Abstract
Background
Peppermint oil has been used for centuries as a treatment for gastrointestinal ailments. It has been shown to have several effects on gastroesophageal physiology relevant to clinical care and management.
Aim
To review the literature on peppermint oil regarding its metabolism, effects on gastrointestinal physiology, clinical use and efficacy, and safety.
Methods
We performed a PubMed literature search using the following terms individually or in combination: peppermint, peppermint oil, pharmacokinetics, menthol, esophagus, stomach, small intestine, gallbladder, colon, transit, dyspepsia, and irritable bowel syndrome. Full manuscripts evaluating peppermint oil that were published through July 15, 2017 were reviewed. When evaluating therapeutic indications, only randomized clinical trials were included. References from selected manuscripts were used if relevant.
Results
It appears that peppermint oil may have several mechanisms of action including: smooth muscle relaxation (via calcium channel blockade or direct enteric nervous system effects); visceral sensitivity modulation (via transient receptor potential cation channels); anti-microbial effects; anti-inflammatory activity; modulation of psychosocial distress. Peppermint oil has been found to affect esophageal, gastric, small bowel, gallbladder, and colonic physiology. It has been used to facilitate completion of colonoscopy and endoscopic retrograde cholangiopancreatography. Placebo controlled studies support its use in irritable bowel syndrome, functional dyspepsia, childhood functional abdominal pain, and postoperative nausea. Few adverse effects have been reported in peppermint oil trials.
Conclusion
Peppermint oil is a natural product which affects physiology throughout the gastrointestinal tract, has been used successfully for several clinical disorders, and appears to have a good safety profile.
Keywords: Menthol, Diet, Irritable Bowel Syndrome, Dysphagia, Smooth muscle, Peppermint oil
INTRODUCTION
Peppermint is a perennial flowering plant that grows throughout Europe and North America. Peppermint (Mentha × piperita) is a (usually) sterile hybrid mint, a cross between Water mint (Mentha aquatica) and Spearmint (Mentha spicata) that is believed to have arisen naturally. Mint plants have a long history of medicinal use, dating to ancient Egypt, Greece, and Rome where they were used as stomach soothers.1 Peppermint oil is obtained by steam distillation from the fresh leaves of peppermint.2 Our goal was to review the literature on peppermint oil regarding its metabolism, effects on gastrointestinal physiology, clinical use and efficacy, and safety.
METHODS
A PubMed literature search was performed using the following terms individually or in combination: peppermint, peppermint oil, pharmacokinetics, menthol, esophagus, stomach, small intestine, gallbladder, colon, transit, dyspepsia, nausea, abdominal pain, and irritable bowel syndrome. Full manuscripts evaluating peppermint oil that were published through July 15, 2017 were reviewed. References from the selected manuscripts also were searched for additional relevant publications. A total of more than 2800 references were initially reviewed. Following removal of references overlapping between searches and those lacking original data, the authors agreed on inclusion of the 96 on which to base the information presented within this manuscript. When evaluating therapeutic trials for clinical disorders (e.g., irritable bowel syndrome), only randomized placebo controlled trials were included.
RESULTS
Pharmacokinetics
Pharmacokinetic data relating to peppermint oil in humans are limited.3 The main constituent and active ingredient of peppermint oil appears to be menthol although it contains a large number (greater than 80) of other components.3, 4 Studies in rats and limited data in humans demonstrate that peppermint oil is rapidly absorbed.3, 5, 6 However, when taken in capsule form designed for delayed release, approximately 70% reaches the colon.5 Though done in a small study (n=13), delayed release formulations of peppermint oil (vs. non-delayed release formulations) altered menthol urinary pharmacokinetics by increasing the apparent lag time and time to peak plasma concentrations. The delayed release formulation did not alter the absorption half-life or total area under the curve.6 In healthy adults, an oral dose of 100 mg of menthol results in an average peak blood concentration of 16.7 ± 5.5 μmol/L and an apparent elimination t1/2 of 56 ± 8 min.7 However, as noted, pharmacokinetics are greatly dependent on the formulation used.6 A L-menthol preparation sprayed directly onto the gastric mucosa was rapidly absorbed with peak concentrations reached within one hour after administration.8 In addition, development (reflected by age) may impact the pharmacokinetics of menthol as we showed in a pilot study in healthy children administered peppermint oil.3
Menthol is primarily metabolized in the liver via hepatic microsomal P450 enzymes and subsequently undergoes biotransformation via UDP-glucuronosyltransferases.2, 3 Menthol is excreted into bile as menthol glucuronide.3, 5 In particular, data show the importance of both CYP2A6 (the major P450 enzyme involved in menthol hydroxylation) and UGT2B7 expression in determining menthol clearance.9, 10 Therefore there is the potential for pharmacogenomics and other substances which impact either CYP2A6 or UGT2B7 activity to alter peppermint oil’s concentration-time curve. Following biliary excretion, menthol glucuronide undergoes enterohepatic circulation. Peppermint oil metabolites are excreted in the urine in part as glucuronic acid conjugates with ≥ 50% of a 100 mg oral dose of menthol appearing in urine as menthol glucuronide.7, 8, 11
Potential Mechanisms of Action
The Figure highlights several preclinical studies which lend insight into peppermint oil’s potential relevance to gastrointestinal tract physiology and gastrointestinal disorders. Peppermint oil’s benefit in functional gastrointestinal disorders such as the irritable bowel syndrome (IBS) has been ascribed primarily to its antispasmodic effect. The degree to which the other gastrointestinal effects of peppermint oil contribute to its clinical benefit remains unclear.
FIGURE. Potential peppermint oil mechanisms of action within the gastrointestinal tract.
