Abstract
First described by Ramon y Cajal as “short-axon” cells over a century ago, inhibitory interneurons in the cerebral cortex make up ~20–30% of the neuronal milieu. A key feature of these interneurons is the striking structural and functional diversity, which allows them to modulate neural activity in diverse ways and ultimately endow neural circuits with remarkable computational power. Here we review our current understanding of the generation of cortical interneurons, with a focus on recent efforts to bridge the gap between progenitor behavior and interneuron production, and how these aspects influence interneuron diversity and organization.
Graphical Abstract
Inhibitory interneurons in the cerebral cortex constitute ~20–30% of the neuronal population. The rich array of interneuron subtypes endows local circuits with a remarkable computational power. How do the developmental origins (i.e. time and place of birth, progenitor behavior, lineage, etc.) influence the production and diversification of cortical interneurons?
INTRODUCTION
Optimal information processing in the nervous system requires the proper balance of excitation and inhibition. Sensory inputs to the brain elicit an increase in excitatory activity with a coordinated change in inhibitory activity. As the levels of excitation change under various stimulus conditions, the inhibitory system responds by adjusting its output to match the excitatory activity. This balance of excitation and inhibition in the brain is maintained by excitatory neurons and inhibitory interneurons, respectively.
Excitatory neurons in the mammalian central nervous system predominantly secrete the neurotransmitter glutamate, which binds to the glutamate receptors at the postsynaptic membrane and drives its depolarization, thereby promoting action potential generation in the target neuron. Inhibitory interneurons, on the other hand, use the neurotransmitter gamma aminobutyric acid (GABA), which binds to the GABA receptors at the post-synaptic membrane and under most circumstances causes hyperpolarization and thus inhibits the target neuron from firing. In the cerebral cortex, the latest evolved part of the mammalian brain, the bulk (~70–80%) of the neuronal population is comprised of glutamatergic excitatory neurons and the remaining (~20–30%) consists of GABAergic inhibitory interneurons.1–4 Glutamatergic excitatory neurons project their axons over long distances both within the cortex as well as to other brain regions and generate the main excitatory output of neural circuits. In comparison, GABAergic interneurons are predominantly locally projecting neurons that are responsible for the inhibitory transmission that refines and shapes circuit output.
INTERNEURON DIVERSITY
Inhibitory interneurons are incredibly diverse. Vastly outnumbered by excitatory projection neurons in the cortex, it is the rich variety of interneuron subtypes that endows the inhibitory system with the ability to sense the appropriate level of excitation across a broad dynamic range and under various stimulus conditions, and ultimately regulate circuit output using a wide range of inhibitory activity. The assortment of interneuron subtypes have been extensively characterized previously based on various criteria such as morphology, molecular marker expression, electrical properties, subcellular synaptic targeting, and connectivity patterns.2, 5–10 Each of these properties influences a given interneuron’s specific role within neural circuits.
Diversity in morphology and molecular marker expression
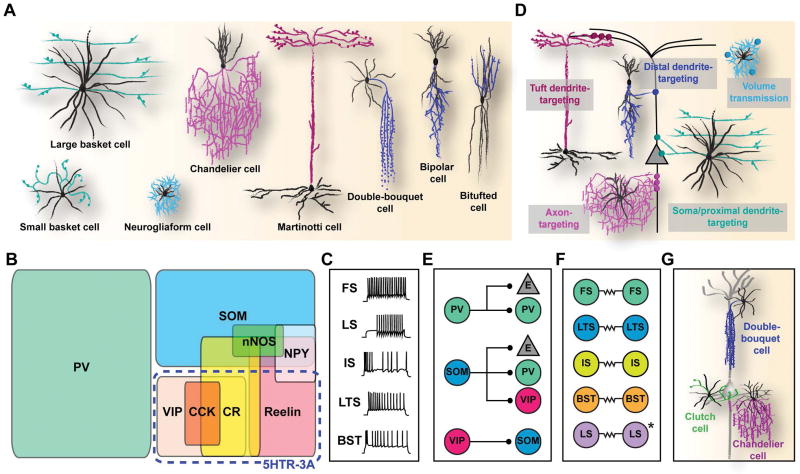
Based on their dendritic and axonal arborization, morphologically distinct subtypes of interneuron include: basket cells, chandelier cells, Martinotti cells, bipolar cells, double-bouquet cells, bitufted cells, and neurogliaform cells (Figure 1A).2, 11 Interneuron subtypes can also be defined by their expression of various molecular markers. These include 1) calcium binding proteins such as parvalbumin (PV), calbindin (CB), and calretinin (CR), 2) neuropeptides such as somatostatin (SOM), vasointestinal protein (VIP), neuropeptide Y (NPY), and cholecystokinin (CCK), and 3) other biochemical markers such as reelin, neuronal nitric oxide synthase (nNOS), and serotonin receptor (5HTR)-3A (Figure 1B).2 Notably, the expression of PV, SOM, or 5HTR-3A is largely found in three, non-overlapping populations of interneurons that account for nearly 100% of GABAergic neurons in the cortex.12, 13
Figure 1. Multiple dimensions of cortical interneuron diversity.
(A) Morphologically defined subtypes of interneurons. Black and colored lines represent dendritic and axonal processes, respectively. Inspired from Schmolesky, M. 1995.149 (B) Classification of subtypes based on molecular marker expression. PV, parvalbumin; SOM, somatostatin; VIP, vasointestinal peptide; CR, calretinin; CCK, cholecystokinin; NPY, neuropeptide Y; 5HTR-3A, serotonin receptor 3A. (C) Electrophysiological classification of interneurons based on the action potential response pattern upon electrical stimulation. Adapted from Markram, H. et al., 2004.2 FS, fast spiking; LS, late spiking; IS, irregular spiking; LTS, low-threshold spiking; BST, bursting. (D) Diversity in subcellular targeting. (E) Diversity in cellular targeting. E, excitatory neuron. (F) Patterns of electrical synapse formation in different interneuron subtypes. (G) Morphologically-defined subtypes that are particularly numerous and refined in primates.
Diversity in electrical properties
Interneurons exhibit a broad spectrum of action potential thresholds and a variety of discharge rates and patterns. Based on the response of a given interneuron to electrical stimulation, it can be categorized as fast-spiking (FS), low threshold spiking (LTS), irregular spiking (IS), bursting (BST), or late-spiking (LS) (Figure 1C).2 This rich array of electrical properties supplies the inhibitory system with sufficient sensitivity, complexity, and dynamic range to regulate neural activity in the brain.
Diversity in subcellular connectivity
Interneurons can also be classified according to the subcellular compartment of the post-synaptic neuron that they target, such as the dendrite (distal, proximal, or tuft), the soma, or the axon (Figure 1D).2, 11, 14 The variety of unique subcellular targeting sites endows interneurons with precise inhibitory control of activity within a single neuron. The most diverse of these is the dendrite-targeting population consisting of interneurons that monitor incoming activity in the dendritic regions of a given post-synaptic neuron. It includes double-bouquet cells, bitufted cells, bipolar cells, and neurogliaform cells, all of which target the distal regions of the dendrite, and Martinotti cells, which preferentially target dendritic tufts.15–17 Soma- (as well as proximal dendrite-) targeting cells consist of basket cells, which sense and regulate global excitatory input in the cell body vicinity of the post-synaptic neuron. The axon-targeting subtype consists of chandelier cells, which synapse exclusively to the axon initial segment of excitatory neurons and modulate action potential generation.
Diversity in cellular connectivity
An additional dimension of interneuron diversity is the variety of specific and complementary neuronal networks they engage in (Figure 1E). Recent studies have made some key observations into the neuronal subtype-specific synaptic connectivity logic of interneurons. First, PV-expressing basket and chandelier cells, which play an essential role in generating network oscillations, target excitatory neurons, and receive strong excitatory inputs from the thalamus and cortex, as well as inhibition from other PV-expressing cells.18–20 Second, SOM-expressing Martinotti cells predominantly receive input from local excitatory cells and in turn inhibit the dendritic tufts of excitatory cells in the cortex.18, 19, 21–23 Interestingly, SOM-expressing cells avoid inhibiting one another and instead strongly inhibit other populations of interneurons.18, 19, 21–23 Third, 5HTR-3A-expressing interneurons consist of two subgroups with distinct synapse connectivity; whereas reelin-expressing, neurogliaform cells release GABA through volume transmission, VIP-expressing cells preferentially form inhibitory synapses with SOM-expressing cells.19, 24 In addition, while some studies show a dense, non-specific inhibitory connectivity between interneurons and nearby excitatory neurons,25–28 others reveal a fine-scale specificity in inhibitory synaptic connections. For example, FS interneurons in layer 2/3 connect preferentially to neighboring excitatory neurons that form reciprocal connections with them.29 Similarly, layer 5 inhibitory interneurons form distinct intralaminar and interlaminar subnetworks with excitatory neurons.30 CCK-expressing basket cells select their postsynaptic targets based on the long-range axonal projection pattern of the principal excitatory neurons.31 Notably, inhibitory synaptic inputs to pyramidal neurons exhibit a broad stereotypical spatial pattern across different neocortical areas.32
A key feature of inhibitory interneurons is their ability to synchronize activity in neuronal networks, which is essential for information transmission across brain regions.33, 34 This feature depends on the unique capability of interneurons to form highly specific, gap-junction mediated electrical synapses with each other. Remarkably, electrical coupling is found almost exclusively amongst interneurons of the same subtype (with the exception of LS neurogliaform cells) (Figure 1F).33–43 A clear example in this regard is PV-expressing FS interneurons which exhibit robust electrical coupling amongst each other to provide large, synchronous inhibition to local excitatory neurons sufficient to induce 20–80 Hz network oscillations.34, 40 To date, pair-wise electrophysiological studies have identified electrically-coupled networks of FS basket cells, FS chandelier cells, LTS Martinotti cells, and LS neurogliaform cells.33–40 The selective electrical coupling between distinct subtypes of interneurons likely promotes cooperative interneuron subnetworks in the cortex.20
Interneuron diversity in the evolution of higher mammals
Since Ramon y Cajal’s discovery of morphologically distinct subtypes of cortical interneurons, it is often argued that the number and diversity of this neuronal population have significantly increased over the course of mammalian evolution.44, 45 Quantitative immunohistochemical studies comparing the proportion of GABAergic neurons in the cortex of different mammals have shown that while inhibitory interneurons constitute ~15% of the total cells in the rat cortex,46–49 they make up ~20–25% of the cells in the visual cortex of macaque monkey, and can reach up to 34–44% of the cells in the supragranular layers of certain areas of the macaque monkey and human cortex.1, 50–52
Furthermore, higher mammals exhibit an increased variety of interneuron subtypes (Figure 1G). For instance, whereas the double bouquet cells are scarce in the mouse or rat cortex, they are increasingly numerous, more elaborate morphologically, and distributed regularly in various areas of the monkey cortex as well as in the human temporal cortex.53–55 Interleaved with pyramidal cells, each double-bouquet cell exhibits a ‘horse-tail’-like long, vertically descending, tightly bundled axon that traverses several cortical layers forming hundreds of synapses onto the basal dendrites of pyramidal cells within a narrow column. This microcolumnar structure formed by double-bouquet cells appears to be a key element in the organization of the primate cortex, inhibiting groups of pyramidal cells located across different layers within the minicolumns.51, 53, 54, 56 Another example of an interneuron introduced during the evolution of higher mammals is a special subtype of small basket cell, namely the clutch cell, which surrounds the cell body of its target cells with large axonal terminals, in layer IV of the visual cortex of cat and monkey.57, 58 It has been suggested that these clutch cells exert their inhibitory effect in an area localized to a single ocular dominance column and may be associated with the afferent axon terminals of thalamic lateral geniculate nucleus cells, perhaps playing a role in the first stage of cortical processing of visual information in higher mammals.57, 58 Chandelier cells, each of which exhibits several hundred axon terminals, are the sole source of inhibitory synapses on the axon initial segment of pyramidal cells and as such play a key role in regulating cortical activity. Interestingly, chandelier cells are morphologically more refined in higher species, with the greatest complexity and abundance found in primates.45, 59 Loss or impaired function of chandelier cells disrupts the inhibitory control of pyramidal cells, and is thought to be a key component in the etiology of temporal lope epilepsy in humans.59 Therefore, while some interneurons can be found across all species, others are characteristic of (or more specialized in) higher mammals, particularly human and non-human primates, and may underlie the increased complexity of cortical function that allows for enhanced cognitive abilities in these species.
