Abstract
The cornea is our window to the world and our vision is critically dependent on corneal clarity and integrity. Its epithelium represents one of the most rapidly regenerating mammalian tissues, undergoing full turnover over the course of approximately one to two weeks. This robust and efficient regenerative capacity is dependent on the function of stem cells residing in the limbus, a structure marking the border between the cornea and the conjunctiva. Limbal stem cells (LSC) represent a quiescent cell population with proliferative capacity residing in the basal epithelial layer of the limbus within a cellular niche. In addition to LSC, this niche consists of various cell populations such as limbal stromal fibroblasts, melanocytes and immune cells as well as a basement membrane, all of which are essential for LSC maintenance and LSC-driven regeneration. The LSC niche’s components are of diverse developmental origin, a fact that had, until recently, prevented precise identification of molecularly defined LSC. The recent success in prospective LSC isolation based on ABCB5 expression and the capacity of this LSC population for long-term corneal restoration following transplantation in preclinical in vivo models of LSC deficiency (LSCD) underline the considerable potential of pure LSC formulations for clinical therapy. Additional studies, including genetic lineage tracing of the developmental origin of LSC will further improve our understanding of this critical cell population and its niche, with important implications for regenerative medicine.
Graphical abstract
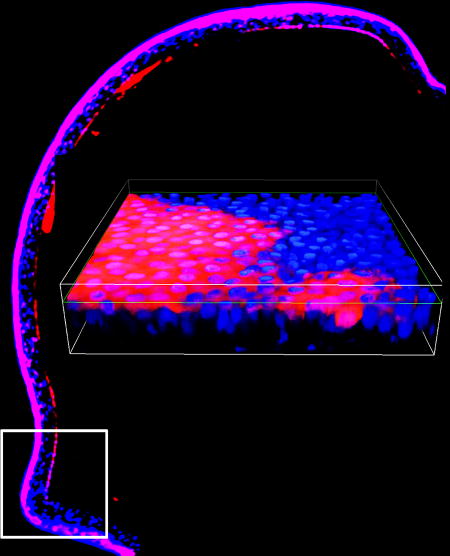
Visual Abstract. Contribution of LSC to corneal epithelial development and homeostasis. Immunofluorescent image of corneal epithelium from a 1 month-old Abcb5/Cre/tdTomato mouse depicting tdTomato-positive Abcb5-derived progeny cells within the entire adult mouse corneal epithelium. The white square on the left indicates the location of the limbus of which a high magnification image is shown on the right.
Limbal Stem Cell Identity
The cornea is essential for normal vision due to its multiple roles that include light refraction and transmission as well as protection of underlying eye structures from environmental injuries. The cornea consists of three layers, i.e. the epithelium, stroma and endothelium, which are separated by two membranes: Bowman’s membrane, located between the epithelial and stromal layers, and Descemet’s membrane, located between the stromal and endothelial layers. A hallmark feature of the corneal epithelium is its high regenerative potential and its capacity for rapid ocular surface repair through proliferation and centripetal migration of progenitor cell populations residing at the border of the cornea and the sclera in a location called limbus (1–3) (Figure 1).
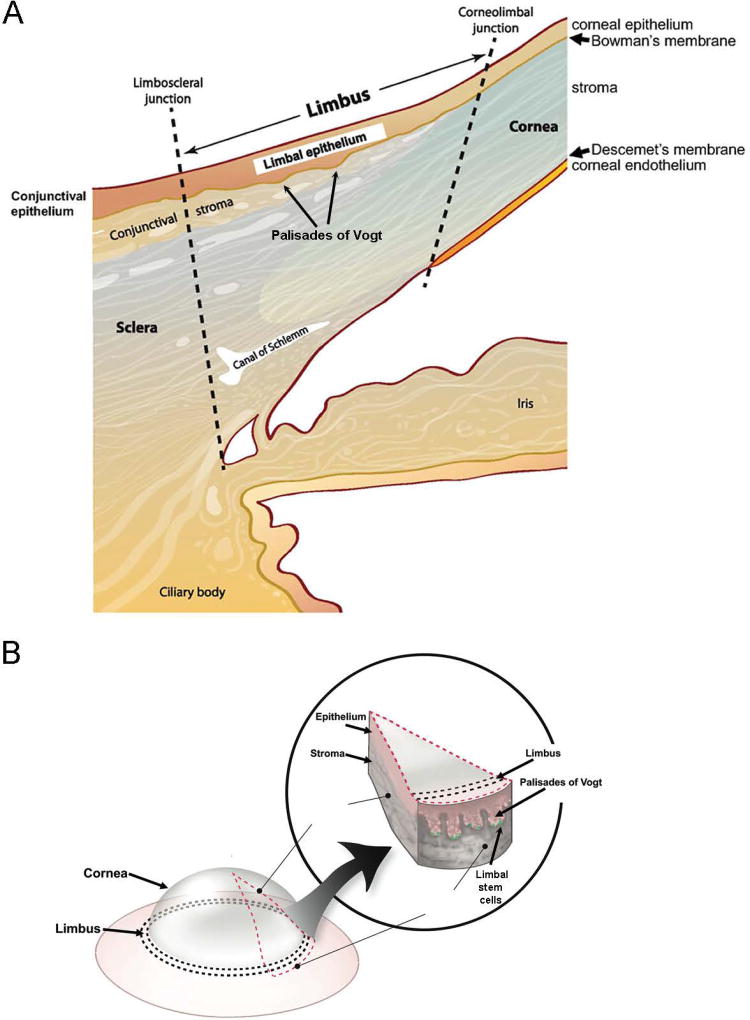
Figure 1. Schematic illustration of the human cornea and limbus.
(A) Cross section illustrating the location and cellular layers of the cornea and limbus. (B) The Palisades of Vogt located at the corneo-limbal-scleral junction of the eye.
Limbal stem cells (LSC) represent a quiescent cell population with high proliferative potential, which enables efficient corneal regeneration and repair (4–14). LSC do not express markers of differentiated mature corneal epithelium (2, 15). This constellation of features led to a decades-long search for a bona fide LSC marker that would enable prospective LSC isolation for therapeutic applications. In 1971, Davanger and Evensen (1) proposed that LSC reside in the palisades of Vogt (POV), a series of radially oriented fibrovascular ridges that are observed in the human limbus (16, 17) and can be detected by the optical coherence tomography (18, 19). In 2005, based on histological examination of the human limbus, Dua et al. reported the presence of limbal epithelial crypts (LEC) and proposed that they also harbor LSC (20). LEC are more frequently detected in the superior or inferior limbus compared to the temporal or nasal limbus (21, 22). In non-human species, only porcine limbus has been reported to share the structure of the human limbus with regard to the location and topography of POV and LEC, while no evidence of POV has been found in the other animals (21, 23). In mice, LSC were first identified as slow-cycling label-retaining cells located in the basal layer of limbal epithelium (5). Despite the lack of the POV structures in mice, lineage-tracing studies clearly have shown that murine corneal stem cells exist in the limbus and that they are capable of producing daughter cells with centripetal migration during corneal regeneration (24–28).
It has been widely accepted that bona fide LSC are defined by their ability to establish and maintain long-term restoration of the corneal epithelium, i.e. properties that are only demonstrated by transplantation experiments (29). Numerous potential LSC markers have been proposed (Table 1), but for most, evidence for successful prospective enrichment of cells capable of long-term corneal restoration is currently lacking. In 2001, Pellegrini et al. proposed that the transcription factor p63 identifies human LSC (30). Following this discovery, Rama et al. evaluated the clinical effectiveness of autologous mixed limbal cell transplants grafted onto patients with unilateral LSC deficiency (LSCD) (29). They concluded that success of the transplants was dependent upon the number of p63+ cells contained within grafts, suggesting that p63 identifies LSC among mixed limbal cultures.
Table 1.
Putative LCS markers
| Marker | Unseparated cells | Enrichment of marker positive cells | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Co- expression with ΔNp63α protein |
Label retention |
Method of enrichment |
Colony forming efficiency (CFE) |
Restoration of corneal epithelium via transplantation |
||||
| Non- human |
Human | IHC | Flow | BrdU | Frequency of holoclones |
Short term |
Long term |
||
| Negative Selection | |||||||||
| Cytokeratin 3 (2) | rabbit | ||||||||
| SSEA14 (131) | + | magnetic beads | + | ||||||
| ALDHdim (40) | + | + | cell sorting | + | |||||
| RHAMMbright (40) | + | + | cell sorting | ||||||
| Connexin-43 (41) | + | + | + | + | cell sorting | ||||
| Positive Selection | |||||||||
| Cytokeratin 15 (39) | mouse | + | |||||||
| Lgr5 (31) | + | + | |||||||
| Tcf4 (32) | + | + | |||||||
| CD157 (33) | + | + | |||||||
| SOD2/CK15 clusters (132) | + | ||||||||
| CD71low/integrin α6high (34) | + | + | cell sorting | + | |||||
| TrkA (35) | + | + | |||||||
| N-Cadherin (36) | + | + | + | + | |||||
| Hoechst efflux (133) | rat | + | side population | ||||||
| Integrins β1-β4 (22) | mouse | + | + | ||||||
| Hoechst efflux (134) | rabbit | side population | + | ||||||
| Abcg2 (37) | rabbit | + | side population | + | |||||
| Abcg2 (37, 38) | + | + | side population | + | |||||
| p63, Abcg2, Integrins (135) | + | + | |||||||
| ABCB5 (42) | mouse | + | + | + | + | cell sorting | + | + | |
In these studies, limbal epithelial cultures used for transplantation generally contained up to 10% p63+ cells, with significant variability observed between individual grafts. When grafts contained less than 3% p63+ cells, the transplants failed; when grafts contained between 3–6% of p63+ cells, a partial transplant success was achieved; and when grafts contained between 5–10% of p63+ cells, the transplants were successful. These findings indicated that human LSC express p63, however, because of the nuclear expression of p63, further enrichment of the limbal grafts for p63 purity was not feasible, leaving unanswered the question whether a pure p63+ population could have resulted in more universal therapeutic success, as might be expected of an autologous LSC graft. Subsequently, several additional potential human LSC markers were described based on their anatomical and immunohistochemical association with p63, including positive selection markers Lgr5 (31), Tcf4 (32), CD157 (33), CD71low/Integrin α6high (34), TrkA (35), N-Cadherin (36), Abcg2 (37, 38) and Cytokeratin15(39), and negative selection markers Cytokeratin 3 (2), ALDHdim (40), RHAMMbright (40) and Connexin-43 (41) (Table 1).
