Abstract
Objective
Tenofovir (TDF) affects bone health and is widely used in pregnancy but data are limited on the effects of TDF exposure in utero. We examined the association between duration of in utero TDF exposure and linear growth in HIV-exposed, uninfected (HEU) infants.
Design
A prospective cohort of pregnant women initiating TDF-containing regimens at primary care services in Cape Town, South Africa were enrolled and followed with their breastfeeding infants through 12 months postpartum.
Methods
Length-for-age z-scores (LAZ) were calculated from infant lengths reported at birth and measured at 6, 12, 24, 36 and 48 weeks, using Fenton and World Health Organization standards. Linear mixed effects models were used to examine the association between duration of TDF exposure and LAZ over time.
Results
In 464 singleton mother-infant pairs (median CD4 at ART initiation, 346 cells/μL; viral load (VL), 4.0 log10 copies/ml), the median duration of in utero TDF exposure was 16.7 weeks (interquartile range, IQR 11.0-22.0) with 31%, 44% and 25% of infants exposed to <12, 12-22 and >22 weeks of TDF respectively. Overall, 12% of children were stunted (LAZ<-2) at 48 weeks. Duration of exposure was not associated with LAZ: adjusted mean difference for >22 vs <12 wks, -0.12 (95% CI: -0.47; 0.23); 12-22 vs <12 wks, -0.06 (95% CI: -0.35; 0.24). Mean LAZ was 0.15 lower per log increase in maternal VL at ART initiation (95% CI: -0.29; -0.0001).
Conclusions
These data suggest no association between duration of TDF exposure in utero and early linear growth.
Keywords: tenofovir, maternal exposure, infant, growth, South Africa
Introduction
In 2014, 66% of the estimated 1.5 million pregnant women living with HIV globally received efficacious antiretroviral regimens for their own health.[1] This number is set to increase substantially over the next decade with the expansion of access to triple-drug antiretroviral therapy (ART) for prevention of mother-to-child HIV transmission (PMTCT) and the promotion of universal treatment for all individuals with HIV infection.[1]
Tenofovir disoproxil fumarate (TDF) is widely used as part of first-line ART during pregnancy. Although generally safe and well-tolerated, TDF has been associated with decreased bone mineral density (BMD) and increased bone turnover in HIV-infected adults and children.[2] Data on the effect of in utero TDF exposure on bone health and growth of HIV-exposed uninfected (HEU) children have been mixed. In a large US cohort, the average length-for-age Z-score (LAZ) for 12-month old HEU infants exposed to TDF-containing ART regimens in utero was 0.14 lower than for those without TDF exposure.[3] In a similar but smaller cohort, TDF-exposed neonates had demonstrably lower bone mineral content (BMC) than their unexposed counterparts. [4] By contrast, preliminary data from the randomized IMPAACT-PROMISE trial conducted in Malawi, Zimbabwe, Uganda and South Africa showed no adverse association between TDF exposure and either birth length or neonatal BMC, although triple ART-exposed infants as a group had lower BMC than those exposed only to short course antiretrovirals for PMTCT.[5] Notably, in this study both ART regimens (with and without TDF) contained the protease inhibitor (PI) lopinavir-ritonavir (LPVr), which has also been associated with bone loss.[6]
Given the increasing number of children exposed to TDF-containing regimens in utero, more longitudinal data are needed on the potential growth effects of early exposure to ART, especially from resource-limited settings where childhood stunting is common and in the absence of concurrent PI use. Previously we found no association between TDF-exposure in utero and fetal long bone growth in a cohort of HIV-infected pregnant women using TDF-containing ART without concurrent PI use in Cape Town, South Africa. [7] Here we report on the postnatal growth of breastfed, HEU infants from the same cohort, specifically examining the association between duration of in utero TDF exposure and linear growth in the first year of life.
Methods
As part of the Maternal-Child Health-Antiretroviral (MCH-ART) study, consecutive HIV-infected, pregnant women initiating TDF-containing ART were followed during pregnancy and with their breastfeeding infants through 12 months (ClinicalTrials.gov NCT01933477).[8] The study was approved by the ethics review committees of the University of Cape Town Faculty of Health Sciences and Columbia University Medical Center.
