Abstract
Objective
Research on executive functions (EFs) has revealed evidence for general abilities that underlie performance across multiple EF tasks and domains. This Common EF factor is highly stable in adolescence through young adulthood, correlates with other important cognitive abilities, and is explained largely by genetic influences. However, little is known about Common EF beyond young adulthood. This study examines three hypotheses regarding the latent structure, genetic/environmental etiology, and cognitive correlates of Common EF in middle-age.
Method
We examined data from 1,284 middle-aged twins (51–60 years) in the Vietnam Era Twin Study of Aging who completed seven neuropsychological measures of EFs, as well as measures of general cognitive ability and processing speed.
Results
Our confirmatory factor analysis indicated that Common EF explained variation across all seven EF tasks. Inhibition and shifting were subsumed entirely under the Common EF factor, and there was an additional working memory span-specific factor. Common EF was heritable in midlife (a2 = .46), with additional evidence for both shared environmental influences (c2 = .41) and nonshared environmental influences (e2 = .13). Higher Common EF was moderately associated with higher general cognitive ability, measured both in early adulthood and midlife, and faster processing speed in midlife. These correlations were primarily driven by shared genetic influences.
Conclusions
These results support the hypothesis that Common EF captures similar EF abilities in midlife as in adolescence and young adulthood. However, environmental influences may explain a larger portion of variance in this construct as individuals age.
Keywords: cognitive control, executive control, heritability, twin study, intelligence
Executive functions (EFs) are all-purpose cognitive control processes that contribute to the regulation of behavior and support active goal maintenance, management, and implementation (Friedman et al., 2008; Miyake & Friedman, 2012; Miyake et al., 2000). There are a number of distinct yet correlated EFs, including prepotent response inhibition (hereafter, inhibition), working memory updating (WM updating), and mental-set shifting (Miyake et al., 2000). These EFs predict a number of important life outcomes including age-related declines in other cognitive abilities, internalizing and externalizing psychopathology, and everyday self-regulation (Caspi et al., 2014; Gustavson, Miyake, Hewitt, & Friedman, 2015; Hasher & Zacks, 1988; Hertzog, Dixon, Hultsch, & MacDonald, 2003; Ito et al., 2015; Young et al., 2009). Importantly, work by Miyake and Friedman in younger adults has highlighted that there is substantial shared variation that underlies each of these distinct EFs, and suggests that a “Common EF” factor is largely responsible for the links between EFs and other outcomes (Friedman & Miyake, 2017; Friedman et al., 2008; Gustavson et al., 2015; Herd et al., 2014; Ito et al., 2015; Miyake & Friedman, 2012; Miyake et al., 2000).
Large-scale studies examining latent EFs in middle-aged or older adults are almost entirely absent (de Frias, Dixon, & Strauss, 2006; Vaughan & Giovanello, 2010). Individual neuropsychological EF tasks such as the Stroop task and Trail Making Test have been studied extensively in aging research, and span a wide range of EF abilities (Demakis, 2004; Ohman, Savikko, Strandberg, & Pitkala, 2014; Vasilopoulos et al., 2012). However, it is unclear the extent to which these different EF abilities are captured by Common EF in later stages of adulthood, whether Common EF is similarly correlated with other well-studied cognitive abilities in midlife, and whether the genetic/environmental etiology of Common EF in midlife is similar to that at earlier ages.
The Unity and Diversity of Executive Functions
The model of EF tested in this study is an extension of the seminal work of Miyake et al (2000), who examined the latent variable associations between three EF constructs: inhibition, WM updating, and shifting. The authors used a battery of nine EF tasks (three for each EF) to show that these three EFs were all substantially correlated with one another at the level of latent variables, but represented separable processes (rs = .42 to .63). Many subsequent studies have examined how these EF latent factors are associated with a wide range of outcomes (for reviews and updates, see Friedman & Miyake, 2017; Miyake & Friedman, 2012). However, a limitation to the approach of studying these three EFs separately is that it is unclear whether associations between any given EF and an outcome are due to the shared abilities that underlie multiple EFs (i.e., Common EF), or abilities specific to individual EF (e.g., Shifting-Specific abilities).
The unity and diversity framework of EFs (Friedman & Miyake, 2017; Friedman et al., 2016; Friedman et al., 2008; Miyake & Friedman, 2012) is a reparameterization of the original correlated-factors model. It decomposes variance of EF tasks into three latent factors: Common EF, Updating-Specific, and Shifting-Specific. Because Common EF represents the shared variation across all EFs, this factor is thought to reflect goal maintenance, management, and implementation (Friedman & Miyake, 2017; Miyake & Friedman, 2012). Updating-Specific is thought to reflect the gating of information in WM, and Shifting-Specific is thought to reflect mental flexibility, or the ability to replace one’s goals after they are no longer necessary. As yet, there is no evidence for an Inhibition-Specific factor, suggesting that inhibition is entirely encapsulated by Common EF (Friedman et al., 2016; Friedman et al., 2008; Ito et al., 2015).
Common EF has been studied in multiple genetically informative samples, and it has been shown to be highly heritable in the first few decades of life. For example, one study showed that a latent factor representing Common EF was explained exclusively by genetic influences (heritability of a2 = 1.0) in a sample of third to eighth grade students (Engelhardt, Briley, Mann, Harden, & Tucker-Drob, 2015). Friedman et al. (2016) also showed that Common EF was highly heritable in late adolescence (a2 = .96; mean age 17 years) and young adulthood (a2 = .81; mean age 23 years), and that the genetic influences were identical across time (genetic correlation [rg] = 1.0). In this same sample of adolescents, the genetic influences that supported greater Common EF were also associated with higher levels of intelligence (rg = .57), and faster processing speed (rg = .67), suggesting that these Common EF abilities are also partially related to, but nevertheless distinct from, these other important cognitive processes (Friedman et al., 2008).
These studies suggest that shared and nonshared environmental influences play little-to-no role in childhood, but explain a combined 4% of the variation in Common EF in adolescence and as much as 20% in young adulthood (Engelhardt et al., 2015; Friedman et al., 2016; Friedman et al., 2008). Therefore, although stable genetic influences on Common EF explain most of its variation in early life, environmental influences may play a larger role in Common EF later in life.
Executive Functions in Mid-to-Late Life
Despite advancements in understanding the etiology and correlates of Common EF in childhood through young adulthood, this framework has not yet been applied to a sample of older adults in mid- or late-life. Nevertheless, this framework is consistent with prevailing views on the structure and organization on EFs in later life. For example, Hasher and Zacks have proposed that general inhibition processes underlie the age-related decline in WM (Hasher & Zacks, 1988; Lustig, Hasher, & Zacks, 2007). This proposal is consistent with the observation that response inhibition is indistinguishable from Common EF in the unity and diversity model of EF, as well as findings that Common EF is substantially recruited in WM and shifting tasks, and is correlated with other general measures of cognition and speed (Friedman et al., 2008). Later work by the same group has revealed that inhibition may also account for the decline in other cognitive abilities in aging, including verbal learning and attention (Persad, Abeles, Zacks, & Denburg, 2002), suggesting that the moderate associations between Common EF and other important cognitive abilities are also stable over time. Similarly, in work on context processing in older adults, others have claimed that general mechanisms underlie the working memory and cognitive control components of context processing (Braver, Satpute, Rush, Racine, & Barch, 2005), and may be executed in the same brain regions thought to support Common EF (Braver, 2012; Friedman & Miyake, 2017).
