Abstract
Importance
Medication adherence is essential to diabetes care. Patient-provider language barriers may impact medication adherence among Latinos.
Objective
Determine the role of patient ethnicity, preferred language, and provider language concordance on adherence to newly prescribed diabetes medications.
Design
Observational study, 2006–2012.
Setting
Large, integrated health care delivery system with professional interpreter services.
Participants
Insured patients with type 2 diabetes, including English-speaking whites, English-speaking Latinos, or Limited English proficiency (LEP) Latinos with newly prescribed diabetes medication.
Exposures
Patient ethnicity, preferred language, and provider self-reported Spanish language fluency.
Main Outcomes and Measures
Primary non-adherence (never dispensed), early stage non-persistence (dispensed only once), late stage non-persistence (received ≥2 dispensings, but discontinued within 24 months), inadequate overall medication adherence (>20% time without sufficient medication supply during 24 months after initial prescription) based on new prescription medication gaps (NPMG).
Results
Participants included 21,878 whites, 5,755 English-speaking Latinos, and 3,205 LEP Latinos with 46,131 prescriptions for new diabetes medications. Among LEP Latinos, 50.2% had a primary care provider reporting high Spanish fluency. For oral medications, early adherence varied substantially: 32.2% of LEP Latinos, 27.2% of English-speaking Latinos and 18.3% of whites were either primary non-adherent or early non-persistent (p<0.05). Inadequate overall adherence was observed in 60.2% of LEP Latinos, 51.7% of English-speaking Latinos and 37.5% of whites. For insulin, early stage non-persistence was 42.8% among LEP Latinos, 34.4% among English-speaking Latinos, and 28.5 % among whites (p<0.05). After adjustment for patient demographic and clinical characteristics and provider demographics, LEP Latinos were more likely to be non-adherent to oral medications and insulin than English-speaking Latinos [RRs 1.11–1.17, p<0.05] or whites [RRs 1.36–1.49, p<0.05]. English-speaking Latinos were more likely to be non-adherent compared to whites [RRs 1.23–1.30. p<0.05]. Patient-provider language concordance was not associated with rates of non-adherence among LEP Latinos.
Conclusions and Relevance
Non-adherence to newly prescribed diabetes medication is substantially greater among Latinos than whites, even among English-speaking Latinos. LEP Latino patients are more likely to be non-adherent than English-speaking Latinos independent of the Spanish language fluency of their providers. Interventions beyond ensuring access to interpreters or patient-provider language concordance will be required to improve medication adherence among Latino patients with diabetes.
INTRODUCTION
Over 3.1 million Latinos in the US have a diagnosis of diabetes and require daily medication use. 1 Adherence to chronic medications is poor in the general population,2,3 and perhaps particularly poor among Latinos.4–6 Poor patient-provider communication has been posited as a key barrier to medication adherence.3 Language barriers in health care are common for Latinos, as over 44 percent have limited English proficiency (LEP) defined as speaking English less than “very well”.7 Language barriers between LEP Latinos and their non-Spanish-speaking providers have been associated with higher risk of poor glycemic control. In a prior study, we found that LEP Latinos whose providers did not speak Spanish were twice as likely as those with Spanish-speaking providers to have poor glycemic control (28% vs 16%).8
Language barriers might lead to poorer glycemic control via several pathways. Medication reconciliation can be difficult across language barriers 9, and providers may be reluctant to initiate or intensify medications when they are uncertain of a patient’s current medication use. Patients are less likely to initiate insulin if they feel their provider did not adequately explain the risks and benefits.10 Encounters carried out across language barriers are often less patient-centered 11 and LEP patients with language discordant providers are less likely to report trust in their providers.12 Providers who lack fluency in the patient’s language may feel stymied when attempting to elicit or address a patient’s medication concerns, 13 and patients lingering questions and lack of comprehension of their medication regimen may lead to poorer medication adherence. However, the extent to which language barriers impact medication adherence for newly prescribed medications, and how these potential barriers play out in health care settings with uniform access to professional interpreter services, is not well understood.
We designed a study to evaluate the role of ethnicity and language barriers, specifically patient-provider language concordance, on Latino patients’ non-adherence to newly prescribed oral hypoglycemic medications or insulin.
We compared medication non-adherence across four groups of insured patients with diabetes: 1) English-speaking, non-Latino whites (whites); 2) English-speaking Latinos; 3) LEP Latinos whose providers were fluent in Spanish (LEP Concordant); 4) LEP Latinos whose providers were not fluent in Spanish (LEP Discordant). Our hypotheses were that LEP discordant patients would have greater medication non-adherence than LEP concordant patients, and that non-adherence among both English-speaking and LEP Latinos would be higher than whites.
METHODS
Study Setting and Population
The study setting was Kaiser Permanente Northern California (KPNC), an integrated healthcare delivery system that provides comprehensive medical services to approximately 3.9 million members. The study population was drawn from the KPNC Diabetes Registry (KPNC-DR), a well-characterized, ethnically diverse diabetic population. 14,15
For this analysis, participants were eligible if they were active health plan members with type 2 diabetes, self-identified as Latino or white, whose preferred language was English or Spanish, and were ≥18 years old on the date they were prescribed a new oral hypoglycemic medication or insulin during 2006–2010 (n=116,802). Patients were excluded if they had end stage renal disease (n=1,744), gaps in KPNC coverage and/or pharmacy benefits for more than 2 months during the 2 years prior through the 2 years after the date of the new medication prescription (n=35,124), or were not empaneled at the time of the new medication prescription with a primary care provider (PCP) for whom Spanish language proficiency data existed (n=49,096), leaving 30,838 patients for the study.
This study was approved by the Institutional Review Boards of KPNC and the University of California, San Francisco.
Measures
Latino patients whose preferred language was Spanish in the electronic health record were considered to have LEP. To determine language concordance, we identified the Spanish fluency of each LEP Latino’s provider using data from two surveys: (1) an administrative survey given to providers upon employment, and (2) a web-based survey we developed specifically to ask KPNC providers about their Spanish language ability conducted in 2012. Both surveys asked providers to indicate their level of Spanish-speaking proficiency as “not at all”, “low”, “moderate”, or “high”. If data from both surveys were available, we used answers from the more recent web-based survey. Providers whose self-reported Spanish-speaking proficiency was “high” were considered to be fluent in Spanish16; their LEP Latino patients were designated as being in language concordant relationships (LEP concordant), LEP Latinos whose providers were not fluent in Spanish were designated as being language discordant (LEP discordant).