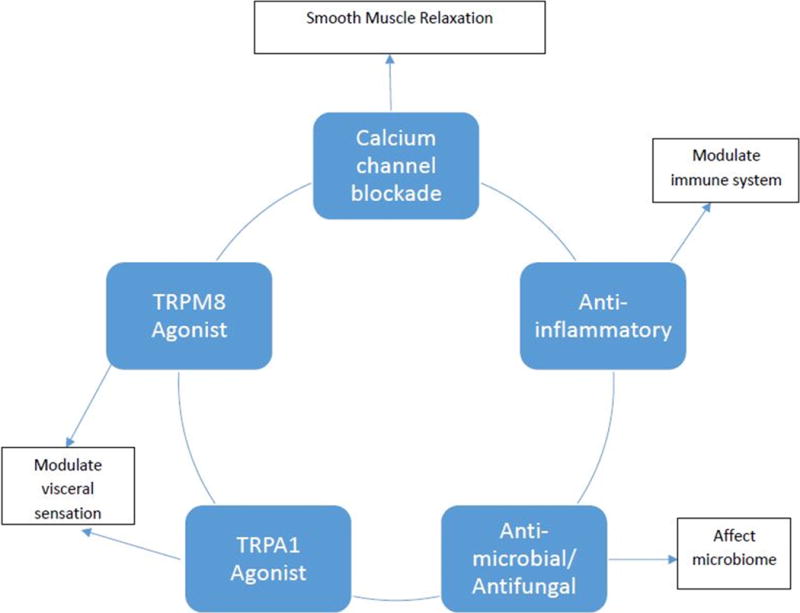
TRPM8 = transient receptor potential cation channel subfamily M member 8; TRPA1 = transient receptor potential cation channel, subfamily A, member 1
Effects on Gastrointestinal Tract Neuromotor Function
Evidence suggests that peppermint oil acts as a smooth muscle relaxant.3 Hawthorn et al. showed in guinea pig ileal smooth muscle in vitro that both peppermint oil and its constituent menthol were capable of blocking calcium channels.12 In vitro studies using guinea pig colon and rabbit jejunum smooth muscle suggest peppermint oil reverses acetylcholine induced contraction and antagonizes serotonin-induced contraction through calcium channel blockade.13 Amato et al., using samples obtained at the time of surgery, observed that menthol induces circular smooth muscle relaxation in human colon by directly inhibiting contractility through the blockade of Ca2+ influx through sarcolemma L-type Ca2+ channels.14 Its action did not involve activation of the transient receptor potential cation channel subfamily M member 8 (TRPM8) channel or nitrous oxide.14
Peppermint oil may also directly affect the enteric nervous system. Using cultured murine small intestine interstitial cells of Cajal, Kim et al. showed via a whole cell patch clamp technique that menthol acts via the transient receptor potential cation channel, subfamily A, member 1 (TRPA1) receptor to induce membrane potential depolarization in a concentration dependent manner.15 G protein stimulation as well as external Ca2+ and release from intracellular stores also appear to be involved.15 Finally, Kim and colleagues also provided evidence that prostaglandin production also is involved in stimulating the effects of menthol on the interstitial cells of Cajal.
Effects on Gastrointestinal Visceral Sensation
Although peppermint oil (via menthol) is a well-known topical analgesic, rodent studies show that peppermint oil can decrease visceral pain when administered orally or intraperitoneally.16–18 Recent studies suggest that the reduction in visceral pain is mediated through the TRPM8 and/or TRPA1 receptor of the transient receptor potential cation channel superfamily located in the gut.19–22
Antimicrobial/Antifungal Actions
Multiple studies have shown that peppermint oil (menthol) is one of the most potent antimicrobial/antifungal/antiviral botanicals.23 Peppermint oil is active against obligate and facultative anaerobes.24 It also is bactericidal to at least 20 common enteric pathogens including Helicobacter pylori, Escherichia coli, Staphylococcus aureus, Klebsiella sp., Salmonella typhi, Shigella boydii, and Shigella flexneri.25–28 More recently it has been shown that menthol can inhibit quorum sensing activity of gram negative pathogens.29 Peppermint oil also appears to have activity against fungal pathogens.30 In a mouse model, a combination of menthol and menthone were effective in reducing the number of Schistosoma mansoni eggs in the feces, liver, and intestine and reducing the number of hepatic granulomas.31
Effects on Inflammation
Studies demonstrate that peppermint oil (menthol) possesses anti-inflammatory activity.32 Oral administration of peppermint oil prevents both xylene induced gut inflammation in mice and acetic acid induced colitis in rats.16, 32 In vitro, menthol suppresses the production of inflammatory mediators from human monocytes.33 Immune cells also contain transient receptor potential cation channels. It is believed that the anti-inflammatory effects of peppermint oil may be mediated, in part, via TRPM8 as its activation down regulates chemically induced colitis in mouse models.34, 35
Effects on Behavior
Studies in humans demonstrated that inhalation of peppermint aroma improves attention but whether it improves mood remains unclear.36–38 Studies in rodents, which suggest menthol has dose dependent anxiolytic effects, implicate involvement of dopamine pathways.39–41 Given the potential role of psychosocial distress in the expression of functional gastrointestinal pain disorders symptoms, this may be another potential mechanism of action.
Peppermint Oil Effects on Gastrointestinal Physiology
Some older studies examining the physiologic effects of peppermint oil describe the administration of “drops” rather than a milligram dosage. Thus, the actual dose of peppermint oil can only be estimated. Our calculations are based on 20 drops/mL and a presumed concentration of 916 mg of peppermint oil per mL.42 A caveat regarding this assumption is the recognition that concentrations may vary as a result of botanical source and/or preparation of the peppermint oil.
Peppermint oil effects on the Esophagus (Table 1)
Table 1.
Peppermint Oil (PMO) Effect on Human Esophageal Function
| Reference | Population | Methods | Findings |
|---|---|---|---|
| Sigmund (1969)43 |
|
|
|
| Pimentel (2001)45 |
|
|
|
| Mizuno (2006)44 |
|
|
|
Unless otherwise indicated data are presented as mean ± standard deviation.
The effects of peppermint oil on esophageal function have been studied in healthy adults. Using esophageal manometry Sigmund et al. demonstrated peppermint oil decreased lower esophageal sphincter pressure.43 Peppermint oil increased the likelihood of reflux by causing equal pressures across the esophageal body, lower esophageal sphincter, and stomach.43 Using double contrast esophageal barium studies Mizuno et al. demonstrated peppermint oil (vs. saline) decreased esophageal spasms.44
Peppermint oil has been used as a spasmolytic agent in esophageal disorders though only in the short term. Using esophageal manometry in patients with diffuse esophageal spasm, Pimentel et al. found peppermint oil did not affect lower esophageal sphincter or esophageal body pressures but improved esophageal manometric findings.45 Differences between Sigmund et al. and Pimentel et al. (e.g., lower esophageal sphincter pressure following peppermint oil) may relate to the larger dose of peppermint oil used in the Sigmund et al. study. Despite these promising data we did not identify long term studies using peppermint oil for hypercontractile disorders of the esophagus.
Peppermint oil Effects on Gastric Physiology and Gastric Emptying (Table 2)
Table 2.
Peppermint Oil (PMO) Effect on Human Gastric Function and Gastric Emptying
| Reference | Population | Methods | Findings |
|---|---|---|---|
| Dalvi (1991)52 |
|
|
|
| Micklefield (2000)49 |
|
|
|
| Hiki (2003)48 |
|
|
|
| Goerg (2003)53 |
|
|
|
| Micklefield (2003)51 |
|
MO: 90 mg and caraway oil infused into the duodenum
|
|
| Mizuno (2006)44 |
|
|
|
| Inamori (2007)86 | Healthy adults (n=10) |
|
|
| Hiki (2012)46 |
|
|
|
| Imagawa (2012)47 |
|
|
|
| Papathanasopoulos (2013)50 |
|
|
|
Unless otherwise indicated data are presented as mean ± standard deviation.