Recently, our laboratories demonstrated that ATP-binding cassette (ABC) superfamily member ABCB5 identifies LSC with the ability to restore and maintain the corneal epithelium upon transplantation to preclinical models of LSCD (42). Specifically, our studies showed that prospectively isolated human ABCB5-positive LSC, but not ABCB5-negative limbal epithelial cells, possessed the capacity to fully restore the corneal epithelium upon grafting to LSC-deficient mice in xenogeneic or syngeneic transplantation models (42). ABCB5 was found in those studies to be preferentially expressed on label-retaining LSC in mice, and on ΔNp63α-positive cells in humans. Consistent with these findings, ABCB5+ LSC frequency was significantly reduced in LSC-deficient patients. Importantly, Abcb5 loss of function in Abcb5 KO mice caused depletion of quiescent LSC due to enhanced proliferation and apoptosis, and resulted in defective corneal differentiation and wound healing, demonstrating that ABCB5 not only marks LSC, but is required for LSC function. These results from gene knockout studies, LSC tracing and transplantation models, as well as phenotypic and functional analyses of human biopsy specimens, provided robust evidence that ABCB5 identifies mammalian LSC. Since this original report, additional studies by independent laboratories have confirmed the presence of ABCB5+ LSC in human and mouse limbal epithelium (43–48). Moreover, our most recent studies utilizing genetic lineage tracing in Abcb5/Cre reporter mice crossed with tdTomato (B6;129S6-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J) mice (49), aimed at further dissecting the origin of LSC and their progeny cell fate, identified tdTomato-positive ABCB5-derived progeny within the entire adult mouse corneal epithelium (Figure 2), identifying at the level of genetic lineage tracing an ABCB5-expressing precursor cell that gives rise to self-renewing corneal epithelium during development and regeneration, consistent with the LSC phenotype.
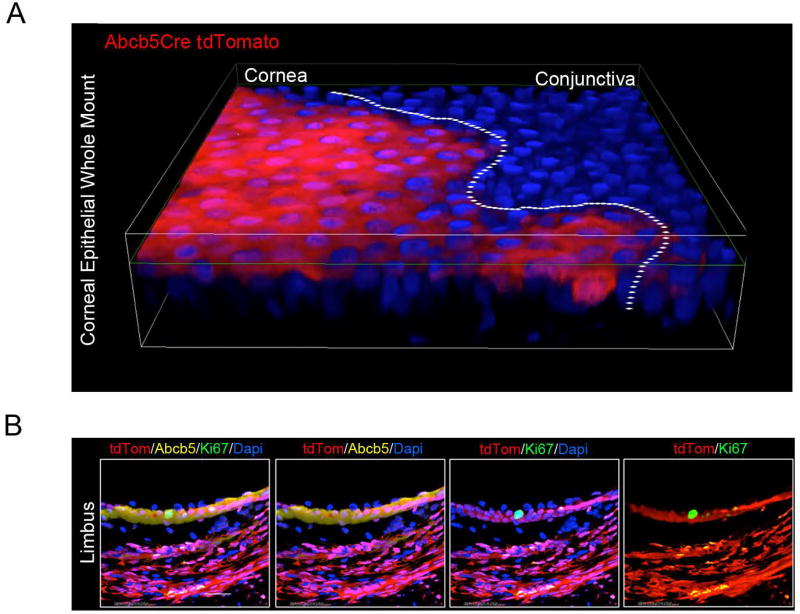
Figure 2. Contribution of ABCB5+ stem cells to corneal epithelial development and homeostasis.
(A) Immunofluorescent image of corneal epithelium from a 1 month-old Abcb5/Cre/tdTomato mouse (whole-mount cornea). Abcb5/Cre transgenic mice were generated by insertion of an IRES-Cre cassette in the Abcb5 3’UTR downstream of the STOP codon located in exon 30. Abcb5/Cre mice were crossed with tdTomato (B6;129S6-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J) mice for genetic lineage tracing studies.
Whole-mount cornea preparations from Abcb5/Cre/tdTomato mice identified tdTomato-positive Abcb5-derived progeny cells within the entire adult mouse corneal epithelium. (B) Immunofluorescent (×60 magnification) images of tdTomato (red), Abcb5 (yellow) and Ki67 (green) co-expression in the limbus of Abcb5/Cre/tdTomato mice. The nuclei are stained with Dapi, 4′,6-diamidino-2-phenylindole (blue).
Developmental Origin of LSC and Their Niche
The LSC niche is one of the few active mammalian stem cell niches preserved in adulthood that enables continuing regeneration and repair of high turnover tissues (50). In addition to LSC themselves, this niche consists of various cell populations such as limbal stromal fibroblasts, melanocytes, immune cells including Langerhans cells, macrophages, vascular endothelial cells, and a basement membrane (36, 51–53) with diverse developmental origins. Limbal stromal fibroblasts originate from neural crest (54), express the mesenchymal stem cell (MSC) markers CD73, CD90 and CD105 (55–57) and possess the ability to differentiate into adipocytes, osteocytes, keratinocyte, vascular endothelial cells, pericytes and cornea-like epithelium in vitro (56, 58–60). While in culture limbal stromal fibroblasts can be induced to express ABCG2 and ABCB5 (48), however, there is no evidence that limbal stromal fibroblasts can give rise to LSC or corneal epithelium in vivo. Nevertheless, it has been shown that stromal stem cells possess the ability to remodel pathological stromal tissue by suppressing inflammation and restoring transparency (57, 61). The role of melanocytes located in close proximity to LSC still remains unclear. It is hypothesized that they protect LSC from oxidative DNA damage and contribute to the maintenance of LSC quiescence (36, 62, 63). Similar to limbal stromal fibroblasts, melanocytes are also derived from neural crest cells (64). The limbal basement membrane consists of specific components such as α1, α2 chains of collagen IV, collagen XVI, laminin α1, laminin γ3, agrin and tenascin C (65–69). Previous studies have suggested that the limbal basement membrane may facilitate stem cell adhesion required for LSC homeostasis and harbor and provide critical LSC growth factors and cytokines released from limbal niche cells (53, 65, 67, 68). In addition to these components, lymphocytes, stromal nerves and blood vessels contribute functionally to the LSC niche (65, 70)
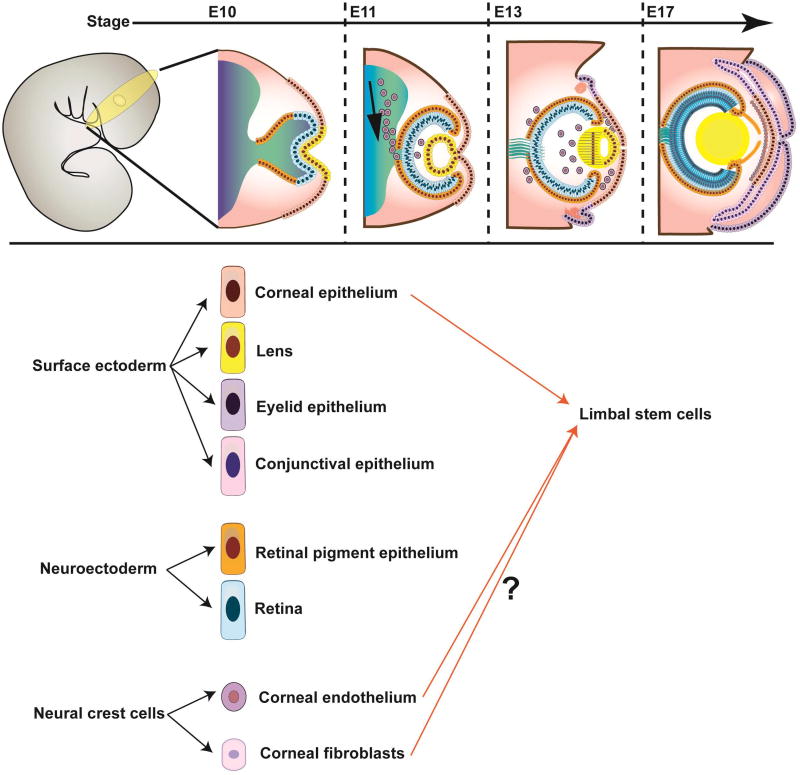
Due to the complexity and heterogeneity of the LSC niche and the relative elusiveness, until recently, of the cellular identity of bona fide LSC, the developmental origin of LSC remains currently enigmatic. To date, it has been established that at least two embryonic layers, the surface ectoderm and the periocular mesenchyme, contribute to the formation of the corneo-limbal-scleral junction, i.e. the area where LSC reside (Figure 3). The surface ectoderm, which separates from neuroectoderm during early eye field formation, gives rise to the corneal epithelium (71). An early eye field transcription factor, PAX6, can be first detected in the surface ectoderm of the developing mouse at embryonic (E) 8 stage (72), and is subsequently expressed in the developing corneal epithelium at E12.5 (73). Recent genetic lineage tracing studies in Pax6/Cre (P0–3.9-GFPCre) mice crossed to ROSAmT/mG reporter mice showed GFP expression in the entire corneal epithelium suggesting that Pax6-expressing progenitors contribute to corneal epithelial generation and regeneration during development and adulthood (73), which raised the possibility that Pax6+ cells within the surface ectoderm represent the cells of origin of LSC. This possibility is also supported by findings in Pax6+/− mice that revealed that Pax6 haploinsufficiency results in reduced expression of the corneal epithelial differentiation marker Krt12 and increased corneal vascularization consistent with a LSCD phenotype (74).
