Abstract
Aberrant HGF/Met signaling promotes tumor migration, invasion, and metastasis through both autocrine and non-autocrine mechanisms; however, the molecular downstream signaling mechanisms by which HGF/Met induces metastasis are incompletely understood. We here report that Ezrin expression is stimulated by HGF and correlates with activated HGF/Met, indicating that HGF/Met signaling regulates the expression of Ezrin. We show that HGF/Met signaling activates the transcription factor Sp1 through the MAPK pathway, and activated Sp1 can in turn directly bind to the promoter of Ezrin gene and regulate its transcription. Notably, knockdown of Ezrin expression by shRNAs inhibits the metastasis induced by either HGF/Met autocrine or non-autocrine signaling in syngeneic wildtype and HGF transgenic mouse hosts. We also used small molecule drugs in preclinical mouse models to confirm that Ezrin is one of the downstream molecules mediating HGF/Met signaling-induced metastasis in melanoma. We conclude that Ezrin is a key downstream factor involved in the regulation of HGF/Met signaling-induced metastasis and demonstrate a link between Ezrin and HGF/Met/MAPK/Sp1 activation in the metastatic process. Our data indicate that Ezrin represents a promising therapeutic target for patients bearing tumors with activated HGF/Met signaling.
Keywords: Ezrin, HGF, metastasis, melanoma, therapeutic target
Introduction
Met, a cell-surface receptor tyrosine kinase encoded by Met gene, is activated by hepatocyte growth factor (HGF) produced from the same cell (autocrine loop), neighboring cells (paracrine loop) or a distant organ (endocrine loop).1–5 Through specific binding to its receptor Met, HGF induces autophosphorylation of tyrosine residues at Tyr 1230/1234/1235 in the catalytic domain of Met, generating phosphotyrosine docking sites able to engage an array of Src-homology-2 domain (SH2 domain)-containing signal transducers, which in turn activate appropriate signaling pathways such as Ras/Erk and PI3K/Akt.1–4 HGF/Met signaling mediates a broad range of cellular physiological activities that are essential for embryonic development, wound healing and tissue regeneration through autocrine and non-autocrine (paracrine or endocrine) mechanisms.1,5 Aberrant HGF/Met signaling propagates an intricate system of signaling cascades that result in a comprehensive rewiring of gene expression patterns and promotes tumor migration, invasion, and metastasis.1–3,5Met is identified as a proto-oncogene that is often amplified and/or overexpressed in most types of solid human tumors including melanoma.2,3 HGF is also found to be upregulated in many types of human tumors.1–3,5,6 The formation of an autocrine HGF/Met signaling loop is thought to play an important role in the genesis and progression of human tumors, including melanoma, rhabdomyosarcoma, osteosarcoma, breast cancer, liver cancer and glioblastoma.2,3,6,7 Notably, experimental activation of autocrine loops through forced co-expression of HGF and Met in cells can confer a tumorigenic and metastatic phenotype.5,8 Constitutive Met activation can stimulate angiogenesis, extracellular matrix dissolution, invasiveness, and metastasis.3,5 Moreover, overexpression of HGF in the tumor microenvironment has been reported to be associated with tumor aggressiveness and invasion.9,10 Previously, we found that constitutive HGF/Met signaling promotes melanoma metastasis through a non-autocrine mechanism in genetically engineered mouse (GEM) models;5 however, the molecular mechanisms downstream of HGF/Met signaling that induces metastasis remain to be resolved. Recent studies reported that Ezrin, a membrane-cytoskeleton linker, is required for the motility and morphogenetic response induced by HGF in epithelial cells,11,12 suggesting that Ezrin may be involved in HGF/Met signaling-mediated tumor metastasis.
Ezrin, encoded by the vil2 gene, is a member of the ERMs (ezrin, radixin, and moesin) family13 and is predominantly expressed in epithelial cells.11 As a key organizer of complex membrane domains, Ezrin links the plasma membrane and cytoskeleton and coordinates the interaction of transmembrane proteins, phospholipids, membrane-associated cytoplasmic proteins, and the cytoskeleton.14,15 As such, Ezrin mediates many cellular processes including cell growth, morphogenesis, adhesion and migration under physiological conditions, as well as in pathological scenarios involving cancer cell invasion and metastasis15 through numerous fundamental signal transduction pathways involving protein kinase A,16 protein kinase C,17 Rho,18,19 PI3K/AKT,20,21 MAPK,22 Src,23 Wnt/β-catenin,24 CD4425 and RTKs such as EGFR26 and Met.11,12 Overexpression of Ezrin has been observed in many human cancers including breast,27 lung 28 and prostate cancers,29 oral squamous cell carcinomas (OSCCs),30 pancreatic carcinomas 31 as well as osteosarcoma 22 and rhabdomyosarcoma,18 compared with normal tissues. An activating Ezrin mutation has also been associated with cell migration and metastasis.18, 22 In contrast, depletion of Ezrin, or overexpression of a dominant-negative (T567A) or non-phosphorylatable (Y353F) mutant significantly reduces invasion and metastasis.18,20,22,32
We recently identified the cytoskeletal organizer Ezrin as a key metastatic regulator in HGF transgenic mouse model system,18 implicating Ezrin in HGF/Met signaling-induced metastasis. We therefore hypothesize and have here demonstrated that Ezrin is a key downstream gene involved in the regulation of HGF/Met signaling-induced metastasis. We found that HGF can stimulate the expression of Ezrin through the transcription factor Sp1. Notably, blocking Ezrin can inhibit metastasis induced by both HGF/Met autocrine and non-autocrine signaling in the syngeneic host and HGF genetically engineered mouse (GEM) hosts. We here demonstrate a link between Ezrin and HGF/Met/MAPK/Sp1 activation in the metastatic process and conclude that Ezrin is a key downstream molecule involved in the regulation of HGF/Met signaling-induced metastasis.
Materials and methods
Plasmids, antibodies, cell lines, and reagents
Plasmids: pcDNA3-HGF, -Met and stably transfected cell lines were described elsewhere.5 Ezrin shRNA-expressing plasmids were purchased from Open Biosystems (GE Dharmacon, Lafayette, CO) and described as in the previous studies.18,33 Antibodies: anti-phospho-AKT (Ser473), anti-AKT, anti-phospho-ERK 1/2 (Thr202/Tyr204) and anti-ERK1/2 antibodies were purchased from Cell Signaling (Danvers, MA, USA); anti-β-actin and Vinculin antibodies were purchased from Santa Cruz (Dallas, TX); anti-Ezrin antibody was purchased from Millipore (Billerica, Ma) and Sigma (St. Louis, MO ); anti-phospho-Ezrin T576 and anti-phospho-Ezrin Y353 were purchased from Sigma (St. Louis, MO ); anti-Sp1 and phospho-Sp1(T453) antibodies were purchased from Abcam (Cambridge, MA). The B16F1 cell line was obtained from American Type Culture Collection (ATCC, Manassas, VA). 37-7 cell line was derived from a neoplasm arising in HGF/SF transgenic mouse.5 Stably expressing cells were established through transfection using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) and selected by antibiotics G418 or puromycin (Sigma, St. Louis, MO). The rHGF was purchased from R &D (Minneapolis, MN). Small molecular inhibitors: PF02341066 and NSC668394 were purchased from Sigma (St. Louis, MO).
Northern blot analysis
Total RNAs were isolated from cells using Trizol (Life Technologies) reagent, according to the manufacturer’s instruction. Northern blot assay was performed as described our previous study (Yan J et al. Oncogene 2004; 23:1939). Briefly, 30 μg of total RNAs were separated on a 1.0% formaldehyde/agarose gel by electrophoresis, transferred to Hybond (Amersham) nylon membrane and hybridized with a-32P-labeled cDNA for Ezrin or β-actin probes using Prime-a-Gene® Labeling System (Promega, Madison, WI, USA). After washing, the blot exposed in a sealed envelope (containing the membrane) at −80 ºC freezer to X-ray film, and adjust the exposure time to get a darker or lighter band pattern.
Western blot analysis
After washing with the cold phosphate-buffered saline (PBS), the cells were lysed in RIPA buffer [50mM Tris (pH 7.4), 150mM NaCl, 1% Triton X–100, 5mM EDTA, plus protease inhibitor cocktail (Boehringer Mannheim)] on ice for 30min. The lysates were centrifuged at 10000 r.p.m. for 10min. The 50 μg protein lysates were fractionated by 4–20% Tris-glycine SDS–polyacrylamide gel electrophoresis and transferred to PVDF membranes. After blocking with 5% milk TBST buffer (TBS buffer + 0.5% Tween-20) for overnight, the membrane blot was incubated with various antibodies at optimum dilution (1:1000 dilution for anit-Ezrin, p-Ezrin, Met, p-Met, Akt, pAkt, Erk1/2, p-Erk1/2, Vinculin; 1:500 dilution for anti- β-actin, Sp1 and p-Sp1) overnight in cold room. The bands were developed by ECL (Amersham). Membranes were stripped using Re-Blotting reagent (Chemicon International) and reprobed as described.5,20
Motility and invasion assay
Cell motility and invasion were measured using a transwell assay as described previously,5 but modified to measure the ability after incubation of 12 hours.
