Abstract
Respiratory syncytial virus (RSV) is a massive medical burden in infants, children and the elderly worldwide, and an effective, safe RSV vaccine remains an unmet need. Here we assess a novel vaccination strategy based on the intradermal delivery of a SynCon® DNA-based vaccine encoding engineered RSV-F using a surface electroporation device (SEP) to target epidermal cells, in clinically relevant experimental models. We demonstrate the ability of this strategy to elicit robust immune responses. Importantly we demonstrate complete resistance to pulmonary infection at a single low dose of vaccine in the cotton rat RSV/A challenge model. In contrast to the formalin-inactivated RSV (FI-RSV) vaccine, there was no enhanced lung inflammation upon virus challenge after DNA vaccination. In summary the data presented outline the pre-clinical development of a highly efficacious, tolerable and safe non-replicating vaccine delivery strategy.
Keywords: Respiratory Syncytial Virus, DNA Vaccines, Electroporation
Introduction
Respiratory syncytial virus (RSV) is the most common cause of lower respiratory tract disease in infants and young children, and is also a major disease burden in the elderly population. Globally RSV causes 3.4 million hospitalizations and 66,000-200,000 deaths annually in children younger than 5 years [1] [2]. While immunoprophylaxis in the form of passive transfer of an anti-RSV-F monoclonal antibody (Palivizumab) reduces hospitalization rates by 50 %, its economic cost limits its use to infants with identified risk factors residing in the developed world [3]. The proven efficacy of passive immunoprophylaxis, human challenge studies [4], epidemiological data [5] and the ability of maternally transferred antibodies to protect infants demonstrates the importance of neutralizing antibodies against RSV [6, 7]. Further studies have highlighted the role of cell-mediated immune responses as well in RSV protection [8, 9]. However, harnessing the immune system in the form of a vaccine to replicate this protection has yet to be fully achieved. There are currently no FDA licensed vaccines for RSV.
Historically, vaccine development for RSV has been impeded by the phenomenon of vaccine-enhanced disease (VED) which was associated with a formalin-inactivated RSV vaccine in the 1960’s [10]. This phenomenon resulted in vaccinated infants developing more severe respiratory disease upon natural RSV exposure, and two patients succumbed to infection. Subsequent studies have associated Formalin-inactivated RSV with Th2 immune responses [11–13], and such responses have been associated with immunopathogenesis of RSV in experimental models [14, 15].
Further understanding of the virus, target antigens and disease mechanisms has led to the development of new vaccine strategies to tackle this unmet medical challenge. Such strategies include gene-based [16], virally-vectored [17], subunit [18], particle-based [19, 20] vaccines targeting the surface RSV fusion (F) and attachment (G) glycoproteins. Vaccination regimens have also targeted pregnant women, with the goal of enhancing transfer of maternal antibody to protect the neonate [21]. We have recently developed a vaccine strategy based on the delivery with electroporation (EP) of pGX2303, a DNA plasmid (pDNA) encoding the engineered synthetic consensus (SynCon®) viral fusion (F) glycoprotein. The RSV-F immunogen protein was not modified in a way to change its stability or other physical properties, rather the consensus sequence strategy was adopted to enhance the breadth of coverage by selecting amino acids which are most common among various RSV strains using our SynCon® design platform. Immunogenicity studies revealed pGX2303 elicited high levels of RSV neutralizing antibodies, robust T cell responses and protection from intranasal viral challenge in mice and cotton rats after a single intramuscular immunization (manuscript in preparation). Thus we have proof of concept concerning the protective efficacy of a RSV DNA vaccine.
To advance this strategy towards a clinical applicable protocol we have proceeded to investigate a less-invasive surface EP (SEP) delivery strategy which specifically targets the vaccine to the epidermis [22, 23]. Our goal was to identify a highly tolerable EP strategy to deliver a DNA-based vaccine which would be widely acceptable in pediatric and geriatric clinical settings. The SEP device has been designed to use low electrical parameters to enhance the uptake of pDNA in the upper layers of the skin, thus avoiding the activation of deep nerves. We believe such an electroporation device will be appropriate for delivery of prophylactic vaccines in all potential target cohorts, including infants, pregnant women and the elderly. In this study we have focused on the EP-enhanced delivery of the pGX2303 vaccine to the skin of rodents using the SEP device. We show this vaccine elicits RSV-F antigen-specific Th1 immune responses, with no evidence of Th2-skewing after skin delivery, as evidenced by strong IFN-γ cellular responses and antibody subclass production favoring IgG2 over IgG1. Furthermore, we demonstrated the development of RSV/A neutralizing antibodies and robust RSV-F binding titers in cotton rats. Importantly, we demonstrate protection from lower respiratory disease in cotton rats after a single intradermal (ID) immunization of a low dose of pGX2303. Histopathology analysis revealed no evidence of vaccine-enhanced disease. Thus, the data indicates our vaccine strategy to be both effective and safe.
Methods
Electroporation devices
The epidermal targeting surface EP (SEP) device consists of an electrode array made up from a 4×4 array of gold-plated trocar needles of 0.43 mm diameter at a 1.5 mm spacing ((Inovio Pharmaceuticals, Plymouth Meeting, PA) Fig. 1a). The SEP array was pressed down on the surface of the skin above the intradermal bleb (or wheel) made by the Mantoux delivery of 50 μl plasmid formulation, in a manner in which all electrodes across the array made contact with the surface of the skin. The electrodes did not penetrate the live skin layers. Three individual 100 ms pulses of 10-50 V were delivered. The CELLECTRA®-3P EP device (Inovio Pharmaceuticals) was employed to assist in the delivery of 200 μl plasmid formulation to Wistar rat quadriceps muscles. It is a triangular three electrode array consisting of 26-gauge solid stainless steel electrodes. In this study, the electrodes were 5 mm in length and penetrated the quadricep muscle of the Wistar rat. Two sets (separated by a one second delay) of two 52 ms 0.2A constant current pulses were delivered.
