Abstract
The aim of this study was to establish an appropriate rat model to study the effect of electroacupuncture (EA) analgesia on acute visceral hyperalgesia. Adult rats received colorectal instillation with different concentrations of acetic acid (AA). Treatment with EA was performed for 30 min at bilateral acupoints of ST-36 and ST-37 in the hind limbs. The visceral sensation of all rats was quantified by scores of abdominal withdrawal reflex (AWR) and discharges of rectus abdominis electromyogram (EMG) in response to colorectal distension (CRD). Two hours after instillation of saline (no AA), 1%, 2%, and 4% AA, there were no, slight, moderate and severe visceral hyperalgesia, respectively. Application of EA significantly relieved the visceral hyperalgesia induced by 2% but not 4% AA. The results suggest that 2% AA acute visceral hyperalgesia in adult rats responds well to EA treatment. This may offer an appropriate model for the investigation of EA effects.
Keywords: acute visceral hyperalgesia, animal model, electroacupuncture analgesia, rats
Introduction
Though roles of acupuncture in various medical practices remain largely controversial worldwide, recent evidences support that acupuncture can relieve pain clinically [1, 14]. Over the past decades, many studies focused on effects and underlying mechanisms of acupuncture through animal experiments, offering scientific evidences and theoretical support to using acupuncture treatment clinically [7, 14, 40]. Most of them were undergone in the field of somatic pain because of the existence of mature animal models [12, 16, 20, 21, 23, 33, 34]. For examples, reports showed that acupuncture can alleviate inflammatory pain under the skin and its surrounding tissues [20, 21, 23], joint pain [33, 34] and some neuropathic pain in the animal’s limbs [12, 16].
Acupuncture has been considered a kind of somatic stimulation that can activate the C and Aδ fibers of the afferent innervated around the treatment area peripherally [40]. The activation then gives rise to a series of neurobiological responses in the central nervous system. One hypothesis for acupuncture’s pain-relieving effect is that it activates endogenous pain modulation system, thereby suppressing the central transmission and perception of noxious stimuli [7, 14, 40]. From this, it follows that the effect of acupuncture is limited or, in other word, that acupuncture cannot completely relieve pain, especially the severe ones.
Visceral pain is a complicated condition of the body: difficult to locate, may be associated with referred pain in different body parts, and may be followed by complex sympathetic and parasympathetic reflexes. These complications have made its understanding difficult. While opioid and nonopioid analgesic techniques are widely accepted medications for the management of acute visceral pain, concerns remain regarding their well-known side effects. Therefore, a nonpharmacologic approach, which can effectively reduce or even replace reliance on agents, would be useful. Acupuncture and related electrical stimulation techniques have been reported to improve pain relief and decrease requirement of opioid agents. They are also shown to reduce opioid-related side effects in the management of acute postoperative abdominal pain [6, 13, 22, 35]. Such effects of electrical stimulations are also reported on other visceral pains such as dysmenorrhea [2] and pain with oocyte retrieval [15].
Compared with somatic pain, however, clinical and experimental studies on acupuncture treatment of acute visceral pain are relatively insufficient. Parameters and mechanisms underlying acupuncture analgesia are mainly obtained from studies on the somatic pain. A major reason of slow progress in visceral pain is the lack of an appropriate animal model. Our recent report [30] has found that electroacupuncture (EA) with different frequencies showed effective analgesia to acute visceral hyperalgesia induced by 2% acetic acid (AA) in adult rats. However, it remains uncertain what concentrations of AA can induce a more appropriate rat model with acute visceral hyperalgesia for investigating the effect and underlying mechanism of EA treatment, and whether EA shows different analgesic effects on visceral sensation after instillation with different concentrations of AA. In this study, we first compared the changes of visceral sensation in adult rats after colorectal instillation of AA with different concentrations (saline, 1%, 2% and 4% AA). Then we observed the analgesic effect of EA on visceral sensation after colorectal instillation of these four concentrations of AA. We expect that the present study can provide an appropriate rat model for the investigation of the therapeutic effects and the mechanism underlying the analgesic effect of EA on acute visceral hyperalgesia.
Materials and Methods
Animals
One hundred twenty-eight male Sprague-Dawley rats, weighing 300–370 g, were obtained from the Experiment Animal Center, Shanghai Medical College, Fudan University. Rats were housed in plastic cages with corn chips bedding and maintained accessing to food and water ad libitum in a cycle of 12 h light on (07:00–19:00) and 12 h light off (19:00–07:00). All rat usage in this study was strictly in accordance with the National Institutions of Health Guide for the Care and Use of Laboratory Animals in order to minimize the number of experiment animals and their suffering.
Experimental process
The study began with colonic instillation of saline to all rats to collect self-control data. Forty-eight hours later, subsequent experiments such as acute visceral hyperalgesia induction, assessment of visceral pain, colonic histological examination and EA treatment were conducted. The visceral sensation of all rats was quantified by scores of abdominal withdrawal reflex (AWR) and discharges of rectus abdominis electromyogram (EMG) in response to colorectal distension (CRD).
Observation of AWR
AWR observation needs to apply a CRD stimulus to the animals. CRD stimulation was applied using an inflatable balloon (constructed from a condom, 6 cm in length, inflated with air) attached to a polyethylene cannula (PE-60) via a Y connector that was also attached to a 20-ml syringe and a sphygmomanometer. The balloon was inserted 7 cm deep from the anus into the descending colon and lightly tied to the root of rat tail to prevent it from sliding out. The rat was then placed in a small transparent cubicle (20 × 8 × 8 cm) on a platform and allowed to adapt for 20 min. Graded strengths of CRD at 20, 40, 60 and 80 mmHg were applied in sequence and kept for 20 s in order to produce different intensities of visceral pain.
