Abstract
For >4 decades, the holy grail in the treatment of acute myocardial infarction has been the mitigation of lethal injury. Despite promising initial results and decades of investigation by the cardiology research community, the only treatment with proven efficacy is early reperfusion of the occluded coronary artery. The remarkable record of failure has led us and others to wonder if cardioprotection is dead. The path to translation, like the ascent to Everest, is certainly littered with corpses. We do, however, highlight a therapeutic principle that provides a glimmer of hope: cellular postconditioning. Administration of cardiosphere-derived cells after reperfusion limits infarct size measured acutely, while providing long-term structural and functional benefits. The recognition that cell therapy may be cardioprotective, and not just regenerative, merits further exploration before we abandon the pursuit entirely.
Keywords: cardiosphere derived cells; cell- and tissue-based therapy; heart failure; myocardial Infarction; ventricular function, left
Since 1937, when Gross et al1 first attempted to reduce myocardial infarct size (by coronary sinus occlusion), many cardioprotective strategies have been devised and tested. Percutaneous coronary intervention is the only such strategy to have withstood the test of time: it has become standard therapy for patients with acute myocardial infarction (AMI), not only reducing immediate mortality and morbidity, but also improving long-term outcomes.2 Nevertheless, some AMI patients, particularly those who end up with a large infarct size, progress to heart failure even with best current therapy. Adverse left ventricular (LV) remodeling after AMI is a precursor to the development of overt heart failure and heralds increased mortality.3,4 In an effort to avert heart failure post–myocardial infarction (MI), numerous adjunctive strategies to reduce infarct size have been tested (>6400 articles on cardioprotection published since 1975). Many have been founded on well-reasoned pathophysiological hypotheses accepted by entire communities of investigators, but, aside from prompt reperfusion, nothing, not one drug or product of potential clinical utility, has emerged. This failure to translate is not attributable to a lack of effort or resources: in the United States alone, the quest for acute cardioprotective therapies has involved a collective investment of several hundred million dollars from the National Heart, Lung, and Blood Institute. Much important work in this area has been done outside the United States, so that the failed expenditure worldwide likely totals ≥$1 billion just on the academic side. Here, we review the dismal history of adjunctive approaches to limit infarct size. Is cardioprotection dead? Or are there new, viable approaches that have the potential to resuscitate this moribund concept?
Sorting the wheat from the chaff is a nontrivial matter when it comes to infarct size–limiting agents. In an effort to validate therapeutic candidates, in 2011 the National Heart, Lung, and Blood Institute established a network called the Consortium for Preclinical Assessment of Cardioprotective Therapies (CAESAR) that was based on the principles of clinical trials (ie, randomization, investigator blinding, exclusion criteria, and appropriate statistical analyses). A number of putatively cardioprotective agents were tested in the CAESAR network, but none was found to be effective under these rigorous experimental conditions. Table 1 summarizes failures to confirm once-promising preclinical data, including those debunked by CAESAR and publicly reported (many others failed in CAESAR but results have not yet been published). Independent of CAESAR, a number of drugs have been tested in patients, with similarly dispiriting results (Table 2). In particular, once the tissue has been reperfused, nothing seems to work. Patients can rarely predict when they will have an AMI, and doctors similarly lack clairvoyance. Ischemic postconditioning (created by cyclic intracoronary balloon inflations) requires immediate manipulation of flow at the time of reperfusion, with loss of benefit if there is delay.29,30 A key consideration of any adjunctive therapy is compatibility with standard clinical practice: in assessing new therapies, it is important to devise interventions that work even after the occluded artery has been successfully opened.
Table 1.
Cardioprotective Agents That Have Been Tested in Preclinical Studies to Reduce Myocardial Infarct Size or Improve Left Ventricular Function and Have Failed
| Agent | Model and Study Authors |
|---|---|
| Allopurinol | Canine (40 min + 4 days); Reimer and Jennings (1985)5 |
| Superoxide dismutase + catalase | Canine (3 h + 24 h); Gallagher et al (1986)6 |
| Superoxide dismutase | Canine (40 min + 4 days); Uraizee et al (1987)7 |
| Oxypurinol | Canine; Puett et al (1987)8 |
| Anti-polymorphonuclear antibody | Canine (3 h + 21 h); Chatelain et al (1987)9 |
| Superoxide dismutase + catalase or oxypurinol | Canine (90 min + 4 days); Richard et al (1988)10 |
| Polyethylene glycol superoxide dismutase | Canine (90 min + 4 days); Tanaka et al (1990)11 |
| Anti-CD18 monoclonal antibody | Canine (90 min + 3 h); Tanaka et al (1993)12 |
| Sildenafil | Porcine (60 min + 48 h); Kukreja et al (2014)* |
| Sodium nitrite | Porcine (60 min + 48 h); Lefer et al (2014)* |
For the outcome of all agents, there was no effect on infarct size.
Experimental Biology Abstract. April 26–30, 2014. San Diego, CA.
Table 2.
Potential Cardioprotective Agents That Have Been Tested in Clinical Trials to Reduce Myocardial Infarct Size or Improve Left Ventricular Function and Have Failed
| Agent | Study | Outcome |
|---|---|---|
| Hyaluronidase | Prethrombolytic era | No effect on infarct size |
| Calcium channel blocker | SPRINT II; Goldbourt et al (1993)13 | Increased mortality |
| Free radical scavenger, human superoxide dismutase | Flaherty et al (1994)14 | No improvement in left ventricular function |
| Antioxidant-trimetazidine | ESPRIM; ESPRIM Group (1994)15 | No effect on mortality or clinical outcomes |
| Fluosol | TAMI-9; Wall et al (1994)16 | No decrease infarct size or increase in left ventricular function |
| Rheoth RX-polaxamer 188 | EMIP-FR; EMIP FR Group (2000)17 | No effect on death, shock, or reinfarction |
| White blood cell inhibitor: Anti-CD18 monoclonal antibody | FESTIVAL; Rusnak et al (2001)18 | No decrease in infarct size |
| Na+/H+ exchange inhibitor | ESCAMI; Zeymer et al (2001)19 CASTEMI; Bar et al (2006)20 |
No effect on infarct size, clinical outcomes, left ventricular ejection fraction |
| Complement inhibitors | COMPLY Trial; Mahaffey et al (2003)21 APEX Trial; Armstrong and Granger (2007)22 |
No decrease in infarct size or decrease in mortality |
| Magnesium | Magnesium in Coronaries Trial Investigators (2002)23 | No effect on mortality, heart failure, or ventricular tachycardia |
| Nicorandil | Kitakaze et al (2007)24 | No effect on mortality or infarct size |
| Cold perfusion | CHILL-MI; Erlinge et al (2014)25 | No effect on infarct size or left ventricular ejection fraction |
| Sodium nitrite | NIAMI; Siddiqi et al (2014)26 | No decrease in infarct size, no effect on left ventricular ejection fraction |
| MPTP inhibitor | MITOCARE; Atar et al (2014)27 | No effect on infarct size or left ventricular ejection fraction |
| Cyclosporine | CIRCUS; Cung et al (2015)28 | No effect on deaths or heart failure |
Adapted and extended from Robert Kloner, MD, National Institutes of Health Workshop on New Horizons in Cardioprotection, 2011. APEX indicates Pexelizumab in Conjunction With Angioplasty in Acute Myocardial Infarction; CASTEMI, Caldaret in ST Elevation MI; CHILL-MI, Rapid endovascular catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction; CIRCUS, Cyclosporine and Prognosis in Acute Myocardial Infarction Patients; COMPLY, Complement inhibition in myocardial infarction treated with thrombolytics; EMIP-FR, European Myocardial Infarction Project - Free Radicals; ESCAMI, Evaluation of the safety and cardioprotective effects of eniporide in acute myocardial infarction; FESTIVAL, An anti-CD11/CD18 monoclonal antibody in patients with acute myocardial infarction having percutaneous transluminal coronary angioplasty; MITOCARE, Effect of intravenous TRO40303 as an adjunct to primary percutaneous coronary intervention for acute ST-elevation myocardial infarction; MPTP, mitochondrial permeability transition pore; NIAMI, intravenous sodium nitrite in acute ST-elevation myocardial infarction; SPRINT II, Systolic Blood Pressure Intervention Trial II; and TAMI-9, Thrombolysis and Angioplasty in Myocardial Infarction-9.