Figure 3. Developmental origin of LSC.
(A) Schematic illustration of corneal development. From left to right: Extending neuroepithelium induces the surface ectoderm to form the lens, cornea, conjunctiva and eyelid. Migrating neural crest cells differentiate into corneal endothelium and stromal fibroblasts. (B) Contribution of diverse embryonic layers to the formation of the LSC niche. Current models suggest that LSC could be developmental descendants of the surface ectoderm as well as the periocular mesenchyme.
Another ectodermal transcription factor, p63, has been detected in the adult human LSC niche and was proposed to mark LSC (30, 75). During embryonic development, p63 expression can be observed in the surface ectoderm at the mouse E6 stage, and, at the time of birth, it is found in the basal cells of the stratified skin and its appendages (76). Comparison of Pax6 and p63 expression in the developing mouse cornea showed that p63 was preceded by Pax6 by two days and, unlike Pax6, was not restricted to the developing eye (73). While loss of function of p63 in knockout mice results in failure of epithelial stratification and marked inhibition of normal limb, tail, facial, and external genital development (76, 77), p63 knockout does not result in LSCD. Human patients with Ectrodactyly-Ectodermal Dysplasia-Clefting syndrome caused by p63 mutations display skeletal malformations, and lacrimal and meibomian gland defects, which, in some cases, are also associated with corneal clouding (78). Genetic lineage tracing studies using ΔNp63+/Cre mice crossed to ROSA26EYFP mice showed selective EYFP expression in developing glandular and stratified epithelia expressing ΔNp63 (e.g. skin, thymus, salivary gland, esophagus and trachea) (79). In findings by the same authors, evaluating this lineage-tracing model in the adult cornea, ΔNp63-derived EYFP-positive progeny cells were detected throughout the corneal epithelium (including the apical layer) and ΔNp63 protein expression was observed in the basal corneal epithelial cell compartment, but was not restricted to the limbus (Pignon, JC and Signoretti, S; personal communication). Consistent with other findings (29, 42), these results show that p63 is expressed by LSC (42), but indicate that it may not represent a specific LSC marker and may not be required for normal LSC function.
As opposed to a possible surface ectodermal origin, LSC might alternatively originate from the periocular mesenchyme. During embryonic development, the periocular mesenchyme gives rise to multiple corneal structures, including the corneal stroma and endothelium, Schlemm’s canal, and the trabecular meshwork. Using a transgenic system allowing distinct binary labeling of mesodermal and neural crest progenitors, Gage et al. showed that both somatic mesoderm and the neural crest contribute to the formation of periocular mesenchyme (54). The majority of cells in the corneal endothelium and stroma hereby appeared to be of neural crest origin. Within the limbal region, the endothelial lining of Schlemm’s canal and the iris stroma were derived mostly from mesoderm, whereas ciliary muscles and trabecular meshwork contained a majority of cells of neural crest origin (54). Mesoderm and neural crest derivatives also exhibited distinct eye transcription factor expression patterns, with PITX2 and FOXC1 preferentially expressed by neural crest-derived progenitors, and PITX1 and MYOG by mesoderm-derived cells. Using a temporal gene knockout approach, Gage at al. demonstrated that neural crest-expressed PITX2 is required for corneal morphogenesis and cell fate specification within the surface ectoderm and the mesenchymal primordia, and is also essential for establishing of the angiogenic privilege of the cornea (80). In addition, Seo et al. reported that neural crest deletion of FOXC1 leads to aberrant vessel growth in the normally avascular mouse cornea due to inhibition of the anti-angiogenic activity of sVEGFR-1 (81, 82). These studies highlight the potential critical role of neural crest derivatives in establishing angiogenic privilege of the central cornea and suggest the possibility that LSC might be of neural crest origin. This notion is also supported by studies of Du et al., which showed that a subpopulation of human corneal neural crest-derived stromal cells expressed mesenchymal stem cell markers and exhibited multipotent differentiation potential (59). Of note, the LSC marker ABCB5 (42) also identifies cells of mesenchymal stem cell molecular phenotype in other tissues (83) suggesting a possible neural crest origin of ABCB5+ LSC. Additional genetic lineage and transplantation studies using clonal cell populations will help to further define the developmental origin of LSC.
LSC in Corneal Homeostasis and Wound Healing – Therapeutic Potential
It has been widely accepted that LSC give rise to transient amplifying cells (TAC), and that TAC migrate centripetally and anteriorly to generate differentiated corneal epithelial cells, which will eventually be shed from the corneal surface, as proposed in the X-Y-Z hypothesis (84, 85) (Figure 4A). Although acute wound healing in the central cornea can be achieved by proliferation and migration of central corneal epithelial cells (86), LSC are essential for corneal homeostasis and normal wound healing (4, 5, 87, 88). The critical role of LSC in corneal repair is further supported by recent studies showing impaired corneal wound healing in Abcb5 knockout mice (42) and suggested by findings of impaired corneal wound healing in diabetic mice with diminished expression of the LSC-expressed genes ABCG2, ΔNp63α and Krt15 (89–92). Lineage-tracing methods have also suggested involvement of LSC in corneal homeostasis and wound healing (24–28). Specifically, Amitai-Lange et al. reported that while central corneal cells had the ability to contribute to mild corneal wound repair, larger corneal injuries required the involvement of LSC (24).
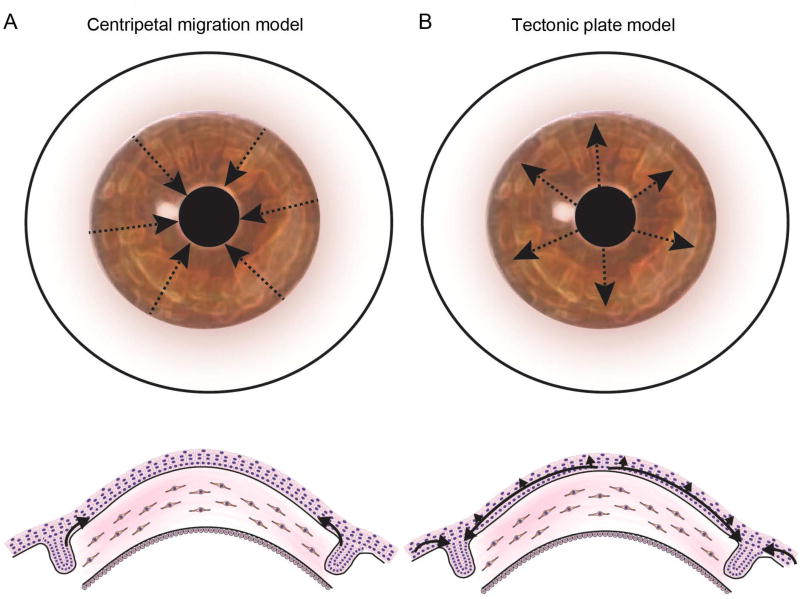
Figure 4. Current models of corneal epithelial migration and maintenance.
(A) The Centripetal Migration Model, also known as the X-Y-Z hypothesis, of corneal regeneration. This model suggests that LSC located in the limbic region undergo centripetal migration and differentiation to maintain the corneal epithelium. (B) An alternative model suggests the existence of stem cells capable of corneal and conjunctival regeneration in the entire corneal epithelium. According to this model, the limbus represents a zone of equilibrium in which the expanding conjunctival and corneal epithelia are confronted in a mechanism reminiscent of tectonic plates; rupture of this equilibrium is suggested to result in migration of LSC onto the cornea.
