Abstract
Angiogenesis is the process of developing new blood vessels from the original vascular network; it is necessary for normal physiological processes, such as embryonic development and wound healing. Angiogenesis is also involved in pathological events, including myocardial ischemia and tumor growth. To investigate the molecular mechanisms of this important process, a variety of methods and models are employed. These strategies can also be used to provide insight into the etiology of angiogenesis-related diseases, thereby contributing to the development of new diagnostics and treatments. Commonly used animal models include the chorioallantoic membrane and yolk sac membrane of chick embryos, the mouse retina and aortic ring, and angiogenesis reactors implanted into mice. These animal models have been instrumental in the study of the angiogenic process. For example, the chorioallantoic membrane undergoes robust angiogenesis during the development of chick embryos, and, because its surface is easily accessible, this membrane provides a convenient model for experimentation. Here, we discuss the methods that employ animal models for the imaging and quantification of angiogenesis. In addition, we propose potential novel directions for future investigations in this area.
Keywords: angiogenesis, animal model, blood vessel, chick embryo, mouse
Introduction
Angiogenesis is the process of forming new vasculature. In the microvasculature and capillaries, newly formed blood vessels branch from the original vascular network in order to adapt to the physiological needs of the location. Due to the importance of normal blood circulation, angiogenesis plays a vital role in the growth, development, and maintenance of the body’s steady state vasculature [8, 25]. The vascular network undergoes dramatic growth and reorganization during embryonic development, and generally remains static in adults. However, it retains the ability to change in response to specific stimuli, such as those associated with wound healing and menstruation [4, 10, 12]. The balance between the factors that promote angiogenesis and the factors that inhibit angiogenesis maintains the resting state of the vasculature network. When this delicate balance is disturbed, excessive or insufficient blood supply may lead to the emergence of various diseases [7, 8, 11].
Vascular endothelial cells, a layer of cells that covers the inner surface of the blood vessel and contacts the bloodstream directly, are vital to angiogenesis [6, 26]. Upon receiving the signal from the adjacent tissue, vascular endothelial cells secrete proteases to digest the basement membrane, followed by subsequent migration, proliferation, differentiation, and, ultimately, reorganization into tubular structures [1, 2, 17]. Through this series of events, new blood vessels form and invade into tissues. Vascular endothelial growth factor (VEGF) is the key regulator of the signaling events controlling this complex process [5, 19, 28]. The body regulates the rate of angiogenesis largely by controlling VEGF messenger RNA stability and transcription rate to meet the body’s requirements [5, 19, 28]. Animal models are critical for the study of angiogenesis because they offer various advantages that facilitate the imaging and quantification of this process. Here, we provide a detailed review of the methods utilized in two common animal models of angiogenesis: chick embryos and mice. We also propose potential new avenues for the use of these animal models in the study of angiogenesis.
Analysis of Angiogenesis in Chick Embryos
The chick embryonic allantois appears on embryonic days 3–4, and subsequently the allantoic membrane reaches and merges with the chorion to form the chorioallantoic membrane, a dense vascular envelope essential for chick embryonic development. Due to the abundance of the vasculature rapidly formed during the embryonic development, and more importantly, the ease of experimental manipulation, the chick embryos have been intensively used for angiogenic investigations [30]. For example, the chorioallantoic membrane model is widely used as an economical in vivo assay for the evaluation of macromolecules and compounds that influence tumor angiogenesis [21, 32]. In addition, the yolk sac membrane model, which is adapted from the chorioallantoic membrane model with advantage in real-time monitoring of the progressive stages of angiogenesis, is used for the assessment of the effects of radiation and anti- or pro-angiogenic agents [21, 32].