Despite the disappointments to date, a new direction has arisen from an unlikely corner: cell therapy. The conventional rationale for cell therapy is to trigger regeneration, not cardioprotection. The dogma is as follows: progenitor cells, if transplanted into the postischemic heart, will implant, proliferate, and differentiate into viable myocardium. Healing occurs by the growth of new, healthy heart muscle, not by preservation of at-risk myocardium. In such a paradigm, cardioprotection plays no role. Recently, we and others have discovered that cell therapy may indeed be effective in limiting injury when given shortly after AMI, but the protection does not require long-term cell implantation, nor does it involve canonical stem cell mechanisms. Instead, transplanted cells recruit cardioprotection. The collective evidence, reviewed here, gives reason to hope that cardioprotection may not be entirely dead after all…perhaps just stunned.
CELL THERAPY FOR AMI
Numerous lines of evidence now support the idea that cells can either be cardioprotective (when administered during or soon after AMI) or regenerative (when administered after scar is well established). Work in a bitransgenic fate-mapping mouse model revealed that the 2 mechanisms are not mutually exclusive: they contribute roughly equally to the long-term (3 weeks post-MI) increase in myocardial viability when cells are given shortly after permanent coronary ligation.31 Three-week end points or longer will lump both contributions together; short-term end points (eg, 48 hours) enable study of the cardioprotective effect in isolation, well before the regenerative mechanisms of cardiomyocyte proliferation and activation of endogenous cardioblasts come into play (on a time scale of weeks). Many animal studies have investigated cells in nonreperfused AMI,32 and several others have targeted chronically scarred myocardium.33–36 Surprisingly little is known about the utility and risks of intra-coronary cell administration soon after (ie, within 20–45 minutes of) reperfusion. No clinical data are available; cell therapy clinical trials have generally infused cells 1 to 14 days post-AMI.37 By that time, cardiomyocytes at risk are already dead, so there is limited potential (if any) for myocardial salvage.38–40 Given the delays intrinsic to autologous tissue harvesting and cell processing, applications in the acute reperfusion phase will require allo-geneic (off-the-shelf donor-derived) products. Preclinical studies of acutely administered allogeneic mesenchymal stem cells41–43 or their precursors44 have yielded variable results. Some studies have questioned the safety of intra-coronary infusion of cells post-MI, with decreased coronary flow and elevation of cardiac enzymes attributed to microvascular plugging.41–43,45 Houtgraaf et al44 had more favorable results with mesenchymal precursor cells after careful attention to cell dosage and infusion rate. These investigators began infusion at 15 minutes of re-flow, and they quantified infarct size only at 8 weeks, at which time longer-term regenerative effects may cloud the evaluation of cardioprotection.46 Table 3 lists all cell types that have been tested in animal models of AMI, along with the following information for each cell type: the most advanced preclinical model tested; the immune match tested (syngeneic, allogeneic, and xenogeneic); whether or not cardioprotection has been demonstrated histologically in 48- to 72-hour follow-up after postreperfusion cell delivery; and clinical testing status (any clinical testing, and clinical testing specifically in AMI adjunctive to reperfusion). The only cell types to have been shown to be cardioprotective are cardiosphere-derived cells, which are discussed further below.
Table 3.
Cell Types Administered in a Potentially Cardioprotective Preclinical Protocol
| Cell Type | Most Advanced Model Tested | Immune Match? (Allo-, Syn-, or Xenogeneic) | Cardioprotection Demonstrated in Realistic Acute Myocardial Infarction Model? | Clinical Testing Status | |
|---|---|---|---|---|---|
| Clinical Testing? | Clinical Testing for Acute Myocardial Infarction? | ||||
| MSCs | Pig | Allo/Syn | No | Yes | No |
| UBMNCs | Pig | Allo/Xeno | No | No | No |
| BMNCs | Pig | Allo/Syn | No | Yes | No |
| EPCs | Pig | Allo/Syn/Xeno | No | Yes | No |
| BATDCs | Rat | Syn | No | No | No |
| CD31+ BMDPCs | Pig | Syn | No | No | No |
| MPAPCs | Rodent | Syn/Allo | No | No | No |
| ESC-Derived CMs | Monkey | Xeno/Syn | No | No | No |
| ADSCs | Pig | Syn | No | Yes | No |
| BM Sca-1 Cells | Rat | Syn | No | No | No |
| CMBs | Mouse | Xeno/Syn | No | No | No |
| iPS-Derived CMs | Monkey | Xeno | No | No | No |
| BMCs | Rat | Syn | No | No | No |
| CDCs | Pig | Allo/Syn/Xeno | Yes | Yes | No |
| TDSCs | Mouse | Syn | No | No | No |
| c-Kit+ Heart Cells | Pig | Syn | No | Yes | No |
| AFSCs | Rat | Syn | No | No | No |
| CD34+ BMCs | Rodent | Syn | No | Yes | No |
| MPCs | Pig | Allo | No | Yes | Yes |
Cell type, most advanced animal model tested, level of immune match, demonstration of cardioprotection in a clinically realistic model of acute myocardial infarction (yes/no) and clinical testing status (yes/no for any studies in patients, and yes/no specifically when tested clinically in acute phase of myocardial infarction [ie, adjunctive to percutaneous intervention]). A PubMed search on “stem cells” and “acute myocardial infarction” yielded 446 preclinical citations (on March 24, 2017). These were sifted to identify studies in which cells were administered in vivo within 3 hours of coronary ligation if not reperfused, or within 2 hours of ischemia/reperfusion if reperfused. For any given cell type, only the earliest citation to have appeared in the search is cited. Variants of cell types subjected to various conditioning protocols or genetic alterations, or from multiple different species, are not parsed out individually. ADSCs55 indicate adipose-derived stem cells; AFSCs,62 amniotic fluid stem cells; BATDCs,51 brown adipose tissue–derived cells; BMNCs,49 bone marrow mononuclear cells; BM?Cs,59 bone marrow? cells; BM Sca-1 cells,56 bone marrow Sca-1 cells; CD31+ BMDPCs,52 CD31+ bone marrow–derived progenitor cells; CD34+ BMCs,63 bone marrow CD34+ cells; CDCs,60 cardiosphere-derived cells; CMBs,57 cardiac mesangioblasts; EPCs,50 endothelial progenitor cells; ESC-derived CMs,54 ESC-derived cardiomyocytes; iPS-derived CMs,58 iPS-derived cardiomyocytes; MPAPCs,53 multipotent adult progenitor cells; MSCs,47 mesenchymal stem cells; TDSCs,61 tongue-derived stem cells; and UBMNCs,48 umbilical cord blood mononuclear cells.
CARDIOSPHERE-DERIVED CELLS
Over the past 12 years, cardiosphere-derived cells (CDCs) have emerged as a candidate cell type for regenerative therapy post-MI.64 Unlike many other cell therapy products, the mechanism of action of CDCs is well understood. These heart-derived cells exhibit multilineage potential and clonogenicity,64 but they work primarily through indirect mechanisms.60 At least 35 independent laboratories worldwide have generated CDCs and verified their therapeutic bioactivity. CDCs were first tested clinically in the CADUCEUS trial (Cardiosphere-Derived Autologous Stem Cells to Reverse Ventricular Dysfunction),65,66 which examined the safety and efficacy of intracoronary autologous CDCs in 17 patients with LV dysfunction and convalescent MI (1.5–3 months prior), in comparison with 8 randomly assigned controls. The results were promising in revealing evidence of therapeutic regeneration with CDCs, but the chronicity of the MI ruled out any contribution from cardioprotection in that study. Although heart-derived stem cells have been tested in both large animals and humans in chronic ischemic settings,36,66 until recently, the only studies using an acute ischemia/reperfusion (I/R) model were in rats,65,67 where structural and functional outcomes were improved dramatically by the intracoronary infusion of allogeneic CDCs 20 minutes post-AMI. However, the 3-week end point in those studies made it impossible to separate cardioprotection from regeneration.