One study has examined the phenotypic associations between latent factors for inhibition, updating, and shifting in a sample of 95 older adults (aged 60–90 years), and revealed strong correlations among the EF factors (rs = .61 to .81), suggesting that common processes continue to underlie the variation in EF in middle or late adulthood (Vaughan & Giovanello, 2010). Here, we extend this work by examining EF in midlife using the unity and diversity model. Additionally, we examine the genetic and environmental etiology of these latent EF factors, and their correlations with other relevant cognitive variables.
The heritabilities of individual neuropsychological tests such as the Stroop, AX-CPT, Trail Making Test, and WM span tests have been established (e.g., Kremen, Moore, Franz, Panizzon, & Lyons, 2014; Kremen et al., 2011; Vasilopoulos et al., 2012), and their genetic correlations with measures of general cognitive ability are similar to those between Common EF and intelligence (Friedman et al., 2008; Kremen et al., 2011). Although no studies have investigated the heritability of a latent Common EF factor in midlife, one study found evidence for common genetic influences underlying general cognitive ability and four EF tasks (from various domains including inhibition, WM span, and shifting) in older adult twins aged 65–88 years (Lee et al., 2012). However, with only four tests, there is very limited ability to detect any EF-specific factors.
Hypotheses of the Current Study
In the current study, we examined three hypotheses regarding Common EF in midlife in 1,284 middle-aged male twins from the Vietnam Era Twin Study of Aging (VETSA). Individuals completed seven standard neuropsychological measures of EF, as well as measures of general cognitive ability and processing speed. To a large extent, our neuropsychological EF tasks overlap with the experimental cognitive tasks used in previous multivariate genetic studies (Friedman et al., 2016; Friedman et al., 2008; Ito et al., 2015). Our WM tasks emphasize WM span more so than WM updating. Therefore, in the present study we refer to a WM factor rather than a WM updating factor.
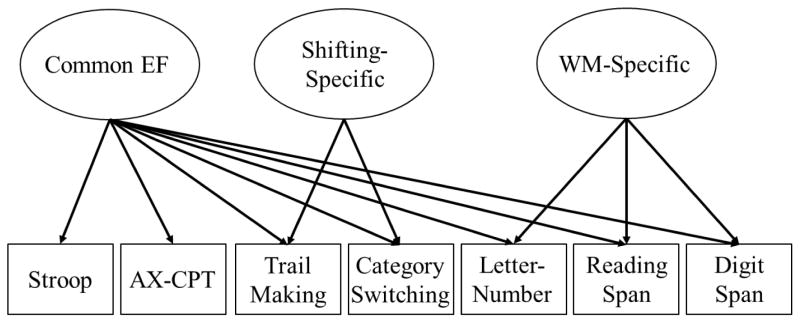
We hypothesized that we would observe evidence for the unity and diversity model of EF across the seven neuropsychological EF tasks. This hypothesized model is displayed in Figure 1. Based on previous work, we expected to observe a Common EF factor as well as evidence for a WM-Specific factor and a Shifting-Specific factor, but not an Inhibition-Specific factor. The second hypothesis is that the latent Common EF factor would be explained substantially by genetic influences. The third hypothesis tested was that Common EF would be moderately correlated with general cognitive ability and faster processing speed, and that these associations would be driven by shared genetic influences. In these analyses, we examined general cognitive ability assessed in both in midlife and in early adulthood.
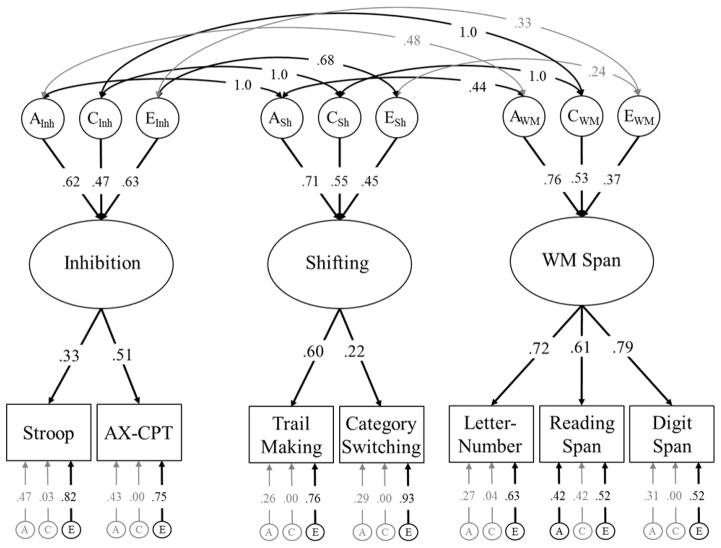
Figure 1.
Unity and diversity model of EF tested in the current study. Ellipses represent latent variables, and rectangles represent measured variables. Based on previous work using this model, we expected that a Common EF factor would underlie performance on all seven neuropsychological tasks. Additionally, there would be orthogonal Shifting-Specific and WM-Specific factors explaining variation in the shifting tasks and WM span tasks (respectively) above and beyond the Common EF factor. As yet, there is no evidence for an Inhibition-Specific factor so we did not expect to observe it here. Task-specific residual variances are estimated but not shown.
Method
Participants
Analyses were based on 1,284 male twins from 364 monozygotic [MZ] twin pairs, 273 dizygotic [DZ] twin pairs, and 10 unpaired twins who completed the first wave of assessment of the longitudinal VETSA study in midlife (M = 55.90 years, SD = 2.44 years). VETSA participants were randomly selected from the Vietnam Era Twin Registry from a previous study on psychological health (Tsuang, Bar, Harley, & Lyons, 2001), and they are largely representative of American men in their age group in terms of health and lifestyle characteristics (Kremen et al., 2006; Schoenborn & Heyman, 2009). All individuals in the study served in the United States military at some point between 1965 and 1975, but nearly 80% reported no combat exposure.
Measures
Each of the dependent measures was residualized after accounting for the effect of age because many of the measures were significantly correlated with age (see Appendix A). However, the results were qualitatively similar to those based on non-age-residualized scores. All cognitive measures were scaled so that higher numbers indicate better performance.
Inhibition
Stroop
We used the Golden and Freshwater (2002) verson of the Stroop task (Stroop, 1935). The dependent measure was a residualized score for the number of correct words identified during the color-word condition (naming colors of words printed in incongruent colors), adjusting for performance on the word condition (reading color words printed in black ink) and the color condition (naming the color of printed strings of Xs).
AX-Continuous Performance Test (CPT)
In the AX-CPT (Braver et al., 2001; Kremen et al., 2011), participants saw a series of letters displayed in the center of a computer screen one at a time. During 70% of the 150 trials, the letter A cue was followed by the letter X probe, for which participants were instructed to press the left mouse button. This task set-up built up a strong prepotent response to respond to all X cues with the target response. On the other 30% of trials (where participants were instructed to respond with the right mouse button), a non-A cue is followed by the X probe (10% BX trials), a valid A cue is followed by a non-X probe (10% AY trials), or a non-A cue is followed by a non-X probe (10% BY trials).
As described in previous work on this sample (Kremen et al., 2011), the key measure of prepotent response inhibition was a signal detection index (d′) comparing the hit rate for AX trials minus the false alarm rate for BX trials, with correction factors to avoid dividing by zero (Corwin, 1994). Here we modified the dependent measure in two ways to improve the distributional characteristics of this measure: (a) we used an arcsine transformation, which is often used to improve measures with ceiling effects, and (b) we trimmed all d′ prime values less than 0 to 0 (46 participants total) to reduce the tail of the distribution. However, we note that these manipulations to improve normality did not affect the patterns of results described below.
Working memory span
Letter-number sequencing
The dependent measure was the total number of trials passed on this Wechsler Memory Scale-III (Wechsler, 1997) subtest in which the task was to re-sequence letter-number strings of increasing size with the numbers in ascending order, followed by the letters in alphabetical order.