While the significance of the limbus as an anatomical niche for corneal epithelial stem cells (i.e., LSC) is relatively established, Majo et al., using a genetic tracing model, identified the existence of additional stem cells capable of corneal and conjunctival regeneration residing in the entire corneal epithelium (93). Consequently, they proposed an alternative theory of corneal regeneration describing the limbus as a zone of equilibrium in which the expanding conjunctival and corneal epithelia are confronted in a mechanism reminiscent of tectonic plates and suggesting that rupture of the corneo-conjunctival equilibrium results in migration of LSC onto the cornea (93) (Figure 4B). The LSC paradigm and this hypothesis advanced by Majo et al. do not appear to be mutually exclusive as recently reported by Lobo et al. (28).
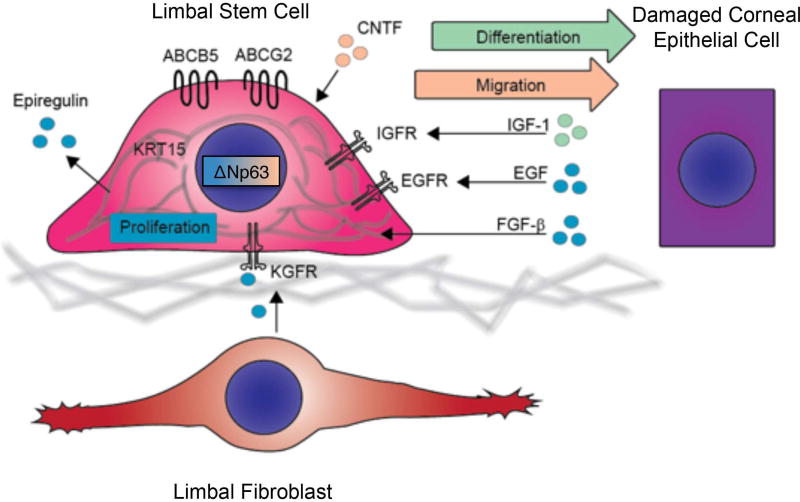
A number of cell signaling pathways have been shown to play a critical role in corneal wound healing (Figure 5). In the diabetic cornea, disease-associated impaired wound healing can be accelerated by overexpression of the hepatocyte growth factor (HGF) receptor c-MET, and by silencing of MMP-10 and cathepsin F (94, 95). Additionally, inhibition of miR-146a, which is pathologically induced in diabetic mice, leads to increased expression of phosphorylated p38 and epidermal growth factor receptor (EGFR) by LSC, resulting in normalization of epithelial wound healing (96). Keratinocyte growth factor (KGF) secreted by limbal fibroblasts increases the mitotic activity of LSC, that express KGF receptor (KGFR), leading to acceleration of corneal epithelial wound healing (97–99). Ciliary neurotrophic factor (CNTF) promotes migration of corneal epithelial stem/progenitor cells through activation of Akt signaling mediated by matrix metalloproteinases (100, 101). In the setting of corneal injury, LSC proliferation can also be stimulated by epidermal growth factor (EGF) and fibroblast growth factor-β (FGF-β) produced by the damaged corneal epithelium (102), whereas LSC differentiation is driven by insulin-like growth factor-I (IGF-I), which induces expression of IGF receptor on LSC (102). A number of EGF family members are expressed by the limbal epithelium, including transforming growth factor-α (TGF-α), hepatocyte binding epidermal growth factor (HB-EGF), epiregulin (EREG) and amphiregulin (AREG) (103). Epiregulin expression is restricted to limbal basal epithelial cells, and is thought to contribute to the high proliferative capacity of these cells in the setting of wound healing (104, 105).
Figure 5. Factors affecting corneal epithelial wound healing.
Normal corneal wound healing is dependent on proliferation (blue), migration (orange) and differentiation (green) of corneal progenitors. Attenuated expression of LSC markers, including ABCB5, ABCG2, ΔNp63α or K15, is associated with abnormal corneal wound healing, which may result in increased corneal fragility, ulceration and clouding. Limbal epithelial cell proliferation is supported by expression of ΔNp63α and epiregulin in limbal basal epithelial cells, by keratinocyte growth factor (KGF) secreted from limbal fibroblasts, and by epidermal growth factor (EGF) and fibroblast growth factor-β (FGF-β) produced by damaged corneal epithelium. Migration is promoted by expression of ΔNp63α and ciliary neurotrophic factor (CNTF). Differentiation is induced by insulin-like growth factor-I (IGF-I), rapidly produced by injured corneal epithelium upon injury. KGFR, KGF receptor; EGFR, EGF receptor; IGFR, IGF receptor.
Loss or deficiency of LSC causes destruction of corneal homeostasis and results in abnormal wound healing (3, 106), a condition known as LSCD. LSCD leads to conjunctival epithelial ingrowth, neovascularization of the corneal stroma, corneal opacification and vision loss (3, 106). The major causes of acquired LSCD are Stevens-Johnson syndrome, ocular cicatricial pemphigoid, chemical or thermal burns and contact lens over-wear (107–110). Limbal tumors, severe corneal infections and iatrogenic causes are more rare etiologies of LSCD (107, 108, 110). In recent decades, significant advances have been made in the development of LSC-based therapies for the treatment of LSCD. First, in 1997, Pellegrini et al. described the use of autologous cultured limbal epithelial cells (CLET) for the treatment of unilateral LSCD (111). In this study, cellular grafts were generated from limbal biopsies of healthy eyes contralateral to the diseased eyes of unilateral LSCD patients and the biopsies were enzymatically digested and expanded as holoclones in vitro, to generate corneal epithelial sheets for transplantation. This cell-based therapeutic approach, containing p63-positive LSC at varying concentrations, recently received conditional approval by the European Medicinal Agency (EMA) based on a reported success rate of 60–70% (29). In another, similar approach, enzymatically digested limbal biopsies cultured on human amniotic membrane were transplanted to patients with unilateral LSCD, with therapeutic success ranging from 50% to 83% (112–114). While these techniques have resulted in long-term restoration of the corneal epithelium, they are, for the most part, only applicable to patients with unilateral disease, but not to the much more frequent group of patients with bilateral disease. Additionally, since such grafts contain variable numbers of LSC, estimated by the expression of p63 in companion cultures (29), they do not represent pure LSC grafts. Thus, there exists a need to further improve LSC-based therapeutic approaches to unilateral and bilateral LSCD therapy. Additionally, several regulatory and logistical barriers need to be overcome in order to further advance widespread approval, availability and acceptance of stem cell-based LSCD therapies: (i) A somatic cell therapeutic should possess a defined composition and purity of the biologically active ingredient for appropriate dosing and potentially required dosing intervals, and prevention of unwanted side effects; (ii) current protocols employ the transfer of non-LSC populations contained within grafts that may be biologically inactive, or might produce unwanted side effects through inauthentic reconstitution of the LSC niche and/or the corneal stroma; and (iii) neither holoclones nor cell/matrix compositions consisting of limbal cells grown on amniotic membrane have been shown to be cryopreservable, presenting considerable challenges in manufacturing, storage, transport, and local transplantation logistics of such therapeutic compositions that have prevented wide-spread availability and adoption of these techniques. We posit that prospective isolation and purification of LSC, for example through use of a cell surface marker such as ABCB5, might have the potential to overcome these obstacles, leading to easier fulfillment of current regulatory requirements and likely further improvements of therapeutic outcomes.
In contrast to unilateral LSCD, treatment of patients with bilateral LSCD poses even greater challenges. Clinical studies using allogeneic limbal tissue transplants provide, at best, transient restoration of the cornea. The failure of these allograft transplants is most likely due to alloantigen-specific immune-mediated rejection of the donor graft. The central cornea is a well-known and established “immune privileged” tissue that allows the survival of fully allogeneic corneal transplants in low-risk recipient patients (115). In contrast, the limbus does not represent the same immune-privileged microenvironment encountered in the central cornea. It contains, in addition to LSC with immune-suppressive roles (116), other cell types such as Langerhans cells, macrophages, and dendritic cells capable of potent induction of rejection responses when grafted to allogeneic recipients. Therefore, such immunogenic cell types contained within mixed limbal allografts that lack purity for relatively non-immunogenic LSC might be primarily responsible for immune-mediated rejection of grafts currently employed for the treatment of patients with bilateral LSCD. Thus, it is very likely that transplantation of purified LSC populations (such as, for example, isolated through the newly available marker ABCB5 (42)) that are devoid of accompanying immunogenic cell populations, might significantly reduce LSC allograft rejection, and hence improve therapeutic outcomes in bilateral LSCD treatment.
It is well established that immune tolerance and privilege, including in allotransplantation, are significantly controlled by negative costimulatory pathway mechanisms, including the molecular interaction of programmed cell death 1 receptor (PD-1) with its ligands, PD-L1 and PD-L2 (117). In experimental model systems of allograft rejection in the cornea, previous studies by Hori et al. (118) and Watson et al. (119) revealed that PD-1 is similarly required for prolonged allograft survival. Specifically, corneal allografts survived when transplanted onto wild-type recipient mice, but were rejected when transplanted onto PD-L1 knock-out recipient mice, validating the critical role of PD-1 in corneal immune privilege (120). Intriguingly, ABCB5+ stem cells derived from other tissues have already been found to express PD-1 and to exhibit immune-privilege with a capacity to engraft across fully mismatched allogeneic barriers (83), warranting examination whether ABCB5+ LSC exhibit similar tolerogenic properties. If so, they might represent a particularly promising cell source for treatment not only of unilateral LSCD, but also as LSC allograft for the treatment of bilateral LSCD.
