging, health
Graphical Abstract
1.1 Introduction
Nearly all living organisms ranging from archaea to mammals display circadian rhythms. Circadian (circa – approximately; dian – day) rhythms are approximately 24 h oscillations that can be found at the molecular, physiological, and behavioral level (Bell-Pedersen et al., 2005; Edgar et al., 2012; Loudon, 2012; Whitehead et al., 2009). The daily rhythms of sleep and activity and the associated rhythms in metabolic states emerge from a complex interplay of endogenous cell autonomous circadian oscillators, daily exposure to light and darkness, and daily patterns of feeding and fasting. These seemingly simple daily behavioral rhythms tune the function of almost all organ systems: digestive system, metabolic organs, immune system, reproductive system, endocrine systems, cardiovascular system, and several brain regions. The cell autonomous circadian oscillator in mammals is based on interlocked transcription-translation feedback loops. This molecular clock reciprocally regulates the cell’s internal environment including, but not limited to, redox state, NAD+ levels, energy state (ATP/AMP ratio), and Ca2+ levels (Peek et al., 2013).
Oscillators modulate the function of a large number of gene products in a tissue specific manner so that the major function of almost every organ is rhythmic (Dibner et al., 2010). A number of signaling molecules produced from different neuroendocrine tissues display a circadian rhythm in their abundance and/or their cognate receptors show circadian modulation of activity (Gamble, K.L., Berry R., Frank, S.J. and Young, 2015; Hastings et al., 2007). Consequently, synchrony within and between tissues demonstrate daily rhythms. The cell autonomous oscillator itself and a significant portion of the genome in each tissue also indirectly respond to food intake and light/dark cycles in a time of the day specific manner (Asher and Sassone-Corsi, 2015; Rusak et al., 1990). As a result, the circadian system is a master integrator of both the internal state of the organism and the organism’s interaction with nutrition and ambient light. Daily oscillations, from individual cells to the whole organism, likely sub-serve a few central purposes. Oscillations temporally separate incompatible biochemical or physiological processes, optimize energy expenditure (as tonic production of several proteins can be costly), and synchronize function of metabolic pathways to reduce the build-up of toxic intermediates. While the circadian system’s plasticity towards change in ambient lighting or food availability has been an advantage in nature to adapt to different seasons, such plasticity can become a liability in modern society where both light and food are available around the clock.
After the invention of electrical lighting, almost all modern humans voluntarily override this natural mechanism of diurnal rhythm by self-selecting a sleep-wake pattern that suits their schedule, which leads to associated alterations in feeding and fasting. Such chronic disruption of diurnal rhythms can compromise health through multiple discrete mechanisms. Reduced sleep can disrupt metabolic homeostasis by mechanisms that are yet to fully understood (Huang et al., 2011; Sharma and Kavuru, 2010). Light at night suppresses sleep and promotes extended wakefulness, thus allowing ingestive behavior to continue late into the night. This extended period of eating may contribute to increased caloric intake that often correlates with modern human lifestyle. Furthermore, eating at a sub-optimal time of the 24 h day can promote excessive energy storage instead of expenditure. Nutrition quality can also impact hunger, satiety, and hedonic drive for food intake and thereby affect daily eating pattern, which in turn can impact the robustness of circadian oscillators in various organs. Chronic circadian disruption due to erratic lifestyle or shift work compromises health and increase the risk of several chronic diseases that are associated with aging (Castanon-Cervantes et al., 2010; Maywood et al., 2006; Qian and Scheer, 2016; Scheer et al., 2009). Conversely, recent work has shown maintaining a defined daily feeding-fasting rhythm, as in time-restricted feeding (TRF), can prevent or attenuate several of these chronic diseases (Chaix et al., 2014). In this review we will discuss the general organization of the circadian system, its role in physiology and metabolism, how these rhythms change with age, and how eating pattern affects circadian regulation. For most of the article, we will focus our discussion largely on rodent models with occasional examples from other model organisms and humans.
1.2 Circadian Clock Machinery
Almost every cell in the body has a clock, each with an approximately 24 h period. However, in the absence of a coordinating signal, small period differences between oscillators leads to desynchrony (Welsh et al., 1995). The suprachiasmatic nucleus (SCN) in the hypothalamus acts as a master clock to coordinate these independent oscillators throughout the body and determine the period of the organism (Guo et al., 2006; Ralph et al., 1990). Unlike peripheral oscillators, the SCN is composed of a network of neurons with intricate intercellular communication (Welsh et al., 2010) to produce robust outputs through both neural and humoral cues (LeSauter and Silver, 1998).
In addition to this internal regulation, the SCN also receives external input, such as light, to help an organism coordinate with their environment. Nutrient consumption also has a large influence on biological rhythms, but has a more direct effect on peripheral oscillators than the SCN. Together, light and nutrients coordinate internal biological rhythms with the environment (Castillo et al., 2004; Daan and Pittendrigh, 1976; Damiola et al., 2000; Emery et al., 1998; Rusak et al., 1990; Schibler et al., 2003; Stokkan et al., 2001).
1.2.1 The Molecular Clock and Transcriptional Regulation
The mammalian molecular clock is comprised of interlocked transcriptional and translational feedback loops. BMAL1 and CLOCK (or NPAS2) heterodimerize and bind to an E-box motif of Period1 (Per1), Per2, Cryptochrome 1 (Cry1), Cry2, Rev-Erba, RORa (Retinoic acid-related orphan nuclear receptors), and other clock controlled genes to drive transcription. As the protein levels of PER and CRY rise, they form heterodimers that deactivate the CLOCK/BMAL1 complex, thus inhibiting their own transcription. REV-ERBα and RORα provide additional regulation by acting on RORE (or RRE, retinoic acid related orphan receptor response element) in the Bmal1 promoter to respectively repress or activate the transcription of Bmal1. Additional layers of post-translational regulation, such as CK1ε/δ and AMPK phosphorylation of PER and CRY, respectively, also play a large role in determining the period the clock (Lamia et al., 2009; Lee et al., 2001). The duration of one full loop is the period of the clock.
1.2.2 Clock Controlled Genes
The molecular clock also regulates the transcription of thousands of clock controlled genes (CCGs) either directly by the CLOCK/BMAL complex binding to an E-box of a promoter and REV-ERB or ROR binding to an RRE of a promotor, or indirectly through other clock output proteins (Bozek et al., 2009; Hirayama and Sassone-Corsi, 2005; Hunt and Sassone-Corsi, 2007). 7–13% of genes are under circadian control (Martino et al., 2004; McCarthy et al., 2007; Storch et al., 2002; Zhang et al., 2014). Additionally, a large number of proteins show daily rhythms, while their respective mRNAs do not (Neufeld-Cohen et al., 2016; Reddy et al., 2006; Robles et al., 2014). These clock controlled genes are involved in a wide range of cellular processes including cell cycle control, inflammation, and metabolism.
