Abstract
The skin is the largest organ of the body and is composed of two layers: the overlying epidermis and the underlying dermis. The dermal fibroblasts originate from distinct locations in the embryo and contain the positional identity and patterning information in the skin. The dermal fibroblast progenitors differentiate into various cell types that are fated to perform specific functions such as hair follicle initiation and scar formation during wound healing. Recent studies have revealed the heterogeneity and plasticity of dermal fibroblasts within skin, which has implications for skin disease and tissue engineering. The objective of this review is to frame our current understanding and provide new insights on the origin and differentiation of dermal fibroblasts and their function during cutaneous development and healing.
Graphical Abstract
Introduction
The skin has served as an excellent model system to understand the complex interplay of different cells types and molecular signaling pathways during development and homeostasis 1–9. Skin organ homeostasis is achieved through constant crosstalk between the major components: dermal fibroblasts, keratinocytes, immune cells, nerves, and intradermal adipocytes 10–13. The epidermis gives rise to the hair and glands and contributes to thermoregulation and barrier formation 1. The dermis provides the structure, strength and flexibility to the skin, and houses other structures such as hair, glands, vessels, and nerves. The main resident cell type of the dermis is the dermal fibroblast, which produces the extracellular matrix (ECM) and contribute to hair follicle initiation and cycling 10,14,15. Early embryonic dermal fibroblast progenitors can potentially differentiate into several cell types such as upper papillary fibroblasts (PF), lower reticular fibroblasts (RF), dermal condensate/dermal papilla (DP), and intradermal adipocytes/dermal white adipose tissue (DWAT) 16. The papillary dermis plays an instructive role for the overlying epidermis in appendage formation 17–21. The adult epidermis and dermis generally lack the capacity to regenerate skin completely after wounding with the exception of the African spiny mouse which has scar-free healing. Gaining deeper insights into dermal fibroblast development can take us one step closer to developing fibroblast-directed therapies for scar-free healing. Here, we will primarily highlight the origin of dermal fibroblasts; genesis of their derivatives; heterogeneity among dermal populations, and their role in wound healing.
Origin of dermal fibroblasts
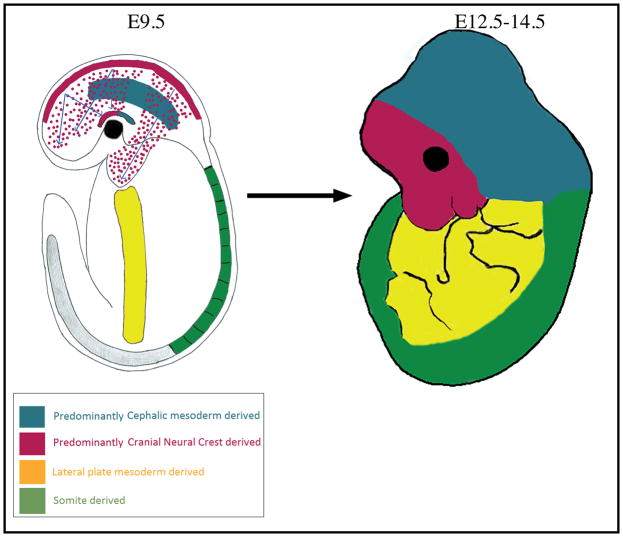
Cell fate-mapping analysis using a variety of techniques in the chick and mouse embryo reveals similarities and differences in the origin of dermal fibroblasts. In the avian and mouse embryo, dorsal dermal fibroblast progenitors originate from the somite 22–24. The ventral and flank trunk dermis, as well as limb dermis, is derived from the lateral plate mesoderm and migrates beneath the ectoderm 25–27 (Figure 1). In birds, craniofacial dermis arises entirely from cranial neural crest that migrates extensively from the dorsal neural tube to populate the skin of the head and face 28,29. In contrast, mammalian craniofacial dermis has a dual embryonic origin of cranial neural crest and cephalic mesoderm 28,30,31. These lineage analysis experiments in both mouse and chick reveal that embryonic dermal fibroblasts originate from different parts of the embryo and populate the skin closest to their origin.
Figure 1.
Emergence of dorsal, ventral, and craniofacial dermal fibroblast progenitors
Our understanding of emergence and early migration of dermal fibroblast precursors and specified progenitors comes from extensive studies in avian models. It is of general consensus that these mechanisms are evolutionarily conserved in other vertebrate models as well.
The chick and mouse dorsal trunk dermal fibroblasts originate from the dermomyotme (DM) of somites 22,32,33. The dermal fibroblast precursors delaminate from the dorsal portion of the DM and migrate short distances to populate the fibrous lattice of subectodermal extracellular space in response to signals from the neural tube and ectoderm 34. Preceding migration, the dorsal DM cells undergo epithelial to mesenchymal transition (EMT) and express Wnt11 34,35. Wnt11 silencing in the chick DM hinders EMT and migration of dorsal dermal precursors 36. Likewise, the Wnt11−/− mutant mouse has reduced dermal volume and hair follicle density in the dermis directly above the neural tube 36. The mechanism through which Wnt11 promotes the movement of dermal fibroblast precursors is unknown. In both chick and mouse embryos, cells delaminate from the dorsal somatopleure of the lateral plate mesoderm (LPM) and give rise to the flank, ventral trunk, and limb dermis 25,26,37. Between E9.5 and E12.5 in the mouse, WNT/β-CATENIN signaling is required for survival of the ventral dermal precursors and their migration to the midline 25.
The mammalian facial dermis is derived from cranial neural crest cells (cNCCs) and the cranial dermis is derived predominantly from the paraxial/cephalic mesoderm 28,30,38 (Figure 1). The avian craniofacial dermis is thought to be derived entirely from cNCC 29. Between E8.5 and E9.5 in the mouse, forebrain and hindbrain cNCCs migrate extensively from the neural plate border to the branchial arches and frontonasal mass 39,40. cNCCs under the ectoderm become the dermal precursors of the face and anterior head (Figure 1). Both attractive and repulsive cues, specifically ephrins and semaphorins, govern the cNCC migratory path 41,42. cNCC proliferation and survival during migration is regulated by Platelet derived growth factor receptor (PDGFR)-αlpha signaling through PI3/Akt signaling 43. The forebrain and midbrain cNCC migrate to the anterior supraorbital arch (SOA) region above the eye by E10.5 and become dermal fibroblast precursors for the forehead skin 28,31. The cephalic mesoderm cells migrate to the posterior SOA by E10.5 (Figure 1) and become dermal fibroblast precursors for most of the cranial skin. From E10.5, the specified cranial dermal fibroblast progenitors expand apically from the SOA to fill the cranial region 28,30,31.
The signals and mechanism of cellular movements underlying the expansion of dorsal, ventral, and cranial dermal progenitors are largely unknown. The next frontier is to understand how intrinsic genetic instructions and extrinsic spatial information guide large scale cellular movements to create the dermis. These results along with more detailed fate maps will be informative for understanding the etiology of congenital dermal hypoplasias such as Setleis syndrome, Goltz syndrome, and Aplasia cutis congenita 44–46.