Non-adherence
Our outcomes of interest were measures of patient non-adherence to each newly prescribed hypoglycemic medication. Diabetes medications were classified as oral or insulin. (See Appendix A for details.) Medication adherence was estimated using medication dispensing data from KPNC pharmacies; patients’ pharmacy benefits were limited to those pharmacies. To be considered a new medication, we required that there was no dispensing of the medication in the prior 24 months; for each new medication (“index therapy”), we measured adherence subsequent to “index date”, defined as the date of first dispensing (or prescribing date if there were zero dispensings). We used four validated measures to assess medication non-adherence to each newly prescribed diabetes medication. 17–19 (See definitions in Table 1.) Three of these measures evaluated non-adherence at specific milestones, while the fourth, a continuous measure of overall adherence (new prescription medication gap (NPMG)), estimated gaps in medication supplies beginning with the index date until the end of follow-up (earlier of 24 months or censorship (date the PCP stopped the medication or switched the patient to an alternative medication)). Following convention, we then categorized patients as demonstrating “adequate” or “inadequate” adherence based on commonly used cut points for gaps in medication supplies (i.e., NPMG < 20% versus ≥ 20% of follow-up time, respectively). 18 We calculated all four measures of medication non-adherence for oral medications. For insulin, we calculated only primary non-adherence and early stage non-persistence. We did not estimate NPMG as insulin use can vary daily with patient requirements, making estimates of this and similar continuous measures of adherence unreliable when based on pharmacy dispensing data alone.
Table 1.
Definitions of Measures of Medication Adherence
| Full name | Definition |
|---|---|
| Primary non-adherence | A dichotomous measure indication that there was no dispensing of a new (index)medication within 60 days of the prescribing date. |
| Early stage non-persistence | A dichotomous measure indicating that the patient did not obtain at least one refill within the period defined by the days’ supply at the first dispensing plus a 90-day grace period. |
| Later stage non-persistence | A dichotomous measure indicating that a newly prescribed medication was refilled at least once, but discontinued before the end of follow-up. Occurs when the patient did not refill the medication within 90 days of end of cumulative days’ supply of previous dispensings. |
| New Prescription Medication Gap (NPMG) | A continuous measure which assesses overall adherence to a newly prescribed medication from the initial prescribing date to the end of follow-up (end of observation period or point of censoring) by estimating gaps in medication supplies. NPMG theoretically ranges from 100% for a patient who obtained no medication (i.e., primary non-adherent) to 0% for a patient who consistently obtained refills of the prescription in a timely fashion and thus maintained adequate medication supplies for the entire follow-up period. Following convention, adherence is categorized as “adequate” versus “inadequate” based on commonly used cut points (i.e.. < 20% versus ≥ 20% of follow-up time, respectively) |
Data Analysis
To guide covariate selection, we constructed a causal directed acyclic graph (DAG) (Appendix B) based on our team’s interpretation of a broad review of the literature and hypothesized causal pathways that could link language barriers to medication adherence.20–22 DAGs are particularly useful in analyses where many factors might influence an outcome, as they clarify the relationships between the factors and assist in defining potential confounders (which should be included as covariates) and mediators (which need not be included in analytic models). From this DAG analysis, we specified the set of covariates required in adjusted regression models to generate causal estimates of the impact of language barriers on adherence. 20,23,24
Based on our DAG, the necessary covariates included patient age, sex, race/ethnicity, socio-economic status and the provider’s race, age, and sex. Neighborhood deprivation index (NDI) 25 was included as a contextual measure of patients’ socio-economic status as socio-economic status has been associated with adherence26. Mediators in our DAG model included comorbidity index, number of non-diabetes medications, out of pocket cost, and depression. (See Appendix A for variable definitions.)
Bivariate analyses comparing covariates and other patient characteristics between specific LEP language groups were performed using chi-square tests for categorical variables, t-tests for interval variables if normally distributed, and Wilcoxon rank sums tests for interval variables not normally distributed.
Repeated measures, multivariate, modified Poisson regression models, with robust error variance and logarithm link27 were generated to calculate adjusted relative risk (RR) of the binary outcomes (i.e.,non-adherence to newly prescribed oral hypoglycemic medications or insulin). We chose this method over logistic regression or binomial regression because: (1) the odds ratio is a biased estimate of relative risk for common outcomes and (2) convergence problems are common in binomial regression models. We used robust variance estimates since log Poisson models tend to give conservative results.27 Our models also adjusted the residual covariance structure for within-subject clustering, as some patients were prescribed >1 new medication during the study. We compared English-speaking and LEP Latinos versus whites, LEP Latinos versus English-speaking Latinos, and LEP discordant versus LEP concordant Latinos.
We conducted a sensitivity analysis incorporating providers who reported “moderate” fluency into the language concordant group.
RESULTS
The study population of 30,838 included 21,878 white, 5,755 English-speaking Latino and 3,205 LEP Latino patients. Among LEP Latinos, 1,610 (50.2%) had a provider who was language concordant) and 1,595 (49.8%) patients had a provider who language discordant). (Table 2)
Table 2.