The chorioallantoic membrane model
Fertilized eggs are incubated in an incubation box at 38°C and 100% humidity, with trays tilted every 90 min. On the fourth day, the eggs are carefully removed and placed (blunt end up) on a tray in a cell incubator at 38°C. Eggs are removed in batches, the surface wiped with 70% alcohol, and then moved onto a clean bench. Trumpet tweezers are used to hold the egg tightly and a small hole is made in the interconnected air chamber; making the aperture as small as possible in order to extend the front end of the forceps into the air chamber. The damaging of other tissues is avoided by carefully using forceps to completely expose and remove the inner shell of the lower surface of the air chamber. After processing, the hole is sealed with clear gum, and the egg placed back into the cell incubator at 38°C and 100% humidity. On day 10, matrigel containing drugs of interest is placed in the eggs. Both the dish containing the matrigel and the eggs are then removed from their respective incubators. With care, the clear gum plug is removed from the egg, and using forceps, the matrigel is placed on the perforated chorioallantoic membrane [16]. Any remaining small pieces of matrigel are placed on the membrane, the clear gum is reinserted into the hole, and the egg is returned to the cell incubator. After 72 h, the transparent gum is opened and the egg is placed under a stereomicroscope to observe and record blood vessels (Fig. 1). For quantitative analysis, the following parameters of blood vessels can be measured: (1) the average number of blood vessels in five different fields; (2) the average length of blood vessels determined with the Image J software; and (3) the number of nodes that comprise at least three branches. In addition, the angiogenic index can be obtained by multiplying the average length of blood vessel with the number of nodes.
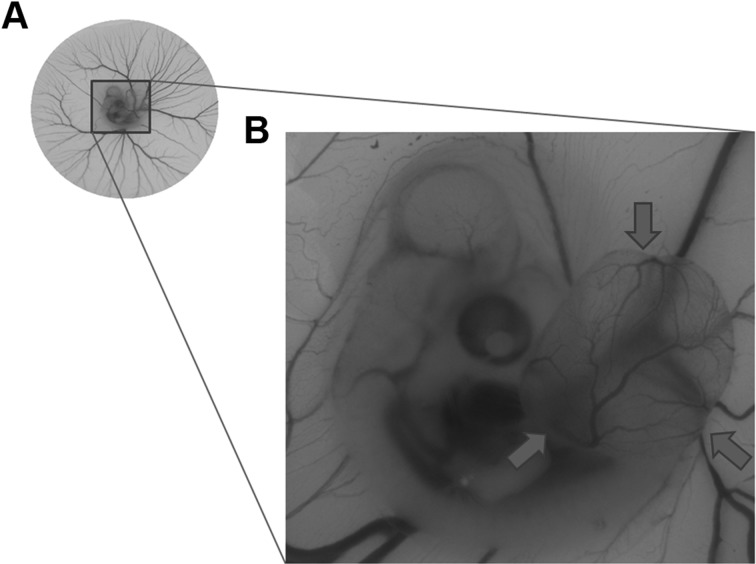
Fig. 1.
Blood vessels in the chick embryo chorioallantoic membrane. (A) Blood vessels of the chicken embryo. (B) An enlarged view of the selected area in Panel A, showing the newly formed chorioallantoic membrane as indicated by the arrows.
The yolk sac membrane model
Fertilized eggs are incubated in an incubation box at 38°C and 100% humidity, with trays tilted every 90 min. On day 3, eggs are removed from the incubator and placed sideways (i.e. blunt ends toward the horizontal direction to ensure the embryo is floating to the top) in a cell incubator at 38°C for about 30 min. The eggs in batches are removed, the surface is wiped with 70% alcohol, and then they are moved to a clean bench. Embryos are removed by breaking the egg shell underneath the embryo using gentle knocking, and the whole embryo is carefully transferred into a 100-mm diameter cell culture dish. The complete embryo is incubated in a cell incubator at 38°C and 100% humidity for 24 h. Dead embryos are cleared from the yolk sacs and, avoiding the main blood vessels, filter paper (6-mm diameter) containing drugs of interest are placed on the surface of the yolk membrane. Samples are returned to the incubator for additional three days. On day 7, the filter papers are carefully removed from the yolk sac. The blood vessel growth in the area previously covered by the filter paper is then observed and recorded using a stereomicroscope [16]. The effect of drugs of interest is determined by measuring the area or diameter of the avascular region at the site of application. However, because of the difficulty in limiting the region on the yolk sac membrane, an accurate quantification of the angiogenic effects with this model is often a serious concern.