Continuous versus categorical changes
Although interneurons are generally categorized based on their morphological, molecular, electrical, and synaptic properties, the extent to which interneuron diversity can be defined as discrete classes as opposed to a continuum of features, is debated.2 Whereas some features of interneuron diversity such as morphology may be classified, other features such as electrical properties are more arbitrary. Future efforts to systematically couple gene expression profiles with electrical and functional properties at the single neuron resolution may reveal more precise characterization of interneurons.60, 61
DEVELOPMENTAL ORIGINS
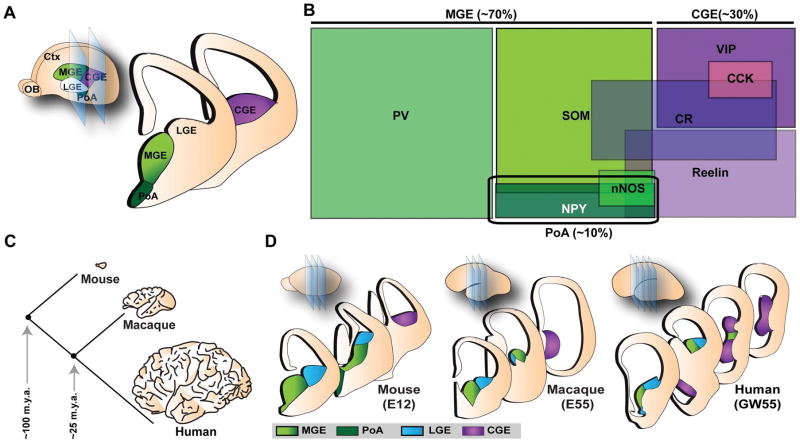
Early lineage tracing experiments at the population level established that excitatory principal neurons and inhibitory interneurons in the cortex are generated separately from each other during development and do not share a common origin.62–65 While excitatory neurons are generated in the dorsal telencephalon, most if not all inhibitory interneurons are born in the ventral telencephalon, in regions defined as the ganglionic eminence (GE) and the preoptic area (PoA). The GE is a transient structure during embryonic development that is further subdivided anatomically into lateral, medial, and caudal domains (LGE, MGE, and CGE, respectively). The PoA is situated ventral to the MGE in the telencephalic stalk. First apparent as protrusions into the lateral ventricles at E11 in the mouse, the morphological boundaries within the GE disappear as embryonic development concludes and are no longer visible in the postnatal brain. In the mouse, all inhibitory interneurons are derived from the MGE, CGE, and PoA (Figure 2A, B). The LGE generates GABAergic inhibitory projection neurons (also called medium spiny neurons) and interneurons destined for the striatum and olfactory bulb, respectively.66–70 Newborn interneurons undergo a long, tangential journey from their birthplace in the subpallium to reach their ultimate destination in the overlying cortex.71
Figure 2. Developmental origins and diversity of cortical interneurons.
(A) Cortical interneurons in mouse are derived from progenitor cells located in the proliferative zones of the ventral telencephalon, specifically in the MGE (medial ganglionic eminence), CGE (caudal ganglionic eminence) and PoA (preoptic area). LGE, lateral ganglionic eminence; Ctx, cortex; OB, olfactory bulb. (B) The major source of interneurons is the MGE, generating ~70% of cortical interneurons comprised of two non-overlapping populations expressing PV (parvalbumin) and SOM (somatostatin). ~30% of cortical interneurons are a heterogenous group of cells generated by the CGE, all of which express 5HTR (serotonin receptor)-3A as well as either VIP (vasointestinal peptide) or reelin. In addition, CGE is the main source of CR (calretinin) and CCK (cholecystokinin)-expressing cells. The PoA generates ~10% of interneurons a fraction of which express NPY (neuropeptide Y), nNOS (neuronal nitric oxide synthase), and SOM. (C) Evolution of the mammalian brain from lower mammals such as mouse to primates such as macaque monkey and human. Mouse and macaque monkey diverged from human ~100 and ~25 m.y.a. (million years ago), respectively. Modified from Rakic, P. 2009.150 (D) Developmental origins of cortical interneurons across mammalian species. Inspired from Molnar, Z. et al., 2013.151 E, embryonic day; GW, gestation week.
Whereas in mice, all cortical interneurons originate in the GE, previous work has suggested that in primates, a significant portion of cortical interneurons are generated in the developing cortex itself.72–75 Based on these reports, some interneuron subtypes that are introduced later in mammalian evolution, such as the double-bouquet cells, may be derived from the unique dorsal niche of interneuron progenitors in primates.74, 75 Subsequent studies, however, re-examined these earlier reports, and found little evidence of interneuron progenitor markers in proliferating cells in the dorsal germinal zones.76, 77 Instead, these studies based on the expression patterns of several transcription factors known to be involved in the specification of interneurons in the mouse suggested that cortical interneurons in the primate or human brain are also generated in the MGE and CGE, and that the ventral origins of interneurons are conserved over mammalian evolution (Figure 2C, D). In addition, using live imaging of fluorescently labeled GE cells in monkey brain slices, it was shown that, similar to the mouse, MGE- and CGE- derived interneurons migrate tangentially through the LGE and into the cortex.77 The spatial configuration of MGE and CGE in primates is also reminiscent of that in rodents. These studies suggest that the majority, if not all, of interneurons in primates are generated by progenitors in the MGE and CGE. Interestingly, however, some features of interneuron development are unique to primates; for instance, the subventricular zone (SVZ) in the subpallium is greatly expanded in the primate fetal brain, and the CGE appears to generate a much greater proportion (as much as half) of cortical interneurons compared to rodents. Future studies further probing the progenitor origins of interneurons in primates will help elucidate how the greatly expanded human cortex acquires the appropriate number and variety of interneurons.
The major source of cortical interneurons is the MGE, generating ~70% of all cortical interneurons in the mouse (Figure 2B). The two prominent non-overlapping populations of cortical interneurons originating in the MGE are the PV-expressing (basket and chandelier) cells, and the SOM-expressing (Martinotti and other) cells.12, 78 PV- and SOM-expressing cells are found throughout different layers of the cortex; in the somatosensory cortex, the ratio of PV- to SOM-expressing interneurons is ~1 to 1 (layer 5/6), ~3 to 1 (layer 4), and ~2.5 to 1 (layer 2/3). Progenitors in the CGE, on the other hand, generate a distinct population of cells (~30% of cortical interneurons) that include bipolar cells, bitufted cells, double-bouquet cells, and neurogliaform cells, all of which express 5HTR-3A, as well as either VIP or reelin.12, 13, 79 The PoA is the source of ~10% of cortical interneurons that frequently express NPY.80, 81
Transcription factor control of interneuron generation
Mouse genetics studies have established the general genetic program of interneuron production. Within the MGE/PoA, the homeobox transcription factorNKX2-1 is critical for the establishment and maintenance of progenitors in the ventricular zone (VZ) and SVZ, as well as for the specification of MGE-derived cortical interneurons.8, 82–84 NKX2-1 is down regulated in post-mitotic interneurons prior to their entry into the cortical plate, but is maintained in cells destined for subpallial structures, such as the striatum. Downstream of NKX2-1, the transcription factor LHX6 is expressed in post-mitotic cells, and is required for the differentiation, migration, and correct laminar positioning of MGE-derived cortical interneurons.85, 86
The DLX family of homeobox transcription factors is broadly expressed in ventral progenitors and critical for the differentiation and migration of GABAergic interneurons.82–84 Specifically, Dlx1 and Dlx2 are functionally redundant genes that repress the expression of Olig2, a transcription factor required for oligodendrocyte precursor (OPC) formation, and promote a GABAergic neuronal fate in a common progenitor. Dlx1/2-null mutants exhibit a severe deficit (~70%) in cortical interneurons. Working together with Dlx1/2, the proneural gene, Ascl1, is also expressed by subpallial progenitors and is required for the production and differentiation of GABAergic interneurons.87, 88 Similar toDLX1/2, loss of ASCL1results in a significant reduction in cortical interneurons.