Cell cycle regulators c-Myc, Cyclin-D1, and Wee-1 are rhythmically expressed. Wee-1 encodes a kinase that phosphorylates the CDC2/Cyclin-B1 complex, which regulates entry into mitosis and is directly regulated by the CLOCK/BMAL complex (Hirayama and Sassone-Corsi, 2005; Hunt and Sassone-Corsi, 2007; Matsuo et al., 2003). Circadian arrhythmic mutant mice demonstrate impaired cell-cycle control in vivo (Matsuo et al., 2003). Thus, through temporal transcriptional control, the clock is able to regulate the timing of the cell cycle. Such circadian gating of cell cycle has practical implication for timing of surgery, radiation therapy, and administration of cell cycle inhibitor drugs for cancer treatments (Dallmann et al., 2016; Levi and Schibler, 2007; Ortiz-Tudela et al., 2016). The clock also regulates inflammation, which is a common cause of metabolic disorders and cancer. CRY regulates the expression of pro-inflammatory cytokines (Narasimamurthy et al., 2012) and chronic behavioral circadian disruption in mice leads to an increase in inflammation (Castanon-Cervantes et al., 2010). Furthermore, mice that lack the circadian gene Per2 exhibit increased incidence of cancer and tumor growth (Fu et al., 2002). Together these studies demonstrate the interconnected and wide reaching role of the circadian clock.
1.3 Diurnal Regulation of Metabolism
Various diseases of aging are associated with metabolic disruption. The anabolic and catabolic metabolism of fat, glucose, cholesterol, and xenobiotics are diurnally regulated by both the endogenous circadian clock and feeding-fasting pattern. These regulatory mechanisms influence metabolism at multiple levels including metabolite concentration, the endocrine system, and the microbiome.
1.3.1 Metabolites
Metabolite production and regulation are under direct (CLOCK/BMAL transcriptional activation) and indirect (CCGs) circadian regulation. Nicotinamide phosphoribosyltransferase (NAMPT) is a clock controlled gene that acts as a rate limiting enzyme for salvage of nicotinamide adenine dinucleotide (NAD+), a metabolite involved in ATP synthesis and oxidation-reduction reactions (Ramsey et al., 2009). NAD+ also modulates activity of protein deacetylases, sirtuins (SIRT), which regulates metabolic enzymes. SIRT1 and NAD both feedback on the core clock to regulate CLOCK/NPAS2, creating an auxiliary feedback loop to further temporally regulate metabolism. SIRT1 feedback plays an important role in clock maintenance as hepatocytes from SIRT1 deficient mice exhibit dysregulated circadian rhythms similar to aged WT mice (Wang et al., 2016). Multiple mitochondrial rate limiting enzymes that are critical parts of the pyruvate metabolism and fatty acid uptake and oxidation are also rhythmically expressed. In circadian mutant mice, enforced feeding-fasting patterns are able to reinstate rhythmic expression of some of these metabolites, such as Acylcarnitine carrier protein and Acyl CoA Dehydrogenase, yet were insufficient to restore rhythms in other enzymes (carnitine palmitoyl transferase 1 and the pyruvate dehydrogenase complex; Manoogian and Panda, 2016; Neufeld-Cohen et al., 2016). These studies illustrate the interaction of nutrient input and circadian control.
1.3.2 The Endocrine System
1.3.2.1 Insulin and Glucagon
The pancreas regulates blood glucose by producing and secreting insulin and glucagon to control release of glucose from the liver when blood glucose levels are too high or too low, respectively. Insulin is produced from β-islet cells in the pancreas, with peak production around 1700 h and a nadir ~0400h in humans (Goel et al., 2009). Temporal control of hormonal production and release is controlled by both feeding-fasting patterns and circadian rhythms. Eating patterns greatly alter nutrient levels in the blood and can therefore act as an acute overriding signal. The circadian system modulates both insulin and glucagon by controlling production and secretion at the cellular level as well as SCN signaling the autonomic nervous system (Kalsbeek et al., 2008; Sadacca et al., 2011; Vieira et al., 2015). In vitro, β-islet cells exhibit robust rhythms of both Bmal1 and Per1 (Mühlbauer et al., 2004; Sadacca et al., 2011). Bmal1−/− in the pancreas had disrupted glucose homeostasis and insulin release despite displaying normal activity and feeding-fasting rhythms (Sadacca et al., 2011). This demonstrates how the molecular clock at the cellular and tissue levels can have significant effects on physiology.
Insulin sensitivity is also influenced by both nutrient state and the clock through SIRT1 (Bass and Takahashi, 2010; Civitarese et al., 2007). Research in both animals and humans has shown that caloric restriction increases levels of SIRT1 (Civitarese et al., 2007). Moreover, mice on an ab lib high-fat diet display decreased SIRT1 levels and impaired insulin sensitivity (Sun et al., 2007).The SCN further regulates blood glucose through the autonomic nervous system. β-islet cells of the pancreas receive parasympathetic input from the ventral PVN, which are circadianly controlled by GABAergic projections from the SCN. Similarly, the SCN has both Glutamatergic and GABAergic projections to the dorsal PVN to modulate sympathetic output to the liver to influence glucose production (Kalsbeek et al., 2008).
1.3.2.2 Cortisol
Cortisol is a steroid hormone in the glucocorticoid family that is involved in metabolism and stress response. Production and secretion of cortisol are rhythmic and regulated by the hypothalamic pituitary axis (HPA) and the autonomic nervous system (ANS). In the HPA axis, corticotropin releasing hormone (CRH) and arginine vasopressin (AVP) are produced in the paraventricular nucleus (PVN) of the hypothalamus. CRH is released from the median eminence and travels to the to the anterior pituitary through the hypophyseal portal system. Hypothalamic AVP neurons project axons directly to the posterior pituitary and release AVP into the circulation. In the anterior pituitary, both CRH and AVP stimulate the release of adrenocorticotropic hormone (ACTH) into the blood. AVP in the adrenal medulla also increases adrenal gland production of ACTH. ACTH acts on the adrenal cortex to stimulate cortisol release. This system is negatively regulated by cortisol in the hypothalamus and the pituitary (Matthews and Challis, 1997). The PVN also sends ANS projections to the intermediolateral column of the spinal cord, which connects to the splanchnic nerve to signal the adrenal gland. Both HPA and ANS regulatory pathways for cortisol are regulated by the SCN at the level of the PVN and upstream in the dorsal medial hypothalamus and the subparaventricular zone. Cells of the adrenal gland also have cellular clocks to temporally influence production and release of cortisol (reviewed in Dickmeis, 2009).
Glucocorticoids are released with both an ultradian (1–2 h) and circadian rhythmicity. Peak levels of glucocorticoids synchronize with the beginning of the active phase to aid in arousal; early morning in diurnal animals and early night in nocturnal animals. Likewise, ACTH exhibits a parallel rhythm as it is also modulated by the SCN (Dickmeis, 2009; Lightman et al., 2008).