Antiretroviral exposure
Our analysis is limited to women who initiated lifelong ART during pregnancy, on a first-line regimen of TDF with efavirenz (EFV) and either emtricitabine (FTC) or lamivudine (3TC). As per provincial guidelines, infants were prescribed nevirapine (NVP) prophylaxis within 72 hours after birth, continued through 4-12 weeks depending on MTCT risk assessment.[9]
Measures
Maternal interviews and serum collection for batched HIV viral load (VL) testing were completed at all study visits. Gestational age was based on research ultrasound at first antenatal visit, maternal recall of last menstrual period (LMP) or fundal height (from clinical records). At the first postnatal visit (within 28 days after birth), breastfeeding mother-infant pairs were recruited for additional study visits at approximately 6 (range, 0-8), 12 (9-20), 24 (21-32), 36 (33-44) and 48 (>44) weeks of age. HIV infection was excluded at 6 weeks and 48 weeks with HIV-PCR testing (Roche COBAS AmpliPrep/COBAS TaqMan HIV-1 qualitative assay; Roche Molecular systems, Branchburg, NJ). Birth length was abstracted from clinical records. Trained research staff measured maternal height at enrolment; and infant length to the nearest 0.5cm using a firm recumbent stadiometer at all subsequent study visits.
We calculated infant length-for-age Z-scores (LAZ) based on Fenton and World Health Organization growth reference standards, using a corrected age for infants born prior to 37 completed weeks of gestation.[10, 11] Stunting was defined as LAZ <-2. Duration of in utero TDF-exposure was expressed in weeks, calculated from number of days between date of ART initiation and date of delivery; for analysis, duration was categorized based on the interquartile range (<12, 12-22 and > 22 weeks). Potential third variables included maternal and infant factors known to affect child growth in general populations (maternal height, haemoglobin, substance abuse and socio-economic factors including education; infant prematurity and feeding) as well as HIV-specific factors (maternal HIV VL and CD4 cell count at ART initiation).[12, 13] A composite socio-economic score was used to categorize participants into one of three groups according to relative levels of disadvantage, as previously described.[14] Maternal smoking was assessed by self-report and the Alcohol Use Disorders Identification Test (AUDIT-C) was used to identify hazardous drinking (score ≥ 3 on questions 1-3) during pregnancy and within the first 6 months postpartum.[15]
Analytic methods
Exploratory data analysis assessed relationships between potential third variables and both the exposure of interest (duration of TDF exposure during pregnancy) and outcome of interest (LAZ over time) using visual plots, basic statistical tests and simple linear regression. Relationships between influential and other third variables were evaluated for descriptive purposes. The proportion of stunted children was compared cross-sectionally by TDF exposure categories using chi-square tests. Mixed-effects linear regression models were used to examine the association between duration of in utero TDF exposure and infant LAZ over time, using a random intercept and slope for categories of TDF exposure duration. Model building included interaction terms where indicated; final selection was based on Akaike's Information Criterion. Analyses were conducted in Stata 12 (StataCorp College Station, TX).
Results
In 464 singleton mother-infant pairs (median CD4 at enrolment, 346 cells/μL; VL, 4.0log10 copies/ml), the median duration of in utero TDF-exposure was 16.7 wks (interquartile range, IQR 11.0-22.0) with 31%, 44% and 25% of infants exposed to <12, 12-22 and >22 weeks of TDF respectively (Table 1). Mothers and infants were followed for a median of 76.0 (IQR 52.6-79.0) weeks postpartum. Fifty-eight (12.5%) did not complete study follow-up, predominantly due to relocation (n=29), maternal or infant demise (n=9) or work/school commitments (n=8). Fifteen mother-infant pairs were considered lost to follow-up (median follow-up time 13.0 weeks, IQR 6.0-26.4), with no difference by TDF exposure (5, 7 and 3 in the <12, 12-22 and > 22 weeks' groups respectively).
Table 1. Characteristics of women at enrolment and infants by duration of in utero exposure to tenofovir.