In addition to the above-mentioned studies, a number of promising therapeutic approaches to LSCD utilizing advanced cell reprogramming techniques have been reported in preclinical models. Corneal epithelial-like cells could be induced from embryonic stem cells (ESCs) (121) and from induced pluripotent stem cells (iPSCs) (122, 123). In this regard, Hayashi et al. created a self-formed ectodermal autonomous multi-zone (SEAM) of ocular cells using human iPSCs from which they successfully isolated corneal epithelial stem/progenitor cells capable of long-term corneal regeneration (122). Other groups showed that corneal epithelial-like cells could be also derived through direct reprogramming of the other cell types such as bone marrow mesenchymal stem cells (BM-MSCs), hair follicle stem cells (HFSCs), skin epithelial stem cells, fibroblasts and oral mucosal epithelial cells (124–128), dental pulp stem cell sheet (129) and nasal mucosal epithelial cell sheet (130) suggesting that these approaches might represent novel options for treatment of LSCD in the future.
Conclusions
Amongst adult stem cell populations that sustain high-turnover mammalian tissues, LSC represent a relatively well-studied entity with proven clinical relevance in human regenerative medicine, based on their capacity for corneal restoration following transplantation to LSCD patients. Recent advances in the identification of LSC markers now promise not only to further enhance their therapeutic potential, but also to allow further dissection of their developmental origin, differentiation plasticity and contributions to the LSC cell niche, as such markers can now be deployed in genetic lineage tracing models capable of documenting ever more primitive precursors and potentially identifying additional cell fates beyond the corneal epithelial lineage alone. Further studies in these respects are urgently needed, as they will refine our current understanding of anterior eye development and homeostasis. In particular, such genetic lineage tracing studies, combined with single cell transplantation studies, will serve to answer the question of identity of a common progenitor for anterior eye chamber development and what its developmental lineage may be. Moreover, further study of LSC and their molecular markers will shed light on the cellular and molecular mechanisms involved in their preservation of undifferentiated phenotype, their high proliferative potential throughout adulthood, their maintenance, replenishment and immunoregulatory functions, thereby informing not only promising novel LSC-based clinical approaches to corneal disease, but also more broadly, based on potential relevance to other adult stem cell niches, the field of adult regenerative medicine as a whole.
Acknowledgments
This work was supported by NIH/NEI 1RO1EY025794-01A1 grant to N.Y.F., B.R.K and M.H.F, NIH/NEI 3R01EY025794-01A1S1 Minority Supplement Award to G.G., NIH/NEI Schepens Core Grant P30EY003790 to B.R.K., VA RR&D 1I01RX000989 Merit Review Award and an HSCI seed grant award to N.Y.F., the Kanae Foundation for the Promotion of Medical Science (Tokyo, Japan), Alcon Japan Ltd. (Tokyo, Japan) and Japan Eye Bank Association (Tokyo, Japan) to Y.S..
Conflict of Interest Statement. M.H.F. and N.Y.F. are inventors or co-inventors of US and international patents assigned to Brigham and Women’s Hospital and/or Boston Children’s Hospital, Boston, MA, and licensed to Ticeba GmbH (Heidelberg, Germany) and Rheacell GmbH & Co. KG (Heidelberg, Germany). M.H.F. serves as a scientific advisor to Ticeba GmbH and Rheacell GmbH & Co. KG‥ M.H.F and B.R.K. participate in corporate sponsored research collaborations with Rheacell GmbH & Co. KG‥
References
- 1.Davanger M, Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971;229(5286):560–1. doi: 10.1038/229560a0. [DOI] [PubMed] [Google Scholar]
- 2.Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103(1):49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng SC. Concept and application of limbal stem cells. Eye (Lond) 1989;3(Pt 2):141–57. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- 4.Lehrer MS, Sun TT, Lavker RM. Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. J Cell Sci. 1998;111(Pt 19):2867–75. doi: 10.1242/jcs.111.19.2867. [DOI] [PubMed] [Google Scholar]
- 5.Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57(2):201–9. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 6.Ebato B, Friend J, Thoft RA. Comparison of limbal and peripheral human corneal epithelium in tissue culture. Invest Ophthalmol Vis Sci. 1988;29(10):1533–7. [PubMed] [Google Scholar]
- 7.Lavker RM, Dong G, Cheng SZ, Kudoh K, Cotsarelis G, Sun TT. Relative proliferative rates of limbal and corneal epithelia. Implications of corneal epithelial migration, circadian rhythm, and suprabasally located DNA-synthesizing keratinocytes. Invest Ophthalmol Vis Sci. 1991;32(6):1864–75. [PubMed] [Google Scholar]
- 8.Lavker RM, Wei ZG, Sun TT. Phorbol ester preferentially stimulates mouse fornical conjunctival and limbal epithelial cells to proliferate in vivo. Invest Ophthalmol Vis Sci. 1998;39(2):301–7. [PubMed] [Google Scholar]
- 9.Lindberg K, Brown ME, Chaves HV, Kenyon KR, Rheinwald JG. In vitro propagation of human ocular surface epithelial cells for transplantation. Invest Ophthalmol Vis Sci. 1993;34(9):2672–9. [PubMed] [Google Scholar]
- 10.Kruse FE, Tseng SC. Growth factors modulate clonal growth and differentiation of cultured rabbit limbal and corneal epithelium. Invest Ophthalmol Vis Sci. 1993;34(6):1963–76. [PubMed] [Google Scholar]
- 11.Kruse FE, Tseng SC. Retinoic acid regulates clonal growth and differentiation of cultured limbal and peripheral corneal epithelium. Invest Ophthalmol Vis Sci. 1994;35(5):2405–20. [PubMed] [Google Scholar]
- 12.Kruse FE. Stem cells and corneal epithelial regeneration. Eye (Lond) 1994;8(Pt 2):170–83. doi: 10.1038/eye.1994.42. [DOI] [PubMed] [Google Scholar]
- 13.Tseng SC, Zhang SH. Limbal epithelium is more resistant to 5-fluorouracil toxicity than corneal epithelium. Cornea. 1995;14(4):394–401. [PubMed] [Google Scholar]
- 14.Tseng SC. Regulation and clinical implications of corneal epithelial stem cells. Mol Biol Rep. 1996;23(1):47–58. doi: 10.1007/BF00357072. [DOI] [PubMed] [Google Scholar]
- 15.Kurpakus MA, Maniaci MT, Esco M. Expression of keratins K12, K4 and K14 during development of ocular surface epithelium. Curr Eye Res. 1994;13(11):805–14. doi: 10.3109/02713689409025135. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg MF, Bron AJ. Limbal palisades of Vogt. Trans Am Ophthalmol Soc. 1982;80:155–71. [PMC free article] [PubMed] [Google Scholar]
- 17.Townsend WM. The limbal palisades of Vogt. Trans Am Ophthalmol Soc. 1991;89:721–56. [PMC free article] [PubMed] [Google Scholar]
- 18.Lathrop KL, Gupta D, Kagemann L, Schuman JS, Sundarraj N. Optical coherence tomography as a rapid, accurate, noncontact method of visualizing the palisades of Vogt. Invest Ophthalmol Vis Sci. 2012;53(3):1381–7. doi: 10.1167/iovs.11-8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin HC, Tew TB, Hsieh YT, Lin SY, Chang HW, Hu FR, et al. Using optical coherence tomography to assess the role of age and region in corneal epithelium and palisades of vogt. Medicine (Baltimore) 2016;95(35):e4234. doi: 10.1097/MD.0000000000004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dua HS, Shanmuganathan VA, Powell-Richards AO, Tighe PJ, Joseph A. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol. 2005;89(5):529–32. doi: 10.1136/bjo.2004.049742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grieve K, Ghoubay D, Georgeon C, Thouvenin O, Bouheraoua N, Paques M, et al. Three-dimensional structure of the mammalian limbal stem cell niche. Exp Eye Res. 2015;140:75–84. doi: 10.1016/j.exer.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Pajoohesh-Ganji A, Pal-Ghosh S, Simmens SJ, Stepp MA. Integrins in slow-cycling corneal epithelial cells at the limbus in the mouse. Stem Cells. 2006;24(4):1075–86. doi: 10.1634/stemcells.2005-0382. [DOI] [PubMed] [Google Scholar]
- 23.Notara M, Schrader S, Daniels JT. The porcine limbal epithelial stem cell niche as a new model for the study of transplanted tissue-engineered human limbal epithelial cells. Tissue Eng Part A. 2011;17(5–6):741–50. doi: 10.1089/ten.TEA.2010.0343. [DOI] [PubMed] [Google Scholar]
- 24.Amitai-Lange A, Altshuler A, Bubley J, Dbayat N, Tiosano B, Shalom-Feuerstein R. Lineage tracing of stem and progenitor cells of the murine corneal epithelium. Stem Cells. 2015;33(1):230–9. doi: 10.1002/stem.1840. [DOI] [PubMed] [Google Scholar]