Dermal fibroblasts cell fate selection
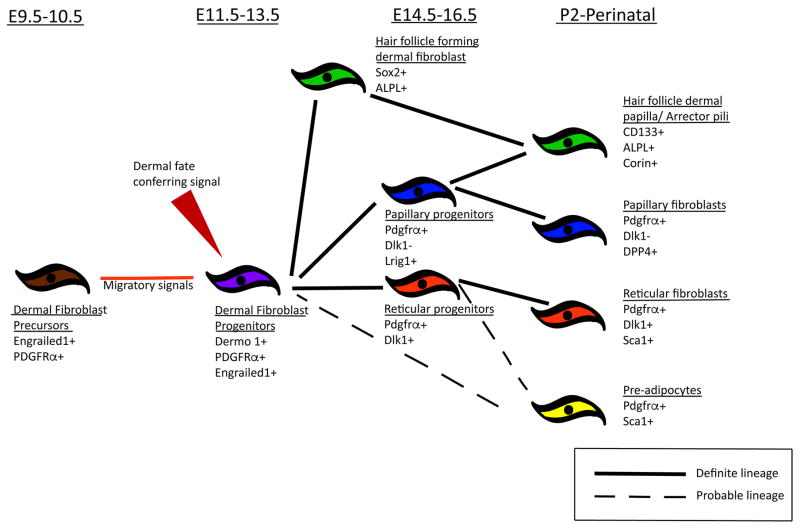
During cell fate selection between E10.5–12.5, WNT/β-CATENINsignaling is the earliest signaling pathway in the dermal fibroblast progenitors, and it is instructive for dermal fibroblast cell fate (Figure 2) 4,22,25,31. Throughout the embryo, the early dermal fibroblast progenitors in the sub-ectodermal regions from E10.5 are WNT signaling responsive and express nuclear β-catenin 4,22,25,31. Ectodermal WNTS are necessary for dermal WNT signaling responsiveness. Conditional deletion of cranial or dorsal ectoderm Wntless, a cargo protein required for all WNT ligand secretion, leads to loss of early dermal lineage markers in the cranial and dorsal regions, respectively 15,47. Conditional ablation of β-catenin in craniofacial and LPM dermal progenitors starting at E9.5 leads to conversion of dermal lineages to cartilage 25,31. Conversely, sustained expression of stabilized β-catenin leads to expansion of dermal progenitors suggesting it is sufficient to elicit dermal fate 15,25,31. Thus, WNT/β-CATENIN signaling is an instructive cue for specifying dermal fibroblast cell fate throughout the embryo and inhibits alternative cell fates by a yet unknown mechanism.
Figure 2.
During dermal fibroblast cell fate selection, WNT/β-CATENIN signaling regulates several of the key known transcription factors such as Twist1, Twist2/Dermo1, Msx1/2, and Engrailed1 in the dermal progenitors 48 (Table 1). Gene deletion of these transcription factors individually does not lead to change in cell fate, suggesting functional redundancy 49–51. Current data suggest that the Twist family of transcription factors are promising effectors of β-catenin signaling in dermal fibroblast cell fate selection in the entire embryo 34,52,53. First, Twist1 and Twist2 are some of earliest markers of chick and mouse dermal fibroblast progenitors and are also direct transcriptional targets of β-catenin 22,25,31,34,54. Second, TWIST1/2 proteins function to inhibit other mesoderm related lineages such as muscle, bone, and cartilage 55–58. Third, Twist1+/−; Twist2+/− compound heterozygotes and Twist2−/− mice have thinner dermis and sparse hair in perinatal skin 44,59, suggesting functional redundancy between the family members during dermal fibroblast development. Fourth, TWIST1 overexpression in epithelial cells leads to EMT due to loss E-cadherin mediated cell-cell adhesion, increase in motility, change in morphology, and increase in expression of fibroblast proteins, such as fibronectin and vimentin 60. Future conditional genetic experiments designed to overcome the functional redundancy of TWIST1 and TWIST2 will be needed to determine their precise role in dermal fibroblast development.
Table 1.
Transcriptional factors in dermal fibroblast precursors and progenitors
| Genes | Spatiotemporal expression in skin | Phenotype (defects in dermal fibroblast, defects in hair/DC/DP) | Reference |
|---|---|---|---|
| Msx1/ Msx2 | Mesenchymal cells underlying the ectoderm of the back of the trunk from E9.5-E13.5 | The number of hair follicles is reduced to one-third that of wild type, a failure of mammary epithelial invagination, development arrests at the lamina or early bud stages in Msx1−/−, Msx2−/− mutant embryos | (Houzelstein et al., 2000;Satokata et al., 2000; Satokata et al., 1994) |
| Prx1/Prx2 | Expression in the somites was confined primarily to the dorsal dermamyotome | No obvious cutaneous abnormality reported | (Cserjesi et al., 1992; ten Berge et al., 1998; Bergwerff et al., 2000) |
| Sim1 | Sim1 is expressed in medial dermamyotome from E9.5 | No obvious cutaneous abnormality reported | (Guillemot et al., 1993) |
| Twist1/ Twist2 | Twist1/2 are expressed in embryonic mesoderm starting at E9.5 | Twist-2−/− mice also showed notable cutaneous abnormalities; with dramatically thin skin and sparse hair. No obvious cutaneous abnormality reported in Twist1 mutants. | (Li et al., 1995; Chen & Behringer, 1995; Sosic et al., 2003) |
| Engrailed1 (En1) | En1 is expressed in dorsal dermamyotome, lateral plate mesenchyme, and cNCC from E9.5 | No obvious cutaneous abnormality reported | (Atit et al., 2006; Ohtola et al., 2008; Tran et al., 2010; Augustine et al., 1995) |
Does positional memory confer regional identity in embryonic and adult dermal fibroblasts?
Though all dermal fibroblasts appear morphologically similar, they have distinct positional identity and function at different anatomical locations 14,17,61–63. Skin patterning with appendages and pigmentation varies regionally in the dorsum, ventrum, face, and cranium. Several studies show that the embryonic origin of fibroblast confers positional identity and memory for skin patterning and function. Heterotopic recombination experiments across anatomical regions or between species reveal that dermal fibroblasts can reprogram the epidermis 17,21. For instance, scale-forming dermis recombined with feather-forming epidermis gives rise to scales in recombinant skin 17. Similarly, quail cranial dermis promotes quail feather formation in a duck embryo 21. Conversely, studies reprogramming epidermal structures between follicular, interfollicular, and glandular fates also report accompanied cellular identity and functional changes in the dermal compartment. Collins et al. 64 found expression of constitutively active β-catenin in the adult epidermis leads to conversion of adult dermis into neonatal dermal fibroblast identity and function. Overexpression of Noggin, a Bone Morphogenetic Protein (BMP) inhibitor, or ablation of Bmpr1a in the ventral foot pad epidermis leads to conversion of eccrine sweat glands to hair follicles that recruit dermal fibroblasts to form ectopic dermal papilla 65.