| Duration of TDF exposure (weeks) | TOTAL (N=464) | |||
|---|---|---|---|---|
| < 12 (n=144) | 12-22 (n=203) | > 22 (n=117) | ||
| MATERNAL | ||||
| Age (years) | 27 (24-32) | 28 (24 – 32) | 27 (25 – 31) | 28 (24-32) |
| Height (cm) | 159 (154 – 163) | 156 (153-160) | 157 (153 – 161) | 157 (153.5 – 161) |
| Anemia prior to ART initiation (hemoglobin < 10 g/dL) | 29.6% (40) | 20.5% (41) | 8.8% (10) | 20.3% (91) |
| HIV viral load (log10 copies/mL) prior to ARTinitiation | 3.9 (3.3 – 4.5) | 4.2 (3.7 – 4.7) | 4.0 (3.5 – 4.3) | 4.0 (3.5 – 4.6) |
| HIV viral load <50 copies/mL at delivery | 59.0% (85) | 78.8% (160) | 90.6% (106) | 75.7% (361) |
| CD4 cell count (cells/mm3) | 356 (255 – 539) | 345 (220 – 492) | 337 (251 – 487) | 346 (235 – 502) |
| SES categories: | ||||
| Lowest | 34.7% (50) | 28.5% (58) | 23% (27) | 29% (135) |
| Moderate | 31.3% (45) | 38% (77) | 35% (41) | 35% (163) |
| Highest | 34% (49) | 33.5% (68) | 42% (49) | 36% (166) |
| AUDIT-C: Above threshold for hazardous drinking during the first 6 months postpartum | 11.9% (14) | 10.1% (19) | 8.2% (8) | 10.2% (41) |
| INFANT | ||||
| Duration of TDF exposure (weeks) | 7.3 (4.2-10.4) | 17.1 (15.1 – 19.1) | 25.3 (23.1 – 26.5) | 16.7 (11.0-21.9) |
| Male sex | 53% (77) | 45% (92) | 53% (62) | 50% (231) |
| Gestational age at birth (weeks) | 39 (37–40) | 39 (38-40) | 39 (38-40) | 39 (38-40) |
| Premature delivery | ||||
| 34-37 completed weeks | 9.0% (13) | 6.9% (14) | 7.7% (9) | 7.8% (36) |
| < 34 completed weeks | 9.0% (13) | 2.5% (5) | 0.0% (0) | 3.9% (18) |
| Duration of any breastfeeding (months) | 7.6 (2.2 – 12.1) | 7.0 (2.2 – 12.2) | 5.3 (2.1 – 12.1) | 6.6 (2.2 – 12.2) |
| Length at birth (cm) | 49 (47 – 51) | 49 (48 – 52) | 49.5 (48 – 51) | 49 (48 – 51) |
| LAZ1 | 0.23 (-0.78 to 1.32) | 0.44 (-0.78 to 1.47) | 0.30 (-0.60 to 1.47) | 0.32 (-0.77 to 1.46) |
| Stunted (LAZ<-2SD) | 5.8% (7) | 4.9% (9) | 9.2% (10) | 6.3% (26) |
| Weight at birth (kg) | 3.08 (2.7 – 3.4) | 3.12 (2.8 – 3.4) | 3.2 (2.9 – 3.4) | 3.15 (2.76 – 3.4) |
| WAZ1 | 0.03 (-0.84 to 0.52) | -0.26 (-0.80 to 0.43) | -0.02 (-0.59 to 0.48) | -0.10 (-0.80 to 0.48) |
| Underweight (WAZ<-2SD) | 3.8% (5) | 2.6% (5) | 2.6% (3) | 2.9% (13) |
Values are median (IQR) or column % (n); TDF, tenofovir (exposure calculated from day of antiretroviral therapy initiation until day of birth); ART, triple antiretroviral therapy; AUDIT-C, Alcohol Use Disorders Identification Test for hazardous drinking (questions 1-3); LAZ – length-for-age Z-score: SD – standard deviation; WAZ – weight-for-age Z-score;
Calculated using Fenton growth charts (adjusted for gestational age and sex)
Log VL at enrolment was similar between TDF exposure groups but a larger proportion of mothers from the >22 weeks' group had achieved viral suppression (≤ 50 copies/mL) by delivery compared to those from the 12-22 and <12 week groups (91% vs. 79% and 59%, respectively). Compared to those with 12-22 weeks and <12 weeks of TDF use, mothers in the >22 weeks' group were also less likely to have anaemia before initiating ART, report hazardous drinking, or be in the lowest SES category (Table 1). Median gestational age at birth did not differ between groups although a larger proportion of infants with <12 weeks of TDF exposure were born prematurely (table 1). Median duration of breastfeeding did not vary substantially between the groups (table 1). Exclusive breastfeeding (EBF) was initiated by 93% (432/464) of mothers; the median duration of EBF was 3.0 months (IQR, 1.3 – 5.7 months).
