Abstract
OBJECTIVE
Adolescents and young adults without childhood ADHD often present to clinics seeking stimulant medication for late-onset Attention-Deficit/Hyperactivity Disorder (ADHD) symptoms. Recent birth-cohort studies support the notion of late-onset ADHD, but these investigations are limited by relying on screening instruments to assess ADHD, not considering alternative causes of symptoms, or failing to obtain complete psychiatric histories. We address these limitations by examining psychiatric assessments administered longitudinally to the local normative comparison group of the Multimodal Treatment Study of ADHD.
METHOD
Individuals without childhood ADHD (N=239) were administered eight assessments from comparison baseline (M age=9.89) to young adulthood (M age=24.40). Diagnostic procedures utilized parent, teacher, and self reports of ADHD symptoms, impairment, substance use, and other mental disorders, with consideration of symptom context and timing.
RESULTS
Approximately 95% of individuals who initially screened positive on symptom checklists were excluded from late-onset ADHD diagnosis. Among individuals with impairing late-onset ADHD symptoms, the most common reason for diagnostic exclusion was symptoms or impairment occurring exclusively in the context of heavy substance use. Most late-onset cases displayed onset in adolescence and an adolescence-limited presentation. There was no evidence for adult-onset ADHD independent of a complex psychiatric history.
CONCLUSIONS
Individuals seeking treatment for late-onset ADHD may be valid cases; however, more commonly, symptoms represent non-impairing cognitive fluctuations, a comorbid disorder, or the cognitive effects of substance use. False positive late-onset ADHD cases are common without careful assessment. Clinicians should carefully assess impairment, psychiatric history, and substance use before treating potential late-onset cases.
In recent years, an influx of adolescents and young adults without documented childhood ADHD have presented to clinics with complaints of inattention and/or hyperactivity/impulsivity symptoms, often inquiring about stimulant medication.1–3 It remains unclear whether this trend is driven by typically developing individuals seeking stimulant medication for cognitive enhancement or by individuals with late-onset ADHD that warrants medical treatment. Recent birth cohort studies support the phenomenon of late-onset ADHD, reporting 2.5%–10.7% prevalence for a form of ADHD that first emerges in adolescence or adulthood.4–7 These studies claim that most adult ADHD cases (67.5%–90.0%) do not experience symptom onset in childhood. This claim is contrary to decades of research characterizing ADHD as a chronic neurodevelopmental disorder with symptoms that appear before age 12.8–11 The authors speculate that late-onset ADHD may appear spontaneously, but critics suggest that these cases may also represent individuals with undetected childhood symptoms (i.e., late-identified rather than late-onset).12–14
Critics also suggest that late-onset ADHD prevalence may be inflated by methodological artifacts, such as reliance on ADHD screening instruments, inability to detect symptoms that emerged in long gaps between assessments, a false-positive paradox, and failure to consider other mental disorders, health problems, or substance abuse as the source of symptoms.12–14 If many late-onset cases are false positives, this may misinform the field’s understanding of ADHD as a chronic disorder and overstate its prevalence. On the other hand, true late-onset ADHD may partially explain the uptick in adolescents and young adults seeking first-time treatment for newly reported difficulties.4–7
This study investigates late-onset ADHD in the local normative comparison group of the Multimodal Treatment Study of ADHD, which was designed to carefully assess ADHD symptoms over time.15–16 For 14 years from childhood to adulthood, comparison participants underwent comprehensive psychiatric evaluations with multi-informant assessment of ADHD symptoms and impairments.17–18 Due to the frequency (8 time-points) and comprehensiveness of these assessments, ADHD symptom onset, other mental disorders, impairments, and substance use can be isolated temporally and considered when determining the history and nature of potential late-onset cases. Through careful review of multi-informant longitudinal psychiatric data using a stepped diagnostic procedure that pinpoints symptom origins, we aimed to: (1) understand what proportion individuals with reported late-onset ADHD symptoms represent true cases of the disorder and (2) provide detailed clinical profiles for identified late-onset ADHD cases. Our procedure complements the epidemiological population studies by exploring the nature of late-onset ADHD after addressing previously noted methodological confounds and illustrating how late-onset ADHD might emerge over time.12–14
METHOD
The Multimodal Treatment Study of ADHD compared effects of 14 months of pharmacological and psychosocial treatments for children (7.0–9.9 years old) with ADHD-Combined Type.15 Two years after baseline, 289 classmates were recruited for the local normative comparison group. The Multimodal Treatment Study of ADHD continued with prospective follow-up until 16 years after baseline.15–18 Informed consent was obtained in childhood and adulthood.
Participants
We identified a comparison group subsample (N=239; see Table 1) who did not meet diagnostic criteria for ADHD during childhood baseline assessment and who had at least one assessment in adolescence (ages 12–17) and adulthood (18 or older). Of the 289 originally- recruited comparison participants, we excluded 31 cases with a baseline Diagnostic Interview Schedule for Children (DISC) diagnosis of ADHD17–19 and 19 participants with insufficient follow-up data. This subsample (N=239) was recruited between 8.19 and 13.85 years of age (M=9.89, SD=1.22) and average age at final adult assessment was 24.40 (SD=1.36).
Table 1.
Baseline Characteristics of the Comparison Subsample (N=239)
| Male Sex (%) | 79.9 | |
| Race/Ethnicity (%) | ||
| White | 66.5 | |
| Black | 11.3 | |
| Hispanic | 12.9 | |
| Other | 9.3 | |
| Median Household Income | $55,000 | |
| M | SD | |
| Age at baseline | 9.89 | 1.22 |
| Intelligence | 109.82 | 18.65 |
| Baseline SNAP Inattention Symptoms Count | 1.70 | 2.61 |
| Baseline SNAP Hyperactivity/Impulsivity Symptom Count | 1.03 | 1.92 |
Procedures
Comparison group recruitment was designed to reflect the local population from which the ADHD sample was drawn. Classes in the schools of the ADHD participants were randomly selected. After obtaining consent from more than 50% of the classmates in the selected classroom, individuals were selected randomly and group-matched for sex. ADHD diagnosis was neither inclusionary nor exclusionary for the comparison group. Study assessments were administered to comparison participants upon recruitment (comparison baseline; two years after ADHD baseline) and at 3, 6, 8, 10, 12, 14, and 16 years after initial baseline by bachelor’s level staff who were trained to be objective.