Cell proliferation
CCK8 kit (Dojindo Molecular Technologies, Inc. Rockville, MD) was used for the measurement of cell growth.33
Experimental and spontaneous metastasis assays
Cells were injected via tail vein into 4- to 6-week-old male mouse hosts: c-Brd and c-Brd HGF transgenic or FVB and FVB HGF transgenic mice. All cell lines were injected at 5×105 into C57/BL6-cBrd or c-Brd HGF transgenic mice. Tumor numbers were obtained by visual inspection of tissues in mice euthanized between 3 and four weeks post-transplantation, and micrometastases were counted by a pathologist after dissection of the lung.18
Immunohistochemistry
Lung tissues were fixed in 10% buffered formalin solution (pH7.2) for overnight, and serially sectioned to 15 μm at 20ºC. Immunohistochemistry was performed as described.33 Immunoreactivity scores were analyzed using Image Scope V 10.0 software from Aperio Technologies (Vista, CA). The size and number of the metastases were also quantified and counted using Image Scope V 10.0 software (Aperio Technologies, Vista, CA).
Preclinical drug treatments
B16F1 or 37-7 cells stably expressing HGF, Met or Ezrin shRNA were transplanted into male c-Brd and c-Brd HGF transgenic or FVB and FVB HGF transgenic mice hosts between 4 and six weeks of age through either intravenously (IV) for experimental metastasis assay or subcutaneously (SQ) for tumor growth. Mice harboring the inoculated tumor cells were randomly assigned to control (Mock), inhibitor PF02341066 treatment (Inh Met) or inhibitor NSC668394 treatment (Inh Ez). The mice were treated with 50 mg/kg of the Met inhibitor PF02341066 (dissolved in 5% DMSO, 30%PEG300 in ddH2O ) or mock (5% DMSO, 30% PEG300 in ddH2O) daily by oral gavage (volume of 100 μl) immediately (experimental metastasis assay) or 7 days (tumor growth) after transplantation of tumor; and treated with 2.226 mg/kg of Ezrin inhibitor NSC668394 (dissolved 2% DMSO in PBS) (Celik et al. JBC 2016, 29:13257–13270; Pore et al. Leukemia 2015, 29(9): 1857–1867) or mock (2% DMSO in PBS) five times per week by intraperitoneal injection (IP) (injection volume of 100 μl). Animals were held for between 3 and four weeks post-injection to obtain accurate metastasis analyses. All mouse procedures were performed according to NIH guidelines [the animal proposal LCBG023, approval by NCI-Bethesda Animal Care and Use Committee (ACUC)].
Luciferase reporter assays
Luciferase assays were performed as described.20
Chromatin immunoprecipitation (ChIP)
ChIP assays were performed as described.20,33 Immunoprecipitated DNA was analyzed by PCR using the following primers for Ezrin promoter: -954 to -764, CCGATCCCAGTTTGTGAAGA and TCCGCAGTCCCGAGTATAAG; −230 to − 21, AATCAACCCTTCCAGTGCAG and CGCGGAATAGTCCAATGTTT.
Statistics
Statistical analyses were performed unpaired t-test (two-tailed) for all column data sets using GraphPad Prism 6 software. The p values of less than 0.05 were considered statistically significant.
Results
HGF stimulates expression of the pro-metastatic gene Ezrin
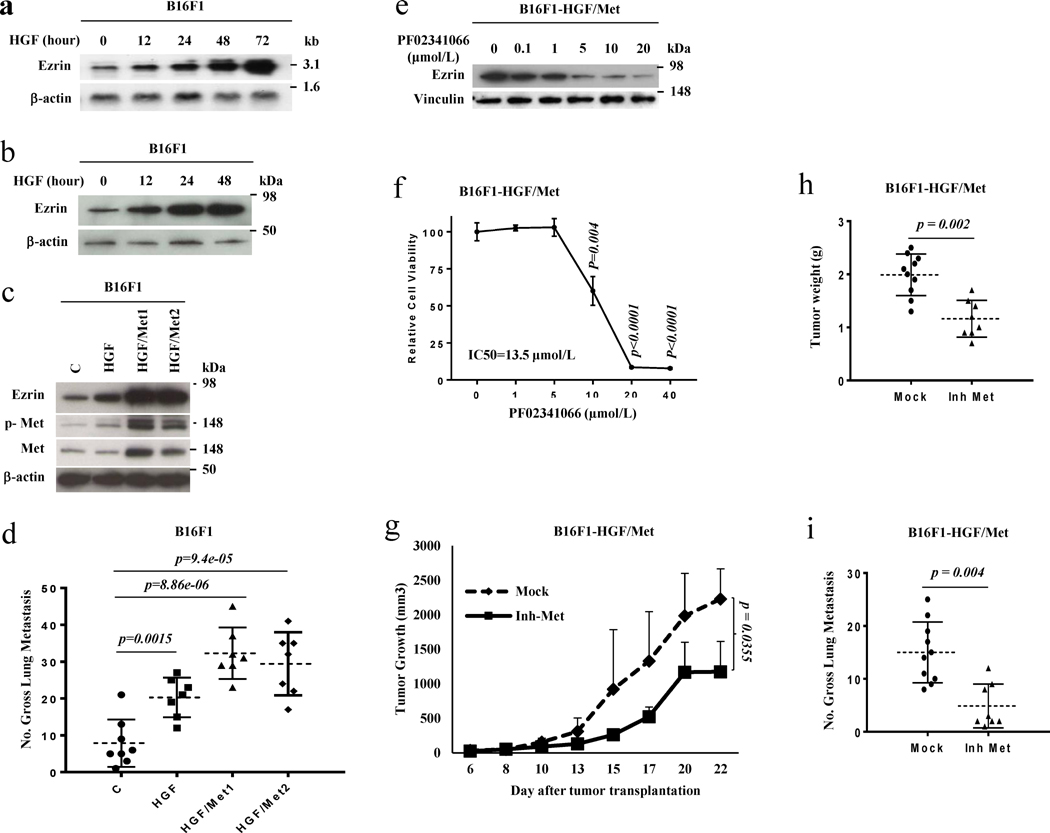
We recently identified the cytoskeletal organizer Ezrin as a critical metastatic regulator using an HGF GEM model.18 To study whether Ezrin is regulated by the HGF/Met signaling pathway, we examined Ezrin expression in B16F1 and 37-7 melanoma cells treated with HGF. When melanoma cells were treated with HGF, Ezrin expression was overtly increased by as early as 12 hours at both RNA (Figure 1a) and protein levels (Figure 1b, S1a). Moreover, under conditions of constitutive intracellular HGF/Met signaling, in previously-established B16F1 and 37-7 cells stably expressing either HGF (HGF) or both HGF and Met (HGF/Met),5 Ezrin expression was greatly enhanced (Figure 1c, S1b). Forced expression of either HGF alone or HGF/Met also promoted experimental pulmonary metastasis (Figure 1d, S1b), raising the possibility that Ezrin helps mediate HGF/Met signaling-associated metastasis. To further confirm the notion that HGF/Met signaling promoted Ezrin expression, we treated B16F1-HGF/Met cells with a Met inhibitor. Figure 1e shows that Ezrin protein levels were decreased by treatment with the Met inhibitor PF02341066 in B16F1-HGF/Met cells in a dose-dependent manner. Moreover, the Met inhibitor significantly inhibited cell growth in vitro (Figure 1f), tumor growth in vivo (Figure 1g, 1h) as well as metastasis (Figure 1i). Our data indicate that HGF/Met signaling regulates Ezrin expression, suggesting that Ezrin is involved in HGF/Met signaling-mediated metastasis.
Figure 1. HGF stimulates the expression of pro-metastatic gene Ezrin and thereby metastasis.
