Abstract
Objective
The primary goal of the current study was to investigate factors contributing to more negative cognitive change at older ages.
Method
Longitudinal data on 12 cognitive tests were examined in 2,637 adults ranging from 18 to 85 years of age. Because both the intervals between measurement occasions and the number of occasions varied across participants, it was possible to investigate effects of interval and number of measurement occasions on cognitive change in adults of different ages. In addition, about one-half of the participants performed alternate versions of the tests on a second and third session on the first occasion, which allowed change to be monitored over intervals of less than one week.
Results
Regression analyses revealed that cognitive change was more negative with increases in the interval between occasions, but was more positive with additional measurement occasions. Both the effects of interval and of number of measurement occasions were similar across adulthood. Increased age was associated with more positive gains over a period of a few days, but was associated with more negative declines when the intervals between occasions averaged about three years.
Conclusions
This combination of results suggests that longitudinal change in cognitive functioning is more negative at older ages not because of greater declines with increases in the interval between measurement occasions, or because of smaller gains with additional measurements. Instead most of the age differences in change may be due to greater losses of benefits associated with the initial assessment over intervals of months or more from the initial assessment.
Keywords: interval effects, retest effects, longitudinal decline, aging cognition
Increased age in adulthood has frequently been found to be associated with more negative cognitive change (e.g., Bielak et al. 2012; Caselli et al. 2009; Ferrer et al. 2004; Finkel et al. 1998; Giambra et al. 1995; Huppert & Whittington, 1993; Lamar et al. 2003; McArdle et al. 2002; Mitchell et al. 2012; Ronnlund & Nilsson, 2006; Ronnlund et al. 2005; Schaie, 2005; Schaie & Hertzog, 1983; Singh-Manoux et al. 2012; van der Elst et al. 2008; van Dijk et al. 2008; Zelinski & Burnight, 1997). To illustrate, correlations between age at the first (T1) occasion and difference scores across the interval from the first (T1) to the second (T2) occasion, derived from tables containing averages in groups ranging from 25 to 88 years of age, were −.93 for episodic memory (Ronnlund et al., 2005, Table 3), −.80 for score on the block design test (Ronnlund et al., 2006, Table 3), and −.89 for an aggregate measure of intellectual ability (Schaie, 2013, Table 5.1). Although the phenomenon of more negative change with increased age is well-established, relatively little is known about the mechanisms involved.
Table 3.
Unstandardized coefficients (and 99% confidence intervals) relating Tn-T1 difference scores to age and the T1-Tn interval in subsamples by number of occasions
| Measure | Age | T1-T | n Interval | Age * Interval |
|---|---|---|---|---|
| Mean z | ||||
| 2 Occasions | −.006* (−.007, −.005) | −.041* (−.051, −.031) | .000 (−.001, .000) | |
| 3 Occasions | −.004* (−.006, −.002) | −.046* (−.064, −.029) | .000 (−.001, .001) | |
| 4 Occasions | −.004* (−.006, −.002) | −.043* (−.061, −.025) | −.001 (−.002, .001) | |
| Memory | ||||
| 2 Occasions | −.008* (−.010, −.006) | −.049* (−.069, −.029) | .000 (−.001, .001) | |
| 3 Occasions | −.005* (−.009, −.002) | −.060* (−.094, −.027) | −.001 (−.003, .002) | |
| 4 Occasions | −.006* (−.010, −.003) | −.026 (−.061, .009) | −.002 (−.004, .001) | |
| Speed | ||||
| 2 Occasions | −.006* (−.008, −.004) | −.056* (−.073, −.039) | .000 (−.001, .001) | |
| 3 Occasions | −.004* (−.006, −.001) | −.060* (−.088, −.032) | −.001 (−.003, .000) | |
| 4 Occasions | −.004* (−.007, −.001) | −.060* (−.091, −.029) | .000 (−.002, .002) | |
| Reasoning | ||||
| 2 Occasions | −.005* (−.007, −.003) | −.028* (−.045, −.010) | −.001 (−.002, .000) | |
| 3 Occasions | −.002 (−.005, .000) | −.034* (−.063, −.004) | .001 (−.001, .003) | |
| 4 Occasions | −.002 (−.005, .001) | −.007 (−.040, .026) | −.001 (−.003, .001) | |
| Space | ||||
| 2 Occasions | −.005* (−.007, −.003) | −.026* (−.043, −.009) | .000 (−.001, .001) | |
| 3 Occasions | −.005* (−.008, −.003) | −.054* (−.083, −.025) | −.001 (−.003, .001) | |
| 4 Occasions | −.004* (−.007, −.001) | −.067* (−.100, −.034) | .000 (−.003, .002) | |
Note:
p<.01.
The coefficients for age represent Tn-T1 differences for each year of age, and those for the T1-Tn interval correspond to differences for each year of T1-Tn interval. The numbers in parentheses are the lower and upper values for the 99% confidence interval. Sample sizes ranged from 809 to 1147.
One factor that could be contributing to the negative age-change relations is more rapid decline in cognitive performance with increases in the interval from the first to the second assessment. That is, the slope of the function relating cognitive change to the interval between measurement occasions may be more negative at older ages. Although plausible, influences of age on the relations between cognitive change and the interval between measurements are difficult to investigate in typical longitudinal studies in which there is little variability in the intervals between occasions, such that increases in the interval between measurement occasions are almost perfectly correlated with increases in age.
The Virginia Cognitive Aging Project (VCAP) is unique in this respect because the intervals between measurement occasions were deliberately varied across participants. Salthouse (2011a) capitalized on this feature in analyses of two-occasion longitudinal data with 1,576 adults between 19 and 95 years of age, and intervals between the two occasions ranging from 0.8 to 8.4 years. The major results were that the functions relating cognitive change to the interval between occasions were nearly parallel in adults of different ages, with little or no interactions of age and interval in the prediction of change.