The median LAZ in the cohort was slightly above zero at birth (0.32, IQR -0.77 to 1.46), and declined thereafter, but did not vary by duration of TDF exposure at any time point (Figure 1). The lowest median LAZ was observed at 48 weeks (-0.65, IQR -1.36 to 0.11). Overall 12% of children were stunted at 48 weeks (Table 2). The prevalence of stunting did not vary between categories of TDF exposure at early study visits (Table 2). At 48 weeks of age, a somewhat higher prevalence of stunting was observed among infants exposed to <12 weeks of TDF compared to those exposed to 12-22 and > 22 weeks (18%, compared to 9% and 10% respectively, p=0.12).
Figure 1. Length-for-age Z-scores of HIV-exposed uninfected infants at birth and during the first 12 months of life, by duration of in utero tenofovir exposure, IQR, inter-quartile range; w, weeks; TDF, tenofovir disoproxil fumarate.
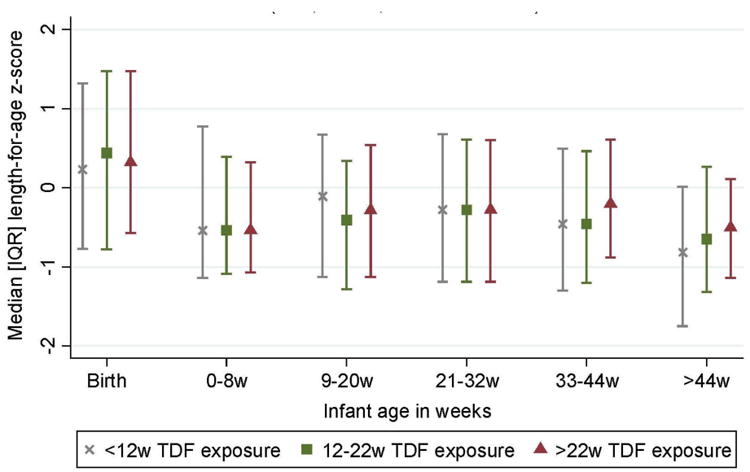
Table 2. Postnatal linear growth by duration of in utero exposure to tenofovir.
| Duration of TDF exposure (weeks) | TOTAL (N=464) | ||||
|---|---|---|---|---|---|
| < 12 (n=144) | 12-22 (n=203) | > 22 (n=117) | p-value | ||
| 1st Postnatal visit (< 2 months, 0-8 wks) | |||||
| Length-for-age Z-score | -0.54 (-1.14 to 0.77) | -0.54 (-1.09 to 0.39) | -0.55 (-1.07 to 0.32) | - | -0.54 (-1.14 to 0.39) |
| Stunted (LAZ<-2SD), (n) 1 | 12.4% (14/113) | 14.7% (27/184) | 9.3% (10/107) | 0.42 | 12.6% (51/404) |
| 2nd Postnatal visit (2-4 months, 9-20 wks) | |||||
| Length-for-age Z-score | -0.11 (-1.13 to 0.67) | -0.41 (-1.28 to 0.34) | -0.28 (-1.13 to 0.54) | - | -0.29 (-1.21 to 0.52) |
| Stunted (LAZ<-2SD), (n)1 | 11.7% (11/94) | 9.1% (15/165) | 8.8% (9/102) | 0.74 | 9.7% (35/361) |
| 3rd Postnatal visit (5-7 months, 21-32 weeks) | |||||
| Length-for-age Z-score | -0.28 (-1.19 to 0.68) | -0.28 (-1.19 to 0.61) | -0.28 (-1.19 to 0.60) | - | -0.28 (-1.19 to 0.66) |
| Stunted (LAZ<-2SD), (n)1 | 9.5% (10/105) | 11.8% (21/178) | 6.5% (6/93) | 0.37 | 9.8% (37/376) |
| 4th Postnatal visit (8-10 months, 33-44 weeks) | |||||
| Length-for-age Z-score | -0.46 (-1.3 to 0.49) | -0.46 (-1.2 to 0.46) | -0.21 (-0.88 to 0.67) | - | -0.44 (-1.2 to 0.48) |
| Stunted (LAZ<-2SD), (n)1 | 10.3% (10/97) | 9.0% (15/166) | 4.6% (4/88) | 0.32 | 8.3% (29/351) |
| 5th Postnatal visit (≥11 months, > 44 weeks) | |||||
| Length-for-age Z-score | -0.82 (-1.75 to 0.01) | -0.65 (-1.32 to 0.26) | -0.51 (-1.14 to 0.11) | - | -0.65 (-1.36 to 0.11) |
| Stunted (LAZ<-2SD), (n)1 | 17.6% (16/91) | 9.15% (14/153) | 9.7% (6/62) | 0.12 | 11.8% (36/306) |
Values are median (IQR) or column % (n); p-values calculated using Chi2 statistic
TDF, tenofovir (exposure calculated from day of antiretroviral initiation until day of birth); LAZ – length-for-age Z-score; calculated using World Health Organization MSGR (adjusted for gestational age and sex)
Missing data for lengths: group denominator (n) for length measures as follows: 1st visit = 404; 2nd visit = 361; 3rd visit = 376: 4th visit = 351; 5th visit = 306
There was no association between duration of in utero TDF-exposure and LAZ in either univariable or multivariable analysis (Table 3): mean adjusted difference in LAZ (aβ) for >22 vs <12 wks, -0.12 (95% CI: -0.47; 0.23); aβ for 12-22 vs <12 wks, -0.06 (95% CI: -0.35; 0.24). The model was adjusted for maternal VL at ART initiation, height, SES, preterm delivery, hazardous drinking in the early postpartum period, and current breastfeeding as a time-varying covariate (Table 3). Maternal height (aβ=0.03 per cm increment, 95% CI: 0.01; 0.05), log VL at ART initiation (aβ= -0.15 per log increase, 95% CI: -0.29; -0.0001) and infant gestational age [aβ=-0.73 (95% CI -1.3; -0.12) for <34 weeks and aβ=-0.1 (95% CI -0.5; 0.32) for 34-37 weeks vs. ≥37 weeks] were strongly associated with infant LAZ.
Table 3. Regression analyses of infant length-for-age Z-scores by duration of in utero exposure to tenofovir.
| Univariable mixed-effects regression (N=461) | Multivariable mixed-effects regression1 (N=323) | |||
|---|---|---|---|---|
| Duration of in utero exposure to tenofovir | Difference in LAZ (95% CI) | p-value | Difference in LAZ (95% CI) | p-value |