(a) The RNA levels of Ezrin were analyzed by Northern blot in B16F1 melanoma cell line treated with 30 nM HGF for the indicated time. (b) The protein levels of Ezrin were analyzed by western blot in B16F1 melanoma cells treated with 30 nM HGF for the indicated time. (c) The Western blot analysis of B16F1 cells stably transfected with HGF (HGF) or HGF plus Met (HGF/Met1 and HGF/Met2) expressing vector; c, as empty vector control. (d) Gross pulmonary metastasis in B16F1 cells stably transfected with HGF (HGF, n=7) or HGF plus Met (HGF/Met1, n=7; HGF/Met2, n=7) expressing vector, as well as empty vector control (C, n=8). (e) The protein levels of Ezrin were analyzed by western blot in B16F1stably transfected with HGF plus Met melanoma cell line (B16F1-HGF/Met) treated with the indicated dose of Met inhibitor PF02341066 for 24 hours. (f) The relative cell proliferation of B16F1-HGF/Met melanoma cells treated with the indicated dose of Met inhibitor PF02341066 for 24 hours. (g, h) Tumor growth curve (g,) and tumor weight (h) of B16F1-HGF/Met cells in xenograft syngeneic C57/BL6-c-Brd mice with (Inh Met, n=10) / without (Mock, n=8) treatment of Met inhibitor PF02341066. (i) Gross pulmonary metastases in B16F1-HGF/Met cells from syngeneic C57/BL6-c-Brd mice treated with Met inhibitor PF02341066 (Inh Met, n=8) and control (Mock, n=10).
Ezrin mediates the regulation of autocrine HGF/Met signaling-induced metastasis
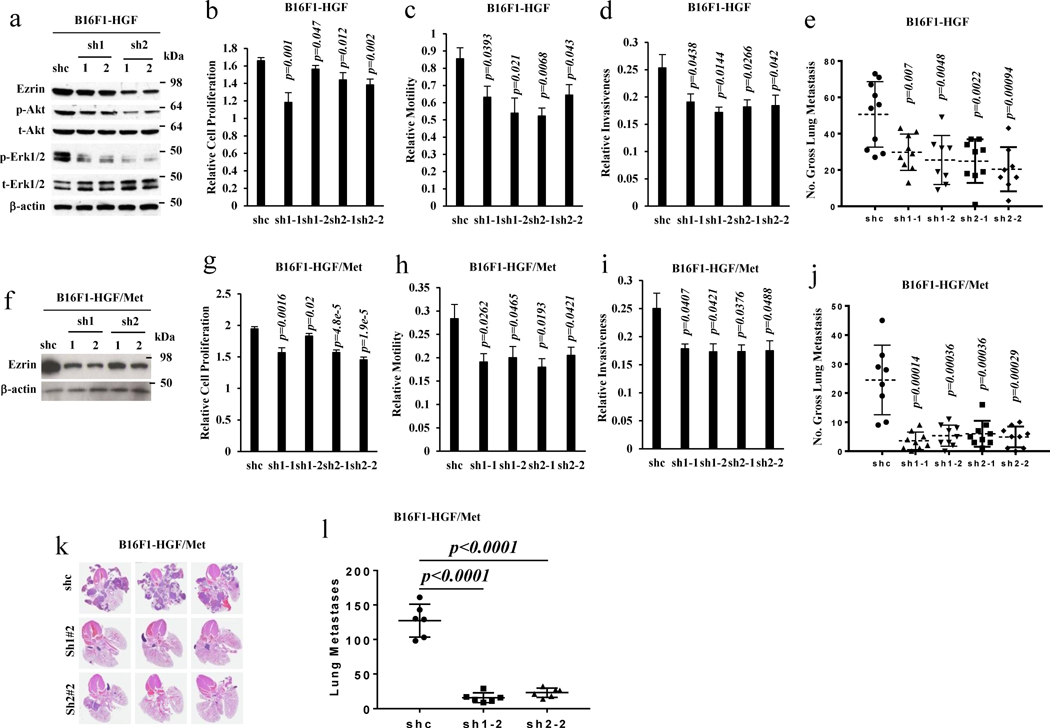
Our data showed that the forced expression of HGF alone or HGF/Met could stimulate Ezrin expression. We and others have reported previously that HGF/Met autocrine signaling promotes melanoma metastasis.5 To determine to what extent HGF/Met signaling-mediated metastasis was dependent on Ezrin, two Ezrin shRNA expression vectors were introduced into stably expressing HGF alone or HGF/Met B16F1 and 37-7 cells, whose metastatic ability had already been enhanced through ectopic HGF or HGF and Met expression (Figure 1d, S1b; our previous study).5 Knockdown of Ezrin in stably expressing HGF cells downregulated the phosphorylation of Akt and Erk (Figure 2a) and inhibited cell proliferation (Figure 2b), motility (Figure 2c) and invasiveness (Figure 2d) in vitro, and significantly blocked HGF-induced metastasis in vivo (Figure 2e, S1c). Similar results also were found when Ezrin was knocked down in stably expressing HGF/Met cells (Figure 2f, S1d), resulting in inhibition of cell proliferation (Figure 2g), motility (Figure 2h) and invasiveness (Figure 2i), and a significant reduction in HGF/Met-promoted macro- and micro-metastasis in vivo (Figure 2j, 2k, 2l and S1d). These results demonstrate that Ezrin can mediate metastasis through autocrine HGF/Met signaling.
Figure 2. Ezrin mediates the regulation of autocrine Met signaling-induced metastasis.
(a) Western blot analysis of B16F1-HGF cells transfected with vector (shc), Ezrin shRNA (sh1, sh2) expressing constructs. sh1-1, sh1-2 are two cell clones from sh1 construct, sh2-1 and sh2-2 are two cell clones from sh2 construct. (b–e) B16F1-HGF cells transfected with vector (shc) and Ezrin shRNAs expressing constructs described in (a) were analyzed with CCK8 to assess cell proliferation (b), with a transwell assay to determine cell motility (c), with a matrigel-coated transwell assay to determine cell invasiveness (d) and with an experimental metastatic assay by tail vein injection to determine gross pulmonary metastasis (e) (shc, n=10; sh1-1, n=9; sh1-2, n=8; sh2-1, n=9; sh2-2, n=8). (f) Western blot analysis of B16F1-HGF/Met cells transfected with vector (shc), Ezrin shRNA (sh1, sh2) expressing constructs. sh1-1, sh1-2 are two cell clones from sh1 construct, sh2-1 and sh2-2 are two cell clones from sh2 construct. (g–l) B16F1-HGF/Met cells transfected with vector (shc) and Ezrin shRNAs expressing constructs described in (f) were analyzed with CCK8 to assess cell proliferation (g), with a transwell assay to determine cell motility (h), with a matrigel-coated transwell assay to determine cell invasiveness (i), with an experimental metastatic assay by tail vein injection to determine gross pulmonary metastasis (j) (shc, n=8; sh1-1, n=9; sh1-2, n=9; sh2-1, n=9; sh2-2, n=9), with representation of lung H&E staining of metastases (k), and with macro- and micro- metastases counted in lung sections (l) (shc, n=6; sh1-2, n=6; sh2-2, n=6).