Recently, Rinkevich et al., performed a classical tissue recombination experiment to demonstrate cell intrinsic function based on embryonic origin 63. In the adult mouse, oral dermis and dorsal dermis were reciprocally-transplanted in the dorsal skin and oral cavity, respectively. Wounding of the dorsal dermal graft in the oral cavity lead to scar formation which is absent in the oral dermal graft in dorsal wild type skin. This experiment demonstrates that scar forming ability is intrinsic and independent of the host microenvironment. The result can be partly explained by the presence of cellular positional memory that can promote specific gene expression patterns and regulate function 61,66.
The positional identity of dermal fibroblasts in humans is regulated by a combinatorial code of Hox genes established in embryonic development and is stably maintained into adulthood and in vitro 61,62. However, it is not known how the different Hox genes regulate dermal fibroblast function of diverse skin patterning. Future studies will need to investigate whether the genes conferring site-specific positional identity also provide inductive cues to pattern the epidermis in vivo. Taken together, these studies show the intrinsic positional identity in the dermal fibroblasts and their ability to reprogram the epidermis 17,21,63. They also reveal that dermal fibroblast identity and function is plastic and can also be reprogrammed 64, thereby demonstrating active cross-talk between the two layers in embryonic and adult skin.
Dermal fibroblast progenitor differentiation into hair inducing and matrix forming fibroblasts, and perhaps intradermal fat
Starting at E14.5, the specified dermal progenitor further differentiates into its derivatives (Figure 2) 16. As early as E12.5, the dermal progenitors express pan-fibroblast markers, PDGFR-alpha and Engrailed1 (En1) 16,22,67. Around E14.5, these dermal progenitors give rise to papillary and reticular dermal lineages which are distinct in their gene expression pattern with varying functions 16. The papillary dermal progenitors gives rise to papillary dermal fibroblasts (PF) and dermal papilla (DP), and the reticular dermal fibroblast progenitors gives rise to reticular fibroblasts (RF) and dermal white adipose tissue (DWAT). PF and DP play a crucial role in hair follicle morphogenesis and cycling 68,69,15. RF secrete dense collagen fibers that provide structural integrity and strength to the skin. DWAT, also called intradermal fat, is a recently defined cell type that is significantly different from subcutaneous fat in terms of developmental origin and contributes to the skin barrier 70,71. The underlying signaling mechanism(s) for differentiation of papillary and reticular dermal fibroblast lineages are yet unidentified. However, we predict WNT signaling activity levels differ and contribute to the differentiation of the papillary and reticular lineages. From E12 to E14.5, the epidermis expresses canonical WNTs and the upper dermal lineage is Wnt-responsive to the ectodermal WNTs 4,15,72. This in turn supports our putative hypothesis that dermal layers are divided into the top WNThigh and deeper WNTlow regions leading to its further differentiation. This hypothesis remains to be rigorously tested in available mesenchymal β-catenin and Wntless mutants 15,22,72.
During embryonic development, the PF cells have a unique capacity to support hair follicle initiation, but the nature of the primary dermal signal is still elusive 73,74. Classical heterotopic tissue recombination experiments led to the idea that an inductive dermal signal is necessary for hair follicle initiation 75–77. Four decades after this concept was defined, the inductive dermal signal remains elusive 10. The primary dermal signal is likely regulated by WNT/β-CATENIN signaling. Ablation of WNT/β-CATENIN signaling in specified dermal fibroblasts prior to epidermal placode formation leads to absence of placodes and hair follicles 15. The downstream effector(s) of the primary dermal WNT/β-CATENIN signaling is unknown and it may be either molecular or mechanical. The mechanical properties of tissue/cells can regulate cell-cycle, stem cell differentiation, disease progression etc. 78–80. In the chick embryo, the formation of high cell density dermis is thought to be required for feather placode initiation 17. And varying the density of dermal fibroblasts in tissue recombination experiments in vitro revealed that a critical density of dermal fibroblasts was necessary for self-organization of placode formation 73. Recently, Shyer et al. show that tissue symmetry is broken by mechanosensation of β-catenin in the dermal progenitors and is critical for initiating the follicle structure and molecular program in the avian skin 81. It remains to be investigated if mammalian dermal fibroblast progenitors form fibroblast microaggregates in the future hair follicle placode fields which then become stable and mechanically signal to the epithelium to form a placode 73,81. Technological improvements in live cell imaging of the dermal layer should aid in observing small scale cellular movements and cellular density changes prior to placode formation.
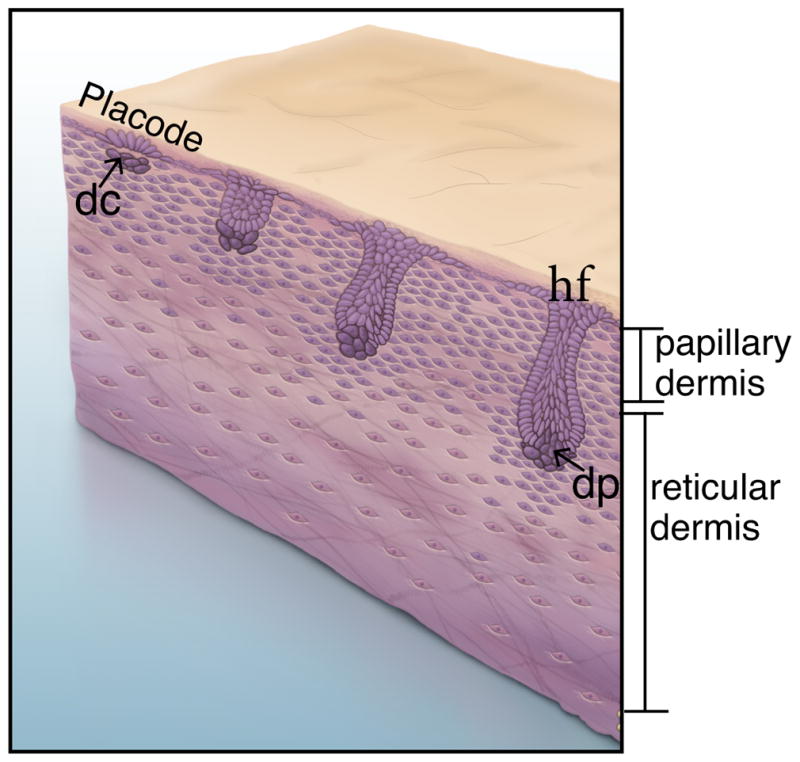
After the epidermal placodes form, clustering of underlying papillary dermal fibroblasts occurs to form the dermal condensate (Figure 3) 73,74,82. Subsequent cross-talk between the epidermal placode and the dermal condensate promotes cutaneous appendage down growth 17. The dermal condensate cells are precursors of the specialized mammalian fibroblasts called the dermal papillae (DP) that reside at the base of the adult hair follicle (Figure. 3) 74,83. Studies in chick embryonic skin suggest that dermal condensates most likely form upon dermal fibroblast rearrangement and migration in response to signals from the ectoderm. During the initial stages of formation, there is plasticity in cell identity of the interfolliclular dermal fibroblasts and fibroblasts in the dermal condensate 73. Dermal condensate formation is dependent on cell migration and cell adhesion, but not cell proliferation 82,84,85. The Scaleless chick mutant shows that placodal signals are required for dermal condensate formation from the papillary dense dermis 86. A variety of secreted molecules are expressed in the placode and can serve as either activators (Shh, FGF, TGFβ2, WNTs, Ectodysplasin) or inhibitors (BMPs) in the placode of the chick and mouse skin 83,87. The target genes of these factors are expressed in dense dermis and later upregulated in the condensate (nuclear β-CATENIN, LEF1) or first appear in the dermal condensate (BMP4/7, NOGGIN, FGF7/10, PDGF-alpha). Signaling factors such as SHH, BMP and TGF-β are shown to be dispensable for hair initiation and dermal condensate formation 5,88,89. Currently, we lack genetic tools to study the emergence of dermal condensate formation in a short interval after placode formation. Thus, questions about how dermal fibroblasts initiate hair and differentiate to dermal condensate fate remain unanswered. Use of single cell RNA-seq could be used to tease out the transcriptional signatures of these rapidly differentiating cell types.