Analysis of Angiogenesis in Mice
The retina model
Aberrant angiogenesis in the retina is involved in various retinopathies, including the retinopathy of prematurity and the age-related macular degeneration. To gain insights into the retinal diseases and therapeutic approaches, the retina model is frequently exploited, which takes advantage of oxygen-induced retinopathy to recapitulate vascular development [9]. Due to the direct reflection of the neovascularization process, and the ease in straightforward analysis of the vascular network, the retinal model is widely used to investigate angiogenesis-related retinopathies.
One-week-old mice are exposed to 75% oxygen for 5 days to induce retinal vascular regression. Mice are then returned to normal air to allow for retinal angiogenesis. Mice are sacrificed, and eyeballs are removed with forceps and immersed in phosphate-buffered saline containing 4% paraformaldehyde overnight at 4°C. The melanin layer is carefully pulled off with pointed tweezers and the lens squeezed out in order to access the retina. The retina is cut into a tampered, petal-like shape with small tweezers; typically, the retina is cut into four petals. Next, a 1-ml pipette tip, which has been cut to create a larger opening, is used to transfer the treated retina to a solution of phosphate-buffered saline containing 1% bovine serum albumin and 5% Triton X-100, and the samples are incubated overnight at 4°C [24]. Retinas are washed twice with phosphate-buffered saline. For staining, the retina is incubated at 4°C in the above buffer containing 20 µg/ml fluorescein-labelled lectin. After retinal staining, samples are treated with antibodies to identify proteins of interest. The retinal blood vessels are observed using upright fluorescence microscopy (Fig. 2). The area of neovascular tufts is quantified with the Adobe Photoshop software as described [9]. In brief, the “magic wand” tool is used to select the region of neovascularization with the following settings: tolerance is 50, and the “add to selection key” and the boxes next to “anti-alias” and “contiguous” are checked. The total number of pixels in the neovascularization region is then recorded.
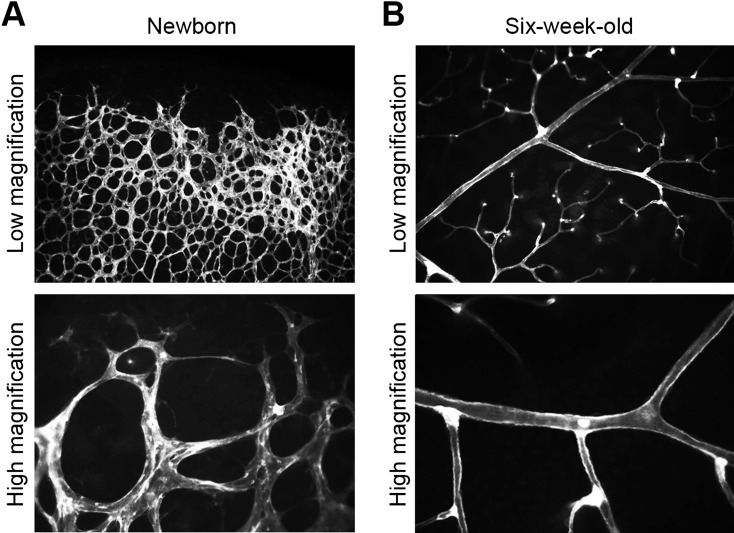
Fig. 2.
Blood vessels in the mouse retina. (A) The edge of retinal blood vessels of the newborn mouse. The vascular network is in the process of dramatic changes. (B) Retinal blood vessels of the six-week-old mouse. The vascular network has a clear structure in a relatively stable state. Vascular endothelial cells are stained with fluorescein-labelled lectin.
The aortic ring model
Angiogenesis is a complex process involving endothelial cell proliferation, migration, and alignment. In vitro angiogenesis assays are limited due to their inability to reflect the physiological angiogenic process, while in vivo angiogenesis assays are to some extent expensive and technically difficult. The aortic ring model bridges the gap between in vivo and in vitro angiogenesis assays, providing an economical way for the evaluation of angiogenic mechanisms and for the identification of novel molecules involved in the angiogenic process.