Similar to negative feedback on the HPA axis, Glucocorticoids influence circadian rhythms by feeding back on the clock. Glucocorticoids bind to a glucocorticoid response element (GRE) in the Per1/2 and Rev-Erbα/β promoter, to activate and suppress transcription respectively (So et al., 2009; Torra et al., 2000; Yamamoto et al., 2005). Conversely, CRY acts as a transcriptional suppressor of glucocorticoid regulated genes (Lamia et al., 2011).
1.3.3 The Microbiome
The microbiome influences metabolism and can contribute to metabolic disorders and obesity. The microbiome is diverse and exhibits daily oscillations in composition. Diet induced obesity and erratic eating patterns can disrupt and dampen these rhythms. Both behavioral and genetically induced circadian disruption have also been shown to decrease the taxonomic diversity and induce intestinal dysbiosis (Voigt et al., 2016, 2014). However, enforced feeding-fasting patterns can restore some of these oscillations (Zarrinpar et al., 2014).
1.4 Circadian Rhythms Dampen with Age
Circadian rhythms deteriorate with age due to multiple factors. Individual SCN neurons are able to maintain a robust amplitude of core clock gene oscillations in aged mice (Wyse and Coogan, 2010) and in vitro (Welsh et al., 1995), however, there is impaired intercellular coupling within the SCN. Although the total number of neurons within the SCN remains the same in aged rats, the number of neurons that contain vasopressin, a coupling factor for oscillators within the SCN, are decreased (Mieda et al., 2015; Roozendaal et al., 1987). GABA, another important coupling factor within the SCN, also displays impaired signaling with age (Nygård and Palomba, 2006). In response to GABA, aged SCN neurons exhibit a decrease in inhibitory post-synaptic potentials (IPSP) compared to young SCN neurons (Farajnia et al., 2012). This decrease in intercellular communication leads to neuronal desynchrony in the SCN and is a likely cause of the overall decrease in electrical activity of the SCN network (Farajnia et al., 2012; Nygård et al., 2005) and SCN output (Nakamura et al., 2011). The dampening of SCN electrical activity begins a cascade effect. Dampening in both neuronal and humoral outputs results in impaired coordination of peripheral oscillators. Peripheral clocks display a decrease in clock gene amplitude with age. This may be due to disruption of intrinsic molecular clock, impaired temporal coordination of oscillators, or a result of overall impaired physiology associated with aging. The robustness of an organism’s biological rhythms is determined by a combination of external cues and internal rhythms. Therefore, as endogenous rhythms dampen with age, the timing of external cues play an increasingly important role in determining the amplitude of an organism’s circadian clock.
1.5 Circadian Rhythm Disruption
In humans, the most obvious consequence of circadian disruption are observed in activity-rest cycles (Dijk et al., 1999; Farajnia et al., 2012; Huang et al., 2002). Changes are also seen in amplitude and phase of temperature and melatonin rhythms, yet some studies show little to no changes (reviewed by Monk, 2005). Sleep quality and consolidation are also greatly disrupted (Dijk et al., 2001; Farajnia et al., 2012). Genetic, behavioral, and lesion induced circadian disruption or arrhythmicity has been shown to result in a wide range of health consequences including increased risk for cancer, cardiovascular disease, obesity, immune disorders, infertility, and affective disorders (Hastings et al., 2003). Genetically arrhythmic mice (Bmal1−/−) exhibit symptoms of early onset aging, including a decrease in muscle and subcutaneous fat, cataracts, and organ shrinkage (Kondratov et al., 2006). Circadian disruption caused by shift work in humans is associated with cardiovascular disease, metabolic disorders, and cancer (Kamdar et al., 2013; Proper et al., 2016; Vyas et al., 2012). In rodents, transplanting the SCN from a young hamster, to an old hamster with weak behavioral rhythms, was sufficient to not only reinstate robust behavioral rhythms in the older hamster, but also increased lifespan by 4 months (normal lifespan is ~2 years; Hurd and Ralph, 1998; Viswanathan and Davis, 1995). This indicates that aging of the SCN also dictates aging of behavioral rhythms and physiology.
In addition to an increased risk for disease, circadian disruption can also lead to cognitive deficits. Rodent models have demonstrated that chronic circadian disruption induces deficits in hippocampal learning and memory, but not in fear conditioning (Antoniadis et al., 2000; Craig and McDonald, 2008). Circadian disruption caused by keeping mice in a short photoperiod (20 h, 10 h light:10 h dark) inhibited cognition measured by cognitive flexibility tests (Karatsoreos et al., 2011). Circadian disruption, induced by keeping mice in a short photoperiod (20 h, 10 h light:10 h dark, a period too short for a WT mouse to entrain), inhibited cognition measured by cognitive flexibility tests (Karatsoreos et al., 2011). These mice also showed a decrease in dendrite length and neuronal complexity in the prelimbic prefrontal cortex, a region of the brain involved in executive functions and emotion (Karatsoreos et al., 2011). Observations of airline cabin crews have shown that chronic jet lag is correlated with decreased cognition and temporal atrophy (Cho, 2001; Cho et al., 2000). Rodent models of jet lag demonstrated an inhibition of neurogenesis in the hippocampus and long term cognitive deficits (Gibson et al., 2010) and shorted lifespan (Davidson et al., 2006). Memory deficits may be explained by the circadian control of brain-derived neurotrophic factor (BDNF) expression in the dentate gyrus, but not in CA1 or CA3 of the hippocampus (Schaaf et al., 2000). It is unclear if this control is direct from the SCN or indirect through corticosterone suppression (Schaaf et al., 2000). BDNF modulates cell growth and survival and in the hippocampus plays in important role spatial and contextual learning and memory (Hall et al., 2000; Mizuno et al., 2000). Memory formation is dependent on long-term potentiation (LTP) to alter synaptic connections. Increases in BDNF expression led to increases in LTP and Bdnf−/− mice exhibit decreased LTP (Figurov et al., 1996; Korte et al., 1995; Patterson et al., 1996). Deficits in LTP can be rescued in Bdnf−/− mice with recombinant BDNF (Patterson et al., 1996). As seen in rodents with circadian disruption, dentate gyrus specific knockdown of neurogenesis in mice impairs spatial memory and object recognition (Jessberger et al., 2009). BDNF is also decreased in the hippocampus of individuals with Alzheimer’s Disease (Phillips et al., 1991).
1.5.1 Circadian Disruption and Neurodegenerative Disease
There many correlations between circadian disruption and neurodegenerative diseases such as Alzheimer’s Disease (AD) and Parkinson’s Disease. However, the causal relationship is unclear. As circadian rhythms have been shown to play a role in neurogenesis (Borgs et al., 2009; Gibson et al., 2010; Tamai et al., 2008), the dampening of circadian rhythms with age is likely to contribute neurodegenerative diseases such as Alzheimer’s disease (AD).