Figure 3.
The DWAT layer in the skin is distinct from the subcutaneous fat layer and can contribute to the skin barrier, wound healing, and hair follicle cycling 11,90. DWAT, is derived from PDGFRα expressing mesenchymal lineage but its precise origin is unknown 67 (Figure 2). The reticular dermal lineage expresses DLK1 from E16.5 16. The preadipocytes at postnatal (P) day2 in the skin express SCA1 and a subset express DLK1. These data suggest that DWAT is derived from the reticular dermal lineage. However, additional in vivo genetic lineage tracing would greatly strengthen our current understanding of the origin of DWAT which has implications for cutaneous homeostasis, healing, and disease. Recently, Plikus et. al. showed alpha-Sma-Cre lineage-marked cells in a wound bed can give rise to DWAT fate 91. The identity of alpha-Sma-Cre lineage marked cells may not be restricted to only myofibroblast cells. The signals required for specifying DWAT fate are also unknown. Sustained activation of dermal WNT/β-CATENIN signaling at E16.5 using the HoxB6CreER or Dlk1CreER lowers the intradermal adipocyte numbers and enhances reticular fibroblast proliferation and ECM production, thereby resulting in dermal fibrosis 92,93. DWAT, unlike other adipose layers, dynamically changes in size during hair cycling and perturbing its ability to regenerate results in cycling defects 90. Thus, there is cross-talk between DWAT, dermal fibroblasts, and follicular cells and it would be interesting to identify other homeostatic mechanisms regulated by DWAT.
Dermal fibroblasts in scar forming and scar-free wound healing
Dermal fibroblasts perform a crucial task in wound healing by populating the wound site to produce ECM which forms a scar 16,94. Selectively eliminating En1 lineage dermal fibroblasts in adult skin leads to delay in wound closure, showing the presence of an intrinsic fibrogenic lineage that is determined during embryonic stages 63. RFs selectively populate wound beds immediately after wounding in comparison to the PFs 16. RFs are naturally selected for the task because of their intrinsic ability to produce thick and organized collagen in comparison to poorly organized and loosely packed ECM of the PFs 95,96. Taken together, these data suggest that the dermal fibroblasts, particularly RFs are important for migrating into and healing the wound. Fibroblast migration to the wound is regulated by inflammatory mediators such as C5a, fibronectin, PDGF, fibroblast growth factor (FGF), and TGF-beta 13,97–99. Recent evidence suggests that DWAT is also necessary. Either absence of mature adipocytes (A-ZIP/F1 transgenic mice 100) in the skin or inhibition of adipogenesis stunts fibroblast recruitment to the wound site leading to delay in wound closure 11.
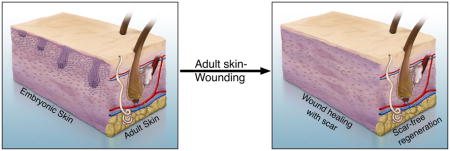
The normal wound healing program results in a scar which is different from normal skin aesthetically, functionally, and morphologically. The scar is composed of poorly reconstituted collagen in dense parallel bundles which affects the homeostatic mechanisms in and around the site. The scar lacks hair, glands, and touch and pressure sensors of the skin (See Graphical Abstract). Thus, it is clinically relevant to understand ways to regenerate scar-free skin.
Wound-induced hair follicle neogenesis (WIHN): partial scar-free wound healing model
Historically, it was presumed that mammals fail to regenerate cutaneous appendages in a wound site, but several reports in the 1940s reported WIHN in rabbits and rats 101. Only in 2007, Ito et al showed hair follicles in the center of large excisional mouse cutaneous wounds occur de novo by recapitulating embryonic hair follicle formation 102. In a recent landmark study, Plikus et al., exploited fibroblast plasticity to regeneration of the intradermal adipocytes in WIHN. They found wound myofibroblast can be reprogrammed to intradermal adipocytes in response to BMPs secreted by de novo hair follicles during WIHN. Thus, WIHN provides a model system to identify the mechanisms regulating hair follicle neogenesis in adult skin and identify cell types to manipulate for complete skin regeneration 91. Both dermal and epidermal WNT/β-CATENIN signaling are crucial in embryonic hair follicle initiation 15,72,102–104. Similarly, either ablation or overexpression of epidermal WNTs in adult mice resulted in absent or enhanced WIHN respectively 102,105. WNT signaling in dermal fibroblasts is active in late wound stages and is induced by FGF9 from γδ T-cells that populate the wound site 12. Based on these observations, it is tempting to speculate that dermal WNT signaling is necessary for WIHN, however conditional ablation of dermal β-catenin lead to enhanced WIHN106 These results raise new questions: (i) What are the downstream effectors of dermal WNT/β-CATENIN signaling regulating WIHN? (ii) How are they similar and different from β-catenin effectors in embryonic hair follicle initiation? (iii) Is there a redundant mechanism for WIHN that is WNT-independent?
Scar-free wound healing and skin regeneration
In the effort to rejuvenate and engineer skin as an organ, mechanisms of scar-free wound healing in adult skin need to be fully understood. A cutaneous wound can regenerate without scar in specific contexts 107. The human embryo is capable of scar-free skin wound healing, but this ability is lost around the third trimester of gestation 108. The difference can be partly explained by the more mature innate immune system response and subsequent gene expression changes in the wound bed of adult skin. 109. Excisional wounds in a neonatal PU.1 null mouse that lacks macrophages and functional neutrophils exhibit scar-less healing as seen in embryos 110. Ablation of macrophages recruited during the initial inflammatory phase of wounds leads to reduction in scar formation 111,112. Similarly, embryonic day 18 mouse wounds lacking mast cells heal with reduced scarring 113. Furthermore, multiple studies have demonstrated that the number of mast cells and dermal dendritic cells that express FXIIIa are significantly more in keloids and hypertrophic scars suggesting that they may play a causative role in scarring 111,114.