The purpose of the current study was to extend the analysis of age differences in cognitive change in two directions. First, only two occasions were considered in the prior study, but it is possible that change is influenced by the number of measurement occasions in addition to the length of the interval between occasions. Because almost 1,500 VCAP participants completed three or more occasions, effects of number of occasions on change were examined in the current study. These effects were investigated by determining the last (i.e., Tn) occasion for each participant (corresponding to the second for those with only 2 occasions, the third for participants with 3 occasions, and the fourth for participants with 4 or more occasions), and the interval from the first (T1) to the last (Tn) occasion. Regression analyses were then conducted with number of measurement occasions (i.e., 2, 3, or 4) serving as a predictor of change across the T1-Tn interval.
A second focus in the study was on age differences in change across short intervals from the initial occasion. Specifically, age differences in change were investigated with T1-T2 intervals averaging about one year, and across very short intervals averaging less than one week. The contrast of change in these two conditions allowed influences operating from a first to a second assessment over a moderate interval to be distinguished from influences operating from a first to a second assessment over a very short interval.
In addition to these new goals, the earlier (Salthouse, 2011a) analyses of the effects of the interval between occasions on cognitive change were extended with a larger sample of adults (N = 2,637), and a range from the first to the last occasion up to 15 years. The prior results of age differences in level of change, but not in the relation between change and interval between occasions, were expected to be replicated.
Methods
Participants
Participants were recruited from the community with advertisements, flyers, and referrals from other participants. The interval between occasions was varied across participants to allow test experience effects to be investigated by reducing the correlation between increase in test experience and increase in age that is high when all participants have the same intervals between occasions (e.g., McArdle et al., 2002; Salthouse et al., 2004). A combination of deliberate variation, and particularly after the second occasion, compatibility of the participant’s schedule with the testing schedule, was used to determine the interval between occasions.
Participation consisted of reporting to the laboratory for three sessions separated by about one week, during which alternate versions of the primary cognitive tests were performed. These three sessions constituted a measurement burst design which allowed the investigation of within-person variability (Salthouse, 2007), and within-occasion change (Salthouse, 2013). In order to investigate other issues, about one-half of the participants performed different cognitive tests on the second and third sessions of the first occasion, but all participants performed alternate versions of the same tests on subsequent occasions. The alternate versions on sessions 2 and 3 involved different items with the same format as the original tests. Scores on all versions were converted to the same scale as that on session 1 by administering the test versions to a sample of participants in a counterbalanced order, and then using regression equations to statistically equate the mean scores across versions (for details see Salthouse, 2007).
Characteristics of the total sample, and in subgroups with each number of longitudinal measurement occasions, and in three different age ranges, are reported in Table 1. The number-of-occasion groups were formed according to the maximum number of occasions completed by the participants, and therefore each group was comprised of different participants. Inspection of the values in Table 1 reveals that the groups were fairly similar in terms of demographic characteristics.
Table 1.
Characteristics of participants with different numbers of occasions
| Number of Occasions | ||||
|---|---|---|---|---|
| Measure | All | 2 | 3 | 4 |
| Number | 2,637 | 1,147 | 676 | 814 |
| Age in years | 53.0 (16.2) | 51.9 (18.0) | 53.9 (15.9) | 53.7 (13.4) |
| Proportion Female | .67 | .66 | .67 | .70 |
| Self Rated Health | 2.2 (0.9) | 2.2 (0.9) | 2.2 (0.9) | 2.1 (0.9) |
| Years of Education | 15.7 (3.5) | 15.4 (4.3) | 15.8 (2.7) | 15.9 (2.7) |
| MMSE | 28.6 (1.5) | 28.5 (1.6) | 28.6 (1.6) | 28.7 (1.4) |
| Estimated IQ | 110.4 (14.1) | 109.2 (13.4) | 110.9 14.4) | 111.8 (14.7) |
| T1-Tn Int. (Mean) | 5.8 (3.1) | 3.4 (2.1) | 6.2 (2.1) | 8.9 (1.9) |
| T1-Tn Int. (Min/Max) | 0.9 / 15.2 | 0.9 / 15.0 | 1.7 / 14.9 | 4.0 / 15.2 |
| Age Group | ||||
| Y (18–44) | M (45–64) | O (65–85) | ||
| Number | 716 | 1264 | 657 | |
| Age in years | 31.6 (8.5) | 54.8 (5.5) | 72.6 (5.5) | |
| Proportion Female | .67 | .71 | .60 | |
| Self-Rated Health | 2.1 (0.8) | 2.1 (0.9) | 2.3 (0.9) | |
| Years of Education | 15.1 (2.5) | 15.9 (2.6) | 16.0 (5.3) | |
| MMSE | 28.6 (1.5) | 28.7 (1.5) | 28.5 (1.6) | |
| Estimated IQ | 108.0 (14.0) | 111.3 (14.7) | 111.3 (12.8) | |
| T1-Tn Int. (Mean) | 5.5 (3.3) | 6.3 (3.1) | 5.2 (2.8) | |
| T1-Tn Int. (Min/Max) | 0.9 / 15.0 | 0.9 / 15.2 | 1.0 / 14.1 | |
Note: Values in parentheses are standard deviations. Health is on a 5-point scale from 1 for “excellent” to 5 for “poor”. MMSE is the Mini Mental State Exam (Folstein, et al., 1975), and the values are from the T2 occasion in which the test was administered to all participants. Estimated IQ is an estimate of IQ based on age-adjusted scores on three tests found to be highly related to Wechsler IV full scale IQ (Salthouse, 2014b). The T1-Tn interval is in years.
Possible differences in cognitive ability of participants with different intervals or numbers of occasions were examined with correlations between the T1-T2 interval and number of occasions and both the mean z-score and the MMSE score at T1. None of the interval correlations, which ranged from .03 to .07, were significant, and thus there was no evidence that participants with different intervals differed in initial level of cognitive ability. Correlations of T1 MMSE score and number of occasions were small (i.e., −.02 to .07) and not significant, but the correlations with T1 mean z-scores were significantly positive in the 45-to-64 (r = .18) and 65-to-85 (r = .25) age groups.