Behavioral responses to visceral pain induced by CRD were assessed by observing the AWR. AWR in response to graded CRD stimulations was observed and recorded as semi-quantitative AWR scores according to the scale of Al-Chaer et al. [3] and our previous studies [24, 30]. To obtain more accurate scores, each graded CRD was repeated three times and the averaged value of scores was taken for analysis.
EMG Recording
As we previously reported [9, 30], adult male rats were initially anesthetized with pentobarbital (35 mg/kg, intraperitoneally) followed by continuous intravenous infusion of pentobarbital (8 mg/kg/h) through a tail vein with a micropump, keeping a mild and stable anesthesia during the entire experiment. After anesthesia, the rat was fixed in a supine position. The body temperature was monitored and kept around 37.5°C by an auto-controlled heat blanket. CRD stimulation was given in a manner similar to that of the behavioral tests. The EMG discharges was continuously recorded by a pair of electrodes placed in the bilateral rectus abdominis of the rat. The change of discharges in response to CRD stimulations at strengths of 20, 40, 60 and 80 mmHg was amplified and fed into a computer via an analog-to-digital converter (Power Lab 8.0, ADInstruments, Australia). The amplified signal was monitored on a screen and analyzed by a polygraph software (Lab Chart 7.0, ADInstruments, Austrilia). The analysis of EMG was done by measuring the area under the curve (AUC) of EMG signal in response to CRD stimulations at strengths of 20, 40, 60 and 80 mmHg. The analytic period is 40 s (20 s during and 20 s after each CRD). The net value for each CRD was calculated by subtracting the AUC of the baseline (40 s interval) before each CRD [30].
Collection of control data
In this study, all rats were first put on fast for 12 h and instilled with saline into the distal colon 48 h before subsequent experiments were conducted. The rats were lightly anesthetized with ether. A polyethylene cannula (PE-60) was lubricated with glycerol and gently inserted into the lumen of the distal colon about 6 cm in depth via the anus. Saline (1.5 ml) was slowly instilled over 1 min. Two hours later, AWR scores were observed and EMG discharges were recorded as self-control data in response to CRD at 20, 40, 60 and 80 mmHg.
Induction of acute visceral hyperalgesia
Similar to the instillation of saline, 64 rats were randomly divided into four groups, and slowly infused with 1.5 ml of saline (no AA), 1% AA, 2% AA and 4% AA into the distal colon. Two hours later, half of the rats in each group were chosen for AWR observations, and the other half were chosen for EMG recordings in each group.
Histological examination of inflammation
After AWR assessments, 2 cm of descending colon was removed from 6 cm proximal to the anus 4 h after instillation of AA, then fixed in 4% paraformaldehyde, and sent for histologic processing. The cross section of each colon wall was cut into 3 µm paraffin sections and stained with hematoxylin-eosin. The severity of lesions in the colon was reviewed by two experienced pathologists as previously reported by Al-Chaer et al. [3].
Application of EA treatment
To assess the analgesic effect of EA on visceral sensation after the infusion of different concentrations of AA, 64 rats were randomly divided into 4 groups: saline + EA, 1% AA + EA, 2% AA + EA and 4% AA + EA. AA was infused as presented above. Half of the rats in each group were chosen for AWR observations, and the other half were chosen for EMG recordings. Treatment with EA was applied 1 h after the infusion of AA in the following way. The rats were slightly restricted in a box with their hind feet bilaterally exposed. Two pairs of stainless steel needles (0.25 mm in diameter) were inserted bilaterally 5 mm deep into two acupoints, Zu-san-li (ST-36, 5 mm lateral to the anterior tubercle of the tibia and 10 mm below the knee joint) and Shang-ju-xu (ST-37, 5 mm lateral to the anterior tubercle of the tibia and 15 mm below the knee joint). Each pair of needles (one in ST-36 and the other in ST-37 ipsilaterally) was connected to the output terminals of an EA apparatus (Model SDZ-IV, Suzhou Medical Appliance Factory, China). Alternating trains of dense-sparse frequencies (5/25 Hz; sparse wave lasted 5 s, and dense wave lasted 10 s, alternately) were selected [24, 31, 32]. The intensity was initially set at 1 mA, then increased stepwisely to 2 mA and 3 mA, and each of them lasted for 10 min. After the application of EA, AWR observations and EMG recordings were immediately performed within 30 min.
Statistical analysis
All averaged values of AWR scores and EMG AUCs in response to each graded CRD stimulation from each rat were directly used for statistical analysis. Data were presented as mean ± SE and analyzed by using a statistical software (SPSS, version 19.0, IBM Co., USA). At each CRD stimulation, differences between self-controls and treatments were compared using the paired t-test. Differences among different groups were analyzed by 2-way repeated-measures analysis of variance (ANOVA) with distention pressure as the repeated measure. S-N-K post hoc test was used where appropriate. P<0.05 implied statistical significance.
Results
Changes of visceral sensation and local injuries after colonic instillation with different concentrations of AA
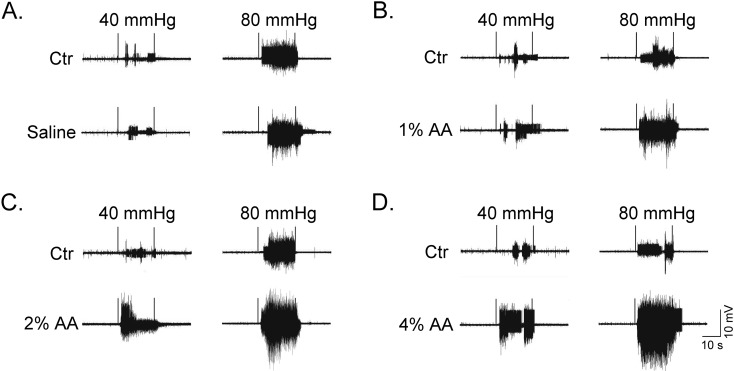
Observation of AWR scores and EMG recordings was undergone in response to graded CRDs after instillation of different concentrations of AA. Figure 1 shows the original EMG discharge samples in response to CRD stimulations at 40 and 80 mmHg. The results of AWR scores and EMG discharges are summarized in Fig. 2 and Fig. 3. A significant increase in AWR scores and EMG discharges in response to graded CRD stimulations was observed in both self-control and AA instillation of all four groups. Comparison among self-control data of different groups demonstrated no obvious differences for all CRD stimulations tested.