In the CGE, the homeobox factor GSX2 appears to be at the top of the hierarchy of transcription factors involved in interneuron specification.89, 90 Enriched in the LGE/CGE neuroepithelium from early development, it controls the expression of genes such as Dlx2, Ascl2, and Olig2 and specifies the LGE/CGE identity. In addition, loss of Gsx2 was shown to cause a selective reduction in CR-expressing interneurons in the cortex.90 Other transcription factors involved in CGE-derived interneuron production include NR2F1, NR2F2, PROX1, and SP8.91–96
Spatial origins of diversity
Based on the combinatorial expression patterns of several transcription factors, it has been suggested that the MGE can be further subdivided into multiple domains, and that each of these spatial domains contains a pool of progenitors that generates distinct subtypes of cortical interneurons (Figure 3A).97, 98 The strongest case in this regard has been made for the dorsal-most region of the MGE, which co-expresses NKX2-1 and NKX6-2, as being the predominant source of SOM/CR-expressing Martinotti cells in the cortex.97–99 However, it is unclear if this domain represents a pure progenitor pool restricted in its potential to generate SOM/CR-expressing interneurons, since the fate-mapping of NKX6-2-expressing progenitors also identifies a significant number of cells expressing other interneuron markers such as PV and NPY.99 In addition, the number of SOM/CR-expressing interneurons is reduced in only a subset of Nkx6-2 null mutant mice (1 out of 4 litters),99 raising the possibility that the subtype specification of this population may not (or only partially) depend the expression of NKX6.2.
Figure 3. Spatial and temporal origins of MGE-derived interneurons.
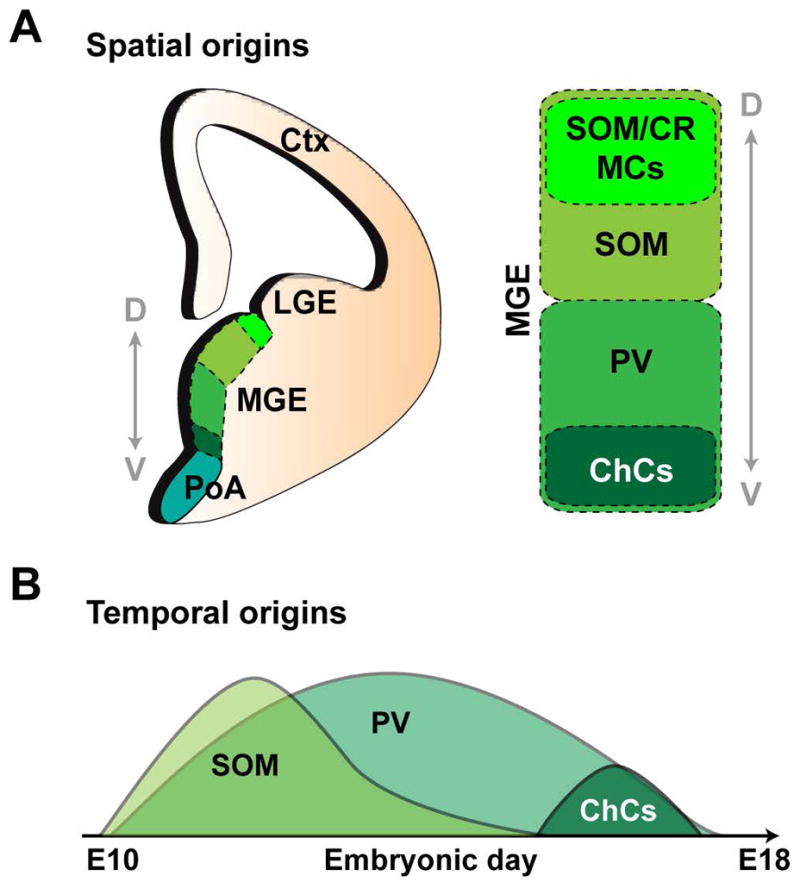
(A) Spatial bias of progenitors to generate SOM (somatostatin)- and PV (parvalbumin)- expressing cells in the dorsal and ventral regions of the MGE (medial ganglionic eminence), respectively. SOM/CR (calretinin)- expressing cells have been suggested to originate in the dorsal-most tip of the MGE, where as ChCs (chandelier cells) are enriched in ventral MGE. LGE, lateral ganglionic eminence; PoA, preoptic area; Ctx, cortex. (B) Temporal bias in MGE fate-specification. Whereas the production of SOM+ cells peaks early and subsequently declines, PV+ cells are continuously generated throughout embryonic neurogenesis. ChCs are preferentially born at the very late embryonic stage. Adapted from Bandler, R.C. et al., 2017.152
Previous fate-mapping and transplantation studies have shown that the dorsal and ventral regions of the MGE are biased towards generating SOM- and PV-expressing cortical interneurons, respectively, arguing for the presence of distinct pools of progenitors in the MGE.97, 100, 101 In particular, chandelier cells were suggested to originate in the ventral most-region of the MGE towards the end of embryonic development.100, 102 Yet, intersectional fate-mapping from dorsal versus ventral MGE progenitor pools defined by NKX2-1/NKX6-2 and NKX2-1/ER81, respectively, showed that both domains are capable of generating chandelier cells.103 Interestingly, however, whereas the dorsal progenitors produced a small number of chandelier cells in both superficial and deep layers, the ventral domain produced a greater number of chandelier cells that were almost exclusively located superficially, suggesting a certain degree of heterogeneity in the fate potential of MGE progenitors. Recently, enhancer-trap transgenic mouse lines were generated to conditionally express CreERT2-IRES-EGFP in different regions of the MGE/PoA for fate-mapping interneuron output.104 Notably, single-cell transcriptome analysis of the MGE failed to reveal the presence of any discrete subgroups of neural progenitors in the region.105 It remains to be determined if at a single cell level, spatially distinct pools of progenitors within the MGE may underlie the production of distinct subtypes of cortical interneurons.
Temporal origins of diversity
In addition to the spatial bias in subtype specification, the time of origin is another important factor in diversifying the interneuron lineage (Figure 3B). MGE-derived interneurons consisting of SOM- and PV- expressing interneurons laminate the cortex in a birthdate-dependent inside-out manner. CGE-derived interneurons are born in a later time window and preferentially occupy the more superficial layers of the cortex.9, 65, 103, 106–108 It has been proposed that distinct cohorts of interneurons are generated by progenitors at different time points of embryonic development. Specifically, the fate-mapping analysis of OLIG2-expressing progenitors using the CreER mice (with tamoxifen induction at E9.5, E10.5, E12.5, and E15.5) showed that ~50% of the labeled cortical interneurons were PV-expressing at any given time point from E9.5 to E15.5. On the other hand, SOM-expressing interneurons exhibited a decline in output; at E9.5, E10.5, and E12.5, ~30% of the labeled interneurons were SOM-expressing, however, by E15.5, only ~10% of the labeled interneurons expressed SOM. These results suggest that SOM-expressing interneurons are predominantly generated earlier whereas PV-expressing interneurons are generated at a relatively constant rate throughout the embryonic neurogenesis.9 Transplantation experiments comparing mitotic progenitors in E13.5 and E15.5 MGE showed a strong tendency for transplants at E15.5 to generate more PV- relative to SOM-expressing interneurons compared with those at E13.5, suggesting that the competence of progenitors to produce distinct subtypes of interneurons changes over the course of neurogenesis.100 Chandelier cells, which often express PV and are located in both superficial and deep layers of the cortex, are preferentially generated at the very late stages of interneuron production by NKX2-1-expressing progenitors. Notably, it is unclear if the early- and late- born interneurons are derived from a common pool of progenitors that proliferates throughout the embryonic period of neurogenesis or from distinct pools of progenitors that enter neurogenesis at different time points.
PROGENITOR BEHAVIOR AND INTERNEURON PRODUCTION
Most of our understanding of cortical neurogenesis has come from studies on excitatory neuron generation. As the precursor to the nervous system, the neural tube begins to close around E8.5 in the mouse and is composed of a single layer of rapidly proliferating cells known as neuroepithelial (NE) cells.109 These cells subsequently transit into a more-fate restricted progenitor type called the radial glial progenitor (RGP) cell that further divides symmetrically to amplify the progenitor pool.110–113 RGPs are the neural progenitor cells in all regions of the central nervous system.114 These cells reside in the VZ that lines the surface of brain ventricles and have a characteristic bipolar morphology, with a short apical process that is anchored onto the ventricular surface and a longer basal radial glial fiber process that extends across the developing cortex to reach the pial surface. As RGPs transition from symmetric proliferative divisions to asymmetric neurogenic divisions (~E11–12 in mice), they can give rise to neurons in two modes: direct or indirect neurogenesis. That is, a given RGP can divide asymmetrically at the VZ surface to generate a self-renewing daughter RGP as well as either 1) a post-mitotic neuron directly (i.e. direct neurogenesis) or 2) an intermediate progenitor cell (IPC) that further divides symmetrically in the SVZ to produce neurons (i.e. indirect neurogenesis via IPC ).113, 115 The basal process of RGPs provides a migration scaffold for the newly born excitatory neurons as they leave the germinal zone and invade the overlying cortical plate (i.e. the future cortex) to laminate it in an inside-out manner. As a result, the early born neurons occupy deep layers whereas the late-born cells migrate past the early-born cells to occupy more superficial layers of the cortex.116 The RGP is, therefore, a pivotal cell type in the developing brain, as it not only serves as the stem cell for the production of the correct number and types of neurons, it also provides a scaffold to guide the newly born neurons to the ultimate location that they are destined to inhabit.