Mizuno et al. in the same study which evaluated esophageal motor function using double contrast barium studies found peppermint oil given orally decreased spasms of the lower stomach.44 During esophagogastroduodenoscopy investigators have found that peppermint oil/menthol sprayed on the mucosa decreased gastric peristalsis and increased pyloric ring diameter.46, 47 Topical mucosa application is potentially appealing because systemic exposure to menthol is much reduced compared with oral administration.2, 6, 8
Peppermint oil sprayed on the mucosa during esophagogastroduodenoscopy was found to decrease peak power frequency on electrogastrography.48 Manometry and/or barostat studies have identified the following peppermint oil effects: decreased intragastric pressure, decreased gastric motility index, no effect on gastric accommodation.49–51 In healthy volunteers peppermint oil taken orally did not affect epigastric symptoms or satiation though there was decreased appetite (vs. placebo) during the fasting period.50
The effect of peppermint oil on gastric emptying has been evaluated with mixed results. Using a test meal of solids and nuclear scintigraphy Dalvi et al. demonstrated peppermint oil (vs. baseline) taken orally with water accelerated gastric emptying in both healthy adults and adults with dyspepsia.52 In contrast, Goerg et al. used ultrasonography to demonstrate peppermint oil (taken orally in a non-enteric capsule) had no effect on gastric emptying of liquids (apple juice) .53 Studying healthy adults via 13C-acetic acid breath testing using a liquid test meal, Inamori et al. found peppermint oil (ingested as a solution with the test meal) caused a more rapid emptying in the early phase following a meal but this did not result in a difference in overall gastric emptying rate. Further investigation of the effect of peppermint oil on gastric emptying is needed given the differences in meal/liquids given and the differing methodologies employed in these previous studies.
Peppermint Oil Effects on Small Bowel Physiology and Transit Time (Table 3)
Table 3.
Peppermint Oil (PMO) Effect on Human Small Bowel Physiology and Transit Time
| Reference | Population | Methods | Findings |
|---|---|---|---|
| Wildgrube (1988)56 |
|
|
|
| Micklefield (2000)49 |
|
|
|
| Goerg(2003)53 |
|
|
|
| Micklefield (2003)51 |
|
|
|
| Mizuno (2006)44 |
|
|
|
Unless otherwise indicated data is presented as mean ± standard deviation.
Peppermint oil appears to decrease small bowel contractility. The double contrast barium study by Mizuno et al. also demonstrated that the oral peppermint oil solution (vs. water) decreased spasm in the duodenal bulb.44 Micklefield et al. found that duodenally instilled peppermint oil decreased duodenal contractions during phase III (but not phase I or II) of the fasting period.51 Investigators using peppermint oil instilled into the duodenum during endoscopic retrograde cholangiopancreatography noted subjective decreases in duodenal contractions and a trend for an overall decrease in duodenal contractions per minute.54, 55 As might be anticipated, the timing of the effect of peppermint oil on duodenal contractility appears related to the peppermint oil formulation used. Using manometry Micklefield and colleagues using an enteric (vs. non-enteric) coated peppermint oil (both mixed with caraway oil) given orally noted that the decrease in duodenal contractions occurred later in the enteric vs the non-enteric coated preparation.49
Based on hydrogen breath testing peppermint oil has been found to slow orocecal transit.53 Wildgrube et al. used carmine red to demonstrate that peppermint oil slowed whole intestinal transit.56
Peppermint Oil Effects on Gallbladder Emptying
Using ultrasound gallbladder volume measurements as a proxy for emptying following a liquid meal (400 mL apple juice) in 12 healthy volunteers, Goerg et al. reported that peppermint oil taken orally inhibited gallbladder emptying vs placebo (44.4% ± 14.0 increase in volume vs 28.6% ± 4.8 decrease in volume, P=0.04).53
Peppermint Oil Effects on Colonic Physiology (Table 4)
Table 4.
Peppermint Oil (PMO) Effect on Human Colonic Function
| Reference | Population | Methods | Findings |
|---|---|---|---|
| Duthie (1981)59 |
|
|
|
| Taylor (1983)87 |
|
|
|
| Rogers (1988)88 |
|
|
|
| Sparks (1995)58 |
|
|
|
| Asao (2003)57 |
|
|
|
Unless otherwise indicated data are presented as mean ± standard deviation.
Using peppermint oil solution mixed with barium, several investigators have found peppermint oil decreased colonic spasm during barium enema.57, 58 Investigators also have found that peppermint oil applied topically to the colonic mucosa inhibits colonic motor activity as measured by colonic manometry and ultrasound.59, 60 Several endoscopy based studies have found peppermint oil (taken orally or administered topically within the colon) decreases colonic peristalsis and/or spasm.61–64 Inoue et al. found a higher adenoma detection rate during colonoscopy in those receiving peppermint oil vs. placebo.64 In contrast, peppermint oil administered intraluminally potentiated the activity of neostigmine given intramuscularly by further increasing the amplitude of sigmoid contractions in adults.65 The dose used in this study by Rogers et al. was half that used in the study by Duthie et al. in which luminal peppermint oil inhibited all sigmoid motor activity following neostigmine injection.53
Peppermint Oil Usage During Endoscopic Procedures
As noted above, the anti-spasmodic properties of peppermint oil have been used successfully during both upper GI endoscopies, colonoscopies, and endoscopic retrograde cholangiopancreatography (ERCP) procedures. These studies are summarized in Table 5.
TABLE 5.
Peppermint Oil Usage During Endoscopic Procedures
| Reference | Population | Design | Findings |
|---|---|---|---|
| Leicester (1982)62 |
|
|
|
| Asao (2001)61 |
|
|
|
| Yamamoto (2006)54 |
|
|
|
| Shavakhi (2012)63 |
|
|
|
| Sola-Bonada (2012)55 |
|
|
|
| Inoue (2014)64 |
|
|
|
Peppermint Oil for Treatment of Gastrointestinal Disorders
Adult Irritable Bowel Syndrome (Table 6)
Table 6.
Peppermint Oil (PMO) Randomized Double Blind Controlled Trials for Adult Irritable Bowel Syndrome (IBS)
| Reference | Population | Design | Findings | Adverse Events |
|---|---|---|---|---|
| Rees (1979)89 |
|
|
|
|
| Dew (1984)90 |
|
|
|
|
| Nash (1986)66 |
|
|
No difference in primary outcomes (raw data not presented) |
|
| Wildgrube (1988)56 |
|
|
|
|
| Liu (1997)91 |
|
|
Higher proportion of subjects with PMO vs. placebo with a marked or moderate improvement in pain [41 (79%) vs. 21 (43%)], distention [43 (83%) vs. 14 (29%)], stool frequency [43 (83%) vs. 16 (33%)], borborygmi [38 (73%) vs. 15 (31%)], and flatulence [41 (79%) vs. 11 (22.5%)], all comparisons P < 0.05. |
|
| Capanni (2005)92 | Adult IBS (n=178); 5 withdrawals |
|
|
|
| Cappello (2007)93 |
|
|
|
|
| Merat (2010)94 |
|
|
|
|
| Alam (2013)95 |
|
|
|
|
| Cash (2016)96 | Adults with Rome III IBS-diarrhea or IBS-mixed (n=72) |
|
|
|
Unless otherwise indicated data are presented as mean ± standard deviation.
A number of trials have investigated peppermint oil for IBS. As can be seen in Table 6, the dosing and formulation for peppermint oil varied significantly. All but one study found peppermint oil more effective than placebo in reducing symptoms.66 Similarly, 5 peppermint oil meta-analyses have found peppermint oil to be effective in IBS.67–71 In the most recent meta-analysis, the number needed to treat was 3.71 It should be noted that given the lack of negative studies there may be potential for publication bias to cause studies showing no benefit to be under-represented in the literature.