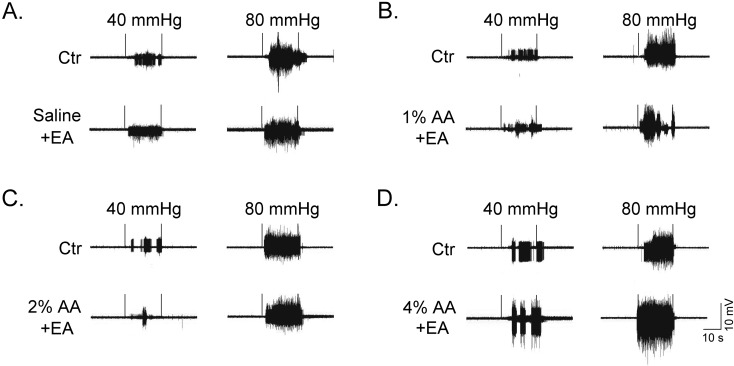
Fig. 1.
Original samples of EMG discharges in response to CRD stimulations at 40 and 80 mmHg after intracolonic instillation with saline (A), 1% AA (B), 2% AA (C) and 4% AA (D). Self-controls (Ctr) of EMG recordings were taken 48 h earlier with instillation of saline. In each original EMG sample, the two vertical trigger lines indicate the interval of 20 s CRD.
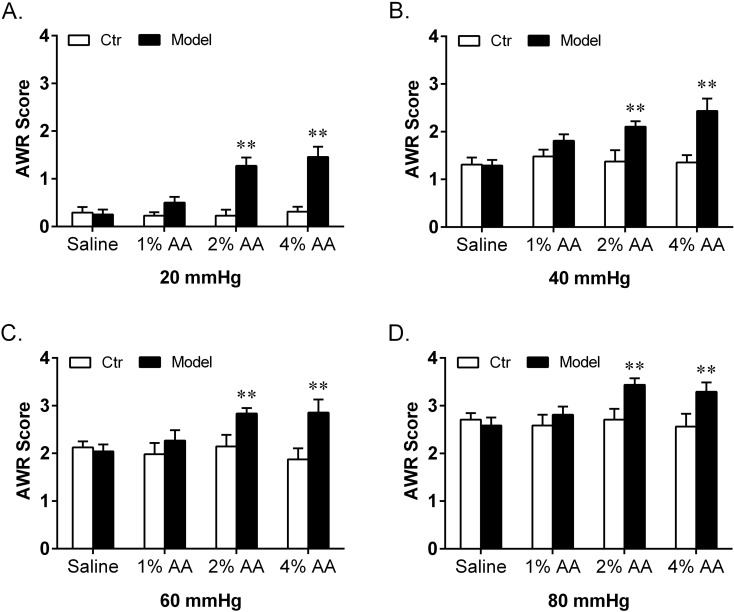
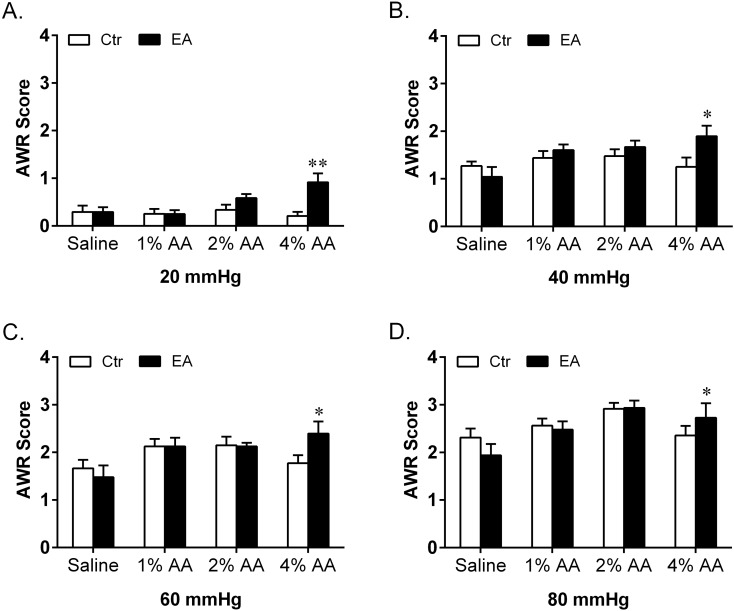
Fig. 2.
Effect of intracolonic instillation with saline, 1% AA, 2% AA and 4% AA on AWR scores in response to CRD stimulations at 20 mmHg (A), 40 mmHg (B), 60 mmHg (C) and 80 mmHg (D). Self-controls (Ctr) of AWR scores were taken 48 h earlier with instillation of saline (n=8 in each group). **P<0.01 vs. Ctr.