In embryonic mice, scar-free wounds express less TGF-beta1 than adult wounds and the ratio of TGF-beta1/TGF-beta3 is higher in a scarring wound 115,116. Consistently, oral cavity wounds that regenerate without scarring in adults exhibit lower immune infiltrate and TGF-beta1/TGF-beta3 ratio compared to external cutaneous wounds. 117,118. Therefore, a recombinant human TGF-beta3, Avotermin, has been escalated to clinical trials in order to prevent scarring post surgeries. It was successful at reducing wound induced scarring in phase I/II studies and improving efficacy at lower dose remains to to be developed 119. These findings suggest that the composition of various TGF-betas isoforms regulate scarring and many other drugs modulating this pathway are under clinical trials in fibrotic and wound healing setting 120. TGF-betas are secreted by immune cells and platelets during early stages of wound healing and later by fibroblasts 121,122. Furthermore, the ratio of COLLAGEN Type 3 to COLLAGEN Type 1 secreted by dermal fibroblasts is higher in fetal wounds than postnatal wounds 123,124. Together, these data suggest if gene expression changes in adult dermal fibroblasts can be altered to resemble early stage embryos, we might achieve scar-less wound healing.
Generally, adult mammals lack the ability to regenerate skin fully, however an upcoming mammalian model, Acomys, the African spiny mouse can regenerate skin from wounds as large as 60% of its body surface complete with follicles, glands and DWAT 125 (See Graphical Abstract). Wounded skin of Acomys exhibit modest inflammation to wounding and the ECM profile resembles early stage human fetal skin with higher levels of COLLAGENS 3α1, 5α1, 5α2 126. There is modest upregulation of CXCL cytokines in Acomys compared to a significant increase in adult murine wounds. Acomys dermal fibroblasts secrete matrix metalloproteinases (MMP) and other enzymes that catabolize collagen, leading to faster ECM remodeling and enhanced cell migration 126,127. Similarly the expression of MMPs correlate with the ability of skin regeneration in fetal rats 128. Alpha-SMA expressing myofibroblasts that are implicated in scar forming ECM are absent in Acomys ear skin wounds in comparison to mice 125. All these factors together may contribute to scar-free wound healing in Acomys. In essence, Acomys skin retains the early embryonic wounding response and achieves scar-free healing throughout its lifetime. By and large, it can be hypothesized that a treatment targeted towards chemokine and TGF-beta1 inhibition, along with increased ECM degradation, is the key to scar-free skin. Though Acomys provides crucial gene candidates, the upstream regulators of the observed effects are unknown. Deeper understanding of skin regeneration in Acomys will move us closer towards developing scar-free wound healing remedies in humans.
Concluding remarks
Dermal fibroblasts serve as a model cell type in understanding various regulatory processes in development and healing. Numerous new insights about them are covered in this review, but there are many more questions that need to be addressed in the future: (i) Though early dermal progenitors give rise to papillary and reticular dermis, the signaling pathways that specify these derivatives are not known. Because multiple cell types express PDGFR-alpha, more accurate lineage maps are needed to understand the differentiation program of dermal derivatives such as DWAT. (ii) We need to identify signals underlying small and large scale cellular movement of dermal fibroblast progenitors during migration and preceding hair follicle initiation. (iii) The HOX code can impart positional memory in adult human fibroblasts, but its role in guiding skin patterning in vivo remains to be tested. (iv) The nature of the primary inductive signal from the dermis to epidermis prior to placode formation needs further investigation. (v) A deeper understanding of the regulatory mechanisms and downstream signals involved in scar-free wound healing models will be needed for treating scars in humans.
Acknowledgments
We thank Derrik Nau (graphical abstract), James Furgeson, and Charlotte Lo for the artwork. We thank all the Atit lab members, Dr. Martin Basch for their critical reading of the manuscript and valuable discussions.
Funding:
RA is funded by the National Institutes of Health-NIDCR (DE-18470)
Footnotes
Competing Interest Statement:
The authors declare no competing financial interest.
References
- 1.Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002;3:199–209. doi: 10.1038/nrg758. [DOI] [PubMed] [Google Scholar]
- 2.Lim X, Nusse R. Wnt Signaling in Skin Development, Homeostasis, and Disease. Cold Spring Harb Perspect Biol. 2012;5 doi: 10.1101/cshperspect.a008029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu XJ, et al. BMP-FGF signaling axis mediates Wnt-induced epidermal stratification in developing mammalian skin. PLoS Genet. 2014;10:e1004687. doi: 10.1371/journal.pgen.1004687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, et al. Reciprocal requirements for EDA/EDAR/NF-kappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Dev Cell. 2009;17:49–61. doi: 10.1016/j.devcel.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genander M, et al. BMP signaling and its pSMAD1/5 target genes differentially regulate hair follicle stem cell lineages. Cell Stem Cell. 2014;15:619–633. doi: 10.1016/j.stem.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouspenskaia T, Matos I, Mertz AF, Fiore VF, Fuchs E. WNT-SHH Antagonism Specifies and Expands Stem Cells prior to Niche Formation. Cell. 2016;164:156–169. doi: 10.1016/j.cell.2015.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes & Development. 2008;22:543–557. doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kandyba E, et al. Competitive balance of intrabulge BMP/Wnt signaling reveals a robust gene network ruling stem cell homeostasis and cyclic activation. Proceedings of the National Academy of Sciences. 2013;110:1351–1356. doi: 10.1073/pnas.1121312110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kretzschmar K, Cottle DL, Schweiger PJ, Watt FM. The Androgen Receptor Antagonizes Wnt/β-Catenin Signaling in Epidermal Stem Cells. J Invest Dermatol. 2015;135:2753–2763. doi: 10.1038/jid.2015.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sennett R, Rendl M. Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Seminars in Cell & Developmental Biology. 2012;23:917–927. doi: 10.1016/j.semcdb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt BA, Horsley V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development. 2013;140:1517–1527. doi: 10.1242/dev.087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gay D, et al. Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat Med. 2013;19:916–923. doi: 10.1038/nm.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shook B, Xiao E, Kumamoto Y, Iwasaki A, Horsley V. CD301b+ Macrophages Are Essential for Effective Skin Wound Healing. J Invest Dermatol. 2016;136:1885–1891. doi: 10.1016/j.jid.2016.05.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Driskell RR, Watt FM. Understanding fibroblastheterogeneity in the skin. Trends Cell Biol. 2015;25:92–99. doi: 10.1016/j.tcb.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Chen D, Jarrell A, Guo C, Lang R, Atit R. Dermal β-catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development. 2012;139:1522–1533. doi: 10.1242/dev.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Driskell RR, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sengel P. Morphogenesis of skin. Cambridge Univ Pr; 1976. [Google Scholar]