Pediatric Functional Abdominal Pain (Table 7)
Table 7.
Peppermint Oil (PMO) Randomized Controlled Trials for Childhood Functional Abdominal Pain
| Reference | Population | Design | Findings | Adverse Events |
|---|---|---|---|---|
| Kline (2001)72 |
|
|
A greater number of subjects receiving PMO vs. placebo achieved a better or much better symptom score [15 (71%) vs. 9 (42.8%), P<0.01] |
|
| Asgarshirazi (2015)73 |
|
|
|
|
FAP = Functional abdominal pain
Two peppermint oil randomized controlled pediatric trials have been carried out, both suggesting benefit. The study by Kline et al. was a double blind, randomized, placebo controlled trial that included children with both IBS and functional abdominal pain (J. Kline, 5/2007, personal communication).72 The other trial was randomized and single blinded comparing peppermint oil to a probiotic and folic acid (presumably as a placebo).72, 73
Functional Dyspepsia (Table 8)
Table 8.
Peppermint Oil (PMO) Randomized Double Blind Controlled Trials for Functional Dyspepsia
| Reference | Population | Design | Findings | Adverse Events |
|---|---|---|---|---|
| Madisch (1999)74 | Adults with functional dyspepsia (n=120); 2 withdrawals |
|
No significant differences between groups (4.6 vs. 4.6) |
|
| May (2000)76 |
|
|
|
|
| Madisch (2004)75 |
|
|
|
|
A number of randomized controlled trials have shown peppermint oil to be effective for functional dyspepsia when used in conjunction with other natural products (primarily caraway).74–77 However, to our knowledge, peppermint oil has not been studied alone in a randomized, double blind trial. As an example, peppermint oil has been used within STW 5-II which also contains extracts from bitter candy tuft, matricaria flower, caraway, licorice root and lemon balm. In adults with functional dyspepsia, Madisch et al. reported that STW5-II was superior to placebo in both improvement of overall gastrointestinal symptom score and complete relief of symptoms (43.3% vs. 3.3%, P<0.001).75
Post-operative Nausea
Peppermint oil aromatherapy when blended with ginger, spearmint, and cardamom oil was found to be superior vs saline for postoperative nausea.78 Peppermint oil aromatherapy was superior to placebo for post-operative nausea following caesarian section.79 However two randomized double-blind trials did not find peppermint oil aromatherapy to be superior to saline or controlled breathing for post-operative nausea.78, 80
Safety
Menthol is listed as generally regarded as safe by the US Food and Drug Administration. The European Medicines Agency recently released their assessment and recommendations regarding peppermint oil (see below).42
Few adverse events have been reported in peppermint trials. In those studies reporting adverse events (see Table 2), no differences were noted between peppermint oil and placebo groups except in the study by Nash et al. in which heartburn was more common in the peppermint oil vs placebo group; however, the efficacy of the enteric coating used for the peppermint oil formulation in this study of 30 years ago is unknown. In theory, enteric coated formulations of peppermint oil facilitate release distal to the stomach – thereby minimizing the risk of gastroesophageal reflux.
The safety of peppermint oil also has been reviewed by the Cosmetic Ingredient Review Expert Panel.81 In rat studies cystlike spaces have been identified in the cerebellum in some rat studies but not others at doses of ≥ 40 mg·kg-1·d-1.81 Subsequently it was shown that the cystlike spaces were artifacts of poor tissue fixation.82
Pulegone, and its metabolite, menthofuran which are present in peppermint oil have been considered to be potentially toxic in high doses. In a rat study, ≥ 80 mg·kg-1·d-1 of pulegone was associated with vacuolization of hepatocytes.81 In a more recent series of studies from the National Toxicology Program, liver necrosis and cytoplasmic vacuolization were only seen in rats given ≥150 mg·kg-1·d-1 of pulegone for 2 weeks.83 In a 3-month administration study in rats, bile duct hyperplasia, hepatocyte focal necrosis and hypertrophy and renal glomerulopathy were only seen at doses ≥75 mg·kg-1·d-1 of pulegone.83 In contrast, mice were more resistant to the effects than were rats with doses of 300 mg·kg-1·d-1 of pulegone required to see hepatic injury at 2 weeks.83 The European Medicines Agency statement points out that “prevailing opinion is that no certain cases of liver toxicity in humans are associated with the use of peppermint oil or mint oil.”42 In a case report of a woman suspected to have ingested peppermint oil in a suicide attempt, although comatose on arrival, she recovered without evidence of hepatic or renal injury.84
In a 2-year study in rats, pulegone was associated with an increased risk of bladder cancer at a 150 mg·kg-1·d-1 dose. In contrast, in a 2-year study of mice, an increased risk for hepatoblastoma was found at the 75 mg·kg-1·d-1 dose but not at the 150 mg·kg-1·d-1 dose in males with no increased risk in females.83 The European Medicines Agency statement posits that “non-relevance of rodent neoplasms to human carcinogenesis seems probable” in part, because of the long term sustained exposure required and doses which are not relevant in human situations.
The amount of pulegone in peppermint oil can be reduced depending on how the peppermint is grown, when it is harvested, and how it is processed.85 Peppermint oil normally contains a maximum of 0.1% pulegone and its metabolite menthofuran according to the European Pharmacopoeia (and more commonly 0.03-0.07%). The European Medicines Agency statement proposes a life-long exposure acceptable dose of 0.75 mg·kg-1·day-1.42 As likely would be used to treat functional gastrointestinal disorders (oral use for less than one year or intermittently over several years) in adults, the European Medicines Agency statement proposes an acceptable exposure of pulegone plus menthofuran to be 75 mg/day.42 The maximum usual dose of peppermint oil used to treat functional gastrointestinal disorders such as IBS is 540 mg/d which would deliver only 0.54 mg of pulegone and menthofuran combined. Pharmacokinetic data are needed from studies in children to understand the true systemic exposure to pulegone and menthofuran after “therapeutic” peppermint oil doses and how the aforementioned recommendations regarding exposure limits might apply to the pediatric population.
SUMMARY
Peppermint oil has been used for centuries to address gastrointestinal ailments. Peppermint oil’s primary active ingredient is menthol which is metabolized in the liver via both P450 (primarily CYP2A6) catalyzed biotransformation and glucuronidation. Peppermint oil effects on neuromotor function and visceral sensation have been studied most extensively. However, more recent data demonstrate its antibacterial/antifungal effects, an ability to downregulate inflammation, and potentially affect attention, and possibly mood.
Peppermint oil affects esophageal, gastric, small bowel, gallbladder, and colonic physiology primarily as a function of its spasmolytic properties, with such effects seen throughout the gastrointestinal tract. These effects appear to facilitate the completion of endoscopic studies (e.g., colonoscopy, endoscopic retrograde pancreatography). Placebo controlled studies support its use in irritable bowel syndrome, functional dyspepsia, childhood functional abdominal pain, and postoperative nausea though significant trial heterogeneity (e.g., peppermint oil dose and formulation) exists. It appears to be safe with few, if any side effects beyond those seen with placebo. Future studies should focus on understanding the pharmacokinetics of peppermint oil in children and its pharmacodynamics in children and adults. A clearer understanding of its modes of action in treating functional gastrointestinal disorders is needed. Finally, studies investigating its clinical utility beyond its spasmolytic effects are warranted.