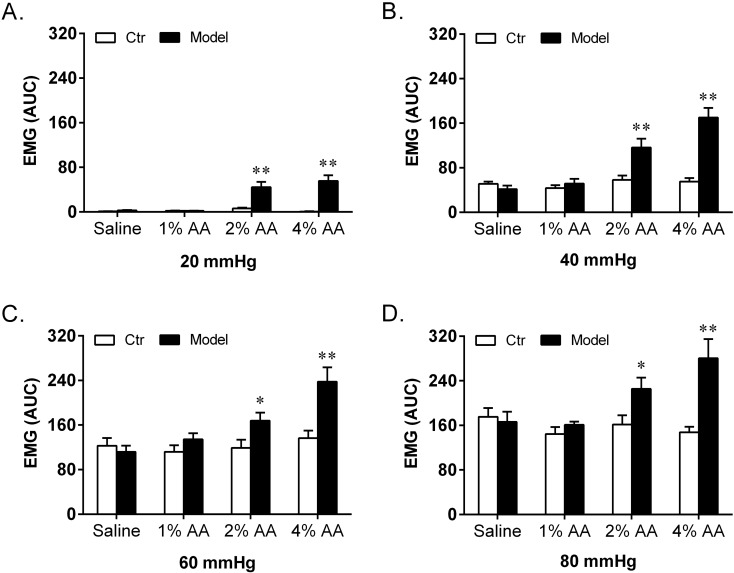
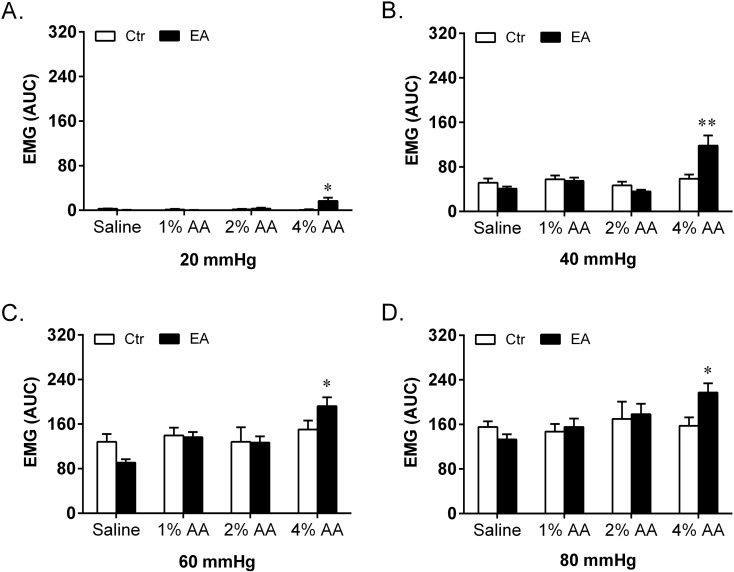
Fig. 3.
Effect of intracolonic instillation with saline, 1% AA, 2% AA and 4% AA on EMG discharges in response to CRD stimulations at 20 mmHg (A), 40 mmHg (B), 60 mmHg (C) and 80 mmHg (D). Self-controls (Ctr) of EMG discharges were taken 48 h earlier with instillation of saline (n=8 in each group). *P<0.05 vs. Ctr; **P<0.01 vs. Ctr.
After instillation with saline (no AA), 1% AA, 2% AA and 4% AA, both the AWR scores and EMG discharges respectively showed no increase, slight increase without statistical significance, significant increase and striking increase at all graded CRD stimulations compared with their self-control data. When compared among different groups, there were significant differences in both AWR and EMG responses. Post hoc analysis showed that the AWR scores and EMG discharges induced by 2% AA and 4% AA were much higher than those of the saline group at each CRD stimulation. Moreover, the AWR scores at 20 and EMG discharges at 40, 60 mmHg of the 4% AA group were significantly higher than those of the 2% AA group.
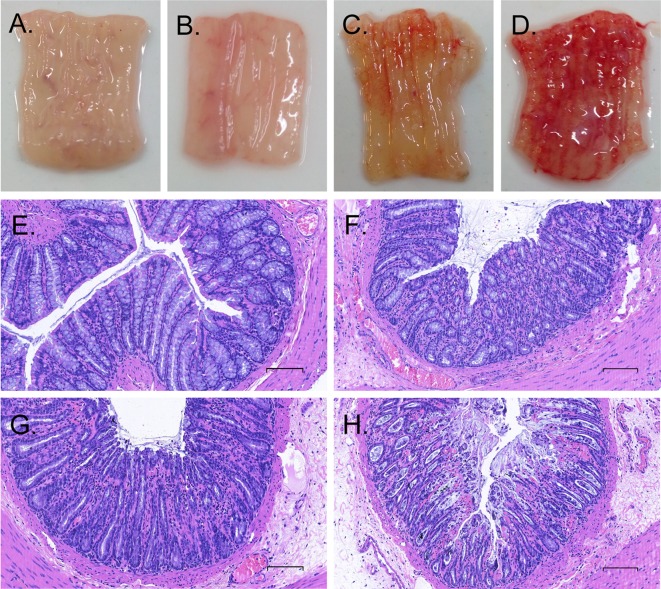
Histological examination was done to investigate the local injuries after instillation of different concentrations of AA in the distal colon (Fig. 4). Consistent with the results of AWR scores and EMG discharges, macroscopic and microscopic observations demonstrated no, mild, moderate and severe inflammation in the distal colons of rats after instillation of saline, 1% AA, 2% AA and 4% AA, respectively.
Fig. 4.
Samples of macroscopic and microscopic photographs (× 200 times) in distal colons after conlorectal instillation of saline (A, E), 1% AA (B, F), 2% AA (C, G) and 4% AA (D, H). The sections were stained by hemetoxylin and eosin, and scale bar represents 30 µm.