The amyloid hypothesis of AD proposes that excess production and accumulation of amyloid β peptide (Aβ) begins a cascade effect that damages the synapse and neurites and leads to neurofibrillary tangles containing tau protein (Hardy and Selkoe, 2002). Prior to aggregation, there are daily fluctuations in Aβ soluble interstitial fluid (ISF) controlled by the sleep-wake cycle. Levels of Aβ in the ISF correlates with the amount of future Aβ aggregation. After Aβ plaques are formed, diurnal rhythms of both the sleep-wake cycle and fluctuations in ISF Aβ are diminished (Roh et al., 2012). In humans, AD patients exhibit a decreased amplitude in activity patterns and a delay in peak activity and body temperature (Volicer et al., 2001). There was also a correlation between circadian disruptions in locomotor activity and “sundowning” (an exacerbation of AD symptoms in the afternoon). This is also associated with a delayed peak of body temperature in the afternoon (Volicer et al., 2001). Further circadian disruption in AD patients can be seen in the SCN. In humans, SCN neurons expressing vasoactive intestinal peptide (VIP; another important intercellular coupling factor in the SCN) were significantly lower in females with AD compared to healthy age-matched controls. Interestingly, this effect was not seen in males. However, young males had more VIP neurons in the SCN compared to older males, whereas young females had less VIP expression compared to healthy older females (Zhou et al., 1995). Although it is unclear if AD leads to circadian disruption or vice versa, some behavioral modifications using bright light exposure have been shown to restore some behavior rhythms in patients with AD and dementia (Ancoli-Israel et al., 2003; Van Someren et al., 1996). These studies indicate that low amplitude circadian rhythms contributes to the AD and dementia pathology.
1.6 Eating Pattern and Health
Extensive reciprocal regulation between the circadian clock and nutrient metabolism (reviewed in Asher and Sassone-Corsi, 2015) suggests that daily eating pattern can affect the amplitude and phase of circadian rhythms. There are a variety of eating patterns that concern ‘fasting’ that are frequently thought of together, including intermittent fasting (IF), periodic fasting (PF), caloric restriction (CR), and time-restricted feeding (TRF). IF, PF, and CR are all based on overtly reducing calories on various timelines. TRF is the only eating pattern that does not require calorie reduction. TRF is based on circadian biology to allow the body a true daily fasting period in which only water is allowed (anything that is more than 5 calories or contains caffeine or artificial sweeteners are excluded).
1.6.1 Time-Restricted Feeding in Rodents
Laboratory mice with ad libitum access to a standard diet typically consume a majority (60–80%) of their daily food intake at nighttime. “Time-restricted feeding” or “restricted feeding” is a term often used in the field of circadian rhythms to examine the role of timing of food access on the circadian clock. In these experiments timing of food access is restricted to anywhere between 2–12 h during the day or night and the effect of this time-restricted access to food on the circadian clock or clock controlled genes are assessed. Within a few days, rodents learn to anticipate the timing of food arrival and consume a large meal during the time-window of food availability. If the period of food access is <6 h, animals cannot eat equivalent amount of food as their ad lib feed (ALF) counterparts. However, with food access of >8 h, they consume an almost equal amount of calories as the ALF cohort. Hence a TRF paradigm, where food access is >8 h is a powerful method to examine the effect of time of food availability independent of nutrition quality or quantity on the circadian clock and animal’s health.
TRF paradigm in combination with high-fat diet has been powerful in elucidating the effect of eating pattern on the prevention and treatment of metabolic diseases. Diet induced obesity (DIO) is a widely used experimental model of obesity, diabetes, and several metabolic diseases in which animals have ad lib access to a diet rich in fat (>11,000 papers in PubMed as of December 2016). After 8–12 weeks, mice on high-fat diet become obese, hence the name. This simple model of obesity without genetic perturbation works well in several strains of mice. Although the metabolic disruption in the DIO model is often ascribed to the high fat diet, the diet also changes the daily eating pattern of mice, such that they continuously snack on the high-fat diet throughout day and night (Pendergast et al., 2013). Such random eating pattern disrupts the circadian oscillator in metabolic organs including the liver. Since genetic and environmental disruption of circadian rhythms, as in shiftwork, perturbs metabolic homeostasis and predisposes to metabolic diseases, disrupted circadian rhythms likely contributes to the disease in the in DIO model. When mice on high-fat diet are granted access to food for 8, 9, 12, or 15 h during the night time, they consume the same amount of total daily calories as the ALF counterparts. However, they are largely protected from several metabolic diseases. Mice with pre-existing obesity due to ALF on a high-fat diet also benefit from the therapeutic effect of TRF (Chaix et al., 2014; Chaix and Zarrinpar, 2015; Sherman et al., 2012).
In mice, TRF exerts pleiotropic effects on multiple organ systems including but not limited to liver, muscle, white adipose tissue, brown adipose tissue, and gut (Zarrinpar et al., 2015). In depth analyses of TRF has largely been done in the liver. Overall TRF exerts profound effect on hepatic gene expression and metabolites (Hatori et al., 2012). Nearly 40% of named metabolites detected in liver change significantly between a DIO and TRF liver. Among them, the largest clusters of metabolites belong to sugar and fat/cholesterol metabolites. Many of these changes correlate with changes in the expression level of corresponding metabolic regulators. In the liver, TRF affects the daily dynamics of major nutrient sensing pathways including CREB, mTOR and AMPK, and thereby impacts their downstream genes or protein products. In DIO liver, the daily rhythm in pCREB and pS6 (readout of mTOR activity) is blunted leading to constitutively elevated pCREB and reduced pS6 levels. In contrast, TRF restores the daytime peak in pCREB and nighttime peak in pS6. The reduction of pCREB in nighttime likely reduces liver gluconeogenesis, while mTOR activation at night can promote glucose utilization in pentose phosphate pathway. Mice on TRF experience extended daily fasting, which likely increases the hepatic AMP levels relative to that seen in the DIO livers. AMP can allosterically activate AMPK, which phosphorylates and deactivates one of the rate-limiting enzymes of fatty acid oxidation, acetyl CoA carboxylase (ACC). Both CREB and AMPK are known to promote the transcription of Per genes and degradation of CRY proteins respectively. These functions likely underlie the effect of TRF on improving the amplitude of oscillation of circadian clock components including that of Bmal1 and Rev-erb. Increased Bmal1 levels correlate with increased expression of its target genes Umps and Tk1, which constitute the rate limiting steps in nucleotide metabolism. Accordingly, TRF livers also display increased levels of nucleotides (Hatori et al., 2012).
DIO livers exhibit elevated level of PPARγ, which is known to promote fatty acid synthesis, elongation, and desaturation. TRF reduces PPARγ expression, which parallels a reduction in long chain fatty acid and unsaturated fatty acids. The combined action of increased fatty acid oxidation and reduced fatty acid synthesis leads to >50% reduction in liver fatty acids and nearly complete absence of fat droplets in hepatocytes. This underlies the reduction in fatty liver disease and liver fibrosis in TRF mice. TRF mice also show a mildly elevated level of ketone bodies, which is now linked to several benefits in metabolism and central nervous system function (Akram, 2013; D’Agostino et al., 2013).