- 25.Di Girolamo N, Bobba S, Raviraj V, Delic NC, Slapetova I, Nicovich PR, et al. Tracing the fate of limbal epithelial progenitor cells in the murine cornea. Stem Cells. 2015;33(1):157–69. doi: 10.1002/stem.1769. [DOI] [PubMed] [Google Scholar]
- 26.Dora NJ, Hill RE, Collinson JM, West JD. Lineage tracing in the adult mouse corneal epithelium supports the limbal epithelial stem cell hypothesis with intermittent periods of stem cell quiescence. Stem Cell Res. 2015;15(3):665–77. doi: 10.1016/j.scr.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasetti RB, Gaddipati S, Tian S, Xue L, Kao WW, Lu Q, et al. Study of corneal epithelial progenitor origin and the Yap1 requirement using keratin 12 lineage tracing transgenic mice. Sci Rep. 2016;6:35202. doi: 10.1038/srep35202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lobo EP, Delic NC, Richardson A, Raviraj V, Halliday GM, Di Girolamo N, et al. Self-organized centripetal movement of corneal epithelium in the absence of external cues. Nat Commun. 2016;7:12388. doi: 10.1038/ncomms12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363(2):147–55. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 30.Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98(6):3156–61. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brzeszczynska J, Ramaesh K, Dhillon B, Ross JA. Molecular profile of organ culture-stored corneal epithelium: LGR5 is a potential new phenotypic marker of residual human corneal limbal epithelial stem cells. Int J Mol Med. 2012;29(5):871–6. doi: 10.3892/ijmm.2012.904. [DOI] [PubMed] [Google Scholar]
- 32.Lu R, Qu Y, Ge J, Zhang L, Su Z, Pflugfelder SC, et al. Transcription factor TCF4 maintains the properties of human corneal epithelial stem cells. Stem Cells. 2012;30(4):753–61. doi: 10.1002/stem.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horenstein AL, Sizzano F, Lusso R, Besso FG, Ferrero E, Deaglio S, et al. CD38 and CD157 ectoenzymes mark cell subsets in the human corneal limbus. Mol Med. 2009;15(3–4):76–84. doi: 10.2119/molmed.2008.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayashi R, Yamato M, Saito T, Oshima T, Okano T, Tano Y, et al. Enrichment of corneal epithelial stem/progenitor cells using cell surface markers, integrin alpha6 and CD71. Biochem Biophys Res Commun. 2008;367(2):256–63. doi: 10.1016/j.bbrc.2007.12.077. [DOI] [PubMed] [Google Scholar]
- 35.Qi H, Li DQ, Shine HD, Chen Z, Yoon KC, Jones DB, et al. Nerve growth factor and its receptor TrkA serve as potential markers for human corneal epithelial progenitor cells. Exp Eye Res. 2008;86(1):34–40. doi: 10.1016/j.exer.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi R, Yamato M, Sugiyama H, Sumide T, Yang J, Okano T, et al. N-Cadherin is expressed by putative stem/progenitor cells and melanocytes in the human limbal epithelial stem cell niche. Stem Cells. 2007;25(2):289–96. doi: 10.1634/stemcells.2006-0167. [DOI] [PubMed] [Google Scholar]
- 37.Budak MT, Alpdogan OS, Zhou M, Lavker RM, Akinci MA, Wolosin JM. Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. J Cell Sci. 2005;118(Pt 8):1715–24. doi: 10.1242/jcs.02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Paiva CS, Chen Z, Corrales RM, Pflugfelder SC, Li DQ. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005;23(1):63–73. doi: 10.1634/stemcells.2004-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida S, Shimmura S, Kawakita T, Miyashita H, Den S, Shimazaki J, et al. Cytokeratin 15 can be used to identify the limbal phenotype in normal and diseased ocular surfaces. Invest Ophthalmol Vis Sci. 2006;47(11):4780–6. doi: 10.1167/iovs.06-0574. [DOI] [PubMed] [Google Scholar]
- 40.Ahmad S, Kolli S, Li DQ, de Paiva CS, Pryzborski S, Dimmick I, et al. A putative role for RHAMM/HMMR as a negative marker of stem cell-containing population of human limbal epithelial cells. Stem Cells. 2008;26(6):1609–19. doi: 10.1634/stemcells.2007-0782. [DOI] [PubMed] [Google Scholar]
- 41.Chen Z, Evans WH, Pflugfelder SC, Li DQ. Gap junction protein connexin 43 serves as a negative marker for a stem cell-containing population of human limbal epithelial cells. Stem Cells. 2006;24(5):1265–73. doi: 10.1634/stemcells.2005-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ksander BR, Kolovou PE, Wilson BJ, Saab KR, Guo Q, Ma J, et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature. 2014;511(7509):353–7. doi: 10.1038/nature13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jongkhajornpong P, Nakamura T, Sotozono C, Nagata M, Inatomi T, Kinoshita S. Elevated expression of ABCB5 in ocular surface squamous neoplasia. Sci Rep. 2016;6:20541. doi: 10.1038/srep20541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kureshi AK, Dziasko M, Funderburgh JL, Daniels JT. Human corneal stromal stem cells support limbal epithelial cells cultured on RAFT tissue equivalents. Sci Rep. 2015;5:16186. doi: 10.1038/srep16186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parfitt GJ, Kavianpour B, Wu KL, Xie Y, Brown DJ, Jester JV. Immunofluorescence tomography of mouse ocular surface epithelial stem cells and their niche microenvironment. Invest Ophthalmol Vis Sci. 2015;56(12):7338–44. doi: 10.1167/iovs.15-18038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaharuddin B, Ahmad S, Md Latar N, Ali S, Meeson A. A human corneal epithelial cell line model for limbal stem cell biology and limbal immunobiology. Stem Cells Transl Med. 2017;6(3):761–6. doi: 10.5966/sctm.2016-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mathan JJ, Ismail S, McGhee JJ, McGhee CN, Sherwin T. Sphere-forming cells from peripheral cornea demonstrate the ability to repopulate the ocular surface. Stem Cell Res Ther. 2016;7(1):81. doi: 10.1186/s13287-016-0339-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaharuddin B, Osei-Bempong C, Ahmad S, Rooney P, Ali S, Oldershaw R, et al. Human limbal mesenchymal stem cells express ABCB5 and can grow on amniotic membrane. Regen Med. 2016;11(3):273–86. doi: 10.2217/rme-2016-0009. [DOI] [PubMed] [Google Scholar]
- 49.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–40. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell. 2007;128(3):445–58. doi: 10.1016/j.cell.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen W, Hara K, Tian Q, Zhao K, Yoshitomi T. Existence of small slow-cycling Langerhans cells in the limbal basal epithelium that express ABCG2. Exp Eye Res. 2007;84(4):626–34. doi: 10.1016/j.exer.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 52.Li W, Hayashida Y, He H, Kuo CL, Tseng SC. The fate of limbal epithelial progenitor cells during explant culture on intact amniotic membrane. Invest Ophthalmol Vis Sci. 2007;48(2):605–13. doi: 10.1167/iovs.06-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polisetti N, Zenkel M, Menzel-Severing J, Kruse FE, Schlotzer-Schrehardt U. Cell adhesion molecules and stem cell niche interactions in the limbal stem cell niche. Stem Cells. 2016;34(1):203–19. doi: 10.1002/stem.2191. [DOI] [PubMed] [Google Scholar]
- 54.Gage PJ, Rhoades W, Prucka SK, Hjalt T. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci. 2005;46(11):4200–8. doi: 10.1167/iovs.05-0691. [DOI] [PubMed] [Google Scholar]
- 55.Li GG, Zhu YT, Xie HT, Chen SY, Tseng SC. Mesenchymal stem cells derived from human limbal niche cells. Invest Ophthalmol Vis Sci. 2012;53(9):5686–97. doi: 10.1167/iovs.12-10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polisetty N, Fatima A, Madhira SL, Sangwan VS, Vemuganti GK. Mesenchymal cells from limbal stroma of human eye. Mol Vis. 2008;14:431–42. [PMC free article] [PubMed] [Google Scholar]
- 57.Vereb Z, Poliska S, Albert R, Olstad OK, Boratko A, Csortos C, et al. Role of human corneal stroma-derived mesenchymal-like stem cells in corneal immunity and wound healing. Sci Rep. 2016;6:26227. doi: 10.1038/srep26227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dravida S, Pal R, Khanna A, Tipnis SP, Ravindran G, Khan F. The transdifferentiation potential of limbal fibroblast-like cells. Brain Res Dev Brain Res. 2005;160(2):239–51. doi: 10.1016/j.devbrainres.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 59.Du Y, Funderburgh ML, Mann MM, SundarRaj N, Funderburgh JL. Multipotent stem cells in human corneal stroma. Stem Cells. 2005;23(9):1266–75. doi: 10.1634/stemcells.2004-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katikireddy KR, Dana R, Jurkunas UV. Differentiation potential of limbal fibroblasts and bone marrow mesenchymal stem cells to corneal epithelial cells. Stem Cells. 2014;32(3):717–29. doi: 10.1002/stem.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Funderburgh JL, Funderburgh ML, Du Y. Stem cells in the limbal stroma. Ocul Surf. 2016;14(2):113–20. doi: 10.1016/j.jtos.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dziasko MA, Tuft SJ, Daniels JT. Limbal melanocytes support limbal epithelial stem cells in 2D and 3D microenvironments. Exp Eye Res. 2015;138:70–9. doi: 10.1016/j.exer.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 63.Higa K, Shimmura S, Miyashita H, Shimazaki J, Tsubota K. Melanocytes in the corneal limbus interact with K19-positive basal epithelial cells. Exp Eye Res. 2005;81(2):218–23. doi: 10.1016/j.exer.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 64.Dupin E, Sommer L. Neural crest progenitors and stem cells: from early development to adulthood. Dev Biol. 2012;366(1):83–95. doi: 10.1016/j.ydbio.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 65.Li W, Hayashida Y, Chen YT, Tseng SC. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007;17(1):26–36. doi: 10.1038/sj.cr.7310137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ljubimov AV, Burgeson RE, Butkowski RJ, Michael AF, Sun TT, Kenney MC. Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest. 1995;72(4):461–73. [PubMed] [Google Scholar]