Reading span
In the reading span task (Daneman & Carpenter, 1980), participants read multiple sentences aloud, presented one at a time on a computer screen. After reading sentences aloud, participants were instructed to recall the last word of each sentence in order. All participants completed 5 trials each of length 2, 3, and 4 sentences. The dependent measure was the total number of correct words recalled across the entire task regardless of order.
Digit span
We used the Wechsler Memory Scale-III (Wechsler, 1997) digit span subtest. The dependent measure was the total number of correct trials across both the forward and backward conditions.1
Shifting
Trail Making Test
From the Delis-Kaplan Executive Function System Trail Making Test (D-KEFS; Delis, Kaplan, & Kramer, 2001), we used condition 2 (number sequencing), condition 3 (letter sequencing), and condition 4 (letter-number switching). The dependent measure was the time for the switching trial after residualizing the time on the single-task trials.
Category switching
From the D-KEFS verbal fluency test (Delis et al., 2001), we used two category fluency trials (animals and boys’ names), and the category switching condition in which participants had to alternate between naming fruits and furniture. Category switching accuracy was scored as the total number of times that the participant correctly switched between categories. The dependent measure was based on a residualized score for category switching accuracy (number of correct switches) after adjusting for the number of correct responses across the category fluency trials.
General cognitive ability
General cognitive ability was measured with the Armed Forces Qualification Test (AFQT; Bayroff & Anderson, 1963). The AFQT is a 100-question multiple choice paper-and-pencil test. The AFQT was administered both in early adulthood (mean age 20) and again in VETSA (mean age 56). AFQT scores were highly stable over the approximately 35-year interval between assessments (r = .74) and correlated highly with traditional measures of IQ and general cognitive ability (Lyons et al., 2009). AFQT percentile scores were transformed based on military norms to normalize the distribution.
Processing speed
Processing speed was assessed with two reaction time (RT)-based computer tasks.
Simple RT
Participants had to press the D key on the computer keyboard as soon as they saw a star on the left side of the screen. They then had to press the L key when they saw as star on the right side of the screen. There were 10 trials on each side. The dependent measure was the average RT.
Choice RT
The choice RT task was completed immediately after the simple RT task. In this task, participants again saw a cue in the center of the screen, but this time the star appeared on either the left of right side of the screen in random order. Participants had to respond with the D or L keys to indicate the location of the star. The dependent measure was the average RT across 21 trials.
Data Analysis
All analyses were conducted with the OpenMX package in R (Boker et al., 2011), which accounts for missing observations using full-information maximum likelihood. Model fit for all common pathway models was determined acceptable if the model did not fit significantly worse than a full genetic Cholesky decomposition by comparing the -2 log-likelihood values (−2LL) using χ2 difference tests (χ2diff). Additionally, we used the Akaike information criterion (AIC), Bayesian information criterion (BIC), and the Root Mean Square Error of Approximation (RMSEA). In all cases, models with lower values are more parsimonious. Additionally, RMSEA values less than .06 indicate good fit (Hu & Bentler, 1998). Significance of individual parameters was also established with χ2 difference tests (by fixing those parameters to zero) or with 95% confidence intervals (95% CI).
All genetic models were based on the standard assumptions in twin designs. First, additive genetic influences (A) are assumed to correlate 1.0 in MZ twin pairs and 0.5 in DZ twin pairs because MZ twins share all their alleles identical-by-descent, whereas DZ twins share, on average, half of their alleles identical-by-decent. Shared environmental influences (C) are assumed to correlate 1.0 for both MZ and DZ twins. These are defined as environmental factors that make twins similar to one another. Nonshared environmental influences (E) do not correlate between MZ or DZ twins. These are defined as environmental factors that make twins uncorrelated, and they also include measurement error. Finally, we assume equal means and variances across twins within pairs and across zygosity. The same assumptions apply to multivariate model that also account for genetic/environmental correlations between constructs. However, variance in latent variables should be virtually free from measurement error (Bollen, 1989), so estimates of nonshared environmental influences on latent variables should reflect true environmental variance rather than a combination of true variance and measurement error.
Results
Preliminary Analyses
Descriptive statistics for all measures are displayed in Table 1 (before being age-residualized based). The full phenotypic correlation matrix between all measures is displayed in Table A1 (below the diagonal). As expected, all EF tasks were correlated positively with one another, rs = .06 – .59, and most were correlated with the measures of general cognitive ability and processing speed, rs = .01 – .40. The univariate heritability estimates for most of the measures described here were reported elsewhere (Kremen et al., 2014) so they are not described in detail here. However, these estimates can be computed from the MZ and DZ cross-twin correlations presented along the diagonal in Table A1 (cross-twin cross-trait correlations are also presented above the diagonal).
Table 1.
Descriptive Statistics for All Measures
| Task | N | M | SD | Range | Skewness | Kurtosis |
|---|---|---|---|---|---|---|
| Age | 1284 | 55.90 | 2.44 | 51.08 – 60.67 | 0.06 | −1.72 |
| Inhibition | ||||||
| Stroop Color-Word | 1255 | 35.95 | 8.33 | 6 – 69 | 0.05 | 0.30 |
| Word Only | 1258 | 93.50 | 14.31 | 49 – 140 | 0.05 | −0.07 |
| Color Only | 1256 | 69.26 | 11.30 | 28 – 105 | 0.14 | 0.05 |
| AX-CPT d′ | 1190 | 0.99 | 0.37 | 0 – 1.39 | −1.35 | 1.06 |
| Updating | ||||||
| Letter-Number | 1280 | 10.13 | 2.36 | 0 – 20 | 0.22 | 1.02 |
| Reading Span | 1248 | 34.14 | 5.33 | 15 – 45 | −0.54 | 0.05 |
| Digit Span | 1276 | 17.11 | 3.90 | 8 – 30 | 0.29 | −0.31 |
| Shifting | ||||||
| Trail-Making Test Trial 4 | 1271 | 89.15 | 35.05 | 31.34 – 240.00 | 1.68 | 3.87 |
| Trial 2 - Number Only | 1273 | 33.45 | 12.36 | 12.98 – 150.00 | 2.46 | 12.77 |
| Trial 3 - Letter Only | 1273 | 33.74 | 13.69 | 12.03 – 150.00 | 2.85 | 15.85 |
| Category Switching Accuracy | 1276 | 11.52 | 3.04 | 1 – 21 | −0.29 | 0.46 |
| Category Fluency | 1277 | 38.29 | 7.53 | 15 – 70 | 0.30 | 0.34 |
| Intelligence | ||||||
| AFQT - Age 20 | 1265 | 0.35 | 0.68 | −1.29 – 2.32 | 0.12 | −0.37 |
| AFQT - Age 55 | 1282 | 0.43 | 0.64 | −1.76 – 2.05 | −0.10 | −0.34 |
| Processing Speed | ||||||
| Simple RT | 1182 | 285.59 | 30.17 | 211.97 – 411.86 | 0.53 | 0.39 |
| Choice RT | 1177 | 345.75 | 35.40 | 254.04 – 596.13 | 0.78 | 2.32 |
Note: All dependent measures are reported before creating standardized residuals controlling for age.
These basic analyses justified fitting the confirmatory common pathway models of EF, including the hypothesized unity and diversity model (from Figure 1). In addition to the unity and diversity model, we also fit two alternative common pathway models: (a) a three factor model akin to Miyake et al.’s (2000) model of Inhibition, Shifting, and WM Updating (WM Span in our model), and (b) a hierarchical model in which the shared variance between the Inhibition, WM Span, and Shifting latent variables is captured by a superordinate Common EF latent variable. These models are displayed in Appendix B. Model fit statistics are described in Table 2, alongside the fit statistics for the full genetic Cholesky decomposition and the unity and diversity models described below. In summary, both alternative models provided an adequate fit to the data. However, the unity and diversity model provided a more parsimonious fit, and was predicted a priori. Therefore, we do not describe these alternative models in further detail.