Different EA analgesic effects on visceral sensation after instillation with different concentrations of AA
Treatment with EA was applied to rats after colorectal instillation of different concentrations of AA. Figure 5 shows the original samples of EMG discharges after EA treatment in all four groups. The results of AWR scores and EMG discharges reflecting EA analgesic effects in all four groups are summarized in Fig. 6 and Fig. 7. Among all four groups, no obvious differences were observed in the self-control data of AWR scores and EMG discharges at each CRD stimulation.
Fig. 5.
Original samples of EMG discharges with EA treatment in response to CRD stimulations at 40 and 80 mmHg after intracolonic instillation with saline (A), 1% AA (B), 2% AA (C) and 4% AA (D). Self-controls (Ctr) of EMG discharges were taken 48 h earlier with instillation of saline. In each original EMG sample, the two vertical trigger lines indicate the interval of 20 s CRD.
Fig. 6.
Effect of EA treatment on AWR scores in response to CRD stimulations at 20 mmHg (A), 40 mmHg (B), 60 mmHg (C) and 80 mmHg (D) after intracolonic instillation with saline, 1% AA, 2% AA and 4% AA. Self-controls (Ctr) of AWR scores were taken 48 h earlier with instillation of saline (n=8 in each group). *P<0.05 vs. Ctr; **P<0.01 vs. Ctr.
Fig. 7.
Effect of EA treatment on EMG discharges in response to CRD stimulations at 20 mmHg (A), 40 mmHg (B), 60 mmHg (C) and 80 mmHg (D) after intracolonic instillation with saline, 1% AA, 2% AA and 4% AA. Self-controls (Ctr) of EMG discharges were taken 48 h earlier with instillation of saline (n=8 in each group). *P<0.05 vs. Ctr; **P<0.01 vs. Ctr.
Immediately after EA treatment, all rats instilled with different concentrations of AA were tested with graded CRD for AWR observations and EMG recordings. Compared with their self-control data, no obvious differences in both AWR scores and EMG discharges were observed in saline + EA, 1% AA + EA and 2% AA + EA groups, respectively. In contrast, the AWR scores and EMG discharges of the 4% AA group remained significantly higher than those of the self-control data. Moreover, significant differences among different groups after EA treatment were found in both AWR scores and EMG discharges. Post hoc analysis exhibited that the AWR scores and EMG discharges of the 4% AA group were significantly higher than those of the saline group at all CRD stimulations.
Comparisons between model and EA treatment after instillation with different concentrations of AA
Compared with the data of their corresponding models, no obvious differences were observed in both AWR scores and EMG discharges of saline + EA and 1% AA + EA groups, respectively. In contrast, the AWR scores and EMG discharges of the 2% AA + EA group were significantly lower than those of the 2% AA group. A decline was also observed in the AWR scores and EMG discharges of the 4% AA + EA group but not statistically different, indicating incomplete analgesia.
Discussion
Among the criteria considered in developing an appropriate acute visceral pain models, the most important are the noxious quality, the reproducibility and control of the stimulus, the quantification of the response, and the ability to use the model in conscious situation. The most widely used animal models are mainly induced by colorectal instillation of irritant chemicals, such as formalin [4, 5, 28, 36, 38, 39], mustard oil [11, 18, 42] and capsaicin [11, 18], or intraperitoneal injection of diluted AA [25, 26, 37]. Such chemical substances cause a visceral pain through injury of abdominal organs. These existing animal models have greatly enhanced our knowledge of visceral nociception or hyperalgesia and some effects and underlying mechanisms of EA [4, 5, 25, 26, 36, 37].
After colorectal instillation of these highly irritating chemicals mentioned above [4, 5, 11, 18, 28, 36, 38, 39, 42], or intraperitoneal injection of diluted AA [25, 26, 37], model rats displayed a series of striking pain behaviors, including fretfulness, abdominal licking, backward extension, contraction of the flanks, whole body contraction and bowing [4, 5, 11, 18, 25, 26, 28, 36,37,38,39, 42]. These complex visceral pain behaviors generally can be evaluated by Miampamba’s scoring system during experiment [28], and makes the observation of subtle pain relief through acupuncture difficult. In the present study, the visceral pain induced by diluted AA is much weaker. With instillation of saline, 1% and 2% AA, the rats showed no visceral pain behaviors, and occasional pain behaviors after 4% AA infusion. These visceral pain behaviors induced by AA infusion, more simple and stable, can be instead observed and assessed by AWR observations and EMG recordings. The rats showed no visceral pain behaviors after instillation of saline, 1% and 2% AA, and occasional pain behaviors after 4% AA infusion. Therefore, Miampamba’s scoring criteria were inappropriate to be used for evaluating the changes of visceral sensation of rats in this study. Instead, the visceral pain behaviors induced by CRD stimulation after AA infusion, are more simple and stable, thus can be observed and assessed by AWR observations and EMG recordings.
Colorectal distension is a method to elicit immediate visceral pain of the animal and have been widely used to measure the alteration of the pain threshold and sensation of the colon in the chronic visceral hyperalgesia studies [3, 9, 24, 29, 41]. Applying different strengths of CRD can generate different involuntary AWRs. The AWRs are reproducible and can be quantified by a semi-quantitative scale, as reported in previous studies [3, 9, 24, 30]. Moreover, the visceral responses to CRD stimulation can be recorded by the EMG discharges under mild anesthesia [9, 30], or in a conscious situation [29, 41], which is more objective and accurate than AWR scores and Miampamba’s scoring system in the assessment of visceral sensation.