| > 22 weeks | 0.05 (-0.22 to 0.31) | 0.72 | -0.12 (-0.47 to 0.23) | 0.49 |
| 12- 22 weeks | -0.02 (-0.25 to 0.21) | 0.87 | -0.06 (-0.35 to 0.24) | 0.71 |
| < 12 weeks (reference) | 0 | - | 0 | - |
95% CI – 95% confidence interval; LAZ – length-for-age Z-scores, calculated using Fenton (birth) and World Health Organization (postnatal) growth reference standards (adjusted for sex and gestational age);
Adjusted for maternal characteristics at initiation of antiretroviral treatment (height, log10 HIV viral load, hemoglobin, cd4 cell count, socio-economic situation), preterm delivery, hazardous drinking in the early postpartum period, and time-varying, current breastfeeding
Discussion
Our findings demonstrate a reassuring lack of association between duration of TDF exposure in utero and linear growth in the first year of life among breastfed HEU infants, and patterns of early linear growth that are broadly comparable to those of the general population. We further demonstrate two independent risk factors for suboptimal linear growth among HEU children, namely high maternal VL at initiation of treatment in pregnancy, and premature delivery.
The lack of a significant effect of TDF-exposure on infant linear growth is consistent with findings from other African data on this association. A recent study in Malawi reported no negative growth effects among breastfed HEU children exposed to TDF vs. non-TDF-containing ART regimens in a setting of universal maternal ART during pregnancy.[16] A reflection of the evolving Malawian national PMTCT guidelines at the time, mothers with infants exposed to TDF-containing regimens were encouraged to breastfeed for 12 months compared to the recommended weaning age of 6 months for the earlier group who comprised all non-TDF-exposed infants. The authors postulate that the higher attained 12-month LAZ among TDF-exposed infants (LAZ at 12 months higher by 0.48, 95% CI 0.25 to 0.71) was mediated through extended breastfeeding. TDF is a highly potent antiretroviral;[17] an additional explanation may be that maternal viral suppression was achieved more effectively and rapidly among women receiving the TDF-containing regimen, limiting fetal exposure to circulating virus and its consequences. In an earlier report from Uganda and Zimbabwe, where ART was restricted to women with advanced disease, TDF-exposed children under 2 years of age also attained higher LAZ than their TDF-unexposed counterparts (LAZ at 48 weeks, -1.13 in TDF-exposed vs. -2.22 in TDF-unexposed group; p=0.03); however, the differences were negated over time, with no residual differences by age 3 years.[18] While the recent IMPAACT-PROMISE results raise concern regarding bone effects of LPVr-containing ART-exposure, no association between TDF and either BMC or neonatal growth was observed.[5]
Reasons for the apparently differential effect of TDF on bone growth in the African compared to US cohorts remain unclear, but may be due in part to differences in prescribing practices and indications for treatment. In contrast to the observational African cohorts, the US cohorts have been followed over several years and in multiple sites. Over time, substantial changes have occurred in prescribing patterns resulting in highly heterogeneous ART exposure, making it difficult to single out the effects of a single drug whilst also increasing the risk for confounding by indication.[3, 4] In particular, the use of TDF with a PI has generally been more common in the US than in African settings including ours,[16, 18, 19] and residual confounding may explain some of the differences observed in observational studies. Differences in co-occurring risk factors for poor child growth may however also contribute to the differential results. For example, the background prevalence of childhood under nutrition – and intergenerational stunting - is generally higher in resource limited settings;[12] potential adverse growth effects resulting from TDF exposure may be difficult to distinguish in background settings of impaired child growth.