Table 2.
Fit Statistics and Model Comparisons for ACE Models
| −2LL | df | AIC | BIC | RMSEA | vs. Saturated Cholesky | vs. Full Unity and Diversity | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| diff (LL) | df | p | diff (LL) | df | p | ||||||
| 1. Saturated ACE Cholesky | 22953.94 | 8697 | 5560 | −34736 | .000 | ||||||
| 2. Three Correlated Factors (Figure B1) | 22996.84 | 8738 | 5517 | −34988 | .000 | 42.90 | 41 | .390 | |||
| 3. Hierarchical Common Factor (Figure B2) | 23000.77 | 8742 | 5521 | −34965 | .000 | 46.83 | 45 | .397 | |||
| Unity and Diversity | |||||||||||
| 4. EF-specific for Inhibition, WM, and Shifting | 22991.25 | 8740 | 5511 | −35003 | .000 | 37.31 | 43 | .716 | |||
| 5. EF-specific for WM and Shifting only | 22992.60 | 8743 | 5507 | −35003 | .000 | 38.66 | 46 | .770 | 1.35 | 3 | 0.717 |
| 6. EF-specific for WM only (Figure 3) | 22993.00 | 8746 | 5501 | −35022 | .000 | 39.06 | 49 | .844 | 1.75 | 6 | 0.941 |
| 7. No EF-specific (Common EF only) | 23074.77 | 8751 | 5573 | −34973 | .022 | 120.83 | 54 | < .001 | 83.52 | 11 | < .001 |
Note: The best fitting model is displayed in bold. For all fit statistics, lower numbers indicate better fit. The last six columns display model comparisons between that model and the Saturated Cholesky (columns 7–9) or the full Unity and Diversity model (columns 10–12). The Three Correlated Factors model refers to a model with Inhibition, Shifting, and WM Span latent variables with separate but correlated ACE components (see Figure B1). The Hierarchical Common Factor Model also has Inhibition, Shifting, and WM Span latent variables, but a higher-order Common EF factor accounts for their covariances (see Figure B2). The Unity and Diversity Model has a Common EF latent variable that directly explains variation in all seven EF tasks, and up to three EF-specific variables that explain variation in those EF tasks not explained by Common EF (see Figure 2 for the best fitting version of this model). −2LL = negative two times the log likelihood.
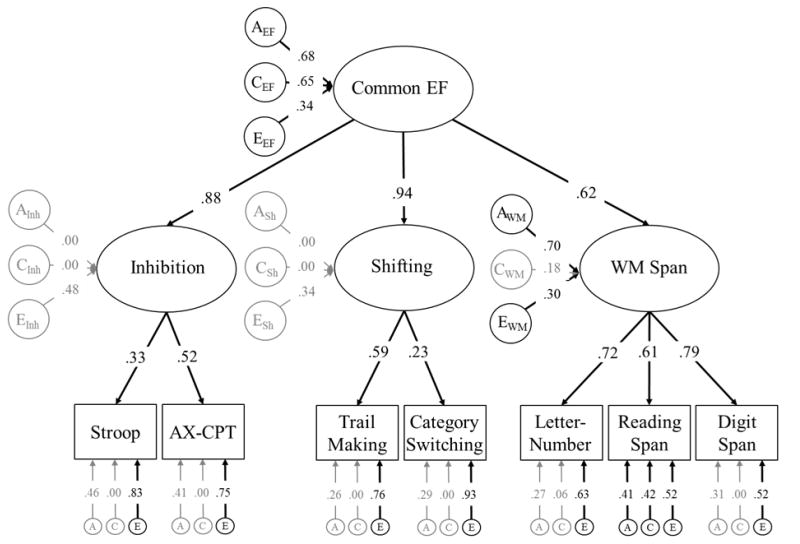
Model of the Executive Function Tasks Alone
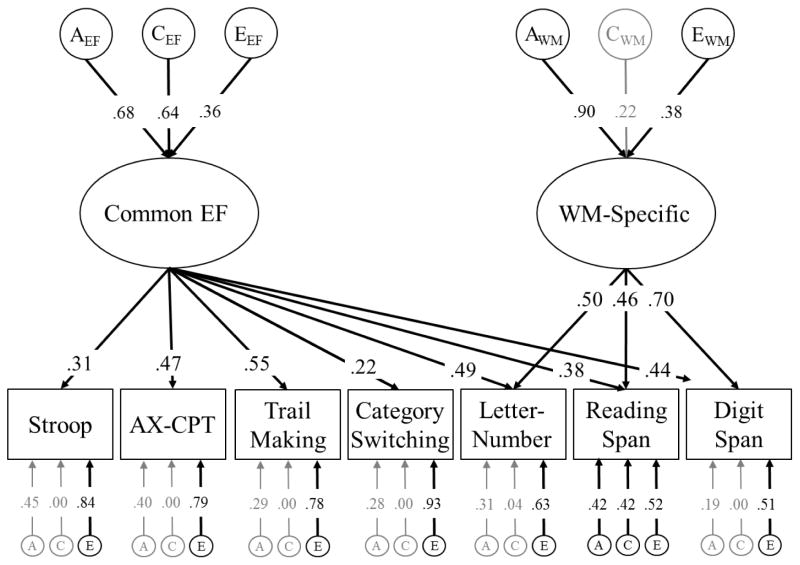
The best fitting model of EF is displayed in Figure 2, −2LL = 22993, df = 8746, AIC = 5501, BIC = −35022, RMSEA = .000. Consistent with our first hypothesis, Common EF accounted for variation in all seven EF tasks. Although some of the factor loadings (λs = .22 to .70) were somewhat low, they were all statistically significant, all χ2diffs(1) > 35.19, ps < .001, and similar to those estimates described for other tasks in previous work (Friedman et al., 2016). Additionally, this model includes a WM-Specific latent factor that accounts for shared variation across the WM span tasks that was not accounted for by the latent factor.
Figure 2.
Genetic unity and diversity model of Common EF and WM-Specific abilities. The ACE factors represent genetic influences (A), shared environmental influences (C), and nonshared environmental influences (E) on each latent construct and individual measure. Latent variables were not modeled for Inhibition-Specific or Shifting-Specific abilities because there was no evidence for these EF-Specific factors in this sample (see Table 2). Circles and ellipses represent latent variables, and rectangles represent measured variables. Variation explained by the latent factors can be computed by squaring the factor loadings. Significant factor loadings are displayed with black text and black lines (p < .05).
As shown in Table 2, we compared this best fitting model of EF to other versions of the unity and diversity model to examine whether it was also necessary to include Inhibition-Specific and/or Shifting-Specific factors. Specifically, we first fit a model with Common EF, Inhibition-Specific, WM-Specific, and Shifting-Specific (Model 4 in Table 2).2 As expected, compared to this full model, the Inhibition-Specific factor could be dropped without a significant decrement in fit, χ2diff(3) = 1.35, p = .717. Next, although we expected to observe evidence for Shifting-Specific (Figure 1, Model 5 in Table 2), this factor could also be dropped without a significant decrement in fit, χ2diff(3) = .40, p = .940. Finally, we could not drop the WM-Specific factor without a significant decrement in fit, χ2diff(5) = 81.77, p < .001. Therefore, we favored the model with only Common EF and WM-Specific factors (Figure 2).