Hepatic fatty acid metabolism is closely linked to cholesterol and bile acid homeostasis. Both feeding and the clock component Rev-erbα participate in the daily production of cholesterol and bile acids through transcriptional regulation of the lipid regulator Srebp1c and several rate-limiting enzymes including Hmgcs2 and Cyp7a1 (Cho et al., 2012; Le Martelot et al., 2009). Distinct feeding-fasting rhythms along with improved Rev-erbα rhythms in the livers of TRF mice corrects the phase of expression of Hmgcs2 and Srebp1c, while increasing the peak levels of Cyp7a1. Elevated Cyp7a1 transcript, which encodes the rate-limiting step in bile acid production from cholesterol, increases hepatic bile acids and contributes to a decrease in serum cholesterol levels in the TRF mice. Increased bile acids can enter the general circulation and activate uncoupling protein 1 (UCP1) mediated energy expenditure in brown adipose tissue by acting through TGR5. In fact, the BAT of TRF mice increased UCP expression, increased mitochondria content, and TRF mice increased oxygen consumption (Hatori et al., 2012).
TRF also alters WAT function and inflammation. In general, the size of adipocyte, macrophage infiltration of WAT, and inflammatory cytokine production are reduced and mitochondria content is increased in the WAT (Hatori et al., 2012).
Benefits of TRF are also seen in mice on other diets including high fructose diet, high fat + high sucrose diet, or a standard diet (Chaix et al., 2014). Mice on a standard diet do not show much body weight loss, rather they show a significant change in body composition under long term TRF of >26 weeks; increased lean mass, and reduced fat mass. ALF mice on a standard diet develop some liver fibrosis with age, which is prevented by TRF. TRF does not profoundly elevate the basal activity level in rodents, but it has an interesting effect on endurance as measured by running on a treadmill. Even after controlling for body weight, DIO mice run for a significantly shorter duration, while TRF mice with access to high-fat diet for 8 or 9 h run >40% longer than the normal chow ALF controls. However, this added endurance is not present in TRF mice with 12 h access to high-fat diet, even though they have comparable adiposity as mice with 8 h access to food (Chaix et al., 2014).
The timing of TRF with relation to day or night seems to have some effect on metabolism. While TRF mice are always leaner than their ALF DIO counterparts, mice with daytime access to high-fat diet (day TRF) fare worse than the night TRF. The underlying changes in metabolism between day- and night-TRF are an exciting area for future investigation. Such difference between day- and night-TRF is exacerbated with high-fat diet, while no such significant difference is generally reported with standard diet (Chaix et al., 2014).
It is worth noting that many caloric restriction studies in rodents inadvertently involve a component of time-restricted access to diet. While many CR studies in rodents are controlled for time of delivering the control standard diet and the reduced caloric diet at a specific time of the morning or evening, some studies are vague about the timing. Nevertheless, CR studies in rodents, irrespective of when the food was delivered usually report positive health outcomes (Lee and Longo, 2016).
1.6.2 Time-Restricted Feeding in Drosophila
While rodents are nocturnal animals, Drosophila are diurnal and they also have a circadian oscillator that coordinates metabolism, physiology and behavior around the clock. With short lifespan and well-established genetic resources, Drosophila offer a powerful model to test TRF on age related pathologies. TRF in Drosophila is done with 12 h access to food during the daytime and access to only 1% agar at night to maintain humidity. TRF flies, like their rodent counterparts, consume the same amount of calories as their ALF cohorts, yet they do not gain body weight and their total activity remains equivalent. As flies age, the diurnal pattern of activity and sleep dampens as in humans. Sleeping duration at night is decreased and increased during the day. However, flies on TRF displayed sustained nocturnal sleep, nearly doubling total sleep duration of the ALF controls. Flies, like humans, also exhibit age dependent deterioration of cardiac function as reflected in increased arrhythmia. Flies on a 12 h TRF show reduced rate of cardiac aging as their heart function is roughly equivalent to cardiac function of ALF flies that are 2 weeks younger. Gene expression profiling of head, body, and heart demonstrated that overnight fasting in flies does not trigger a gene expression signature characteristic of caloric restriction (Gill et al., 2015).
Taken together these studies demonstrate the potential for simple behavior modifications to enhance the biological clock and rhythmic gene expression to optimize an individual’s health.
1.7 Conclusion
Circadian rhythms are an integral part of physiology that seem to be essential for health. Erratic lifestyle associated with modern society (aberrant eating and sleep patterns, inappropriate light exposure, jet-lag, and shift work) contribute to circadian rhythm disruption. This disruption compromises multiple levels of physiology (ex. metabolism, inflammation) and increases the risk for non-infectious chronic diseases such as metabolic disorder, diabetes, cardiovascular disease, and cancer. Unfortunately, biological rhythms naturally dampen with age, which may contribute to a wide variety of age related diseases. Although further research is still needed to determine the optimal eating duration and efficacy of time-restricted feeding in humans, research in rodents suggests that maintaining a regular daily feeding-fasting pattern may be sufficient to restore robust circadian rhythms to optimize an individual’s physiology and decrease their risk of disease to extend healthspan.
Highlights.
Internal circadian rhythms play a large role in physiology and overall health.
Circadian rhythms naturally dampen with age.
Lifestyle (eating and sleeping pattern) can promote or disrupt circadian rhythms.
Disrupted circadian rhythms compromise health and increase the risk of disease.
Feeding/fasting patterns may restore robust circadian rhythms and improve health.