Measures
ADHD Symptoms
Symptoms in childhood and adolescence were measured using the SNAP rating scale completed by parents, teachers, and adolescents.20–21 Symptoms in adulthood were measured using the Conners Adult ADHD Rating Scale completed by participants and parents.22 The SNAP and Conner’s scale both list DSM-IV-TR ADHD symptoms. Respondents indicated the extent to which participants displayed each symptom on a scale from “0-not at all” to “3-very much”. Scores of “2” and “3” indicated symptom presence, as is standard practice when using these scales to detect clinically meaningful ADHD symptoms.23
Impairment
In adolescence, impairment was measured using the parent version of the Columbia Impairment Scale.24 Because the Columbia Impairment Scale assesses impairment across multiple domains, including several that are unrelated to ADHD (e.g., feeling nervous/afraid), we examined impairment scores for four central domains of ADHD-related impairment: “getting along with kids own age,” “schoolwork,” “behavior at home,” and “behavior at school.” The scale utilizes a 0–4 severity scale and scores ≥ 3 in at least one of the four domains was considered sufficient to meet the impairment threshold.25 In adulthood, parent-and self-versions of the Impairment Rating Scale were used to measure impairment globally and in eleven domains of functioning.26 Response options ranged from 0=no problem to 6=extreme problem. The Impairment Rating Scale is a measure of general impairment and has strong psychometric properties for identifying ADHD-related impairment. An empirically validated cutoff of ≥ 3 on any item was used to define clinically significant impairment.26
Substance Use
Heavy substance use was measured using the DISC and Substance Use Questionnaire.19,27–28 Substance use disorders reported on the DISC by either the parent or self were considered when determining late onset ADHD. Self-reported marijuana or other drug use on the Substance Use Questionnaire more than twice per week was classified as heavy substance use.
Mental Disorders
On the DISC,19 parent or self-report that indicated the presence of a mental disorder that better accounted for ADHD symptoms was exclusionary for a late-onset ADHD diagnosis. All disorders assessed by the DISC were considered (see Supplement). Eight experienced, licensed clinicians (three psychiatrists, five clinical psychologists) reviewed onset and chronicity of all mental symptoms and each voted whether a case should be excluded based on ADHD symptoms or impairment being attributable to another disorder (e.g., effects of anxiety symptoms on concentration). A case was excluded if agreed upon by a majority. Most decisions were unanimous (see Supplement).
Analytic Plan
There is a considerable risk for both false negative and false positive ADHD diagnoses in adolescents and adults.29 Regarding false negatives, there is established underreporting of ADHD symptoms in non-self-referred children, adolescents, and adults, concern that informants do not fully observe the functioning of adolescents and adults, and evidence that wording of some DSM ADHD symptoms may not be developmentally relevant for adolescents and adults.21,29–32 Regarding false positives, normative variations in attention can be mistaken for ADHD symptoms and ADHD symptoms often overlap with features of other disorders.33 To optimize sensitivity and specificity, our strategy to assess adolescent- and adult-onset ADHD took the stepped approach outlined by Sibley et al.,34 which first casts an intentionally wide net for ADHD symptoms to protect against false negatives (using a version of an “or rule” that allows all reported symptoms to be considered). The second step protects against false positives by carefully assessing and requiring meaningful impairment, establishing symptoms across settings, and ruling out substance abuse or other mental disorders as the source of ADHD-like symptoms.
Symptom Criteria
At each assessment, SNAP (parent, teacher, and adolescent) or Conners (parent and adult) ratings were combined at the item-level using an “or rule,” such that if a symptom was endorsed by any rater, it was deemed present. Symptom count was determined separately for the inattention and hyperactivity/impulsivity. After calculating combined symptom count, DSM-5 symptom thresholds were applied considering current age (six symptoms for 12– 16; five symptoms for 17 and over) for either inattention or hyperactivity/impulsivity.35
Impairment
Next, parent- and self-ratings from the Impairment Rating Scale were combined at the item level using an “or” rule to designate clinically significant impairment. If a participant who met symptom threshold for ADHD also had clinically significant impairment according to the parent Columbia Impairment Scale (adolescents) or combined Impairment Rating Scale (adults), he or she was retained as a potential case of late-onset ADHD.
Onset
We examined SNAP symptom data at all assessments for those cases with symptoms and impairment in adolescence (ages 12–17) or adulthood (18 or older). If a case was younger than 12 when symptom criteria for ADHD were first met, the case was not considered to be late-onset.
Substance Use
All retained cases were examined to determine whether heavy substance use was a probable source of ADHD symptoms. If ADHD symptoms occurred exclusively in the context of heavy substance use, we designated substance use to be the source of ADHD symptoms.
Other Mental Disorders
Next, retained cases were examined to determine whether ADHD symptoms or impairments were better explained by another mental disorder. Cases with comorbidities were retained as potential cases of late-onset ADHD if there was low likelihood that the comorbid disorder could account for ADHD symptoms or impairments.
Cross-situational Symptoms
DSM-5 ADHD diagnosis requires several symptoms to be present in two or more settings.35 Therefore, cross-situational symptoms were required at the time DSM-5 symptom thresholds were met. Cross-situational symptoms were defined as: (1) at least two symptoms reported each by the parent and teacher or (2) at least two symptoms endorsed each by the self and another informant. Because symptoms endorsed on self reports might occur in the same setting as parent or teacher reports, we consulted interview questions about symptom setting to ensure self-reported symptoms represented a second context.
Onset and Chronicity
Among cases that met criteria for late-onset ADHD, we calculated the average age of onset and examined chronicity by plotting ADHD symptoms by rater at each assessment point. To consider whether included cases were late-onset vs. late-identified, we compared childhood ADHD symptom severity for included cases to sample (N=239) means at baseline in childhood (see Table 1).