Genetic influences accounted for about 46% of the variation in Common EF (i.e., the a factor loading of .682), a2 = .46, 95% CI [.10, .85]. The rest of the variation in EF was accounted for largely by shared environmental influences, c2 = .41, 95% CI [.05, .72], but also by nonshared environmental influences, e2 = .13, 95% CI [.04, .24].
Individual differences in WM-Specific were explained mostly by genetic influences, a2 = .81, 95% CI [.59, .94]. Shared environmental influences accounted for about 5% of the variation in WM-Specific, but these influences were not significant, c2 = .05, 95% CI [.00, .38], and nonshared environmental influences accounted for the remaining 14% of the variation in WM-Specific, e2 = .14, 95% CI [.06, .21].
With respect to the residual task-specific variance components, most of the remaining variation in the neuropsychological EF tasks was captured by the nonshared environmental factors (which includes measurement error). Residual genetic and shared environmental influences were only significant for Reading Span (residual a2 = .18, residual c2 = .18), suggesting few genetic and/or shared environmental influences on the EF tasks above and beyond those captured by Common EF and WM-Specific.
Executive Function and Other Cognitive Abilities
The genetic/environmental models involving general cognitive ability are displayed in Figure 3 for general cognitive ability in midlife, −2LL = 25936, df = 10019, AIC = 5898, BIC = −40523, RMSEA = .000, and general cognitive ability in early adulthood, −2LL = 26089, df = 10005, AIC = 6079, BIC = −40277, RMSEA = .008.
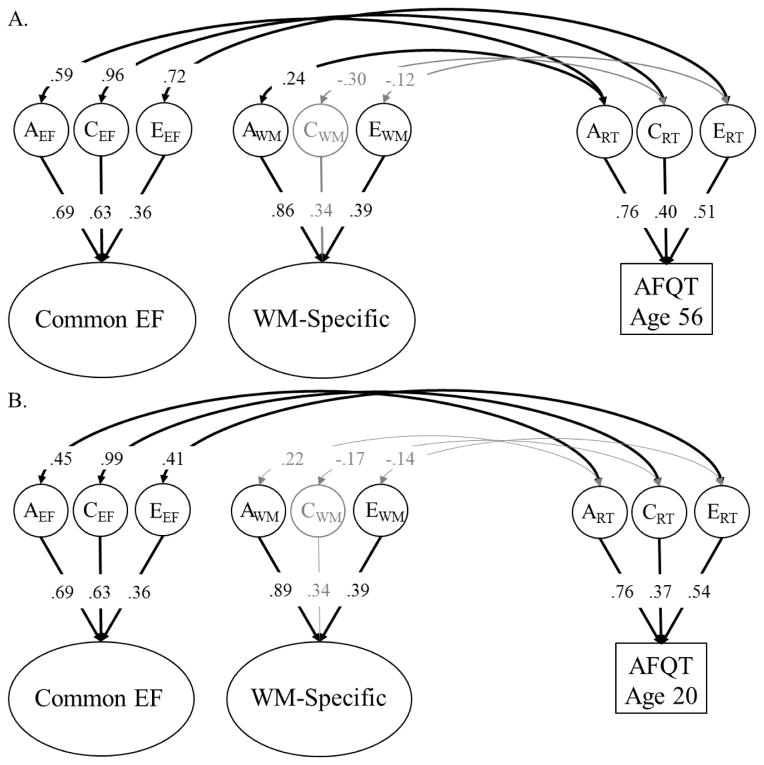
Figure 3.
Correlations between the genetic (A), shared environmental (C), and nonshared environmental (E) variance components on Common EF and WM-Specific in midlife, and general cognitive ability in midlife (Panel A) or young adulthood (Panel B). Common EF and WM-Specific are not correlated, by definition. Circles and ellipses represent latent variables, and rectangles represent measured variables. Factor loadings and residual variance components for the individual EF tasks are not displayed here, but were nearly identical to those displayed in Figure 2. Significant factor loadings and correlations are displayed with black text and black lines (p < .05).
As shown in Figure 3A, Common EF was moderately-to-highly correlated with general cognitive ability in midlife; phenotypic r = .68, 95% CI [.61, .75]. The genetic correlation, rg = .59, 95% CI [.33, .88], shared environmental correlation, rc = .96, 95% CI [.56, 1.0], and nonshared environmental correlation, re = .72, 95% CI [.45, 1.0], were all statistically significant, all χ2diff(1) > 6.51, ps < .011. Although the estimate of the genetic correlation was smaller in magnitude than that for the shared and nonshared environmental correlations, it explained the largest portion of the phenotypic correlation (45%, compared to 35% for shared environment and 19% for nonshared environment) because both Common EF and general cognitive ability were explained most strongly by genetic influences.
As shown in Figure 3B, similar correlations were observed between Common EF in midlife and AFQT scores at age 20 at the phenotypic, r = .54, 95% CI [.45, .62], genetic, rg = .45, 95% CI [.14, .78], shared environmental, rc = .99, 95% CI [.39, 1.0], and nonshared-environmental levels, re = .41, 95% CI [.14, .79]. Again, the genetic correlation explained the largest portion of the phenotypic correlation (43%, compared to 42% for shared environmental and 15% for nonshared environment). These similar genetic correlations with EF factors in Figures 3A and 3B were expected given that previous work on this sample has revealed that the genetic correlation was not different from 1.0 between AFQT scores in young adulthood and midlife (Lyons et al., 2009).
WM-Specific was also associated with general cognitive ability, though somewhat less so than Common EF. When both constructs were measured in midlife, WM-Specific was only weakly (but significantly) genetically correlated with general cognitive ability, rg = .24, 95% CI [.01, .48]. Although this genetic correlation was similar in magnitude for AFQT scores at age 20, it was not statistically significant, rg = .22, 95% CI [−.04, .49]. There were no environmental correlations between WM-Specific and general cognitive ability at either wave, all χ2diff(1) < 1.08, ps > .298. Because the environmental correlations were estimated as slightly negative at both timepoints, the total phenotypic correlation between WM-Specific and general cognitive ability was small (estimated phenotypic r = .09 for both models).
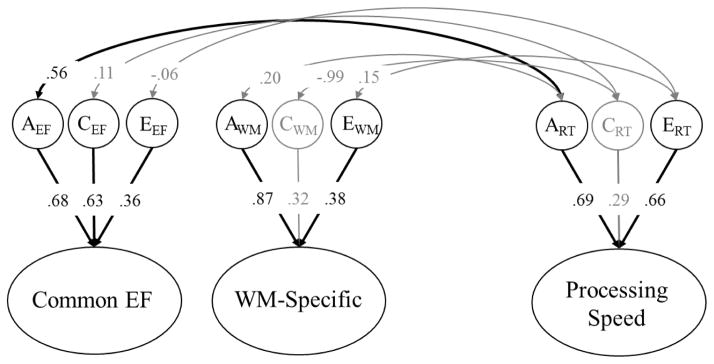
The model involving processing speed is displayed in Figure 4, −2LL = 28876, df = 11087, AIC = 6702, BIC = −44668, RMSEA = .006. In contrast to the results for general cognitive ability, the overall phenotypic association between Common EF and processing speed (r = .26) appeared to be entirely driven by a moderate genetic correlation, rg = .56, 95% CI [.01, 1.0]. The shared environmental, rc = .11, 95% CI [−1.0, 1.0], and nonshared environmental correlations, re = −.06, 95% CI [−.46, .29], with Common EF were small and nonsignificant, both χ2diff(1) < .11, ps > .740. WM-Specific was not associated with the processing speed at the genetic or environmental levels, both χ2diff(1) < 1.17, ps > .279.