Acknowledgments
Funding
Research in SP lab is supported in part by funding from the NIH (EY016807, CA014195) Glenn Foundation for Aging Research, American Foundation for Aging Research, Helmsley charitable trust, World Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akram M. A Focused Review of the Role of Ketone Bodies in Health and Disease. J. Med. Food. 2013;0:1–3. doi: 10.1089/jmf.2012.2592. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Gehrman P, Martin JL, Shochat T, Marler M, Corey-Bloom J, Levi L. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients. Behav. Sleep Med. 2003;1:22–36. doi: 10.1207/S15402010BSM0101_4. [DOI] [PubMed] [Google Scholar]
- Antoniadis EA, Ko CH, Ralph MR, McDonald RJ. Circadian rhythms, aging and memory. Behav. Brain Res. 2000;111:25–37. doi: 10.1016/s0166-4328(00)00145-5. [DOI] [PubMed] [Google Scholar]
- Asher G, Sassone-Corsi P. Time for food: The intimate interplay between nutrition, metabolism, and the circadian clock. Cell. 2015;161:84–92. doi: 10.1016/j.cell.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat. Rev. Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgs L, Beukelaers P, Vandenbosch R, Nguyen L, Moonen G, Maquet P, Albrecht U, Belachew S, Malgrange B. Period 2 regulates neural stem/progenitor cell proliferation in the adult hippocampus. BMC Neurosci. 2009;10:30. doi: 10.1186/1471-2202-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozek K, Relógio A, Kielbasa SM, Heine M, Dame C, Kramer A, Herzel H. Regulation of clock-controlled genes in mammals. PLoS One. 2009:4. doi: 10.1371/journal.pone.0004882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. J. Immunol. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo MR, Hochstetler KJ, Tavernier RJ, Greene DM, Bult-Ito A. Entrainment of the master circadian clock by scheduled feeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R551–R555. doi: 10.1152/ajpregu.00247.2004. [DOI] [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A. The effects of time-restricted feeding on lipid metabolism and adiposity. Adipocyte. 2015;4:319–324. doi: 10.1080/21623945.2015.1025184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20:991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong L-W, DiTacchio L, Atkins AR, Glass CK, Liddle C, Auwerx J, Downes M, Panda S, Evans RM. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485:123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. Chronic “jet lag” produces temporal lobe atrophy and spatial cognitive deficits. Nat. Neurosci. 2001;4:567–568. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- Cho K, Ennaceur A, Cole JC, Suh CK. Chronic Jet Lag Produces Cognitive Deficits. J. Neurosci. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-06-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:485–494. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig LA, McDonald RJ. Chronic disruption of circadian rhythms impairs hippocampal memory in the rat. Brain Res. Bull. 2008;76:141–151. doi: 10.1016/j.brainresbull.2008.02.013. [DOI] [PubMed] [Google Scholar]
- D’Agostino DP, Pilla R, Held HE, Landon CS, Puchowicz M, Brunengraber H, Ari C, Arnold P, Dean JB. Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;304:R829–R836. doi: 10.1152/ajpregu.00506.2012. [DOI] [PubMed] [Google Scholar]
- Daan S, Pittendrigh CS. A Functional analysis of circadian pacemakers in nocturnal rodents - II. The variability of phase response curves. J. Comp. Physiol. A. 1976;106:253–266. [Google Scholar]
- Dallmann R, Okyar A, Lévi F. Dosing-Time Makes the Poison: Circadian Regulation and Pharmacotherapy. Trends Mol. Med. 2016 doi: 10.1016/j.molmed.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Damiola F, Le Minli N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Sellix MT, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Curr. Biol. 2006;16:R914–R916. doi: 10.1016/j.cub.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annual review of physiology. 2010 doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- Dickmeis T. Glucocorticoids and the circadian clock. J. Endocrinol. 2009 doi: 10.1677/JOE-08-0415. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Czeisler Ca. Age-related increase in awakenings: impaired consolidation of nonREM sleep at all circadian phases. Sleep. 2001;24:565–577. doi: 10.1093/sleep/24.5.565. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Kiel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J. Physiol. 1999;516:611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RS, Green EW, Zhao Y, van Ooijen G, Olmedo M, Qin X, Xu Y, Pan M, Valekunja UK, Feeney KA, Maywood ES, Hastings MH, Baliga NS, Merrow M, Millar AJ, Johnson CH, Kyriacou CP, O’Neill JS, Reddy AB. Peroxiredoxins are conserved markers of circadian rhythms. Nature. 2012;485:459–464. doi: 10.1038/nature11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P, So WV, Kaneko M, Hall JC, Rosbash M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95:669–679. doi: 10.1016/s0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- Farajnia S, Michel S, Deboer T, vanderLeest HT, Houben T, Rohling JHT, Ramkisoensing A, Yasenkov R, Meijer JH. Evidence for neuronal desynchrony in the aged suprachiasmatic nucleus clock. J. Neurosci. 2012;32:5891–5899. doi: 10.1523/JNEUROSCI.0469-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- Gamble KL, Berry R, Frank SJA, Young ME. Circadian Clock Control of Endocrine Factors. Nat Rev Endocrinol. 2014 Aug;10(8):466–475. doi: 10.1038/nrendo.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Wang C, Tjho S, Khattar N, Kriegsfeld LJ. Experimental “jet lag” inhibits adult neurogenesis and produces long-term cognitive deficits in female hamsters. PLoS One. 2010:5. doi: 10.1371/journal.pone.0015267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, Le HD, Melkani GC, Panda S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science. 2015;347(80):1265–1269. doi: 10.1126/science.1256682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Stunkard AJ, Rogers NL, Van Dongen HPA, Allison KC, O’Reardon JP, Ahima RS, Cummings DE, Heo M, Dinges DF. Circadian rhythm profiles in women with night eating syndrome. J. Biol. Rhythms. 2009;24:85–94. doi: 10.1177/0748730408328914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Guo H, Brewer JM, Lehman MN, Bittman EL. Suprachiasmatic regulation of circadian rhythms of gene expression in hamster peripheral organs: effects of transplanting the pacemaker. J. Neurosci. 2006;26:6406–6412. doi: 10.1523/JNEUROSCI.4676-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat. Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hastings M, O’Neill JS, Maywood ES. Circadian clocks: Regulators of endocrine and metabolic rhythms. J. Endocrinol. 2007 doi: 10.1677/JOE-07-0378. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat. Rev. Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JAJ, Ellisman MH, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama J, Sassone-Corsi P. Structural and functional features of transcription factors controlling the circadian clock. Curr. Opin. Genet. Dev. 2005;15:548–556. doi: 10.1016/j.gde.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J. Clin. Invest. 2011 doi: 10.1172/JCI46043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Liu R, Wang Q, Someren EJW, Van, Xu H, Zhou J. Age-associated difference in circadian sleep – wake and rest – activity rhythms. Physiol. Behav. 2002;76:597–603. doi: 10.1016/s0031-9384(02)00733-3. [DOI] [PubMed] [Google Scholar]