Progenitor types
Using a combination of mouse genetics and in utero retroviral labeling to mark individual dividing progenitors and their progeny in the MGE and PoA, it has been shown that progenitors in the subpallium, similar to their pallial counterparts, are radial glial cell in nature.117 During the neurogenesis period, similar to dorsal RGPs, they undergo interkinetic nuclear migration and divide asymmetrically at the VZ surface to self-renew and produce either a post-mitotic daughter neuron or an IPC. The IPC migrates into the SVZ and undergoes further symmetric divisions before producing post-mitotic daughter neurons that migrate away. The progressively generated progeny of the dividing RGP closely associates with the basal radial glial fiber to form a radial clone in the embryonic MGE/PoA.117
Progenitor origin of neuronal diversity
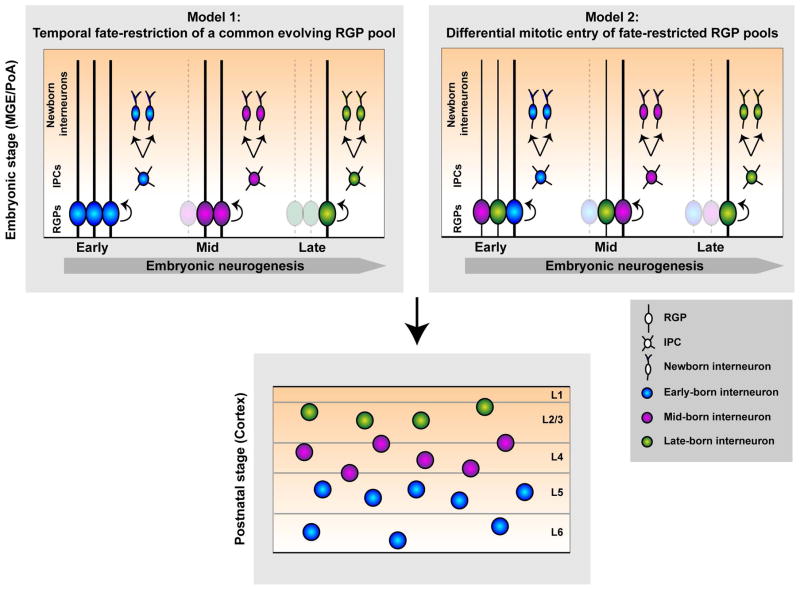
Developmental studies probing the origins of the retina and the spinal cord have provided important insights into the mechanisms that allow for the generation of a rich array of cell types from a given progenitor population (Figure 4). The first possibility is of a common pool of progenitors that continuously undergoes asymmetric neurogenesis and becomes progressively fate-restricted over time, thereby generating distinct interneuron subtypes at different times. This is the case for the seven principal types of neurons found in the mature vertebrate retina as they are generated successively in a defined chronological order from a common population of multipotent retinal progenitor cells.118 The second possibility of generating a diverse output of neurons from progenitors is via the existence of multiple pools of fate-restricted progenitors that may be spatially, temporally, or molecularly segregated. This model is reminiscent of the spinal cord, where different populations of neurons arise from progenitor domains that are distinct from each other based on the restricted expression of transcription factors.119
Figure 4. Two models of generating diversity from RGPs.
A rich variety of neuronal subtypes can arise from either temporal fate restriction of a common pool of dividing RGPs (radial glial progenitors, Model 1) or via multiple distinct pools of fate-restricted RGPs that are spatially segregated or enter mitosis at different times during embryonic neurogenesis (Model 2). The changes in neuronal progeny density may arise from the depletion of RGPs (dotted line). IPC, intermediate progenitor cell; L, layer.
In the case of excitatory neurons in the cortex, multiple lines of evidence suggest that diversity is established predominantly via the first model described above; that is, excitatory neurons are sequentially generated from a common pool of RGPs that undergoes progressive fate restriction. Classic transplantation experiments in the ferret showed that early progenitors in the dorsal VZ are multipotent as they can produce excitatory neurons destined to inhabit both deep and superficial layers of the cortex, when transplanted into older hosts.120–122 On the other hand, late progenitors are only capable of generating neurons in the superficial layers (even when transplanted into younger hosts), suggesting a progressive restriction in potential of these progenitors. Consistent with these observations, lineage-tracing experiments by genetically or virally labeling dividing RGPs and their progeny have shown that early in neurogenesis, RGPs are capable of producing excitatory neurons that occupy all cortical layers, whereas at later embryonic stages the neuronal progeny are restricted to the superficial layers,123, 124 suggesting that excitatory neuron diversity is achieved at the progenitor level via progressive fate restriction of common (i.e. multipotent) RGPs.
A recent genetic fate-mapping study of cortical excitatory cells proposed that a subpopulation of dorsal RGPs in the VZ expressing CUX2, which is also expressed in superficial layer excitatory neurons, exclusively generates superficial layer excitatory neurons, raising the possibility of fate-restricted pallial progenitors.125 Subsequently, however, conflicting results were reported to show that CUX2-expressing progenitors generated excitatory neurons in all layers, suggesting CUX2-expressing RGPs are multipotent.126 In addition, lineage tracing of RGPs expressing FEZF2, a transcription factor also expressed in layer 5 excitatory neurons, revealed that these RGPs sequentially generate excitatory neurons in all layers (in a birthdate-dependent inside-out manner) as well as glia.126 Related to this, using the mosaic analysis with double markers (MADM) that provides unprecedented resolution on progenitor division pattern and potential, it has been demonstrated that individual RGPs in the dorsal VZ of the mouse progress through a predictable and deterministic program during which their proliferative potential progressively diminishes, generating ~8–9 excitatory neurons that are vertically aligned spanning both the superficial and deep layers of the cortex.127
Interestingly, lineage (or the developmental origin) of excitatory neurons not only influences their structural organization, but also plays a fundamental role in the functional development of the cortex. During embryonic development, clonally related excitatory neurons arising from individual asymmetrically dividing RGPs preferentially form gap-junction mediated electrical coupling with each other compared to nearby non-clonally related excitatory neurons, a feature that is dependent on the birthdate-dependent inside-out migration of clonally related neurons along the basal radial glial fiber of their mother RGP.128, 129 This lineage-related preferential electrotonic communication promotes specific formation of chemical synapses between sister excitatory neurons as well as their functional similarities.130, 131 Therefore, the sequential generation of excitatory neurons destined to inhabit diverse cortical layers from a common RGP pool is not only critical for laminar formation of the cortex, but also underlies the functional columnar circuit assembly of the cortex.
While significant progress has been made in the field of excitatory cortical neurogenesis, our understanding of interneuron neurogenesis and, more specifically, how progenitor behavior influences interneuron diversity in the cortex, has really lagged.
Unique RGP-vessel interaction in the ventral telencephalon
It has recently been shown that RGPs in the MGE come in two varieties with distinct basal radial glial fiber characteristics.132 While the apical fiber of both populations is anchored onto the VZ surface, one population of RGPs projects a long basal radial glial fiber all the way to the pial surface whereas the other exhibits a relatively shorter basal radial glial fiber that is anchored onto the blood vessels in the periventricular region. The relative proportion of the two types of RGPs depends on the embryonic time point examined. At the early stages (<E12.5), a majority of the RGPs extend to the pial surface, very similar to those in the dorsal telencephalon responsible for generating excitatory neurons. However, as development proceeds, unlike dorsal RGPs whose basal fibers remain attached to the pial surface throughout neurogenesis, in the MGE there is a progressive decrease in the relative fraction of pial surface-anchored RGPs with a concomitant increase in the fraction of blood vessel-anchored RGPs. By E16.5, vessel-anchored RGPs are the predominant population of dividing RGPs in this region. Notably, this vascular-progenitor interaction is actively maintained even as RGPs undergo interkinetic nuclear migration to divide at the VZ surface.
The timing of this pial- to vessel-anchorage switch of RGP fibers appears to coincide with the change in migration routes of cortical interneurons. Born in the ventral telencephalon, interneurons destined for the cortex undergo a lengthy tangential migration which is segregated into two main routes: a superficial route along the marginal zone (MZ) close to the pial surface, and a deep route along the SVZ and the intermediate zone (IZ) of the GE region.133 While the majority of early born interneurons (<E12.5) take the superficial route, interneurons born later (>E12.5) transition to taking the deep route until it becomes the main route after ~E14.5. It is possible that due to the relatively short glial fiber of vessel-anchored RGPs, there is a lack of scaffold available for the newborn interneurons to reach the pial surface, and so they take the deep route to the cortex upon detaching from the RGP fiber. This also raises the possibility that the switch in RGP fiber anchorage influences the progenitors to transition from generating interneuron subtypes that are early-born to late-born populations. Consistent with this, disruption of RGP fiber anchorage onto periventricular vasculature in the MGE leads to a decrease in the progenitor divisions during the late embryonic stages, as well as a substantial loss of PV-expressing and, to a lesser extent, of SOM-expressing interneurons in the adult cortex.132 Progenitor behavior, in terms of RGP interaction with pial surface versus periventricular vasculature, therefore affects the production of different subtypes of interneurons. Notably, this progenitor-vessel interaction is highly reminiscent of the vascular niche of adult neurogenesis in the subependymal zone (SEZ).134–137 The similar organization between stem/progenitor cells and vessels in the embryonic and adult stages raises the possibility that a majority of adult SEZ stem cells may originate from the vessel-anchored RGPs in the ventral, but not dorsal, telencephalon.
Interestingly, several studies suggest that the microenvironment during embryonic neurogenesis, such as the vasculature, plays a crucial role in the expansion of the proliferative capacity in higher mammals.138–140 It has also been observed that the progenitors in the outer SVZ in the human MGE lack a prominent long radial glial fiber.76 It is thus tempting to think that RGP-vessel interaction observed in the mouse ventral telencephalon may be another conserved feature of interneuron progenitors during mammalian evolution.
Robust IPC divisions in the ventral telencephalon
Live imaging and clonal labeling showed that IPCs generated from RGPs in the MGE via asymmetric divisions frequently undergo multiple rounds of symmetric divisions in the SVZ.117 This is different from the dorsal IPCs, which mostly undergo a single round of symmetric terminal division in the SVZ.141, 142 Notably, the number of RGPs in the dorsal developing cortex remains largely constant throughout embryonic neurogenesis, indicating that a majority of RGPs sustain the entire neurogenesis program. On the other hand, the number of RGPs in the MGE appears to progressively decrease as development proceeds, suggesting a gradual cell cycle exit and depletion of RGPs. Accompanying this, the SVZ in the MGE is greatly expanded. In line with these differences, the developing dorsal cortex contains significantly more RGP divisions at the VZ surface than IPC divisions in the SVZ, whereas the MGE contains a much greater number of dividing cells in the SVZ compared to the VZ surface. The robust amplification of SVZ divisions in the ventral relative to dorsal region of the telencephalon may reflect an increased influence of IPCs in the generation of the appropriate numbers and diversity of cortical interneurons. The coupling between RGP depletion and IPC amplification raises an intriguing possibility of a brief and rapid (i.e. burst) production of certain interneuron subtypes. Notably, the ganglionic eminences of human and non-human primates exhibit a massive expansion in the area occupied by the SVZ with cells outside the VZ generating large numbers of inhibitory interneurons,76 suggesting an ever-increasing role of IPCs in the production of interneurons in the evolution of higher mammals.