The HEU infants in our study demonstrated linear growth comparable to the general South African population; the estimated prevalence of stunting in our study (12% at 1 year) is roughly half the national South African prevalence of stunting among children <3 years.[20] In keeping with findings from other settings in sub-Saharan Africa, the average LAZ of our cohort decreased over time, highlighting the need for better complementary feeding practices in resource-limited settings where food insecurity and low dietary diversity are common.[16]
Of note, our data suggest a confounding effect of gestational age at delivery on the relationship between duration of TDF exposure and infant linear growth.[21] Although prematurity is a well described, independent predictor of childhood stunting, the association seen in our data is partly artificial as a result of how our exposure variable was defined; infants born at earlier stages of pregnancy were necessarily exposed to ART for shorter periods of time. Nonetheless, maternal HIV infection is a known risk factor for preterm delivery, which in turn substantially increases the risk of child mortality and morbidity including suboptimal growth.[22, 23] As such, identifying and providing appropriate care to premature infants should be integral to strategies aimed at optimizing HEU child health.
We found higher pre-treatment maternal VL to be associated with reduced postnatal linear growth. Several possible mechanisms can be hypothesized. Firstly, higher HIV VL is strongly associated with increased immune activation.[24, 25] Substantial immune activation in maternal/fetal units – commonly seen in chronic viral infections during pregnancy - has been associated with long-term risk of neurodevelopmental disorders; fetal programming for linear growth might be similarly affected.[26, 27] Although severe immune reconstitution inflammatory syndrome (IRIS) is not common in pregnancy, the fetal effects of maternal immune reconstitution during pregnancy (particularly among women with advanced disease initiating potent ART) are not clear. The risk of ART-related bone resorption in HIV-infected adults appears to be highest among those initiating treatment at advanced disease stages,[6, 28] and a similar relationship may exist between disease severity at ART initiation in pregnancy and subsequent infant health outcomes. HIV disease severity is also associated with increased risk of maternal cytomegalovirus (CMV) reactivation, which increases the risk for congenital CMV and in turn, growth restriction. [26, 29, 30] Finally, HEU children born to women with advanced HIV disease are known to be at higher risk for common childhood infections;[12] in turn, recurrent infections substantially increase the risk of childhood stunting.[21]
Our data are unique in providing longitudinal clinical growth data on a well-characterized cohort of TDF-exposed HEU children not exposed to PI-containing regimens, in a setting with universal maternal ART and breastfeeding. However, our study is limited by the lack of a non-TDF exposed comparison group, the lack of serum bone markers and radiographic measures of BMC. Moreover, these observational data could not control for possible unobserved systematic differences between TDF exposure categories, and data from randomized clinical trials comparing child growth outcomes following in utero exposure to different drug combinations remain critical.
Conclusion
As HIV-free survival continues to increase dramatically across Africa, optimizing the health of HEU children is a growing priority. Long-term monitoring of potential adverse growth effects of in utero ART exposure is a crucial step towards finding optimal drug regimens for pregnant and breast feeding women. We found no evidence of adverse linear growth following in utero TDF exposure, and demonstrate maternal HIV disease severity and premature birth as risk factors for poor infant growth. These findings provide reassuring safety data in support of currently recommended ART regimens for pregnant women, and support prevention of premature delivery with early diagnosis and treatment of HIV-infected women as crucial strategies to optimize the health of their uninfected children.