INDIRECT EFFECTS OF CDCS
In the vast majority of experimental studies, the number of differentiated myocytes derived from transplanted stem cells is too small to account for the observed improvements in cardiac function.60 Thus, the prevailing concept of stem cell efficacy has shifted toward the paracrine hypothesis, which proposes that transplanted cells produce soluble factors beneficial to the infarcted heart.68 Potential cardioprotective effects of paracrine factors include antiapoptotic effects on resident myocytes,67,69 upregulation of angiogenesis,60,70 modulation of inflammatory processes resulting in better infarct healing,71 promotion of cardiomyocyte cell cycle reentry,31 and induction of secondary humoral effects in the host tissue.72,73 Recent findings implicate exosomes as critical agents of the indirect effects of CDCs, likely attributable, at least in part, to the transfer of cardio-protective and regenerative microRNAs (eg, miR-146a) from CDCs to surrounding heart tissue.74
ROLE OF INFLAMMATION AND MACROPHAGES
Innate immunity pathways are recruited to deal with sterile inflammation, as occurs in AMI. The first step is an intense influx of neutrophils within minutes of injury. Macrophages (Mϕ) are then mobilized to clear necrotic debris, antagonize further neutrophil entry, and begin wound healing.75–77 Although there is ample evidence that neutrophils exacerbate I/R injury by killing damaged (but salvageable) cardiomyocytes,78 nonselective inhibition of inflammation has not proven to be useful therapeutically.79–82 Targeting of distinct immune cell populations and subpopulations may be a more viable strategy. Mϕ, in particular, are an important potential target; they can originate within the heart (tissue-resident Mϕ) or from a blood-borne influx of monocytes, which then differentiate into Mϕ in the tissue.83 Despite the common classification of Mϕ into either M1 or M2 subpopulations (with proinflammatory or reparative properties, respectively), Mϕ are highly plastic and can assume a variety of activated states in response to microenvironmental cues.83,84 In fact, at least 4 distinct resident Mϕ subsets exist within the adult heart under normal conditions.85 Following AMI, both resident and monocyte-derived Mϕ expand their populations to regulate repair with several distinct phenotypes, modulating phagocytosis, antigen presentation, and T-cell activation.85,86 It is interesting to note that in the neonatal heart, Mϕ are essential for cardiac regeneration, a function lost within days of birth.87
CELLULAR POSTCONDITIONING
In 2014, the laboratory of one of the authors (E.M.) demonstrated the phenomenon of cellular postconditioning: CDCs are cardioprotective when given within a reasonable delay after I/R in AMI. In pigs subjected to 90 minutes of ischemia and 30 minutes of reflow, the intracoronary infusion of CDCs decreased infarct size and also reduced the extent of microvascular occlusion measured at 48 hours.88 Cyclic sham interruptions of coronary flow starting 30 minutes post-I/R were not cardioprotective, distinguishing CDC-related cardioprotection from ischemic postconditioning. To be absolutely certain that ischemic postconditioning did not confound the results, we performed a new set of experiments using nonocclusive continuous-flow methods to deliver CDCs into the infarct-related artery 30 minutes after reflow in AMI pigs. Figure 1 confirms robust infarct size reduction measured histologically, and preservation of LV ejection fraction, wall thickness, and wall motion using MRI, as well. These new data provide additional evidence of the protective effects of CDC postconditioning. After the initial report in pigs, we published a follow-on mechanistic study in rats with AMI.89 This work confirmed and extended the initial findings: intracoronary infusion of CDCs at 20 minutes of reperfusion reduced infarct size and improved functional recovery. CDCs decreased the number of myocardial CD68+ Mϕ, and these CDCs secreted factors that polarized Mϕ toward a distinctive cardio-protective phenotype. Systemic depletion of Mϕ with clodronate abolished CDC-mediated cardioprotection. Post-I/R adoptive transfer of CDC-conditioned Mϕ also reduced infarct size, recapitulating cellular postconditioning. Thus, CDCs appear to limit acute injury by polarizing an effector Mϕ population within the heart.
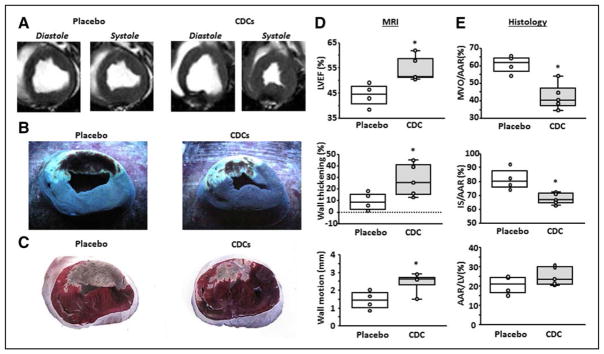
Figure 1. Validation of cellular postconditioning in pigs.
A, MR short-axis images from a placebo and CDC-treated pig. Transverse cardiac slices stained with Thioflavin T and Gentian Violet (B), and triphenyl tetrazolium chloride (TTC) (C) in the same representative sample. B, The area of MVO appears nonfluorescent under UV light, whereas the area-at-risk (AAR) is unstained with Gentian Violet. C, Viable myocardium appears red and scar appears white/yellow. LVEF (D, top), infarct wall thickening (D, middle), and infarct wall motion (D, lower) are improved following CDC treatment. MVO/AAR (E, top), and infarct size (IS)/AAR (E, middle) are decreased following CDC treatment, whereas AAR is not different between groups (E, lower). Graphs depict mean±SEM. Statistical significance was determined by using the Student t test. *P<0.05. CDC indicates cardiosphere-derived cell; LVEF, left ventricular ejection fraction; MVO, microvascular occlusion; and SEM, standard error of the mean.
Given the concerns articulated earlier, any putative new cardioprotective mechanism will understandably be greeted skeptically. Thus, independent validation of the basic phenomena is highly desirable. Using blinded analysis and randomization, the Lefer laboratory (one of the principals in the CAESAR network, and an author here) has now independently reproduced the findings of robust cardioprotection by CDCs. Figure 2 shows the results of a study in which allogeneic rat CDCs were administered 20 minutes following reperfusion in the spontaneously hypertensive rat AMI model. Placebo (phosphate-buffered saline)–injected spontaneously hypertensive rats exhibit very large areas of infarction (ie, >50% of the area-at-risk) following coronary I/R. CDC postconditioning significantly attenuated myocardial infarct size and plasma cardiac troponin I levels at 48 hours postreperfusion. It its interesting to note that LV structure and function were preserved in CDC-treated spontaneously hypertensive rats at 28 days post-AMI in comparison with rats that had received phosphate-buffered saline (Figure 2), verifying that the effects are durable (as the Marbán laboratory had shown in another pig study).90
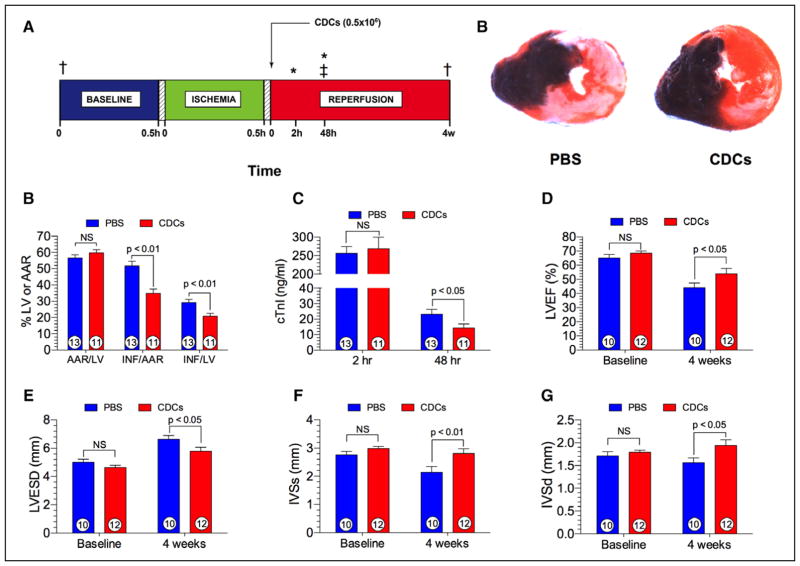
Figure 2. Cellular postconditioning in spontaneously hypertensive rats.