RESULTS
Adolescent Onset ADHD
Table 2 provides an outline of the multistep assessment process and displays the proportion of cases included at each step.
Table 2.
Results of stepped procedure for evaluating the validity of late-onset ADHD cases
| Adolescent-Onset | Adult-Onset | |||
|---|---|---|---|---|
| % | n | % | n | |
| Meets DSM-5 ADHD symptom criteria | 40.6% | 97 | 19.7% | 47 |
| + clinically significant impairment | 13.4% | 32 | 16.7% | 40 |
| + late-onset | 8.8% | 21 | 10.0% | 24 |
| + not due to substance abuse | 7.5% | 18 | 4.1% | 10 |
| + not attributable to other mental disorder | 5.4% | 13 | 1.3% | 3 |
| + cross-situational symptoms | 2.5% | 6 | 0.8% | 2 |
| Absence of subthreshold childhood symptoms (less than 3 childhood symptoms of IN and H/I) | 1.3% | 3 | 0.8% | 2a |
Note. Symptom criteria were counted using an “or” rule that considered information from all available informants (e.g., parent, self, teacher). Designated period was either adolescence or adulthood. Cross-situationality was inferred from multiple raters and consulting interview questions about context as needed.
One case was first assessed at age 12, at which point there were not subthreshold symptoms.
Symptom Criteria
Out of 239 comparison cases without ADHD at baseline, 97 (40.6%) met DSM-5 symptom threshold for ADHD based on combined parent, teacher, and self-reports using an item level “or rule” during at least one adolescent follow-up assessment. (If a stricter “or rule” was applied requiring a single rater to endorse symptoms above the DSM-5 threshold, 93 adolescents met DSM-5 ADHD symptom count).
Impairment
Of the 97 cases who met symptom criteria for ADHD in adolescence, 32 (33.0%) experienced clinically significant impairment at the time they met the DSM-5 symptom count. In total, 13.4% of the 239 comparison cases without ADHD at baseline met both symptom and impairment criteria for ADHD at an adolescent follow-up assessment.
Adolescent Onset
Among these 32 cases, eleven were under age 12 when they first met DSM ADHD symptom count according to at least one source and were considered childhood-onset cases. Thus, only 21 cases actually had onset during adolescence.
Ruling out Substance Use
Among the 21 cases that showed adolescent-onset ADHD symptoms and impairment, three had a marijuana use disorder that better accounted for the ADHD symptoms. In total, 18 cases of adolescent onset ADHD with significant impairment were not attributable to heavy substance use.
Ruling out Other Disorders
Of these 18 cases, nine had a history of pre-existing or concurrent mental disorders and were reviewed by the clinical panel. The panel voted to exclude five cases based on evidence that symptoms better reflected another mental disorder (see Supplement). Thus, 13 cases appeared to have onset of elevated ADHD symptoms and impairment in adolescence that was not attributable to other mental disorders.
Cross-situational Symptoms
Of the 13 cases that had onset of elevated ADHD symptoms and impairment in adolescence, six had symptoms that were only reported by a teacher. One had symptoms that were reported by the teacher and the self, but self-reported symptoms occurred only in the classroom. Thus, 6 cases (2.5% of the comparison without ADHD at baseline) appeared to have an onset of elevated ADHD symptoms and impairment in adolescence that were present in more than one setting (see Table 2).