When acute visceral hyperalgesia was induced by highly irritant chemicals, such as formalin [4, 5, 28, 36, 38, 39], severe transmural inflammation occurs, which may produce simultaneously somatic pain due to involvement of the abdominal wall structures. In this case, these models are no longer truly or purely representing visceral pain. Intraperitoneal injection of diluted AA is also widely used as a visceral pain model [25, 26, 37]. This model provokes a widespread range of inflammatory responses that affect visceral organs and the abdominal wall tissues as well, and thus is also not an ideal visceral pain model. The histological examinations of present study showed that the local inflammations induced by instillation of concentrations of AA from 1% to 4% (not more than 4%) were almost confined to the mucosa and submucosa of the colon and no abdominal wall structures were involved, suggesting a true and pure acute visceral hyperalgesia model.
It is challenging to choose an appropriate animal model with acute visceral hyperalgesia for acupuncture study. One must consider that acupuncture may be effective for mild to moderate pain, such as irritable bowel syndrome [9, 24, 31, 32], dysmenorrhea [2] and pain with oocyte retrieval [15]. However, it is not potent enough to match the robust effects of pharmaceutical agents as morphine. Therefore, acupuncture only can be regarded as an alternative to conventional treatment for severe pain, for example, postoperative visceral pain by decreasing the opioid requirement with patient-controlled analgesia after intra-abdominal surgery [6, 13, 22, 35] and EA-induced analgesia remains controversial [8, 10]. Consistent with the results from clinical studies, the experimental studies showed that the analgesic effect of EA treatment is incomplete in acute visceral pain induced by colorectal instillation of formalin [4, 5, 36] and intraperitoneal injection of diluted AA [25, 26, 37]. In a severe somatic pain model with cancer induced bone pain, the experimental results also demonstrated that EA worked little or incomplete [17, 19, 27].
Alternating frequency of 5/25 Hz EA is widely used in clinical practice in China. Our previous studies [24, 31, 32] have already demonstrated that chronic visceral hyperalgesia can be well relieved by this pattern of electrical stimulation. Based on the above reasons, we chose these parameters for EA treatment in the present study. In this study, the results showed that visceral hyperalgesia induced by instillation of 1% was too mild to show the effect of EA analgesia. The hyperalgesia induced by 4% AA, on the other end, was only partially relieved by EA and could be too severe for the model. In contrast, 2% AA induced a moderate visceral hyperalgesia that was highly effectively relieved by EA, as the increased AWR scores and EMG discharges were almost completely reduced. For these reasons, the acute visceral hyperalgesia model induced by 2% AA can be chosen subsequently for investigating the possible involvement of endogenous neurophysiological mechanisms, such as opioid or other neurotransmitter pathways, underlying EA analgesia. With this acute visceral hyperalgesia rat model, our recent report [30] has showed that frequency-specific EA analgesia to acute visceral hyperalgesia is mediated via different endogenous opioid pathways.
In conclusion, our present findings provide evidence that moderate acute visceral hyperalgesia induced by colorectal instillation of 2% AA in adult rats is an appropriate model for the investigation of the therapeutic effects and neurobiological mechanisms underlying EA analgesia on acute visceral hyperalgesia.
Conflict of Interest
The authors declare that they have no competing interests.
Acknowledgments
This work was supported by the research fund entitled “A Proposal to Establish A Programme to Facilitate Clinical Implementation of Traditional Medicine” from Canada.
References
- 1.[No authors listed]1998. NIH Consensus Conference. Acupuncture. JAMA 280: 1518–1524. [PubMed] [Google Scholar]
- 2.Abaraogu U.O., Tabansi-Ochuogu C.S.2015. As acupressure decreases pain, acupuncture may improve some aspects of quality of life for women with primary dysmenorrhea: a systematic review with meta-analysis. J. Acupunct. Meridian Stud. 8: 220–228. doi: 10.1016/j.jams.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 3.Al-Chaer E.D., Kawasaki M., Pasricha P.J.2000. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology 119: 1276–1285. doi: 10.1053/gast.2000.19576 [DOI] [PubMed] [Google Scholar]
- 4.Cao F.Y., Chen G.B., Yin G.F., Ru L.Q.2006. Effect of electroacupuncture on the activity of nitric oxide synthase in the colon, dorsal root ganglia and spinal cord in rats with pelvic pain. Zhen Ci Yan Jiu 31: 15–18(In Chinese). [Google Scholar]
- 5.Chen G.B., Yin G.F., Cao F.Y., Ru L.Q.2012. Effect of Electroacupuncture on the Ach in the colon, dorsal root ganglia and sacral spinal cord in the pelvic visceral pain models of rats. J. Clin. Acupunct. Moxibustion 28: 51–53(Chinese journal with English abstract). [Google Scholar]