- 18.Sengel P, Dhouailly D. Tissue Interactions in Amniote skin Development in Cell Interactions in Differentiation. Vol. 51. Academic Press; 1977. pp. 153–169. [Google Scholar]
- 19.Dhouailly D, Rogers GE, Sengel P. The specification of feather and scale protein synthesis in epidermal-dermal recombinations. Developmental Biology. 1978;65:58–68. doi: 10.1016/0012-1606(78)90179-3. [DOI] [PubMed] [Google Scholar]
- 20.Dhouailly D. Wilhelm Roux’s archives of developmental biology. Vol. 181. Springer Verlag; 1977. pp. 3–10. [Google Scholar]
- 21.Eames BF, Schneider RA. Quail-duck chimeras reveal spatiotemporal plasticity in molecular and histogenic programs of cranial feather development. Development. 2005;132:1499–1509. doi: 10.1242/dev.01719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atit R, et al. β-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Developmental Biology. 2006;296:164–176. doi: 10.1016/j.ydbio.2006.04.449. [DOI] [PubMed] [Google Scholar]
- 23.Mauger A. The role of somitic mesoderm in the development of dorsal plumage in chick embryos. I. Origin regulative capacity and determination of the plumage-forming mesoderm. J Embryol Exp Morphol. 1972;28:313–341. [PubMed] [Google Scholar]
- 24.Sengel P, Mauger A. Peridermal cell patterning in the feather-forming skin of the chick embryo. Developmental Biology. 1976;51:166–171. doi: 10.1016/0012-1606(76)90132-9. [DOI] [PubMed] [Google Scholar]
- 25.Ohtola J, et al. β-catenin has sequential roles in the survival and specification of ventral dermis. Development. 2008;135:2321–2329. doi: 10.1242/dev.021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nowicki JL, Takimoto R, Burke AC. The lateral somitic frontier: dorso-ventral aspects of anterio-posterior regionalization in avian embryos. Mechanisms of Development. 2003;120:227–240. doi: 10.1016/s0925-4773(02)00415-x. [DOI] [PubMed] [Google Scholar]
- 27.Christ B, Jacob HJ, Jacob M, Wachtler F. On the origin, distribution and determination of avian limb mesenchymal cells. 1982;110(Pt B):281–291. [PubMed] [Google Scholar]
- 28.Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Developmental Biology. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- 29.Couly G, Le Douarin NM. The fate map of the cephalic neural primordium at the presomitic to the 3-somite stage in the avian embryo. Development. 1988;103(Suppl):101–113. doi: 10.1242/dev.103.Supplement.101. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida T, Vivatbutsiri P, Morriss-Kay G, Saga Y, Iseki S. Cell lineage in mammalian craniofacial mesenchyme. Mechanisms of Development. 2008;125:797–808. doi: 10.1016/j.mod.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Tran TH, et al. Role of canonical Wnt signaling/β-catenin via Dermo1 in cranial dermal cell development. Development. 2010;137:3973–3984. doi: 10.1242/dev.056473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scaal M, Christ B. Formation and differentiation of the avian dermomyotome. Anat Embryol. 2004;208:411–424. doi: 10.1007/s00429-004-0417-y. [DOI] [PubMed] [Google Scholar]
- 33.Ben-Yair R, Kahane N, Kalcheim C. Coherent development of dermomyotome and dermis from the entire mediolateral extent of the dorsal somite. Development. 2003;130:4325–4336. doi: 10.1242/dev.00667. [DOI] [PubMed] [Google Scholar]
- 34.Olivera-Martinez I, Thélu J, Dhouailly D. Molecular mechanisms controlling dorsal dermis generation from the somitic dermomyotome. Int J Dev Biol. 2004;48:93–101. doi: 10.1387/ijdb.15272374. [DOI] [PubMed] [Google Scholar]
- 35.Brill G, et al. Epithelial-mesenchymal conversion of dermatome progenitors requires neural tube-derived signals: characterization of the role of Neurotrophin-3. Development. 1995;121:2583–2594. doi: 10.1242/dev.121.8.2583. [DOI] [PubMed] [Google Scholar]
- 36.Morosan-Puopolo G, et al. Wnt11 is required for oriented migration of dermogenic progenitor cells from the dorsomedial lip of the avian dermomyotome. PLoS ONE. 2014;9:e92679. doi: 10.1371/journal.pone.0092679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fliniaux I, Viallet JP, Dhouailly D. Signaling dynamics of feather tract formation from the chick somatopleure. Development. 2004;131:3955–3966. doi: 10.1242/dev.01263. [DOI] [PubMed] [Google Scholar]
- 38.Baker CV, Bronner-Fraser M, Le Douarin NM, Teillet MA. Early- and late-migrating cranial neural crest cell populations have equivalent developmental potential in vivo. Development. 1997;124:3077–3087. doi: 10.1242/dev.124.16.3077. [DOI] [PubMed] [Google Scholar]
- 39.Trainor PA, Krumlauf R. Patterning the cranial neural crest: hindbrain segmentation and Hox gene plasticity. Nat Rev Neurosci. 2000;1:116–124. doi: 10.1038/35039056. [DOI] [PubMed] [Google Scholar]
- 40.Minoux M, Rijli FM. Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development. 2010;137:2605–2621. doi: 10.1242/dev.040048. [DOI] [PubMed] [Google Scholar]
- 41.Davy A, Soriano P. Ephrin-B2 forward signaling regulates somite patterning and neural crest cell development. Developmental Biology. 2007;304:182–193. doi: 10.1016/j.ydbio.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gammill LS, Gonzalez C, Bronner-Fraser M. Neuropilin 2/semaphorin 3F signaling is essential for cranial neural crest migration and trigeminal ganglion condensation. Dev Neurobiol. 2007;67:47–56. doi: 10.1002/dneu.20326. [DOI] [PubMed] [Google Scholar]
- 43.He F, Soriano P. A critical role for PDGFRα signaling in medial nasal process development. PLoS Genet. 2013;9:e1003851. doi: 10.1371/journal.pgen.1003851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tukel T, et al. Homozygous nonsense mutations in TWIST2 cause Setleis syndrome. Am J Hum Genet. 2010;87:289–296. doi: 10.1016/j.ajhg.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grzeschik KH, et al. Deficiency of PORCN, a regulator of Wnt signaling, is associated with focal dermal hypoplasia. Nat Genet. 2007;39:833–835. doi: 10.1038/ng2052. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, et al. Mutations in X-linked PORCN, a putative regulator of Wnt signaling, cause focal dermal hypoplasia. Nat Genet. 2007;39:836–838. doi: 10.1038/ng2057. [DOI] [PubMed] [Google Scholar]
- 47.Goodnough LH, et al. Distinct Requirements for Cranial Ectoderm and Mesenchyme-Derived Wnts in Specification and Differentiation of Osteoblast and Dermal Progenitors. PLoS Genet. 2014;10:e1004152. doi: 10.1371/journal.pgen.1004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Budnick I, et al. Defining the identity of mouse embryonic dermal fibroblasts. genesis. 2016;54:415–430. doi: 10.1002/dvg.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Satokata I, et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;24:391–395. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- 50.Houzelstein D, Chéraud Y, Auda-Boucher G, Fontaine-Pérus J, Robert B. The expression of the homeobox gene Msx1 reveals two populations of dermal progenitor cells originating from the somites. Development. 2000;127:2155–2164. doi: 10.1242/dev.127.10.2155. [DOI] [PubMed] [Google Scholar]