Table 9.
Peppermint Aromatherapy Randomized Controlled Trials for Post-operative Nausea
| Reference | Population | Design | Findings | Adverse Events |
|---|---|---|---|---|
| Lane (2012) | Women following a caesarian section (n=35) |
|
|
|
| Hunt (2013) |
|
|
|
|
| Sites (2014) |
|
|
|
|
Acknowledgments
Declaration of personal interests:
Bruno Chumpitazi serves as a consultant for Mead Johnson Nutrition and receives research funding from the National Institutes of Health (NIH). Robert Shulman is a consultant for Nutrinia Inc. and receives research funding support from the National Institutes of Health (NIH).
Declaration of funding interests:
This study was supported in part by R01 NR013497 and R21 AT009101 from the National Institutes of Health, the Daffy’s Foundation, the USDA/ARS under Cooperative Agreement No. 6250-51000-043 (RJS), and K23 DK101688 (BPC) from the National Institutes of Health, and P30 DK56338 from the National Institutes of Health which funds the Texas Medical Center Digestive Disease Center (RJS, BPC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work is a publication of the USDA/ARS Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine and Texas Children’s Hospital. The contents do not necessarily reflect the views or policies of the USDA, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Abbreviations
- PMO
Peppermint Oil
- IBS
Irritable Bowel Syndrome
Footnotes
Guarantor of the article: Bruno P. Chumpitazi
Author contributions: BPC, KG and RJS: conception and design, literature search and data collection, data interpretation, critical revision of the manuscript for important intellectual content, and final approval of the version to be published. BPC drafted the original manuscript.
References
- 1.Ulbricht C, Costa D, J MGS, et al. An evidence-based systematic review of spearmint by the natural standard research collaboration. Journal of dietary supplements. 2010;7(2):179–215. doi: 10.3109/19390211.2010.486702. [DOI] [PubMed] [Google Scholar]
- 2.Kearns GL, Chumpitazi BP, Abdel-Rahman SM, et al. Systemic exposure to menthol following administration of peppermint oil to paediatric patients. BMJ open. 2015;5(8):e008375. doi: 10.1136/bmjopen-2015-008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grigoleit HG, Grigoleit P. Pharmacology and preclinical pharmacokinetics of peppermint oil. Phytomedicine. 2005;12(8):612–6. doi: 10.1016/j.phymed.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Vassallo S, Ford M, Delaney Ka LLET. Clinical Toxicology. Philadelphia, PA: WB Saunders; 2001. Essential Oils; pp. 343–52. [Google Scholar]
- 5.Somerville KW, Richmond CR, Bell GD. Delayed release peppermint oil capsules (Colpermin) for the spastic colon syndrome: a pharmacokinetic study. BrJ ClinPharmacol. 1984;18(4):638–40. doi: 10.1111/j.1365-2125.1984.tb02519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White DA, Thompson SP, Wilson CG, et al. A pharmacokinetic comparison of two delayed release peppermint oil preparations, Colpermin and Mintec, for treatment of the irritable bowel syndrome. Int J Pharm. 1987;40:151–4. [Google Scholar]
- 7.Gelal A, Jacob P, 3rd, Yu L, et al. Disposition kinetics and effects of menthol. Clinical pharmacology and therapeutics. 1999;66(2):128–35. doi: 10.1053/cp.1999.v66.100455001. [DOI] [PubMed] [Google Scholar]
- 8.Hiki N, Kaminsky L, Hasunuma T, et al. A phase I study evaluating tolerability, pharmacokinetics, and preliminary efficacy of L-menthol in upper gastrointestinal endoscopy. Clinical pharmacology and therapeutics. 2011;90(2):221–8. doi: 10.1038/clpt.2011.110. [DOI] [PubMed] [Google Scholar]
- 9.Miyazawa M, Marumoto S, Takahashi T, et al. Metabolism of (+)- and (−)-menthols by CYP2A6 in human liver microsomes. Journal of oleo science. 2011;60(3):127–32. doi: 10.5650/jos.60.127. [DOI] [PubMed] [Google Scholar]
- 10.de Wildt SN, Kearns GL, Leeder JS, et al. Cytochrome P450 3A: ontogeny and drug disposition. Clin Pharmacokinet. 1999;37(6):485–505. doi: 10.2165/00003088-199937060-00004. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi T, Caldwell J, Farmer PB. Metabolic fate of [3H]-l-menthol in the rat. Drug metabolism and disposition: the biological fate of chemicals. 1994;22(4):616–24. [PubMed] [Google Scholar]
- 12.Hawthorn M, Ferrante J, Luchowski E, et al. The actions of peppermint oil and menthol on calcium channel dependent processes in intestinal, neuronal and cardiac preparations. AlimentPharmacolTher. 1988;2(2):101–18. doi: 10.1111/j.1365-2036.1988.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 13.Hills JM, Aaronson PI. The mechanism of action of peppermint oil on gastrointestinal smooth muscle. An analysis using patch clamp electrophysiology and isolated tissue pharmacology in rabbit and guinea pig. Gastroenterology. 1991;101(1):55–65. doi: 10.1016/0016-5085(91)90459-x. [DOI] [PubMed] [Google Scholar]
- 14.Amato A, Liotta R, Mule F. Effects of menthol on circular smooth muscle of human colon: analysis of the mechanism of action. European journal of pharmacology. 2014;740:295–301. doi: 10.1016/j.ejphar.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Wie J, So I, et al. Menthol Modulates Pacemaker Potentials through TRPA1 Channels in Cultured Interstitial Cells of Cajal from Murine Small Intestine. Cell Physiol Biochem. 2016;38(5):1869–82. doi: 10.1159/000445549. [DOI] [PubMed] [Google Scholar]
- 16.Atta AH, Alkofahi A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J Ethnopharmacol. 1998;60(2):117–24. doi: 10.1016/s0378-8741(97)00137-2. [DOI] [PubMed] [Google Scholar]
- 17.Taher YA. Antinociceptive activity of Mentha piperita leaf aqueous extract in mice. Libyan J Med. 2012;7 doi: 10.3402/ljm.v7i0.16205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adam B, Liebregts T, Best J, et al. A combination of peppermint oil and caraway oil attenuates the post-inflammatory visceral hyperalgesia in a rat model. ScandJ Gastroenterol. 2006;41(2):155–60. doi: 10.1080/00365520500206442. [DOI] [PubMed] [Google Scholar]