A number of issues related to longitudinal change in this project have been investigated in previous publications. For example, Salthouse (2012) found that the rate of cognitive change was similar among participants with different levels of initial ability after considering effects associated with regression to the mean. Selective attrition was investigated in another study (Salthouse, 2014b) by comparing actual change in longitudinal participants with imputed change in non-returning participants. The results indicated that selective attrition primarily affected level of functioning rather than rate of change. Several studies (i.e., Salthouse, 2014c, 2015b, 2016a) investigated effects of test experience on change by comparing new participants tested for the first time with longitudinal participants of the same age tested for the second time, and comparing longitudinal (i.e., same participants tested at different ages and different times) and quasi-longitudinal (i.e., different participants tested at different ages and different times) age trends. In each case the inferred test experience effects were positive, and smaller at older ages. Finally, statistical control procedures have revealed that large proportions of cognitive change appear to be general, and shared across different cognitive abilities, rather than specific to particular cognitive domains (Salthouse, 2016b; 2017).
Measures
The analyses were conducted on scores from 12 tests representing four cognitive abilities: memory, speed, reasoning, and spatial visualization. Memory was assessed with tests of word recall (Wechsler, 1997b), paired associate learning (Salthouse et al. 1996), and logical memory (Wechsler, 1997b). Speed was assessed with tests of digit symbol substitution (Wechsler, 1997a), and pattern comparison and letter comparison (Salthouse & Babcock, 1991). Reasoning was assessed with tests of matrix reasoning (Raven, 1962), series completion (Zachary, 1986), and letter sets (Ekstrom et al., 1976). Spatial visualization ability was assessed with tests of spatial relations (Bennett et al. 1997), paper folding (Ekstrom et al. 1976), and form boards (Ekstrom et al. 1976). Tests of vocabulary were also performed, but they were not analyzed here because they have different age trends than the other measures, and can be postulated to reflect an achievement based on the interaction of abilities and experience rather than an ability. Most participants completed all tests, although some tests were not administered in the early years of the project, and certain tests were not completed by participants with sensory or motor limitations.
All scores were converted to z-scores based on the mean and standard deviation of scores on the first (T1) occasion. Means of the three z-scores for each ability were computed at each occasion to assess the separate abilities, and the mean of all 12 z-scores was computed to represent general cognitive ability.
The primary analyses were conducted on difference scores created by subtracting the score at the first (T1) occasion from the score at a later (Tn) occasion. Difference scores have the advantage of directly representing change compared to regression residuals which reflect relative, rather than absolute, change. However, the data were also analyzed with latent change models (using the model portrayed in Figure 2 in Salthouse, 2015a), which minimize measurement error because the latent variables are defined by shared systematic variance, and control influences on the level of performance when examining effects on change. The results with difference scores and with the latent change analyses were very similar, but only results with difference scores are reported because they are simpler and more familiar.
Results1
The first two sets of analyses focused on the T2-T1 differences in the total sample of 2,637 participants, and in subgroups with different numbers of occasions. The initial analyses with the total sample investigated linear and non-linear age and interval effects across the T1-T2 interval for the mean z-score. The non-linear (quadratic) trend was not significant for either age or interval. Although there was no evidence of nonlinear age trends in the entire sample, another set of analyses explored possible nonlinear trends by comparing the 429 adults between 65 and 74 years of age (mean age 69) with the 228 adults between 75 and 85 years of age (mean age 79). The two groups did not differ in T2 MMSE scores (i.e., 28.6 vs. 28.3), but the coefficient relating change to T1-T2 interval effect was significantly more negative in the group with a mean age of 79 than in the group with a mean age of 69 (−.100 vs. −036, d = −.31). This combination of results suggests that there may be an accelerated decline of interval effects in adults over 65 years of age who, based on their MMSE scores, have little evidence of dementia.
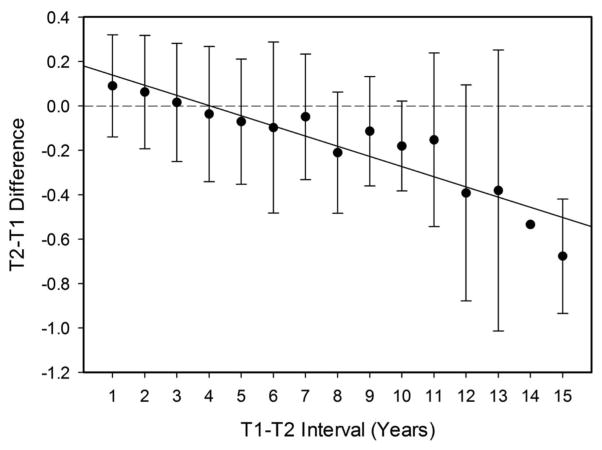
Figure 1 portrays the mean and standard deviation of the T2-T1 difference in mean z-score as a function of the interval between the T1 and T2 occasions. The interval comparisons involve different individuals, and thus it is not surprising that the standard deviations were large. Nevertheless, a clear linear trend is apparent in which longer intervals between the two occasions were associated with more negative change.
Figure 1.
T2-T1 difference in mean z-score by T1-T2 interval. The number of participants in successive intervals from 1 to 15 years were, respectively: 224, 1036, 767, 236, 150, 73, 65, 32, 19, 16, 7, 5, 3, 1, and 2. Bars represent standard deviations.
Regression equations were used to predict the T2-T1 difference score from age, T1-T2 interval, and the interaction of age and interval (examined with the product of centered age and interval variables). Results of these analyses with the mean z-score across all 12 measures, and with the composite scores for each ability domain, are presented in Table 2. The age and interval relations were all significant, with smaller age effects than interval effects. Importantly, none of the interactions of age and interval was significant, and thus there was no evidence that the interval effects on cognitive change varied as a function of age.
Table 2.
Unstandardized coefficients (and 99% confidence intervals) relating T2-T1 difference to age and to the T1-T2 interval in the total sample of 2,367 participants
| Measure | Age | T1-T2 Interval | Age * Interval |
|---|---|---|---|
| Mean z | −.005* (−.006, −.004) | −.042* (−.050, −.035) | .000 (−.001, .000) |
| Memory | −.007* (−.008, −.005) | −.047* (−.062, −.032) | .000 (−.001, .000) |
| Speed | −.005* (−.006, −.004) | −.056* (−.069, −.043) | .000 (−.001, .000) |
| Reasoning | −.004* (−.005, −.002) | −.028* (−.041, −.015) | −.001 (−.001, .000) |
| Space | −.005* (−.006, −.003) | −.036* (−.050, −.023) | .000 (−.001, .000) |
Note:
p<.01.