Direct versus indirect neurogenesis
In the MGE, there is some evidence to suggest that direct and indirect neurogenesis play a role in interneuron fate specification. First, cyclin D1 (CCND1) and cyclin D2 (CCND2) are two cell cycle regulators that are expressed in distinct progenitor niches, with CCND1 predominating in the VZ and CCND2 in the SVZ of cerebral cortex and GE.143, 144 Ccnd2null mice exhibit a significant reduction in the density of PV-expressing interneurons in the cortex, while the density of SOM-expressing interneurons is less affected, suggesting a role of CCND2 in promoting indirect neurogenesis in the SVZ to generate the appropriate number of PV-expressing interneurons.144 Second, forcing asymmetrically dividing RGPs at the VZ surface of the MGE to relocate to the SVZ and undergo symmetric divisions alters the output of the interneurons from SOM- to PV-expressing.145 Together, these results suggest that SOM-expressing cells are predominantly generated through direct neurogenesis at the VZ surface, whereas production of the correct number of PV-expressing neurons may largely occur via indirect neurogenesis in the SVZ of the MGE.
CLONAL PRODUCTION AND ORGANIZATION
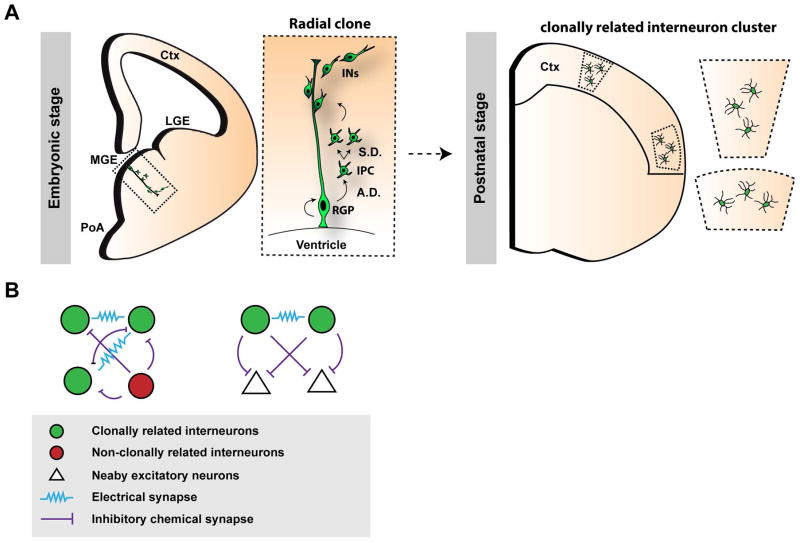
An ultimate resolution of understanding the developmental origin of cortical interneuron diversity and functionality is to decipher the interneuron output and organization at the single RGP level. Labeling individual RGPs in the MGE and PoA using EGFP-expressing retroviruses showed that dividing RGPs undergo asymmetric neurogenesis to generate a self-renewing RGP and simultaneously a post-mitotic interneuron or an IPC which further divides to produce post-mitotic interneurons. The newly born daughter IPCs/neurons associate with the mother RGP fiber and form a clonal unit in the embryonic MGE/PoA before embarking on tangential migration (Figure 5A).117, 146–148
Figure 5. Lineage related production, organization and functional development of cortical interneurons.
(A) RGPs (radial glial progenitors) at the ventricular surface of the MGE (medial ganglionic eminence) and PoA (preoptic area) undergo asymmetric divisions (A.D.) to self-renew and simultaneously generate a post-mitotic IN (interneuron) or an IPC (intermediate progenitor cell), which can further undergo symmetric divisions (S.D.) in the subventricular zone to produce differentiating interneurons. In the mature cortex, clonally-related interneurons do not randomly disperse, but are frequently organized in spatially isolated intra- or inter-laminar clusters. Adapted from Sultan, K.T. et al., 2013 and 2014.153, 154 LGE, lateral ganglionic eminence; Ctx, cortex. (B) Clonally-labeled interneurons in clusters (green) preferentially form electrical, but not chemical, synapses with each other compared to nearby, non-clonally related interneurons (red). This lineage-related preferential electrical coupling promotes the coordinated formation of inhibitory synapses between clonally-labeled interneurons and the same nearby excitatory cells in the cortex.
In the mature cortex, interneurons derived from dividing RGPs in the MGE/PoA labeled at a clonal density were shown to frequently form spatially isolated intra- and inter-laminar clusters 117, 146. These observations of the clustering of clonally related cortical interneurons were challenged in two subsequent studies where barcoded retrovirus libraries were used to provide single cell resolution of clonal identity.147, 148 Notwithstanding the over-representation of certain barcodes and ~54% failure rate in barcode recovery, it was concluded that interneuron clones randomly disperse across the forebrain. Further systematic and in-depth analysis of the original barcoded datasets, however, revealed that spatial clustering is in fact a reliable feature of clonally related interneurons in the forebrain or only the cortex, supporting the contribution of lineage relationship in spatial distribution and organization of interneurons in the forebrain as well as in the cortex.
Notably, the analysis of the subtype composition of interneurons within individual clonal clusters can provide important insights into the potential of individual RGPs to generate interneuron diversity. The two prominent and distinct classes of interneurons generated by the NKX2-1-expressing progenitors in the MGE/PoA are the PV- and SOM-expressing cells. For cortical clusters that contained at least three interneurons, a majority was composed of mixed subtypes of PV- and SOM-expressing cells. Based on the electrophysiological properties and neurochemical marker expression, at least 50% of the clonally labeled interneuron clusters in the cortex were comprised of cells of mixed subtypes (i.e. FS and non-FS, or PV+ and SOM+). Similar observations were also reported in the barcoded datasets.147, 148 These results clearly suggest that individual RGPs in the MGE/PoA are capable of producing distinct subtypes of interneurons, and that interneuron diversity in the cortex may be generated via the progressive fate-restriction of a common (i.e. multipotent) pool of dividing RGPs. Nonetheless, a fraction of the clonally labeled interneuron clusters were of a relatively pure subtype (either SOM or PV only), leaving open the possibility that some RGPs may be more limited than others in their interneuron subtype output potential. In comparison to dorsal RGPs which predominantly undergo a progressively deterministic program to produce excitatory neurons that laminate different layers of the cortex, it is possible that the RGP population in the ventral telencephalon responsible for generating interneurons is more heterogenous in mitotic behaviors that diversify the interneuron lineage.
Interestingly, it has recently been discovered that clonally labeled interneuron clusters in the cortex derived from the MGE/PoA exhibit remarkable functional organization (Figure 5B). They preferentially form electrical, but not chemical, synapses with each other compared to nearby non-clonally related interneurons. This lineage-related electrical coupling facilitates action potential generation and synchronous firing. Moreover, it promotes correlated inhibitory synapse formation between interneurons and the same nearby excitatory neurons. These results suggest that progenitor origin and lineage relationship influence microcircuit assembly of inhibitory interneurons in the cortex.
CONCLUDING REMARKS
Mounting evidence has shown that the developmental (i.e. time and place of birth, progenitor behavior, lineage, etc.) and genetic (i.e. transcription factors, signaling pathways, etc.) programs control the production and diversification of cortical interneurons. Despite such progress, the fundamental molecular and cellular framework of the generation of diverse interneurons with distinct and defined properties, localizations, and functions is still largely missing. The rapid advances in single cell analysis techniques open the possibility of dissecting progenitor behavior and interneuron development systematically at the single cell resolution. These efforts will provide unprecedented insights into the production and specification of cortical interneurons. It is worth mentioning that interneuron development can also be influenced by various environmental factors such as local and distant chemical signals as well as synaptic inputs. Therefore, it will be interesting to explore the active interplay between the cell intrinsic program and local environment in the generation and maintenance of the remarkably diverse interneurons in the cortex.
Acknowledgments
Our research was supported by grants from the National Institutes of Health (P01NS048120, R01DA024681, R01 NS085004, and R01MH101382), the Human Frontier Science Program (RGP0053/2014), the Memorial Sloan Kettering Cancer Center Functional Genomics Initiative (14658), the Howard Hughes Medical Institute, and the New York State Stem Cell Science (NYSTEM) (C030137).