- 6.Chen L., Tang J., White P.F., Sloninsky A., Wender R.H., Naruse R., Kariger R.1998. The effect of location of transcutaneous electrical nerve stimulation on postoperative opioid analgesic requirement: acupoint versus nonacupoint stimulation. Anesth. Analg. 87: 1129–1134. [PubMed] [Google Scholar]
- 7.Chen S., Wang S., Rong P., Wang J., Qiao L., Feng X., Liu J., Zhang J.2014. Acupuncture for visceral pain: neural substrates and potential mechanisms. Evid. Based Complement. Alternat. Med. 2014: 609594. doi: 10.1155/2014/609594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen P.A., Rotne M., Vedelsdal R., Jensen R.H., Jacobsen K., Husted C.1993. Electroacupuncture in anaesthesia for hysterectomy. Br. J. Anaesth. 71: 835–838. doi: 10.1093/bja/71.6.835 [DOI] [PubMed] [Google Scholar]
- 9.Cui K.M., Li W.M., Gao X., Chung K., Chung J.M., Wu G.C.2005. Electro-acupuncture relieves chronic visceral hyperalgesia in rats. Neurosci. Lett. 376: 20–23. doi: 10.1016/j.neulet.2004.11.018 [DOI] [PubMed] [Google Scholar]
- 10.El-Rakshy M., Clark S.C., Thompson J., Thant M.2009. Effect of intraoperative electroacupuncture on postoperative pain, analgesic requirements, nausea and sedation: a randomised controlled trial. Acupunct. Med. 27: 9–12. doi: 10.1136/aim.2008.000075 [DOI] [PubMed] [Google Scholar]
- 11.Fichna J., Lapointe T., Chapman K., Janecka A., Vergnolle N., Altier C., Storr M.A.2012. New neostigmine-based behavioral mouse model of abdominal pain. Pharmacol. Rep. 64: 1146–1154. doi: 10.1016/S1734-1140(12)70911-8 [DOI] [PubMed] [Google Scholar]
- 12.Gao Y.H., Li C.W., Wang J.Y., Kan Y., Tan L.H., Jing X.H., Liu J.L.2016. Activation of hippocampal MEK1 contributes to the cumulative antinociceptive effect of electroacupuncture in neuropathic pain rats. BMC Complement. Altern. Med. 16: 517. doi: 10.1186/s12906-016-1508-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamza M.A., White P.F., Ahmed H.E., Ghoname E.A.1999. Effect of the frequency of transcutaneous electrical nerve stimulation on the postoperative opioid analgesic requirement and recovery profile. Anesthesiology 91: 1232–1238. doi: 10.1097/00000542-199911000-00012 [DOI] [PubMed] [Google Scholar]
- 14.Han J.S.2011. Acupuncture analgesia: areas of consensus and controversy. Pain 152:(Suppl): S41–S48. doi: 10.1016/j.pain.2010.10.012 [DOI] [PubMed] [Google Scholar]
- 15.Humaidan P., Brock K., Bungum L., Stener-Victorin E.2006. Pain relief during oocyte retrieval--exploring the role of different frequencies of electro-acupuncture. Reprod. Biomed. Online 13: 120–125. doi: 10.1016/S1472-6483(10)62025-1 [DOI] [PubMed] [Google Scholar]
- 16.Jiang H., Yu X., Ren X., Tu Y.2016. Electroacupuncture alters pain-related behaviors and expression of spinal prostaglandin E2 in a rat model of neuropathic pain. J. Tradit. Chin. Med. 36: 85–91. doi: 10.1016/S0254-6272(16)30013-9 [DOI] [PubMed] [Google Scholar]
- 17.Kuai L., Chen H., Zhang T.T., Yang H.Y.2012. [Study on dose-effect relationship of electroacupuncture with different current intensities alleviating tibial cancer pain and inhibition of expression of spinal GFAP in rats]. Zhongguo Zhenjiu 32: 331–337(In Chinese). [PubMed] [Google Scholar]
- 18.Laird J.M., Martinez-Caro L., Garcia-Nicas E., Cervero F.2001. A new model of visceral pain and referred hyperalgesia in the mouse. Pain 92: 335–342. doi: 10.1016/S0304-3959(01)00275-5 [DOI] [PubMed] [Google Scholar]
- 19.Lee H.J., Lee J.H., Lee E.O., Lee H.J., Kim K.H., Lee K.S., Lee C.H., Nam D.W., Kim S.H., Lee H.J., Ahn K.S.2009. Substance P and beta endorphin mediate electroacupuncture induced analgesic activity in mouse cancer pain model. Acupunct. Electrother. Res. 34: 27–40. doi: 10.3727/036012909803861095 [DOI] [PubMed] [Google Scholar]
- 20.Li W.M., Cui K.M., Li N., Gu Q.B., Schwarz W., Ding G.H., Wu G.C.2005. Analgesic effect of electroacupuncture on complete Freund’s adjuvant-induced inflammatory pain in mice: a model of antipain treatment by acupuncture in mice. Jpn. J. Physiol. 55: 339–344. doi: 10.2170/jjphysiol.RP001505 [DOI] [PubMed] [Google Scholar]
- 21.Liao H.Y., Hsieh C.L., Huang C.P., Lin Y.W.2017. Electroacupuncture Attenuates CFA-induced Inflammatory Pain by suppressing Nav1.8 through S100B, TRPV1, Opioid, and Adenosine Pathways in Mice. Sci. Rep. 7: 42531. doi: 10.1038/srep42531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin J.G., Lo M.W., Wen Y.R., Hsieh C.L., Tsai S.K., Sun W.Z.2002. The effect of high and low frequency electroacupuncture in pain after lower abdominal surgery. Pain 99: 509–514. doi: 10.1016/S0304-3959(02)00261-0 [DOI] [PubMed] [Google Scholar]
- 23.Liu B., Tai Y., Caceres A.I., Achanta S., Balakrishna S., Shao X., Fang J., Jordt S.E.2016. Oxidized phospholipid OxPAPC activates TRPA1 and contributes to chronic inflammatory pain in mice. PLoS One 11: e0165200. doi: 10.1371/journal.pone.0165200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu H., Zhang Y., Qi D., Li W.2017. Downregulation of the spinal NMDA receptor NR2B subunit during electro-acupuncture relief of chronic visceral hyperalgesia. J. Physiol. Sci. 67: 197–206. doi: 10.1007/s12576-016-0456-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J., Fu W., Yi W., Xu Z., Liao Y., Li X., Chen J., Liu X., Xu N.2011. Extrasegmental analgesia of heterotopic electroacupuncture stimulation on visceral pain rats. Brain Res. 1373: 160–171. doi: 10.1016/j.brainres.2010.12.013 [DOI] [PubMed] [Google Scholar]