Acknowledgments
The authors thank the study staff at the Gugulethu Midwife & Obstetric Unit and the mothers and infants who participated in the study. This research was supported by PEPFAR through NICHD under Cooperative Agreement 1R01HD074558. Additional funding comes from the Elizabeth Glaser Pediatric AIDS Foundation, South African Medical Research Council, the Fogarty Foundation (NIH Fogarty International Center Grant #5R25TW009340) and the Office of AIDS Research. J.J. is supported by NICHD K23HD070760.
SLR assisted with collection of data, conducted the analysis and wrote the first draft of the manuscript. JJ provided consultation on the concept, manuscript drafting and analysis. LM and EJA conceived the MCH-ART study, and were responsible for study design, funding, implementation and overall leadership. TKP was the study coordinator. TKP and KB were responsible for data management and oversight. KB contributed to the data analysis. SO was responsible for data cleaning processes. AR and AZ were the senior study managers and provided oversight of all study administration processes.
Sources of support: Research supported by PEPFAR through NICHD under Cooperative Agreement 1R01HD074558. Additional funding comes from the Elizabeth Glaser Pediatric AIDS Foundation, South African Medical Research Council, the Fogarty Foundation (NIH Fogarty International Center Grant #5R25TW009340) and the Office of AIDS Research. J.J. is supported by NICHD K23HD070760.
Footnotes
All authors contributed to and approved the final manuscript.
References
- 1.WHO. Global health sector response to HIV, 2000-2015. [accessed 18 April 2016];Focus on innovations in Africa: progress report. 2015 http://apps.who.int/iris/bitstream/10665/198065/1/9789241509824_eng.pdf.
- 2.Grant PM, Cotter AG. Tenofovir and bone health. Curr Opin HIV AIDS. 2016;11:326–332. doi: 10.1097/COH.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siberry GK, Williams PL, Mendez H, Seage GR, 3rd, Jacobson DL, Hazra R, et al. Safety of tenofovir use during pregnancy: early growth outcomes in HIV-exposed uninfected infants. AIDS. 2012;26:1151–1159. doi: 10.1097/QAD.0b013e328352d135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siberry GK, Jacobson DL, Kalkwarf HJ, Wu JW, DiMeglio LA, Yogev R, et al. Lower Newborn Bone Mineral Content Associated With Maternal Use of Tenofovir Disoproxil Fumarate During Pregnancy. Clin Infect Dis. 2015;61:996–1003. doi: 10.1093/cid/civ437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siberry G, C T, Stranix-Chibanda L, Marr C, Shepherd JA, Browning R, et al. Impact of Maternal Tenofovir Use on HIV-Exposed Newborn Bone Mineral. Abstract #36; Conference on Retroviruses and Opportunistic Infections (CROI); Boston, Massachusetts. 2016. [Google Scholar]
- 6.Moran CA, Weitzmann MN, Ofotokun I. The protease inhibitors and HIV-associated bone loss. Curr Opin HIV AIDS. 2016;11:333–342. doi: 10.1097/COH.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jao J, Abrams EJ, Phillips T, Petro G, Zerbe A, Myer L. In Utero Tenofovir Exposure Is not Associated With Fetal Long Bone Growth. Clin Infect Dis. 2016;62:1604–1609. doi: 10.1093/cid/ciw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myer L, Phillips TK, Zerbe A, Ronan A, Hsiao NY, Mellins CA, et al. Optimizing Antiretroviral Therapy (ART) for Maternal and Child Health (MCH): Rationale and Design of the MCH-ART Study. JAIDS. 2016;72 Suppl 2:S189–196. doi: 10.1097/QAI.0000000000001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.PAWC. PMTCT Clinical Guidelines Update. [accessed 6 October 2016];2013 https://www.westerncape.gov.za/assets/departments/health/wcp_2013_pmtct_clinical_guidelines_update_final_replacement_2.pdf.