Ezrin is required for non-autocrine HGF/Met signaling-induced metastasis
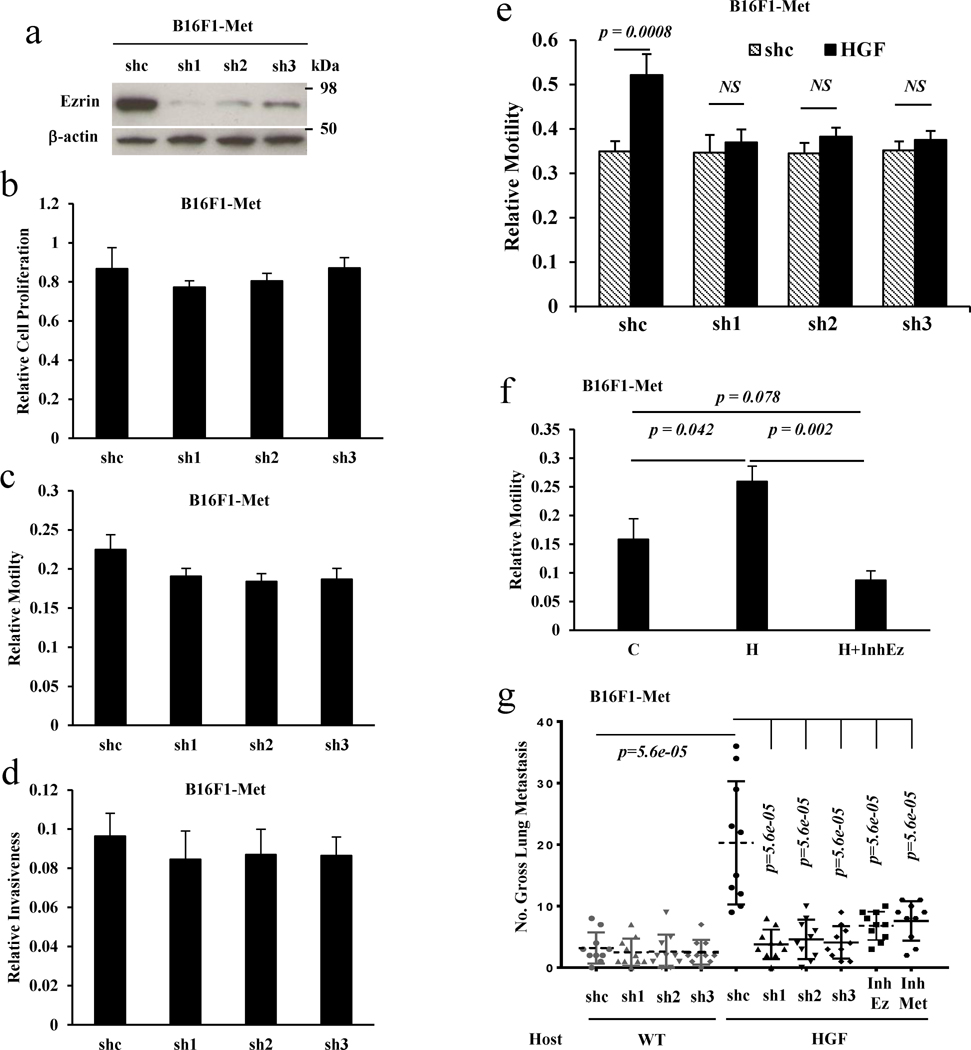
We previously reported that non-autocrine HGF/Met signaling also plays an essential role in promoting melanoma metastasis in GEM models.5, 8 To determine whether Ezrin is necessary for non-autocrine HGF/Met signaling-induced cell metastasis, we introduced three Ezrin shRNA constructs into stably expressing Met B16F1 cells (B16F1-Met).5Figure 3a shows that all three Ezrin shRNAs successfully downregulated endogenous Ezrin. Unlike in stably expressing HGF or HGF/Met cells, knockdown of Ezrin in stably expressing Met cells did not significantly inhibit cell proliferation (Figure 3b) or motility (Figure 3c) as well as cell invasiveness (Figure 3d). As expected, treatment of B16F1-Met cells with HGF increased cell motility (Figure 3e, 3f). However, knockdown of Ezrin by shRNAs abrogated this HGF-mediated increase in motility (Figure 3e). An Ezrin inhibitor also blocked the HGF-induced increase in motility (Figure 3f). When B16F1-Met cells carrying a shRNA vector control were injected into the tail vein of syngeneic wildtype or HGF transgenic mice, they exhibited a significantly higher number of pulmonary metastases in HGF transgenic mouse hosts compared with wildtype hosts through non-autocrine HGF/Met signaling, as we reported previously.5 However, the metastatic potential of the B16F1-Met cells bearing an Ezrin shRNA vector was low and not significantly different from wildtype host mice (Figure 3g). Interestingly, the B16F1-Met cells carrying shRNA Ezrin exhibited significantly fewer metastases compared with Met-expressing cells with empty control vector in HGF transgenic host mice (Figure 3g). Similar results also were found when Ezrin was knocked down in stably expressing Met 37-7 (37-7 Met) cells, resulting in a significant reduction of metastasis in FVB HGF transgenic mouse hosts (Figure S1e), suggesting that blocking Ezrin could abrogate the ability of non-autocrine-associated HGF/Met signaling-induced metastasis. These results indicate that Ezrin is also required for non-autocrine HGF/MET signaling-induced cell metastasis.
Figure 3. Ezrin is required for non-autocrine HGF/Met signaling-induced cell metastasis.
(a) Western blot analysis of B16F1 cells stably expressing Met (B16F1-Met) transfected with control vector (shc) and Ezrin shRNA (sh1, sh2, sh3) expressing constructs. (b–d) B16F1-Met cells transfected with control vector (shc) or the Ezrin shRNAs expressing constructs described in (a) were analyzed with CCK8 to assess cell proliferation (b), with a transwell assay to determine cell motility (c), and with a matrigel-coated transwell assay to determine cell invasiveness (d). (e) Inhibition of Ezrin by shRNA blocked HGF-induced cell motility in B16F1-Met cells; shc, vector control. (f) Cell motility was determined by transwell assay in B16F1-Met cells treated with (H) or without (C) HGF and HGF plus the Ezrin inhibitor NSC668394 (H+InhEz). (g) Gross pulmonary metastasis of B16F1-Met cells stably transfected with Ezrin shRNA (sh1, sh2, sh3) or control shRNA (shc) was determined using an experimental metastasis assay employing tail vein injections in wildtype (wt) (shc, n=10; sh1, n=10; sh2, n=10; sh3, n=10) or HGF transgenic (HGF) (shc, n=10; sh1, n=10; sh2, n=10; sh3, n=10) syngeneic host mice; and gross pulmonary metastasis of B16F1-Met cells stably transfected with control shRNA was determined with an experimental metastasis assay using tail vein injection in HGF transgenic (HGF) syngeneic host mice administrated with Ezrin (InhEz, n=10) or Met (InhMet, n=10) inhibitors.
A small molecule inhibitor of Ezrin blocks HGF/Met-induced tumor growth and metastasis
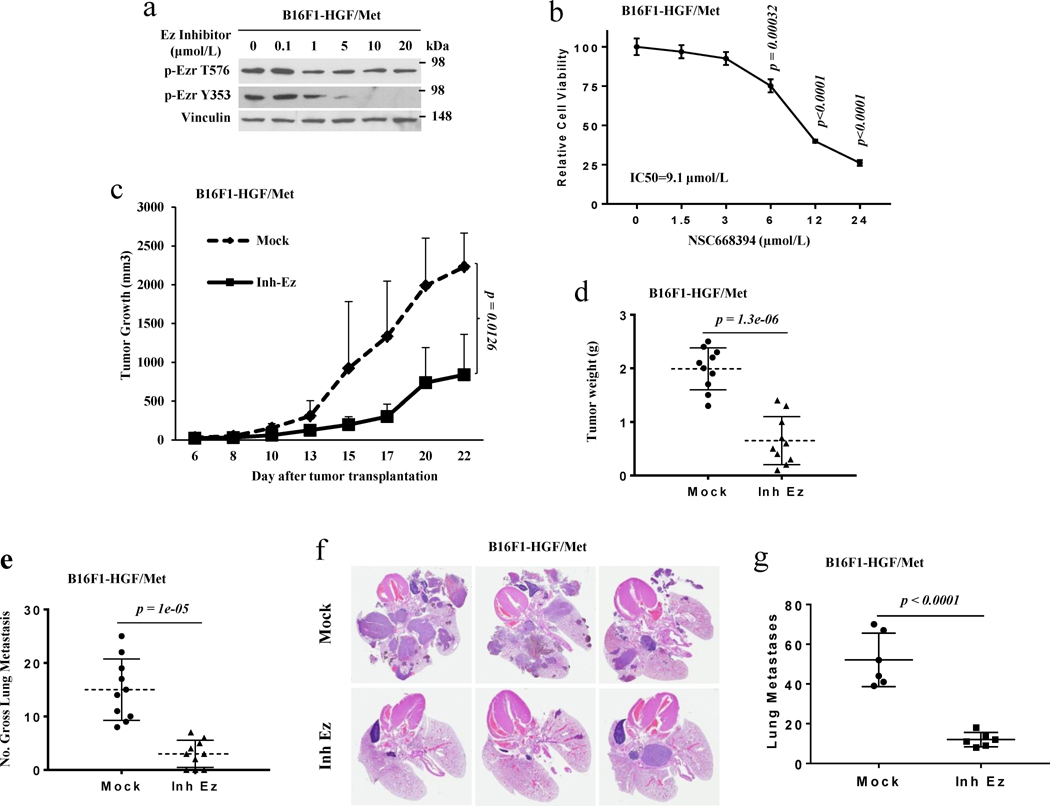
Our data show that Ezrin is required for both autocrine and non-autocrine HGF/Met signaling-induced melanoma metastases. To further confirm the importance of Ezrin in HGF/Met signaling-associated metastasis and examine its potential as a therapeutic target, we tested the efficacy of a small molecular inhibitor of Ezrin34 both in vitro and in vivo. Figure 4a shows that phosphorylated Ezrin T576 and Y353 in HGF/Met-expressing B16F1 (B16F1-HGF/Met) cells were decreased following treatment with the Ezrin inhibitor. The Ezrin inhibitor also blocked the growth of B16F1-HGF/Met cells (Figure 4b). To further examine the consequences of Ezrin inhibition in a more relevant preclinical mouse model in vivo, we designed and performed animal studies in xenograft and experimental metastasis models. Seven days after transplantation of B16F1-HGF/Met cells by subcutaneous injection into syngeneic mice, Ezrin inhibitor was administered to mice five times per week over two weeks by intraperitoneal injection. The tumors in the mice treated with Ezrin inhibitor grew significantly more slowly and weighed less than those in mice treated with vehicle control (Figure 4c, d). In the experimental metastasis model, immediately following transplantation of B16F1-HGF/Met cells into C57/BL6-c-Brd mice, the Ezrin inhibitor was administered to mice five times per week over three weeks by intraperitoneal injection. The mice treated with Ezrin inhibitor bore a significantly lower number of pulmonary macro and micro-metastases (Figure 4e, 4f and 4g). Moreover, the Ezrin inhibitor could also reduce the metastatic potential of B16F1 Met cells growing in HGF transgenic mouse hosts (Figure 3g). These data demonstrate that an Ezrin inhibitor can block Ezrin activation to reduce melanoma cell growth effectively and metastasis in cells stimulated by either autocrine or non-autocrine HGF/Met signaling. Our results further suggest that disturbing Ezrin inhibits the metastasis of melanoma with activation of HGF/Met signaling.