The coefficients for age represent differences in T2-T1 difference for each year of age, and those for the T1-T2 interval correspond to T2-T1 differences for each year of T1-T2 interval. The numbers in parentheses are the lower and upper values for the 99% confidence interval. Sample sizes ranged from 2,600 to 2,637.
Effects of interval and number of measurement occasions on the difference scores were investigated in two sets of analyses. The strong correlation (r = .75) between total interval (i.e., T1-Tn) and number of occasions (i.e,, n) resulted in distortion of the regression coefficients when both predictor variables were considered simultaneously. Separate analyses were therefore conducted of age and interval with each number of occasions, and of age and number of occasions with statistical control of interval, and with intervals below and above the median T1-Tn interval.
Unstandardized coefficients for the effects of interval on the Tn-T1 difference score with each number of occasions are reported in Table 3. Inspection of the entries in the table reveals that the same pattern was apparent in the analyses of the mean z-score, and in the analyses for each ability domain. That is, the majority of the age and interval relations, but none of the interactions, were significantly different from zero.
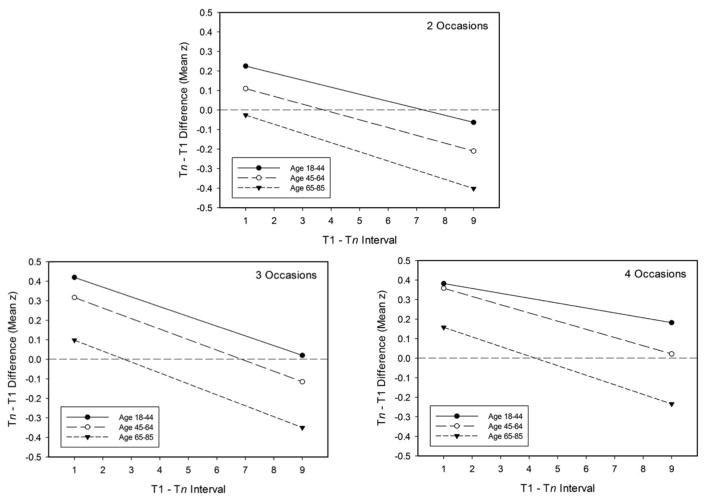
Regression lines relating change to the interval between occasions, with change expressed as the Tn-T1 difference in mean z-score, are portrayed in the three panels of Figure 2. Several points should be noted about the data in this figure. First, the difference scores at short intervals were positive, which likely reflects influences of prior test experience. Second, in each age group the difference scores were more negative with longer intervals between occasions. Third, the difference scores were more negative at older ages with each number of occasions. And fourth, with the exception of flatter functions relating difference score to interval with four occasions in the young group, the regression lines were nearly parallel in each age group.
Figure 2.
Regression lines relating T2-T1 change in mean z-score units to interval in years between occasions for adults in three age groups. The three panels portray results with 2, 3, or 4 occasions.
A second set of regression analyses examined Tn-T1 differences as a function of the number of measurement occasions. Three separate analyses were conducted with each cognitive measure, one based on all participants with the T1-Tn interval as a covariate, one based only on data of participants with T1-Tn intervals below the median (5.1 years) interval, and a third based only on data of participants with T1-Tn intervals above the median. Results of these analyses are summarized in Table 4, where it can be seen that in most of the analyses the difference score representing cognitive change was more positive with additional measurement occasions. Several interactions of age and number of occasions were significant, in each case corresponding to smaller effects of number of occasions at older ages.
Table 4.
Unstandardized coefficients (and 99% confidence intervals) relating Tn-T1 difference scores to age and number of occasions
| Measure | Age | NumOcc | Age * NumOcc |
|---|---|---|---|
| Mean z | |||
| T1-Tn as covariate | −.008* (−.009, −.007) | .111* (.086, .136) | −.002* (−.003, −.001) |
| Short T1-Tn | −.006* (−.008, −.004) | .057* (.020, .094) | −.001 (−.002, .002) |
| Long T1-Tn | −.008* (−.010, −.006) | .077* (.041, .112) | −.002 (−.004, .000) |
| Memory | |||
| T1-Tn as covariate | −.010* (−.012, −.008) | .153* (.105, .200) | −.002 (−.004, .000) |
| Short T1-Tn | −.009* (−.012, −.005) | .103* (.030, .176) | .000 (−.004, .005) |
| Long T1-Tn | −.009* (−.013, −.006) | .127* (.063, .190) | −.003 (−.007, .001) |
| Speed | |||
| T1-Tn as covariate | −.008* (−.009, −.006) | .117* (.077, .157) | −.002 (−.004, .000) |
| Short T1-Tn | −.005* (−.008, −.002) | .034 (−.029, .098) | .002 (−.002, .006) |
| Long T1-Tn | −.007* (−.010, −.004) | .069* (.016, .122) | −.002 (−.006, .001) |
| Reasoning | |||
| T1-Tn as covariate | −.007* (−.009, −.006) | .070* (.029, .110) | −.002* (−.004, .000) |
| Short T1-Tn | −.006* (−.010, −.003) | .038 (−.025, .100) | −.002 (−.006, .002) |
| Long T1-Tn | −.009* (−.013, −.006) | .042 (−.012, .096) | .000 (−.003, .004) |
| Space | |||
| T1-Tn as covariate | −.007* (−.009, −.006) | .096* (.057, .134) | −.002* (−.004, .000) |
| Short T1-Tn | −.006* (−.009, −.003) | .054 (−.006, .113) | −.001 (−.004, .003) |
| Long T1-Tn | −.007* (−.010, −.004) | .078* (.028, .129) | −.002 (−.005, .001) |
Note:
p<.01.