After sacrificing the mouse, the abdominal cavity is cut open, the internal organs are removed, and the thoracic aorta is located. Pointed tweezers are positioned between the aorta and the spine, and the aorta is gradually stripped from the spine and removed in one long piece. In a laminar flow hood, small blood vessels are cut from both ends of the aorta, then tissue surrounding the aorta is removed by gently peeling with forceps; the aorta is placed into a centrifuge tube containing phosphate-buffered saline. Using a surgical blade, the aorta is cut into 2-mm segments, and a syringe needle is used to transfer these small pieces (arterial rings) into a new centrifuge tube containing sterile phosphate-buffered saline. The arterial rings are washed five times with phosphate-buffered saline; contact between the pipette tip and the arterial ring is carefully avoided. The arterial rings are transferred into culture medium and placed in an incubator. A 96-well plate with matrigel is prepared and, after solidification, the arterial rings are transferred to each well containing the solidified matrigel. Another layer of matrigel is added, and after solidification, complete culture medium is added to each well. After approximately 72 h, the arterial ring begins to bud new vessels, with maximum vessel growth occurring after one week [20, 29]. The mouse arterial rings are analyzed using inverted fluorescence microscopy. The sprout length can be quantified with the Image J software. Meanwhile, the number of sprouts that branch from the main ring and the individual branches can be either manually counted or analyzed with computer-assisted image analysis methods as described [3]. However, manual counting is optimal when there are numerous sprouts; in this case, individual sprouts are unable to be distinguished by using computer-assisted image analysis methods.
The angiogenesis reactor
Angiogenesis requires a coordinated interplay between endothelial cells with other cell types, such as pericytes. To fully reproduce this complex process, the angiogenesis reactor implanted in mice is adopted. This method is usually recommended to validate the potential compounds that show potent pro- or anti-angiogenic activities in other experimental models.
The extracellular matrix extract containing angiogenic factors (e.g. heparin, fibroblast growth factor 2, and VEGF) is added to a sterile, semi-open angiogenesis reactor, and the reactor is incubated at 37°C for 1 h to allow the matrix gel to polymerize. Reactors are implanted into athymic nude mice (females,6 to 8 weeks of age). After 11 days, the reactor is removed from the mice and photographed [13]. To further examine the structure of the newly formed blood vessels, the matrix is extracted from the reactors, and the samples are frozen and analyzed using immunofluorescence microscopy. Endothelial cells are detected using an anti-CD31 antibody; mural cells are detected using an anti-desmin antibody [27]. To quantify the angiogenesis induction response, cells are collected from the angiogenesis reactor by digesting the matrix with dispase and isolating the cells by centrifugation. The cells are stained with fluorescein-labeled lectin-1, and the fluorescence intensity is measured using a fluorimeter.
Conclusions and Perspectives
Animal models, specifically chicken embryos and mouse organs, are vital to the study of angiogenesis. These systems are useful for understanding the molecular mechanisms underlying angiogenesis, angiogenesis-related diseases and for screening drugs that can be used to treat these diseases [14, 15, 18, 22, 23, 31]. Chick embryos and mice have long been used as research models in developmental biology because they have the advantages of being relatively inexpensive to maintain, easy to manipulate, and they have a short life-cycle. Here, based on ten years of experience studying angiogenesis, we describe the use of chick embryo chorioallantoic membrane, chick embryo yolk sac membrane, mouse retina, mouse aortic ring, and angiogenesis reactors implanted into mice in the study of this process. We systematically describe the protocols used in these animal models with the goal of helping researchers in the investigation of angiogenesis, especially those investigators who are new to the field. Furthermore, we hope to promote the research and development of drugs for the treatment of angiogenesis-related diseases using these convenient models as a platform for in vitro drug screening.
Acknowledgments
We thank Mr. Yuan Ren for preparing the figures. This work was supported by grants from the National Natural Science Foundation of China (31471262 and 31671403).