A, Experimental protocol involving male spontaneously hypertensive rats (SHRs) subjected to 30 minutes of left coronary artery ischemia followed by either 48 hours (h) or 4 weeks (w) of reperfusion. Myocardial area-at-risk and infarct size were determined at 48 h postreperfusion. Plasma levels of cardiac troponin I (cTnI) was measured at 2 h and 48 h of reperfusion. At 20 minutes of reperfusion, rat CDCs (0.5×106) or phosphate-buffered saline (PBS) were injected directly into the left ventricular lumen following aortic cross-clamping. B, Representative photomicrographs of SHRs receiving either PBS or CDCs at 20 minutes of reperfusion. Myocardial infarct size is significantly attenuated in the CDC-treated heart. C, Myocardial area-at-risk (AAR) as a percentage of the left ventricle (LV), infarct size (INF) per AAR, and INF as a percent of the LV in rats receiving either PBS or CDCs. Myocardial infarct size per area-at-risk or LV was significantly (P<0.01) reduced in the CDC group. D, Plasma cardiac troponin I (cTnI) levels at 2 and 48 h following reperfusion. cTnI levels are significantly (P<0.05) reduced at 48 h postreperfusion. E, Left ventricular ejection fraction (LVEF) at baseline and at 4 weeks following reperfusion. LVEF is similar at baseline and significantly (P<0.05) greater in animals receiving CDCs. F, Left ventricular end-systolic dimension (LVESD) at baseline and 4 weeks of reperfusion in the PBS and CDC groups. LVESD is significantly (P<0.05) reduced in the CDC group in comparison with PBS. G, Interventricular septal dimension at end-systole (IVSs) at baseline and 4 weeks following reperfusion. IVSs was significantly (P<0.01) greater in hearts treated with CDCs than with PBS. H, Interventricular septal dimension at end-diastole (IVSd) at baseline and 4 weeks postreperfusion. Similar to IVSs, IVSd was significantly (P<0.01) greater in the CDC group than in the PBS group. Numbers inside the bars represent the number of animals in each group. Statistical significance was determined by using the Student t test. CDC indicates cardiosphere-derived cell; and 2,3,5-TTC, 2,3,5-triphenyltetrazolium chloride. *Plasma samples for cTnI. ‡Myocardial infarct size analysis. †2-D Echocardiography, Visual Sonics Vevo 2100.
Figure 3 summarizes our current understanding of the mechanisms of CDC-mediated postconditioning. Extensive evidence supports the notion that extracellular nanovesicles called exosomes are secreted by CDCs and mediate their salient effects, likely via cell-cell transfer of noncoding RNAs, including microRNAs (although exosomes contain a redundancy of other bio-active molecules, including proteins and transcripts).91 The effects of CDCs on macrophages are replicated by CDC-secreted exosomes (CDCexo), and CDCexo themselves mimic cellular postconditioning.92 Although the cascade of microRNA transfer and target gene suppression might seem too slow to mediate a process that necessarily must be rapid to prevent substantial cardiomyocyte death, microRNAs are known to be capable of suppressing proinflammatory gene expression in just 1 hour.93 A host of acute and longer-term salutary effects ensue. Within just 2 hours, cardiomyocyte apoptosis in the postischemic heart is inhibited by ≈60%.89 Meanwhile, macrophages are altered so as to become cytoprotective. CDCexo-polarized macrophages exhibit enhanced phagocytosis; our working hypothesis posits that such macrophages become more efficient in clearing necrotic debris (thereby enhancing the healing process known as efferocytosis).94,95 The net effects are a reduction of infarct size evident early, with sustained structural and functional benefits. The recognition of a central mechanistic role for CDCexo begs the question of whether cell-free therapeutics may be able to recruit benefits equivalent to cellular postconditioning. In the long run, this possibility seems likely: as we come to recognize the key bioactive components within CDCexo, they may become effective therapeutic agents on their own, either naked or packaged within designer exosomes. In the immediate future, however, CDCexo themselves may not be a realistic, alternative therapeutic candidate to CDCs. Although CDCexo reproduce the salient benefits of CDCs, we have recently reported, in a porcine model of cellular postconditioning, that intramyocardial injection is required for efficacy.96 The intracoronary route is far preferable clinically, especially in the setting of recent reperfusion when the heart can be particularly susceptible to ventricular arrhythmias.97 Thus, CDCs, which are effective after intracoronary delivery (Figures 1 and 2, and references 55–57), continue to be the prime therapeutic candidate for reducing infarct size translationally, as discussed further below.
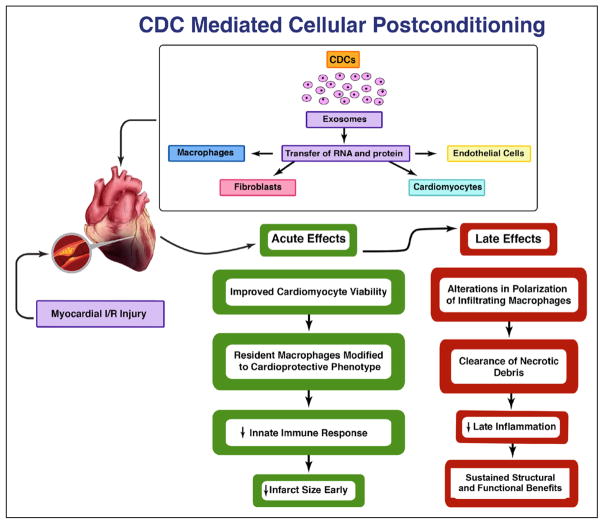
Figure 3. Mechanisms of CDC-mediated cellular postconditioning.
Cardiosphere-derived cells (CDCs) release exosomes resulting in the transfer of RNA and proteins to macrophages, fibroblasts, endothelial cells, and cardiomyocytes, in turn, leading to both acute and late cardioprotective actions. In the acute phase of reperfusion injury, CDCs improve cardiomyocyte viability and reduce myocardial infarct size by the conversion of resident macrophages to a cardioprotective phenotype and dampening the innate immune response. During the later phases of myocardial re-perfusion injury, CDC therapy results in sustained infarct size reduction by alterations in polarization of infiltrating macrophages, accelerated clearance of necrotic debris, and significant attenuation of the late inflammatory response in the myocardium.
NEW CONCEPTS SUGGEST NEW APPROACHES
The discovery that CDCs work in AMI despite being administered with some delay after reperfusion is notable, because it avoids the need for pretreatment and immediate intervention on reopening the affected artery.98 The concept of cellular postconditioning is novel, and merits comparison with other cardioprotective processes that can be recruited pharmacologically and by transient ischemia (preconditioning and ischemic postconditioning). Unlike those phenomena, however, cellular postconditioning has the unique advantage of being recruitable 30 minutes after reperfusion (and perhaps even longer; the precise limits of the cardioprotective window remain to be defined). The idea that cell therapy may mitigate ischemic injury by modulating Mϕ is supported by recent work,89 and is consistent with the immunomodulatory properties described for CDCs.99,100 Although inflammation figures prominently in AMI, there has been little by way of targeted intervention to take advantage of our exploding knowledge of innate immunity pathways and Mϕ biology. Although not originally conceived as selectively targeting inflammation to reduce infarct size, CDCs may turn out to achieve this long-elusive goal.
PROSPECTS FOR TRANSLATION
Few of the cell types tested preclinically in AMI model have progressed to clinical testing (Table 3). As summarized in Table 4, allogeneic CDCs are already in advanced clinical testing; they have proven safe to date in >100 patients treated by coronary infusion.
Table 4.
Summary of Allogeneic CDC Clinical Trials to Date
| Study Name | Study Design | No. of Subjects | Study Results |
|---|---|---|---|
| ALLSTAR | Phase 1 open-label allogeneic CDC in patients after myocardial infarction; single-vessel occlusive intracoronary delivery101 | 14 | Allogeneic CDCs safe, possibly effective in reducing scar size |
| Phase 2 multicenter randomized double-blind placebo-controlled trial of allogeneic CDCs in patients after myocardial infarction; single-vessel occlusive intracoronary delivery | 142 | Enrollment complete in follow-up | |
| DYNAMIC | Patients with open-label heart failure with reduced ejection fraction, allogeneic CDCs; triple-vessel nonocclusive intracoronary delivery102 | 14 | Improved left ventricular ejection fraction and clinical status |
| HOPE-Duchenne* | Muscular dystrophy; randomized allogeneic CDCs vs controls; triple-vessel nonocclusive intracoronary delivery | 25 | Enrollment complete in follow-up |
| Regress-HFpEF† | Randomized double-blind placebo-controlled trial; allogeneic CDCs vs placebo; triple-vessel nonocclusive intracoronary delivery | 40 | Enrollment underway |
| ALPHA‡ | Pulmonary hypertension; allogeneic CDCs vs placebo | 26 | Enrollment underway |
ALLSTAR indicates the Allogeneic Heart Stem Cells to Achieve Myocardial Regeneration; ALPHA, Allogeneic CDCs for Pulmonary Hypertension Therapy; CDC, cardiosphere-derived cell; DYNAMIC, the dilated cardiomyopathy intervention with allogeneic myocardially-regenerative cells; HOPE-Duchenne, Halt Cardiomyopathy Progression in Duchenne; and Regress-HFpEF, Regression-Heart Failure with Preserved Ejection Fraction.