References
- 1.Hendry SH, Schwark HD, Jones EG, Yan J. Numbers and proportions of GABA-immunoreactive neurons in different areas of monkey cerebral cortex. J Neurosci. 1987;7:1503–1519. doi: 10.1523/JNEUROSCI.07-05-01503.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 3.Sherwood CC, Raghanti MA, Stimpson CD, Spocter MA, Uddin M, Boddy AM, Wildman DE, Bonar CJ, Lewandowski AH, Phillips KA, et al. Inhibitory interneurons of the human prefrontal cortex display conserved evolution of the phenotype and related genes. Proc Biol Sci. 2010;277:1011–1020. doi: 10.1098/rspb.2009.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- 5.Maass W, Natschlager T, Markram H. Fading memory and kernel properties of generic cortical microcircuit models. J Physiol Paris. 2004;98:315–330. doi: 10.1016/j.jphysparis.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeFelipe J, Lopez-Cruz PL, Benavides-Piccione R, Bielza C, Larranaga P, Anderson S, Burkhalter A, Cauli B, Fairen A, Feldmeyer D, et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci. 2013;14:202–216. doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 9.Miyoshi G, Butt SJ, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci. 2007;27:7786–7798. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat. 1997;14:1–19. doi: 10.1016/s0891-0618(97)10013-8. [DOI] [PubMed] [Google Scholar]
- 12.Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010;30:16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- 15.Gentet LJ. Functional diversity of supragranular GABAergic neurons in the barrel cortex. Front Neural Circuits. 2012;6:52. doi: 10.3389/fncir.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci. 2006;26:5069–5082. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Toledo-Rodriguez M, Gupta A, Wu C, Silberberg G, Luo J, Markram H. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J Physiol. 2004;561:65–90. doi: 10.1113/jphysiol.2004.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci. 2007;10:462–468. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- 19.Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16:1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karnani MM, Jackson J, Ayzenshtat I, Tucciarone J, Manoocheri K, Snider WG, Yuste R. Cooperative Subnetworks of Molecularly Similar Interneurons in Mouse Neocortex. Neuron. 2016;90:86–100. doi: 10.1016/j.neuron.2016.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adesnik H, Bruns W, Taniguchi H, Huang ZJ, Scanziani M. A neural circuit for spatial summation in visual cortex. Nature. 2012;490:226–231. doi: 10.1038/nature11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu H, Jeong HY, Tremblay R, Rudy B. Neocortical somatostatin-expressing GABAergic interneurons disinhibit the thalamorecipient layer 4. Neuron. 2013;77:155–167. doi: 10.1016/j.neuron.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang X, Shen S, Cadwell CR, Berens P, Sinz F, Ecker AS, Patel S, Tolias AS. Principles of connectivity among morphologically defined cell types in adult neocortex. Science. 2015;350:aac9462. doi: 10.1126/science.aac9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olah S, Fule M, Komlosi G, Varga C, Baldi R, Barzo P, Tamas G. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature. 2009;461:1278–1281. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69:1188–1203. doi: 10.1016/j.neuron.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci. 2011;31:13260–13271. doi: 10.1523/JNEUROSCI.3131-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bock DD, Lee WC, Kerlin AM, Andermann ML, Hood G, Wetzel AW, Yurgenson S, Soucy ER, Kim HS, Reid RC. Network anatomy and in vivo physiology of visual cortical neurons. Nature. 2011;471:177–182. doi: 10.1038/nature09802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofer SB, Ko H, Pichler B, Vogelstein J, Ros H, Zeng H, Lein E, Lesica NA, Mrsic-Flogel TD. Differential connectivity and response dynamics of excitatory and inhibitory neurons in visual cortex. Nat Neurosci. 2011;14:1045–1052. doi: 10.1038/nn.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshimura Y, Callaway EM. Fine-scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nat Neurosci. 2005;8:1552–1559. doi: 10.1038/nn1565. [DOI] [PubMed] [Google Scholar]
- 30.Otsuka T, Kawaguchi Y. Cortical inhibitory cell types differentially form intralaminar and interlaminar subnetworks with excitatory neurons. J Neurosci. 2009;29:10533–10540. doi: 10.1523/JNEUROSCI.2219-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armstrong C, Wang J, Yeun Lee S, Broderick J, Bezaire MJ, Lee SH, Soltesz I. Target-selectivity of parvalbumin-positive interneurons in layer II of medial entorhinal cortex in normal and epileptic animals. Hippocampus. 2016;26:779–793. doi: 10.1002/hipo.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katzel D, Zemelman BV, Buetfering C, Wolfel M, Miesenbock G. The columnar and laminar organization of inhibitory connections to neocortical excitatory cells. Nat Neurosci. 2011;14:100–107. doi: 10.1038/nn.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galarreta M, Hestrin S. Electrical synapses between GABA-releasing interneurons. Nat Rev Neurosci. 2001;2:425–433. doi: 10.1038/35077566. [DOI] [PubMed] [Google Scholar]
- 34.Wang XJ, Buzsaki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci. 1996;16:6402–6413. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galarreta M, Erdelyi F, Szabo G, Hestrin S. Electrical coupling among irregular-spiking GABAergic interneurons expressing cannabinoid receptors. J Neurosci. 2004;24:9770–9778. doi: 10.1523/JNEUROSCI.3027-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blatow M, Rozov A, Katona I, Hormuzdi SG, Meyer AH, Whittington MA, Caputi A, Monyer H. A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron. 2003;38:805–817. doi: 10.1016/s0896-6273(03)00300-3. [DOI] [PubMed] [Google Scholar]
- 37.Chu Z, Galarreta M, Hestrin S. Synaptic interactions of late-spiking neocortical neurons in layer 1. J Neurosci. 2003;23:96–102. doi: 10.1523/JNEUROSCI.23-01-00096.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deans MR, Gibson JR, Sellitto C, Connors BW, Paul DL. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron. 2001;31:477–485. doi: 10.1016/s0896-6273(01)00373-7. [DOI] [PubMed] [Google Scholar]
- 39.Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 2000;3:904–910. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- 40.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon A, Olah S, Molnar G, Szabadics J, Tamas G. Gap-junctional coupling between neurogliaform cells and various interneuron types in the neocortex. J Neurosci. 2005;25:6278–6285. doi: 10.1523/JNEUROSCI.1431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 43.Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- 44.Yanez IB, Munoz A, Contreras J, Gonzalez J, Rodriguez-Veiga E, DeFelipe J. Double bouquet cell in the human cerebral cortex and a comparison with other mammals. J Comp Neurol. 2005;486:344–360. doi: 10.1002/cne.20533. [DOI] [PubMed] [Google Scholar]
- 45.DeFelipe J. Cortical interneurons: from Cajal to 2001. Changing Views of Cajal’s Neuron. 2002;136:215–238. doi: 10.1016/s0079-6123(02)36019-9. [DOI] [PubMed] [Google Scholar]
- 46.Beaulieu C. Numerical data on neocortical neurons in adult rat, with special reference to the GABA population. Brain Res. 1993;609:284–292. doi: 10.1016/0006-8993(93)90884-p. [DOI] [PubMed] [Google Scholar]
- 47.Gabbott PL, Dickie BG, Vaid RR, Headlam AJ, Bacon SJ. Local-circuit neurones in the medial prefrontal cortex (areas 25, 32 and 24b) in the rat: morphology and quantitative distribution. J Comp Neurol. 1997;377:465–499. doi: 10.1002/(sici)1096-9861(19970127)377:4<465::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 48.Meinecke DL, Peters A. GABA immunoreactive neurons in rat visual cortex. J Comp Neurol. 1987;261:388–404. doi: 10.1002/cne.902610305. [DOI] [PubMed] [Google Scholar]
- 49.Micheva KD, Beaulieu C. Postnatal development of GABA neurons in the rat somatosensory barrel cortex: a quantitative study. Eur J Neurosci. 1995;7:419–430. doi: 10.1111/j.1460-9568.1995.tb00338.x. [DOI] [PubMed] [Google Scholar]
- 50.Beaulieu C, Kisvarday Z, Somogyi P, Cynader M, Cowey A. Quantitative distribution of GABA-immunopositive and -immunonegative neurons and synapses in the monkey striate cortex (area 17) Cereb Cortex. 1992;2:295–309. doi: 10.1093/cercor/2.4.295. [DOI] [PubMed] [Google Scholar]
- 51.del Rio MR, DeFelipe J. Double bouquet cell axons in the human temporal neocortex: relationship to bundles of myelinated axons and colocalization of calretinin and calbindin D-28k immunoreactivities. J Chem Neuroanat. 1997;13:243–251. doi: 10.1016/s0891-0618(97)00050-1. [DOI] [PubMed] [Google Scholar]
- 52.Gabbott PL, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey: II. Quantitative areal and laminar distributions. J Comp Neurol. 1996;364:609–636. doi: 10.1002/(SICI)1096-9861(19960122)364:4<609::AID-CNE2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 53.DeFelipe J, Hendry SH, Hashikawa T, Molinari M, Jones EG. A microcolumnar structure of monkey cerebral cortex revealed by immunocytochemical studies of double bouquet cell axons. Neuroscience. 1990;37:655–673. doi: 10.1016/0306-4522(90)90097-n. [DOI] [PubMed] [Google Scholar]
- 54.del Rio MR, DeFelipe J. A light and electron microscopic study of calbindin D-28k immunoreactive double bouquet cells in the human temporal cortex. Brain Res. 1995;690:133–140. doi: 10.1016/0006-8993(95)00641-3. [DOI] [PubMed] [Google Scholar]
- 55.Peters A, Sethares C. The organization of double bouquet cells in monkey striate cortex. J Neurocytol. 1997;26:779–797. doi: 10.1023/a:1018518515982. [DOI] [PubMed] [Google Scholar]
- 56.Favorov OV, Kelly DG. Minicolumnar organization within somatosensory cortical segregates: II. Emergent functional properties. Cereb Cortex. 1994;4:428–442. doi: 10.1093/cercor/4.4.428. [DOI] [PubMed] [Google Scholar]
- 57.Kisvarday ZF, Cowey A, Somogyi P. Synaptic relationships of a type of GABA-immunoreactive neuron (clutch cell), spiny stellate cells and lateral geniculate nucleus afferents in layer IVC of the monkey striate cortex. Neuroscience. 1986;19:741–761. doi: 10.1016/0306-4522(86)90296-4. [DOI] [PubMed] [Google Scholar]
- 58.Kisvarday ZF, Martin KA, Whitteridge D, Somogyi P. Synaptic connections of intracellularly filled clutch cells: a type of small basket cell in the visual cortex of the cat. J Comp Neurol. 1985;241:111–137. doi: 10.1002/cne.902410202. [DOI] [PubMed] [Google Scholar]