The analyses with the T1-Tn interval as a covariate were based on the entire sample, and the analyses with short and long intervals involved participants with T1-Tn intervals below, and above, the median (5.6 years) interval. Coefficients for age represent Tn-T1 differences for each year of age, and those for number of occasions (NumOcc) correspond to Tn-T1 differences associated with additional measurement occasions. The numbers in parentheses are the lower and upper values for the 99% confidence interval. Sample sizes ranged from 1,267 to 2,636.
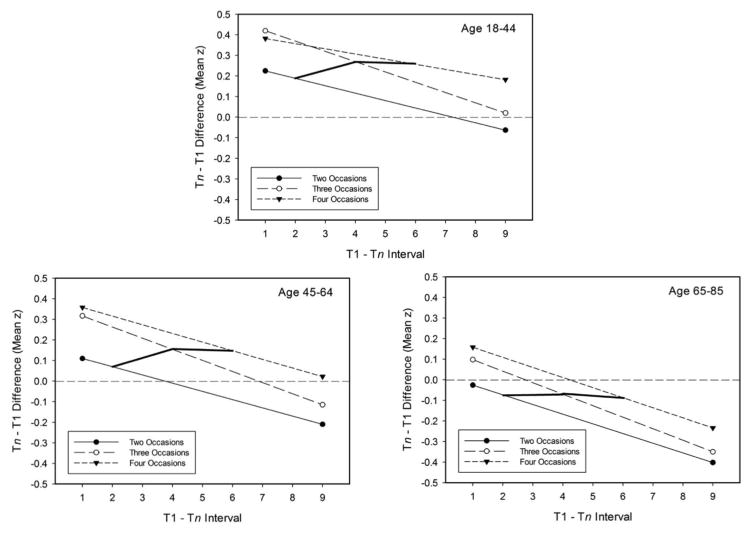
Figure 3 portrays the same data as Figure 2, but with the panels portraying results by age group instead of by number of occasions. This organization of the data reveals that successive occasions were characterized by distinct interval-change functions, in which the functions with more occasions were associated with similar slopes but higher intercepts. The Tn-T1 mean z-score differences, controlling variation in age and T1-Tn interval, were −.11, .04, and .15 standard deviations with two, three, and four occasions, respectively.
Figure 3.
Regression lines relating T2-T1 change in mean z-score units to interval in years between occasions with either 2, 3, or 4 occasions. The three panels portray results with adults in three age groups. The solid lines portray the values of expected change if measurements were obtained every 2 years.
Thick solid lines are portrayed in Figure 3 to illustrate the relation of cognitive change that would be expected if the assessments were spaced two years apart. Notice that even though longer intervals are associated with more negative change, the thick lines indicate that negative interval effects would not be detected if additional assessments occurred between the first and last measurement occasion.
Another set of analyses involved comparisons of mean z-score change across discrete age groups instead of treating age as a continuous predictor in multiple regression. Results of these analyses are reported in Table 5, in the form of means and 99% confidence intervals, and effect sizes in d units for the differences between age groups. Entries in the top two panels, consisting of regression coefficients for the interval and number-of-occasions effects, confirm the lack of age differences on the effects of T1-T2 interval and number of occasions on cognitive change found in the regression analyses.
Table 5.
Age differences in components of mean-z score change
| d units | ||||||
|---|---|---|---|---|---|---|
| Y | M | O | Y-M | M-O | Y-O | |
| 18–44 | 45–64 | 65–85 | ||||
| Change/T11-T21 year | ||||||
| N | 715 | 1264 | 657 | |||
| Estimate | −.036* | −.041* | −.048* | −.04 | −.04 | −.07 |
| 99% CI | (−.049, −.024) | (−.052, −.030) | (−.068, −.028) | |||
| Tn1-T11 Change/Occasion | ||||||
| N | 715 | 1264 | 657 | |||
| Estimate | .116* | .121* | .082* | −.01 | .07 | .06 |
| 99% CI | (.072, .161) | (.087, .155) | (.018, .145) | |||
| Short T2-T1 Change (T11-T21 interval < 1.5 years, Avg. = 1.1 years) | ||||||
| N | 56 | 102 | 67 | |||
| Estimate | .133* | .116* | .018 | .07 | .44* | .57* |
| 99% CI | (.076, .190) | (.067, .164) | (−.031, .067) | |||
| Long T2-T1 Change (T11-T21 interval > 1.49 years, Avg. = 3.2 years) | ||||||
| N | 659 | 1162 | 590 | |||
| Estimate | .106* | .010 | −.088* | .36* | .36* | .72* |
| 99% CI | (.079, .132) | (−.011, .031) | (−.117, −.058) | |||
| Within Occasion | ||||||
| T12-T11 Change (Avg. T11-T12 interval = 5.6 days) | ||||||
| N | 362 | 699 | 374 | |||
| Estimate | .120* | .199* | .279* | −.32* | −.33* | −.66* |
| 99% CI | (.087, .153) | (.175, .224) | (.247, .310) | |||
| T13-T12 Change (Avg. T12-T13 interval = 5.0 days) | ||||||
| N | 357 | 698 | 373 | |||
| Estimate | .070* | .076* | .075* | −.04 | .01 | −.03 |
| 99% CI | (.047, .092) | (.059, .093) | (.054, .095) | |||
Note:
p<.01.
T11, T12, and T13 refer to the first, second, and third sessions, respectively, on the first (T1) measurement occasion. Age group differences were evaluated with an independent groups t-test, and effect sizes are in Cohen’s d units.
The middle two panels report results contrasting age groups on change across short and long T1-T2 intervals. With short T1-T2 intervals averaging about 1 year the change was positive and nearly equivalent in the young and middle groups, but the change was not different from zero in the old group. However, there was a monotonic negative relation between cognitive change and age with T1-T2 intervals greater than 1.5 years, and all of the differences between age groups were significant.
As noted in the introduction, approximately one-half of the participants performed different versions of the cognitive tests on the second and third sessions of the first occasion. The bottom two panels of Table 5 report age differences for these participants in the mean z-score change across successive sessions separated by about one week. Note that there were positive changes from the first to the second session in each age group, with larger gains at older ages. The gains were less pronounced from the second to the third session, and none of the age group differences in the magnitude of change were significant in this contrast.