- 26.Liu J.H., Fu W.B., Xu Z.H., Liao Y., Li X.R.2010. [Effect of transection or pretreatment of the infraorbital nerve with snake venom on the analgesia induced by electroacupuncture of “Sibai” (ST 2) acupoint in visceral pain rats]. Zhen Ci Yan Jiu 35: 281–286(In Chinese). [PubMed] [Google Scholar]
- 27.Mao-Ying Q.L., Ren D.H., Mi W.L., Liu Q., Wang Y.Q.2008. [Analgesic effects of electroacupuncture combined with Celebrex on rats with tibial cancer pain]. Zhong Xi Yi Jie He Xue Bao 6: 830–835(In Chinese). doi: 10.3736/jcim20080812 [DOI] [PubMed] [Google Scholar]
- 28.Miampamba M., Chéry-Croze S., Gorry F., Berger F., Chayvialle J.A.1994. Inflammation of the colonic wall induced by formalin as a model of acute visceral pain. Pain 57: 327–334. doi: 10.1016/0304-3959(94)90008-6 [DOI] [PubMed] [Google Scholar]
- 29.Miranda A., Mickle A., Schmidt J., Zhang Z., Shaker R., Banerjee B., Sengupta J.N.2011. Neonatal cystitis-induced colonic hypersensitivity in adult rats: a model of viscero-visceral convergence. Neurogastroenterol. Motil. 23: 683–e281. doi: 10.1111/j.1365-2982.2011.01724.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi D., Wu S., Zhang Y., Li W.2016. Electroacupuncture analgesia with different frequencies is mediated via different opioid pathways in acute visceral hyperalgesia rats. Life Sci. 160: 64–71. doi: 10.1016/j.lfs.2016.06.025 [DOI] [PubMed] [Google Scholar]
- 31.Qi D.B., Li W.M.2012a. Effects of electroacupuncture on expression of c-fos protein and N-methyl-D-aspartate receptor 1 in the rostral ventromedial medulla of rats with chronic visceral hyperalgesia. Zhong Xi Yi Jie He Xue Bao 10: 416–423. doi: 10.3736/jcim20120410 [DOI] [PubMed] [Google Scholar]
- 32.Qi D.B., Li W.M.2012b. Effects of electroacupuncture on expression of c-fos protein in the spinal dorsal horn of rats with chronic visceral hyperalgesia. Zhong Xi Yi Jie He Xue Bao 10: 1490–1496. doi: 10.3736/jcim20121224 [DOI] [PubMed] [Google Scholar]
- 33.Seo B.K., Park D.S., Baek Y.H.2013. The analgesic effect of electroacupuncture on inflammatory pain in the rat model of collagenase-induced arthritis: mediation by opioidergic receptors. Rheumatol. Int. 33: 1177–1183. doi: 10.1007/s00296-012-2502-5 [DOI] [PubMed] [Google Scholar]
- 34.Seo B.K., Sung W.S., Park Y.C., Baek Y.H.2016. The electroacupuncture-induced analgesic effect mediated by 5-HT1, 5-HT3 receptor and muscarinic cholinergic receptors in rat model of collagenase-induced osteoarthritis. BMC Complement. Altern. Med. 16: 212. doi: 10.1186/s12906-016-1204-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang B., Tang J., White P.F., Naruse R., Sloninsky A., Kariger R., Gold J., Wender R.H.1997. Effect of the intensity of transcutaneous acupoint electrical stimulation on the postoperative analgesic requirement. Anesth. Analg. 85: 406–413. [DOI] [PubMed] [Google Scholar]
- 36.Xu K.D., Liang T., Wang K., Tian D.A.2010. Effect of pre-electroacupuncture on p38 and c-Fos expression in the spinal dorsal horn of rats suffering from visceral pain. Chin. Med. J. (Engl.) 123: 1176–1181. [PubMed] [Google Scholar]
- 37.Yu W.C., Huang G.Y., Zhang M.M.2008. [Influence of connexin 43 gene knockout on the analgesic effect of acupuncture in visceral pain mice]. Zhen Ci Yan Jiu 33: 3–6(In Chinese). [PubMed] [Google Scholar]
- 38.Zhang Y., Gong K., Zhou W., Shao G., Li S., Lin Q., Li J.2011. Involvement of subtypes γ and ε of protein kinase C in colon pain induced by formalin injection. Neurosignals 19: 142–150. doi: 10.1159/000328311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y.B., Guo Z.D., Li M.Y., Fong P., Zhang J.G., Zhang C.W., Gong K.R., Yang M.F., Niu J.Z., Ji X.M., Lv G.W.2015. Gabapentin effects on PKC-ERK1/2 signaling in the spinal cord of rats with formalin-induced visceral inflammatory pain. PLoS One 10: e0141142. doi: 10.1371/journal.pone.0141142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Z.Q.2008. Neural mechanism underlying acupuncture analgesia. Prog. Neurobiol. 85: 355–375. doi: 10.1016/j.pneurobio.2008.05.004 [DOI] [PubMed] [Google Scholar]
- 41.Zhou Y.Y., Wanner N.J., Xiao Y., Shi X.Z., Jiang X.H., Gu J.G., Xu G.Y.2012. Electroacupuncture alleviates stress-induced visceral hypersensitivity through an opioid system in rats. World J. Gastroenterol. 18: 7201–7211. doi: 10.3748/wjg.v18.i48.7201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zielińska M., Ben Haddou T., Cami-Kobeci G., Sałaga M., Jarmuż A., Padysz M., Kordek R., Spetea M., Husbands S.M., Fichna J.2015. Anti-inflammatory effect of dual nociceptin and opioid receptor agonist, BU08070, in experimental colitis in mice. Eur. J. Pharmacol. 765: 582–590. doi: 10.1016/j.ejphar.2015.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]