Figure 1. Development of a SEP intradermal delivery protocol for pGX2303.
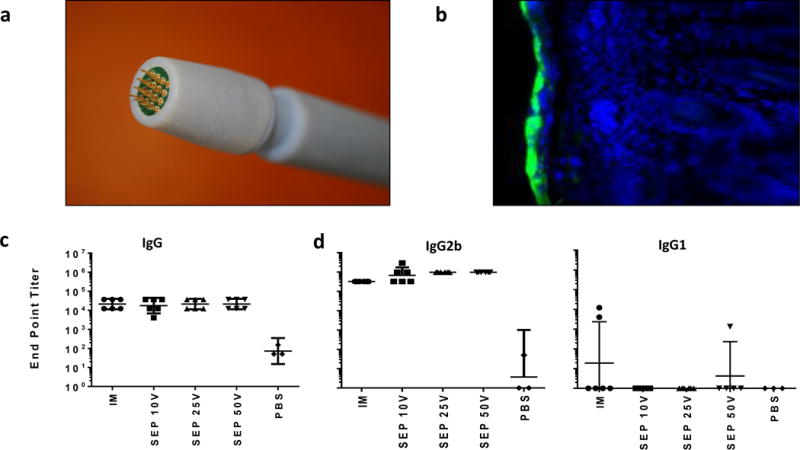
Images of the hand held surface electroporation device electrode array (a). Epidermal expression of the reporter gene GFP is shown 24 hours after ID delivery with SEP of pGFP in cotton rat skin (b). Green: GFP. Blue: DAPI stain. Endpoint Wistar rat IgG (c), IgG2b and IgG1 (d) RSV-F antigen binding titers were measured by ELISA on day 21 after a day 0 and 14 immunization regimen with 60 μg pGX2303 delivered ID with SEP or IM with CELLECTRA®-3P. Geometric mean with 95% +/− CL depicted.
Animals
Wistar rats (8-10 weeks old) were purchased from Charles River Laboratories (Wilmington, MA). Animals were group housed with ad libitum access to food and water. Wistar rats were housed and handled at BTS (San Diego, CA) according to the standards of the Institutional Animal Care and Use Committee (IACUC). Female Sigmodon hispidus cotton rats between 6 and 8 weeks of age (Source: Sigmovir Biosystems, Inc., Rockville MD) were maintained and handled under veterinary supervision in accordance with National Institutes of Health guidelines and Sigmovir IACUC approved animal study protocol. The cotton rats were group housed and provided with standard rodent chow (Harlan #7004) and tap water ad libitum.
Plasmid DNA
The vaccine plasmid pGX2303 contains an insert which is a consensus sequence of the RSV fusion glycoprotein of subtype A and B viruses. Sequences for the consensus strategy were obtained from GenBank. Consensus RSV-F was synthetically codon and RNA-optimized and then subcloned by GenScript (Piscataway Township, NJ) into pGX001, a modified pVAX1 mammalian expression vector. gWiz-GFP reporter gene plasmid was purchased from Aldevron (Fargo, ND). All plasmids were diluted in 1xPBS (Phosphate buffered saline) before injection.
pGX2303 RSV-F immunogen insert amino acid sequence:
MELPILKTNAITTILAAVTLCFASSQNITEEFYQSTCSAVSKGYLSALRTGWYTSVITIELSNIKENKCNGTDAKVKLIKQELDKYKNAVTELQLLMQSTPAANNRARRELPRFMNYTLNNTKNTNVTLSKKRKRRFLGFLLGVGSAIASGIAVSKVLHLEGEVNKIKSALLSTNKAVVSLSNGVSVLTSKVLDLKNYIDKQLLPIVNKQSCSISNIETVIEFQQKNNRLLEITREFSVNAGVTTPVSTYMLTNSELLSLINDMPITNDQKKLMSNNVQIVRQQSYSIMSIIKEEVLAYVVQLPLYGVIDTPCWKLHTSPLCTTNTKEGSNICLTRTDRGWYCDNAGSVSFFPQAETCKVQSNRVFCDTMNSLTLPSEVNLCNIDIFNPKYDCKIMTSKTDVSSSVITSLGAIVSCYGKTKCTASNKNRGIIKTFSNGCDYVSNKGVDTVSVGNTLYYVNKQEGKSLYVKGEPIINFYDPLVFPSDEFDASISQVNEKINQSLAFIRKSDELLHNVNAGKSTTNIMITTIIIVIIVILLSLIAVGLLLYCKARSTPVTLSKDQLSGINNIAFSNX
Immunizations
For all skin immunizations animals were first sedated using isoflurane anesthesia, the left abdominal flank was shaved and 50 μl of pGX2303 was injected ID using a Mantoux-like injection. SEP-EP array was immediately placed over the bleb and the electrical pulses were applied at the injection site using the Elgen pulse generator (Inovio Pharmaceuticals). For IM delivery, 200 μl (0.25 mg/ml) of pGX2303 was injected into the quadriceps muscle. For IM immunizations, CELLECTRA-3P array was inserted into the muscle at the site of DNA injection and the electrical pulses applied immediately. For cotton rat control group immunizations, Formalin-inactivated-RSV (FI-RSV) Lot#100 [10] was diluted 1:100 in PBS, and 100 μl was injected IM into the left quadriceps muscle; or 100 μl Live RSV/A virus ((1×105 pfu) (ATCC, Manassas, VA)) was instilled intranasally.