Figure 4. A small molecular inhibitor of Ezrin inhibits the growth and metastasis of melanoma cells.
(a) Western blot analysis of B16F1-HGF/Met cells treated with the Ezrin inhibitor NSC668394 for 2 hours with the indicated dosage. (b) The relative cell proliferation of B16F1-HGF/Met melanoma cells treated with the indicated dose of the Ezrin inhibitor NSC668394 for 24 hours. (c, d) Tumor growth curve (c) and tumor weight (d) of B16F1-HGF/Met cells in xenograft syngeneic C57/BL6-c-Brd mice with (Inh Ez, n=10)/without (Mock, n=10) treatment of Ezrin inhibitor NSC668394. (e) Gross pulmonary metastases in B16F1-HGF/Met cells from syngeneic C57/BL6-c-Brd mice treated with Ezrin inhibitor NSC668394 (Inh Ez, n=10) and control (Mock, n=10). (f) Representation of lung H&E staining of metastases of B16F1-HGF/Met cells in syngeneic C57/BL6-c-Brd mice treated with the Ezrin inhibitor (Inh Ez) or PBS (Mock). (g) Number of macro- and micro- metastases of B16F1-HGF/Met cells in syngeneic C57/BL6-c-Brd mice treated with the Ezrin inhibitor (Inh Ez, n=6) or control (Mock, n=6) in lung sections.
HGF stimulates Ezrin expression by upregulating the expression of the transcription factor Sp1
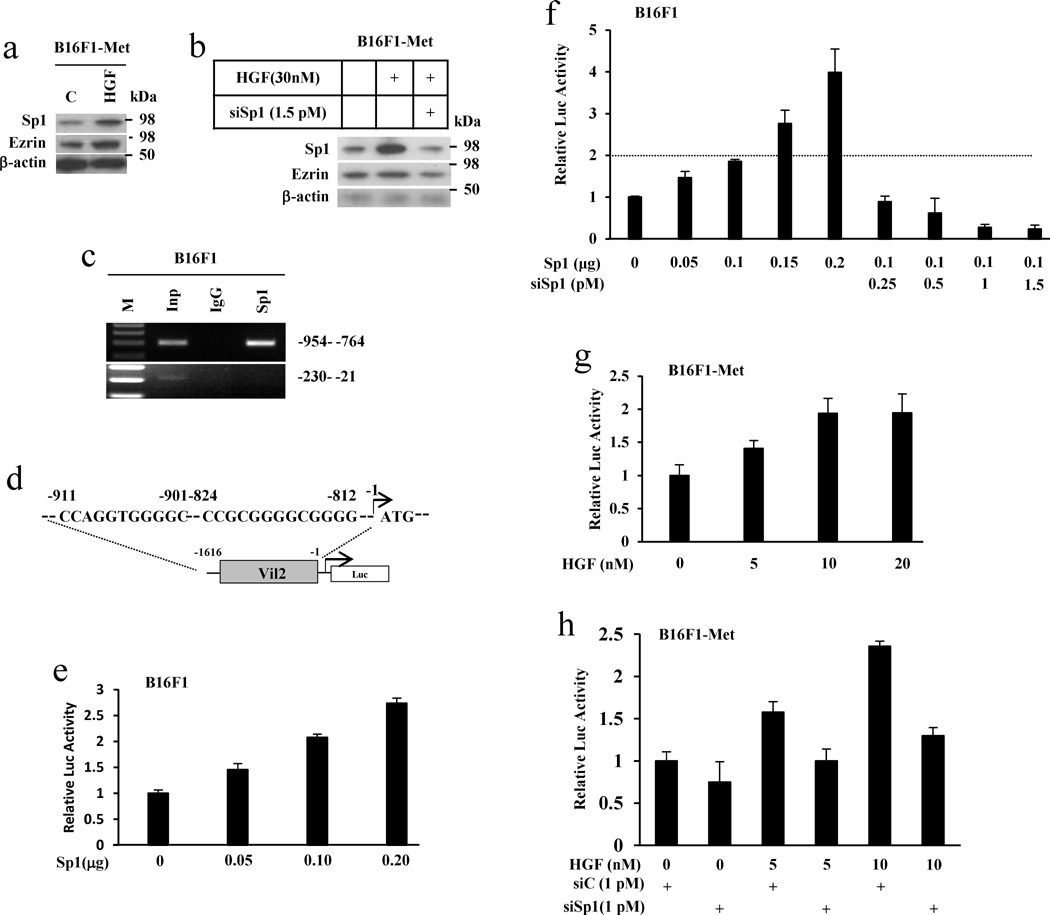
Our data now show that HGF/Met signaling stimulates Ezrin expression and Ezrin functions as a downstream effector in HGF/Met signaling-mediated melanoma metastasis. To understand the mechanism by which HGF/Met signaling regulates Ezrin expression, we analyzed the potential transcription factor binding sites within the mouse Ezrin gene (Vil2) promoter region (www.gene-regulation.com/pup/programs/alibaba2). Sp1 was previously reported to regulate the expression of the human Ezrin gene (Vil2),35 and several Sp1 binding sites were identified in the mouse vil2 promoter region (Figure S2). We hypothesized that HGF/Met signaling elevates the expression of Ezrin by regulating the Sp1 transcription factor. To test this, we first treated the melanoma B16F1-Met cells with HGF to determine whether HGF could stimulate Sp1 expression and whether there is a relationship between HGF/Met signaling, Sp1 and Ezrin. Results from a Western blot showed that treatment of B16F1-Met cells with HGF increased the expression of Sp1, as well as Ezrin (Figure 5a). Notably, a small interfering RNA of Sp1 could abrogate HGF-simulated Ezrin expression (Figure 5b). These results indicate that HGF can stimulate Sp1 expression, thereby upregulating Ezrin expression, and suggest that Sp1 is required for HGF/Met signaling-mediated expression of Ezrin. To confirm that Sp1 directly binds to the mouse Ezrin gene promoter, we performed a ChIP assay. Figure 5c shows that Sp1 can bind to the Ezrin gene promoter between −954 and −764, a region containing several Sp1 binding sequences (Figure S2), but not to an irrelevant site between −230 and −21. We next determined the effect of Sp1 on the luciferase activity using an Ezrin gene promoter-driven luciferase reporter construct (ref 20, Figure 5d). Figure 5e and 5f show that Sp1 can stimulate luciferase activity in B16F1 cells harboring an Ezrin gene promoter-luciferase expression vector in a dose-dependent fashion, an effect that can be reversed with a Sp1 siRNA (Figure 5f). To further confirm the role of Sp1 as a link between HGF and Ezrin, we treated the B16F1-Met cells harboring an Ezrin gene promoter-driven luciferase expression vector with HGF and found that luciferase activities were increased following increasing doses of HGF (Figure 5g). Notably, consistent with the result from Figure 5b, the siRNA for Sp1 blocked the HGF-stimulated Ezrin gene promoter-driven luciferases activity (Figure 5h). Together, the results show that Sp1, which is stimulated by HGF, directly binds to Ezrin gene promoter and transcriptionally regulates Ezrin expression. Our data demonstrate that Sp1 is a functional link between HGF and Ezrin, indicating that HGF-induced Ezrin expression acts through regulation of the transcription factor Sp1.
Figure 5. HGF stimulates the expression of Ezrin through activation of transcription factor Sp1.