J.L. Jefferies et al, unpublished data, 2017 (ClinicalTrials.gov. Unique identifier: NCT02485938).
E. Marban et al, unpublished data, 2017 (ClinicalTrials.gov. Unique identifier: NCT02941705).
M. Lewis et al, unpublished data, 2017.
Thus, from a product readiness viewpoint, it should be straightforward to initiate clinical testing of the hypothesis that CDCs induce cellular postconditioning, targeting end points including infarct size and LV ejection fraction. Demonstration of efficacy in humans would comprise the ultimate proof of concept that cellular postconditioning is genuine. Nevertheless, some cautionary notes are worth considering before launching into clinical trials. First, dosing of the CDCs needs to be carefully adjusted. CDCs are large cells that can be microcclusive.34 In the setting of AMI, where microvascular occlusion already can occur, intermediate dosing may be required: too few infused cells will be ineffective, while too many may actually worsen preexisting microvascular occlusion. Even in the highly controlled pig model, we have found that excessively high doses result in decreased efficacy, consistent with the Goldilocks caveat. In humans with highly variable degrees of I/R injury on presentation with AMI, it will be even more challenging to estimate a safe-but-effective dose. A second consideration is the fact that it is exceedingly difficult, in the AMI setting, to determine which patients will go on to develop large infarcts. The results of early percutaneous intervention are so overwhelmingly positive, even for patients presenting with hypotension and tombstone Ts, that entry criteria are now difficult to establish reliably for any cardioprotective protocol. On balance, proceeding with relatively low CDC doses and broad inclusion criteria seems most prudent, recognizing that the number of patients one must treat to see benefit will necessarily be increased by such a conservative approach. The ongoing AMICI trial (Safety Study of Allogeneic Mesenchymal Precursor Cell Infusion in MyoCardial Infarction) of allogeneic mesenchymal precursor cells in AMI may provide helpful safety data and insights into dosage to help guide future trials (Clinicaltrials.gov NCT01781390). Indeed, mesenchymal precursor cells are the first cells to be tested clinically as adjunctive therapy to percutaneous intervention in AMI (Table 3).
CONCLUSIONS
Despite >40 years of effort and thousands of reports of therapies claiming to limit myocardial infarct size in the setting of AMI, there are no approved treatments to supplement the unambiguous efficacy of early reflow. Recent data reviewed here demonstrate the powerful effects of cellular postconditioning with CDCs administered following reperfusion. Here we additionally provide compelling unpublished data from 2 different laboratories in 2 different animal species demonstrating robust cardioprotection when CDCs are administered 20 to 30 minutes following reperfusion. These studies demonstrate major reductions in myocardial infarct size in the spontaneously hypertensive rat and in the Yucatan miniswine model. Reductions in infarct size are accompanied by improved LV function and preservation of myocardial blood flow with attenuated no-reflow. Cellular postconditioning may be clinically tractable, providing new hope that myocardial reperfusion injury can be effectively targeted. The jury is still out, but we conclude that cardioprotection against AMI is not dead…at least, not yet.
Acknowledgments
The authors thank David J. Polhemus, PhD, and Rishi Trivedi, MS, for performing the studies of CDC postconditioning in spontaneously hypertensive rats, and Romain Gallet, MD, and James Dawkins, VMD, for having performed and analyzed the pig experiments.
SOURCES OF FUNDING
General laboratory support was provided by the National Institutes of Health (to Drs Lefer and Marbán), the California Institute for Regenerative Medicine (to Dr Marbán), and the US Department of Defense (to Dr Marbán).
Footnotes
DISCLOSURES
Dr Marbán owns founder’s equity in Capricor, Inc. Dr Lefer reports no conflicts.
Circulation is available at http://circ.ahajournals.org.
References
- 1.Gross L, Blum L, Silverman G. Experimental attempts to increase the blood supply to the dog’s heart by means of coronary sinus occlusion. J Exp Med. 1937;65:91–108. doi: 10.1084/jem.65.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 4.St John Sutton M, Pfeffer MA, Plappert T, Rouleau JL, Moyé LA, Dagenais GR, Lamas GA, Klein M, Sussex B, Goldman S. Quantitative two-dimensional echocardiographic measurements are major predictors of adverse cardiovascular events after acute myocardial infarction. The protective effects of captopril. Circulation. 1994;89:68–75. doi: 10.1161/01.cir.89.1.68. [DOI] [PubMed] [Google Scholar]
- 5.Reimer KA, Jennings RB. Failure of the xanthine oxidase inhibitor allopurinol to limit infarct size after ischemia and reperfusion in dogs. Circulation. 1985;71:1069–1075. doi: 10.1161/01.cir.71.5.1069. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher KP, Buda AJ, Pace D, Gerren RA, Shlafer M. Failure of super-oxide dismutase and catalase to alter size of infarction in conscious dogs after 3 hours of occlusion followed by reperfusion. Circulation. 1986;73:1065–1076. doi: 10.1161/01.cir.73.5.1065. [DOI] [PubMed] [Google Scholar]
- 7.Uraizee A, Reimer KA, Murry CE, Jennings RB. Failure of superoxide dismutase to limit size of myocardial infarction after 40 minutes of ischemia and 4 days of reperfusion in dogs. Circulation. 1987;75:1237–1248. doi: 10.1161/01.cir.75.6.1237. [DOI] [PubMed] [Google Scholar]
- 8.Puett DW, Forman MB, Cates CU, Wilson BH, Hande KR, Friesinger GC, Virmani R. Oxypurinol limits myocardial stunning but does not reduce infarct size after reperfusion. Circulation. 1987;76:678–686. doi: 10.1161/01.cir.76.3.678. [DOI] [PubMed] [Google Scholar]
- 9.Chatelain P, Latour JG, Tran D, de Lorgeril M, Dupras G, Bourassa M. Neutrophil accumulation in experimental myocardial infarcts: relation with extent of injury and effect of reperfusion. Circulation. 1987;75:1083–1090. doi: 10.1161/01.cir.75.5.1083. [DOI] [PubMed] [Google Scholar]
- 10.Richard VJ, Murry CE, Jennings RB, Reimer KA. Therapy to reduce free radicals during early reperfusion does not limit the size of myocardial infarcts caused by 90 minutes of ischemia in dogs. Circulation. 1988;78:473–480. doi: 10.1161/01.cir.78.2.473. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka M, Stoler RC, FitzHarris GP, Jennings RB, Reimer KA. Evidence against the “early protection-delayed death” hypothesis of superoxide dismutase therapy in experimental myocardial infarction. Polyethylene glycol-superoxide dismutase plus catalase does not limit myocardial infarct size in dogs. Circ Res. 1990;67:636–644. doi: 10.1161/01.res.67.3.636. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka M, Brooks SE, Richard VJ, FitzHarris GP, Stoler RC, Jennings RB, Arfors KE, Reimer KA. Effect of anti-CD18 antibody on myocardial neutrophil accumulation and infarct size after ischemia and reperfusion in dogs. Circulation. 1993;87:526–535. doi: 10.1161/01.cir.87.2.526. [DOI] [PubMed] [Google Scholar]
- 13.Goldbourt U, Behar S, Reicher-Reiss H, Zion M, Mandelzweig L, Kaplinsky E. Early administration of nifedipine in suspected acute myocardial infarction. The Secondary Prevention Reinfarction Israel Nifedipine Trial 2 Study. Arch Intern Med. 1993;153:345–353. [PubMed] [Google Scholar]
- 14.Flaherty JT, Pitt B, Gruber JW, Heuser RR, Rothbaum DA, Burwell LR, George BS, Kereiakes DJ, Deitchman D, Gustafson N. Recombinant human superoxide dismutase (h-SOD) fails to improve recovery of ventricular function in patients undergoing coronary angioplasty for acute myocardial infarction. Circulation. 1994;89:1982–1991. doi: 10.1161/01.cir.89.5.1982. [DOI] [PubMed] [Google Scholar]