Endpoint-binding titer ELISA
The endpoint-binding titer assay was performed on a 96-well assay plate (Corning cat# 9018). Plates were coated with RSV Protein F (Sino Biological cat# 11049-V08B) at a concentration of 0.3 μg/ml overnight at 4°C. In brief, after washing, plates were blocked for 1hr at 37°C. Plates were washed. and serum samples (diluted to 1:50 in PBS) were added to row A and serially diluted (1:3) to a final concentration of 1:36450 (row G). Samples were run in triplicates. Plates were incubated for 2hr at 37°C. Plates were washed and HRP-labeled secondary antibody was added. In this study we used goat anti-rat IgG-HRP (Sigma cat# A9037), goat anti-rat IgG1-HRP (ThermoFisher Scientific cat# PA1-84708), goat anti-rat IgG2b-HRP (ThermoFisher Scientific cat# PA1-84710) and chicken anti-Cotton rat IgG-HRP (MyBioSource cat# MBS560029). After 1hr at 37°C incubation with the secondary antibody plates were developed with TMB Substrate Solution (KPL cat#5 0-76-11). OD values at 450nm were read immediately on a SpectraMAX Plus plate reader from Molecular Devices. The endpoint binding titer was defined as highest sample dilution with a signal greater than or equal to the background signal. Background was determined for each assay plate as the signal in the absence of serum sample.
GFP in vivo expression detection
Abdominal flanks of anaesthetized Wistar rats were shaved and depilated. 50μl of 1mg/ml GFP-reporter DNA plasmid were injected ID using a Mantoux-like injection. Immediately following the injection, the 4×4 SEP-EP array was placed over the formed bleb and the EP-treatment was administered at the injection site. 24hrs after the treatment the rat was euthanized and the skin was harvested. Skin biopsies of the treatment site were collected and immersed into 4% paraformaldehyde in 1xPBS (made from 20% paraformaldehyde, Electron Microscopy Sciences cat# 15713) and incubated for 12hrs at 4°C. Biopsies were washed three times for five minutes in 1xPBS and immersed in 30% (w/v) sucrose (Sigma, cat# S0389) in D.I. water. After at least 24hr incubation at 4°C the skin biopsies were embedded into optimal cutting temperature (OCT) compound (Sakura Tissue-Tek cat# 4583) and snap-frozen. Frozen tissue blocks were sectioned to a thickness of 15 μm on a Hacker-Bright Cryostat. Sections were transferred to Superfrost Plus Microslides (VWR cat# 48311-703), mounted with DAPI Fluoromount-G (SouthernBiotech cat#0100-20), which also provided the nuclear counterstain, and covered with micro cover glasses (VWR cat# 48404-452). Sections were imaged on an Olympus BX51 fluorescent microscope equipped with a QImaging Retiga3000 monochromatic camera. Images were processed with Q-capture Pro7 software.
Challenge Virus
The prototype Long strain of RSV/A was propagated in HEp-2 cells (ATCC) after serial plaque-purification to reduce defective-interfering particles. A pool of virus designated as hRSV/A/Long Lot# 021413, prepared in sucrose stabilizing media, and containing approximately 2 × 107 pfu/ml was used. This stock of virus was stored under −80°C condition and has been characterized at Sigmovir Biosystems, MD in vivo using the Cotton rat model for upper and lower respiratory tract replication. For the challenge experiment animals were infected intra-nasally (IN) with RSV/A at 1 × 105 pfu per animal in a 0.1 ml PBS volume.
Lung viral titration
Lung homogenates were clarified by centrifugation and diluted 1:10 and 1:100 in EMEM. Confluent HEp-2 monolayers were infected in duplicates with 50 μl per well starting with undiluted samples and followed by diluted homogenates in 24-well plates. After one hour incubation at 37°C in a 5% CO2 incubator, wells were overlaid with 0.75% methylcellulose medium and plates were stored into the 37°C incubator. After 4 days of incubation the overlay was removed and the cells were fixed with 0.1% crystal violet stain for one hour, then rinsed and air dried. Plaques were counted and virus titers were expressed as plaque forming units per gram of tissue. Viral titers in a group were calculated as the geometric mean ± standard error for all animals in that group at a given time. The RT-PCR method can be used to quantify lung RSV viral gene levels, but for proven protection the standard plaque assay was used to quantify infectious particles, and not just viral genes of interest.
Pulmonary histopathology
Lungs were dissected, and the right lobes were inflated with 10% neutral buffered formalin, and immersed in the same fixative solution. Following fixation, the lungs were embedded in paraffin, sectioned and stained with hematoxylin and eosin (H&E). Four parameters of pulmonary inflammation were evaluated: peribronchiolitis (inflammatory cell infiltration around the bronchioles), perivasculitis (inflammatory cell infiltration around the small blood vessels), interstitial pneumonia (inflammatory cell infiltration and thickening of alveolar walls), and alveolitis (cells within the alveolar spaces). Slides were scored blind on a 0-4 severity scale (0=no; 1=minimal; 2=mild; 3=moderate; 4=maximum inflammation). The scores were subsequently converted to a 0-100% histopathology scale (0 = 0, 1 = 5, 2 = 25, 3 = 75, and 4 = 100)[24].
RSV neutralizing antibody assay (60% reduction)
Heat-treated sera samples were diluted 1:10 with EMEM and further serially diluted 1:4. Diluted sera samples were incubated with hRSV/A/Long Lot# 021413(25-50 PFU) for 1 hour at room temperature and inoculated in duplicates onto confluent HEp-2 monolayers in 24 well plates. After one hour incubation at 37°C in a 5% CO2 incubator, the wells were overlaid with 0.75% Methylcellulose medium. After 4 days of incubation the overlay was removed and the cells were fixed with 0.1% crystal violet stain for one hour and then rinsed and air dried. The corresponding reciprocal neutralizing antibody titers were determined at the 60% reduction end-point of the virus control using the statistics program “plqrd.manual.entry” [25]. The geometric means ± standard error for all animals in a group were calculated.
Statistical analysis
Data were expressed as mean +/− standard error of mean (SEM) or geometric mean +/− 95% confidence limit (CL) for each group. Statistical differences between groups were evaluated using a two-tailed Mann-Whitney test to compare between two groups as data were not always normally distributed. GraphPad Prism 5.0b software was used. P<0.05 was considered statistically significant.