(a) Whole cell lysates from B16F1-Met cells treated with 30 nM HGF for 24 hours were analyzed by western blot. (b) Western blot analysis of B16F1-Met cells treated with HGF only or plus 1.5 pmol of Sp1 siRNA at the indicated dosage for 24 hours. (c) The physical interaction of Sp1 and the Ezrin gene promoter was assessed by ChIP assay. Native Sp1 in B16F1 cells was analyzed using an anti-Sp1 antibody. Sp1 bound to the −954 to −764, but not the −230 to −21 regions. Inp, input; M, markers. (d) Two candidate Sp1 binding sites consisting of the 5′-(G/T)GGGCGG(G/A)(G/A)(C/T)-3′ were found within the 5′-flanking region (between −911 and −812 bp) of the Ezrin gene promoter. (e) Ezrin gene promoter (−1616 to −1) activity was shown to be responsive to increasing amounts of a Sp1 expression vector using a firefly luciferase (Luc) reporter. (f) Using the same luciferase assay, the addition of siRNA for Sp1 with a Sp1-expression vector inhibited Sp1–induced luciferase activity driven by the Ezrin gene promoter. (g) Ezrin gene promoter (−1616 to −1) activity was stimulated by increasing amounts of HGF using a firefly luciferase (Luc) reporter. (h) Using the same luciferase assay, the addition of siRNA for Sp1 inhibited HGF-stimulated luciferase activity driven by the Ezrin gene promoter in a co-transfection assay.
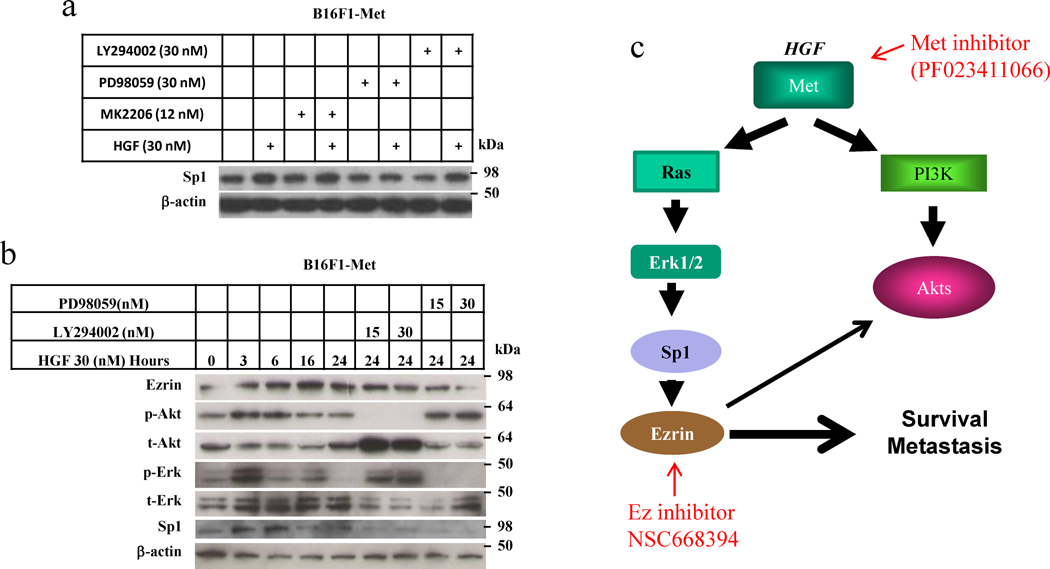
HGF binds to its receptor Met and induces autophosphorylation of Met, activating MAPK and PI3K/AKT and thereby many other signaling downstream pathways. To determine which downstream signaling pathway is involved in upregulating Sp1 and thus enhancing Ezrin expression, we treated the B16F1-Met cells with HGF, and with or without an inhibitor of MAPK or PI3K. Figure 6a shows that the MAPK inhibitor PD98059 blocked the HGF-induced Sp1 activity in early, but the PI3K (LY294002) and AKT (MK2206) inhibitors did not, indicating that activation of Sp1 requires Erk1/2 MAPK signaling but not PI3K/AKT signaling. To further confirm this finding, we treated the B16F1-Met cells with HGF and with/without the MAPK inhibitor PD98059 or the PI3K inhibitor LY294002 for 24 hours and used Western blotting to analyze the whole lysate from the cells. As expected, HGF activated Sp1 and increased the expression of Ezrin after 3 hours of treatment, whereas a MAPK inhibitor blocked the HGF-mediated Sp1 activity and expression of Ezrin (Figure 6b). These results show that inhibition of MAPK activity blocks the activation of Sp1 and expression of Ezrin-mediated by HGF/Met signaling. Our data demonstrate that Sp1 is downstream of MAPK stimulation in the HGF/Met signaling pathway and that upon activation by HGF/Met signaling binds to the Ezrin gene promoter and upregulates Ezrin expression (Figure 6c).
Figure 6. Inhibition of MAPK activity blocks activation of Sp1 and expression of Ezrin.
(a) Western blot analysis of Sp1 expression in B16F1-Met cells treated with HGF plus MAPK inhibitor PD89059, PI3K inhibitor LY294002 or AKT inhibitor MK2206 for 8 hours. (b) Western blot analysis of B16F1-Met cells treated with HGF plus MAPK inhibitor PD89059 or PI3K inhibitor LY294002 for from 0 to 24 hours. (c) Mechanical pathway of Ezrin transactivation by HGF/Met signaling through transcription Sp1.
Discussion
We and others have previously shown that autocrine and/or non-autocrine HGF/Met signaling not only play a pleiotropic role in cell proliferation, migration, and invasion but also participate in metastatic progression in melanoma and other cancers.1–5 In our current study, we uncovered a novel molecular mechanism by which HGF/Met signaling regulates melanoma metastasis. Our findings show that HGF/Met signaling promotes expression of the pro-metastatic Ezrin gene and that blocking Ezrin by shRNA and small molecular inhibitors inhibits autocrine and non-autocrine HGF/Met signaling-induced melanoma metastasis. We also show that HGF stimulates the expression of the transcription factor Sp1, which binds directly to the Ezrin gene promoter and regulates Ezrin expression, linking HGF/Met signaling to Ezrin in melanoma metastasis.
HGF, as a scatter factor, activates the receptor tyrosine kinase Met, triggering downstream signal pathways such as GAB1, GRB2, Src, PI3K/AKT, JNK and p38MAPK and enhancing cell proliferation, survival, motility, anchorage-independent growth, migration, and invasiveness.1–4 As an invasive growth stimulus, HGF/Met signaling has been reported to affect the activation of several cell adhesion and extracellular matrix (ECM) molecules that regulate the cytoskeletal network; for instance, activation of Met results in the phosphorylation of E-cadherin.36 HGF was also shown to induce phosphorylation of focal adhesion kinase (FAK) and promote cell motility.37,38 After phosphorylation on tyrosine 1349, Met becomes a docking site for recruiting Gab1, which further activates downstream FAK and PAK. Activation of both Met/Gab1/FAK and Met/Gab1/PAK signaling promotes tumor cell motility and migration.3 Dysregulation of HGF/Met signaling has emerged as a crucial feature of many human metastatic tumors,2,3 and numerous efforts have been directed towards the development of small, low-molecular-weight biological inhibitors targeting HGF and Met.2 Although HGF/Met is broadly expressed in adult tissue and has been implicated in potential physiological and pathophysiological processes, there is concern about the possibility that inhibition of broad HGF/Met signaling may influence normal physiological functions.1,2 Therefore, the identification of specific downstream targets responsible for HGF/Met signaling-mediated metastasis may facilitate the development of novel and more effective therapies for patients with advanced cancer.
The determination and significance of Ezrin in downstream of HGF/Met signaling explain the high metastatic potential that has been associated with the Met receptor. Indeed, as a linker between cell membrane and cytoskeleton, Ezrin has been reported to be overexpressed in numerous tumor types and to participate in the regulation of progression to the metastatic state.15 In accord with our finding that Ezrin is a downstream effector of HGF/Met signaling, previous studies have shown that HGF/Met signaling can activate Ezrin by phosphorylation of sites Y145 and Y353, thereby enhancing epithelial cell migration and tubulogenesis; Ezrin when truncated or mutated at sites Y145 and Y353 exhibits impaired morphogenic and mitogenic responses to HGF/Met signaling.11,12 It is worth noting that the inhibition of Ezrin by shRNA or small molecular inhibitor resulted in reduced proliferation and inhibited tumor growth, therefore, they could affect the actual measurement of cell motility and invasion as well as metastasis. To adequately determine the cell motility and invasion, however, we examined the cell ability of motility and invasion at 12 hours using transwell assay. We also assessed the micrometastases in lung sections under the microscope. Our findings are the first evidence that HGF/Met signaling also upregulates Ezrin expression in melanoma for promoting melanoma cell migration, invasion in vitro, and metastasis in vivo.
We previously found that the homeoprotein Six1 transcriptionally activates Ezrin gene expression in rhabdomyosarcoma.20 However, Six1 is typically not expressed in melanoma cells, indicating that other transcription factors may be involved in the regulation of Ezrin in melanoma. Using Ezrin gene promoter sequences and transcription array analyses, we found several CG-rich Sp1 binding sites within the Ezrin gene promoter. Consistent with previous findings that Sp1 regulates expression of the human Ezrin gene (Vil2) in esophageal carcinoma cells,35 we confirmed that Sp1 directly (physically) binds to the Ezrin gene promoter and promotes the transcriptional expression of the Ezrin gene. Notably, we found that HGF activates the Ezrin gene promoter through Sp1; HGF/Met-mediated Ezrin gene promoter activation was blocked by siRNA for Sp1 in our study.