- 15.ESPRIM Group. The ESPRIM trial: short-term treatment of acute myocardial infarction with molsidomine. European Study of Prevention of Infarct with Molsidomine (ESPRIM) Group. Lancet. 1994;344:91–97. [PubMed] [Google Scholar]
- 16.Wall TC, Califf RM, Blankenship J, Talley JD, Tannenbaum M, Schwaiger M, Gacioch G, Cohen MD, Sanz M, Leimberger JD. Intravenous Fluosol in the treatment of acute myocardial infarction. Results of the Thrombolysis and Angioplasty in Myocardial Infarction 9 Trial. TAMI 9 Research Group. Circulation. 1994;90:114–120. doi: 10.1161/01.cir.90.1.114. [DOI] [PubMed] [Google Scholar]
- 17.EMIP-FR Group. Effect of 48-h intravenous trimetazidine on short- and long-term outcomes of patients with acute myocardial infarction, with and without thrombolytic therapy; A double-blind, placebo-controlled, randomized trial. The EMIP-FR Group. European Myocardial Infarction Project—Free Radicals. Eur Heart J. 2000;21:1537–1546. doi: 10.1053/euhj.1999.2439. [DOI] [PubMed] [Google Scholar]
- 18.Rusnak JM, Kopecky SL, Clements IP, Gibbons RJ, Holland AE, Peterman HS, Martin JS, Saoud JB, Feldman RL, Breisblatt WM, Simons M, Gessler CJ, Jr, Yu AS. An anti-CD11/CD18 monoclonal antibody in patients with acute myocardial infarction having percutaneous transluminal coronary angioplasty (the FESTIVAL study) Am J Cardiol. 2001;88:482–487. doi: 10.1016/s0002-9149(01)01723-4. [DOI] [PubMed] [Google Scholar]
- 19.Zeymer U, Suryapranata H, Monassier JP, Opolski G, Davies J, Rasmanis G, Linssen G, Tebbe U, Schröder R, Tiemann R, Machnig T, Neuhaus KL ESCAMI Investigators. The Na(+)/H(+) exchange inhibitor eniporide as an adjunct to early reperfusion therapy for acute myocardial infarction. Results of the evaluation of the safety and cardioprotective effects of eniporide in acute myocardial infarction (ESCAMI) trial. J Am Coll Cardiol. 2001;38:1644–1650. doi: 10.1016/s0735-1097(01)01608-4. [DOI] [PubMed] [Google Scholar]
- 20.Bär FW, Tzivoni D, Dirksen MT, Fernández-Ortiz A, Heyndrickx GR, Brachmann J, Reiber JH, Avasthy N, Tatsuno J, Davies M, Hibberd MG, Krucoff MW CASTEMI Study Group. Results of the first clinical study of adjunctive CAldaret (MCC-135) in patients undergoing primary percutaneous coronary intervention for ST-Elevation Myocardial Infarction: the randomized multicentre CASTEMI study. Eur Heart J. 2006;27:2516–2523. doi: 10.1093/eurheartj/ehl304. [DOI] [PubMed] [Google Scholar]
- 21.Mahaffey KW, Granger CB, Nicolau JC, Ruzyllo W, Weaver WD, Theroux P, Hochman JS, Filloon TG, Mojcik CF, Todaro TG, Armstrong PW COMPLY Investigators. Effect of pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to fibrinolysis in acute myocardial infarction: the COMPlement inhibition in myocardial infarction treated with thromboLYtics (COMPLY) trial. Circulation. 2003;108:1176–1183. doi: 10.1161/01.CIR.0000087404.53661.F8. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong PW, Granger CB. Pexelizumab and the APEX AMI trial. JAMA. 2007;297:1881. doi: 10.1001/jama.297.17.1881-b. author reply 1881–1881; author reply 1882. [DOI] [PubMed] [Google Scholar]
- 23.Magnesium in Coronaries (MAGIC) Trial Investigators. Early administration of intravenous magnesium to high-risk patients with acute myocardial infarction in the Magnesium in Coronaries (MAGIC) Trial: a randomised controlled trial. Lancet. 2002;360:1189–1196. doi: 10.1016/s0140-6736(02)11278-5. [DOI] [PubMed] [Google Scholar]
- 24.Kitakaze M, Asakura M, Kim J, Shintani Y, Asanuma H, Hamasaki T, Seguchi O, Myoishi M, Minamino T, Ohara T, Nagai Y, Nanto S, Watanabe K, Fukuzawa S, Hirayama A, Nakamura N, Kimura K, Fujii K, Ishihara M, Saito Y, Tomoike H, Kitamura S J-WIND investigators. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370:1483–1493. doi: 10.1016/S0140-6736(07)61634-1. [DOI] [PubMed] [Google Scholar]
- 25.Erlinge D, Götberg M, Lang I, Holzer M, Noc M, Clemmensen P, Jensen U, Metzler B, James S, Bötker HE, Omerovic E, Engblom H, Carlsson M, Arheden H, Ostlund O, Wallentin L, Harnek J, Olivecrona GK. Rapid endovascular catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. The CHILL-MI trial: a randomized controlled study of the use of central venous catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. J Am Coll Cardiol. 2014;63:1857–1865. doi: 10.1016/j.jacc.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 26.Siddiqi N, Neil C, Bruce M, MacLennan G, Cotton S, Papadopoulou S, Feelisch M, Bunce N, Lim PO, Hildick-Smith D, Horowitz J, Madhani M, Boon N, Dawson D, Kaski JC, Frenneaux M NIAMI investigators. Intravenous sodium nitrite in acute ST-elevation myocardial infarction: a randomized controlled trial (NIAMI) Eur Heart J. 2014;35:1255–1262. doi: 10.1093/eurheartj/ehu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atar D, Arheden H, Berdeaux A, Bonnet JL, Carlsson M, Clemmensen P, Cuvier V, Danchin N, Dubois-Randé JL, Engblom H, Erlinge D, Firat H, Halvorsen S, Hansen HS, Hauke W, Heiberg E, Koul S, Larsen AI, Le Corvoisier P, Nordrehaug JE, Paganelli F, Pruss RM, Rousseau H, Schaller S, Sonou G, Tuseth V, Veys J, Vicaut E, Jensen SE. Effect of intravenous TRO40303 as an adjunct to primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: MITOCARE study results. Eur Heart J. 2015;36:112–119. doi: 10.1093/eurheartj/ehu331. [DOI] [PubMed] [Google Scholar]
- 28.Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D, Bonnefoy-Cudraz E, Guérin P, Elbaz M, Delarche N, Coste P, Vanzetto G, Metge M, Aupetit JF, Jouve B, Motreff P, Tron C, Labeque JN, Steg PG, Cottin Y, Range G, Clerc J, Claeys MJ, Coussement P, Prunier F, Moulin F, Roth O, Belle L, Dubois P, Barragan P, Gilard M, Piot C, Colin P, De Poli F, Morice MC, Ider O, Dubois-Randé JL, Unterseeh T, Le Breton H, Béard T, Blanchard D, Grollier G, Malquarti V, Staat P, Sudre A, Elmer E, Hansson MJ, Bergerot C, Boussaha I, Jossan C, Derumeaux G, Mewton N, Ovize M. Cyclosporine before PCI in Patients with Acute Myocardial Infarction. N Engl J Med. 2015;373:1021–1031. doi: 10.1056/NEJMoa1505489. [DOI] [PubMed] [Google Scholar]
- 29.Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera JS, Halkos ME, Kerendi F, Guyton RA, Vinten-Johansen J. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res. 2004;62:74–85. doi: 10.1016/j.cardiores.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Yang XM, Proctor JB, Cui L, Krieg T, Downey JM, Cohen MV. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol. 2004;44:1103–1110. doi: 10.1016/j.jacc.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 31.Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, Sun B, Aminzadeh M, Marbán E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med. 2013;5:191–209. doi: 10.1002/emmm.201201737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 33.Bolli R, Tang XL, Sanganalmath SK, Rimoldi O, Mosna F, Abdel-Latif A, Jneid H, Rota M, Leri A, Kajstura J. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation. 2013;128:122–131. doi: 10.1161/CIRCULATIONAHA.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, Lardo AC, Lai S, Steenbergen C, Gerstenblith G, Lange R, Marbán E. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–83. doi: 10.1161/CIRCULATIONAHA.108.816058. 7 p following 1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee ST, White AJ, Matsushita S, Malliaras K, Steenbergen C, Zhang Y, Li TS, Terrovitis J, Yee K, Simsir S, Makkar R, Marbán E. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J Am Coll Cardiol. 2011;57:455–465. doi: 10.1016/j.jacc.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 36.Malliaras K, Smith RR, Kanazawa H, Yee K, Seinfeld J, Tseliou E, Dawkins JF, Kreke M, Cheng K, Luthringer D, Ho CS, Blusztajn A, Valle I, Chowdhury S, Makkar RR, Dharmakumar R, Li D, Marbán L, Marbán E. Validation of contrast-enhanced magnetic resonance imaging to monitor regenerative efficacy after cell therapy in a porcine model of convalescent myocardial infarction. Circulation. 2013;128:2764–2775. doi: 10.1161/CIRCULATIONAHA.113.002863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kreke M, Smith RR, Marbán L, Marbán E. Cardiospheres and cardiosphere-derived cells as therapeutic agents following myocardial infarction. Expert Rev Cardiovasc Ther. 2012;10:1185–1194. doi: 10.1586/erc.12.102. [DOI] [PubMed] [Google Scholar]