- 59.DeFelipe J. Chandelier cells and epilepsy. Brain. 1999;122(Pt 10):1807–1822. doi: 10.1093/brain/122.10.1807. [DOI] [PubMed] [Google Scholar]
- 60.Zeisel A, Munoz-Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A, Marques S, Munguba H, He L, Betsholtz C, et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347:1138–1142. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- 61.Tasic B, Menon V, Nguyen TN, Kim TK, Jarsky T, Yao Z, Levi B, Gray LT, Sorensen SA, Dolbeare T, et al. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci. 2016;19:335–346. doi: 10.1038/nn.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parnavelas JG, Barfield JA, Franke E, Luskin MB. Separate progenitor cells give rise to pyramidal and nonpyramidal neurons in the rat telencephalon. Cereb Cortex. 1991;1:463–468. doi: 10.1093/cercor/1.6.463. [DOI] [PubMed] [Google Scholar]
- 63.Luskin MB, Parnavelas JG, Barfield JA. Neurons, astrocytes, and oligodendrocytes of the rat cerebral cortex originate from separate progenitor cells: an ultrastructural analysis of clonally related cells. J Neurosci. 1993;13:1730–1750. doi: 10.1523/JNEUROSCI.13-04-01730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan SS, Kalloniatis M, Sturm K, Tam PP, Reese BE, Faulkner-Jones B. Separate progenitors for radial and tangential cell dispersion during development of the cerebral neocortex. Neuron. 1998;21:295–304. doi: 10.1016/s0896-6273(00)80539-5. [DOI] [PubMed] [Google Scholar]
- 65.Anderson SA, Kaznowski CE, Horn C, Rubenstein JL, McConnell SK. Distinct origins of neocortical projection neurons and interneurons in vivo. Cereb Cortex. 2002;12:702–709. doi: 10.1093/cercor/12.7.702. [DOI] [PubMed] [Google Scholar]
- 66.Deacon TW, Pakzaban P, Isacson O. The lateral ganglionic eminence is the origin of cells committed to striatal phenotypes: neural transplantation and developmental evidence. Brain Res. 1994;668:211–219. doi: 10.1016/0006-8993(94)90526-6. [DOI] [PubMed] [Google Scholar]
- 67.Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- 68.Olsson M, Bjorklund A, Campbell K. Early specification of striatal projection neurons and interneuronal subtypes in the lateral and medial ganglionic eminence. Neuroscience. 1998;84:867–876. doi: 10.1016/s0306-4522(97)00532-0. [DOI] [PubMed] [Google Scholar]
- 69.Olsson M, Campbell K, Wictorin K, Bjorklund A. Projection neurons in fetal striatal transplants are predominantly derived from the lateral ganglionic eminence. Neuroscience. 1995;69:1169–1182. doi: 10.1016/0306-4522(95)00325-d. [DOI] [PubMed] [Google Scholar]
- 70.Stenman J, Toresson H, Campbell K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci. 2003;23:167–174. doi: 10.1523/JNEUROSCI.23-01-00167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marin O, Rubenstein JL. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- 72.Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- 73.Petanjek Z, Berger B, Esclapez M. Origins of cortical GABAergic neurons in the cynomolgus monkey. Cereb Cortex. 2009;19:249–262. doi: 10.1093/cercor/bhn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jakovcevski I, Mayer N, Zecevic N. Multiple origins of human neocortical interneurons are supported by distinct expression of transcription factors. Cereb Cortex. 2011;21:1771–1782. doi: 10.1093/cercor/bhq245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Radonjic NV, Ayoub AE, Memi F, Yu X, Maroof A, Jakovcevski I, Anderson SA, Rakic P, Zecevic N. Diversity of cortical interneurons in primates: the role of the dorsal proliferative niche. Cell Rep. 2014;9:2139–2151. doi: 10.1016/j.celrep.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hansen DV, Lui JH, Flandin P, Yoshikawa K, Rubenstein JL, Alvarez-Buylla A, Kriegstein AR. Non-epithelial stem cells and cortical interneuron production in the human ganglionic eminences. Nat Neurosci. 2013;16:1576–1587. doi: 10.1038/nn.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma T, Wang C, Wang L, Zhou X, Tian M, Zhang Q, Zhang Y, Li J, Liu Z, Cai Y, et al. Subcortical origins of human and monkey neocortical interneurons. Nat Neurosci. 2013;16:1588–1597. doi: 10.1038/nn.3536. [DOI] [PubMed] [Google Scholar]
- 78.Xu Q, Tam M, Anderson SA. Fate mapping Nkx2. 1-lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- 79.Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJ, Battiste J, Johnson JE, Machold RP, Fishell G. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30:1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gelman D, Griveau A, Dehorter N, Teissier A, Varela C, Pla R, Pierani A, Marin O. A wide diversity of cortical GABAergic interneurons derives from the embryonic preoptic area. J Neurosci. 2011;31:16570–16580. doi: 10.1523/JNEUROSCI.4068-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gelman DM, Martini FJ, Nobrega-Pereira S, Pierani A, Kessaris N, Marin O. The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J Neurosci. 2009;29:9380–9389. doi: 10.1523/JNEUROSCI.0604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anastasiades PG, Butt SJ. Decoding the transcriptional basis for GABAergic interneuron diversity in the mouse neocortex. Eur J Neurosci. 2011;34:1542–1552. doi: 10.1111/j.1460-9568.2011.07904.x. [DOI] [PubMed] [Google Scholar]
- 83.Gelman DM, Marin O, Rubenstein JLR. The Generation of Cortical Interneurons. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. 4. Bethesda (MD): 2012. [PubMed] [Google Scholar]
- 84.Hernandez-Miranda LR, Parnavelas JG, Chiara F. Molecules and mechanisms involved in the generation and migration of cortical interneurons. ASN Neuro. 2010;2:e00031. doi: 10.1042/AN20090053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Flandin P, Zhao Y, Vogt D, Jeong J, Long J, Potter G, Westphal H, Rubenstein JL. Lhx6 and Lhx8 coordinately induce neuronal expression of Shh that controls the generation of interneuron progenitors. Neuron. 2011;70:939–950. doi: 10.1016/j.neuron.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vogt D, Hunt RF, Mandal S, Sandberg M, Silberberg SN, Nagasawa T, Yang Z, Baraban SC, Rubenstein JL. Lhx6 directly regulates Arx and CXCR7 to determine cortical interneuron fate and laminar position. Neuron. 2014;82:350–364. doi: 10.1016/j.neuron.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Long JE, Cobos I, Potter GB, Rubenstein JL. Dlx1&2 and Mash1 transcription factors control MGE and CGE patterning and differentiation through parallel and overlapping pathways. Cereb Cortex. 2009;19(Suppl 1):i96–106. doi: 10.1093/cercor/bhp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Long JE, Swan C, Liang WS, Cobos I, Potter GB, Rubenstein JL. Dlx1&2 and Mash1 transcription factors control striatal patterning and differentiation through parallel and overlapping pathways. J Comp Neurol. 2009;512:556–572. doi: 10.1002/cne.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Waclaw RR, Wang B, Pei Z, Ehrman LA, Campbell K. Distinct temporal requirements for the homeobox gene Gsx2 in specifying striatal and olfactory bulb neuronal fates. Neuron. 2009;63:451–465. doi: 10.1016/j.neuron.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu Q, Guo L, Moore H, Waclaw RR, Campbell K, Anderson SA. Sonic hedgehog signaling confers ventral telencephalic progenitors with distinct cortical interneuron fates. Neuron. 2010;65:328–340. doi: 10.1016/j.neuron.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lodato S, Tomassy GS, De Leonibus E, Uzcategui YG, Andolfi G, Armentano M, Touzot A, Gaztelu JM, Arlotta P, Menendez de la Prida L, et al. Loss of COUP-TFI alters the balance between caudal ganglionic eminence- and medial ganglionic eminence-derived cortical interneurons and results in resistance to epilepsy. J Neurosci. 2011;31:4650–4662. doi: 10.1523/JNEUROSCI.6580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ma T, Zhang Q, Cai Y, You Y, Rubenstein JL, Yang Z. A subpopulation of dorsal lateral/caudal ganglionic eminence-derived neocortical interneurons expresses the transcription factor Sp8. Cereb Cortex. 2012;22:2120–2130. doi: 10.1093/cercor/bhr296. [DOI] [PubMed] [Google Scholar]
- 93.Cai Y, Zhang Q, Wang C, Zhang Y, Ma T, Zhou X, Tian M, Rubenstein JL, Yang Z. Nuclear receptor COUP-TFII-expressing neocortical interneurons are derived from the medial and lateral/caudal ganglionic eminence and define specific subsets of mature interneurons. J Comp Neurol. 2013;521:479–497. doi: 10.1002/cne.23186. [DOI] [PubMed] [Google Scholar]
- 94.Miyoshi G, Young A, Petros T, Karayannis T, McKenzie Chang M, Lavado A, Iwano T, Nakajima M, Taniguchi H, Huang ZJ, et al. Prox1 Regulates the Subtype-Specific Development of Caudal Ganglionic Eminence-Derived GABAergic Cortical Interneurons. J Neurosci. 2015;35:12869–12889. doi: 10.1523/JNEUROSCI.1164-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rubin AN, Kessaris N. PROX1: a lineage tracer for cortical interneurons originating in the lateral/caudal ganglionic eminence and preoptic area. PLoS One. 2013;8:e77339. doi: 10.1371/journal.pone.0077339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Touzot A, Ruiz-Reig N, Vitalis T, Studer M. Molecular control of two novel migratory paths for CGE-derived interneurons in the developing mouse brain. Development. 2016;143:1753–1765. doi: 10.1242/dev.131102. [DOI] [PubMed] [Google Scholar]