Results
Development of a non-invasive EP strategy to deliver pGX2303 a SynCon® RSV-F vaccine and elicit robust host Th-1 immune responses
Studies have demonstrated efficient delivery of reporter genes and pDNA vaccines to the epidermis with the surface electroporation device [22, 23, 26]. SEP is a hand held device consisting of an electrode array head which is pressed against the skin over the site of the ID injection bleb following injection of the formulated plasmid (Fig. 1a). The shallow electrical field produced by the SEP specifically permits transfection of only the epidermal skin layer (Fig. 1b).
We investigated the optimal SEP voltage for delivery of our SynCon® RSV-F vaccine (pGX2303). Our goal was to identify a low voltages, previously associated with low level injection site lesion by histology [27], which elicited of robust host immune responses. Preliminary reporter gene expression studies in guinea pig and rat skin suggested SEP voltages above 5 V were required for consistent gene expression in rat or guinea pig skin. We proceeded to determine the host immune response elicited after pGX2303 delivery with SEP at 10, 25 and 50 V. Sixty μg pGX2303 was delivered to the skin or muscle of Wistar rats on days 0 and 14, and the animals were bled on Day 22 for immune analysis. Day 22 native RSV-F protein end point binding titers in the serum are displayed in Figure 1c. There was no significant difference in the total IgG serum levels across all the treated groups. At day 22 binding end point titers [Geometric Mean] were 21,044 for IM, 17,523 for 10 V SEP, 21,044 for 25 V SEP, and 21,044 for 50 V SEP delivery. Previous studies in mice had revealed strong Th-1 responses had been associated with IM+EP delivery of pGX2303 vaccine in mice (unpublished data). To determine whether this Th1-skewed immunological phenotype was also elicited after ID+SEP pGX2303 delivery in rats we analyzed the serum levels of IgG1 and IgG2b, Th-2 and Th-1 associated rat antibody classes [28, 29], respectively. Robust IgG2b RSV-F binding titers were detected in all groups, with a trend to high titers in the ID treated groups compared to IM, and no significant difference between the 10, 25 and 50 V SEP groups (Fig. 1d). The rat IgG1 levels were low or below the limit of detection of the assay in all the SEP-treated rats. In summary, low voltage SEP delivery of the pGX2303 vaccine to the rat epidermis was associated with robust immune responses. At 10 or 25 V EP parameters the associated skin damage was minimal and there was no evidence of host Th2 responses being raised.
Elicitation of host humoral immunity after pGX2303 SynCon™ RSV-F vaccination
Due to being highly permissive to infection with non-adapted human RSV, and the ability to observe the pathological mechanisms associated with VED with the FI-RSV vaccine, the cotton rat (S.hispidus) is the benchmark small animal model of RSV research [30, 31]. To assess the level of humoral immunity elicited after SEP delivery of pGX2303 in cotton rats, we assayed antibody binding titers and viral neutralizing activity. Table 1 displays the vaccine groups tested in this study. Cotton rats were immunized with 10 μg (Groups A&B) or 60 μg (Groups C-D) doses of pGX2303 vaccine receiving one (Groups A&C) or two (Groups B&D) doses. Control groups included animals intranasally (denoted IN in Table 1) inoculated with 105 PFU of live RSV/A virus (Group E), 2 doses of FI-RSV Lot#100 delivered IM (Group F) or PBS (Group G).
Table 1.
Vaccine Groups for cotton rat RSV/A challenge
| Group | Delivery Method | Day 0 | Day 14 | Challenge Day 35 |
|---|---|---|---|---|
| A | ID 10V SEP | 10 μg pGX2303 | − | + |
| B | ID 10V SEP | 10 μg pGX2303 | 10 μg pGX2303 | + |
| C | ID 10V SEP | 60 μg pGX2303 | − | + |
| D | ID 10V SEP | 60 μg pGX2303 | 60 μg pGX2303 | + |
| E | IN Instillation | 105 pfu RSV/A | − | + |
| F | IM | FI-RSV | FI-RSV | + |
| G | ID | PBS | − | + |
Abbreviations: ID, intradermal, IM, intramuscular; SEP, surface electroporation; IN, intranasal; pfu, plaque forming unit; PBS, phosphate buffered saline.
On day 28 RSV-F antigen end point titers (Geometric Mean) following a single dose (day 0) of pGX2303 were 15,136 for 10 μg dose and 23,488 for 60 μg dose. Day 28 the end point titers following two doses (days 0 & 14) of pGX2303 were 36,450 for both the 10 and 60 μg groups (Fig. 2a). In comparison to the control groups, pGX2303-treated animals harbored equivalent binding titer levels at day 28 to the FI-RSV vaccinated group, and wild-type RSV/A inoculated group. Post-vaccination rise in RSV/A neutralizing antibodies was detected in all the groups treated with pGX2303 delivered SEP (Fig. 2b). Together, this data highlighted the ability of ID + SEP delivered pGX2303 to induce robust RSV-specific binding antibodies, along with viral neutralizing antibody activity.
Figure 2. ID delivery of pGX2303 elicits strong humoral immune responses in the Cotton rats.
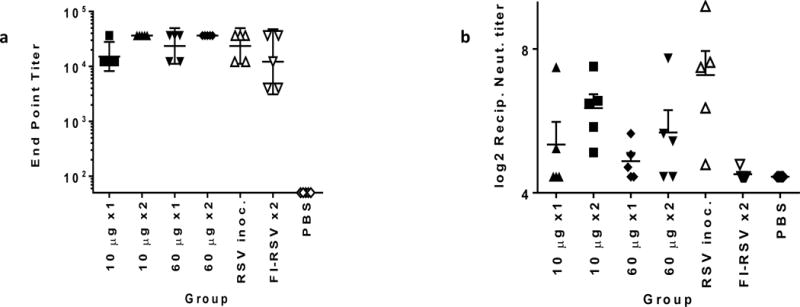
Cotton rats in groups A-G were treated as outlined in Table 1. The levels of RSV-F binding antibody were measured by ELISA in the serum collected from individual Cotton rats on day 28 (a). Geometric mean with 95% +/− CL depicted. RSV/A neutralizing antibody titers in the serum were determined in a HEp2 plaque assay (b). The log2 reciprocal neutralizing antibody titers are plotted for each vaccine group on day 28 along with error bars + SEM.