Onset and Chronicity
Average age of onset among the 6 adolescent onset cases of ADHD was 14.22 (SD=1.50, range: 12.09–16.08). Figures 1 & 2 display the chronicity of ADHD across assessment points for all adolescent-onset ADHD cases. Four of six cases met symptom criteria only during the teenage years. These four remitting cases did not receive any medication or behavioral treatments for ADHD during the follow-up period. Two cases had symptoms that persisted into their twenties. Five of six adolescent-onset cases (83.3%) had childhood ADHD symptoms that exceeded sample baseline means (see Table 1; Figures 1&2). Average number of childhood symptoms among the six included cases was 2.5 for inattention (range=0–5, SD=2.26, d=.31) and 1.67 for hyperactivity/impulsivity (range=0–3, SD=1.21, d=.33).
Figure 1. Adolescence-Limited ADHD Cases: Symptom Counts according to Parent, Teacher, Self, and Combined Reports at each Available Assessment Point.
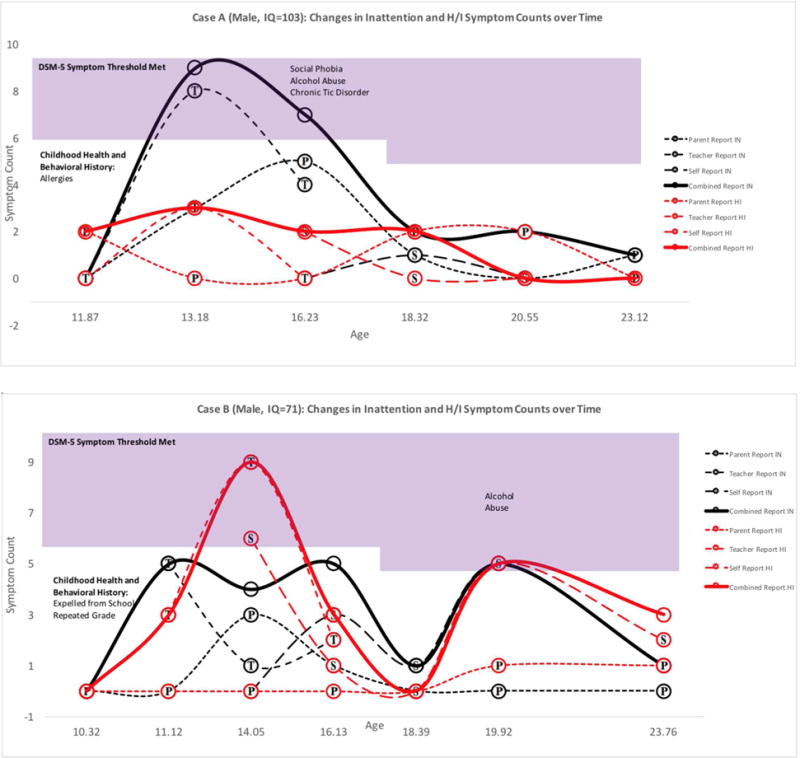
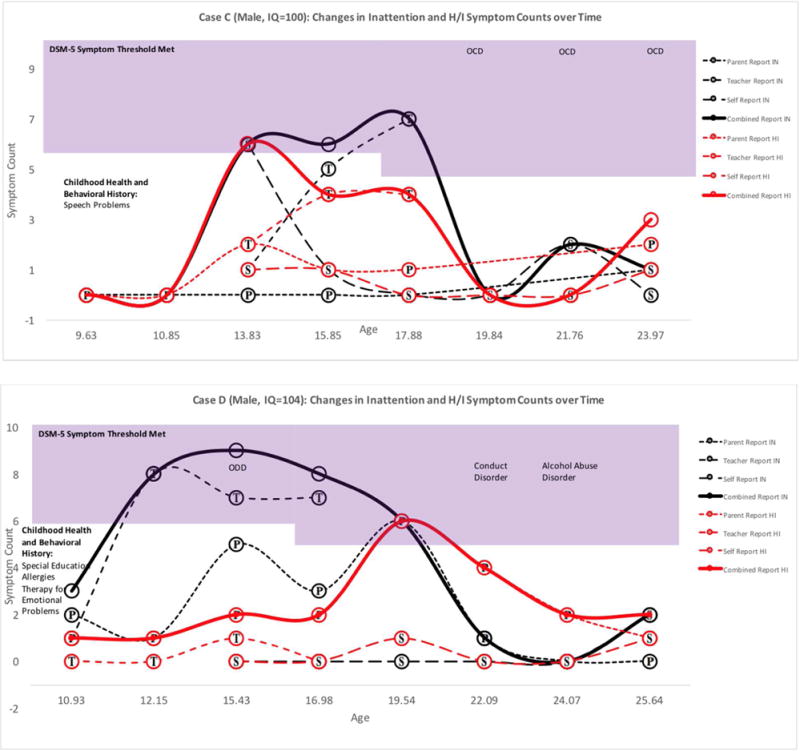
Note. Symptoms in the shaded region exceed DSM-5 age-specific symptom thresholds. Childhood Health and Behavioral History was reported retrospectively at baseline. Substance use and mental health diagnoses were obtained from the parent and self DISC interview. P=Parent report, T=Teacher Report, S=Self Report. Bold lines represent combined report across raters using an “or” rule. For Case B, symptom duration was assessed by consulting the self-DISC interview.
Figure 2. Adolescent-Onset Persistent ADHD Cases: Symptom Counts according to Parent, Teacher, Self, and Combined Reports at each Available Assessment Point.
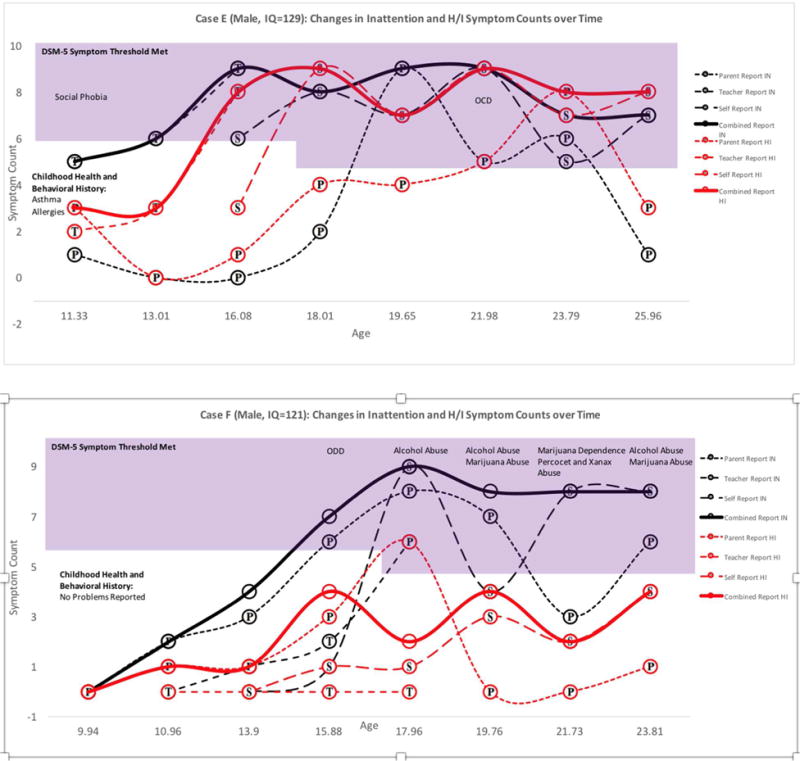
Note. Symptoms in the shaded region exceed DSM-5 age-specific symptom thresholds. Childhood Health and Behavioral History was reported retrospectively at baseline. Substance use and mental health diagnoses were obtained from the parent and self DISC interview. P=Parent report, T=Teacher Report, S=Self Report. Bold lines represent combined report across raters using an “or” rule. One voter dissented for the inclusion of Case E.
Adult-Onset ADHD
Symptom Criteria
Out of 239 comparison cases without ADHD at baseline, 19.7% (n=47) met DSM-5 symptom criteria for ADHD during at least one adult assessment based on combined parent and self-report using an item-level OR rule. (If a stricter “or rule” was applied requiring a single rater to endorse symptoms above the DSM-5 threshold, 43 adults met DSM-5 ADHD symptom criteria).
Impairment
Among 47 cases who met symptom criteria, 40 (85.1%) experienced clinically significant impairment. In total, 16.7% of the 239 comparison cases without ADHD at baseline met both symptom and impairment criteria for ADHD during at least one adult assessment.
Adult Onset
Of the 40 cases with both ADHD symptoms and impairment in adulthood, 12 showed symptom onset during childhood, 18 during adolescence, and 10 during adulthood. Four were previously deemed adolescent-onset cases. Thus, 24 of 239 cases first met impairment criteria for ADHD in adulthood, though 14 saw initial symptom onset in adolescence and 10 saw initial symptom onset in adulthood.