Characteristics of 30,838 Insured Patients with Diabetes by Ethnicity, English Language Proficiency, and Patient-Physician Language Concordance
| Patient/Physician Characteristics | Ethnicity and English Language Proficiency | Among LEP Latino | |||
|---|---|---|---|---|---|
|
| |||||
| White | English-speaking Latino | LEP Latino | Language concordant | Language discordant | |
| n=21878 | n=5755 | n=3205 | n=1610 | n=1595 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
|
| |||||
| Patient Characteristics | |||||
|
| |||||
| Age, mean (SD) | 62.7 (12.3) | 56.2 (13.6)a | 55.5 (13.3)bc | 55.6 (13.3) | 55.5 (13.2) |
|
| |||||
| Men | 11702 (53.5) | 3060 (53.2) | 1711 (53.4) | 841 (52.2) | 870 (54.5) |
|
| |||||
| Neighborhood Deprivation Index | |||||
| 1st Quartile (least deprived) | 5337 (24.8) | 752 (13.2)a | 216 (6.8)bc | 116 (7.3) | 100 (6.3) |
| 2nd Quartile | 6775 (31.4) | 1332 (23.4) | 511 (16.1) | 256 (16.1) | 255 (16.1) |
| 3rd Quartile | 6080 (28.2) | 1724 (30.3) | 948 (29.9) | 451 (28.4) | 497 (31.4) |
| 4th Quartile (most deprived) | 3357 (15.6) | 1874 (33.0) | 1495 (47.2) | 765 (48.2) | 730 (46.1) |
|
| |||||
| Charlson co-morbidity index | |||||
| < 1 | 5231 (23.9) | 1705 (29.6)a | 1033 (32.2)bc | 496 (30.8) | 537 (33.7) |
| 1 to < 2 | 7146 (32.7) | 2084 (36.2) | 1288 (40.2) | 652 (40.5) | 636 (39.9) |
| 2 to < 3 | 4894 (22.4) | 1208 (21.0) | 594 (18.5) | 303 (18.8) | 291 (18.2) |
| 3 or more | 4607 (21.1) | 758 (13.2) | 290 (9.0) | 159 (9.9) | 131 (8.2) |
|
| |||||
| Depression | 5046 (23.1) | 894 (15.5)a | 418 (13.0)bc | 243 (15.1) | 175 (11.0)d |
|
| |||||
| # Chronic (non-diabetic) medications dispensed, mean (SD) | 4.8 (3.1) | 3.6 (2.8)a | 3.0 (2.4)bc | 3.1 (2.5) | 3.0 (2.4) |
|
| |||||
| Out-of-pocket medication cost, mean (SD) | 113.5 (153.2) | 70.5 (104.1)a | 61.1 (88.2)bc | 63.9 (98.1) | 58.3 (76.7) |
|
| |||||
| Oral hypoglycemics | |||||
| Sulfonylureas | 8578 (39.2) | 2140 (37.2)a | 1178 (36.8)b | 608 (37.8) | 570 (35.7) |
| Biguanides | 7932 (36.3) | 2241 (38.9)a | 1275 (39.8)b | 663 (41.2) | 612 (38.4) |
| Other | 1905 (8.7) | 427 (7.4)a | 224 (7.0)b | 116 (7.2) | 108 (6.8) |
|
| |||||
| Insulin | 1510 (6.9) | 364 (6.3) | 132 (4.1)bc | 73 (4.5) | 59 (3.7) |
|
| |||||
| No diabetes medications in year prior to index date | 9733 (44.5) | 2569 (44.6) | 1445 (45.1) | 698 (43.4) | 747 (46.8)d |
|
| |||||
| Hemoglobin A1c, mean (SD) | 8.11 (1.82) | 8.65 (2.03)a | 8.76 (2.10)bc | 8.76 (2.09) | 8.76 (2.11) |
|
| |||||
| Hemoglobin A1c ≥ 9.0% | 4899 (24.4) | 1885 (35.9)a | 1075 (37.0)b | 545 (37.1) | 530 (36.9) |
|
| |||||
| Provider Characteristics | |||||
|
| |||||
| Age, mean (SD) | 45.1 (8.1) | 44.6 (7.8)a | 43.6 (7.9)bc | 43.4 (7.3) | 43.8 (8.6) |
|
| |||||
| Men | 12950 (59.2) | 3184 (55.3)a | 1733 (54.1)b | 827 (51.4) | 906 (56.8)d |
|
| |||||
| Race/Ethnicity | |||||
| White | 10337 (47.2) | 1914 (33.3)a | 890 (27.8)bc | 291 (18.1) | 599 (37.6)d |
| Hispanic/Latino | 1567 (7.2) | 768 (13.3) | 1356 (42.3) | 1163 (72.2) | 193 (12.1) |
| African American | 915 (4.2) | 275 (4.8) | 86 (2.7) | 0 (0.0) | 86 (5.4) |
| Asian | 8622 (39.4) | 2634 (45.8) | 826 (25.8) | 155 (9.6) | 671 (42.1) |
| Other/Unknown | 437 (2.0) | 164 (2.8) | 47 (1.5) | 1 (0.1) | 46 (2.9) |
Neighborhood Deprivation Index, oral hypoglycemics, insulin, and no diabetes medication use were measured for the year prior to the first index date.
Abbreviations: LEP: limited English proficient; Language concordant: LEP patient with language concordant primary care provider; Language discordant: LEP patient with language discordant primary care provider.
Whites vs. English-speaking Latinos: p-value < 0.05
Whites vs. LEP Latinos: p-value < 0.05
English-speaking Latino vs. LEP Latino: p-value < 0.05
LEP concordant vs. LEP discordant: p-value < 0.05
For categorical variables, p-values are reflective across all comparison groups.
Compared to whites, Latino patients were somewhat younger, and lived in poorer neighborhoods. They also had fewer comorbid illnesses, fewer chronic non-diabetes medications, lower out of pocket costs and were also less likely to be diagnosed with depression. However, Latino patients had higher rates of poor glycemic control (A1c>9%) than whites, with LEP Latinos at 37%, English-speaking Latinos 36% and whites 24%. LEP concordant and LEP discordant patients were similar, though depression was more commonly diagnosed among the LEP concordant (15.1% vs. 11.0%, p<0.05). (Table 2)
A total of 696 providers cared for the 30,838 patients, with each provider caring for 45 patients on average (std. dev. 33; range 1 to 156). Providers differed slightly across groups by age, gender and race, with providers caring for LEP Latino patients more likely to identify as Latino than providers caring for whites or English-speaking Latinos (42.3% vs. 7.2% and 13.3%). There were 46,131 new prescriptions for diabetes medications (39,157 oral medications, 6,974 insulin) over the five-year study period. At each adherence stage, LEP Latinos had greater non-adherence to oral medication prescriptions than English-speaking Latinos, who in turn had greater non-adherence than white patients. (Table 3) Initiation of treatment to oral medications was poor: 32.2% of LEP Latinos, 27.2% of English-speaking Latinos and 18.3% of whites were either primary non-adherent or early non-persistent. Over half (54.2%) of LEP Latinos were non-adherent to treatment with oral diabetes medications before the end of follow-up.
Table 3.