Several checks on the robustness of the results were conducted. For example, all of the analyses were repeated with the latent change model portrayed in Figure 2 of Salthouse (2015a). In addition, because many of the participants performed alternate versions of the tests on the second and third sessions at each occasion (Salthouse, 2007), and also performed sequential and alternating versions of the Connections Test (Salthouse, 2011b), similar analyses were conducted on these data. In all cases the pattern closely resembled that in the primary analyses with significant effects of age, interval, and number of occasions, but little or no interactions of age and interval, or age and number of occasions.
Discussion
The results summarized in all three figures, and the coefficients for interval in Tables 2 and 3, indicate that the change in cognitive functioning between a first and second measurement occasion was more negative with increases in the interval between occasions. As noted in the introduction, increased age has been found to be associated with more negative cognitive change, and thus steeper declines in the change-interval function might have been expected at older ages. However, the results of this study confirmed and extended the results reported by Salthouse (2011a) of little or no age differences in the slopes of the functions relating cognitive change to the interval between the first and second measurement occasions, and no interactions of age and interval on the T2-T1 difference scores.
A second focus in the study involved investigating effects of number of measurement occasions on cognitive change. The results in Table 2 and Figures 2 and 3 indicate that the functions relating change to the interval between occasions had similar slopes, but higher levels, with more measurement occasions. Furthermore, regression analyses revealed that although the mean z-score difference was more negative by about −.05 standard deviations per year of interval, it was more positive by about .10 standard deviations with each additional measurement occasion. In addition to supporting a distinction between different components of change, the discovery of positive effects of number of cognitive assessments suggests that, contrary to intuition, sensitivity to detect cognitive change may be reduced, rather than enhanced, with increases in the number of assessments (Salthouse, 2014a).
The possibility that participants with different intervals between occasions, or with different numbers of occasions, might have differed with respect to their initial levels of functioning was examined with correlations between Time 1 cognitive functioning and interval duration and number of occasions. The correlations with interval were all small and not significantly different from zero, which implies that although the variations in intervals occurred for a variety of reasons, the effects of interval on change were not confounded with differences in cognitive functioning. There were positive correlations between the T1 mean z-score and number of occasions in the middle and old groups, which suggests that participants over the age of 45 who returned for more occasions had higher levels of cognitive functioning than the participants who did not return. It is therefore possible that the positive effects of number of occasions on change may have been overestimated relative to what would have been found had the participants not differed in initial level of functioning.
Change was also examined across short intervals between assessments. As expected from the discovery of age differences in the level of the functions relating change to interval, cognitive change was less positive with increased age when the T1-T2 intervals averaged a little more than one year. Factors operating within the first 12 to 18 months after the initial occasion can therefore be inferred to contribute to the age differences in cognitive change.
At least two types of influences could be contributing to change over short intervals; time-related processes associated with the interval between assessments, and experience-related processes originating in the first assessment and manifested in the second assessment. Because many participants performed alternate versions of the tests on sessions separated by about one week, it was possible to investigate performance in a second assessment with minimal involvement of time-related influences. The results in Table 5 indicated that the change over an interval of about one year was more negative with increased age, but that change over an interval of about one week was more positive with increased age. This combination of results implies that it is the presence of an interval of months or more from the first assessment that is the major factor contributing to negative age differences in cognitive change.
It is instructive to compare the results of this study with those of other longitudinal studies. Some studies, such as those cited in the introduction, focused on healthy adults across a wide age range. Consistent with the current results, those studies have typically found more negative change at older ages, frequently manifested in a shift from positive to negative change with increased age.
Other longitudinal studies have been restricted to older adults, and have been primarily focused on distinguishing between healthy and pathological aging. These studies have often reported a period of relatively stable cognitive functioning, followed by substantial decline prior to dementia diagnosis (e.g., Johnson et al., 2009; Machulda et al., 2013; Wilson et al., 2012). As an example, Wilson et al. (2012) reported a study involving adults with an average age of about 80 years and a mean MMSE of 28.3 at the initial occasion who were evaluated annually for an average of 10 years. All of the participants were eventually diagnosed with dementia, and had an average MMSE of 21.1 at the time of diagnosis. There was no cognitive decline in these participants until about 7.5 years prior to the diagnosis of dementia, but the decline accelerated in the years immediately preceding the diagnosis.
In contrast to the studies limited to older adults, the participants in the current study spanned a wide age range, with a mean age of 53 years, and had high levels of functioning throughout the period of observation, as the average MMSE at the second occasion was over 28. Another difference from the studies just mentioned is that the frequent evaluations in the earlier studies may have obscured cognitive decline because the results portrayed in the bottom right panel of Figure 3 indicate that little decline would be expected when assessments are separated by intervals of two years or less.
All of the previous longitudinal studies focused on overall change, but the findings in this and other recent studies (i.e., Salthouse, 2011a; 2013), suggest that longitudinal change can conceptualized as involving at least four distinct components. One component can be postulated to operate over an interval of years between occasions. Although the age and interval variables are both scaled in units of time, the dimensions differ in important respects. To illustrate, the interval dimension corresponds to time since the initial assessment, and thus the negative change can be postulated to reflect decline from the first measurement in the relevant ability. In contrast, the age dimension refers to time from birth, and because it is meaningful in the absence of prior assessments, it may reflect neurobiological processes that operate independent of specific experience. Importantly, the lack of interactions in the regression analyses suggests that the influences of age and interval on change are largely additive, and independent of one another.
A second change component can be postulated to be related to the number of measurement occasions between the first and last occasion. The higher average level of change associated with each additional measurement occasion is likely attributable to benefits of repeated experience with the tests. Although prior research (e.g., Salthouse, 2014c, 2015b, 2016a) suggests that there may be smaller benefits with increased age on change from a first to a second occasion, the current results suggest that there were little or no age differences in the effects of additional occasions on cognitive change.
A third cognitive change component can be postulated to operate over an interval of days. The changes over this time period are positive, and may reflect performance improvements associated with reductions in anxiety, or acquisition of more effective strategies. Because the gains from the first to the second session were greater at older ages, the initial performance of older adults may have been more limited by anxiety and ineffective strategies than younger adults.