- 51.Kimmel RA, et al. Two lineage boundaries coordinate vertebrate apical ectodermal ridge formation. Genes & Development. 2000;14:1377–1389. [PMC free article] [PubMed] [Google Scholar]
- 52.Chen ZF, Behringer RR. twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes & Development. 1995;9:686–699. doi: 10.1101/gad.9.6.686. [DOI] [PubMed] [Google Scholar]
- 53.Soo K, et al. Twist function is required for the morphogenesis of the cephalic neural tube and the differentiation of the cranial neural crest cells in the mouse embryo. Developmental Biology. 2002;247:251–270. doi: 10.1006/dbio.2002.0699. [DOI] [PubMed] [Google Scholar]
- 54.Goodnough LH, et al. Twist1 mediates repression of chondrogenesis by beta-catenin to promote cranial bone progenitor specification. Development. 2012;139:4428–4438. doi: 10.1242/dev.081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cserjesi P, et al. MHox: a mesodermally restricted homeodomain protein that binds an essential site in the muscle creatine kinase enhancer. Development. 1992;115:1087–1101. doi: 10.1242/dev.115.4.1087. [DOI] [PubMed] [Google Scholar]
- 56.Gong XQ, Li L. Dermo-1, a multifunctional basic helix-loop-helix protein, represses MyoD transactivation via the HLH domain, MEF2 interaction, and chromatin deacetylation. J Biol Chem. 2002;277:12310–12317. doi: 10.1074/jbc.M110228200. [DOI] [PubMed] [Google Scholar]
- 57.Scaal M, Füchtbauer EM, Brand-Saberi B. cDermo-1 expression indicates a role in avian skin development. Anat Embryol. 2001;203:1–7. doi: 10.1007/pl00008244. [DOI] [PubMed] [Google Scholar]
- 58.Li L, Cserjesi P, Olson EN. Dermo-1: a novel twist-related bHLH protein expressed in the developing dermis. Developmental Biology. 1995;172:280–292. doi: 10.1006/dbio.1995.0023. [DOI] [PubMed] [Google Scholar]
- 59.Sosic D, Richardson JA, Yu K, Ornitz DM, Olson EN. Twist regulates cytokine gene expression through a negative feedback loop that represses NF-kappaB activity. Cell. 2003;112:169–180. doi: 10.1016/s0092-8674(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 60.Yang J, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 61.Chang HY, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang HY. Anatomic demarcation of cells: genes to patterns. Science. 2009;326:1206–1207. doi: 10.1126/science.1175686. [DOI] [PubMed] [Google Scholar]
- 63.Rinkevich Y, et al. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science. 2015;348:aaa2151–aaa2151. doi: 10.1126/science.aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Collins CA, Kretzschmar K, Watt FM. Reprogramming adult dermis to a neonatal state through epidermal activation of β-catenin. Development. 2011;138:5189–5199. doi: 10.1242/dev.064592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu CP, Polak L, Keyes BE, Fuchs E. Spatiotemporal antagonism in mesenchymal-epithelial signaling in sweat versus hair fate decision. Science. 2016;354 doi: 10.1126/science.aah6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rinn JL, Bondre C, Gladstone HB, Brown PO, Chang HY. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006;2:e119. doi: 10.1371/journal.pgen.0020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15:302–308. doi: 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou L, Yang K, Wickett RR, Andl T, Zhang Y. Dermal sheath cells contribute to postnatal hair follicle growth and cycling. Journal of Dermatological Science. 2016;82:129–131. doi: 10.1016/j.jdermsci.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 69.Fujiwara H, et al. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. 2011;144:577–589. doi: 10.1016/j.cell.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Driskell RR, Jahoda CAB, Chuong CM, Watt FM, Horsley V. Defining dermal adipose tissue. Exp Dermatol. 2014;23:629–631. doi: 10.1111/exd.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jahoda CAB, Gilmore AC. What Lies Beneath: Wnt/β-Catenin Signaling and Cell Fate in the Lower Dermis. J Invest Dermatol. 2016;136:1084–1087. doi: 10.1016/j.jid.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 72.Fu J, Hsu W. Epidermal Wnt Controls Hair Follicle Induction by Orchestrating Dynamic Signaling Crosstalk between the Epidermis and Dermis. Journal of Investigative Dermatology. 2012 doi: 10.1038/jid.2012.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang TX, Jung HS, Widelitz RB, Chuong CM. Self-organization of periodic patterns by dissociated feather mesenchymal cells and the regulation of size, number and spacing of primordia. Development. 1999;126:4997–5009. doi: 10.1242/dev.126.22.4997. [DOI] [PubMed] [Google Scholar]
- 74.Hardy MH. The secret life of the hair follicle. Trends in genetics : TIG. 1992;8:55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- 75.Kollar EJ. The induction of hair follicles by embryonic dermal papillae. J Invest Dermatol. 1970;55:374–378. doi: 10.1111/1523-1747.ep12260492. [DOI] [PubMed] [Google Scholar]
- 76.Dhouailly D. Dermo-epidermal interactions between birds and mammals: differentiation of cutaneous appendages. J Embryol Exp Morphol. 1973;30:587–603. [PubMed] [Google Scholar]
- 77.Millar SE. An Ideal Society? Neighbors of Diverse Origins Interact to Create and Maintain Complex Mini-Organs in the Skin. Plos Biol. 2005;3:e372. doi: 10.1371/journal.pbio.0030372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Klein EA, et al. Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr Biol. 2009;19:1511–1518. doi: 10.1016/j.cub.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Engler AJ, Sweeney HL, Discher DE, Schwarzbauer JE. Extracellular matrix elasticity directs stem cell differentiation. J Musculoskelet Neuronal Interact. 2007;7:335. [PubMed] [Google Scholar]
- 80.Janmey PA, Miller RT. Mechanisms of mechanical signaling in development and disease. Journal of Cell Science. 2011;124:9–18. doi: 10.1242/jcs.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shyer AE, et al. Emergent cellular self-organization and mechanosensation initiate follicle pattern in the avian skin. Science. 2017;357:811–815. doi: 10.1126/science.aai7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Michon F, Charveron M, Dhouailly D. Dermal condensation formation in the chick embryo: requirement for integrin engagement and subsequent stabilization by a possible notch/integrin interaction. Dev Dyn. 2007;236:755–768. doi: 10.1002/dvdy.21080. [DOI] [PubMed] [Google Scholar]
- 83.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 84.Wessells NK. Morphology and proliferation during early feather development. Developmental Biology. 1965;12:131–153. doi: 10.1016/0012-1606(65)90025-4. [DOI] [PubMed] [Google Scholar]
- 85.Jiang TX, Chuong CM. Mechanism of skin morphogenesis. I. Analyses with antibodies to adhesion molecules tenascin, N-CAM, and integrin. Developmental Biology. 1992;150:82–98. doi: 10.1016/0012-1606(92)90009-6. [DOI] [PubMed] [Google Scholar]
- 86.Sengel P, ABBOTT UK. In vitro studies with the Scaleless mutant. Interactions during Feather and scale differentiation. J Hered. 1963;54:255–262. doi: 10.1093/oxfordjournals.jhered.a107261. [DOI] [PubMed] [Google Scholar]