Protection from lower and upper respiratory infection after pGX2303 SynCon® RSV-F vaccination
To determine whether pGX2303 delivered ID with SEP conferred cotton rats with protective immunity in a RSV/A challenge model, cotton rats (Groups A-G – Table 1) were challenged with 105 pfu of RSV/A IN on day 28. Five days post infection lung and nasal passages were collected, homogenized and viral titrations were determined in a HEp-2 cell plaque assay.
Lung viral titrations revealed protection (Log10 titers < LOD) in all the pGX2303 vaccinated animals (Fig. 3a: Groups A-D Table 1). Importantly one 10 μg dose of pGX2303 delivered ID with SEP provided a reduction of viral load in the lungs by 80 fold in comparison to nonimmunized animals. Control animals previously inoculated with RSV were fully protected, whereas only partial protection was observed in the FI-RSV group. Viral titers from the nasal passage revealed significant disease protection in all the pGX2303-vaccinated animals compared to PBS-treated cotton rats (Fig. 3b). There was a nonsignificant trend for greater disease protection in animals receiving two doses compared to one dose, as evidenced by Groups B and D harboring lower viral titers compared to Groups A and C. Alternatively, inter-group analysis revealed no significant difference between groups related pGX2303 dose used (10 or 60 μg). Intranasal inoculation with live RSV/A virus on day 0 conferred the animals with complete upper respiratory tract protection in a follow-on intranasal challenge, whereas no significant protection was observed in the FI-RSV-treated animals.
Figure 3. ID delivery of pGX2303 provides protection against upper and lower respiratory tract disease.
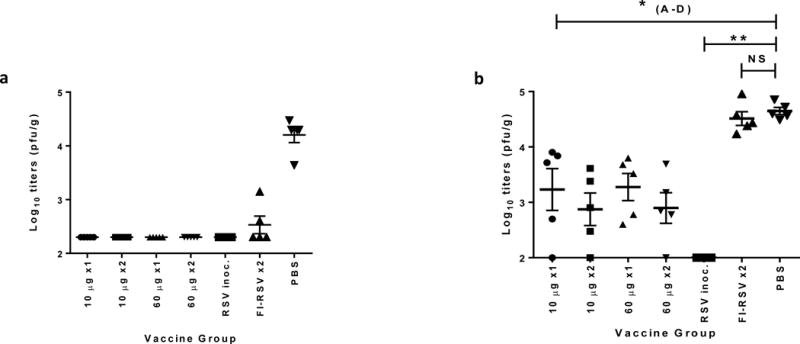
Groups A-G (Cotton rats (5 per group)) were treated as outlined in Table 1. Group I received PBS IM as a negative control. All animals were challenge with 1×105 pfu of RSV/A delivered IN on day 28. On day 33 animals were sacrificed and lung (a) and nasal (b) viral titers were measured in tissue homogenates by HEp2 cell line plaque assay. Data points represent pfu’s per g of lung tissue expressed as Log10 titers. Horizontal lines represent mean titer per group and error bars +/− SEM. * p<0.05 in comparison to negative control (Grp. G).
Abbreviations: Pfu, plaque-forming unit; g, gram; SEM, standard error of the mean; ns, non-significant.
Pulmonary histopathology in Cotton rats after RSV challenge
The phenomenon of FI-RSV vaccine-enhanced disease (VED) is characterized by bronchoconstriction and severe lung inflammation with peribronchiolar cell infiltrates including mononuclear cells and eosinophils. The cotton rat serves as a replicate model displaying the pathophysiology consequences of VED after RSV challenge following FI-RSV vaccination [32]. We employed the cotton rat to determine whether pGX2303 vaccination was associated with VED. Five days following RSV/A challenge lungs were dissected and formaldehyde-fixed. Lung tissue was dissected, and sections were stained with hematoxylin and eosin (H&E). We evaluated four parameters of lung inflammation: peribronchiolitis (PB), perivasculitis (PV), alveolitis (A) and interstitial pneumonia (IP). The severity of each parameter was scored on a scale of 0 to 100 as described in the methods section.
In a blinded study, the severity of each parameter was scored on a scale of 0 to 100, as described in the methods section. Figure 4a shows the scores received for each vaccine group. The hallmarks of high level peribronchiolitis (mean score: 75) and alveolitis (mean score: 45) of VED were clearly observed in the FI-RSV Group F (Fig. 4a). The H&E stained image in Figure 4c is a representative section showing a high level inflammation surrounding the bronchioles in cotton rat lungs in the FI-RSV group compared to naïve (N) lung tissue (Fig. 4b). The lung histopathology profile in the pGX2303 vaccinated groups were unremarkable, and showed no significant difference to Day 0 RSV/A inoculated animals in comparisons of any of the four parameters evaluated (Fig. 4a). Similarly, representative H&E images from RSV/A inoculated (Fig. 4d) and pGX2303 vaccinated (Fig. 4e) cotton rats show similar levels of cellular infiltrates five days post challenge. In summary there was no evidence of VED after pGX2303 immunization of cotton rats.
Figure 4. Cotton rat lung pulmonary histopathology after RSV/A challenge.