- 38.Jennings RB, Murry CE, Steenbergen C, Jr, Reimer KA. Development of cell injury in sustained acute ischemia. Circulation. 1990;82(3 suppl):II2–I12. [PubMed] [Google Scholar]
- 39.Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985;76:1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hochman JS, Choo H. Limitation of myocardial infarct expansion by reperfusion independent of myocardial salvage. Circulation. 1987;75:299–306. doi: 10.1161/01.cir.75.1.299. [DOI] [PubMed] [Google Scholar]
- 41.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 42.Perin EC, Silva GV, Assad JA, Vela D, Buja LM, Sousa AL, Litovsky S, Lin J, Vaughn WK, Coulter S, Fernandes MR, Willerson JT. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J Mol Cell Cardiol. 2008;44:486–495. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Vulliet PR, Greeley M, Halloran SM, MacDonald KA. Kittleson Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet. 2004;363:783–784. doi: 10.1016/S0140-6736(04)15695-X. [DOI] [PubMed] [Google Scholar]
- 44.Houtgraaf JH, de Jong R, Kazemi K, de Groot D, van der Spoel TI, Arslan F, Hoefer I, Pasterkamp G, Itescu S, Zijlstra F, Geleijnse ML, Serruys PW, Duckers HJ. Intracoronary infusion of allogeneic mesenchymal precursor cells directly after experimental acute myocardial infarction reduces infarct size, abrogates adverse remodeling, and improves cardiac function. Circ Res. 2013;113:153–166. doi: 10.1161/CIRCRESAHA.112.300730. [DOI] [PubMed] [Google Scholar]
- 45.Lim SY, Kim YS, Ahn Y, Jeong MH, Hong MH, Joo SY, Nam KI, Cho JG, Kang PM, Park JC. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc Res. 2006;70:530–542. doi: 10.1016/j.cardiores.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 46.Malliaras K, Ibrahim A, Tseliou E, Liu W, Sun B, Middleton RC, Seinfeld J, Wang L, Sharifi BG, Marbán E. Stimulation of endogenous cardioblasts by exogenous cell therapy after myocardial infarction. EMBO Mol Med. 2014;6:760–777. doi: 10.1002/emmm.201303626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, Yamagishi M, Mori H, Kangawa K, Kitamura S. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004;287:H2670–H2676. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- 48.Henning RJ, Abu-Ali H, Balis JU, Morgan MB, Willing AE, Sanberg PR. Human umbilical cord blood mononuclear cells for the treatment of acute myocardial infarction. Cell Transplant. 2004;13:729–739. doi: 10.3727/000000004783983477. [DOI] [PubMed] [Google Scholar]
- 49.Li TS, Hayashi M, Ito H, Furutani A, Murata T, Matsuzaki M, Hamano K. Regeneration of infarcted myocardium by intramyocardial implantation of ex vivo transforming growth factor-beta-preprogrammed bone marrow stem cells. Circulation. 2005;111:2438–2445. doi: 10.1161/01.CIR.0000167553.49133.81. [DOI] [PubMed] [Google Scholar]
- 50.Boyle AJ, Schuster M, Witkowski P, Xiang G, Seki T, Way K, Itescu S. Additive effects of endothelial progenitor cells combined with ACE inhibition and beta-blockade on left ventricular function following acute myocardial infarction. J Renin Angiotensin Aldosterone Syst. 2005;6:33–37. doi: 10.3317/jraas.2005.004. [DOI] [PubMed] [Google Scholar]
- 51.Yamada Y, Wang XD, Yokoyama S, Fukuda N, Takakura N. Cardiac progenitor cells in brown adipose tissue repaired damaged myocardium. Biochem Biophys Res Commun. 2006;342:662–670. doi: 10.1016/j.bbrc.2006.01.181. [DOI] [PubMed] [Google Scholar]
- 52.Grøgaard HK, Sigurjonsson OE, Brekke M, Kløw NE, Landsverk KS, Lyberg T, Eriksen M, Egeland T, Ilebekk A. Cardiac accumulation of bone marrow mononuclear progenitor cells after intracoronary or intravenous injection in pigs subjected to acute myocardial infarction with subsequent reperfusion. Cardiovasc Revasc Med. 2007;8:21–27. doi: 10.1016/j.carrev.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Van’t Hof W, Mal N, Huang Y, Zhang M, Popovic Z, Forudi F, Deans R, Penn MS. Direct delivery of syngeneic and allogeneic large-scale expanded multipotent adult progenitor cells improves cardiac function after myocardial infarct. Cytotherapy. 2007;9:477–487. doi: 10.1080/14653240701452065. [DOI] [PubMed] [Google Scholar]
- 54.Rajasingh J, Bord E, Hamada H, Lambers E, Qin G, Losordo DW, Kishore R. STAT3-dependent mouse embryonic stem cell differentiation into cardiomyocytes: analysis of molecular signaling and therapeutic efficacy of cardiomyocyte precommitted mES transplantation in a mouse model of myocardial infarction. Circ Res. 2007;101:910–918. doi: 10.1161/CIRCRE-SAHA.107.156786. [DOI] [PubMed] [Google Scholar]
- 55.Valina C, Pinkernell K, Song YH, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28:2667–2677. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- 56.Lu G, Haider HK, Jiang S, Ashraf M. Sca-1+ stem cell survival and engraftment in the infarcted heart: dual role for preconditioning-induced connexin-43. Circulation. 2009;119:2587–2596. doi: 10.1161/CIRCULATIONAHA.108.827691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gálvez BG, Covarello D, Tolorenzi R, Brunelli S, Dellavalle A, Crippa S, Mohammed SA, Scialla L, Cuccovillo I, Molla F, Staszewsky L, Maisano F, Sampaolesi M, Latini R, Cossu G. Human cardiac mesoangioblasts isolated from hypertrophic cardiomyopathies are greatly reduced in proliferation and differentiation potency. Cardiovasc Res. 2009;83:707–716. doi: 10.1093/cvr/cvp159.MD. [DOI] [PubMed] [Google Scholar]
- 58.Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–416. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goodchild TT, Robinson KA, Pang W, Tondato F, Cui J, Arrington J, Godwin L, Ungs M, Carlesso N, Weich N, Poznansky MC, Chronos NA. Bone marrow-derived B cells preserve ventricular function after acute myocardial infarction. JACC Cardiovasc Interv. 2009;2:1005–1016. doi: 10.1016/j.jcin.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 60.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, Marbán E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shibuya M, Miura T, Fukagawa Y, Akashi S, Oda T, Kawamura S, Ikeda Y, Matsuzaki M. Tongue muscle-derived stem cells express connexin 43 and improve cardiac remodeling and survival after myocardial infarction in mice. Circ J. 2010;74:1219–1226. doi: 10.1253/circj.cj-10-0033. [DOI] [PubMed] [Google Scholar]
- 62.Bollini S, Cheung KK, Riegler J, Dong X, Smart N, Ghionzoli M, Loukogeorgakis SP, Maghsoudlou P, Dubé KN, Riley PR, Lythgoe MF, De Coppi P. Amniotic fluid stem cells are cardioprotective following acute myocardial infarction. Stem Cells Dev. 2011;20:1985–1994. doi: 10.1089/scd.2010.0424. [DOI] [PubMed] [Google Scholar]
- 63.Gunetti M, Noghero A, Molla F, Staszewsky LI, de Angelis N, Soldo A, Russo I, Errichiello E, Frasson C, Rustichelli D, Ferrero I, Gualandris A, Berger M, Geuna M, Scacciatella P, Basso G, Marra S, Bussolino F, Latini R, Fagioli F. Ex vivo-expanded bone marrow CD34(+) for acute myocardial infarction treatment: in vitro and in vivo studies. Cytotherapy. 2011;13:1140–1152. doi: 10.3109/14653249.2011.597559. [DOI] [PubMed] [Google Scholar]