Sp1 is a transcription factor that can activate or repress the transcription of genes responsive to physiologic and pathological stimuli, and can, therefore, help regulate a diverse array of cellular processes, such as cell growth, differentiation, apoptosis, angiogenesis, carcinogenesis, and metastasis.39–42 Sp1 has been reported to regulate the expression of genes that play pivotal roles in cancer cell behavior, including IGF1R,43 EGFR,44 VEGF,45 PDGF,46 uPA,47 MMP9 48 and MMP2.49 Sp1 is overexpressed in many cancers and is associated with tumor progression as well as poor prognosis. 40–42 Clinically, a high level of Sp1 protein has been associated with a variety of cancers.40–42,50 Growing evidence also indicates that posttranslational modifications of Sp1 such as phosphorylation, acetylation, sumoylation, ubiquitination, and glycosylation could influence the transcriptional activity and stability of Sp1.50 For example, HGF activates the VEGF gene promoter by inducing the phosphorylation of Sp1.41 We show here that HGF/Met signaling not only rapidly phosphorylates Sp1 (Figure S3) but also enhances its expression later on. Notably, these effects were blocked by the MAPK inhibitor, suggesting that HGF/Met signaling may mediate activation of Sp1 at both transcriptional and post-translational modification levels through the MAPK pathway. Consistent with our findings, previous studies have shown that Sp1 is activated by the p42/p44 MAPK signaling cascade in other cancers.47,50 In contrast, these effects were not inhibited by the PI3K/AKT inhibitor, implying that this downstream pathway of HGF/Met signaling is not involved in the regulation of Sp1 in melanoma.
In summary, we here provide the first evidence that HGF/Met signaling promotes Ezrin expression through the transcription factor Sp1. The inhibition of Ezrin function by either the siRNAs or an Ezrin inhibitor blocks HGF/Met signaling-mediated melanoma cell motility and metastasis. Our data demonstrate that Ezrin is a key downstream molecule involved in the regulation of HGF/Met signaling-induced metastasis, and introduce the notion that Ezrin represents a potentially effective therapeutic target for metastatic melanomas activated by aberrant HGF/Met signaling.
Supplementary Material
Novelty and Impact.
The study provides the first evidence that HGF/Met signaling promotes Ezrin expression through the transcription factor Sp1. The determination that Ezrin is located downstream of HGF/Met signaling helps to explain the high metastatic potential that has long been associated with the Met receptor. The identification of specific downstream targets responsible for HGF/Met signaling-mediated metastasis may facilitate the development of novel and more effective therapies for patients with advanced cancer.
Acknowledgments
Funding
This project was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health. The institute had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Footnotes
The authors have no conflict of interest to declare.
Author contribution statement
LH and YY designed experiments; LH, QZ, KB, JX, and YY performed experiments and analyzed data; YY wrote the manuscript, and GM revised the manuscript. All authors read and approved the manuscript.
References
- 1.Trusolino L, Bertotti A, Comoglio PM. MET signaling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–48. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 2.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 3.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–25. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 4.Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, Graziani A, Panayotou G, Comoglio PM. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–71. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 5.Yu Y, Merlino G. Constitutive c-Met signaling through a nonautocrine mechanism promotes metastasis in a transgenic transplantation model. Cancer Res. 2002;62:2951–6. [PubMed] [Google Scholar]
- 6.Sharp R, Recio JA, Jhappan C, Otsuka T, Liu S, Yu Y, Liu W, Anver M, Navid F, Helman LJ, DePinho RA, Merlino G. Synergism between INK4a/ARF inactivation and aberrant HGF/SF signaling in rhabdomyosarcomagenesis. Nat Med. 2002;8:1276–80. doi: 10.1038/nm787. [DOI] [PubMed] [Google Scholar]
- 7.Jiang W, Hiscox S, Matsumoto K, Nakamura T. Hepatocyte growth factor/scatter factor, its molecular, cellular and clinical implications in cancer. Crit Rev Oncol Hematol. 1999;29:209–48. doi: 10.1016/s1040-8428(98)00019-5. [DOI] [PubMed] [Google Scholar]
- 8.Rong S, Segal S, Anver M, Resau JH, Vande Woude GF. Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc Natl Acad Sci U S A. 1994;91:4731–5. doi: 10.1073/pnas.91.11.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–9. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura T, Matsumoto K, Kiritoshi A, Tano Y, Nakamura T. Induction of hepatocyte growth factor in fibroblasts by tumor-derived factors affects invasive growth of tumor cells: in vitro analysis of tumor-stromal interactions. Cancer Res. 1997;57:3305–13. [PubMed] [Google Scholar]
- 11.Crepaldi T, Gautreau A, Comoglio PM, Louvard D, Arpin M. Ezrin is an effector of hepatocyte growth factor-mediated migration and morphogenesis in epithelial cells. J Cell Biol. 1997;138:423–34. doi: 10.1083/jcb.138.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naba A, Reverdy C, Louvard D, Arpin M. Spatial recruitment and activation of the Fes kinase by ezrin promotes HGF-induced cell scattering. EMBO J. 2008;27:38–50. doi: 10.1038/sj.emboj.7601943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato N, Funayama N, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites. J Cell Sci. 1992;103(Pt 1):131–43. doi: 10.1242/jcs.103.1.131. [DOI] [PubMed] [Google Scholar]
- 14.Lan M, Kojima T, Murata M, Osanai M, Takano K, Chiba H, Sawada N. Phosphorylation of ezrin enhances microvillus length via a p38 MAP-kinase pathway in an immortalized mouse hepatic cell line. Exp Cell Res. 2006;312:111–20. doi: 10.1016/j.yexcr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11:276–87. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou R, Cao X, Watson C, Miao Y, Guo Z, Forte JG, Yao X. Characterization of protein kinase A-mediated phosphorylation of ezrin in gastric parietal cell activation. J Biol Chem. 2003;278:35651–9. doi: 10.1074/jbc.M303416200. [DOI] [PubMed] [Google Scholar]
- 17.Ng T, Parsons M, Hughes WE, Monypenny J, Zicha D, Gautreau A, Arpin M, Gschmeissner S, Verveer PJ, Bastiaens PI, Parker PJ. Ezrin is a downstream effector of trafficking PKC-integrin complexes involved in the control of cell motility. EMBO J. 2001;20:2723–41. doi: 10.1093/emboj/20.11.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Y, Khan J, Khanna C, Helman L, Meltzer PS, Merlino G. Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat Med. 2004;10:175–81. doi: 10.1038/nm966. [DOI] [PubMed] [Google Scholar]
- 19.Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol. 2007;178:517–27. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Y, Davicioni E, Triche TJ, Merlino G. The homeoprotein six1 transcriptionally activates multiple protumorigenic genes but requires ezrin to promote metastasis. Cancer Res. 2006;66:1982–9. doi: 10.1158/0008-5472.CAN-05-2360. [DOI] [PubMed] [Google Scholar]
- 21.Gautreau A, Poullet P, Louvard D, Arpin M. Ezrin, a plasma membrane-microfilament linker, signals cell survival through the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci U S A. 1999;96:7300–5. doi: 10.1073/pnas.96.13.7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, Yeung C, Gorlick R, Hewitt SM, Helman LJ. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004;10:182–6. doi: 10.1038/nm982. [DOI] [PubMed] [Google Scholar]
- 23.Heiska L, Melikova M, Zhao F, Saotome I, McClatchey AI, Carpén O. Ezrin is key regulator of Src-induced malignant phenotype in three-dimensional environment. Oncogene. 2011;30:4953–62. doi: 10.1038/onc.2011.207. [DOI] [PubMed] [Google Scholar]