References
- 1.Bentley K., Chakravartula S.2017. The temporal basis of angiogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372: 20150522. doi: 10.1098/rstb.2015.0522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betz C., Lenard A., Belting H.G., Affolter M.2016. Cell behaviors and dynamics during angiogenesis. Development 143: 2249–2260. doi: 10.1242/dev.135616 [DOI] [PubMed] [Google Scholar]
- 3.Blacher S., Devy L., Burbridge M.F., Roland G., Tucker G., Noël A., Foidart J.M.2001. Improved quantification of angiogenesis in the rat aortic ring assay. Angiogenesis 4: 133–142. doi: 10.1023/A:1012251229631 [DOI] [PubMed] [Google Scholar]
- 4.Bodnar R.J.2015. Chemokine regulation of angiogenesis during wound healing. Adv. Wound Care (New Rochelle) 4: 641–650. doi: 10.1089/wound.2014.0594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruce D., Tan P.H.2011. Vascular endothelial growth factor receptors and the therapeutic targeting of angiogenesis in cancer: where do we go from here? Cell Commun. Adhes. 18: 85–103. [DOI] [PubMed] [Google Scholar]
- 6.Caporali A., Martello A., Miscianinov V., Maselli D., Vono R., Spinetti G.2017. Contribution of pericyte paracrine regulation of the endothelium to angiogenesis. Pharmacol. Ther. 171: 56–64. doi: 10.1016/j.pharmthera.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 7.Chen D.B., Zheng J.2014. Regulation of placental angiogenesis. Microcirculation 21: 15–25. doi: 10.1111/micc.12093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung A.S., Lee J., Ferrara N.2010. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat. Rev. Cancer 10: 505–514. doi: 10.1038/nrc2868 [DOI] [PubMed] [Google Scholar]
- 9.Connor K.M., Krah N.M., Dennison R.J., Aderman C.M., Chen J., Guerin K.I., Sapieha P., Stahl A., Willett K.L., Smith L.E.2009. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat. Protoc. 4: 1565–1573. doi: 10.1038/nprot.2009.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demir R., Yaba A., Huppertz B.2010. Vasculogenesis and angiogenesis in the endometrium during menstrual cycle and implantation. Acta Histochem. 112: 203–214. doi: 10.1016/j.acthis.2009.04.004 [DOI] [PubMed] [Google Scholar]
- 11.Elshabrawy H.A., Chen Z., Volin M.V., Ravella S., Virupannavar S., Shahrara S.2015. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis 18: 433–448. doi: 10.1007/s10456-015-9477-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flegg J.A., Menon S.N., Maini P.K., McElwain D.L.2015. On the mathematical modeling of wound healing angiogenesis in skin as a reaction-transport process. Front. Physiol. 6: 262. doi: 10.3389/fphys.2015.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao J., Sun L., Huo L., Liu M., Li D., Zhou J.2010. CYLD regulates angiogenesis by mediating vascular endothelial cell migration. Blood 115: 4130–4137. doi: 10.1182/blood-2009-10-248526 [DOI] [PubMed] [Google Scholar]
- 14.Goveia J., Stapor P., Carmeliet P.2014. Principles of targeting endothelial cell metabolism to treat angiogenesis and endothelial cell dysfunction in disease. EMBO Mol. Med. 6: 1105–1120. doi: 10.15252/emmm.201404156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lankhorst S., Saleh L., Danser A.J., van den Meiracker A.H.2015. Etiology of angiogenesis inhibition-related hypertension. Curr. Opin. Pharmacol. 21: 7–13. doi: 10.1016/j.coph.