- 64.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marbán E. Regenerative potential of cardio-sphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 65.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, Marbán L, Mendizabal A, Cingolani E, Johnston PV, Gerstenblith G, Schuleri KH, Lardo AC, Marbán E. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) J Am Coll Cardiol. 2014;63:110–122. doi: 10.1016/j.jacc.2013.08.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng K, Malliaras K, Li TS, Sun B, Houde C, Galang G, Smith J, Matsushita N, Marbán E. Magnetic enhancement of cell retention, engraftment, and functional benefit after intracoronary delivery of cardiac-derived stem cells in a rat model of ischemia/reperfusion. Cell Transplant. 2012;21:1121–1135. doi: 10.3727/096368911X627381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li TS, Cheng K, Lee ST, Matsushita S, Davis D, Malliaras K, Zhang Y, Matsushita N, Smith RR, Marbán E. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells. 2010;28:2088–2098. doi: 10.1002/stem.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tseliou E, de Couto G, Terrovitis J, Sun B, Weixin L, Marbán L, Marbán E. Angiogenesis, cardiomyocyte proliferation and anti-fibrotic effects underlie structural preservation post-infarction by intramyocardially-injected cardiospheres. PLoS One. 2014;9:e88590. doi: 10.1371/journal.pone.0088590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tseliou E, Pollan S, Malliaras K, Terrovitis J, Sun B, Galang G, Marbán L, Luthringer D, Marbán E. Allogeneic cardiospheres safely boost cardiac function and attenuate adverse remodeling after myocardial infarction in immunologically mismatched rat strains. J Am Coll Cardiol. 2013;61:1108–1119. doi: 10.1016/j.jacc.2012.10.052. [DOI] [PubMed] [Google Scholar]
- 72.Cho HJ, Lee N, Lee JY, Choi YJ, Ii M, Wecker A, Jeong JO, Curry C, Qin G, Yoon YS. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med. 2007;204:3257–3269. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perez-Ilzarbe M, Agbulut O, Pelacho B, Ciorba C, San Jose-Eneriz E, Desnos M, Hagège AA, Aranda P, Andreu EJ, Menasché P, Prósper F. Characterization of the paracrine effects of human skeletal myoblasts transplanted in infarcted myocardium. Eur J Heart Fail. 2008;10:1065–1072. doi: 10.1016/j.ejheart.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 74.Ibrahim AG, Cheng K, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2:606–619. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159–173. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255–265. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 78.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 79.Graham DJ, Campen D, Hui R, Spence M, Cheetham C, Levy G, Shoor S, Ray WA. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet. 2005;365:475–481. doi: 10.1016/S0140-6736(05)17864-7. [DOI] [PubMed] [Google Scholar]
- 80.McGettigan P, Henry D. Cardiovascular risk with non-steroidal anti-inflammatory drugs: systematic review of population-based controlled observational studies. PLoS Med. 2011;8:e1001098. doi: 10.1371/journal.pmed.1001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patrono C, Baigent C. Nonsteroidal anti-inflammatory drugs and the heart. Circulation. 2014;129:907–916. doi: 10.1161/CIRCULATIONAHA.113.004480. [DOI] [PubMed] [Google Scholar]
- 82.Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, Egger M, Jüni P. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086. doi: 10.1136/bmj.c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ben-Mordechai T, Holbova R, Landa-Rouben N, Harel-Adar T, Feinberg MS, Abd Elrahman I, Blum G, Epstein FH, Silman Z, Cohen S, Leor J. Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J Am Coll Cardiol. 2013;62:1890–1901. doi: 10.1016/j.jacc.2013.07.057. [DOI] [PubMed] [Google Scholar]
- 87.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014;124:1382–1392. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kanazawa H, Tseliou E, Malliaras K, Yee K, Dawkins JF, De Couto G, Smith RR, Kreke M, Seinfeld J, Middleton RC, Gallet R, Cheng K, Luthringer D, Valle I, Chowdhury S, Fukuda K, Makkar RR, Marbán L, Marbán E. Cellular postconditioning: allogeneic cardiosphere-derived cells reduce infarct size and attenuate microvascular obstruction when administered after reperfusion in pigs with acute myocardial infarction. Circ Heart Fail. 2015;8:322–332. doi: 10.1161/CIRCHEARTFAILURE.114.001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Couto G, Liu W, Tseliou E, Sun B, Makkar N, Kanazawa H, Arditi M, Marbán E. Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction. J Clin Invest. 2015;125:3147–3162. doi: 10.1172/JCI81321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kanazawa H, Tseliou E, Dawkins JF, De Couto G, Gallet R, Malliaras K, Yee K, Kreke M, Valle I, Smith RR, Middleton RC, Ho CS, Dharmakumar R, Li D, Makkar RR, Fukuda K, Marban L, Marban E. Durable benefits of cellular postconditioning: long-term effects of allogeneic cardiosphere-derived cells infused after reperfusion in pigs with acute myocardial infarction. J Am Heart Assoc. 2016;5:e002796. doi: 10.1161/JAHA.115.002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ibrahim A, Marbán E. Exosomes: fundamental biology and roles in cardiovascular physiology. Annu Rev Physiol. 2016;78:67–83. doi: 10.1146/annurev-physiol-021115-104929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Couto G, Makkar N, Marbán E. Cardiosphere-derived cell exosomes confer acute cardioprotection following ischemia-reperfusion injury through macrophage polarization. Circulation. 2015;132(suppl 3):A16991–A16991. [Google Scholar]
- 93.Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, Hunninghake GM, Vera MP, Blackwell TS, Baron RM, Feinberg MW MICU Registry. MicroRNA-181b regulates NF-κB-mediated vascular inflammation. J Clin Invest. 2012;122:1973–1990. doi: 10.1172/JCI61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Greenlee-Wacker MC. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol Rev. 2016;273:357–370. doi: 10.1111/imr.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.de Couto G, Jaghatspanyan E, Kravets E, Marbán E. Cardiosphere-derived cell exosomes reduce proinflammatory gene expression and enhance efferocytosis of macrophages to reduce infarct size following ischemia-reperfusion injury. Circulation. 2016;134(suppl 1):A16656–A16656. [Google Scholar]
- 96.Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, Tseliou E, Luthringer D, Kreke M, Smith RR, Marban L, Ghaleh B, Marban E. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 2017;38:201–211. doi: 10.1093/eurheartj/ehw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gorenek B. Tachyarrhythmias in percutaneous coronary interventions. J Electrocardiol. 2006;39:412e1–412.e5. doi: 10.1016/j.jelectrocard.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 98.Kloner RA. Current state of clinical translation of cardioprotective agents for acute myocardial infarction. Circ Res. 2013;113:451–463. doi: 10.1161/CIRCRESAHA.112.300627. [DOI] [PubMed] [Google Scholar]
- 99.Aminzadeh MA, Tseliou E, Sun B, Cheng K, Malliaras K, Makkar RR, Marbán E. Therapeutic efficacy of cardiosphere-derived cells in a transgenic mouse model of non-ischaemic dilated cardiomyopathy. Eur Heart J. 2015;36:751–762. doi: 10.1093/eurheartj/ehu196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lauden L, Boukouaci W, Borlado LR, López IP, Sepúlveda P, Tamouza R, Charron D, Al-Daccak R. Allogenicity of human cardiac stem/progenitor cells orchestrated by programmed death ligand 1. Circ Res. 2013;112:451–464. doi: 10.1161/CIRCRESAHA.112.276501. [DOI] [PubMed] [Google Scholar]
- 101.Chakravarty T, Makkar RR, Ascheim DD, Traverse JH, Schatz R, DeMaria A, Francis GS, Povsic TJ, Smith RR, Lima JA, Pogoda JM, Marbán L, Henry TD. ALLogeneic Heart STem Cells to Achieve Myocardial Regeneration (ALLSTAR) Trial: rationale and design. Cell Transplant. 2017;26:205–214. doi: 10.3727/096368916X692933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tseliou E, Kanazawa H, Dawkins J, Gallet R, Kreke M, Smith R, Middleton R, Valle J, Marbán L, Kar S, Makkar R, Marbán E. Widespread myocardial delivery of heart-derived stem cells by nonocclusive triple-vessel intra-coronary infusion in porcine ischemic cardiomyopathy: superior attenuation of adverse remodeling documented by magnetic resonance imaging and histology. PLoS One. 2016;11:e0144523. doi: 10.1371/journal.pone.0144523. [DOI] [PMC free article] [PubMed] [Google Scholar]