Ruling out Substance Use
Of the 24 cases meeting symptom and impairment criteria, 14 had impairing symptoms exclusively in the context of heavy substance use (see Supplement). In total, ten adult-onset ADHD cases were not attributable to heavy substance use.
Ruling out Other Mental Disorders
Of the ten remaining cases, five were excluded because symptoms or impairment were attributable to another mental health disorder. Two cases did not possess DISC interviews for adulthood and these cases were deemed inconclusive. Thus, three cases appeared to have onset of elevated ADHD symptoms and impairment in adolescence that was not attributable to other mental disorders. One of the included adult cases was excluded in adolescence due to anxiety and mania, but included in adulthood because comorbid disorders had remitted when ADHD symptoms returned (see Figure 3).
Figure 3. Adult-Onset ADHD Case: Symptom Counts according to Parent, Teacher, Self, and Combined Reports at each Available Assessment Point.
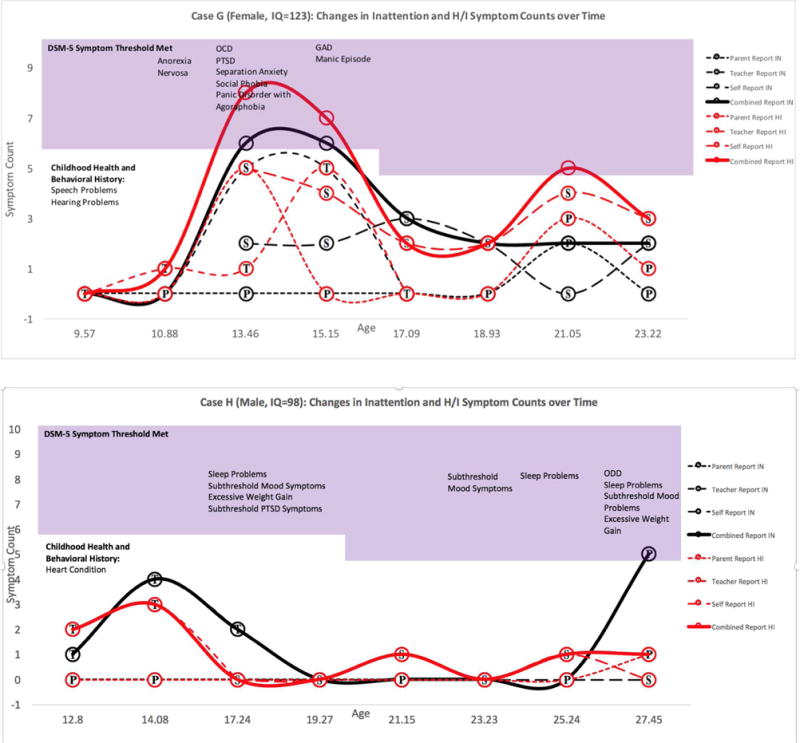
Note. For Case G, ADHD symptoms reported at age 13.46 and 15.15 were deemed by a panel of clinical experts to be attributable to other mental disorders (anxiety disorders and mania). As a result, onset of symptoms that appear unattributable to other disorders occurs at 21.05 years. Symptoms in the shaded region exceed DSM-5 age-specific symptom thresholds. Childhood Health and Behavioral History was reported retrospectively at baseline. Substance use and mental health diagnoses were obtained from the parent and self DISC interview. P=Parent report, T=Teacher Report, S=Self Report. Bold lines represent combined report across raters using an “or” rule. Two voters dissented for the inclusion of Case G based on symptom presence at age 21.05. For cases G and H, symptom duration was reported to be over six months on the DISC interview.
Cross-situational Symptoms
One of the three remaining adult-onset ADHD cases possessed symptoms in only one setting. Thus, of 239 comparison cases without ADHD at baseline, only 2 (0.8%) showed evidence of adult-onset ADHD (see Table 2).
Onset and Chronicity
The adult-onset cases reported onset at age 21.05 and 27.45, respectively. Both cases met criteria for ADHD at only one adult assessment. One case’s childhood symptoms (0 inattention, 1 hyperactivity/impulsivity) were below the baseline sample average. The other was first assessed at age 12, reporting one inattention and two hyperactivity/impulsivity symptoms at that time (see Figure 3).
The supplement depicts cases with late-onset ADHD symptoms and impairment who were excluded from diagnosis.
DISCUSSION
The local normative comparison group of the Multimodal Treatment Study of ADHD provided a unique opportunity to study detailed fluctuations in ADHD symptoms over time in adolescents and young adults without a childhood history of ADHD. After using a stepped diagnostic procedure that carefully considered multi-informant data, longitudinal symptom patterns from childhood to adulthood, impairment, co-occurring mental disorders, and substance use, approximately 95% of cases that initially screened positive for late-onset ADHD were excluded from diagnosis (see Table 2). These data indicate that when assessing adolescents and young adults for first-time ADHD diagnoses, clinicians should obtain a thorough psychiatric history and assessment of current functioning. Furthermore, 53% of adolescents and 83% of adults who met all symptom, impairment, and late-onset criteria for ADHD were excluded because symptoms or impairment were better explained by heavy substance use or another mental disorder (see Table 2, Supplement). Therefore, previously reported late-onset ADHD prevalence rates (2.5%–10.7%) may be overestimated due to limited ability to consult multiinformant data, track symptoms in extended gaps between assessment points, and review detailed patterns of substance use and comorbidity over time when determining diagnosis.4–7
Six adolescent-onset ADHD cases appeared in the comparison group. One form of adolescent-onset ADHD (n=4) was adolescence-limited (see Figure 1) and characterized by above-average childhood symptoms, borderline to average intelligence, and symptom remission by age 19. In all four of these cases, the preponderance of symptoms was reported by teachers, though corroborated by parents and adolescents. One explanation for this pattern is developmental misfit that mimics or facilitates inattention symptoms. Mounting environmental demands in adolescence may temporarily exacerbate above-average but subthreshold childhood ADHD symptoms (see Figure 1) or create cognitive overload for adolescents with slower developing prefrontal regions.36–37 In absence of mature executive functions, some adolescents may also display deficient self-control in socially or emotionally salient contexts, leading to adolescence-limited behavior problems that may be perceived as hyperactive/impulsive symptoms by raters.38 Further work is needed to better understand this adolescence-limited presentation and the influence of cognitive development on ADHD-like symptoms in adolescents without childhood ADHD.