- 10.WHO. WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. [accessed 2 May 2016];2006 http://www.who.int/childgrowth/standards/technical_report/en/
- 11.Fenton TR, Nasser R, Eliasziw M, Kim JH, Bilan D, Sauve R. Validating the weight gain of preterm infants between the reference growth curve of the fetus and the term infant. BMC Pediatr. 2013;13:92. doi: 10.1186/1471-2431-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prendergast AJ, Humphrey JH. The stunting syndrome in developing countries. Paediatr Int Child Health. 2014;34:250–265. doi: 10.1179/2046905514Y.0000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhn L, Kasonde P, Sinkala M, Kankasa C, Semrau K, Scott N, et al. Does severity of HIV disease in HIV-infected mothers affect mortality and morbidity among their uninfected infants? Clinical Infectious Diseases. 2005;41:1654–1661. doi: 10.1086/498029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brittain K, Mellins CA, Phillips T, Zerbe A, Abrams EJ, Myer L, et al. Social Support, Stigma and Antenatal Depression Among HIV-Infected Pregnant Women in South Africa. AIDS Behav. 2016 doi: 10.1007/s10461-016-1389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.2nd. World Health Organization Department of Mental Health and Substance Dependence; 2001. [accessed 23 June 2016]. Guidelines for Use in Primary Care. WHO. AUDIT. The Alcohol Use Disorders Identification Test. http://apps.who.int/iris/bitstream/10665/67205/1/WHO_MSD_MSB_01.6a.pdf. [Google Scholar]
- 16.Liotta G, Floridia M, Andreotti M, Jere H, Sagno JB, Marazzi MC, et al. Growth indices in breastfed infants pre and postnatally exposed to tenofovir compared with tenofovir-unexposed infants. AIDS. 2016;30:525–527. doi: 10.1097/QAD.0000000000000944. [DOI] [PubMed] [Google Scholar]
- 17.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 18.Gibb DM, Kizito H, Russell EC, Chidziva E, Zalwango E, Nalumenya R, et al. Pregnancy and infant outcomes among HIV-infected women taking long-term art with and without tenofovir in the DART trial. [accessed 3 July 2016];2012 :e1001217. doi: 10.1371/journal.pmed.1001217. http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1001217. [DOI] [PMC free article] [PubMed]
- 19.Pintye J, Langat A, Singa B, Kinuthia J, Odeny B, Katana A, et al. Maternal Tenofovir Disoproxil Fumarate Use in Pregnancy and Growth Outcomes among HIV-Exposed Uninfected Infants in Kenya. Infect Dis Obstet Gynecol. 2015;2015:276851. doi: 10.1155/2015/276851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Said-Mohamed R, Micklesfield LK, Pettifor JM, Norris SA. Has the prevalence of stunting in South African children changed in 40 years? A systematic review BMC Public Health. 2015;15:534. doi: 10.1186/s12889-015-1844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 22.March of Dimes, PMNCH, Save the Children, WHO. Born Too Soon: The Global Action Report on Preterm Birth. [accessed 3 July 2016];2012 http://apps.who.int/iris/bitstream/10665/44864/1/9789241503433_eng.pdf?ua=1.
- 23.Xiao PL, Zhou YB, Chen Y, Yang MX, Song XX, Shi Y, et al. Association between maternal HIV infection and low birth weight and prematurity: a meta-analysis of cohort studies. BMC Pregnancy Childbirth. 2015;15:246. doi: 10.1186/s12884-015-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2015;29:463–471. doi: 10.1097/QAD.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borges AH, O'Connor JL, Phillips AN, Ronsholt FF, Pett S, Vjecha MJ, et al. Factors Associated With Plasma IL-6 Levels During HIV Infection. J Infect Dis. 2015;212:585–595. doi: 10.1093/infdis/jiv123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dauby N, Goetghebuer T, Kollmann TR, Levy J, Marchant A. Uninfected but not unaffected: chronic maternal infections during pregnancy, fetal immunity, and susceptibility to postnatal infections. Lancet Infect Dis. 2012;12:330–340. doi: 10.1016/S1473-3099(11)70341-3. [DOI] [PubMed] [Google Scholar]
- 27.Khandaker GM, Zimbron J, Lewis G, Jones PB. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med. 2013;43:239–257. doi: 10.1017/S0033291712000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ofotokun I, Titanji K, Vunnava A, Roser-Page S, Vikulina T, Villinger F, et al. Antiretroviral therapy induces a rapid increase in bone resorption that is positively associated with the magnitude of immune reconstitution in HIV infection. AIDS. 2016;30:405–414. doi: 10.1097/QAD.0000000000000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filteau S, Rowland-Jones S. Cytomegalovirus Infection May Contribute to the Reduced Immune Function, Growth, Development, and Health of HIV-Exposed, Uninfected African Children. Front Immunol. 2016;7:257. doi: 10.3389/fimmu.2016.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gompels UA, Larke N, Sanz-Ramos M, Bates M, Musonda K, Manno D, et al. Human cytomegalovirus infant infection adversely affects growth and development in maternally HIV-exposed and unexposed infants in Zambia. Clinical Infectious Diseases. 2012;54:434–442. doi: 10.1093/cid/cir837. [DOI] [PMC free article] [PubMed] [Google Scholar]