- 19.Karashima Y, Damann N, Prenen J, et al. Bimodal action of menthol on the transient receptor potential channel TRPA1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(37):9874–84. doi: 10.1523/JNEUROSCI.2221-07.2007. Epub 2007/09/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahieu F, Owsianik G, Verbert L, et al. TRPM8-independent menthol-induced Ca2+ release from endoplasmic reticulum and Golgi. J Biol Chem. 2007;282(5):3325–36. doi: 10.1074/jbc.M605213200. Epub 2006/12/05. [DOI] [PubMed] [Google Scholar]
- 21.Liu B, Fan L, Balakrishna S, et al. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain. 2013;154(10):2169–77. doi: 10.1016/j.pain.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrington AM, Hughes PA, Martin CM, et al. A novel role for TRPM8 in visceral afferent function. Pain. 2011;152(7):1459–68. doi: 10.1016/j.pain.2011.01.027. Epub 2011/04/15. [DOI] [PubMed] [Google Scholar]
- 23.Kamatou GP, Vermaak I, Viljoen AM, et al. Menthol: a simple monoterpene with remarkable biological properties. Phytochemistry. 2013;96:15–25. doi: 10.1016/j.phytochem.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro S, Meier A, Guggenheim B. The antimicrobial activity of essential oils and essential oil components towards oral bacteria. Oral MicrobiolImmunol. 1994;9(4):202–8. doi: 10.1111/j.1399-302x.1994.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 25.Imai H, Osawa K, Yasuda H, et al. Inhibition by the essential oils of peppermint and spearmint of the growth of pathogenic bacteria. Microbios. 2001;106(Suppl 1):31–9. [PubMed] [Google Scholar]
- 26.Pattnaik S, Subramanyam VR, Kole C. Antibacterial and antifungal activity of ten essential oils in vitro. Microbios. 1996;86(349):237–46. [PubMed] [Google Scholar]
- 27.Pattnaik S, Subramanyam VR, Rath CC. Effect of essential oils on the viability and morphology of Escherichia coli (SP-11) Microbios. 1995;84(340):195–9. [PubMed] [Google Scholar]
- 28.Thompson A, Meah D, Ahmed N, et al. Comparison of the antibacterial activity of essential oils and extracts of medicinal and culinary herbs to investigate potential new treatments for irritable bowel syndrome. BMC complementary and alternative medicine. 2013;13:338. doi: 10.1186/1472-6882-13-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Husain FM, Ahmad I, Khan MS, et al. Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of Gram-negative bacteria. Front Microbiol. 2015;6:420. doi: 10.3389/fmicb.2015.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hossain F, Follett P, Dang Vu K, et al. Evidence for synergistic activity of plant-derived essential oils against fungal pathogens of food. Food Microbiol. 2016;53(Pt B):24–30. doi: 10.1016/j.fm.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Zaia MG, Cagnazzo T, Feitosa KA, et al. Anti-Inflammatory Properties of Menthol and Menthone in Schistosoma mansoni Infection. Frontiers in pharmacology. 2016;7:170. doi: 10.3389/fphar.2016.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghasemi-Pirbaluti M, Motaghi E, Bozorgi H. The effect of menthol on acute experimental colitis in rats. European journal of pharmacology. 2017;805:101–7. doi: 10.1016/j.ejphar.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Juergens UR, Stober M, Vetter H. The anti-inflammatory activity of L-menthol compared to mint oil in human monocytes in vitro: a novel perspective for its therapeutic use in inflammatory diseases. European journal of medical research. 1998;3(12):539–45. [PubMed] [Google Scholar]
- 34.Perraud AL, Knowles HM, Schmitz C. Novel aspects of signaling and ion-homeostasis regulation in immunocytes. The TRPM ion channels and their potential role in modulating the immune response. Molecular immunology. 2004;41(6-7):657–73. doi: 10.1016/j.molimm.2004.04.013. Epub 2004/06/29. [DOI] [PubMed] [Google Scholar]
- 35.Ramachandran R, Hyun E, Zhao L, et al. TRPM8 activation attenuates inflammatory responses in mouse models of colitis. Proc Natl Acad Sci U S A. 2013;110(18):7476–81. doi: 10.1073/pnas.1217431110. Epub 2013/04/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moss M, Hewitt S, Moss L, et al. Modulation of cognitive performance and mood by aromas of peppermint and ylang-ylang. Int J Neurosci. 2008;118(1):59–77. doi: 10.1080/00207450601042094. [DOI] [PubMed] [Google Scholar]
- 37.Ilmberger J, Heuberger E, Mahrhofer C, et al. The influence of essential oils on human attention. I: alertness. Chem Senses. 2001;26(3):239–45. doi: 10.1093/chemse/26.3.239. [DOI] [PubMed] [Google Scholar]
- 38.Itai T, Amayasu H, Kuribayashi M, et al. Psychological effects of aromatherapy on chronic hemodialysis patients. Psychiatry Clin Neurosci. 2000;54(4):393–7. doi: 10.1046/j.1440-1819.2000.00727.x. [DOI] [PubMed] [Google Scholar]
- 39.Umezu T. Evidence for dopamine involvement in ambulation promoted by menthone in mice. Pharmacology, biochemistry, and behavior. 2009;91(3):315–20. doi: 10.1016/j.pbb.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 40.Umezu T, Morita M. Evidence for the involvement of dopamine in ambulation promoted by menthol in mice. J Pharmacol Sci. 2003;91(2):125–35. doi: 10.1254/jphs.91.125. [DOI] [PubMed] [Google Scholar]
- 41.da Silveira NS, de Oliveira-Silva GL, Lamanes Bde F, et al. The aversive, anxiolytic-like, and verapamil-sensitive psychostimulant effects of pulegone. Biol Pharm Bull. 2014;37(5):771–8. doi: 10.1248/bpb.b13-00832. [DOI] [PubMed] [Google Scholar]
- 42.European Medicines Agency. Public statement on the use of herbal medicinal products containing pulegone and menthofuran. 2016 Jul 12; ( http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/07/WC500211079.pdf). 2016.