Non-adherence‡ to Newly Prescribed Diabetes Medications by Ethnicity, English Language Proficiency, and Patient-Provider Language Concordance
| Ethnicity and English Language Proficiency | Among LEP Latino | ||||
|---|---|---|---|---|---|
|
| |||||
| White | English-speaking Latino | LEP Latino | Language concordant | Language discordant | |
| Oral diabetes prescriptions | % | % | % | % | % |
|
| |||||
| Primary non-adherence | 3.6 | 5.3a | 6.5bc | 6.7 | 6.3 |
| Early stage non-persistence† | 14.7 | 21.9a | 25.7bc | 25.7 | 25.7 |
| Later stage non-persistence† | 32.4 | 45.7a | 54.2bc | 53.9 | 54.6 |
|
| |||||
| Insulin prescriptions | % | % | % | % | % |
|
| |||||
| Primary non-adherence | 6.8 | 8.0 | 8.3 | 9.4 | 7.3 |
| Early stage non-persistence† | 28.5 | 34.4a | 42.8bc | 42.2 | 43.3 |
Unadjusted probabilities from repeated measures Poisson regression models with identity link. Models adjusted for within-subject clustering, as some patients were prescribed >1 new medication during the study.
Abbreviations: LEP: limited English proficient; Language concordant: LEP patient with language concordant primary care provider; Language discordant: LEP patient with language discordant primary care physician.
Percentages for early stage non-persistence are among patients with new prescriptions that were dispensed (i.e., primary adherent). Percentages for later stage non-persistence are among patients with new prescriptions that were dispensed at least twice (i.e., among early persistent).
English-speaking Latinos vs Whites: p-value < 0.05
LEP Latinos vs Whites: p-value < 0.05
LEP Latino vs English-speaking Latino: p-value < 0.05
LEP discordant vs. LEP concordant: p-value < 0.05
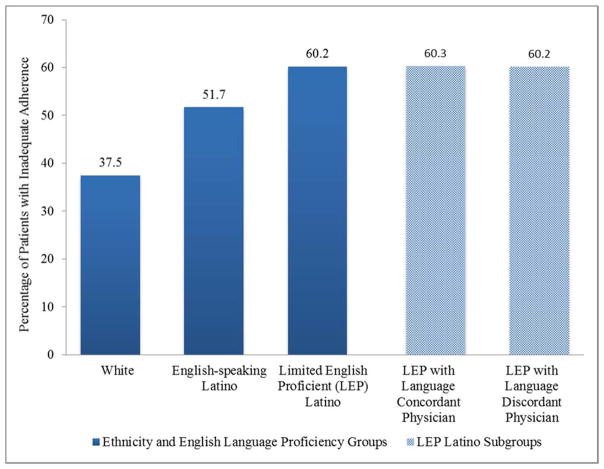
The prevalence of inadequate adherence for oral medications based on NPMG was substantially greater for Latinos than whites, and greater still for LEP Latinos (60.2% LEP Latinos, 51.7% English-speaking Latinos, 37.5% whites) (p<0.05 for each comparison) (Figure 1.).
Figure 1.
Inadequate Adherence to Newly Prescribed Oral Hypoglycemic Medications Based on New Prescription Medication Gap (NPMG) by Ethnicity, English Language Proficiency, and Patient-Physician Language Concordance. Following convention, patients are categorized as demonstrating “adequate” or “inadequate” adherence based on commonly used cut points for gaps in medication supplies (i.e., NPMG < 20% versus ≥ 20% of follow-up time, respectively).
The ethnic-language patterns for primary non-adherence for the far fewer new insulin prescriptions were similar to those of oral medications, though the differences were not statistically significant. Early stage non-persistence with insulin varied substantially: 42.8 % among LEP Latinos, 34.4% among English-speaking Latinos, and 28.5% among whites (p<0.05 for each comparison).
There were no significant differences between the LEP Concordant and LEP Discordant groups for any of the measures. The patterns described above persisted after adjustment for variables guided by our DAG (Appendix C) and in fuller models incorporating potential mediators (comorbidity index, number of non-diabetes medications, out of pocket cost, depression) (Table 4). Relative to whites, English-speaking Latinos had adjusted relative risks ranging from 1.19 to 1.30 for non-adherence to oral medications and insulin, while LEP Latinos had somewhat larger ARRs ranging from 1.36 to 1.49. LEP concordant and LEP discordant patients had similar risks of non-adherence relative to whites ranging from a low of 1.33 to a high of 1.52. The differences in primary non-adherence of insulin were smaller and not significant (Table 4).
Table 4.
Adjusted Relative Risk (95% CI) of Non-adherence to Newly Prescribed Diabetes Medications for Latino Groups Compared to Whites (N = 30,838) and for Limited English Proficient Latinos Compared to English-speaking Latinos (N = 8960)
| White (reference) | English-speaking Latino (reference) | ||||
|---|---|---|---|---|---|
|
| |||||
| English-speaking Latino | LEP Latino | LEP Concordant | LEP Discordant | LEP Latino | |
|
| |||||
| Oral diabetes prescriptions | |||||
| Primary non-adherence | 1.39 (1.23,1.58)* | 1.66 (1.43,1.92)* | 1.72 (1.40,2.12)* | 1.61 (1.35,1.93)* | 1.19 (1.02,1.39) † |
| Early stage non-persistence | 1.36 (1.28,1.45)* | 1.58 (1.47,1.70)* | 1.59 (1.43,1.76)* | 1.58 (1.44,1.72)* | 1.16 (1.07,1.26) † |
| Later stage non-persistence | 1.28 (1.23,1.33)* | 1.49 (1.42,1.56)* | 1.47 (1.37,1.56)* | 1.50 (1.42,1.59)* | 1.16 (1.11,1.22) † |
| Inadequate adherence based on NPMG | 1.27 (1.23,1.31)* | 1.43 (1.37,1.48)* | 1.40 (1.32,1.47)* | 1.45 (1.38,1.52)* | 1.13 (1.08,1.17) † |
|
| |||||
| Insulin prescriptions | |||||
| Primary non-adherence | 1.28 (1.02,1.60)* | 1.21 (0.89,1.63) | 1.23 (0.84,1.82) | 1.16 (0.77,1.75) | 0.94 (0.68,1.31) |
| Early stage non-persistence | 1.30 (1.17,1.43)* | 1.54 (1.36,1.74)* | 1.51 (1.27,1.81)* | 1.56 (1.35,1.81)* | 1.19 (1.04,1.36) † |
Note: Adjusted for patient age, sex, and neighborhood deprivation index; and provider age, sex, and race/ethnicity.