Finally, a fourth change component can be postulated to operate over intervals of months to years since the initial occasion. This component may be associated with the maintenance of positive effects, possibly related to anxiety reduction or strategy acquisition, originating in the initial assessment. These short interval changes were nearly equivalent in magnitude in the young and middle groups, but were smaller in the old group.
To summarize, no age differences were evident in the effects on cognitive change associated with the interval between occasions, and there were only small age differences associated with the number of occasions. Age was positively related to change over an interval of about one week, but older adults had more negative change than young adults over an interval averaging about one year. This combination of results suggests that processes operating in a period of months after the initial assessment, and possibly associated with losses in the benefits associated with the first assessment, are primarily responsible for age differences in cognitive change.
The four hypothesized change components varied in their sensitivity to age in healthy adults, but the profile could be different in various clinical conditions. For example, certain pathologies may affect the relations of change to interval or to number of measurement occasions, or affect the amount of change occurring across short intervals. Although challenging to investigate because of the design requirements of variable retest intervals and both within-occasion and between-occasion assessments, examination of the components of change in patient groups could prove informative about the nature of cognitive change in specific pathological conditions.
Several limitations of the research should be noted. First, many of the participants were functioning at a relatively high level, with an average estimated IQ above 110, and the results may not generalize to individuals with lower levels of cognitive functioning. Second, the majority of the participants reported themselves to be in very good to excellent health, and it is possible that the nature of cognitive change differs in individuals with lower levels of health, or with various diseases. Third, relatively few of the participants were over age 75, and there is some evidence that the pattern of change components might differ among very old adults. Fourth, the analyses of difference scores were not optimal because difference scores are correlated with the scores contributing to the difference. Nevertheless, it is worth noting that very similar results were obtained with the latent difference score analyses in which regression procedures were used to control the level of the initial score when assessing change. And fifth, because of the moderately high correlation between interval duration and number of occasions, the effects of one variable could be meaningfully examined only while controlling the value of the other variable.
Despite these limitations, the results of this study were consistent in replicating and extending earlier findings that more negative cognitive change with increased age is not attributable to a greater rate of decline as a function of the time between measurement occasions. The current study also revealed that cognitive change was more positive with additional measurement occasions, and that the benefits of more occasions were similar at different ages. However, increased age was associated with less positive change from a first to a second occasion when the assessments were separated by about one year, which suggests that age differences in cognitive change are primarily attributable to processes operating across an interval of months between occasions.
Although the results of this study are not directly relevant to age differences in single-occasion cross-sectional comparisons, they have important implications for the interpretation of cognitive change in multi-occasion longitudinal studies. Specifically, these findings suggest that longitudinal change does not reflect a unitary process associated with the mere passage of time, but instead is a product of multiple influences operating in different directions and across different time frames. Before interpreting longitudinal changes in cognitive functioning, therefore, researchers should consider influences of factors such as the number of measurement occasions and the length of the interval between occasions in addition to characteristics such as age of the participant.
Public Significance.
Longitudinal comparisons often reveal that cognitive functioning is lower on subsequent occasions, but prior research has not been able to distinguish influences associated with increased age from other influences such as those associated with the interval between measurement occasions or the number of measurement occasions. A unique contribution of this research was the derivation of separate estimates of different components of change. The results revealed that effects of age were largely independent of effects of longitudinal interval and number of measurement occasions, but were pronounced when the second measurement occasion occurred within about a year of the first occasion. This type of decomposition of change could prove valuable in understanding cognitive decline associated with pathological conditions.
Acknowledgments
This research was supported by National Institute on Aging Grant RO1AG024270.
Footnotes
Because of the moderate number of statistical comparisons, a significance level of .01 was used to minimize chance findings. The reduction in power associated with a conservative significance level was considered acceptable because of the relatively large sample sizes.
The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
There are no conflicts of interest.
References
- Bennett GK, Seashore HG, Wesman AG. Differential Aptitude Test. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Bielak AAM, Anstey KJ, Christensen H, Windsor TD. Activity engagement is related to level, but not change in cognitive ability across adulthood. Psychology and Aging. 2012;27:219–22. doi: 10.1037/a0024667. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Osborn D, Sabbagh MN, Connor DJ, Ahern GL, Baxter LC, Rapcsak SZ, Shi J, Woodruff BK, Locke DEC, Snyder CH, Alexander GE, Rademakers R, Reiman EM. Longitudinal modeling of age-related memory decline and the APOE e4 effect. New England Journal of Medicine. 2009;361:255–263. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harman HH, Dermen D. Manual for Kit of Factor-Referenced Cognitive Tests. Princeton, NJ: Educational Testing Service; 1976. [Google Scholar]
- Ferrer E, Salthouse TA, Stewart W, Schwartz B. Modeling age and retest processes in longitudinal studies of cognitive abilities. Psychology and Aging. 2004;19:243–259. doi: 10.1037/0882-7974.19.2.243. [DOI] [PubMed] [Google Scholar]
- Finkel D, Pedersen NL, Plomin R, McClearn GE. Longitudinal and cross-sectional twin data on cognitive abilities in adulthood: The Swedish Adoption/Twin Study of Aging. Developmental Psychology. 1998;34:1400–1413. doi: 10.1037//0012-1649.34.6.1400. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Giambra LM, Arenberg D, Zonderman AB, Kawas C, Costa PT. Adult life span changes in immediate visual memory and verbal intelligence. Psychology and Aging. 1995;10:123–139. doi: 10.1037//0882-7974.10.1.123. [DOI] [PubMed] [Google Scholar]