Figure 4.
Correlations between the genetic (A), shared environmental (C), and nonshared environmental (E) variance components on Common EF, WM-Specific, and processing speed. Circles and ellipses represent latent variables, and rectangles represent measured variables (circles are not shown around residual ACE latent variables). Factor loadings and residual variance components for individual tasks are not displayed here. For EF tasks, they were nearly identical to those displayed in Figure 2. For processing speed, factor loadings were significant (.76 for simple RT; .88 for choice RT), and residual genetic/environmental components were estimated, but only significant for nonshared environmental components (residual paths: a = .27, c = .00, e = .62 for simple RT; a = .00, c = .21, e = .41, for choice RT). Significant factor loadings and correlations are displayed with black text and black lines (p < .05).
Discussion
Our results support each of the three hypotheses regarding the factor structure, correlates, and etiology of Common EF in middle-aged adults. First, we showed that a Common EF ability explained performance across a set of seven commonly used neuropsychological measures of EFs. Second, we showed that the variation in Common EF is explained most strongly by genetic influences (a2 = .46), but also by shared environmental influences (c2 = .38) and nonshared environmental influences (e2 = .13). Third, we demonstrated the Common EF factor was moderately associated with better general cognitive ability and faster processing speed. Shared genetic influences primarily accounted for the association of Common EF with general cognitive ability and processing speed. To our knowledge, this is the first study to examine the unity and diversity hypothesis of EF beyond young adulthood, especially in a genetically informative sample.
Theoretical Implications for the Unity and Diversity of Executive Function
These findings extend our knowledge of the cognitive control and goal management processes comprising Common EF in midlife. We confirmed that Common EF processes underlie variation in a number of neuropsychological EF tasks spanning three domains. These results suggest that the structure of EF in midlife is similar to that in adolescence and young adulthood (Miyake & Friedman, 2012), although there were two ability-specific EF factors in the younger samples but only one in our midlife sample (discussed below). These findings are also highly consistent with existing theories of EFs in older adults (Braver et al., 2005; Hasher & Zacks, 1988; Lustig et al., 2007).
Perhaps the most interesting finding concerns the implications for age-related change in the genetic and environmental etiology of Common EF. Previous work has suggested that Common EF is explained entirely by genetic influences in childhood and early adolescence (Engelhardt et al., 2015), whereas environmental influences start to explain some variance in Common EF by late adolescence (4%; Friedman et al., 2008) and more so in young adulthood (19%; Friedman et al., 2016). Genetic influences explained 46% of the variance in Common EF in midlife, but 85% in early adulthood (Friedman et al., 2016). However, the confidence interval for these two estimates did overlap.
Surprisingly, most of the environmental influences on Common EF were due to shared environmental influences (c2 = .41). This result was interesting given that there was evidence for minimal or no shared environmental influences explaining variation across EFs in previous work across the lifespan (Friedman et al., 2016). We observed a significant correlation between the shared environmental influences on Common EF in midlife and AFQT in early adulthood (rc = .96), which accounted for 35% of the phenotypic correlation. That finding suggests that these shared environmental influences on Common EF were not new to midlife but were shared with general cognitive ability at age 20 or earlier. Thus, the difference from previous studies does not appear to be aging-related. Further research will be needed to determine whether the difference is due to task, cohort, or other differences.
The results concerning general cognitive ability and processing speed help support the relationship between Common EF abilities and other aspects of cognitive function that have been observed in adolescence (Friedman et al., 2008). Despite having different tests than the previous work, the genetic correlations were very similar.
Furthermore, Common EF in midlife was correlated with general cognitive ability assessed in early adulthood to almost the same degree as the correlation between Common EF and general cognitive ability in midlife. These results suggest that the genetic influences that link Common EF to other cognitive abilities in early stages of life are largely the same genetic influences that link Common EF and other cognitive abilities in midlife. This is not surprising given that the genetic correlation between AFQT scores in early and middle adulthood reported here were not different from 1.0 across the 35-year interval (Lyons et al., 2009), and that the genetic influences on Common EF also show perfect stability between adolescence and early adulthood (Friedman et al., 2016).
Theoretical Implications for EF-Specific Processes
Consistent with previous research, we did not observe any evidence for an Inhibition-Specific factor, providing more evidence that inhibition is entirely encapsulated by Common EF (Friedman et al., 2016; Friedman et al., 2008; Ito et al., 2015). A key component to Hasher and Zacks (1988) view of EF in older adulthood is that general inhibition deficits underlie age-related decline in other cognitive abilities (Lustig et al., 2007; Persad et al., 2002). However, existing views of individual differences in Common EF highlight that these abilities are related to differences in goal maintenance and implementation, and do not necessarily invoke an active inhibition process directly (Miyake & Friedman, 2012). Thus, although an important outstanding question concerns what aspects of Common EF reflect inhibition processes versus cognitive control and goal management processes more generally, these findings provide further evidence that individual differences in response inhibition are wholly captured by the Common EF abilities that underlie performance on a wide range of EFs.
These results also have some interesting implications for WM-Specific processes. The complex WM span tasks used here involve storage, manipulation, and retrieval, but did not involve the within-trial removal or replacement of irrelevant information in WM, and only one task (Reading Span) required participants to processes irrelevant information in between memorizing stimuli. Nevertheless, we were still able to model a latent WM-Specific factor that was highly heritable and positively genetically correlated with general cognitive ability, although this genetic correlation was significantly lower than the correlation between Updating-Specific and intelligence observed in adolescence (Friedman et al., 2008). These differences from previous work might be partly due to the differences in types of WM tasks, but suggest that WM span and WM updating are quite similar even at the genetic level (Friedman & Hewitt, 2013; Schmiedek, Hildebrandt, Lövdén, Wilhelm, & Lindenberger, 2009).
Interestingly, we did not observe any evidence of a Shifting-Specific factor in the current study. These results suggest that, like inhibition, shifting may be entirely encapsulated by Common EF in midlife. However, we must acknowledge that an equally likely possibility is that we could not examine Shifting-Specific due to limitations of the shifting measures described here. We only had two indicators of shifting (and inhibition) in this study, and semantic shifting in the category switching measure may have been different from the shifting abilities measured by other studies. For example, the experimental cognitive shifting tasks in previous work require much more rapidly-paced switching than the neuropsychological tasks used here (Friedman et al., 2016). Also, both measures of switching were based on single trials (controlling for baseline trials), making it impossible to assess reliability of these measures, and likely causing them to have more measurement error than the WM span tasks. Based on these limitations, we do not conclude that Shifting-Specific is necessarily absent in midlife, but rather that future work will be necessary to examine the correlates and genetic/environmental etiology of Shifting-Specific, perhaps using the other task switching paradigms (Vaughan & Giovanello, 2010). Nevertheless, the two shifting tasks included here were correlated with the other five neuropsychological EF tasks, and loaded significantly on the latent Common EF factor, suggesting that they were still useful in modeling the latent associations between Common EF and other outcomes.
More generally, we focused on EF tasks assessing inhibition, shifting, and WM span processes, but these are not the only types of EFs. Other EF tasks assess dual tasking, planning, or verbal fluency, which may have their own specific variance components (Friedman & Miyake, 2017). Additionally, there are self-reported measures of everyday EFs that do not necessarily correspond directly to performance on laboratory EF tasks (Duckworth & Kern, 2011; Toplak, West, & Stanovich, 2013), perhaps because in the real world the effective use of EFs may require more self-direction than in guided laboratory-based tasks (Munakata, Snyder, & Chatham, 2012). Therefore, it will be useful to examine other EF-specific processes during midlife both in laboratory and real-world settings, as these findings would grant insight into the ecological validity of the results described here and shed light into the possibly different genetic/environmental architecture of other EF processes.