Abbreviations: LEP: limited English proficient; LEP Concordant: LEP Latino patient with language concordant primary care provider; LEP Discordant: LEP Latino patient with language discordant primary care physician. NPMG: New Prescription Medication Gap (adherence defined as “adequate” or “inadequate” based on gaps in medication supplies of < 20% or ≥ 20% of the time, respectively, starting from the prescribing date or date of first dispensing).
Compared to Whites: p-value <0.05
Compared to English-speaking Latino: p-value <0.05
Differences in non-adherence between LEP Latinos and English-speaking Latinos, while smaller than those noted between Latino groups and whites, also persisted after adjustment for patient and provider factors (Table 4). As in the unadjusted analyses, no differences in non-adherence were noted between LEP concordant versus LEP discordant patients at any stage of medication use.(data not shown).
A sensitivity analysis incorporating providers reporting “moderate” Spanish into the language concordant group resulted in a slight change in patient non-adherence by NPMG: LEP concordant 52.6% vs LEP discordant 55.3%, p= 0.13.
DISCUSSION
In this study of non-adherence to newly prescribed diabetes medications among insured patients in an integrated healthcare delivery system with uniform access to interpreter services, we found substantive, graded differences in non-adherence among whites, English-speaking Latinos and LEP Latinos. While the greatest non-adherence was among LEP Latinos, large differences in adherence between whites and Latino groups existed irrespective of English language proficiency. Contrary to our hypothesis that language discordance in the patient-provider encounter would be associated with greater medication non-adherence for LEP patients, we found no difference in non-adherence measures among the LEP Latinos when examined by the Spanish fluency of their provider.
The relationship between Latino ethnicity, English language proficiency, and medication non-adherence has been difficult to study. Although a population level study found no differences between elderly Latinos and whites in self-report of diabetes medication non-adherence,31 the association between Latino ethnicity and medication non-adherence has been observed in various clinical settings and with diverse illnesses. 28,29–31 Using this same Kaiser diabetes registry, a previous study of ongoing medication adherence (employing other measures of adherence and models with different covariates) found that a combined lipid, blood pressure and diabetes medication adherence outcome varied by ethnicity of the patient and by the language capability of the providers, though diabetes medications alone did not. 28 Multiple explanations have been posited for greater non-adherence among Latinos, including issues of pharmacy logistics32, financial barriers33, numeracy and literacy barriers34, cultural attitudes toward medications35, and language barriers36. Latinos, particularly immigrants, also have high rates of depression, which may contribute to medication non-adherence.37
Our study adds to this literature in several ways. First, it offers a clinically relevant measurement of the substantial difference in newly prescribed medication adherence between Latinos and white patients struggling with the same disease, in a setting conducive to medication adherence, and irrespective of Latino language preference. Despite favorable clinical characteristics (i.e. fewer comorbidities and less medication burden), approximately a third of LEP Latinos never started their prescribed treatment to oral medications and half never started prescribed insulin. Between 50–60% of Latino patients (English-speaking or LEP) did not have adequate supply of a newly prescribed diabetes medication during follow-up (e.g., were without medication at least 4.8 out of 24 months) while the same was true for about 37% of white patients.
Second, we also observed that language concordance between clinician and patient is insufficient to eliminate disparities in adherence for LEP Latinos with diabetes. Prior work has consistently demonstrated that LEP patients with language discordant providers report less comprehension of diagnosis and treatment, including medication instructions. 36,38 LEP patients report more adverse medication events, less trust in provider, and less satisfaction with the medical encounter. 12,36,39 Providers caring for LEP patients report that language barriers make it difficult to elicit symptoms, reconcile medications, and establish rapport.13 Why then, did we find no differences in medication adherence by patient-provider language concordance and only small differences in adherence between LEP and English-speaking Latinos? Several facts are worth considering. Medical homes that harness the skills of ethnically and linguistically diverse medical staff (e.g., nurse care managers) may play a large role, as support personnel may supplant the central role of the primary care provider in medication coaching. Uniform access to professional interpreter systems, testing and certification of bilingual staff,40 and greater provider awareness of language-associated disparities may also help mitigate language barriers in the clinical encounter. Yet, the high, non-adherence rate observed suggests that even these programs are insufficient to overcome barriers to medication adherence among LEP and English speaking Latinos; more research, using qualitative as well as quantitative methods is clearly needed to determine key factors and enable successful interventions.
Our study of medication non-adherence should not be taken to imply that language concordance is not associated with better glycemic control among Latinos. Glycemic control is dictated by a range of lifestyle factors (e.g., diet, exercise, weight control, stress) in addition to use of pharmacotherapy. In a recent study, we found that LEP Latino patients who switched from a language discordant provider to a language concordant provider improved glycemic control more than those who switched to another language discordant provider.41 Even in the absence of adherence differences, improved attention to lifestyle or intensification of insulin, which we could not capture, could account for the improvement in glycemic control observed when patients switched to language concordant providers.
Strengths and Limitations
Our study has additional strengths. To our knowledge, this the first study to determine ethnic-language medication adherence differences by examining all new diabetes medication prescriptions in the clinical record. Most studies have relied on estimates of ongoing medication adherence. The use of detailed electronic records allowed us to use multiple, validated measures of medication non-adherence to examine oral diabetes medication and insulin adherence overall and at different stages of medication use, including primary non-adherence which is typically not captured. We have shown previously that because Kaiser Permanente maintains a closed pharmacy system, ascertainment of pharmacy utilization in a sample with pharmacy benefits is quite complete.42 We were able to include a direct measure of provider Spanish fluency via a provider self-report survey in addition to administrative data. Sensitivity analysis showed little variation when we altered our cut-off for provider fluency. Finally, in what may well be a “best case” scenario for many Latinos (who often lack ongoing health care access) the integrated healthcare system setting in this study affords all patients relatively uniform access and quality of care, relatively small financial and logistical barriers to medication use, and access to bilingual staff and interpreter services, thereby increasing the internal validity of our findings.