A second adolescent-onset ADHD presentation was characterized by above-average childhood ADHD symptoms and superior intellect (see Figure 2). Two males with superior IQs exhibited a persistent form of late-onset ADHD with slowly escalating symptoms from childhood through young adulthood. This profile echoes previous findings that childhood ADHD symptoms may be masked in individuals with cognitive strengths, delaying initial ADHD diagnosis.1 Since symptoms were likely present but mitigated in childhood, these individuals might better be characterized as late-identified, rather than late-onset, ADHD cases.39
The Multimodal Treatment Study of ADHD comparison group did not support adult-onset ADHD independent of a complex psychiatric history. The two cases identified as adult-onset both possessed a variety of past or current mental health symptoms (see Figure 3). In both cases, it was difficult to disentangle the etiology of these individuals’ symptoms, so the panel conservatively voted to retain the cases. In line with the false-positive paradox,8 the vast majority of cases who initially met late-onset symptom and impairment criteria were excluded from diagnosis because of clear evidence that heavy substance use or another mental disorder better accounted for symptoms or impairment (Table 2). In fact, the majority of impairing late-onset ADHD symptoms in young adulthood could be traced to heavy substance use (see Table 2 and Supplement). There are still other potential sources for late-onset symptoms, such as brain injury, illness, or trauma that should also be considered in future investigations. Without clear exclusionary guidelines for ADHD in adolescents and adults, there is risk that ADHD may become a catchall diagnosis for executive dysfunction stemming from any source. It is unclear whether ADHD-like presentations stemming from non-traditional sources should be differentiated from a chronic form of ADHD with developmental origins, though treatment may be similar.40 Despite many strengths to birth-cohort samples, they are limited because they do not possess the detailed and frequent data collection required to carefully follow psychiatric functioning over time. One of the studies also did not perform full childhood diagnostic assessments, which may have led to missed childhood symptoms in some cases.5 Of course, average age at comparison baseline was approximately ten years, limiting our study’s ability to consider detailed symptom records before this assessment.
The comparison group was drawn from the same local school, sex, and age/grade pool as the ADHD sample, which may over-represent certain characteristics, such as male sex or slightly above-average family income. During adolescence, impairment ratings were only available from parents. Some cases may have met impairment criteria in adolescence if teacher or self ratings had been available. We assessed cases only to the mid-to-late 20s. New late-onset cases might appear later in development. We also did not collect comprehensive data on physical health or personality disorders with impulsive features that may better explain late-onset cases. Because only eight late onset cases were detected, we were insufficiently powered to conduct analyses comparing late-onset cases to other subgroups.
Conclusion
Some adolescents and young adults who present for first-time ADHD diagnoses may represent valid late-onset cases. However, the most common source of impairing late-onset ADHD symptoms in adolescence and young adulthood was substance use. Prior to diagnosing or treating ADHD in late-onset cases, clinicians should carefully assess and treat substance use and comorbid mental health disorders as a potential source of symptoms. The majority of adolescent-onset cases possessed transient symptoms. Thus, it may be appropriate to give provisional firsttime ADHD diagnoses in adolescence and to monitor symptoms over time as remission may occur within a few years. Further research is needed to understand how cognitive immaturity or adolescent neurocognitive changes might mimic or facilitate emerging ADHD symptoms.
Supplementary Material
Acknowledgments
Dr. Hechtman has received research support, served on advisory boards and has been a speaker for Ely Lilly, IronShore, Ortho Janssen, Purdue and Shire. Dr. Arnold has received research funding from Curemark, Forest, Lilly, Neuropharm, Novartis, Noven, Shire, and YoungLiving (as well as NIH and Autism Speaks) and has consulted with or been on advisory boards for Gowlings, Neuropharm, Novartis, Noven, Organon, Otsuka, Pfizer, Roche, Seaside Therapeutics, Sigma Tau, Shire, and Tris Pharma and received travel support from Noven. Dr. Swanson acknowledges research support, advisory board membership, speaker’s bureau membership, and/or consulting for Alza, Richwood, Shire, Celgene, Novartis, Celltech, Gliatech, Cephalon, Watson, CIBA, UCB, Janssen, McNeil and Lilly. Dr. Arnold has received research funding from Curemark, Forest, Lilly, Neuropharm, Novartis, Noven, Shire, Supernus, Roche, and YoungLiving (as well as NIH and Autism Speaks), has consulted with Gowlings, Neuropharm, Organon, Pfizer, Sigma Tau, Shire, Tris Pharma, and Waypoint, been on advisory boards for Arbor, Ironshore, Novartis, Noven, Otsuka, Pfizer, Roche, Seaside Therapeutics, Sigma Tau, Shire, and received travel support from Noven. Dr. Rohde has received grant or research support from, served as a consultant to, and served on the speakers’ bureau of Eli Lilly and Co., Janssen, Medice, Novartis and Shire. The ADHD and Juvenile Bipolar Disorder Outpatient Programs chaired by Dr. Rohde have received unrestricted educational and research support from the following pharmaceutical companies: Eli Lilly and Co., Janssen, Novartis, and Shire. Dr. Rohde has received travel grants from Shire to take part in the 2014 APA, 2015 WFADHD and 2016 AACAP congresses. He has received royalties from Artmed Editora and Oxford University Press. Dr. Mitchell has received royalties from New Harbinger Press.