Concluding Remarks
We examined the structure, etiology, and correlates of Common EF in a sample of middle-aged male twins. To our knowledge, this is the first study to examine the unity and diversity model, including its genetic architecture, in older adults. Common EF was related to other cognitive abilities to a similar degree as the previously reported associations in earlier periods of life, and driven by similar genetic influences. Although Common EF was still moderately heritable in midlife, shared and nonshared environmental influences appeared to play a larger role than they did in other samples focusing on children and young adults. The present study also supported the unity and diversity model of EF in middle-aged adults, although there was no evidence for a Shifting-Specific factor as there was in studies of younger cohorts. Despite some differences, the similarity of the genetic architecture and correlations with other cognitive abilities in midlife to those observed in the younger cohorts is striking considering there was only a single overlapping task from previous research in the present study (the Stroop test).
Our use of neuropsychological tests, as opposed to the much lengthier experimental tasks used in the prior studies, also suggests that these Common EF and WM-Specific factors can be fruitfully applied to more clinically-oriented research. Future work should continue to examine Common EF with other important outcomes, as this unity and diversity model effectively controls for task-specific variance and measurement error in individual neuropsychological tasks, thereby providing stronger and more accurate estimates of the associations between general EF processes and important outcomes. Given the associations between Common EF and important social and clinical outcomes in adolescence and early adulthood (Miyake & Friedman, 2012), Common EF is likely to play strong role in a number of outcomes related to cognitive aging.
Public Significance Statement.
Executive functions are important higher-level cognitive abilities that control and regulate behavior. Understanding the structure, etiology, and correlates of individual differences in executive functions in midlife is vital to further elucidating why and how cognitive abilities decline in later stages of life.
Acknowledgments
This research was supported by Grants AG050595, AG018386, AG018384, AG022381, AG047903, and MH63207 from the National Institutes of Health.
This material was, in part, the result of work supported with resources of the VA San Diego Center of Excellence for Stress and Mental Health Healthcare System. The content is solely the responsibility of the authors and does not necessarily represent official views of the NIA, NIH, or VA. The VA has provided financial support and maintenance of the Vietnam Era Twin Registry. The Registry and numerous organizations provided invaluable assistance in the conduct of this study. The authors gratefully acknowledge the continued participation of the members of the VET Registry and their families.
Appendix A
Table A1.
Phenotypic and Cross-Twin (MZ/DZ) Correlations
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Stroop | .28 / .15 | .10 / .04 | .13 / .11 | .01 / .06 | .15 / .12 | .13 / .06 | .12 / .10 | .16 / .10 | .16 / .13 | .01 / .00 | .03 / .06 |
| 2. AX-CPT | .16 | .36 / .18 | .23 / .18 | .09 / .07 | .21 / .12 | .17 / .15 | .20 / .16 | .24 / .18 | .27 / .24 | .14 / .10 | .11 / .13 |
| 3. Trail-Making Test | .16 | .27 | .37 / .21 | .13 /−.03 | .26 / .27 | .16 / .17 | .27 / .25 | .21 / .20 | .28 / .25 | .15 / .00 | .15 / .06 |
| 4. Category Switching | .06 | .11 | .13 | .14 / .00 | .07 / .07 | .06 / .05 | .12 / .00 | .10 / .10 | .14 / .09 | .06 / .01 | .03 / −.01 |
| 5. Letter-Number | .16 | .22 | .27 | .12 | .54 / .37 | .40 / .26 | .50 / .36 | .30 / .22 | .35 / .23 | .12 / −.03 | .14 / .04 |
| 6. Reading Span | .15 | .21 | .18 | .10 | .43 | .69 / .49 | .46 / .29 | .36 / .24 | .36 / .23 | .08 / .08 | .09 / .06 |
| 7. Digit Span | .14 | .18 | .26 | .10 | .59 | .51 | .66 / .42 | .33 / .23 | .38 / .26 | .14 / .00 | .13 / .06 |
| 8. AFQT - Age 20 | .18 | .24 | .25 | .12 | .31 | .35 | .30 | .72 / .43 | .69 / .41 | .01 / .01 | .03 / .04 |
| 9. AFQT - Ag 55 | .22 | .35 | .31 | .14 | .37 | .40 | .37 | 0.73 | .75 / .46 | .04 / .04 | .07 / .08 |
| 10. Simple RT | .01 | .13 | .07 | .02 | .10 | .13 | .14 | .02 | .10 | .39 / .10 | .34 / .15 |
| 11. Choice RT | .08 | .14 | .14 | .04 | .13 | .13 | .16 | .04 | .14 | .66 | .42 / .28 |
| 12. Age | −.16 | −.01 | −.05 | −.04 | −.03 | .09 | −.04 | .11 | .02 | .08 | .12 |
Note: Phenotypic correlations between all measures of the study are displayed below the diagonal. Twin 1 – Twin 2 correlations are displayed along and above the diagonal. Correlations for MZ twins are displayed on the left and correlations for DZ twins are displayed on the right. All correlations were based on age-residualized measures, except for those with age, which are the correlations between age and the non-age-residualized measures. Significant correlations are displayed in bold (p < .05).
Appendix B – Alternative Genetic Models of Executive Function
Figure B1.
Three factor model of EF with correlated genetic and environmental influences on Inhibition (AInh, CInh, EInh), Shifting (ASh, CSh, ESh), and WM Span (AWM, CWM, EWM). This model also fit the data well, and corresponds closely to the correlated factors model presented by Miyake et al. (2000). However, we favored the unity and diversity model presented in Figure 2 because it had the most parsimonious fit (see Table 2) and highlights Common EF vs. EF-specific variance components. Significant factor loadings and correlations are displayed with black text and black lines (p < .05).
Figure B2.
Hierarchical genetic/environmental model in which Common EF is modeled as a hierarchical latent variable above Inhibition, Shifting, and WM Span. ACEs for EF-Specific processes (e.g., WM-Specific) are modeled directly on their latent variables (e.g., AWM, CWM, EWM). This model also fit the data well, but we favored the unity and diversity model presented in Figure 2 because it had a more parsimonious fit (see Table 2) and corresponds to the parameterization used in current work (Friedman & Miyake, 2017; Miyake & Friedman, 2012). Significant factor loadings and correlations are displayed with black text and black lines (p < .05).
Footnotes
We also analyzed data using only the backward span, because this more complex measure of WM span involved the manipulation of information in WM instead of simple storage and recall. These analyses revealed the same pattern of results as the combined forward and backward span, so we favored the total score reported here.
To identify these Inhibition-Specific and Shifting-Specific factors with only two indicators, it was necessary to equate the factor loadings (e.g., for Stroop and AX-CPT on the Inhibition-Specific factor).