- 97.Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marin O. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci. 2007;27:9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fogarty M, Grist M, Gelman D, Marin O, Pachnis V, Kessaris N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci. 2007;27:10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sousa VH, Miyoshi G, Hjerling-Leffler J, Karayannis T, Fishell G. Characterization of Nkx6-2-derived neocortical interneuron lineages. Cereb Cortex. 2009;19(Suppl 1):i1–10. doi: 10.1093/cercor/bhp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Inan M, Welagen J, Anderson SA. Spatial and temporal bias in the mitotic origins of somatostatin- and parvalbumin-expressing interneuron subgroups and the chandelier subtype in the medial ganglionic eminence. Cereb Cortex. 2012;22:820–827. doi: 10.1093/cercor/bhr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wonders CP, Taylor L, Welagen J, Mbata IC, Xiang JZ, Anderson SA. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev Biol. 2008;314:127–136. doi: 10.1016/j.ydbio.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taniguchi H, Lu J, Huang ZJ. The spatial and temporal origin of chandelier cells in mouse neocortex. Science. 2013;339:70–74. doi: 10.1126/science.1227622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.He M, Tucciarone J, Lee S, Nigro MJ, Kim Y, Levine JM, Kelly SM, Krugikov I, Wu P, Chen Y, et al. Strategies and Tools for Combinatorial Targeting of GABAergic Neurons in Mouse Cerebral Cortex. Neuron. 2016;91:1228–1243. doi: 10.1016/j.neuron.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Silberberg SN, Taher L, Lindtner S, Sandberg M, Nord AS, Vogt D, McKinsey GL, Hoch R, Pattabiraman K, Zhang D, et al. Subpallial Enhancer Transgenic Lines: a Data and Tool Resource to Study Transcriptional Regulation of GABAergic Cell Fate. Neuron. 2016;92:59–74. doi: 10.1016/j.neuron.2016.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen YJ, Friedman BA, Ha C, Durinck S, Liu J, Rubenstein JL, Seshagiri S, Modrusan Z. Single-cell RNA sequencing identifies distinct mouse medial ganglionic eminence cell types. Sci Rep. 2017;7:45656. doi: 10.1038/srep45656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cavanagh ME, Parnavelas JG. Development of vasoactive-intestinal-polypeptide-immunoreactive neurons in the rat occipital cortex: a combined immunohistochemical-autoradiographic study. J Comp Neurol. 1989;284:637–645. doi: 10.1002/cne.902840410. [DOI] [PubMed] [Google Scholar]
- 107.Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 108.Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- 109.Breunig JJ, Haydar TF, Rakic P. Neural stem cells: historical perspective and future prospects. Neuron. 2011;70:614–625. doi: 10.1016/j.neuron.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Gotz M. Neuronal or glial progeny: regional differences in radial glia fate. Neuron. 2003;37:751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 111.Malatesta P, Hartfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- 112.Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 113.Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 114.Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- 115.Kowalczyk T, Pontious A, Englund C, Daza RA, Bedogni F, Hodge R, Attardo A, Bell C, Huttner WB, Hevner RF. Intermediate neuronal progenitors (basal progenitors) produce pyramidal-projection neurons for all layers of cerebral cortex. Cereb Cortex. 2009;19:2439–2450. doi: 10.1093/cercor/bhn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Angevine JB, Jr, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- 117.Brown KN, Chen S, Han Z, Lu CH, Tan X, Zhang XJ, Ding L, Lopez-Cruz A, Saur D, Anderson SA, et al. Clonal production and organization of inhibitory interneurons in the neocortex. Science. 2011;334:480–486. doi: 10.1126/science.1208884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marquardt T. Transcriptional control of neuronal diversification in the retina. Prog Retin Eye Res. 2003;22:567–577. doi: 10.1016/s1350-9462(03)00036-3. [DOI] [PubMed] [Google Scholar]
- 119.Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 120.Desai AR, McConnell SK. Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development. 2000;127:2863–2872. doi: 10.1242/dev.127.13.2863. [DOI] [PubMed] [Google Scholar]
- 121.Frantz GD, McConnell SK. Restriction of late cerebral cortical progenitors to an upper-layer fate. Neuron. 1996;17:55–61. doi: 10.1016/s0896-6273(00)80280-9. [DOI] [PubMed] [Google Scholar]
- 122.McConnell SK, Kaznowski CE. Cell cycle dependence of laminar determination in developing neocortex. Science. 1991;254:282–285. doi: 10.1126/science.254.5029.282. [DOI] [PubMed] [Google Scholar]
- 123.Luskin MB, Pearlman AL, Sanes JR. Cell lineage in the cerebral cortex of the mouse studied in vivo and in vitro with a recombinant retrovirus. Neuron. 1988;1:635–647. doi: 10.1016/0896-6273(88)90163-8. [DOI] [PubMed] [Google Scholar]
- 124.Walsh C, Cepko CL. Clonal dispersion in proliferative layers of developing cerebral cortex. Nature. 1993;362:632–635. doi: 10.1038/362632a0. [DOI] [PubMed] [Google Scholar]
- 125.Franco SJ, Gil-Sanz C, Martinez-Garay I, Espinosa A, Harkins-Perry SR, Ramos C, Muller U. Fate-restricted neural progenitors in the mammalian cerebral cortex. Science. 2012;337:746–749. doi: 10.1126/science.1223616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guo C, Eckler MJ, McKenna WL, McKinsey GL, Rubenstein JL, Chen B. Fezf2 expression identifies a multipotent progenitor for neocortical projection neurons, astrocytes, and oligodendrocytes. Neuron. 2013;80:1167–1174. doi: 10.1016/j.neuron.2013.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gao P, Postiglione MP, Krieger TG, Hernandez L, Wang C, Han Z, Streicher C, Papusheva E, Insolera R, Chugh K, et al. Deterministic progenitor behavior and unitary production of neurons in the neocortex. Cell. 2014;159:775–788. doi: 10.1016/j.cell.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.He S, Li Z, Ge S, Yu YC, Shi SH. Inside-Out Radial Migration Facilitates Lineage-Dependent Neocortical Microcircuit Assembly. Neuron. 2015;86:1159–1166. doi: 10.1016/j.neuron.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yu YC, He S, Chen S, Fu Y, Brown KN, Yao XH, Ma J, Gao KP, Sosinsky GE, Huang K, et al. Preferential electrical coupling regulates neocortical lineage-dependent microcircuit assembly. Nature. 2012;486:113–117. doi: 10.1038/nature10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li Y, Lu H, Cheng PL, Ge S, Xu H, Shi SH, Dan Y. Clonally related visual cortical neurons show similar stimulus feature selectivity. Nature. 2012;486:118–121. doi: 10.1038/nature11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yu YC, Bultje RS, Wang X, Shi SH. Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature. 2009;458:501–504. doi: 10.1038/nature07722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tan X, Liu WA, Zhang XJ, Shi W, Ren SQ, Li Z, Brown KN, Shi SH. Vascular Influence on Ventral Telencephalic Progenitors and Neocortical Interneuron Production. Dev Cell. 2016;36:624–638. doi: 10.1016/j.devcel.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lavdas AA, Grigoriou M, Pachnis V, Parnavelas JG. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci. 1999;19:7881–7888. doi: 10.1523/JNEUROSCI.19-18-07881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- 135.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 136.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 137.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fietz SA, Lachmann R, Brandl H, Kircher M, Samusik N, Schroder R, Lakshmanaperumal N, Henry I, Vogt J, Riehn A, et al. Transcriptomes of germinal zones of human and mouse fetal neocortex suggest a role of extracellular matrix in progenitor self-renewal. Proc Natl Acad Sci U S A. 2012;109:11836–11841. doi: 10.1073/pnas.1209647109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Goldman SA, Chen Z. Perivascular instruction of cell genesis and fate in the adult brain. Nat Neurosci. 2011;14:1382–1389. doi: 10.1038/nn.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pollen AA, Nowakowski TJ, Chen J, Retallack H, Sandoval-Espinosa C, Nicholas CR, Shuga J, Liu SJ, Oldham MC, Diaz A, et al. Molecular identity of human outer radial glia during cortical development. Cell. 2015;163:55–67. doi: 10.1016/j.cell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Noctor SC, Martinez-Cerdeno V, Kriegstein AR. Neural stem and progenitor cells in cortical development. Novartis Found Symp. 2007;288:59–73. discussion 73–58, 96–58. [PubMed] [Google Scholar]
- 142.Noctor SC, Martinez-Cerdeno V, Kriegstein AR. Contribution of intermediate progenitor cells to cortical histogenesis. Arch Neurol. 2007;64:639–642. doi: 10.1001/archneur.64.5.639. [DOI] [PubMed] [Google Scholar]
- 143.Glickstein SB, Monaghan JA, Koeller HB, Jones TK, Ross ME. Cyclin D2 is critical for intermediate progenitor cell proliferation in the embryonic cortex. J Neurosci. 2009;29:9614–9624. doi: 10.1523/JNEUROSCI.2284-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Glickstein SB, Moore H, Slowinska B, Racchumi J, Suh M, Chuhma N, Ross ME. Selective cortical interneuron and GABA deficits in cyclin D2-null mice. Development. 2007;134:4083–4093. doi: 10.1242/dev.008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Petros TJ, Bultje RS, Ross ME, Fishell G, Anderson SA. Apical versus Basal Neurogenesis Directs Cortical Interneuron Subclass Fate. Cell Rep. 2015;13:1090–1095. doi: 10.1016/j.celrep.2015.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ciceri G, Dehorter N, Sols I, Huang ZJ, Maravall M, Marin O. Lineage-specific laminar organization of cortical GABAergic interneurons. Nat Neurosci. 2013;16:1199–1210. doi: 10.1038/nn.3485. [DOI] [PubMed] [Google Scholar]
- 147.Harwell CC, Fuentealba LC, Gonzalez-Cerrillo A, Parker PR, Gertz CC, Mazzola E, Garcia MT, Alvarez-Buylla A, Cepko CL, Kriegstein AR. Wide Dispersion and Diversity of Clonally Related Inhibitory Interneurons. Neuron. 2015;87:999–1007. doi: 10.1016/j.neuron.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mayer C, Jaglin XH, Cobbs LV, Bandler RC, Streicher C, Cepko CL, Hippenmeyer S, Fishell G. Clonally Related Forebrain Interneurons Disperse Broadly across Both Functional Areas and Structural Boundaries. Neuron. 2015;87:989–998. doi: 10.1016/j.neuron.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schmolesky M. The Primary Visual Cortex. In: Kolb H, Fernandez E, Nelson R, editors. Webvision: The Organization of the Retina and Visual System. Salt Lake City (UT): 1995. [PubMed] [Google Scholar]
- 150.Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Molnar Z, Butt SJ. Best-laid schemes for interneuron origin of mice and men. Nat Neurosci. 2013;16:1512–1514. doi: 10.1038/nn.3557. [DOI] [PubMed] [Google Scholar]
- 152.Bandler RC, Mayer C, Fishell G. Cortical interneuron specification: the juncture of genes, time and geometry. Curr Opin Neurobiol. 2017;42:17–24. doi: 10.1016/j.conb.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sultan KT, Brown KN, Shi SH. Production and organization of neocortical interneurons. Frontiers in Cellular Neuroscience. 2013:7. doi: 10.3389/fncel.2013.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sultan KT, Shi W, Shi SH. Clonal origins of neocortical interneurons. Current Opinion in Neurobiology. 2014;26:125–131. doi: 10.1016/j.conb.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]