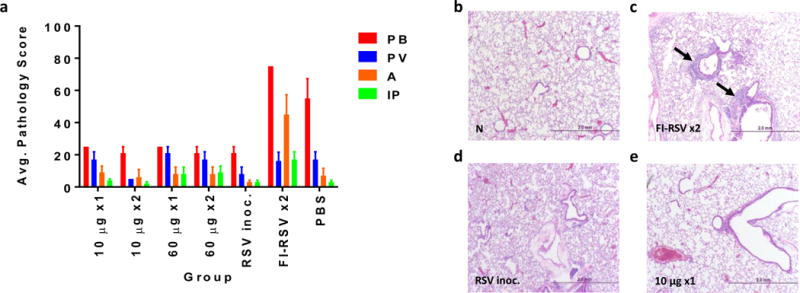
The lungs of Cotton rats in groups A-G were harvested 5 days after 1×105 RSV/A challenge. Lungs were formalin fixed, sectioned and H&E stained. Slides were scored on a 0-4 severity scale in a blinded manner. Scores were converted onto a 0-100% histopathology scale (0=no; 25=minimal, 50=mild; 75=moderate; 100=maximal inflammation). The parameters evaluated were peribronchiolitis (PB), perivasculitis (PV), alveolitis (A) and interstitial pneumonia (IP). Bars represent mean pathology scores + SEM (a). Representative lung H&E images non-treated animal (b), and 5 days post challenge from FI-RSV vaccinated animal (c), day 0 RSV/A infected animals (d) and from pGX2303 immunized animal from (e). Magnification x40.
Discussion
In this study we outlined a vaccination strategy based on the intradermal delivery of a gene-based vaccine against RSV enhanced by surface electroporation, and successfully demonstrated protection against lower respiratory tract disease after RSV/A challenge. Importantly, we observed protection after a single dose of the synthetic consensus RSV-F vaccine delivered to the skin using SEP at low voltage parameters shown to cause no visible skin damage. Furthermore, histopathology studies performed in parallel with the formalin-inactivated RSV vaccine revealed no VED was associated with our pGX2303 vaccine formulation.
DNA vaccines are a promising solution to unmet medical needs. Pre-clinical studies have clearly shown DNA vaccines can raise strong humoral and cellular immune responses against viral antigens which correlate with disease protection [16, 23, 33–36]. Furthermore, in humans, optimized EP-delivered DNA vaccines targeting HPV E6&E7 oncoproteins have proved to be therapeutically efficacious in regressing high grade cervical lesions and clearing HPV infection [37, 38]. Historically most DNA-based vaccines have been delivered to the muscle tissue, but the skin offers an alternative and attractive delivery target. Both conventional and gene-based vaccines have been effectively delivered to the skin to drive robust immune responses in pre-clinical and clinical settings [23, 39–44]. The skin is an attractive delivery site for several reasons. Primarily the skin is a highly immunocompetent tissue populated with a high concentration of resident professional APCs. In a recent study, we identified epidermal dendritic cells that were directly transfected by plasmid DNA following EP-enhanced delivery to the skin with the SEP device [22]. We proceeded to describe a mechanism in which transfected epidermal DCs mediated a host immune response which developed significantly more rapidly in comparison to an IM-EP DNA vaccination [22]. The induction of a rapid host immune response has obvious important implications in a successful RSV vaccine. RSV infection is extremely prevalent - 50% of infants infected by one year of age, and severe lower respiratory disease leading to hospitalization especially prevalent in infants below 6 months of age. Thus, the ability of a vaccine to drive a rapid protective immune response is of great importance in the pediatric population. Furthermore, RSV is recognized as a significant disease burden in elderly adults, similar to that of influenza. Analysis of US health care databases and viral surveillance results indicated in persons older than 64 years of age RSV infection causes approximately 10,000 deaths annually [45].
Importantly in this study, and to our knowledge the first study to report this, we demonstrated full protection against lower respiratory tract disease after a single dose of a non-replicating RSV vaccine delivered to the skin in the cotton rat model. While two doses of the pRSV-F vaccine provided increased protection against upper respiratory disease in the nasal passage, significant levels of protection were still observed in the cotton rats receiving a single dose. All cotton rats that received one or two doses were fully protected from lower respiratory disease in the lung (Fig. 3). Furthermore at day 14 after immunization robust antibody binding levels were observed in all the pGX2303 vaccine groups and neutralizing antibody activity was detected in the majority of the groups (Fig. 2). This data suggests the potential to generate rapid protective immunity using this SynCon™ RSV-F vaccine plus SEP ID delivery protocol.
In addition to the skin being a highly immunocompetent organ, it is also easily accessible, and vaccine delivery methods targeting this tissue can therefore be minimally invasive and thus potentially more tolerable. This is a very important consideration in the development of highly tolerable prophylactic vaccine strategies which incorporate EP, rather than therapeutic indications were a higher level of treatment discomfort may be acceptable. We have previously demonstrated an improvement in tolerability of skin EP using the CELLECTRA®-3P minimally invasive intradermal DNA vaccine delivery clinical EP platform compared to our intramuscular CELLECTRA®-5P device [46]. In this study, we employed the noninvasive SEP device to enhance DNA vaccine delivery to the skin (Fig. 1). This device is currently in pre-clinical development and we aim to move forward to clinical translation in humans in the near future, and we believe SEP has the potential to become an acceptable EP delivery methodology in the pediatric setting. We were encouraged by the lack of any visible damage at the treatment site upon its use at 10V to deliver the pGX2303 vaccine in rats and guinea pigs, and the data strongly supports the benefits of this delivery platform in terms of tolerability for clinical use.
The cotton rat was chosen as the relevant animal model in which to test our vaccine protocol due to being highly permissive to infection with non-adapted human RSV, and the pathological mechanisms associated VED with the FI-RSV vaccine can be closely recapitulated in this animal. Thus the cotton rat offers an appropriate model to evaluate the potential of VED associated with the pGX2303 vaccine protocol alongside a FI-RSV vaccine. Blinded histopathology analysis of lung sections harvested 5 days after viral challenge revealed no evidence of VED in the SynCon™ RSV-F vaccinated groups or animals previously infected with RSV (Fig. 4). In contrast, animals receiving the FI-RSV vaccine displayed the hallmarks of VED which include severe alveolitis and peribronchiolitis. Furthermore, analysis of the host immune response elicited after pGX2303 vaccination revealed strong Th1 rather than Th2 responses being raised (Fig. 1). IgG subclass analysis revealed robust IgG2 levels (Th1-associated) after ID delivery of pGX2303 vaccine in Wistar rats, while IgG1 (Th2-associated) levels were low or below the LOD of the assay (Fig. 1). Other studies with pGX2303 also report strong IFN-γ T cell responses elicited by pGX2303 in mice, guinea pig and NHP models [47] (manuscript in preparation). Preliminary T cell epitope mapping in the guinea pig revealed responses to native RSV-F antigen epitopes, however more exhaustive studies will have to be conducted to determine whether immune responses are raised to neo-epitopes which could be potentially presented after SynCon® RSV-F immunization. Further characterization of the antibody response is being undertaken, specifically investigating the neutralizing capacity of antibodies in terms of pre- and postfusion antigen targeting. Future studies will also investigate the ability of the antibodies raised by pGX2303 to compete against known RSV neutralizing antibodies. In summary the immunological data suggested we were driving protective Th1 immune responses in multiple animal models by delivering a RSV vaccine targeting the F antigen to the skin with SEP. Such an observation is important in consideration of the immunopathology of RSV in experimental models which has been associated with Th2 responses [11–15].