- 43.Sigmund CJ, McNally EF. The action of a carminative on the lower esophageal sphincter. Gastroenterology. 1969;56(1):13–8. [PubMed] [Google Scholar]
- 44.Mizuno S, Kato K, Ono Y, et al. Oral peppermint oil is a useful antispasmodic for double-contrast barium meal examination. J Gastroenterol Hepatol. 2006;21(8):1297–301. doi: 10.1111/j.1440-1746.2006.04131.x. [DOI] [PubMed] [Google Scholar]
- 45.Pimentel M, Bonorris GG, Chow EJ, et al. Peppermint oil improves the manometric findings in diffuse esophageal spasm. J Clin Gastroenterol. 2001;33(1):27–31. doi: 10.1097/00004836-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Hiki N, Kaminishi M, Yasuda K, et al. Multicenter phase II randomized study evaluating dose-response of antiperistaltic effect of L-menthol sprayed onto the gastric mucosa for upper gastrointestinal endoscopy. Digestive endoscopy : official journal of the Japan Gastroenterological Endoscopy Society. 2012;24(2):79–86. doi: 10.1111/j.1443-1661.2011.01163.x. [DOI] [PubMed] [Google Scholar]
- 47.Imagawa A, Hata H, Nakatsu M, et al. Peppermint oil solution is useful as an antispasmodic drug for esophagogastroduodenoscopy, especially for elderly patients. Dig Dis Sci. 2012;57(9):2379–84. doi: 10.1007/s10620-012-2194-4. [DOI] [PubMed] [Google Scholar]
- 48.Hiki N, Kurosaka H, Tatsutomi Y, et al. Pepperint oil reduces gastric spasm during upper endoscopy: a randomized, double-blind, double-dummy controlled trial. GastrointestEndosc. 2003;57:475–82. doi: 10.1067/mge.2003.156. [DOI] [PubMed] [Google Scholar]
- 49.Micklefield GH, Greving I, May B. Effects of peppermint oil and caraway oil on gastroduodenal motility. PhytotherRes. 2000;14(1):20–3. doi: 10.1002/(sici)1099-1573(200002)14:1<20::aid-ptr542>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 50.Papathanasopoulos A, Rotondo A, Janssen P, et al. Effect of acute peppermint oil administration on gastric sensorimotor function and nutrient tolerance in health. Neurogastroenterol Motil. 2013;25(4):e263–71. doi: 10.1111/nmo.12102. Epub 2013/03/16. [DOI] [PubMed] [Google Scholar]
- 51.Micklefield G, Jung O, Greving I, et al. Effects of intraduodenal application of peppermint oil (WS(R) 1340) and caraway oil (WS(R) 1520) on gastroduodenal motility in healthy volunteers. PhytotherRes. 2003;17(2):135–40. doi: 10.1002/ptr.1089. [DOI] [PubMed] [Google Scholar]
- 52.Dalvi SS, Nadkarni PM, Pardesi R, et al. Effect of peppermint oil on gastric emptying in man: a preliminary study using a radiolabelled solid test meal. Indian J Physiol Pharmacol. 1991;35(3):212–4. [PubMed] [Google Scholar]
- 53.Goerg JK, Spilker T. Effect of peppermint oil and caraway oil on gastrointestinal motility in healthy volunteers: a pharmacodynamic study using simultaneous determination of gastric and gall-bladder emptying and orocaecal transit time. Aliment PharmacolTher. 2003;17(3):445–51. doi: 10.1046/j.1365-2036.2003.01421.x. [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto N, Nakai Y, Sasahira N, et al. Efficacy of peppermint oil as an antispasmodic during endoscopic retrograde cholangiopancreatography. J Gastroenterol Hepatol. 2006;21(9):1394–8. doi: 10.1111/j.1440-1746.2006.04307.x. [DOI] [PubMed] [Google Scholar]
- 55.Sola-Bonada N, de Andres-Lazaro AM, Roca-Massa M, et al. 1.6% peppermint oil solution as intestinal spasmolytic in retrograde endoscopic cholangiopancreatography. Farmacia hospitalaria : organo oficial de expresion cientifica de la Sociedad Espanola de Farmacia Hospitalaria. 2012;36(4):256–60. doi: 10.1016/j.farma.2011.08.003. Esencia de menta al 1,6% como espasmolitico intestinal en la colangiopancreatografia retrograda endoscopica. [DOI] [PubMed] [Google Scholar]
- 56.Wildgrube HJ. Untersuchungen zur Wirksamkeit von Pfefferminzöl auf Beschwerdebild und funktionelle Parameter bei Patienten mit Reizdarm-Syndrom (Studie) Naturheilpraxis. 1988;41:591–6. [Google Scholar]
- 57.Asao T, Kuwano H, Ide M, et al. Spasmolytic effect of peppermint oil in barium during double-contrast barium enema compared with Buscopan. ClinRadiol. 2003;58(4):301–5. doi: 10.1016/s0009-9260(02)00532-9. [DOI] [PubMed] [Google Scholar]
- 58.Sparks MJ, O’Sullivan P, Herrington AA, et al. Does peppermint oil relieve spasm during barium enema? Br J Radiol. 1995;68(812):841–3. doi: 10.1259/0007-1285-68-812-841. [DOI] [PubMed] [Google Scholar]
- 59.Duthie HL. The effect of peppermint oil on colonic motility in man. Br J Surg. 1981;68:820. - [Google Scholar]
- 60.Taylor BA. Ultrasound used to measure the response of colonic motility to essential oils. Proceedings of the International Symposium on Gastrointestinal Motility. 1983:441–8. [Google Scholar]
- 61.Asao T, Mochiki E, Suzuki H, et al. An easy method for the intraluminal administration of peppermint oil before colonoscopy and its effectiveness in reducing colonic spasm. Gastrointestinal endoscopy. 2001;53(2):172–7. doi: 10.1067/mge.2000.108477. [DOI] [PubMed] [Google Scholar]
- 62.Leicester RJ, Hunt RH. Peppermint oil to reduce colonic spasm during endoscopy. Lancet. 1982;2(8305):989. doi: 10.1016/s0140-6736(82)90191-x. [DOI] [PubMed] [Google Scholar]
- 63.Shavakhi A, Ardestani SK, Taki M, et al. Premedication with peppermint oil capsules in colonoscopy: a double blind placebo-controlled randomized trial study. Acta gastro-enterologica Belgica. 2012;75(3):349–53. [PubMed] [Google Scholar]
- 64.Inoue K, Dohi O, Gen Y, et al. L-menthol improves adenoma detection rate during colonoscopy: a randomized trial. Endoscopy. 2014;46(3):196–202. doi: 10.1055/s-0034-1365035. [DOI] [PubMed] [Google Scholar]
- 65.Rogers J, Tay HH, Misiewicz JJ. Peppermint Oil [Letter to the Editor] Lancet. 1988;332(8602):98–9. [Google Scholar]
- 66.Nash P, Gould SR, Barnardo DE. Peppermint oil does not relieve the pain of irritable bowel syndrome. Br J ClinPract. 1986;40(7):292–3. [PubMed] [Google Scholar]
- 67.Ruepert L, Quartero AO, de Wit NJ, et al. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;(8):CD003460. doi: 10.1002/14651858.CD003460.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huertas-Ceballos A, Logan S, Bennett C, et al. Pharmacological interventions for recurrent abdominal pain (RAP) and irritable bowel syndrome (IBS) in childhood. CochraneDatabaseSystRev. 2008;(1):CD003017. doi: 10.1002/14651858.CD003017.pub2. [DOI] [PubMed] [Google Scholar]
- 69.Pittler MH, Ernst E. Peppermint oil for irritable bowel syndrome: a critical review and metaanalysis. AmJ Gastroenterol. 1998;93(7):1131–5. doi: 10.1111/j.1572-0241.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 70.Ford AC, Talley NJ, Spiegel BM, et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ. 2008;337:a2313. doi: 10.1136/bmj.a2313. Epub 2008/11/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48(6):505–12. doi: 10.1097/MCG.0b013e3182a88357. [DOI] [PubMed] [Google Scholar]