- 87.Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27:247–261. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- 88.Woo WM, Zhen HH, Oro AE. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes & Development. 2012;26:1235–1246. doi: 10.1101/gad.187401.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jamora C, et al. A signaling pathway involving TGF-beta2 and snail in hair follicle morphogenesis. Plos Biol. 2005;3:e11. doi: 10.1371/journal.pbio.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Festa E, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Plikus MV, et al. Regeneration of fat cells from myofibroblasts during wound healing. Science. 2017;355:748–752. doi: 10.1126/science.aai8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hamburg EJ, Atit RP. Sustained Beta-Catenin Activity in Dermal Fibroblasts Is Sufficient for Skin Fibrosis. Journal of Investigative Dermatology. 2012;132:2469–2472. doi: 10.1038/jid.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mastrogiannaki M, et al. β-Catenin Stabilization in Skin Fibroblasts Causes Fibrotic Lesions by Preventing Adipocyte Differentiation of theReticular Dermis. J Invest Dermatol. 2016;136:1130–1142. doi: 10.1016/j.jid.2016.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Darby IA, Laverdet B, Bonté F, Desmoulière A. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol. 2014;7:301–311. doi: 10.2147/CCID.S50046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sorrell JM, Caplan AI. Fibroblast heterogeneity: more than skin deep. Journal of Cell Science. 2004;117:667–675. doi: 10.1242/jcs.01005. [DOI] [PubMed] [Google Scholar]
- 96.Sorrell JM, Baber MA, Caplan AI. Clonal characterization of fibroblasts in the superficial layer of the adult human dermis. Cell Tissue Res. 2007;327:499–510. doi: 10.1007/s00441-006-0317-y. [DOI] [PubMed] [Google Scholar]
- 97.Steed DL. The role of growth factors in wound healing. Surg Clin North Am. 1997;77:575–586. doi: 10.1016/s0039-6109(05)70569-7. [DOI] [PubMed] [Google Scholar]
- 98.Bryan D, Walker KB, Ferguson M, Thorpe R. Cytokine gene expression in a murine wound healing model. Cytokine. 2005;31:429–438. doi: 10.1016/j.cyto.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 99.Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg. 2004;187:11S–16S. doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 100.Moitra J, et al. Life without white fat: a transgenic mouse. Genes & Development. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang X, et al. Principles and mechanisms of regeneration in the mouse model for wound-induced hair follicle neogenesis. Regeneration. 2015;2:169–181. doi: 10.1002/reg2.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ito M, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 103.Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- 104.Tsai SY, et al. Developmental Biology. Developmental Biology. 2014;385:179–188. doi: 10.1016/j.ydbio.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Myung PS, Takeo M, Ito M, Atit RP. Epithelial Wnt Ligand Secretion Is Required for Adult Hair Follicle Growth and Regeneration. Journal of Investigative Dermatology. 2012 doi: 10.1038/jid.2012.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rognoni E, et al. Inhibition of β-catenin signalling in dermal fibroblasts enhances hair follicle regeneration during wound healing. Development. 2016;143:2522–2535. doi: 10.1242/dev.131797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 108.Bullard KM, Longaker MT, Lorenz HP. Fetal wound healing: current biology. World J Surg. 2003;27:54–61. doi: 10.1007/s00268-002-6737-2. [DOI] [PubMed] [Google Scholar]
- 109.Mescher AL, Neff AW. Regenerative capacity and the developing immune system. Adv Biochem Eng Biotechnol. 2005;93:39–66. doi: 10.1007/b99966. [DOI] [PubMed] [Google Scholar]
- 110.Martin P, et al. Wound healing in the PU.1 null mouse--tissue repair is not dependent on inflammatory cells. Curr Biol. 2003;13:1122–1128. doi: 10.1016/s0960-9822(03)00396-8. [DOI] [PubMed] [Google Scholar]
- 111.Lucas T, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 112.Brancato SK, Albina JE. Wound macrophages as key regulators of repair: origin, phenotype, and function. Am J Pathol. 2011;178:19–25. doi: 10.1016/j.ajpath.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Smith CJ, Smith JC, Finn MC. The possible role of mast cells (allergy) in the production of keloid and hypertrophic scarring. J Burn Care Rehabil. 1987;8:126–131. doi: 10.1097/00004630-198703000-00008. [DOI] [PubMed] [Google Scholar]
- 114.Ong CT, et al. Comparative proteomic analysis between normal skin and keloid scar. Br J Dermatol. 2010;162:1302–1315. doi: 10.1111/j.1365-2133.2010.09660.x. [DOI] [PubMed] [Google Scholar]
- 115.Krummel TM, et al. Transforming growth factor beta (TGF-beta) induces fibrosis in a fetal wound model. J Pediatr Surg. 1988;23:647–652. doi: 10.1016/s0022-3468(88)80638-9. [DOI] [PubMed] [Google Scholar]
- 116.Hsu M, Peled ZM, Chin GS, Liu W, Longaker MT. Ontogeny of expression of transforming growth factor-beta 1 (TGF-beta 1), TGF-beta 3, and TGF-beta receptors I and II in fetal rat fibroblasts and skin. Plastic and Reconstructive Surgery. 2001;107:1787–94. doi: 10.1097/00006534-200106000-00023. discussion 1795–6. [DOI] [PubMed] [Google Scholar]
- 117.Schrementi ME, Ferreira AM, Zender C, DiPietro LA. Site-specific production of TGF-beta in oral mucosal and cutaneous wounds. Wound Repair Regen. 2008;16:80–86. doi: 10.1111/j.1524-475X.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- 118.Szpaderska AM, Zuckerman JD, DiPietro LA. Differential injury responses in oral mucosal and cutaneous wounds. J Dent Res. 2003;82:621–626. doi: 10.1177/154405910308200810. [DOI] [PubMed] [Google Scholar]
- 119.Little JA, et al. TGF β 3 immunoassay standardization: comparison of NIBSC reference preparation code 98/608 with avotermin lot 205-0505-005. J Immunoassay Immunochem. 2012;33:66–81. doi: 10.1080/15321819.2011.600402. [DOI] [PubMed] [Google Scholar]
- 120.Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 122.Plasari G, et al. Nuclear factor I-C links platelet-derived growth factor and transforming growth factor beta1 signaling to skin wound healing progression. Mol Cell Biol. 2009;29:6006–6017. doi: 10.1128/MCB.01921-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Beanes SR, et al. Confocal microscopic analysis of scarless repair in the fetal rat: defining the transition. Plastic and Reconstructive Surgery. 2002;109:160–170. doi: 10.1097/00006534-200201000-00026. [DOI] [PubMed] [Google Scholar]
- 124.Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast Reconstr Surg. 2010;126:1172–1180. doi: 10.1097/PRS.0b013e3181eae781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Seifert AW, et al. Skin shedding and tissue regeneration in African spiny mice (Acomys) Nature. 2012;489:561–565. doi: 10.1038/nature11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brant JO, Lopez MC, Baker HV, Barbazuk WB, Maden M. A Comparative Analysis of Gene Expression Profiles during Skin Regeneration in Mus and Acomys. PLoS ONE. 2015;10:e0142931. doi: 10.1371/journal.pone.0142931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lauer-Fields JL, Juska D, Fields GB. Matrix metalloproteinases and collagen catabolism. Biopolymers. 2002;66:19–32. doi: 10.1002/bip.10201. [DOI] [PubMed] [Google Scholar]
- 128.Dang CM, et al. Scarless fetal wounds are associated with an increased matrix metalloproteinase-to-tissue-derived inhibitor of metalloproteinase ratio. Plastic and Reconstructive Surgery. 2003;111:2273–2285. doi: 10.1097/01.PRS.0000060102.57809.DA. [DOI] [PubMed] [Google Scholar]