- Hunt T, Sassone-Corsi P. Riding Tandem: Circadian Clocks and the Cell Cycle. Cell. 2007;129:461–464. doi: 10.1016/j.cell.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Hurd MW, Ralph MR. The significance of circadian organization for longevity in the golden hamster. J. Biol. Rhythms. 1998;13:430–436. doi: 10.1177/074873098129000255. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn. Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Foppen E, Schalij I, Van Heijningen C, van der Vliet J, Fliers E, Buijs RM. Circadian control of the daily plasma glucose rhythm: An interplay of GABA and glutamate. PLoS One. 2008:3. doi: 10.1371/journal.pone.0003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamdar BB, Tergas AI, Mateen FJ, Bhayani NH, Oh J. Night-shift work and risk of breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2013;138:291–301. doi: 10.1007/s10549-013-2433-1. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc. Natl. Acad. Sci. 2011;108:1657–1662. doi: 10.1073/pnas.1018375108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, Carrolltt P, Wolf E, Brem G, Thoenent H, Bonhoeffer T. Brain-Derived Neurotrophic. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, Evans RM. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia Ka, Papp SJ, Yu RT, Barish GD, Uhlenhaut NH, Jonker JW, Downes M, Evans RM. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature. 2011;480:552–556. doi: 10.1038/nature10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Martelot G, Claudel T, Gatfield D, Schaad O, Kornmann B, Lo Sasso G, Moschetta A, Schibler U. REV-ERBα participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009:7. doi: 10.1371/journal.pbio.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Etchegaray JP, Cagampang FRA, Loudon ASI, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107:855–867. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- Lee C, Longo V. Dietary restriction with and without caloric restriction for healthy aging. F1000Research. 2016;5:1–7. doi: 10.12688/f1000research.7136.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSauter J, Silver R. Output signals of the SCN. Chronobiol. Int. 1998 doi: 10.3109/07420529808998706. [DOI] [PubMed] [Google Scholar]
- Levi F, Schibler U. Circadian rhythms: mechanisms and therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 2007;47:593–628. doi: 10.1146/annurev.pharmtox.47.120505.105208. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Wiles CC, Atkinson HC, Henley DE, Russell GM, Leendertz JA, McKenna MA, Spiga F, Wood SA, Conway-Campbell BL. The significance of glucocorticoid pulsatility. Eur. J. Pharmacol. 2008 doi: 10.1016/j.ejphar.2007.11.073. [DOI] [PubMed] [Google Scholar]
- Loudon ASI. Circadian biology: A 2.5 billion year old clock. Curr. Biol. 2012:22. doi: 10.1016/j.cub.2012.06.023. [DOI] [PubMed] [Google Scholar]
- Manoogian ENC, Panda S. Circadian clock, nutrient quality, and eating pattern tune diurnal rhythms in the mitochondrial proteome. Proc. Natl. Acad. Sci. 2016:10–12. doi: 10.1073/pnas.1601786113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino T, Arab S, Straume M, Belsham DD, Tata N, Cai F, Liu P, Trivieri M, Ralph M, Sole MJ. Day/night rhythms in gene expression of the normal murine heart. J. Mol. Med. 2004;82:256–264. doi: 10.1007/s00109-003-0520-1. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003;302:255–259. doi: 10.1126/science.1086271. [DOI] [PubMed] [Google Scholar]
- Matthews SG, Challis JR. CRH and AVP-induced changes in synthesis and release of ACTH from the ovine fetal pituitary in vitro: negative influences of cortisol. Endocrine. 1997;6:293–300. doi: 10.1007/BF02820506. [DOI] [PubMed] [Google Scholar]
- Maywood ES, O’Neill J, Wong GKY, Reddy AB, Hastings MH. Circadian timing in health and disease. Prog. Brain Res. 2006;153:253–269. doi: 10.1016/S0079-6123(06)53015-8. [DOI] [PubMed] [Google Scholar]
- McCarthy JJ, Andrews JL, McDearmon EL, Campbell KS, Barber BK, Miller BH, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol. Genomics. 2007;31:86–95. doi: 10.1152/physiolgenomics.00066.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M, Ono D, Hasegawa E, Okamoto H, Honma K, ichi Honma S, Sakurai T. Cellular clocks in AVP neurons of the scn are critical for interneuronal coupling regulating circadian behavior rhythm. Neuron. 2015;85:1103–1116. doi: 10.1016/j.neuron.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J. Neurosci. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk TH. Aging human circadian rhythms: conventional wisdom may not always be right. J. Biol. Rhythms. 2005;20:366–374. doi: 10.1177/0748730405277378. [DOI] [PubMed] [Google Scholar]
- Mühlbauer E, Wolgast S, Finckh U, Peschke D, Peschke E. Indication of circadian oscillations in the rat pancreas. FEBS Lett. 2004;564:91–96. doi: 10.1016/S0014-5793(04)00322-9. [DOI] [PubMed] [Google Scholar]
- Nakamura TJ, Nakamura W, Yamazaki S, Kudo T, Cutler T, Colwell CS, Block GD. Age-related decline in circadian output. J. Neurosci. 2011;31:10201–10205. doi: 10.1523/JNEUROSCI.0451-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proin fl ammatory cytokines. Proc Natl Acad Sci U S A. 2012;109:12662–12667. doi: 10.1073/pnas.1209965109. www.pnas.org/cgi/doi/10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld-Cohen A, Robles MS, Aviram R, Manella G, Adamovich Y, Ladeuix B, Nir D, Rousso-Noori L, Kuperman Y, Golik M, Mann M, Asher G. Circadian control of oscillations in mitochondrial rate-limiting enzymes and nutrient utilization by PERIOD proteins. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E1673–E1682. doi: 10.1073/pnas.1519650113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygård M, Hill RH, Wikström MA, Kristensson K. Age-related changes in electrophysiological properties of the mouse suprachiasmatic nucleus in vitro. Brain Res. Bull. 2005;65:149–154. doi: 10.1016/j.brainresbull.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Nygård M, Palomba M. The GABAergic network in the suprachiasmatic nucleus as a key regulator of the biological clock: does it change during senescence? Chronobiol. Int. 2006;23:427–435. doi: 10.1080/07420520500545938. [DOI] [PubMed] [Google Scholar]
- Ortiz-Tudela E, Innominato PF, Rol MA, Lévi F, Madrid JA. Relevance of internal time and circadian robustness for cancer patients. BMC Cancer. 2016;16:285. doi: 10.1186/s12885-016-2319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TAS, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Peek CB, Affinati AH, Ramsey KM, Kuo H-Y, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, Levine DC, Bacsik DJ, Gius D, Newgard CB, Goetzman E, Chandel NS, Denu JM, Mrksich M, Bass J. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342:1243417. doi: 10.1126/science.1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast JS, Branecky KL, Yang W, Ellacott KLJ, Niswender KD, Yamazaki S. High-fat diet acutely affects circadian organisation and eating behavior. Eur. J. Neurosci. 2013;37:1350–1356. doi: 10.1111/ejn.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- Proper KI, van de Langenberg D, Rodenburg W, Vermeulen RC, van der Beek AJ, van Steeg H, van Kerkhof LW. The Relationship Between Shift Work and Metabolic Risk Factors: A Systematic Review of Longitudinal Studies. Am J Prev Med. 2016:1–11. doi: 10.1016/j.amepre.2015.11.013. [DOI] [PubMed] [Google Scholar]