- 67.Mei H, Gonzalez S, Deng SX. Extracellular matrix is an important component of limbal stem cell niche. J Funct Biomater. 2012;3(4):879–94. doi: 10.3390/jfb3040879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schlotzer-Schrehardt U, Dietrich T, Saito K, Sorokin L, Sasaki T, Paulsson M, et al. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp Eye Res. 2007;85(6):845–60. doi: 10.1016/j.exer.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 69.Tuori A, Uusitalo H, Burgeson RE, Terttunen J, Virtanen I. The immunohistochemical composition of the human corneal basement membrane. Cornea. 1996;15(3):286–94. doi: 10.1097/00003226-199605000-00010. [DOI] [PubMed] [Google Scholar]
- 70.Vantrappen L, Geboes K, Missotten L, Maudgal PC, Desmet V. Lymphocytes and Langerhans cells in the normal human cornea. Invest Ophthalmol Vis Sci. 1985;26(2):220–5. [PubMed] [Google Scholar]
- 71.Hay ED, Linsenmayer TF, Trelstad RL, von der Mark K. Origin and distribution of collagens in the developing avian cornea. Curr Top Eye Res. 1979;1:1–35. [PubMed] [Google Scholar]
- 72.Grindley JC, Davidson DR, Hill RE. The role of Pax-6 in eye and nasal development. Development. 1995;121(5):1433–42. doi: 10.1242/dev.121.5.1433. [DOI] [PubMed] [Google Scholar]
- 73.Li G, Xu F, Zhu J, Krawczyk M, Zhang Y, Yuan J, et al. Transcription factor PAX6 (Paired Box 6) controls limbal stem cell lineage in development and disease. J Biol Chem. 2015;290(33):20448–54. doi: 10.1074/jbc.M115.662940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramaesh T, Collinson JM, Ramaesh K, Kaufman MH, West JD, Dhillon B. Corneal abnormalities in Pax6+/− small eye mice mimic human aniridia-related keratopathy. Invest Ophthalmol Vis Sci. 2003;44(5):1871–8. doi: 10.1167/iovs.02-0576. [DOI] [PubMed] [Google Scholar]
- 75.Di Iorio E, Barbaro V, Ruzza A, Ponzin D, Pellegrini G, De Luca M. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci U S A. 2005;102(27):9523–8. doi: 10.1073/pnas.0503437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398(6729):714–8. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 77.Ince TA, Cviko AP, Quade BJ, Yang A, McKeon FD, Mutter GL, et al. p63 Coordinates anogenital modeling and epithelial cell differentiation in the developing female urogenital tract. Am J Pathol. 2002;161(4):1111–7. doi: 10.1016/S0002-9440(10)64387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Di Iorio E, Kaye SB, Ponzin D, Barbaro V, Ferrari S, Bohm E, et al. Limbal stem cell deficiency and ocular phenotype in ectrodactyly-ectodermal dysplasia-clefting syndrome caused by p63 mutations. Ophthalmology. 2012;119(1):74–83. doi: 10.1016/j.ophtha.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 79.Pignon JC, Grisanzio C, Geng Y, Song J, Shivdasani RA, Signoretti S. p63-expressing cells are the stem cells of developing prostate, bladder, and colorectal epithelia. Proc Natl Acad Sci U S A. 2013;110(20):8105–10. doi: 10.1073/pnas.1221216110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gage PJ, Kuang C, Zacharias AL. The homeodomain transcription factor PITX2 is required for specifying correct cell fates and establishing angiogenic privilege in the developing cornea. Dev Dyn. 2014;243(11):1391–400. doi: 10.1002/dvdy.24165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seo S, Singh HP, Lacal PM, Sasman A, Fatima A, Liu T, et al. Forkhead box transcription factor FoxC1 preserves corneal transparency by regulating vascular growth. Proc Natl Acad Sci U S A. 2012;109(6):2015–20. doi: 10.1073/pnas.1109540109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443(7114):993–7. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schatton T, Yang J, Kleffel S, Uehara M, Barthel SR, Schlapbach C, et al. ABCB5 identifies immunoregulatory dermal cells. Cell Rep. 2015;12(10):1564–74. doi: 10.1016/j.celrep.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24(10):1442–3. [PubMed] [Google Scholar]
- 85.Yoon JJ, Ismail S, Sherwin T. Limbal stem cells: Central concepts of corneal epithelial homeostasis. World J Stem Cells. 2014;6(4):391–403. doi: 10.4252/wjsc.v6.i4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chang CY, Green CR, McGhee CN, Sherwin T. Acute wound healing in the human central corneal epithelium appears to be independent of limbal stem cell influence. Invest Ophthalmol Vis Sci. 2008;49(12):5279–86. doi: 10.1167/iovs.07-1260. [DOI] [PubMed] [Google Scholar]
- 87.Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Prog Retin Eye Res. 2015;49:17–45. doi: 10.1016/j.preteyeres.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao J, Mo V, Nagasaki T. Distribution of label-retaining cells in the limbal epithelium of a mouse eye. J Histochem Cytochem. 2009;57(2):177–85. doi: 10.1369/jhc.2008.952390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chikama T, Wakuta M, Liu Y, Nishida T. Deviated mechanism of wound healing in diabetic corneas. Cornea. 2007;26(9 Suppl 1):S75–81. doi: 10.1097/ICO.0b013e31812f6d8e. [DOI] [PubMed] [Google Scholar]
- 90.Kramerov AA, Saghizadeh M, Maguen E, Rabinowitz YS, Ljubimov AV. Persistence of reduced expression of putative stem cell markers and slow wound healing in cultured diabetic limbal epithelial cells. Mol Vis. 2015;21:1357–67. [PMC free article] [PubMed] [Google Scholar]
- 91.Lutty GA. Effects of diabetes on the eye. Invest Ophthalmol Vis Sci. 2013;54(14):ORSF81–7. doi: 10.1167/iovs.13-12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saghizadeh M, Soleymani S, Harounian A, Bhakta B, Troyanovsky SM, Brunken WJ, et al. Alterations of epithelial stem cell marker patterns in human diabetic corneas and effects of c-met gene therapy. Mol Vis. 2011;17:2177–90. [PMC free article] [PubMed] [Google Scholar]
- 93.Majo F, Rochat A, Nicolas M, Jaoude GA, Barrandon Y. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature. 2008;456(7219):250–4. doi: 10.1038/nature07406. [DOI] [PubMed] [Google Scholar]
- 94.Kramerov AA, Saghizadeh M, Ljubimov AV. Adenoviral gene therapy for diabetic keratopathy: effects on wound healing and stem cell marker expression in human organ-cultured corneas and limbal epithelial cells. J Vis Exp. 2016;(110):e54058. doi: 10.3791/54058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saghizadeh M, Dib CM, Brunken WJ, Ljubimov AV. Normalization of wound healing and stem cell marker patterns in organ-cultured human diabetic corneas by gene therapy of limbal cells. Exp Eye Res. 2014;129:66–73. doi: 10.1016/j.exer.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Winkler MA, Dib C, Ljubimov AV, Saghizadeh M. Targeting miR-146a to treat delayed wound healing in human diabetic organ-cultured corneas. PLoS One. 2014;9(12):e114692. doi: 10.1371/journal.pone.0114692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sotozono C, Kinoshita S, Kita M, Imanishi J. Paracrine role of keratinocyte growth factor in rabbit corneal epithelial cell growth. Exp Eye Res. 1994;59(4):385–91. doi: 10.1006/exer.1994.1122. [DOI] [PubMed] [Google Scholar]
- 98.Wilson SE, Walker JW, Chwang EL, He YG. Hepatocyte growth factor, keratinocyte growth factor, their receptors, fibroblast growth factor receptor-2, and the cells of the cornea. Invest Ophthalmol Vis Sci. 1993;34(8):2544–61. [PubMed] [Google Scholar]