Our study also has several limitations. We were unable to measure individual level socio-economic indicators such as education and income, health literacy or acculturation level of patients, all potentially important factors in medication adherence. Instead, we relied on the neighborhood deprivation index, a contextual measure of socioeconomic status. Second, we may have misclassification error in adjudication of patient English proficiency status using administrative data. Data from our own work and others indicates that about 20–30% of patients indicate a non-English language preference despite speaking English well.43 If patients classified as LEP with language discordant clinicians were actually English speaking, a small difference between the LEP groups may have been obscured. Adoption of current recommendations to include an English language proficiency question when capturing race, ethnicity and language data would strengthen studies of this type. Third, we measured adherence after each new prescription in the electronic medical record. If a patient refused a medication during the clinical encounter, the provider would most likely not write a prescription. In our study, lower rates of insulin use among Latinos than whites, along with higher rates of poor glycemic control among Latinos, suggests that Latino patients might be refusing insulin within the clinical encounter, resulting in no prescription and no opportunity to measure adherence. Our findings thus likely underestimate the adherence gap between Latinos and whites. Fourth, our findings may not generalize to other settings with less access to interpreters or with different Latino groups. Fifth, we were unable to systematically measure interpreter use. Sixth, we had no objective measure of provider Spanish proficiency. Finally, we have no direct measure of medication adherence beyond dispensing.
Conclusion
Our study among insured patients suggests that much more needs to be done to improve adherence to newly prescribed medications among Latino patients at all levels of English proficiency. Latinos, regardless of their language ability, had much lower rates of initiating newly prescribed diabetes medications, suggesting that early interventions should be attempted and evaluated. Given the lack of evidence of substantive differences in adherence between language concordant vs. discordant LEP patients, these interventions need to go beyond simply addressing language barriers. More research on non-adherence in this vulnerable subgroup is needed. Finally, interventions both within and outside the patient-provider interaction will likely be required to adequately support English and Spanish speaking Latino patients with the challenges of diabetes medication adherence.
Supplementary Material
Appendix A
Oral medications included alpha-glucosidase inhibitors, sulfonylureas, biguanides, thiazolidinediones, meglitinides, Dipeptidyl peptidase-4 inhibitors (DPP-4 Inhibitors), and combination oral diabetes therapies.
Insulin medication included all insulin except inhaled insulin. Injected glucagon-like peptide-1 agonists (pramlintide, exenatide, or liraglutide) were excluded from the analysis.
As a measure of socioeconomic status, we calculated the neighborhood deprivation index (NDI) based on 2010 census tract boundaries using 2005–2009 American Community Survey census data, and applied to the first recorded patient address in the period 1/1/2006–12/31/2010. 25 NDI was then categorized, based on quartiles of the distribution among all census tracts used in the creation of the NDI measure.
Patient characteristics not used as covariates (because the DAG analysis did not require them), but shown in Table 2 to further describe the patient population, included co-morbidities, depression, number of chronic (non-diabetic) medications dispensed, out-of-pocket medication cost in the 6 months prior to index date, use of oral hypoglycemics or insulin, no diabetes medication use, and hemoglobin A1c. The Charlson co-morbidity index was used to measure co-morbidities (not including diabetes) in the calendar year prior to the index date year, and was grouped into four levels (< 1, 1 to < 2, 2 to < 3, and ≥ 3). Depression was defined by physician diagnosis (ICD-9 code: 296.2x, 296.3x, 298.0, 300.4, 309.0, 309.28, 311) or prescribed medications (citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, escitalopram, bupropion, isocarboxazid, maprotiline, mirtazapine, nefazodone, phenelzine, tranylcypromine, trazodone, venlafaxine, duloxetine, desvenlafaxine) in the calendar year prior to the index date year. Number of chronic (non-diabetic) medications dispensed and out-of-pocket medication cost, use of oral hypoglycemics or insulin, or no diabetes medication use, was measured during the 12 months prior to the index date. Hemoglobin A1c was measured as the latest measure within 6 months prior to the index date and was used to indicate poor glycemic control, defined as hemoglobin A1c ≥ 9.0%.
Appendix B
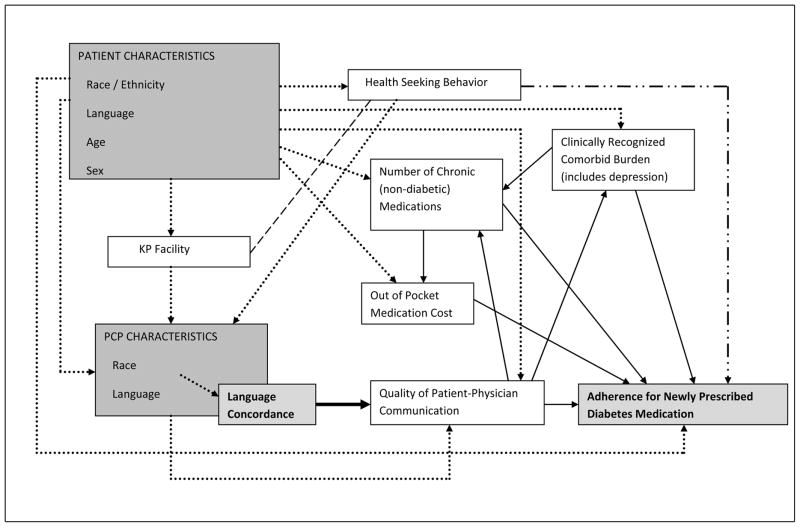
Figure 1.
Directed acyclic graph illustrating covariate selection to obtain an unconfounded estimate of the indirect effect of patient-provider language concordance on adherence for newly prescribed diabetes medications. Relationships between variables associated with patient-provider language concordance and adherence are displayed. Employing established methods for analysis of a directed acyclic graph (DAG), variables are identified as potential confounders of the association between language concordance and adherence. The analysis indicates that variables in the shaded boxes are to be included in multivariate analysis, with adjustments only for patient and provider (PCP) characteristics being necessary for inclusion as confounders, and thus covariates, in the multivariate model. Variables in the white boxes are to be excluded as potential confounders and covariates. Based on DAG analysis, dotted arrows indicate causal pathways between language concordance and medication adherence which contain confounders that have been blocked by adjustment; the dashed line represents a spurious association that does not confound the association between language concordance and medication adherence; the dash-double dotted arrow displays open causal pathway and possible residual confounding, and solid arrows identify causal pathways for the indirect effects. The resulting multivariate model estimates the indirect effect of patient-provider language concordance on adherence.
Appendix C
Results of a sensitivity analysis incorporating multiple potential mediators and confounders.
Table 4.