Funding/Support: The work reported was supported by cooperative agreement grants and contracts from NIMH and the National Institute on Drug Abuse (NIDA) to the following: University of California–Berkeley: U01 MH50461, N01MH12009, and HHSN271200800005-C; DA-8-5550; Duke University: U01 MH50477, N01MH12012, and HHSN271200800009-C; DA-8-5554; University of California– Irvine: U01MH50440,N01MH12011,and HHSN271200800006- C; DA-8-5551; Research Foundation for Mental Hygiene (New York State Psychiatric Institute/Columbia University): U01 MH50467, N01 MH12007, and HHSN271200800007-C; DA-8-5552; Long Island–Jewish Medical Center U01 MH50453; New York University: N01MH 12004, and HHSN271200800004-C; DA-8-5549; University of Pittsburgh: U01 MH50467, N01 MH 12010, and HHSN271200800008-C; DA-8-5553; DA039881; and McGill University N01MH12008, and HHSN271200800003-C; DA-8-5548. Additional funding support provided by NIDA (K23DA032577 to J.T.M.
Footnotes
Conflict of Interest Disclosures: The remaining authors have no conflicts to disclose.
Additional Contributions: The Multimodal Treatment Study of Children with ADHD (MTA) was a National Institute of Mental Health (NIMH) cooperative agreement randomized clinical trial, continued under an NIMH contract as a follow-up study and finally under a National Institute on Drug Abuse (NIDA) contract. Collaborators from NIMH: Benedetto Vitiello, M.D. (Child & Adolescent Treatment and Preventive Interventions Research Branch), Joanne B. Severe, M.S. (Clinical Trials Operations and Biostatistics Unit, Division of Services and Intervention Research), Peter S. Jensen, M.D. (currently at REACH Institute and Mayo Clinic), L. Eugene Arnold, M.D., M.Ed. (currently at Ohio State University), Kimberly Hoagwood, Ph.D. (currently at Columbia); previous contributors from NIMH to the early phases: John Richters, Ph.D. (currently at National Institute of Nursing Research); Donald Vereen, M.D. (currently at NIDA). Principal investigators and co-investigators from the sites are: University of California, Berkeley/San Francisco: Stephen P. Hinshaw, Ph.D. (Berkeley), Glen R. Elliott, Ph.D., M.D. (San Francisco); Duke University: Karen C. Wells, Ph.D., Jeffery N. Epstein, Ph.D. (currently at Cincinnati Children’s Hospital Medical Center), Desiree W. Murray, Ph.D.; previous Duke contributors to early phases: C. Keith Conners, Ph.D. (former PI); John March, M.D., M.P.H.; University of California, Irvine: James Swanson, Ph.D., Timothy Wigal, Ph.D.; previous contributor from UCLA to the early phases: Dennis P. Cantwell, M.D. (deceased); New York University: Howard B. Abikoff, Ph.D.; Montreal Children’s Hospital/ McGill University: Lily Hechtman, M.D.; New York State Psychiatric Institute/Columbia University/Mount Sinai Medical Center: Laurence L. Greenhill, M.D. (Columbia), Jeffrey H. Newcorn, M.D. (Mount Sinai School of Medicine). University of Pittsburgh: Brooke Molina, Ph.D., Betsy Hoza, Ph.D. (currently at University of Vermont), William E. Pelham, Ph.D. (PI for early phases, currently at Florida International University). Follow-up phase statistical collaborators: Robert D. Gibbons, Ph.D. (University of Illinois, Chicago); Sue Marcus, Ph.D. (Mt. Sinai College of Medicine); Kwan Hur, Ph.D. (University of Illinois, Chicago). Original study statistical and design consultant: Helena C. Kraemer, Ph.D. (Stanford University). Collaborator from the Office of Special Education Programs/US Department of Education: Thomas Hanley, Ed.D. Collaborator from Office of Juvenile Justice and Delinquency Prevention/Department of Justice: Karen Stern, Ph.D.
Clinical Trial Number: NCT00000388, Multimodal Treatment Study of Children with Attention Deficit and Hyperactivity Disorder (MTA). https://clinicaltrials.gov/ct2/show/NCT00000388
References
- 1.Olfson M, Blanco C, Wang S, Laje G, Correll CU. National trends in the mental health care of children, adolescents, and adults by office-based physicians. JAMA psychiatry. 2014;71(1):81–90. doi: 10.1001/jamapsychiatry.2013.3074. [DOI] [PubMed] [Google Scholar]
- 2.McGough JJ. Treatment Controversies in Adult ADHD. Am J Psychiat. 2016;173(10):960–6. doi: 10.1176/appi.ajp.2016.15091207. [DOI] [PubMed] [Google Scholar]
- 3.Benson K, Flory K, Humphreys KL, Lee SS. Misuse of stimulant medication among college students: a comprehensive review and meta-analysis. Clin child and fam psyc rev. 2015 Mar;18(1):50–76. doi: 10.1007/s10567-014-0177-z. [DOI] [PubMed] [Google Scholar]
- 4.Agnew-Blais JC, Polanczyk GV, Danese A, Wertz, et al. Evaluation of the persistence, remission, and emergence of attention-deficit/hyperactivity disorder in young adulthood. JAMA psychiatry. 2016;73(7):713–20. doi: 10.1001/jamapsychiatry.2016.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caye A, Rocha TB, Anselmi L, et al. Attention-deficit/hyperactivity disorder trajectories from childhood to young adulthood: evidence from a birth cohort supporting a late-onset syndrome. JAMA psychiatry. 2016;73(7):705–12. doi: 10.1001/jamapsychiatry.2016.0383. [DOI] [PubMed] [Google Scholar]
- 6.Moffitt TE, Houts R, Asherson P, et al. Is adult ADHD a childhood-onset neurodevelopmental disorder? Evidence from a four-decade longitudinal cohort study. Am J Psychiat. 2015;172(10):967–77. doi: 10.1176/appi.ajp.2015.14101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riglin L, Collishaw S, Thapar AK, et al. Association of genetic risk variants with attention-deficit/hyperactivity disorder trajectories in the general population. Jama psychiatry. 2016;73(12):1285–92. doi: 10.1001/jamapsychiatry.2016.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li D, Sham PC, Owen MJ, He L. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD) Hum Mol Genet. 2006;15(14):2276–2284. doi: 10.1093/hmg/ddl152. [DOI] [PubMed] [Google Scholar]