- Qian J, Scheer FAJL. Circadian System and Glucose Metabolism: Implications for Physiology and Disease. Trends Endocrinol. Metab. 2016;27:282–293. doi: 10.1016/j.tem.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong H-K, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, Bass J. Circadian Clock Feedback Cycle Through NAMPT-Mediated NAD(+) Biosynthesis. Science. 2009;324(80):651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AB, Karp NA, Maywood ES, Sage EA, Deery M, O’Neill JS, Wong GKY, Chesham J, Odell M, Lilley KS, Kyriacou CP, Hastings MH. Circadian Orchestration of the Hepatic Proteome. Curr. Biol. 2006;16:1107–1115. doi: 10.1016/j.cub.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Robles MS, Cox J, Mann M. In-Vivo Quantitative Proteomics Reveals a Key Contribution of Post-Transcriptional Mechanisms to the Circadian Regulation of Liver Metabolism. PLoS Genet. 2014:10. doi: 10.1371/journal.pgen.1004047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, Holtzman DM. Sleep-wake cycle and diurnal fluctuation of amyloid-β as biomarkers of brain amyloid pathology. Sci. Transl. Med. 2012;4:1–20. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, van Gool WA, Swaab DF, Hoogendijk JE, Mirmiran M. Changes in vasopressin cells of the rat suprachiasmatic nucleus with aging. Brain Res. 1987;409:259–264. doi: 10.1016/0006-8993(87)90710-4. [DOI] [PubMed] [Google Scholar]
- Rusak B, Robertson Ha, Wisden W, Hunt SP. Light pulses that shift rhythms induce gene expression in the suprachiasmatic nucleus. Science. 1990;248:1237–1240. doi: 10.1126/science.2112267. [DOI] [PubMed] [Google Scholar]
- Sadacca LA, Lamia KA, DeLemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2011;54:120–124. doi: 10.1007/s00125-010-1920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf MJ, Duurland R, de Kloet ER, Vreugdenhil E. Circadian variation in BDNF mRNA expression in the rat hippocampus. Brain Res. Mol. Brain Res. 2000;75:342–344. doi: 10.1016/s0169-328x(99)00314-9. [DOI] [PubMed] [Google Scholar]
- Scheer F, a JL, Hilton MF, Mantzoros CS, Shea Sa. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. U. S. A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U, Schibler U, Ripperger J, Ripperger J, Brown Sa, Brown Sa. Peripheral Circadian Oscillators in Mammals: Time and Food. J. Biol. Rhythms. 2003;18:250–260. doi: 10.1177/0748730403018003007. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kavuru M. Sleep and metabolism: An overview. Int. J. Endocrinol. 2010 doi: 10.1155/2010/270832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012;26:3493–3502. doi: 10.1096/fj.12-208868. [DOI] [PubMed] [Google Scholar]
- So AY-L, Bernal TU, Pillsbury ML, Yamamoto KR, Feldman BJ. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc. Natl. Acad. Sci. U. S. A. 2009;106:17582–17587. doi: 10.1073/pnas.0909733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokkan K, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the Circadian Clock in the Liver by Feeding. Science. 2001;291(80):490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- Storch K-F, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 Improves Insulin Sensitivity under Insulin-Resistant Conditions by Repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Tamai SI, Sanada K, Fukada Y. Time-of-day-dependent enhancement of adult neurogenesis in the hippocampus. PLoS One. 2008:3. doi: 10.1371/journal.pone.0003835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torra IP, Tsibulsky V, Delaunay F, Saladin R, Laudet V, Fruchart JC, Kosykh V, Staels B. Circadian and glucocorticoid regulation of Rev-erbalpha expression in liver. Endocrinology. 2000;141:3799–3806. doi: 10.1210/endo.141.10.7708. [DOI] [PubMed] [Google Scholar]
- Van Someren EJW, Hagebeuk EEO, Lijzenga C, Scheltens P, De Rooij SEJA, Jonker C, Pot AM, Mirmiran M, Swaab DF. Circadian rest-activity rhythm disturbances in Alzheimer’s disease. Biol. Psychiatry. 1996;40:259–270. doi: 10.1016/0006-3223(95)00370-3. [DOI] [PubMed] [Google Scholar]
- Vieira E, Merino B, Quesada I. Role of the clock gene Rev-erbα in metabolism and in the endocrine pancreas. Diabetes, Obes. Metab. 2015 doi: 10.1111/dom.12522. [DOI] [PubMed] [Google Scholar]
- Viswanathan N, Davis FC. Suprachiasmatic nucleus grafts restore circadian function in aged hamsters. Brain Res. 1995;686:10–16. doi: 10.1016/0006-8993(95)00423-n. [DOI] [PubMed] [Google Scholar]
- Voigt RM, Forsyth CB, Green SJ, Mutlu E, Engen P, Vitaterna MH, Turek FW, Keshavarzian A. Circadian Disorganization Alters Intestinal Microbiota. 2014:9. doi: 10.1371/journal.pone.0097500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt RM, Summa KC, Forsyth CB, Green SJ, Engen P, Naqib A, Vitaterna MH, Turek FW, Keshavarzian A. The Circadian Clock Mutation Promotes Intestinal Dysbiosis. Alcohol. Clin. Exp. Res. 2016;40:335–347. doi: 10.1111/acer.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A. Sundowning and circadian rhythms in Alzheimer’s disease. Am. J. Psychiatry. 2001;158:704–711. doi: 10.1176/appi.ajp.158.5.704. [DOI] [PubMed] [Google Scholar]
- Vyas MV, Garg aX, Iansavichus aV, Costella J, Donner a, Laugsand LE, Janszky I, Mrkobrada M, Parraga G, Hackam DG. Shift work and vascular events: systematic review and meta-analysis. Bmj. 2012;345:e4800–e4800. doi: 10.1136/bmj.e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Zhao T, Cui K, Hu G, Chen Q, Chen W, Wang X, Soto-gutierrez A, Zhao K, Deng C. Negative reciprocal regulation between Sirt1 and Per2 modulates the circadian clock and aging. Sci. Rep. 2016:1–15. doi: 10.1038/srep28633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual Neurons Dissociated from Rat Suprachiasmatic Nucleus Express Independently Phased Circadian Firing Rhythms. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu. Rev. Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead K, Pan M, Masumura KI, Bonneau R, Baliga NS. Diurnally entrained anticipatory behavior in archaea. PLoS One. 2009:4. doi: 10.1371/journal.pone.0005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse CA, Coogan AN. Impact of aging on diurnal expression patterns of CLOCK and BMAL1 in the mouse brain. Brain Res. 2010;1337:21–31. doi: 10.1016/j.brainres.2010.03.113. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nakahata Y, Tanaka M, Yoshida M, Soma H, Shinohara K, Yasuda A, Mamine T, Takumi T. Acute physical stress elevates mouse Period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. J. Biol. Chem. 2005;280:42036–42043. doi: 10.1074/jbc.M509600200. [DOI] [PubMed] [Google Scholar]
- Zarrinpar A, Chaix A, Panda S. Daily Eating Patterns and Their Impact on Health and Disease. Trends Endocrinol. Metab. 2015:1–15. doi: 10.1016/j.tem.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014;20:1006–1017. doi: 10.1016/j.cmet.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc. Natl. Acad. Sci. U. S. A. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JN, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer’s disease. Neurobiol. Aging. 1995;16:571–576. doi: 10.1016/0197-4580(95)00043-e. [DOI] [PubMed] [Google Scholar]