Sensitivity Analysis: _ Adjusted Relative Risk (95% CI) of Non-adherence to Newly Prescribed Diabetes Medications for Latino Groups Compared to Whites (N = 30,838) and for Limited English Proficient Latinos Compared to English-speaking Latinos (N = 8960)
| White (reference) | English-speaking Latino (reference) | ||||
|---|---|---|---|---|---|
|
| |||||
| English-speaking Latino | LEP Latino | LEP Concordant | LEP Discordant | LEP Latino | |
|
| |||||
| Oral diabetes prescriptions | |||||
| Primary non-adherence | 1.19 (0.98,1.43) | 1.4 (1.19,1.64)* | 1.48 (1.2,1.81)* | 1.35 (1.1,1.66)* | 1.18 (0.99,1.4) |
| Early stage non-persistence | 1.3 (1.23,1.39)* | 1.47 (1.37,1.59)* | 1.48 (1.33,1.64)* | 1.47 (1.34,1.61)* | 1.13 (1.05,1.22) † |
| Later stage non-persistence | 1.24 (1.19,1.29)* | 1.42 (1.36,1.49)* | 1.39 (1.31,1.49)* | 1.44 (1.36,1.52)* | 1.15 (1.09,1.2) † |
| Inadequate adherence based on NPMG | 1.23 (1.19,1.27)* | 1.36 (1.31,1.41)* | 1.33 (1.26,1.4)* | 1.38 (1.32,1.45)* | 1.11 (1.06,1.15) † |
|
| |||||
| Insulin prescriptions | |||||
| Primary non-adherence | 1.21 (0.96,1.51) | 1.05 (0.78,1.43) | 1.07 (0.73,1.58) | 1.01 (0.67,1.53) | 0.87 (0.63,1.21) |
| Early stage non-persistence | 1.28 (1.16,1.41)* | 1.49 (1.32,1.69)* | 1.47 (1.23,1.75)* | 1.52 (1.3,1.76)* | 1.17 (1.02,1.34) † |
Note: Adjusted for patient age, sex, neighborhood deprivation index, co-morbidities, depression, number of meds dispensed, and out-of-pocket costs; and provider age, sex, and race/ethnicity.
Abbreviations: LEP: limited English proficient; LEP Concordant: LEP Latino patient with language concordant primary care provider; LEP Discordant: LEP Latino patient with language discordant primary care physician. NPMG: New Prescription Medication Gap (adherence defined as “adequate” or “inadequate” based on gaps in medication supplies of < 20% or ≥ 20% of the time, respectively, starting from the prescribing date or date of first dispensing).
Compared to Whites: p-value <0.05
Compared to English-speaking Latino: p-value <0.05
Footnotes
Conflict of Interest Disclosures: None of the study authors have any conflicts of interest to disclose.
Author Contributions:
Alicia Fernandez, MD contributed to the study’s conception and design, drafting of manuscript, obtainment of funding, project supervision, and the acquisition, analysis and interpretation of the data. Judy Quan, PhD contributed to the statistical analysis of the data and drafting of manuscript. Howard Moffet, MPH contributed to the study’s conception and design, drafting of manuscript, and the acquisition, analysis and interpretation of the data. Melissa Parker, MS contributed to the statistical analysis of the data and drafting of manuscript. Dean Schillinger, MD, provided critical revision of the manuscript for important intellectual content. Andy J Karter, PhD, contributed to the study’s conception and design, drafting of manuscript, obtainment of funding, project supervision, and the acquisition, analysis and interpretation of the data.
References
- 1.Centers for Disease Control and Prevention NCfHS, Division of Health Interview Statistics, data from the National Health Interview Survey. 2011. 2015 http://www.cdc.gov/diabetes/statistics/prev/national/tnumhage.htm.
- 2.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2):CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez F, Cannon CP, Steg PG, et al. Predictors of long-term adherence to evidence-based cardiovascular disease medications in outpatients with stable atherothrombotic disease: findings from the REACH Registry. Clin Cardiol. 2013;36(12):721–727. doi: 10.1002/clc.22217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palacio AM, Uribe C, Hazel-Fernandez L, et al. Can phone-based motivational interviewing improve medication adherence to antiplatelet medications after a coronary stent among racial minorities? A randomized trial. J Gen Intern Med. 2015;30(4):469–475. doi: 10.1007/s11606-014-3139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Servellen G, Lombardi E. Supportive relationships and medication adherence in HIV-infected, low-income Latinos. Western Journal of Nursing Research. 2005;27(8):1023–1039. doi: 10.1177/0193945905279446. [DOI] [PubMed] [Google Scholar]
- 7.Ryan C. Language use in the United States 2011 American Community Survey Reports. 2013. [Google Scholar]
- 8.Fernandez A, Schillinger D, Warton EM, et al. Language barriers, physician-patient language concordance, and glycemic control among insured Latinos with diabetes: the Diabetes Study of Northern California (DISTANCE) J Gen Intern Med. 2011;26(2):170–176. doi: 10.1007/s11606-010-1507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clay BJ, Halasyamani L, Stucky ER, Greenwald JL, Williams MV. Results of a medication reconciliation survey from the 2006 Society of Hospital Medicine national meeting. J Hosp Med. 2008;3(6):465–472. doi: 10.1002/jhm.370. [DOI] [PubMed] [Google Scholar]
- 10.Karter AJ, Subramanian U, Saha C, et al. Barriers to insulin initiation: the translating research into action for diabetes insulin starts project. Diabetes Care. 2010;33(4):733–735. doi: 10.2337/dc09-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivadeneyra R, Elderkin-Thompson V, Silver RC, Waitzkin H. Patient centeredness in medical encounters requiring an interpreter. Am J Med. 2000;108(6):470–474. doi: 10.1016/s0002-9343(99)00445-3. [DOI] [PubMed] [Google Scholar]
- 12.Schenker Y, Karter AJ, Schillinger D, et al. The impact of limited English proficiency and physician language concordance on reports of clinical interactions among patients with diabetes: the DISTANCE study. Patient Educ Couns. 2010;81(2):222–228. doi: 10.1016/j.pec.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karliner LS, Perez-Stable EJ, Gildengorin G. The language divide. The importance of training in the use of interpreters for outpatient practice. J Gen Intern Med. 2004;19(2):175–183. doi: 10.1111/j.1525-1497.2004.30268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. Jama. 2002;287(19):2519–2527. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 15.Karter AJ, Schillinger D, Adams AS, et al. Elevated rates of diabetes in Pacific Islanders and Asian subgroups: The Diabetes Study of Northern California (DISTANCE) Diabetes Care. 2013;36(3):574–579. doi: 10.2337/dc12-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenthal A, Wang F, Schillinger D, Perez Stable EJ, Fernandez A. Accuracy of physician self-report of Spanish language proficiency. J Immigr Minor Health. 