References
- Bayroff AG, Anderson AA. Development of Literacy Screening Scales for AFQT 7 and 8 Failures. Washington DC: 1963. [Google Scholar]
- Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, … Fox J. OpenMx: An Open Source Extended Structural Equation Modeling Framework. Psychometrika. 2011;76:306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen K. Structural equations with latent variables. New York: John Wiley; 1989. pp. 612–621. [Google Scholar]
- Braver TS. The variable nature of cognitive control: A dual mechanisms framework. Trends in Cognitive Sciences. 2012;16:106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Keys BA, Carter CS, Cohen JD, Kaye JA, … Reed BR. Context processing in older adults: Evidence for a theory relating cognitive control to neurobiology in healthy aging. Journal of Experimental Psychology: General. 2001;130:746–763. [PubMed] [Google Scholar]
- Braver TS, Satpute AB, Rush BK, Racine CA, Barch DM. Context processing and context maintenance in healthy aging and early stage dementia of the Alzheimer’s type. Psychology and Aging. 2005;20:33–46. doi: 10.1037/0882-7974.20.1.33. [DOI] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, … Moffitt TE. The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science. 2014;2:119–137. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin J. On measuring discrimination and response bias: Unequal numbers of targets and distractors and two classes of distractors. Neuropsychology. 1994;8:110. [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior. 1980;19:450–466. [Google Scholar]
- de Frias CM, Dixon RA, Strauss E. Structure of four executive functioning tests in healthy older adults. Neuropsychology. 2006;20:206–214. doi: 10.1037/0894-4105.20.2.206. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system (D-KEFS) Psychological Corporation; 2001. [Google Scholar]
- Demakis GJ. Frontal lobe damage and tests of executive processing: a meta-analysis of the category test, stroop test, and trail-making test. Journal of Clinical and Experimental Neuropsychology. 2004;26:441–450. doi: 10.1080/13803390490510149. [DOI] [PubMed] [Google Scholar]
- Duckworth AL, Kern ML. A Meta-Analysis of the Convergent Validity of Self-Control Measures. Journal of Research in Personality. 2011;45:259–268. doi: 10.1016/j.jrp.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt LE, Briley DA, Mann FD, Harden KP, Tucker-Drob EM. Genes Unite Executive Functions in Childhood. Psychological Science. 2015;26:1151–1163. doi: 10.1177/0956797615577209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Hewitt JK. Do working memory span and updating tasks measure the same thing? A twin study. Paper presented at the Behavioral Genetics Annual Meeting.2013. [Google Scholar]
- Friedman NP, Miyake A. Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex. 2017;86:186–204. doi: 10.1016/j.cortex.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Altamirano LJ, Corley RP, Young SE, Rhea SA, Hewitt JK. Stability and change in executive function abilities from late adolescence to early adulthood: A longitudinal twin study. Developmental Psychology. 2016;52:326–340. doi: 10.1037/dev0000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, Defries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden CJ, Freshwater SM. The Stroop Color and Word Test: A Manual for Clinical and Experimental Uses;[adult Version] Stoelting; 2002. [Google Scholar]
- Gustavson DE, Miyake A, Hewitt JK, Friedman NP. Understanding the cognitive and genetic underpinnings of procrastination: Evidence for shared genetic influences with goal management and executive function abilities. Journal of Experimental Psychology: General. 2015;144:1063–1079. doi: 10.1037/xge0000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new view. Psychology of Learning and Motivation. 1988;22:193–225. [Google Scholar]
- Herd SA, O’Reilly RC, Hazy TE, Chatham CH, Brant AM, Friedman NP. A neural network model of individual differences in task switching abilities. Neuropsychologia. 2014;62:375–389. doi: 10.1016/j.neuropsychologia.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog C, Dixon RA, Hultsch DF, MacDonald SW. Latent change models of adult cognition: Are changes in processing speed and working memory associated with changes in episodic memory? Psychology and Aging. 2003;18:755–769. doi: 10.1037/0882-7974.18.4.755. [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler PM. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods. 1998;3:424–453. doi: 10.1037//1082-989x.3.4.424. [DOI] [Google Scholar]
- Ito TA, Friedman NP, Bartholow BD, Correll J, Loersch C, Altamirano LJ, Miyake A. Toward a comprehensive understanding of executive cognitive function in implicit racial bias. Journal of Personality and Social Psychology. 2015;108:187–218. doi: 10.1037/a0038557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Moore CS, Franz CE, Panizzon MS, Lyons MJ. Behavior genetics of cognition across the lifespan. Springer; New York: 2014. Cognition in middle adulthood; pp. 105–134. [Google Scholar]
- Kremen WS, Panizzon MS, Xian H, Barch DM, Franz CE, Grant MD, … Lyons MJ. Genetic architecture of context processing in late middle age: More than one underlying mechanism. Psychology and Aging. 2011;26:852–863. doi: 10.1037/a0025098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Thompson-Brenner H, Leung YM, Grant MD, Franz CE, Eisen SA, … Lyons MJ. Genes, environment, and time: the Vietnam Era Twin Study of Aging (VETSA) Twin Research and Human Genetics. 2006;9:1009–1022. doi: 10.1375/183242706779462750. [DOI] [PubMed] [Google Scholar]
- Lee T, Mosing MA, Henry JD, Trollor JN, Ames D, Martin NG, … Team OR. Genetic influences on four measures of executive functions and their covariation with general cognitive ability: The Older Australian Twins Study. Behavior Genetics. 2012;42:528–538. doi: 10.1007/s10519-012-9526-1. [DOI] [PubMed] [Google Scholar]
- Lustig C, Hasher L, Zacks RT. Inhibitory deficit theory: Recent developments in a “new view”. In: Gorfein DS, editor. Inhibition in cognition. Washington, DC: American Psychological Association; 2007. pp. 145–162. [Google Scholar]
- Lyons MJ, York TP, Franz CE, Grant MD, Eaves LJ, Jacobson KC, … Xian H. Genes determine stability and the environment determines change in cognitive ability during 35 years of adulthood. Psychological Science. 2009;20:1146–1152. doi: 10.1111/j.1467-9280.2009.02425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP. The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science. 2012;21:8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Snyder HR, Chatham CH. Developing cognitive control: Three key transitions. Current Directions in Psychological Science. 2012;21:71–77. doi: 10.1177/0963721412436807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman H, Savikko N, Strandberg TE, Pitkala KH. Effect of physical exercise on cognitive performance in older adults with mild cognitive impairment or dementia: A systematic review. Dementia and Geriatric Cognitive Disorders. 2014;38:347–365. doi: 10.1159/000365388. [DOI] [PubMed] [Google Scholar]
- Persad CC, Abeles N, Zacks RT, Denburg NL. Inhibitory changes after age 60 and their relationship to measures of attention and memory. Journals of Gerontology Series B-Psychological Sciences and Social Sciences. 2002;57:223–232. doi: 10.1093/geronb/57.3.P223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedek F, Hildebrandt A, Lövdén M, Wilhelm O, Lindenberger U. Complex span versus updating tasks of working memory: the gap is not that deep. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35:1089. doi: 10.1037/a0015730. [DOI] [PubMed] [Google Scholar]
- Schoenborn CA, Heyman KM. Health characteristics of adults aged 55 years and over: United States, 2004–2007. National Health Statistics Report. 2009;16:1–31. [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. doi: 10.1037/0096-3445.121.1.15. [DOI] [Google Scholar]
- Toplak ME, West RF, Stanovich KE. Practitioner Review: Do performance - based measures and ratings of executive function assess the same construct? Journal of Child Psychology and Psychiatry. 2013;54:131–143. doi: 10.1111/jcpp.12001. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Bar JL, Harley RM, Lyons MJ. The harvard twin study of substance abuse: What we have learned. Harvard Review of Psychiatry. 2001;9:267–279. doi: 10.1093/hrp/9.6.267. [DOI] [PubMed] [Google Scholar]
- Vasilopoulos T, Franz CE, Panizzon MS, Xian H, Grant MD, Lyons MJ, … Kremen WS. Genetic architecture of the delis-kaplan executive function system trail making test: Evidence for distinct genetic influences on executive function. Neuropsychology. 2012;26:238–250. doi: 10.1037/a0026768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan L, Giovanello K. Executive function in daily life: Age-related influences of executive processes on instrumental activities of daily living. Psychology and Aging. 2010;25:343–355. doi: 10.1037/a0017729. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WMS-III: Wechsler memory scale administration and scoring manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]