- 24.Hiscox S, Jiang WG. Ezrin regulates cell-cell and cell-matrix adhesion, a possible role with E-cadherin/beta-catenin. J Cell Sci. 1999;112(Pt 18):3081–90. doi: 10.1242/jcs.112.18.3081. [DOI] [PubMed] [Google Scholar]
- 25.Legg JW, Lewis CA, Parsons M, Ng T, Isacke CM. A novel PKC-regulated mechanism controls CD44 ezrin association and directional cell motility. Nat Cell Biol. 2002;4:399–407. doi: 10.1038/ncb797. [DOI] [PubMed] [Google Scholar]
- 26.Haag A, Gutierrez P, Bühler A, Walser M, Yang Q, Langouët M, Kradolfer D, Fröhli E, Herrmann CJ, Hajnal A, Escobar-Restrepo JM. An in vivo EGF receptor localization screen in C. elegans Identifies the Ezrin homolog ERM-1 as a temporal regulator of signaling. PLoS Genet. 2014;10:e1004341. doi: 10.1371/journal.pgen.1004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruce B, Khanna G, Ren L, Landberg G, Jirström K, Powell C, Borczuk A, Keller ET, Wojno KJ, Meltzer P, Baird K, McClatchey A, Bretscher A, Hewitt SM, Khanna C. Expression of the cytoskeleton linker protein ezrin in human cancers. Clin Exp Metastasis. 2007;24:69–78. doi: 10.1007/s10585-006-9050-x. [DOI] [PubMed] [Google Scholar]
- 28.Deng X, Tannehill-Gregg SH, Nadella MV, He G, Levine A, Cao Y, Rosol TJ. Parathyroid hormone-related protein and ezrin are up-regulated in human lung cancer bone metastases. Clin Exp Metastasis. 2007;24:107–19. doi: 10.1007/s10585-007-9059-9. [DOI] [PubMed] [Google Scholar]
- 29.Chuan YC, Iglesias-Gato D, Fernandez-Perez L, Cedazo-Minguez A, Pang ST, Norstedt G, Pousette A, Flores-Morales A. Ezrin mediates c-Myc actions in prostate cancer cell invasion. Oncogene. 2010;29:1531–42. doi: 10.1038/onc.2009.442. [DOI] [PubMed] [Google Scholar]
- 30.Madan R, Brandwein-Gensler M, Schlecht NF, Elias K, Gorbovitsky E, Belbin TJ, Mahmood R, Breining D, Qian H, Childs G, Locker J, Smith R, Haigentz M, Jr, Gunn-Moore F, Prystowsky MB. Differential tissue and subcellular expressionof ERM proteins in normal and malignant tissues: cytoplasmic ezrin expression has prognostic signficance for head and neck squamous cell carcinoma. Head Neck. 2006;28:1018–27. doi: 10.1002/hed.20435. [DOI] [PubMed] [Google Scholar]
- 31.Oda Y, Aishima S, Morimatsu K, Hayashi A, Shindo K, Fujino M, Mizuuchi Y, Hattori M, Tanaka M, Oda Y. Differential ezrin and phosphorylated ezrin expression profiles between pancreatic intraepithelial neoplasia, intraductal papillary mucinous neoplasm, and invasive ductal carcinoma of the pancreas. Hum Pathol. 2013;44(8):1487–98. doi: 10.1016/j.humpath.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Chuan YC, Pang ST, Cedazo-Minguez A, Norstedt G, Pousette A, Flores-Morales A. Androgen induction of prostate cancer cell invasion is mediated by ezrin. J Biol Chem. 2006;281:29938–48. doi: 10.1074/jbc.M602237200. [DOI] [PubMed] [Google Scholar]
- 33.Yu Y, Zeng P, Xiong J, Liu Z, Berger SL, Merlino G. Epigenetic drugs can stimulate metastasis through enhanced expression of the pro-metastatic Ezrin gene. PLoS One. 2010;5:e12710. doi: 10.1371/journal.pone.0012710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bulut G, Hong SH, Chen K, Beauchamp EM, Rahim S, Kosturko GW, Glasgow E, Dakshanamurthy S, Lee HS, Daar I, Toretsky JA, Khanna C, Uren A. Small molecule inhibitors of ezrin inhibit the invasive phenotype of osteosarcoma cells. Oncogene. 2012;31:269–81. doi: 10.1038/onc.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao SY, Li EM, Cui L, Lu XF, Meng LY, Yuan HM, Xie JJ, Du ZP, Pang JX, Xu LY. Sp1 and AP-1 regulate expression of the human gene VIL2 in esophageal carcinoma cells. J Biol Chem. 2009;284:7995–8004. doi: 10.1074/jbc.M809734200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matteucci E, Ridolfi E, Desiderio MA. Hepatocyte growth factor differently influences Met-E-cadherin phosphorylation and downstream signaling pathway in two models of breast cells. Cell Mol Life Sci. 2006;63:2016–26. doi: 10.1007/s00018-006-6137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu ZX, Yu CF, Nickel C, Thomas S, Cantley LG. Hepatocyte growth factor induces ERK-dependent paxillin phosphorylation and regulates paxillin-focal adhesion kinase association. J Biol Chem. 2002;277:10452–8. doi: 10.1074/jbc.M107551200. [DOI] [PubMed] [Google Scholar]
- 38.Lai JF, Kao SC, Jiang ST, Tang MJ, Chan PC, Chen HC. Involvement of focal adhesion kinase in hepatocyte growth factor-induced scatter of Madin-Darby canine kidney cells. J Biol Chem. 2000;275:7474–80. doi: 10.1074/jbc.275.11.7474. [DOI] [PubMed] [Google Scholar]
- 39.Opitz OG, Rustgi AK. Interaction between Sp1 and cell cycle regulatory proteins is important in transactivation of a differentiation-related gene. Cancer Res. 2000;60:2825–30. [PubMed] [Google Scholar]
- 40.Jiang Y, Wang L, Gong W, Wei D, Le X, Yao J, Ajani J, Abbruzzese JL, Huang S, Xie K. A high expression level of insulin-like growth factor I receptor is associated with increased expression of transcription factor Sp1 and regional lymph node metastasis of human gastric cancer. Clin Exp Metastasis. 2004;21:755–64. doi: 10.1007/s10585-005-1198-2. [DOI] [PubMed] [Google Scholar]
- 41.Wang L, Wei D, Huang S, Peng Z, Le X, Wu TT, Yao J, Ajani J, Xie K. Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin Cancer Res. 2003;9:6371–80. [PubMed] [Google Scholar]
- 42.Yao JC, Wang L, Wei D, Gong W, Hassan M, Wu TT, Mansfield P, Ajani J, Xie K. Association between expression of transcription factor Sp1 and increased vascular endothelial growth factor expression, advanced stage, and poor survival in patients with resected gastric cancer. Clin Cancer Res. 2004;10(12 Pt 1):4109–17. doi: 10.1158/1078-0432.CCR-03-0628. [DOI] [PubMed] [Google Scholar]
- 43.Beitner-Johnson D, Werner H, Roberts CT, Jr, LeRoith D. Regulation of insulin-like growth factor I receptor gene expression by Sp1: physical and functional interactions of Sp1 at GC boxes and at a CT element. Mol Endocrinol. 1995;9:1147–56. doi: 10.1210/mend.9.9.7491107. [DOI] [PubMed] [Google Scholar]
- 44.Xu K, Shu HK. EGFR activation results in enhanced cyclooxygenase-2 expression through p38 mitogen-activated protein kinase-dependent activation of the Sp1/Sp3 transcription factors in human gliomas. Cancer Res. 2007;67:6121–9. doi: 10.1158/0008-5472.CAN-07-0141. [DOI] [PubMed] [Google Scholar]
- 45.Shi Q, Le X, Abbruzzese JL, Peng Z, Qian CN, Tang H, Xiong Q, Wang B, Li XC, Xie K. Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Res. 2001;61(10):4143–54. [PubMed] [Google Scholar]
- 46.Rafty LA, Khachigian LM. von Hippel-Lindau tumor suppressor protein represses platelet-derived growth factor B-chain gene expression via the Sp1 binding element in the proximal PDGF-B promoter. J Cell Biochem. 2002;85:490–5. doi: 10.1002/jcb.10152. [DOI] [PubMed] [Google Scholar]
- 47.Benasciutti E, Pagès G, Kenzior O, Folk W, Blasi F, Crippa MP. MAPK and JNK transduction pathways can phosphorylate Sp1 to activate the uPA minimal promoter element and endogenous gene transcription. Blood. 2004;104:256–62. doi: 10.1182/blood-2003-08-2661. [DOI] [PubMed] [Google Scholar]
- 48.Murthy S, Ryan AJ, Carter AB. SP-1 regulation of MMP-9 expression requires Ser586 in the PEST domain. Biochem J. 2012;445:229–36. doi: 10.1042/BJ20120053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sze KM, Wong KL, Chu GK, Lee JM, Yau TO, Ng IO. Loss of phosphatase and tensin homolog enhances cell invasion and migration through AKT/Sp-1 transcription factor/matrix metalloproteinase 2 activation in hepatocellular carcinoma and has clinicopathologic significance. Hepatology. 2011;53:1558–69. doi: 10.1002/hep.24232. [DOI] [PubMed] [Google Scholar]
- 50.Beishline K, Azizkhan-Clifford J. Sp1 and the ‘hallmarks of cancer’. FEBS J. 2015;282:224–58. doi: 10.1111/febs.13148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.