- 9.Nigg JT. Is ADHD a disinhibitory disorder? Psychol Bull. 2001;127(5):571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- 10.Zang YF, He Y, Zhu CZ, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29(2):83–91. doi: 10.1016/j.braindev.2006.07.002. (Castellanos and. [DOI] [PubMed] [Google Scholar]
- 11.Faraone SV, Biederman J. Can Attention-Deficit/Hyperactivity Disorder Onset Occur in Adulthood? JAMA psychiatry. 2016 doi: 10.1001/jamapsychiatry.2016.0400. [DOI] [PubMed] [Google Scholar]
- 12.Faraone SV, Perlis RH, Doyle AE, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol psychiat. 2005;57(11):1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Castellanos FX. Is Adult-onset ADHD a distinct entity? Am J Psychiat. 2015;172(10):929–931. doi: 10.1176/appi.ajp.2015.15070988. [DOI] [PubMed] [Google Scholar]
- 14.Solanto MV. Child vs Adult Onset of Attention-Deficit/Hyperactivity Disorder. JAMA psychiatry. 2017 doi: 10.1001/jamapsychiatry.2016.2741. [DOI] [PubMed] [Google Scholar]
- 15.MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- 16.Jensen PS, Arnold LE, Swanson JM, et al. 3-year follow-up of the NIMH MTA study. J Am Acad Child Psy. 2007;46:989–1002. doi: 10.1097/CHI.0b013e3180686d48. [DOI] [PubMed] [Google Scholar]
- 17.Molina BS, Hinshaw SP, Swanson JM, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Psy. 2009;48:484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swanson JM, Arnold LE, Molina BSG, et al. Young Adult Outcomes in the Follow-up of the Multimodal Treatment Study of Attention-Deficit/Hyperactivity Disorder: Symptom Persistence, Source Discrepancy, and Height Suppression. Journal Child Psychol Psyc. doi: 10.1111/jcpp.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaffer D, Fisher P, Lucas CP, et al. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Psy. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Swanson JM. School-based Assessments and Interventions for ADD Students. Irvine Calif: KC Publications; 1992. [Google Scholar]
- 21.Sibley MH, Swanson JM, Arnold LE, et al. Defining ADHD symptom persistence in adulthood: optimizing sensitivity and specificity. Journal Child Psychol Psyc. 2016 doi: 10.1111/jcpp.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conners CK, Erhardt D, Sparrow E. Conner’s Adult ADHD Rating Scales: CAARS. Toronto: MHS; 1999. [Google Scholar]
- 23.Swanson JM, Kraemer HC, Hinshaw SP, et al. Clinical relevance of the primary findings of the MTA: success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Psy. 2001;40(2):168–79. doi: 10.1097/00004583-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Bird HR, Shaffer D, Fisher P, et al. The Columbia Impairment Scale (CIS): Pilot findings on a measure of global impairment for children and adolescents. Int J Meth Psy Res. 1993 [Google Scholar]
- 25.Bird HR, Andrews H, Schwab-Stone M, et al. Global measures of impairment for epidemiologic and clinical use with children and adolescents. Int J Meth Psy Res. 1996 [Google Scholar]
- 26.Fabiano GA, Pelham WE, Jr, Waschbusch DA, et al. A practical measure of impairment: Psychometric properties of the impairment rating scale in samples of children with attention deficit hyperactivity disorder and two school-based samples. J Clin Child Adolesc. 2006;35:369–385. doi: 10.1207/s15374424jccp3503_3. [DOI] [PubMed] [Google Scholar]
- 27.Molina BS, Pelham WE., Jr Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J Ab Psy. 2003;112(3):497. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- 28.Molina BS, Hinshaw SP, Arnold LE, et al. Adolescent substance use in the multimodal treatment study of attention-deficit/hyperactivity disorder (ADHD)(MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. J Am Acad Child Psy. 2013;52(3):250–63. doi: 10.1016/j.jaac.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molina BSG, Sibley MH. The Case for Including Informant Reports in the Assessment of Adulthood ADHD. ADHD Report. 2014;22:1–7. [Google Scholar]
- 30.Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J abnorm psychol. 2002;111(2):279–289. [PubMed] [Google Scholar]
- 31.Evans SW, Allen J, Moore S, Strauss V. Measuring symptoms and functioning of youth with ADHD in middle schools. J Abnorm Child Psych. 2005 Dec 1;33(6):695–706. doi: 10.1007/s10802-005-7648-0. [DOI] [PubMed] [Google Scholar]
- 32.Barkley RA, Murphy KR, Fischer M. ADHD in Adults: What the Science Says. New York: Guilford; 2008. [Google Scholar]
- 33.Murphy K, Gordon M, Barkley RA. To what extent are ADHD symptoms common? A reanalysis of standardization data from a DSM-IV checklist. The ADHD Report. 2000;8(3):1–5. [Google Scholar]
- 34.Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psyc. 2016 Dec 31;3(12):1157–65. doi: 10.1016/S2215-0366(16)30190-0. [DOI] [PubMed] [Google Scholar]
- 35.American Psychiatric Association. Diagnostic and Statistical Manual, Version 5. Washington DC: 2013. [Google Scholar]
- 36.Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psyc. 2006 Mar 1;47(3–4):296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- 37.Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann NY Acad Sci. 2008 Mar 1;1124(1):111–26. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moffitt TE, Caspi A. Childhood predictors differentiate life-course persistent and adolescence-limited antisocial pathways among males and females. Dev psychopathol. 2001;13(02):355–375. doi: 10.1017/s0954579401002097. [DOI] [PubMed] [Google Scholar]
- 39.Chandra S, Biederman J, Faraone SV. Assessing the Validity of the Age at Onset Criterion for Diagnosing ADHD in DSM-5. Journal of attention disorders. 2016 doi: 10.1177/1087054716629717. [DOI] [PubMed] [Google Scholar]
- 40.McAllister TW, Zafonte R, Jain S, et al. Randomized placebo-controlled trial of methylphenidate or galantamine for persistent emotional and cognitive symptoms associated with PTSD and/or traumatic brain injury. Neuropsychopharmacology. 2016;41(5):1191–8. doi: 10.1038/npp.2015.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