- Huppert FA, Whittington JE. Changes in cognitive function in a population sample. In: Cox BD, Huppert FA, Whichelow MJ, editors. The Health and Lifestyle Survey: Seven Years On. Aldershot, England: Dartmouth Publishing Co; 1993. [Google Scholar]
- Johnson DK, Storandt M, Morris JC, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer Disease. Archives of Neurology. 2009;66:1254–1259. doi: 10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar M, Resnick SM, Zonderman AB. Longitudinal changes in verbal memory in older adults: Distinguishing the effects of age from repeat testing. Neurology. 2003;60:82–86. doi: 10.1212/wnl.60.1.82. [DOI] [PubMed] [Google Scholar]
- Machulda MM, Pankratz VS, Christianson TJ, Ivnik RJ, Mielke MM, Roberts RO, Knopman DS, Boeve BF, Petersen RC. Practice effects and longitudinal cognitive change in normal aging vs. incident mild cognitive impairment and dementia in the Mayo Clinic Study of Aging. Clinical Neuropsychology. 2013;27:1247–1264. doi: 10.1080/13854046.2013.836567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ, Ferrer-Caja E, Hamagami F, Woodcock RW. Comparative longitudinal structural analyses of the growth and decline of multiple intellectual abilities over the life span. Developmental Psychology. 2002;38:115–142. [PubMed] [Google Scholar]
- Mitchell MB, Cimino CR, Benitez A, Brown CL, Gibbons LE, et al. Cognitively stimulating activities: Effects of cognition across four studies with up to 21 years of longitudinal data. Journal of Aging Research. 2012 doi: 10.1155/2012/461592. Article ID 461592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J. Advanced Progressive Matrices, Set II. London: H.K Lewis; 1962. [Google Scholar]
- Ronnlund M, Nilsson LG. Adult life-span patterns in WAIS-R Block Design performance: Cross-sectional versus longitudinal age gradients and relations to demographic factors. Intelligence. 2006;34:63–78. [Google Scholar]
- Ronnlund M, Nyberg L, Backman L, Nilsson L-G. Stability, growth, and decline in adult life span development of declarative memory: Cross-sectional and longitudinal data from a population-based study. Psychology and Aging. 2005;20:3–18. doi: 10.1037/0882-7974.20.1.3. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Implications of within-person variability in cognitive and neuropsychological functioning on the interpretation of change. Neuropsychology. 2007;21:401–411. doi: 10.1037/0894-4105.21.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Effects of age on time-dependent cognitive change. Psychological Science. 2011a;22:682–688. doi: 10.1177/0956797611404900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. What cognitive abilities are involved in trail-making performance? Intelligence. 2011b;39:222–232. doi: 10.1016/j.intell.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Does the direction and magnitude of cognitive change depend on initial level of ability? Intelligence. 2012;40:352–361. doi: 10.1016/j.intell.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Effects of age and ability on components of cognitive change. Intelligence. 2013;41:501–511. doi: 10.1016/j.intell.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Frequent assessments may obscure cognitive decline. Psychological Assessment. 2014a;26:1063–1069. doi: 10.1037/pas0000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Selectivity of attrition in longitudinal studies of cognitive functioning. Journals of Gerontology: Series B. Psychological Science. 2014b;69:567–574. doi: 10.1093/geronb/gbt046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Why are there different age relations in cross-sectional and longitudinal comparisons of cognitive functioning? Current Directions in Psychological Science. 2014c;23:252–256. doi: 10.1177/0963721414535212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Do cognitive interventions alter the rate of age-related cognitive change? Intelligence. 2015a;53:86–91. doi: 10.1016/j.intell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Test experience effects in longitudinal comparisons of adult cognitive functioning. Developmental Psychology. 2015b;51:1262–1270. doi: 10.1037/dev0000030. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Aging cognition unconfounded by prior test experience. Journal of Gerontology: Psychological Sciences. 2016a;71:49–58. doi: 10.1093/geronb/gbu063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Little relation of adult age on cognition after controlling general influences. Developmental Psychology. 2016b;52:1545–1554. doi: 10.1037/dev0000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Shared and unique influences on age-related cognitive change. Neuropsychology. 2017;31:11–19. doi: 10.1037/neu0000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Developmental Psychology. 1991;27:763–776. [Google Scholar]
- Salthouse TA, Fristoe N, Rhee SH. How localized are age-related effects on neuropsychological measures? Neuropsychology. 1996;10:272–285. [Google Scholar]
- Salthouse TA, Schroeder DH, Ferrer E. Estimating retest effects in longitudinal assessments of cognitive functioning in adults between 18 and 60 years of age. Developmental Psychology. 2004;40:813–822. doi: 10.1037/0012-1649.40.5.813. [DOI] [PubMed] [Google Scholar]
- Schaie KW. Developmental influences on adult intelligence: The Seattle Longitudinal Study. 2. New York, NY: Oxford University Press; 2013. [Google Scholar]
- Schaie KW, Hertzog C. Fourteen-year cohort-sequential analyses of adult intellectual development. Developmental Psychology. 1983;19:531–543. [Google Scholar]
- Singh-Manoux A, Kivimaki M, Glymour AM, Elbaz A, Berr C, Ebmeier KP, Ferrie JE, Dugravot A. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. British Medical Journal. 2012;344:d7622. doi: 10.1136/bmj.d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Elst W, van Boxtel MPJ, van Breukelen GJP, Jolles J. Detecting the significance of changes in performance on the Stroop Color-Word Test, Rey’s Verbal Learning Test, and the Letter Digit Substitution Test: The regression-based change approach. Journal of the International Neuropsychological Society. 2008;14:71–80. doi: 10.1017/S1355617708080028. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, van Gerven PWM, van Boxtel MPJ, van der Elst W, Jolles J. No protective effects of education during normal cognitive aging: Results from the 6-year follow-up of the Maastricht Aging Study. Psychology and Aging. 2008;23:119–130. doi: 10.1037/0882-7974.23.1.119. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: The Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3. San Antonio, TX: The Psychological Corporation; 1997b. [Google Scholar]
- Wilson RS, Segawa E, Boyle PA, Anagnos SE, Hizel LP, Bennett DA. The natural history of cognitive decline in Alzheimer’s Disease. Psychology and Aging. 2012;27:1008–1017. doi: 10.1037/a0029857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary RA. Shipley Institute of Living Scale—Revised. Los Angeles, CA: Western Psychological Services; 1986. [Google Scholar]
- Zelinski EM, Burnight KP. Sixteen-year longitudinal and time lag changes in memory and cognition in older adults. Psychology and Aging. 1997;12:503–513. doi: 10.1037//0882-7974.12.3.503. [DOI] [PubMed] [Google Scholar]