- 99.Miyashita H, Yokoo S, Yoshida S, Kawakita T, Yamagami S, Tsubota K, et al. Long-term maintenance of limbal epithelial progenitor cells using rho kinase inhibitor and keratinocyte growth factor. Stem Cells Transl Med. 2013;2(10):758–65. doi: 10.5966/sctm.2012-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen J, Chen P, Backman LJ, Zhou Q, Danielson P. Ciliary Neurotrophic Factor promotes the migration of corneal epithelial stem/progenitor cells by up-regulation of MMPs through the phosphorylation of Akt. Sci Rep. 2016;6:25870. doi: 10.1038/srep25870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou Q, Chen P, Di G, Zhang Y, Wang Y, Qi X, et al. Ciliary neurotrophic factor promotes the activation of corneal epithelial stem/progenitor cells and accelerates corneal epithelial wound healing. Stem Cells. 2015;33(5):1566–76. doi: 10.1002/stem.1942. [DOI] [PubMed] [Google Scholar]
- 102.Trosan P, Svobodova E, Chudickova M, Krulova M, Zajicova A, Holan V. The key role of insulin-like growth factor I in limbal stem cell differentiation and the corneal wound-healing process. Stem Cells Dev. 2012;21(18):3341–50. doi: 10.1089/scd.2012.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Polisetti N, Agarwal P, Khan I, Kondaiah P, Sangwan VS, Vemuganti GK. Gene expression profile of epithelial cells and mesenchymal cells derived from limbal explant culture. Mol Vis. 2010;16:1227–40. [PMC free article] [PubMed] [Google Scholar]
- 104.Morita S, Shirakata Y, Shiraishi A, Kadota Y, Hashimoto K, Higashiyama S, et al. Human corneal epithelial cell proliferation by epiregulin and its cross-induction by other EGF family members. Mol Vis. 2007;13:2119–28. [PubMed] [Google Scholar]
- 105.Zhou M, Li XM, Lavker RM. Transcriptional profiling of enriched populations of stem cells versus transient amplifying cells. A comparison of limbal and corneal epithelial basal cells. J Biol Chem. 2006;281(28):19600–9. doi: 10.1074/jbc.M600777200. [DOI] [PubMed] [Google Scholar]
- 106.Chen JJ, Tseng SC. Corneal epithelial wound healing in partial limbal deficiency. Invest Ophthalmol Vis Sci. 1990;31(7):1301–14. [PubMed] [Google Scholar]
- 107.Baylis O, Figueiredo F, Henein C, Lako M, Ahmad S. 13 years of cultured limbal epithelial cell therapy: a review of the outcomes. J Cell Biochem. 2011;112(4):993–1002. doi: 10.1002/jcb.23028. [DOI] [PubMed] [Google Scholar]
- 108.Bobba S, Di Girolamo N, Mills R, Daniell M, Chan E, Harkin DG, et al. Nature and incidence of severe limbal stem cell deficiency in Australia and New Zealand. Clin Exp Ophthalmol. 2016 doi: 10.1111/ceo.12813. [DOI] [PubMed] [Google Scholar]
- 109.Chan CC, Holland EJ. Severe limbal stem cell deficiency from contact lens wear: patient clinical features. Am J Ophthalmol. 2013;155(3):544–9. e2. doi: 10.1016/j.ajo.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 110.Lim P, Fuchsluger TA, Jurkunas UV. Limbal stem cell deficiency and corneal neovascularization. Semin Ophthalmol. 2009;24(3):139–48. doi: 10.1080/08820530902801478. [DOI] [PubMed] [Google Scholar]
- 111.Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349(9057):990–3. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 112.Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343(2):86–93. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- 113.Prabhasawat P, Ekpo P, Uiprasertkul M, Chotikavanich S, Tesavibul N. Efficacy of cultivated corneal epithelial stem cells for ocular surface reconstruction. Clin Ophthalmol. 2012;6:1483–92. doi: 10.2147/OPTH.S33951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cheng J, Zhai H, Wang J, Duan H, Zhou Q. Long-term outcome of allogeneic cultivated limbal epithelial transplantation for symblepharon caused by severe ocular burns. BMC Ophthalmol. 2017;17(1):8. doi: 10.1186/s12886-017-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3(11):879–89. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 116.Holan V, Pokorna K, Prochazkova J, Krulova M, Zajicova A. Immunoregulatory properties of mouse limbal stem cells. J Immunol. 2010;184(4):2124–9. doi: 10.4049/jimmunol.0903049. [DOI] [PubMed] [Google Scholar]
- 117.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2(2):116–26. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 118.Hori J, Wang M, Miyashita M, Tanemoto K, Takahashi H, Takemori T, et al. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J Immunol. 2006;177(9):5928–35. doi: 10.4049/jimmunol.177.9.5928. [DOI] [PubMed] [Google Scholar]
- 119.Watson MP, George AJ, Larkin DF. Differential effects of costimulatory pathway modulation on corneal allograft survival. Invest Ophthalmol Vis Sci. 2006;47(8):3417–22. doi: 10.1167/iovs.05-1597. [DOI] [PubMed] [Google Scholar]
- 120.Shen L, Jin Y, Freeman GJ, Sharpe AH, Dana MR. The function of donor versus recipient programmed death-ligand 1 in corneal allograft survival. J Immunol. 2007;179(6):3672–9. doi: 10.4049/jimmunol.179.6.3672. [DOI] [PubMed] [Google Scholar]
- 121.Ahmad S, Stewart R, Yung S, Kolli S, Armstrong L, Stojkovic M, et al. Differentiation of human embryonic stem cells into corneal epithelial-like cells by in vitro replication of the corneal epithelial stem cell niche. Stem Cells. 2007;25(5):1145–55. doi: 10.1634/stemcells.2006-0516. [DOI] [PubMed] [Google Scholar]
- 122.Hayashi R, Ishikawa Y, Sasamoto Y, Katori R, Nomura N, Ichikawa T, et al. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature. 2016;531(7594):376–80. doi: 10.1038/nature17000. [DOI] [PubMed] [Google Scholar]
- 123.Shalom-Feuerstein R, Serror L, De La Forest Divonne S, Petit I, Aberdam E, Camargo L, et al. Pluripotent stem cell model reveals essential roles for miR-450b-5p and miR-184 in embryonic corneal lineage specification. Stem Cells. 2012;30(5):898–909. doi: 10.1002/stem.1068. [DOI] [PubMed] [Google Scholar]
- 124.Gu S, Xing C, Han J, Tso MO, Hong J. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Mol Vis. 2009;15:99–107. [PMC free article] [PubMed] [Google Scholar]
- 125.Blazejewska EA, Schlotzer-Schrehardt U, Zenkel M, Bachmann B, Chankiewitz E, Jacobi C, et al. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells. 2009;27(3):642–52. doi: 10.1634/stemcells.2008-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ouyang H, Xue Y, Lin Y, Zhang X, Xi L, Patel S, et al. WNT7A and PAX6 define corneal epithelium homeostasis and pathogenesis. Nature. 2014;511(7509):358–61. doi: 10.1038/nature13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kitazawa K, Hikichi T, Nakamura T, Mitsunaga K, Tanaka A, Nakamura M, et al. OVOL2 maintains the transcriptional program of human corneal epithelium by suppressing epithelial-to-mesenchymal transition. Cell Rep. 2016;15(6):1359–68. doi: 10.1016/j.celrep.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 128.Sasamoto Y, Hayashi R, Park SJ, Saito-Adachi M, Suzuki Y, Kawasaki S, et al. PAX6 isoforms, along with reprogramming factors, differentially regulate the induction of cornea-specific genes. Sci Rep. 2016;6:20807. doi: 10.1038/srep20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gomes JA, Geraldes Monteiro B, Melo GB, Smith RL, Cavenaghi Pereira da Silva M, Lizier NF, et al. Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Invest Ophthalmol Vis Sci. 2010;51(3):1408–14. doi: 10.1167/iovs.09-4029. [DOI] [PubMed] [Google Scholar]
- 130.Kobayashi M, Nakamura T, Yasuda M, Hata Y, Okura S, Iwamoto M, et al. Ocular surface reconstruction with a tissue-engineered nasal mucosal epithelial cell sheet for the treatment of severe ocular surface diseases. Stem Cells Transl Med. 2015;4(1):99–109. doi: 10.5966/sctm.2014-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nakatsu MN, Deng SX. Enrichment of human corneal epithelial stem/progenitor cells by magnetic bead sorting using SSEA4 as a negative marker. Methods Mol Biol. 2013;1014:71–7. doi: 10.1007/978-1-62703-432-6_5. [DOI] [PubMed] [Google Scholar]
- 132.Lyngholm M, Vorum H, Nielsen K, Ostergaard M, Honore B, Ehlers N. Differences in the protein expression in limbal versus central human corneal epithelium--a search for stem cell markers. Exp Eye Res. 2008;87(2):96–105. doi: 10.1016/j.exer.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 133.Umemoto T, Yamato M, Nishida K, Kohno C, Yang J, Tano Y, et al. Rat limbal epithelial side population cells exhibit a distinct expression of stem cell markers that are lacking in side population cells from the central cornea. FEBS Lett. 2005;579(29):6569–74. doi: 10.1016/j.febslet.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 134.Umemoto T, Yamato M, Nishida K, Yang J, Tano Y, Okano T. Limbal epithelial side-population cells have stem cell-like properties, including quiescent state. Stem Cells. 2006;24(1):86–94. doi: 10.1634/stemcells.2005-0064. [DOI] [PubMed] [Google Scholar]
- 135.Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li DQ. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22(3):355–66. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]