Together, the results from this preclinical development program support the continued advancement of this pGX2303 vaccine candidate paired with skin surface EP delivery device. In the clinically relevant cotton rat model, we successfully demonstrated that an electroporation-assisted skin delivery vaccine protocol drives protective immune responses in the absence of VED. Furthermore, protection from lower respiratory disease could be achieved with one low dose of vaccine.
Acknowledgments
We acknowledge the members of the Inovio Pharmaceuticals R&D department for significant technical assistance.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
- EP
electroporation
- SEP
surface electroporation
- RSV
respiratory syncytial virus
- VED
vaccine-enhanced disease
- ID
intradermal
- pfu
plaque-forming unit
Footnotes
Conflict of interest statement
TRFS, KS, MPM, KAK, JRM, LH, NYS, and KEB are employees of Inovio Pharmaceuticals, and own shares or have been awarded stock options in the company.
References
- 1.Nair H, et al. An evaluation of the emerging interventions against Respiratory Syncytial Virus (RSV)-associated acute lower respiratory infections in children. BMC Public Health. 2011;11(Suppl 3):S30. doi: 10.1186/1471-2458-11-S3-S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro JM, Jean RE. Respiratory syncytial virus. N Engl J Med. 2001;345(15):1132–3. doi: 10.1056/NEJM200110113451515. [DOI] [PubMed] [Google Scholar]
- 3.Homaira N, et al. Effectiveness of Palivizumab in Preventing RSV Hospitalization in High Risk Children: A Real-World Perspective. Int J Pediatr. 2014;2014:571609. doi: 10.1155/2014/571609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee FE, et al. Experimental infection of humans with A2 respiratory syncytial virus. Antiviral Res. 2004;63(3):191–6. doi: 10.1016/j.antiviral.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Luchsinger V, et al. Role of neutralizing antibodies in adults with community-acquired pneumonia by respiratory syncytial virus. Clin Infect Dis. 2012;54(7):905–12. doi: 10.1093/cid/cir955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roca A, et al. Prevalence of respiratory syncytial virus IgG antibodies in infants living in a rural area of Mozambique. J Med Virol. 2002;67(4):616–23. doi: 10.1002/jmv.10148. [DOI] [PubMed] [Google Scholar]
- 7.Ochola R, et al. The level and duration of RSV-specific maternal IgG in infants in Kilifi Kenya. PLoS One. 2009;4(12):e8088. doi: 10.1371/journal.pone.0008088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fishaut M, Tubergen D, McIntosh K. Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. J Pediatr. 1980;96(2):179–86. doi: 10.1016/s0022-3476(80)80799-2. [DOI] [PubMed] [Google Scholar]
- 9.Mbawuike IN, et al. HLA-restricted CD8+ cytotoxic T lymphocyte, interferon-gamma, and interleukin-4 responses to respiratory syncytial virus infection in infants and children. J Infect Dis. 2001;183(5):687–96. doi: 10.1086/318815. [DOI] [PubMed] [Google Scholar]
- 10.Kim HW, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89(4):422–34. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 11.Graham BS, et al. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151(4):2032–40. [PubMed] [Google Scholar]
- 12.Graham BS. Immunological determinants of disease caused by respiratory syncytial virus. Trends Microbiol. 1996;4(7):290–3. doi: 10.1016/0966-842x(96)10032-9. [DOI] [PubMed] [Google Scholar]
- 13.Moghaddam A, et al. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat Med. 2006;12(8):905–7. doi: 10.1038/nm1456. [DOI] [PubMed] [Google Scholar]
- 14.Hussell T, et al. CD8+ T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur J Immunol. 1997;27(12):3341–9. doi: 10.1002/eji.1830271233. [DOI] [PubMed] [Google Scholar]
- 15.Hussell T, et al. Th1 and Th2 cytokine induction in pulmonary T cells during infection with respiratory syncytial virus. J Gen Virol. 1996;77(Pt 10):2447–55. doi: 10.1099/0022-1317-77-10-2447. [DOI] [PubMed] [Google Scholar]
- 16.Grunwald T, et al. Novel vaccine regimen elicits strong airway immune responses and control of respiratory syncytial virus in nonhuman primates. J Virol. 2014;88(8):3997–4007. doi: 10.1128/JVI.02736-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson TR, et al. Genetic vaccine for respiratory syncytial virus provides protection without disease potentiation. Mol Ther. 2014;22(1):196–205. doi: 10.1038/mt.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patton K, et al. Enhanced immunogenicity of a respiratory syncytial virus (RSV) F subunit vaccine formulated with the adjuvant GLA-SE in cynomolgus macaques. Vaccine. 2015;33(36):4472–8. doi: 10.1016/j.vaccine.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Smith G, et al. Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PLoS One. 2012;7(11):e50852. doi: 10.1371/journal.pone.0050852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glenn GM, et al. A Randomized, Blinded, Controlled, Dose-Ranging Study of a Respiratory Syncytial Virus Recombinant Fusion (F) Nanoparticle Vaccine in Healthy Women of Childbearing Age. J Infect Dis. 2015 doi: 10.1093/infdis/jiv406. [DOI] [PubMed] [Google Scholar]
- 21.Glenn GM, et al. A Randomized, Blinded, Controlled, Dose-Ranging Study of a Respiratory Syncytial Virus Recombinant Fusion (F) Nanoparticle Vaccine in Healthy Women of Childbearing Age. J Infect Dis. 2016;213(3):411–22. doi: 10.1093/infdis/jiv406. [DOI] [PubMed] [Google Scholar]