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 16.Li D., Xie S., Ren Y., Huo L., Gao J., Cui D., Liu M., Zhou J.2011. Microtubule-associated deacetylase HDAC6 promotes angiogenesis by regulating cell migration in an EB1-dependent manner. Protein Cell 2: 150–160. doi: 10.1007/s13238-011-1015-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J., Sun X., Wang Z., Chen L., Li D., Zhou J., Liu M.2012. Regulation of vascular endothelial cell polarization and migration by Hsp70/Hsp90-organizing protein. PLoS One 7: e36389. doi: 10.1371/journal.pone.0036389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu K., Bhat M., Basu S.2016. Plants and their active compounds: natural molecules to target angiogenesis. Angiogenesis 19: 287–295. doi: 10.1007/s10456-016-9512-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mittal K., Ebos J., Rini B.2014. Angiogenesis and the tumor microenvironment: vascular endothelial growth factor and beyond. Semin. Oncol. 41: 235–251. doi: 10.1053/j.seminoncol.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 20.Peng G., Ren Y., Sun X., Zhou J., Li D.2012. Inhibition of farnesyltransferase reduces angiogenesis by interrupting endothelial cell migration. Biochem. Pharmacol. 83: 1374–1382. doi: 10.1016/j.bcp.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 21.Plasswilm L., Höper J., Cordes N., Tannapfel A.1999. Investigation of microvessel density after irradiation. Radiat. Res. 151: 454–460. doi: 10.2307/3579833 [DOI] [PubMed] [Google Scholar]
- 22.Rao N., Lee Y.F., Ge R.2015. Novel endogenous angiogenesis inhibitors and their therapeutic potential. Acta Pharmacol. Sin. 36: 1177–1190. doi: 10.1038/aps.2015.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reinmuth N., Heigener D., Reck M.2015. Novel angiogenesis inhibitors in nonsmall cell lung cancer. Curr. Opin. Oncol. 27: 79–86. doi: 10.1097/CCO.0000000000000166 [DOI] [PubMed] [Google Scholar]
- 24.Rezzola S., Belleri M., Gariano G., Ribatti D., Costagliola C., Semeraro F., Presta M.2014. In vitro and ex vivo retina angiogenesis assays. Angiogenesis 17: 429–442. doi: 10.1007/s10456-013-9398-x [DOI] [PubMed] [Google Scholar]
- 25.Sato Y.2015. Novel link between inhibition of angiogenesis and tolerance to vascular stress. J. Atheroscler. Thromb. 22: 327–334. doi: 10.5551/jat.28902 [DOI] [PubMed] [Google Scholar]
- 26.Sewduth R., Santoro M.M.2016. “Decoding” angiogenesis: new facets controlling endothelial cell behavior. Front. Physiol. 7: 306. doi: 10.3389/fphys.2016.00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi X., Liu M., Li D., Wang J., Aneja R., Zhou J.2012. Cep70 contributes to angiogenesis by modulating microtubule rearrangement and stimulating cell polarization and migration. Cell Cycle 11: 1554–1563. doi: 10.4161/cc.19954 [DOI] [PubMed] [Google Scholar]
- 28.Shibuya M.2013. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J. Biochem. 153: 13–19. doi: 10.1093/jb/mvs136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun X., Li F., Dong B., Suo S., Liu M., Li D., Zhou J.2013. Regulation of tumor angiogenesis by the microtubule-binding protein CLIP-170. Protein Cell 4: 266–276. doi: 10.1007/s13238-013-3007-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yadgary L., Yair R., Uni Z.2011. The chick embryo yolk sac membrane expresses nutrient transporter and digestive enzyme genes. Poult. Sci. 90: 410–416. doi: 10.3382/ps.2010-01075 [DOI] [PubMed] [Google Scholar]
- 31.Ye W.2016. The complexity of translating anti-angiogenesis therapy from basic science to the clinic. Dev. Cell 37: 114–125. doi: 10.1016/j.devcel.2016.03.015 [DOI] [PubMed] [Google Scholar]
- 32.Zhou Q., Qi C.L., Li Y., He X.D., Li J.C., Zhang Q.Q., Tian L., Zhang M., Han Z., Wang H., Yang X., Wang L.J.2013. A novel four-step system for screening angiogenesis inhibitors. Mol. Med. Rep. 8: 1734–1740. doi: 10.3892/mmr.2013.1704 [DOI] [PubMed] [Google Scholar]