- 72.Kline RM, Kline JJ, Di Palma J, et al. Enteric-coated, pH-dependent peppermint oil capsules for the treatment of irritable bowel syndrome in children. JPediatr. 2001;138:125–8. doi: 10.1067/mpd.2001.109606. [DOI] [PubMed] [Google Scholar]
- 73.Asgarshirazi M, Shariat M, Dalili H. Comparison of the Effects of pH-Dependent Peppermint Oil and Synbiotic Lactol (Bacillus coagulans + Fructooligosaccharides) on Childhood Functional Abdominal Pain: A Randomized Placebo-Controlled Study. Iran Red Crescent Med J. 2015;17(4):e23844. doi: 10.5812/ircmj.17(4)2015.23844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Madisch A, Heydenreich CJ, Wieland V, et al. Treatment of functional dyspepsia with a fixed peppermint oil and caraway oil combination preparation as compared to cisapride. A multicenter, reference-controlled double-blind equivalence study. Arzneimittelforschung. 1999;49(11):925–32. doi: 10.1055/s-0031-1300528. [DOI] [PubMed] [Google Scholar]
- 75.Madisch A, Holtmann G, Mayr G, et al. Treatment of functional dyspepsia with a herbal preparation. A double-blind, randomized, placebo-controlled, multicenter trial. Digestion. 2004;69(1):45–52. doi: 10.1159/000076546. [DOI] [PubMed] [Google Scholar]
- 76.May B, Kohler S, Schneider B. Efficacy and tolerability of a fixed combination of peppermint oil and caraway oil in patients suffering from functional dyspepsia. Aliment Pharmacol Ther. 2000;14(12):1671–7. doi: 10.1046/j.1365-2036.2000.00873.x. [DOI] [PubMed] [Google Scholar]
- 77.Rich G, Shah A, Koloski N, et al. A randomized placebo-controlled trial on the effects of Menthacarin, a proprietary peppermint- and caraway-oil-preparation, on symptoms and quality of life in patients with functional dyspepsia. Neurogastroenterol Motil. 2017 doi: 10.1111/nmo.13132. [DOI] [PubMed] [Google Scholar]
- 78.Hunt R, Dienemann J, Norton HJ, et al. Aromatherapy as treatment for postoperative nausea: a randomized trial. Anesthesia and analgesia. 2013;117(3):597–604. doi: 10.1213/ANE.0b013e31824a0b1c. [DOI] [PubMed] [Google Scholar]
- 79.Lane B, Cannella K, Bowen C, et al. Examination of the effectiveness of peppermint aromatherapy on nausea in women post C-section. Journal of holistic nursing : official journal of the American Holistic Nurses’ Association. 2012;30(2):90–104. doi: 10.1177/0898010111423419. quiz 5-6. [DOI] [PubMed] [Google Scholar]
- 80.Sites DS, Johnson NT, Miller JA, et al. Controlled breathing with or without peppermint aromatherapy for postoperative nausea and/or vomiting symptom relief: a randomized controlled trial. Journal of perianesthesia nursing : official journal of the American Society of PeriAnesthesia Nurses. 2014;29(1):12–9. doi: 10.1016/j.jopan.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 81.Nair B. Final report on the safety assessment of Mentha Piperita (Peppermint) Oil, Mentha Piperita (Peppermint) Leaf Extract, Mentha Piperita (Peppermint) Leaf, and Mentha Piperita (Peppermint) Leaf Water. IntJToxicol. 2001;20(Suppl 3):61–73. [PubMed] [Google Scholar]
- 82.Molck AM, Poulsen M, Tindgard Lauridsen S, et al. Lack of histological cerebellar changes in Wistar rats given pulegone for 28 days. Comparison of immersion and perfusion tissue fixation. Toxicology letters. 1998;95(2):117–22. doi: 10.1016/s0378-4274(98)00029-0. [DOI] [PubMed] [Google Scholar]
- 83.National Toxicology P. Toxicology and carcinogenesis studies of pulegone (CAS No. 89-82-7) in F344/N rats and B6C3F1 mice (gavage studies) Natl Toxicol Program Tech Rep Ser. 2011;(563):1–201. [PubMed] [Google Scholar]
- 84.Nath SS, Pandey C, Roy D. A near fatal case of high dose peppermint oil ingestion- Lessons learnt. Indian journal of anaesthesia. 2012;56(6):582–4. doi: 10.4103/0019-5049.104585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rios-Estepa R, Turner GW, Lee JM, et al. A systems biology approach identifies the biochemical mechanisms regulating monoterpenoid essential oil composition in peppermint. Proc Natl Acad Sci U S A. 2008;105(8):2818–23. doi: 10.1073/pnas.0712314105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Inamori M, Akiyama T, Akimoto K, et al. Early effects of peppermint oil on gastric emptying: a crossover study using a continuous real-time 13C breath test (BreathID system) J Gastroenterol. 2007;42(7):539–42. doi: 10.1007/s00535-007-2067-3. [DOI] [PubMed] [Google Scholar]
- 87.Taylor BA, D HL, Oliveira RB, Rhodes J. Ultrasound used to measure the response of colonic motility to essential oils. In: Roman C, editor. Gastrointestinal Motility Proceedings of the 9th International Symposium on Gastrointestinal Motility held in Aix-en-Provence; France. September 12–16, 1983; Netherlands: Springer; 1983. pp. 441–8. [Google Scholar]
- 88.Rogers J, Tay HH, Misiewicz JJ. Peppermint Oil. 1988:98–9. [Google Scholar]
- 89.Rees WD, Evans BK, Rhodes J. Treating irritable bowel syndrome with peppermint oil. Br MedJ. 1979;2(6194):835–6. doi: 10.1136/bmj.2.6194.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dew MJ, Evans BK, Rhodes J. Peppermint oil for the irritable bowel syndrome: a multicentre trial. Br J ClinPract. 1984;38(11–12):394–8. [PubMed] [Google Scholar]
- 91.Liu JH, Chen GH, Yeh HZ, et al. Enteric-coated peppermint-oil capsules in the treatment of irritable bowel syndrome: a prospective, randomized trial. J Gastroenterol. 1997;32(6):765–8. doi: 10.1007/BF02936952. [DOI] [PubMed] [Google Scholar]
- 92.Capanni M, S E, Biagini MR, Milani S, Surrenti C, Galli A. Efficacy of peppermint oil in the treatment of irritable bowel syndrome: a randomized, controlled trial. Gazz Med Ital. 2005;164:119–26. [Google Scholar]
- 93.Cappello G, Spezzaferro M, Grossi L, et al. Peppermint oil (Mintoil((R))) in the treatment of irritable bowel syndrome: A prospective double blind placebo-controlled randomized trial. DigLiver Dis. 2007;39(6):530–6. doi: 10.1016/j.dld.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 94.Merat S, Khalili S, Mostajabi P, et al. The effect of enteric-coated, delayed-release peppermint oil on irritable bowel syndrome. Dig Dis Sci. 2010;55(5):1385–90. doi: 10.1007/s10620-009-0854-9. [DOI] [PubMed] [Google Scholar]
- 95.Alam MS, Roy PK, Miah AR, et al. Efficacy of Peppermint oil in diarrhea predominant IBS - a double blind randomized placebo - controlled study. Mymensingh Med J. 2013;22(1):27–30. Epub 2013/02/19. [PubMed] [Google Scholar]
- 96.Cash BD, Epstein MS, Shah SM. A Novel Delivery System of Peppermint Oil Is an Effective Therapy for Irritable Bowel Syndrome Symptoms. Dig Dis Sci. 2016;61(2):560–71. doi: 10.1007/s10620-015-3858-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