- 22.Smith TR, et al. DNA vaccination strategy targets epidermal dendritic cells, initiating their migration and induction of a host immune response. Mol Ther Methods Clin Dev. 2014;1:14054. doi: 10.1038/mtm.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broderick KE, et al. Prototype development and preclinical immunogenicity analysis of a novel minimally invasive electroporation device. Gene Ther. 2011;18(3):258–65. doi: 10.1038/gt.2010.137. [DOI] [PubMed] [Google Scholar]
- 24.Prince GA, et al. Vaccine-enhanced respiratory syncytial virus disease in cotton rats following immunization with Lot 100 or a newly prepared reference vaccine. J Gen Virol. 2001;82(Pt 12):2881–8. doi: 10.1099/0022-1317-82-12-2881. [DOI] [PubMed] [Google Scholar]
- 25.Coates HV, Alling DW, Chanock RM. An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am J Epidemiol. 1966;83(2):299–313. doi: 10.1093/oxfordjournals.aje.a120586. [DOI] [PubMed] [Google Scholar]
- 26.Mendoza JM, et al. Elucidating the Kinetics of Expression and Immune Cell Infiltration Resulting from Plasmid Gene Delivery Enhanced by Surface Dermal Electroporation. Vaccines (Basel) 2013;1(3):384–97. doi: 10.3390/vaccines1030384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin F, et al. Optimization of electroporation-enhanced intradermal delivery of DNA vaccine using a minimally invasive surface device. Hum Gene Ther Methods. 2012;23(3):157–68. doi: 10.1089/hgtb.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saoudi A, et al. TH2 activated cells prevent experimental autoimmune uveoretinitis, a TH1-dependent autoimmune disease. Eur J Immunol. 1993;23(12):3096–103. doi: 10.1002/eji.1830231208. [DOI] [PubMed] [Google Scholar]
- 29.Gracie JA, Bradley JA. Interleukin-12 induces interferon-gamma-dependent switching of IgG alloantibody subclass. Eur J Immunol. 1996;26(6):1217–21. doi: 10.1002/eji.1830260605. [DOI] [PubMed] [Google Scholar]
- 30.Niewiesk S, Prince G. Diversifying animal models: the use of hispid cotton rats (Sigmodon hispidus) in infectious diseases. Lab Anim. 2002;36(4):357–72. doi: 10.1258/002367702320389026. [DOI] [PubMed] [Google Scholar]
- 31.Prince GA, et al. Efficacy and safety studies of a recombinant chimeric respiratory syncytial virus FG glycoprotein vaccine in cotton rats. J Virol. 2000;74(22):10287–92. doi: 10.1128/jvi.74.22.10287-10292.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prince GA, et al. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactiva ted virus. J Virol. 1986;57(3):721–8. doi: 10.1128/jvi.57.3.721-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulmer JB, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259(5102):1745–9. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 34.Laddy DJ, et al. Heterosubtypic protection against pathogenic human and avian influenza viruses via in vivo electroporation of synthetic consensus DNA antigens. PLoS One. 2008;3(6):e2517. doi: 10.1371/journal.pone.0002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shedlock DJ, et al. Induction of broad cytotoxic T cells by protective DNA vaccination against Marburg and Ebola. Mol Ther. 2013;21(7):1432–44. doi: 10.1038/mt.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muthumani K, et al. A synthetic consensus anti-spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci Transl Med. 2015;7(301):301ra132. doi: 10.1126/scitranslmed.aac7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim TJ, et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat Commun. 2014;5:5317. doi: 10.1038/ncomms6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trimble CL, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bins AD, et al. A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nat Med. 2005;11(8):899–904. doi: 10.1038/nm1264. [DOI] [PubMed] [Google Scholar]
- 40.Song JM, et al. Improved protection against avian influenza H5N1 virus by a single vaccination with virus-like particles in skin using microneedles. Antiviral Res. 2010;88(2):244–7. doi: 10.1016/j.antiviral.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bakari M, et al. Broad and potent immune responses to a low dose intradermal HIV-1 DNA boosted with HIV-1 recombinant MVA among healthy adults in Tanzania. Vaccine. 2011;29(46):8417–28. doi: 10.1016/j.vaccine.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drabick JJ, et al. Cutaneous transfection and immune responses to intradermal nucleic acid vaccination are significantly enhanced by in vivo electropermeabilization. Mol Ther. 2001;3(2):249–55. doi: 10.1006/mthe.2000.0257. [DOI] [PubMed] [Google Scholar]
- 43.Glenn GM, et al. Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nat Med. 2000;6(12):1403–6. doi: 10.1038/82225. [DOI] [PubMed] [Google Scholar]
- 44.Brave A, et al. Plasmid DNA vaccination using skin electroporation promotes poly-functional CD4 T-cell responses. Immunol Cell Biol. 2011;89(3):492–6. doi: 10.1038/icb.2010.109. [DOI] [PubMed] [Google Scholar]
- 45.Falsey AR, et al. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–59. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 46.Diehl MC, et al. Tolerability of intramuscular and intradermal delivery by CELLECTRA((R)) adaptive constant current electroporation device in healthy volunteers. Hum Vaccin Immunother. 2013;9(10):2246–52. doi: 10.4161/hv.24702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schultheis K, et al. Characterization of guinea pig T cell responses elicited after EP-assisted delivery of DNA vaccines to the skin. Vaccine. 2017;35(1):61–70. doi: 10.1016/j.vaccine.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]