2011;13(2):239–243. doi: 10.1007/s10903-010-9320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker MM, Moffet HH, Adams A, Karter AJ. An algorithm to identify medication nonpersistence using electronic pharmacy databases. J Am Med Inform Assoc. 2015;22(5):957–961. doi: 10.1093/jamia/ocv054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karter AJ, Parker MM, Moffet HH, Ahmed AT, Schmittdiel JA, Selby JV. New prescription medication gaps: a comprehensive measure of adherence to new prescriptions. Health Serv Res. 2009;44(5 Pt 1):1640–1661. doi: 10.1111/j.1475-6773.2009.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raebel MA, Schmittdiel J, Karter AJ, Konieczny JL, Steiner JF. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care. 2013;51(8 Suppl 3):S11–21. doi: 10.1097/MLR.0b013e31829b1d2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 21.Robins JM. Data, design, and background knowledge in etiologic inference. Epidemiology. 2001;12(3):313–320. doi: 10.1097/00001648-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Hernan MA, Hernandez-Diaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. American journal of epidemiology. 2002;155(2):176–184. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 23.Pearl J. Causality: Models, Reasoning, and Inference. 2. New York, NY: Cambridge University Press; 2009. [Google Scholar]
- 24.Pearl J. Causal diagrams for empirical research. Biometrika. 1995;82(4):669–710. [Google Scholar]
- 25.Laraia BA, Karter AJ, Warton EM, Schillinger D, Moffet HH, Adler N. Place matters: neighborhood deprivation and cardiometabolic risk factors in the Diabetes Study of Northern California (DISTANCE) Soc Sci Med. 2012;74(7):1082–1090. doi: 10.1016/j.socscimed.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alsabbagh MH, Lemstra M, Eurich D, et al. Socioeconomic status and nonadherence to antihypertensive drugs: a systematic review and meta-analysis. Value Health. 2014;17(2):288–296. doi: 10.1016/j.jval.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 28.Traylor AH, Schmittdiel JA, Uratsu CS, Mangione CM, Subramanian U. Adherence to cardiovascular disease medications: does patient-provider race/ethnicity and language concordance matter? J Gen Intern Med. 2010;25(11):1172–1177. doi: 10.1007/s11606-010-1424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu D, Juarez DT, Yeboah M, Castillo TP. Interventions to increase medication adherence in African-American and Latino populations: a literature review. Hawaii J Med Public Health. 2014;73(1):11–18. [PMC free article] [PubMed] [Google Scholar]
- 30.McQuaid EL, Everhart RS, Seifer R, et al. Medication adherence among Latino and non-Latino white children with asthma. Pediatrics. 2012;129(6):e1404–1410. doi: 10.1542/peds.2011-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heisler M, Faul JD, Hayward RA, Langa KM, Blaum C, Weir D. Mechanisms for Racial and Ethnic Disparities in Glycemic Control in Middle-aged and Older Americans in the Health and Retirement Study. Arch Intern Med. 2007;167(17):1853–1860. doi: 10.1001/archinte.167.17.1853. [DOI] [PubMed] [Google Scholar]
- 32.Compton S, Haack S, Phillips CR. Identification of barriers to medication adherence in a Latino population. Res Social Adm Pharm. 2010;6(4):365–371. doi: 10.1016/j.sapharm.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Lyles CR, Seligman HK, Parker MM, et al. Financial Strain and Medication Adherence among Diabetes Patients in an Integrated Health Care Delivery System: The Diabetes Study of Northern California (DISTANCE) Health Serv Res. 2015 doi: 10.1111/1475-6773.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gazmararian JA, Kripalani S, Miller MJ, Echt KV, Ren J, Rask K. Factors associated with medication refill adherence in cardiovascular-related diseases: a focus on health literacy. J Gen Intern Med. 2006;21(12):1215–1221. doi: 10.1111/j.1525-1497.2006.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conn VS, Enriquez M, Ruppar TM, Chan KC. Cultural relevance in medication adherence interventions with underrepresented adults: systematic review and meta-analysis of outcomes. Prev Med. 2014;69:239–247. doi: 10.1016/j.ypmed.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson E, Chen AH, Grumbach K, Wang F, Fernandez A. Effects of limited English proficiency and physician language on health care comprehension. J Gen Intern Med. 2005;20(9):800–806. doi: 10.1111/j.1525-1497.2005.0174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wassertheil-Smoller S, Arredondo EM, Cai J, et al. Depression, anxiety, antidepressant use, and cardiovascular disease among Hispanic men and women of different national backgrounds: results from the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2014;24(11):822–830. doi: 10.1016/j.annepidem.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gany F, Leng J, Shapiro E, et al. Patient satisfaction with different interpreting methods: a randomized controlled trial. J Gen Intern Med. 2007;22(Suppl 2):312–318. doi: 10.1007/s11606-007-0360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slean GR, Jacobs EA, Lahiff M, Fisher L, Fernandez A. Aspects of culturally competent care are associated with less emotional burden among patients with diabetes. Med Care. 2012;50(9 Suppl 2):S69–73. doi: 10.1097/MLR.0b013e3182641127. [DOI] [PubMed] [Google Scholar]
- 40.Meyers K, Tang G, Fernandez A. Responding to the Language Challenge: Kaiser Permanente's Approach. Perm J. 2009;13(3):77–83. doi: 10.7812/tpp/08-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker MM, Fernandez A, Moffet HH, Grant RW, AT, Karter A. Switching to a Language Concordant Primary Care Provider Improves Glycemic Control among Limited English Proficiency Latinos with Type 2 Diabetes. JAMA Intern Med. 2016 doi: 10.1001/jamainternmed.2016.8648. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karter AJ, Parker MM, Moffet HH, Ahmed AT, Schmittdiel JA, Selby JV. New prescription medication gaps: a comprehensive measure of adherence to new prescriptions. Health services research. 2009;44(5 Pt 1):1640–1661. doi: 10.1111/j.1475-6773.2009.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gee GC, Walsemann KM, Takeuchi DT. English proficiency and language preference: testing the equivalence of two measures. Am J Public Health. 2010;100(3):563–569. doi: 10.2105/AJPH.2008.156976. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.